Abstract
Human herpes viruses have latency and lytic replication phases in their lifecycle. Proper regulation of herpes viral lifecycle is essential for the evasion of host immune surveillance and development of their related diseases. Recent advancements indicate a role of a novel class of viral non-coding RNAs, microRNAs (miRNAs), in the fine-tuning of herpes viral lifecycle. So far, herpes viral miRNAs have been shown to promote viral latency by inhibiting viral lytic replication either through direct targeting of key viral replication genes or through manipulation of host pathways that regulate viral lifecycle. The oncogenic Kaposi's sarcoma-associated herpes virus (KSHV) has adapted both strategies to control viral latency. Our recent study has identified a KSHV miRNA that inhibits viral lytic replication by upregulating the NF-κB pathway.
MicroRNAs (miRNAs) are ∼22 nucleotide (nt) long non-coding RNAs that primarily regulate gene expression at the post-transcriptional level by binding to the 3′ untranslated region (3′UTR) of target messenger RNAs (mRNAs).Citation1 miRNAs genes are first transcribed into primary miRNAs (pri-miRNAs) by RNA polymerase II. The RNase III enzyme Drosha then recognizes and cleaves the pri-miRNAs into precursor miRNAs (pre-miRNAs), which are transported into cytoplasm and further processed by another RNase III enzyme named Dicer to produce mature miRNAs. One strand of the mature miRNAs is incorporated into the RNA-induced silencing complex (RISC) to inhibit the translation of target mRNAs.Citation1 miRNAs-mediated degradation and epigenetic silencing of the target mRNAs, while not common, have also been described.Citation2 So far, miRNAs have been found to regulate diverse biological processes including cell cycle, development, differentiation and metabolism.Citation1 Interestingly, several viruses, mostly herpesviruses, also encode miRNAs.Citation3 Recent studies have shown that these viral miRNAs participate in the regulation of herpesvirus lifecycle.
Herpesviruses are enveloped, double-stranded DNA viruses. There are 8 human herpesviruses: the alpha-subfamily includes herpes simplex virus type 1 and 2 (HSV-1 and HSV-2) and varicella zoster virus (VZV); the beta-subfamily include cytomegalovirus (CMV) and human herpesvirus type 6 and 7 (HHV-6 and HHV-7); and the gamma-subfamily includes Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV). Human herpesviruses are associated with a variety of important diseases particularly in immunocompromised hosts such as those with HIV infection and those undergoing iatrogenic organ transplantation.Citation4 Herpesviruses have two distinct phases in their lifecycle: latency and lytic replication. Following primary infection, herpesvirus establishes a persistent latent infection in the host cells. During latency, the virus persists as an episome in the nucleus and expresses no or only a limited number of genes. Therefore, latency allows for the virus to effectively evade host immune surveillance and establish a lifelong persistent infection. Under certain conditions such as immunosuppression, the virus can reactivate into lytic replication resulting in the expression of viral lytic genes and production of infectious virions.Citation4
Interestingly, the majority of viral miRNAs identified are from herpesviruses, suggesting that viral miRNAs may play important roles in the unique lifecycle of these viruses. A possible role of miRNAs in regulating viral lytic replication has been suggested for miRNAs encoded CMV and HSVs ().Citation3 Indeed, the CMV-encoded miRNAs were found to regulate the virus transactivator for lytic replication while the HSV-1-encoded miRNAs downregulates the viral immediate early (IE) gene products to inhibit lytic replication.Citation5–Citation7 Grey and colleagues applied a computational approach to predict the potential binding sites within the 3′UTRs of CMV transcripts. Three of the 14 CMV miRNA targets including the major IE gene encoding IE72 (UL123, IE1), UL112/113 and UL120/121 were validated.Citation5 Transfection of a synthetic miR-UL112-1 RNA duplex into cells prior to viral infection inhibit the expression of IE72 and viral DNA replication.Citation5 Murphy and colleagues also used a computational approach to predict the potential viral targets of herpesviral miRNAs.Citation6 By using reverse genetic approach, they confirmed that CMV miR-UL112-1 targets IE1 mRNA to inhibit viral lytic replication. HSV-1 and HSV-2 miR-H2 are transcribed antisense to IE gene ICP0 and downregulate ICP0 protein expression.Citation7,Citation8 ICP0 is required for efficient initiation of lytic infection and reactivation from latency. Additionally, HSV-1 miR-H6 inhibits the second HSV-1 transcription factor ICP4.Citation7 Another IE gene product ICP34.Citation5 of HSV-2, a key viral neurovirulence factor, is also targeted by the miR-I, which is situated antisense to the gene.Citation9 These results indicate that miRNAs encoded by HSVs facilitate the establishment and maintenance of viral latency by functioning as a molecular switch to turn on and off of the IE genes. The EBV-encoded miR-BART2 transcribed antisense to the BALF5 transcript is perfectly complementary to the BALF5 3′ UTR, thus may target this mRNA for degradation. The EBV-encoded BALF5 is a viral DNA polymerase that is essential for viral DNA replication. Barth and colleagues confirmed that miR-BART2 indeed inhibits the activity of a luciferase reporter containing the BALF5 3′UTR, and reduces the endogenous BALF5 protein level in EBV-infected cells.Citation10 The expression levels of miR-BART2 and BALF5 were inversely correlated. While miR-BART2 is expressed in latency, BALF5 is not present during latent infection. In contrast, an increase in the miR-BART2 level in EBV-infected cells undergoing lytic replication resulted in a ∼50% reduction in the BLAF5 protein level and ∼20% reduction of virus production.Citation10 Collectively, these results illustrate a strategy by which herpesviruses maintain viral latency by encoding specific miRNAs to inhibit the expression of viral lytic genes.
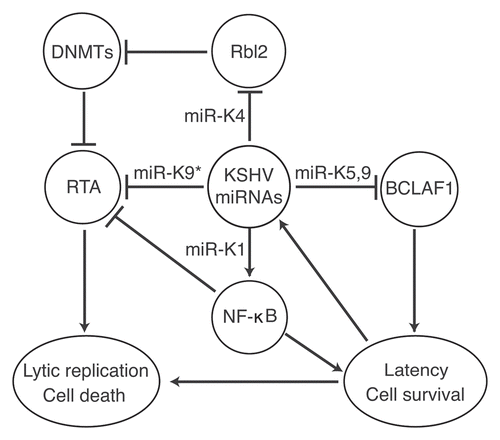
KSHV is an oncogenic herpesvirus etiologically associated with Kaposi's sarcoma (KS), the most common tumor in untreated AIDS patients. KSHV is also associated with primary effusion lymphoma (PEL) and a subset of multicentric Castleman's disease (MCD), two rare lymphoproliferative malignancies.Citation4 KSHV encodes 12 miRNAs genes (miR-K1–12) located in the latency-associated region. miR-K1–9 and miR-K11 are clustered together in an noncoding region while miR-K10 and -K12 are located in the coding region and 3′-UTR of ORFK12, respectively.Citation3 Similar to other herpesviruses, KSHV has two phases of lifecycle. In KSHV-related malignancies, a small number of KSHV-infected cells undergo spontaneous lytic replication; however, the majority of the tumor cells are in latent phase of replication, indicating the essential roles of both viral latency and lytic replication in the development of KSHV-induced malignancies.Citation4 Consequently, the mechanism by which KSHV achieves latency has been the focus of intensive research in the last decade. All the 12 KSHV miRNA genes are expressed during latency. With the exception of miR-K10 and -K12, they are not inducted upon lytic induction, suggesting their potential roles in regulating viral lifecycle. Ganem and colleagues recently showed that miR-K9* targets RTA and thereby antagonizes KSHV reactivation.Citation11 The same group has previously used tandem array-based expression screens to identify Bcl-2-associated factor (BCLAF1) as a target of miR-K5, K9 and K10a/b.Citation12 Knockdown of BCLAF1 resulted in an increase in lytic KSHV replication, indicating that KSHV miR-Ks may control latency by targeting host genes. We and Lieberman's group have recently used reverse genetic approach to define the role of KSHV miRNAs in viral lifecycle by generating a similar mutant virus with a deletion of the cluster of the 10 pre-miRNAs.Citation13,Citation14 In both cases, the mutant virus has increased level of lytic activity in latent cells and in cells induced for lytic replication. The expression levels of IE gene RTA (ORF50) and late lytic protein major capsid protein (MCP, ORF25) transcripts and viral early lytic protein ORF59 as well as the production of infectious virions were significantly higher in mutant virus cells than in wild-type virus (WT) cells, indicating suppression of KSHV lytic replication by the miRNA cluster.Citation13 Lieberman's group went on to show that miR-K4-5p epigenetically regulates KSHV lytic replication by targeting retinoblastoma (Rb)-like protein 2 (Rbl2) to increase the mRNA levels of DNA methyl transferase 1, 3a and 3b (DNMT1, -3a and -3b).Citation14 Our results showed that mutant virus cells had lower NFκB activity than WT cells and overexpression of the miRNA cluster in mutant virus cells can rescue the NFκB activity.Citation13 Further analysis of the NKκB pathway led to the identification of the inhibitor of NFκB (IκBα) as the target of miR-K1. Expression of miR-K1 in the mutant virus cells is sufficient to rescue NFκB activity and inhibit viral lytic replication while suppression of miR-K1 in KSHV-infected BCP-1 cells increased the IκBα protein level, suppressed the NFκB activity and increased the expression of viral lytic genes.Citation13 The NFκB pathway is involved in cell growth and survival, immunity, inflammation and tumor development. Several studies have shown that this pathway also promotes latency of gammaherpesviruses.Citation15 Our findings suggest KSHV miRNAs manipulate this host survival pathway to regulate viral latency and lytic replication.Citation13
In summary, recent advancements have shown that miRNAs encoded by herpesviruses play a key role in promoting virus latency by either directly targeting the expression of key viral lytic genes or indirectly targeting cellular regulatory pathways. These findings have provided insights into the biological functions of virus-encoded miRNAs as well as the mechanism controlling herpesviral latency and lytic replication.
Figures and Tables
Acknowledgements
This work was supported by grants from American Cancer Society (#RSG-04-195) and National Institute of Health (CA096512, CA124332, CA132637 and DE017333) to S.J.G. and the National Science Foundation (CCF-0546345) to Y.F.H.
Addendum to:
References
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism and function. Cell 2004; 116:5 - 7
- Iorio MV, Piovan C, Croce CM. Interplay between microRNAs and the epigenetic machinery: An intricate network. Biochim Biophys Acta 2010; In press
- Cullen BR. Viral and cellular messenger RNA targets of viral microRNAs. Nature 2009; 457:421 - 425
- Greene W, Kuhne K, Ye FC, Chen JG, Zhou FC, Lei XF, Gao SJ. Molecular biology of KSHV in relation to AIDS-associated oncogenesis. Cancer Treat Res 2007; 133:69 - 127
- Grey F, Meyers H, White EA, Spector DH, Nelson J. A human cytomegalovirus-encoded microRNA regulates expression of multiple viral genes involved in replication. PLoS Pathog 2007; 3:163
- Murphy E, Vanicek J, Robins H, Shenk T, Levine AJ. Suppression of immediate-early viral gene expression by herpesvirus-coded microRNAs: implications for latency. Proc Natl Acad Sci USA 2008; 105:5453 - 5458
- Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, Cullen BR. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature 2008; 454:780 - 783
- Tang S, Patel A, Krause PR. Novel less-abundant viral microRNAs encoded by herpes simplex virus 2 latency-associated transcript and their roles in regulating ICP34.5 and ICP0 mRNAs. J Virol 2009; 83:1433 - 1442
- Tang S, Bertke AS, Patel A, Wang K, Cohen JI, Krause PR. An acutely and latently expressed herpes simplex virus 2 viral microRNA inhibits expression of ICP34.5, a viral neurovirulence factor. Proc Natl Acad Sci USA 2008; 105:10931 - 10936
- Barth S, Pfuhl T, Mamiani A, Ehses C, Roemer K, Kremmer E, Jaker C, et al. Epstein-Barr virus-encoded microRNA miR-BART2 downregulates the viral DNA polymerase BALF5. Nucleic Acids Res 2008; 36:666 - 675
- Bellare P, Ganem D. Regulation of KSHV lytic switch protein expression by a virus-encoded microRNA: an evolutionary adaptation that fine-tunes lytic reactivation. Cell Host Microbe 2009; 6:570 - 575
- Ziegelbauer JM, Sullivan CS, Ganem D. Tandem array-based expression screens identify host mRNA targets of virus-encoded microRNAs. Nat Genet 2009; 41:130 - 134
- Lei XF, Bai ZQ, Ye FC, Xie JP, Kim CG, Huang YF, Gao SJ. Regulation of NFkappaB inhibitor IkappaBalpha and viral replication by a KSHV microRNA. Nat Cell Biol 2010; 12:193 - 199
- Lu F, Stedman W, Yousef M, Renne R, Lieberman PM. Epigenetic regulation of Kaposi's sarcoma-associated herpesvirus latency by virus-encoded microRNAs that target Rta and the cellular Rbl2-DNMT pathway. J Virol 2010; 84:2697 - 2706
- Ye FC, Zhou FC, Xie JP, Kang T, Greene W, Kuhne K, et al. Kaposi's sarcoma-associated herpesvirus latent gene vFLIP inhibits viral lytic replication through NFkappaB-mediated suppression of the AP-1 pathway: a novel mechanism of virus control of latency. J Virol 2008; 82:4235 - 4249