Abstract
In the preceding report, moderately lived-mice fed dietary 2-mercaptoethanol (2-Me) had their life extended, whereas long-lived mice were found to have the quality of life improved, but not extended, and did not develop high fat-diet obesity. In the present report, alteration of longevity of mice prone to develop spontaneous, systemic lupus erythematosus (SLE) by dietary 2-Me was determined. NZB, NZW, (NZW x NZB) F1-hybrid, BXSB/MpJ, BXSB-Yaa+/J, MRL/MpJ and MRL/MpJ-Faslpr mice received drinking water, without or with 2-Me at concentrations of 10-3 or 10-2 M. Therapeutic benefit was assessed by changes in longevity. The median survival of MRL/MpJ males was increased from 443 to 615 days and those of (NZW x NZB) F1 and NZB males and females were increased approximately 2-fold. The most unexpected finding was that longevity of F1 males was significantly extended irrespective of whether dietary exposure to 2-Me was initiated at 28 days of age, at 50 days of age, or initiated during gestation (and then terminated at weaning--28 days of age). Survival of F1-hybrids in which treatment was initiated in utero or at 28 days of age was not significantly different, whereas if initiation was delayed until 50 days of age, survival was >200 days shorter. Survival of male MRL/MpJ-Fas lpr and BXSB/MpJ (Yaa-), two strains with genetically controlled accelerated SLE, was not altered by 2-Me when started at 50 days. Various alternatives are discussed regarding potential long-lasting mechanisms imprinted early in life. Even though present day treatments of rodent SLE are generally aimed at controlling specific immunological events, with or without survival benefits, or are procedures presently unsuitable for therapeutic use in humans, the findings presented herein seem worthy of clinical evaluation.
Introduction
We previously demonstratedCitation1–Citation6 that optimization of murine immunological reactivity in tissue culture required a sulfhydryl compound; the most effective being 2-mercaptoethanol (2-Me). Since these reports, 2-Me was found beneficial for both growth/function of other cell-types in vitro including those of other species and when fed orally, impeded and/or reversed some in situ physiological changes associated with aging. More recently, thiolcontaining compounds possessing oxidation-reduction potentials weaker than 2-Me were found to impart beneficial effects for human diseases (reviewed in ref. Citation7, in preceding report). Based on these effects, the research herein addressed the question: What consequences might dietary 2-Me impart on health and disease of mice other than those associated with aging?
Previous investigations indicate that (a) potential chronic and lethal toxicityCitation8 attributes of daily oral consumption of 2-Me did not negatively alter longevity,Citation7,Citation9,Citation10 most likely because the maximum average intake of 16 ugm per gm body weight spread over 24 hours was less than a LD50 bolus of 345 ugm per gm body weight,Citation8 (b) in situ age-related functions were prevented/reversed,Citation9–Citation12 (c) appearance of cancer was slowed,Citation10,Citation11 (d) survival of long-lived miceCitation10 and moderately lived mice was extended,Citation7 (e) high-fat diet obesity was curtailed,Citation7 and (f) a high quality of life was maintained by preventing recumbent, emaciation and cachectic health aspects associated with aging.Citation7 The present report is an extension of in situ investigations on 2-Me; namely, to assess alteration of longevity of mice that are prone to develop spontaneous, autoimmune-like, systemic lupus erythematosus (SLE).
Results
Because of potential toxic effects of 2-Me and since it is generally accepted that death of SLE-prone mice is a consequence of autoimmune-incited renal failure, the only SLE parameter monitored was survival. As a reference for presentation, median life spans found for strains treated with 2-Me are shown in . The results shown in are those found for (NZW × NZB) F1-hybrids started on treatment at the time they were weaned (28 days of age). As shown in , mean survival of females (parents were not on 2-Me) was significantly increased (p = 0.019 and p = 0.006) by both 10−3 and 10−2 M 2-Me (median time also increased, ). Mean survival at the two 2-Me concentrations was not significantly different. Likewise, mean survival of males (parents were not on 2-Me) was also significantly increased by 10−3 and 10−2 M 2-Me (p = 0.0006 and 0.0002). Again, median survival increased and survival at the two 2-Me concentrations was not significantly different.
Survival shown in is that of offspring of parents/grandparents that were on 10−3 M 2-Me water their entire lives. The F1-hybrids for this experiment were potentially exposed to 2-Me in utero, during lactation, and to any water consumed prior to weaning at 28 days. At weaning, offspring from these treated parents were continued on 10−3 M (n = 10) or switched to either untreated water (n = 6) or to 10−2 M water (n = 7). Surprisingly, mean survival (and medians) of offspring that were switched to untreated water (median 693 days) was statistically indistinguishable (p = 0.29 and 0.35) from that of those switched to 10−2 M or those continued on 10−3 M (medians of 704 and 723).
Natural demise of untreated (NZB × NZW) F1-hybrid and NZB mice is generally accepted to be due to renal failure. Since survival of untreated NZB and the reverse F1-cross used herein is similar to those published, it is assumed that their deaths were also due to renal failure. Further, no visible tumors were noted in untreated animals. In contrast, two of 17 F1-hybrid animals treated with 10−3 M 2-Me starting at 28 days of age and two of 22 treated with 10−2 M died with cancer (shown by “+” in the Figures); all but one at >500 days.
Longevity of treated/non-treated, F1 parental strains and two other unrelated, autoimmune-SLE-prone strains was determined. As shown in and C, mean survivals of NZB females (p = 0.010) and males (p < 0.0009) were significantly increased by 2-Me; median survivals were also increased (). No tumors were observed for treated or non-treated NZB animals.
In contrast to NZB and F1-hybrid animals, NZW females () and males () treated with 10−3 M had shorter mean survival times (significant for males, p = 0.021, but not for females, p = 0.085) than non-treated controls (median survivals were also shorter, ), whereas mean and median survival of those treated with 10−2 M were similar to those of non-treated animals. Survival of NZW females and males treated with 10−2 M was extended compared to that of those treated with 10−3 M (however only females were significantly extended). A low incidence of cancer was found for this strain, irrespective of treatment—controls (1 of 20), 10−3 M (3 of 15) and 10−2 M (1 of 18).
As shown in , the amount of a 6% fat diet consumed by control and 10−3 M 2-Me-treated C57BL/10 males was similar. However, these mice consumed considerably more 2-Me water relative to normal water. Similar results were found with (NZW × NZB) F1 mice at a similar age; mice on 2-Me consumed the same amount of food (same 6% fat diet fed B10) and more 2-Me water than control mice. The average ugm of 2-Me consumed/day/gm body weight by F1-hybrids was similar to that consumed by B10 mice.
and show the survival of males of the autoimmune accelerated SLE-prone strains, MRL/MpJ-Faslpr/J and BXSB/MpJ, and their congeneic, control strains, MRL/MpJ (which develops a late, mild-form of SLECitation13) and BXSB-Yaa+/J (which does not develop SLE), started on 10−2 M 2-Me at 50 days of age (the day they arrived from Jackson Laboratory). As shown in , 2-Me had no significant beneficial or detrimental effect on survival of mice with the mutant lpr gene. Even though median survival of the congeneic control, MRL/MpJ, was increased by 2-Me, the mean survival was not significantly different (p = 0.11); primarily due to the survival of a single control animal and to the small number of animals tested.
shows survival changes that occurred in male BXSB and its congeneic partner treated or not treated with 10−2 M 2-Me (treatment started at 50 days). The accelerated demise of BXSB mice, carrying the Yaa gene on the Y chromosome was not altered, whereas survival of the congeneic partner was dramatically increased (p = 0.006).
An explanation as to why 2-Me was ineffective for the two accelerated models of SLE is that treatment started at 50 days of age is just too late relative to initiation of disease. This was tested by determining survival of F1-hybrid males (for which 2-Me was effective) started on treatment at different ages. Shown in , starting in utero, at 28 days or 50 days of age resulted in significant increases in survival (p of 0.007 to <0.0001) vs. that of controls. However, 2-Me treatment instituted early increased longevity more, with no significant difference between in utero and 28 days of age.
Discussion
Systemic lupus erythematosus in mice and humansCitation14,Citation15 is a chronic systemic autoimmune disease that is influenced by undefined environmental factors and is under control of a multitude of genetic loci that contribute to susceptibility by epistatic interactions.Citation16–Citation19 Moreover, even though SLE disease may involve multiple organ systems, glomerulonephritis, which can lead to fatal renal failure, is considered the most serious. Presently, NZB, (NZB × NZW) F1-hybrid, MRL/MpJ-Faslpr and BXSB (males only) strains of mice have been the most extensively studied as models for human SLE; other congeneic, transgenic, F1-hybrids and knockout models are increasingly being studied. In the present report, only these four strains, plus the other F1 parental strain, NZW and where appropriate congeneic strains were studied.
Because of potential toxicity of 2-Me and because certain SLE phenotypic parameters are not always associated with survival,Citation20–Citation22 survival, presumed to be a consequence of renal failure, was the only phenotypic change chosen to be monitored in the present investigation. It was reasoned that if any significant increase in longevity occurred, additional research could be undertaken to define which other phenotypes might be altered by 2-Me and their role in longevity changes. Findings of significance were: (a) longevity was increased when 2-Me treatment (i) was started at 28 days of age for NZB and for (NZW × NZB) F1-hybrids, (ii) was started at 50 days of age for (NZW × NZB) F1-hybrids and for MRL/MpJ, (the latter strain develops SLE more slowlyCitation13 than the other two), but was ineffective (started at 50 days) at extending survival of animals with genetically-controlled, accelerated SLE; (b) longevity of F1-hybrids was inversely associated with the age at which treatment was started—treatment started in utero or at 28 days of age resulted in similar increases in longevity, whereas when started at 50 days, survival was >200 days less; (c) 2-Me imprinted a long-term survival benefit for F1-hybrid males potentially exposed to 2-Me only in utero, via lactation (similar to the maternally transmitted autoantibodies that curtailed diabetes in NOD progenyCitation23), or days prior to weaning; (d) median survival of NZW mice, the F1-parental strain that does not develop nephritic SLE even though it carries SLE susceptibility genes, was shortened by 10−3 M 2-Me—for females from 756 to 527 days and for males from 916 to 760 days. In contrast, treatment with 10−2 M 2-Me did not significantly shorten or extend survival of NZW of either sex.
These findings raise a number of intriguing questions regarding 2-Me's mechanism of action. First, was the increased longevity associated with 2-Me treatment a consequence of (a) preventing glomerulonephritis, (b) merely slowing progression of disease or (c) alteration of some other event that was lethal? Second, by what mechanism(s) did 2-Me extend survival of strains with relatively moderate or slow developing SLE but not those possessing the accelerating, lpr or Yaa genes? Third, what mechanism was imprinted within 28 days of birth by 2-Me that persisted for a lifetime? Fourth, how did 2-Me shorten the life span of NZW at 10−3 M but not at 10−2 M.
Based on the magnitude of change in longevity, it is presumed that it was in some manner a manifestation of extensively reduced nephritis (perhaps completely). Assessment of genes that are common to the various strains was not informative to explain the selectively extended survival. Possibilities being considered are; (a) an effective 2-Me treatment must be initiated prior to triggering the disease process (for strains raised in house, this was at least 28 days or earlier) and for the two accelerated SLE models, treatment started at 50 days (when they arrived from Jackson Labs) is simply too late, (b) products/factors/pathways controlled by the two accelerating genes, lpr and Yaa, resist/bypass 2-Me imparted survival mechanisms of the other strains (is death due to failure of a different organ system as suggested for Crry-Ig-treated lpr animalsCitation24 or to dysregulated lymphoproliferation) and (c) others yet to be defined. Support for the first postulate is the 2-Me extended survival of the slower-development of SLE in the lpr-parental strain, MRL/MpJ (treatment started at 50 days), as well as the much poorer extension of F1-hybrids started at 50 days compared to that of those started in utero or at 28 days of age. Further analysis with other SLE-prone lpr-strains started on treatment at different ages and monitoring various SLE phenotypic markers should be informative.
The third finding, perhaps of greater significance, is that longevity of treated F1-hybrid males (median survival of 349 for nontreated) was independent of whether treatment was started at 28 days of age (medians of 664/750 days at 10−3/10−2 M) or started in utero and stopped at 28 days of age (median of 693 days), or continued post weaning (medians of 723/704 days at 10−3/10−2 M). Interesting mechanisms to consider for this prenatal/early life, long-lasting imprinting, which may also underlie the increased longevity of animals started on treatment at weaning are, 2-Me: (a) alters parental (with emphasis on the maternal parent) SLE-inducing environmental factor/etiologic agent(s) or commensal intestinal floraCitation25,Citation26 resulting in flora (or products) of offspring that mimics that of the parents; (b) interferes with pathogen recognition (initiation phase) via T or B cell receptors, and/or innate receptors, such as TLR7 and TLR9,Citation27–Citation31 (c) alters a specific innate or adoptive pathway component post etiologic recognition and (d) replacesCitation32 or programsCitation33–Citation35 antigen presenting dendritic cellsCitation36 so that an homeostatic change (dysfunctionalCitation33 or deficientCitation37–Citation40 correction) occurs in regulatory cells to favor tolerance relative to effector autoimmune T cells as a consequence of IL-2 deprivation.Citation39 Any of these postulates, especially the one in which regulatory cells are induced to play a toleragenic role, are plausible. Indeed, any could easily be achieved by reduction of essential disulfide bonds (or curtailing formation of new ones) by 2-Me. For example, alterations that create molecular and/or cellular modifications, such as cleavage of autoantigens by presumed cysteinic proteasesCitation41–Citation43 during the process of apoptosis, could prevent the presentation of alternative epitopesCitation44 to which tolerance is absent. Such changes could alter SLE-initiation, or for that matter, any other autoimmune disease that has an environmental link. Experiments with appropriate strains of mice on defined diets and with specific flora should answer whether this has any relevance for extension of the life span of SLE-prone mice by 2-Me.
And finally, by what means was survival of NZW mice shortened by 10−3 M but not by 10−2 M 2-Me drinking water? A similar shortening was found for leukemic-prone, AKR/Cum, mice treated with 5 × 10−4 M, but not those treated with 10−3 or 10−2 M (Click RE, unpublished). An interesting possibility is that alteration of crucial protein SH/disulfide moieties could lead to activation/inactivation of endogenous viruses and/or their products.Citation45
No tumors were observed in long-lived (treated) or short-lived (untreated) NZB animals; the latter most likely because of its short life span. In contrast, 2-Me treatment did not alter the low incidence of tumors in long-lived NZW and F1-hybrids; although fewer animals with tumors were observed in those treated with 10−2 M than with 10−3 M 2-Me. These results support the findings of others in which the development of tumors was slowed by 2-Me exposure.Citation10,Citation11 How 2-Me effectively prevented cancer in other cancer-prevalent strains is the focus of a future report.
Although numerous gene manipulations or immunotherapy regimens, deemed by many as potential targets, alter development of SLE,Citation46–Citation48 limited progress has been achieved towards controlling autoimmunity in general.Citation49 Indeed, at present, there are two clinically applicable protocols that result in long-term survival (“cures”) of SLE mice; allogeneic bone marrow transplantationCitation50,Citation51 and a diet of fish oil containing a high concentration of the n-3 fatty acid, docosahexaenoic acid.Citation52 Other treatments, such as retinoic acid supplementation,Citation53 alteration of IFNγ, IFNγ receptorsCitation54 and/or FcγRIIB,Citation55 addition of a class II, I-E molecule to strains that lack this gene,Citation56 B cell depletionCitation57,Citation58 are designed as interventions aimed at controlling specific immune aspects of the disease, with or without long-term survival benefits or are protocols presently unsuitable for therapeutic use in humans. Thus, there remains a need for clinically applicable protocols that prevent development of SLE as well as cure-established SLE. The findings presented herein add a simple modality, presumably preventive, that intervenes with an early event triggered by an etiologic SLE-inducing-agent(s), worthy of clinical evaluation. This is especially intriguing in that a comparable genetic defect in fas/apo-1 of lpr mice (in which 2-Me was ineffective) is absent in humans with SLE.Citation59
Conclusions
As therapeutic, nontoxic, in situ levels of 2-Me become better defined, it is anticipated that a more thorough understanding of the mechanisms of its alteration of fat-induced obesity,Citation7 maintenance of end-stage high-quality of life,Citation7 increase in longevity of non-SLECitation7,Citation10 and SLE-prone strains and slowing the development of cancerCitation10,Citation11 will be extended with the multitude of genetically defined strains of mice and available specific blocking and activating factors. It will be of interest to establish whether 2-Me (a) acts via a systemic metabolic pathway, such as maintenance of immune homeostasis systems, (b) alters environmental factor(s) that are associated (causative) with disease, (c) scavengers free radicals associated with autooxidation and aging, (d) acts by some combination of these or (e) by other presently undefined, alternatives. In addition, whether 2-Me can effectively treat established SLE, as well as prevent/alter other autoimmune diseases, such as diabetes, Crohn's and arthritis is of considerable interest.
Materials and Methods
Mice and their husbandry.
Inbred strains of NZB, NZW, BXSB/MpJ, BXSB-Yaa+/J, MRL/MpJ and MRL/MpJ-Faslpr were purchased from Jackson Laboratory, Bar Harbor, ME. From these strains, experimental male and female NZB, NZW and (NZW × NZB) F1-hybrids (note this cross is the reverse of that routinely used by others) were derived from our breeding colony. All mice were housed in standard Plexiglas ventilated conventional boxes (4–5 animals/box) within a facility that maintained a 12 hour light/12 hour dark cycle. All animals in the study succumbed from natural causes and had free access to food (Harlan Teklad mouse/rat 6% fat diet) and to autoclaved distilled/deionized water with or without added 2-Me. All experiments were performed in accordance with institutional animal research guidelines approved by the VA IACUC.
Water.
Sterile, deionized/distilled water for drinking was supplied in glass bottles, which were changed, cleaned and autoclaved twice a week. For animals treated, 2-Me was added to each bottle to obtain a final concentration of 10−3 or 10−2 M on the day the bottles were changed. Consumption of water was measured over a 3.5-day interval, corrected for the number of animals per cage and expressed as average ml/day/mouse.
Feed.
The amount of feed consumed over a seven day period was measured, corrected for the number of animals per cage and expressed as average gms/day/mouse.
Statistics.
The Mann-Whitney U test was used to assess differences in mean survival. For all comparisons, p values less than 0.05 were considered to be statistically significant.
Figures and Tables
Figure 1 Longevity of (NZW × NZB) F1-hybrid male and female mice exposed to normal, 10−3 M or 10−2 M 2-Me drinking water. (A) Females. Treatment started at 28 days of age. Nontreated water shown in black (n = 9), 10−3 M 2-Me water in red (n = 8) and 10−2 M 2-Me water in blue (n = 11). (B) Males. Treatment started at 28 days of age. Control (n = 15), 10−3 M 2-Me (n = 9), 10−2 M 2-Me (n = 11). (C) Males. Grandparents and parents on 10−3 M 2-Me their entire lives. At 28 days of age, water of offspring of these parents/grandpaarents was switched to: nontreated water (n = 6), 10−2 M 2-Me (n = 7), or continued on 10−3 M 2-Me (n = 10) for the remainder of their lives. Animals with solid tumors or ascites are designated by +.
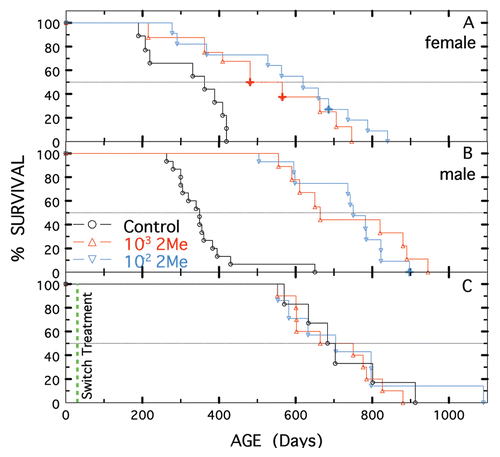
Figure 2 Longevity of NZB and NZW mice not treated or treated with 10−3 or 10−2 M 2-Me. (A and B) Females. (C and D) Males. Treatment started at 28 days of age for control shown in black, 10−3 M 2-Me in red and 10−2 M 2-Me in blue. Animals with solid tumors or ascites are designated by +.
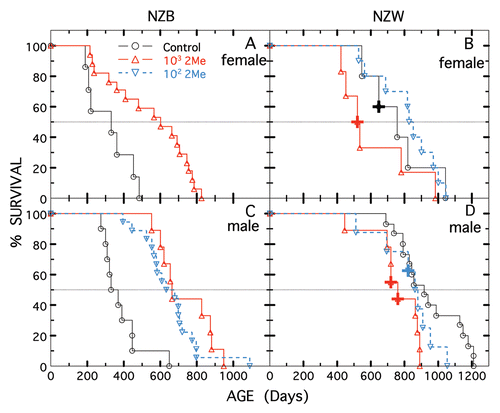
Figure 3 Longevity of MRL/MpJ (shown in black) and MRL/MpJ-Faslpr/J (shown in red) males not treated (open triangles) or treated with 10−2 M 2-Me starting at 50 days (closed triangles). Five mice per treatment.
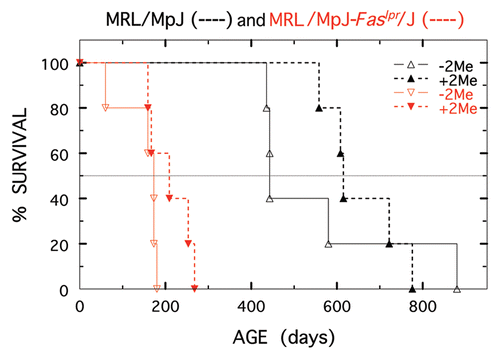
Figure 4 Longevity of BXSB-Yaa+/J (shown in black) and BXSB/MpJ (shown in red) males not treated (open triangles) or treated with 10−2 M 2-Me starting at 50 days (closed triangles). Five mice per treatment.
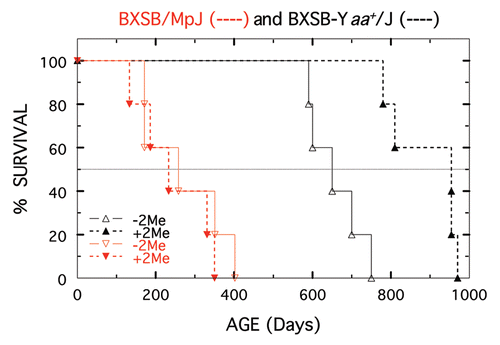
Figure 5 Survival of (NZW × NZB) F1 males started on 10−3 M 2-Me at different ages. Not treated shown in black (n = 15). Started in utero shown in blue, (n = 10). Started at 28 days of age shown in red (n = 9) and started at 50 days shown in green (n = 6).
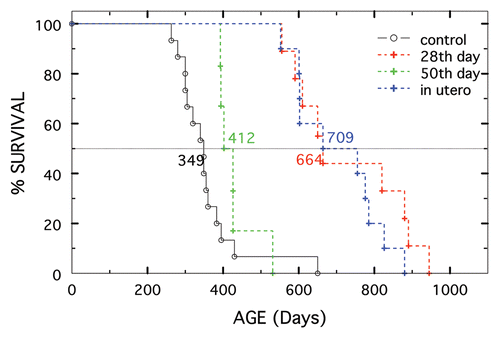
Table 1 Median life span of 2-Me-treated/non-treated strains of mice
Table 2 Body weight and average daily intake of food, water and 2-Me by male (NZW × NZB) F1 and C57BL10 mice at 210 days of age
Acknowlededgements
A special thank you to Dr. Ellen Heber-Katz for excellent suggestions during the preparation of the manuscript. NIH Grants R01CA023678 and R01AI019643 funded this research, in part.
References
- Click RE, Benck L, Alter BJ. Enhancement of antibody synthesis in vitro by mercaptoethanol. Cell Immunol 1972; 3:156 - 160
- Click RE, Benck L, Alter BJ. Immune responses in vitro. (I) Culture conditions for antibody synthesis. Cell Immunol 1972; 3:264 - 276
- Heber-Katz E, Click RE. Immune responses in vitro. (V) Role of mercaptoethanol in the mixed-leukocyte reaction. Cell Immunol 1972; 3:410 - 418
- Katz-Heber E, Peck AB, Click RE. Immune responses in vitro. (II) Mixed leukocyte interaction in a protein-free medium. Eur J Immunol 1973; 3:379 - 385
- Peck AB, Click RE. Immune responses in vitro. (III) Enhancement of the mouse mixed lymphocyte interaction by isologous and homologous sera. Eur J Immunol 1973; 3:385 - 392
- Peck AB, Katz-Heber E, Click RE. Immune response in vitro. (IV) Comparison of mouse mixed lymphocyte interactions in protein-free culture medium and in medium supplemented with isologous serum. Eur J Immunol 1973; 3:516 - 519
- Click RE. Obesity, longevity, quality of life: Alteration by dietary 2-mercaptoethanol. Virulence 2010; 1:512 - 518
- White K, Bruckner JV, Guess WL. Toxicological studies of 2-mercaptoethanol. J Pharm Sci 1973; 62:237 - 241
- Chang MP, Tamaka JL, Stosic-Grujicic S, Yamamoto EK, Perkins EH, Strehier BL, et al. Restoration of impaired immune functions in aging animals. (VI) Differential potentiating effect of 2-mercaptoethanl on young and old murine spleen cells. Int J Immunopharmacol 1982; 4:429 - 436
- Heidrick ML, Hendricks LC, Cook DE. Effect of dietary 2-mercaptoethanl on the life span, immune system, tumor incidence and lipid peroxidation damage in spleen lymphocytes of aging BC3F1 mice. Mech Ageing Dev 1984; 31:341 - 356
- Beregi E, Regius O, Rajczy K, Boross M, Penzes L. Effect of cigarette smoke and 2-mercaptoethanol administration on age-related alterations and immunological parameters. Gerontology 1991; 37:326 - 334
- Fischer HD, Wustmann C, Rudolph E, Oehler J, Jahkel M, Rostock A, et al. Effect of 2-mercaptoethaol on posthypoxic and age related biochemical and behavioral changes in mice and rats. Biomed Biochim Acta 1990; 49:1085 - 1090
- Altman A, Theofilopoulos AN, Weiner R, Katz DH, Dixon FJ. Analysis of T cell function in autoimmune murine strains. Defects in production and responsiveness to interleukin 2. J Exp Med 1981; 154:791 - 808
- Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med 1978; 148:1198 - 1215
- Foster MH. Relevance of systemic lupus erythematosus nephritis animal models to human disease. Semin Nephrol 1999; 19:12 - 24
- Reichin M, Harley JB, Lockshin MD. Serologic studies of monozygotic twins with systemic lupus erythematosus. Arthritis Rheum 1992; 35:457 - 464
- Santiago-Raber ML, Laporte C, Reininger L, Izui S. Genetic basis of murine lupus. Autoimmunity Rev 2004; 3:33 - 39
- Gatev V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL 10 as risk loci for systemic lupus erythematosus. Nature Genetics 2009; 41:1228 - 1233
- Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nature Genetics 2009; 41:1234 - 1237
- Bagavant H, Fu SM. New insights from murine lupus: disassociation of autoimmunity and end organ damage and the role of T cells. Curr Opin Rheumatol 2005; 17:523 - 529
- Bagavant H, Fu SM. Pathogenesis of kidney disease in systemic lupus erythematosus. Curr Opin Rheumatol 2009; 21:489 - 494
- Fujii T, Okada M, Fujita Y, Sato T, Tanaka M, Usui T, et al. Vaccination with autoreactive CD4(+)Th1 clones in lupus-prone MRL/Mp-Fas(lpr/lpr) mice. Autoimmun 2009; 33:125 - 134
- Greeley SAW, Katsumata M, Yu L, Eisenbarth GS, Moore DJ, Goodarzi H, et al. Elimination of maternally transmitted autoantibodies prevents diabetes in nonobese diabetic mice. Nat Med 2002; 8:399 - 402
- Bao L, Haas M, Kraus DM, Hack BK, Rakstang JK, Holers VM, et al. Administration of a soluble recombinant complement C3 inhibitor protects against renal disease in MRL/lpr mice. J Am Soc Nephrol 2003; 14:670 - 679
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci 2010; 107:12204 - 12209
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci 2010; In press
- Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffman RL. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol 2007; 37:3582 - 3586
- Santiago-Raber ML, Baudino L, Izui S. Emerging roles of TLR7 and TLR9 in murine SLE. J Autoimmunity 2009; 33:231 - 238
- Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med 2009; 15:401 - 409
- Hayashi T, Gray CS, Chan M, Tawatao RI, Ronacher L, McGargill MA, et al. Prevention of autoimmune disease by induction of tolerance to Toll-like receptor 7. Proc Natl Acad Sci USA 2009; 106:2764 - 2769
- Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci 2010; 107:11942 - 11947
- Chen C, Hirsch JG. Restoration of antibody-forming capacity in cultures of nonadherent spleen cells by mercaptoethanol. Science 1972; 176:60 - 61
- Yan B, Ye S, Chen G, Kuang M, Shen N, Chen S. Dysfunctional CD4+,CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferonalpha-producing antigen-presenting cells. Arthritis Rheum 2008; 58:801 - 812
- Jensen SS, Gad M. Differential induction of inflammatory cytokines by dendritic cells treated with novel TLR-cytokine based cocktails: targeting dendritic cells in autoimmunity. J Inflam 2010; 7:37 - 49
- Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce TH2 and tolerogenic responses. Nature Immun 2010; 11:647 - 655
- Monrad SU, Desch KC, Kaplan MJ. Role of Dendritic dells in the pathogenesis of systemic lupus erythematosus. Future Rheumatol 2008; 3:269 - 279
- Wu HY, Staines NA. A deficiency of CD4+,CD25+ T cells permits the development of spontaneous lupus-like disease in mice, and can be reversed by induction of mucosal tolerance to histone peptide autoantigen. Lupus 2004; 13:192 - 200
- Kang HK, Datta SK. Regulatory T cells in lupus. Int Rev Immunol 2006; 25:5 - 25
- Humrich JY, Morbach H, Undeutsch R, Enghard P, Rosenberger S, Weigert O, et al. Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus. Proc Natl Acad Sci 2010; 107:204 - 209
- Benkhoucha M, Santiago-Raber ML, Schneiter G, Chofflon M, Funakoshi H, Nakamura T, et al. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc Natl Acad Sci USA 2010; 107:6424 - 6429
- Casciola Rosen LA, Miller DK, Anhalt GJ, Rosen A. Specific cleavage of the 70 kDa protein component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell death. J Biol Chem 1994; 269:30757 - 30760
- Casciola Rosen L, Nicholson DW, Chong T, Rowan KR, Thornberry NA, Miller DK, et al. Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med 1996; 183:1957 - 1964
- Casiano CA, Martin SJ, Green DR, Tan EM. Selective cleavage of nuclear auto-antigens during CD95 (Fas/Apo-19)-mediated T cell apoptosis. J Exp Med 1996; 184:765 - 770
- Deng H, Apple R, Clare-Salzler M, Trembleau S, Mathis D, Adorini L, et al. Determinant capture as a possible mechanism of protection afforded by major histocompatibility complex class II molecules in autoimmune disease. J Exp Med 1993; 178:1675 - 1680
- Yoshiki T, Mellors RC, Strand M, August JT. The viral envelope glycoprotein of murine leukemia virus and the pathogenesis of immune complex glomerulonephritis of New Zealand mice. J Exp Med 1974; 140:1011 - 1027
- Karim MY, Pisoni CN, Khamashta MA. Update on immunotherapy for Systemic lupus erythematosus—What's hot and what's not. Rheum 2009; 48:332 - 341
- Monneaux F, Muller S. Molecular therapies for systemic lupus erythematosus: Clinical trials and future prospects. Arthritis Res Ther 2009; 11:234 - 244
- Crow MK. Developments in the clinical understanding of lupus. Arthritis Res Ther 2009; 11:245 - 256
- Delaleu N, Peck AB. Autoimmunity: limited progress for the patient, despite decades of research. Scand J Immunol 2009; 70:411 - 414
- Sun L, Aklyama K, Zhang H, Yamaza T, Hou Y, Zhao S, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 2009; 27:1421 - 1432
- Ikehara S. The future of stem cell transplantation in autoimmune disease. Clin Rev Allergy Immunol 2009; 38:292 - 297
- Halade GV, Rahman MM, Bhattacharya A, Barnes JL, Chandrasekar B, Fernandes G. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupusprone mice. J Immunol 2010; 184:5280 - 5286
- Kinoshita K, Yoo BS, Nozaki Y, Sugiyama M, Ikoma S, et al. Retinoic acid reduces autoimmune renal injury and increases survival in NZB/W F1. mice. J Immunol 2003; 170:5793 - 5798
- Ozmen L, Roman D, Fountoulakis M, Schmid G, Ryffel B, Garotta G. Experimental therapy of systemic lupus erythematosus: the treatment of NZB/W mice with mouse soluble interferongamma-receptor inhibits the onset of glomerulonephritis. Eur J Immunol 1995; 25:6 - 12
- Bolland S, Yim YS, Tus K, Wakeland EK, Ravetch JV. Genetic modifiers of systemic lupus erythematosus in FcγRIIB-/- mice. J Exp Med 2002; 195:1167 - 1174
- Merino R, Iwamoto M, Fossati L, Muniesa P, Araki K, Takahashi S. Prevention of systemic lupus erythematosus in autoimmune BXSB mice by a transgene encoding I-Eα chain. J Exp Med 1993; 178:1189 - 1197
- Coca A, Sanz I. B cell depletion in lupus and Sjogren's Syndrome: An update. Curr Opin Rheumatol 2009; 21:483 - 488
- Favas C, Isenberg DA. B-cell-depletion therapy in SLE—what are the current prospects for its acceptance?. Nat Rev Rheumatol 2009; 5:711 - 716
- Mysler E, Bini P, Drappa J, Ramos P, Friedman SM, Krammer PH, et al. The apoptosis-1/Fas protein in human systemic lupus erythematosus. J Clin Invest 1994; 93:1029 - 1034