Abstract
Bacterial cell wall components, such LPS and LTA, are potent initiators of an inflammatory response that can lead to septic shock. The advances in the past were centered around membrane-bound receptors and intracellular events, but our understanding of the initial interactions of these bacterial components with serum proteins as they enter the bloodstream remain unclear. In this study we identified several serum proteins, which are involved in the innate recognition of bacterial products. Using affinity chromatography and mass spectrometry we performed proteomic analysis of LPS- and LTA-binding serum proteins. We isolated proteins from normal serum that can interact with LPS and LTA. Fluorescent binding experiments and cytokine assays revealed that serum proteins, such as apolipoprotein, LDL, transferrin and holotransferrin could neutralize LPS/LTA binding as well as the subsequent inflammatory response, suggesting that serum proteins modulate LPS/LTA-induced responses. When compared with the proteomic profile of serum from septic patients it was shown that these proteins were in lower abundance. Investigation of serum proteins in 25 critically ill patients with a mortality rate of 40% showed statistically higher levels of these proteins in survivors. Patients surviving sepsis had statistically significant higher levels of apolipoprotein, albumin, LDL, transferrin and holotransferrin than individuals that succumbed, suggesting that these proteins have an inhibitory effect on LPS/LTA-induced inflammatory responses and in their absence there might be an augmented inflammatory response in sepsis.
Keywords: :
Introduction
Patients with severe sepsis and septic shock are at significant risk of a fatal outcome as a result of their own immune system’s response to bacterial infection. It is widely accepted that the excess reaction of the host occurs at the level of the innate immune system and is directly linked to the recognition of bacterial cell wall components, such as lipopolysaccharide (LPS) from Gram-negative bacteria or lipoteichoic acid (LTA) from Gram-positive bacteria. As part of its mechanism of activation, the innate immune system employs germ-lined encoded receptors, Toll-like receptors (TLRs), in order to “sense” pathogens.
The TLRs are able to detect microbial signatures and trigger activation leading to cytokine secretion. Each member of the family recognizes a restricted collection of microbial signatures, able to sense different types of microbial pathogens ranging from bacteria and viruses to fungi and spirochetes. As far as sepsis and bacterial recognition is concerned, TLR4 is the central sensor of Gram-negative bacterial products,Citation1,Citation2 whereas TLR2 is the key receptor in activating the immune system against Gram-positive bacteria.Citation3 In addition to the involvement of TLRs, other accessory molecules are involved. Recent data suggests that multiple membrane-based and intracellular molecules interact upon activationCitation4,Citation5 and additional serum proteins are needed to mount an ideal response against the invading pathogen. Most research has focused on the interaction of bacterial products with TLRs, but the initial interactions of the bacterial products is not with TLRs, but with serum proteins which seem to be crucial in delivering them to their cellular targets and in triggering the subsequent inflammatory response.
Most research has previously focused on the interaction of bacterial LPS with serum proteins, thus very little is known of the LTA interactions. In the case of LPS, in addition to lipopolysaccharide-binding protein (LBP), LPS has been shown to bind bactericidal/permeability-increasing protein (BPI), various types of lipoproteins such as high density lipoprotein (HDL),Citation6 low density lipoprotein (LDL)Citation7 and very low density lipoprotein (VLDL).Citation8
In this study, we set out to identify and characterize serum proteins that bind LPS and LTA in order to gain insight into the initial events that lead to the formation of an efficient receptor complex and the subsequent inflammatory response. Using affinity chromatography and proteomics we have identified several serum proteins that bind LPS and LTA: LDL, apolipoprotein, transferrin, holotransferrin, hemoglobin and albumin, that serve a specific role for innate immunity that has gone unrecognized in the past. Characterization of the interaction of these serum proteins with LPS and LTA revealed that some could inhibit their delivery to their cellular targets, suggesting that these serum proteins serve dual roles and contribute to specific elimination of bacterial cell wall components in addition to other physiological functions. Investigation of serum proteins in 25 critically ill patients with a mortality rate of 40% showed statistically higher levels of these proteins in survivors. Our data underscores the importance of serum proteins and defines novel and specific roles for LDL, apolipoprotein, transferrin, holotransferrin, hemoglobin and albumin in innate immunity and suggests that these proteins have inhibitory effects against LPS/LTA and in their absence there might be an augmented inflammatory response in sepsis.
Results
Cell activation of human monocytes by LPS or LTA is attenuated in the presence of serum
In order to investigate the involvement of serum proteins in the LPS/LTA-induced immune response, human monocytes were stimulated with LPS (100 ng/ml) or LTA (10 μg/ml) in the presence and absence of 5% human pooled serum (HPS) (). TNF-α was measured in supernatants using a flow cytometric cytokine bead array system (Becton Dickinson). Human serum was found to be able to reduce the amount of TNF-α released from human monocytes in response to LTA significantly (p < 0.05), whereas in the case of LPS the effect was less pronounced (, black bar-charts). These results suggested that at least one compound in serum attenuates the response induced by LPS/LTA.
Figure 1. Effect of human serum/LBP on LPS/LTA-induced cytokine secretion. Cytokine secretion was measured after stimulation using either 100 ng/ml LPS or 10 μg/ml of LTA in the presence and absence of human serum or LBP. TNF-α was measured in the cell supernatant using a flow cytometric cytokine bead array system (Becton Dickinson). The results are representative from three independent experiments. Asterisks denote statistical significance (p < 0.001).
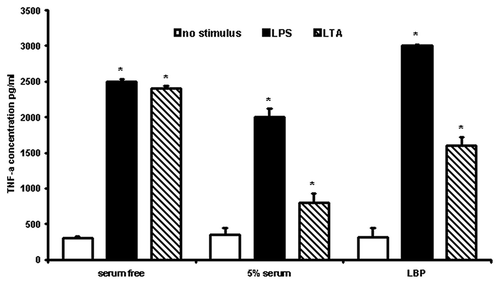
Since LBP is an important serum protein involved in the innate immune response to a variety of bacterial products, we investigated whether LBP was responsible for the inhibitory effects observed in the presence of serum. Thus human monocytes were stimulated with either LPS or LTA in the presence of 1 μg/ml LBP (, gray bar-charts). It was shown the LBP could significantly (p < 0.001) inhibit LTA-induced TNF-α secretion. In contrast, in the case of LPS, it significantly (p < 0.001) enhanced LPS-induced responses, suggesting that LBP plays a differential role in Gram-positive and Gram-negative infections.
Isolation of LPS/LTA-binding serum proteins
Since serum components seemed to attenuate the immune response both in the case of LPS, but most significantly in the case of LTA, we proceeded to investigate which serum proteins are involved in binding LPS and/or LTA. We performed a complete and unbiased proteomic analysis of normal human serum components that bind to LPS/LTA. Affinity chromatography, protein purification and peptide fingerprinting were utilized. The two dimensional map revealed 16 protein spots (), and in the case of LPS and LTA were almost identical, thus suggesting that both bacterial components bind to the same serum proteins. The spots of interest were excised, tryptic digested and sent for MALDI-TOF analysis. The peptide fingerprint was used to search Swiss-Prot database in order to identify the proteins. Mass spec analysis identified the protein spots () as albumin (, spot 1), transferrin (, spot 2), hemoglobin (, spot 3), Apoliprotein A-I (ApoAI) (, spot 4), fragment of low density lipoprotein (LDL) (, spot 5), Apolipoprotein D (, spot 8), Apolipoprotein A-IV (ApoAIV) (, spot 10,11), Apolipoprotein E (, spot 12) and TRIM5, a member of the tripartite motif family (, spot 9). Most of the lower molecular weight spots were identified as fragments of apolipoprotein A-I (, spots 6, 7, 13 and 14) and apolipoprotein D (, spots 15 and 16). Surprisingly, we didn’t isolate any plasma soluble CD14, LBP, BPI] or anti-core glycolipid antibodies. Since there are mainly acute phase reactants their levels (concentration) in serum samples may be too low in order to be detected by proteomics.
Figure 2. Two-dimensional gel electrophoresis of LPS/LTA-binding serum proteins. LPS (A) or LTA (B) was covalently coupled to NHS-activated columns. Normal human serum was passed through the columns. Unbound proteins were washed away, bound proteins were eluted, concentrated and analyzed by two-dimensional gel electrophoresis. The proteins that were excised for tryptic digestion are indicated by the numbers.
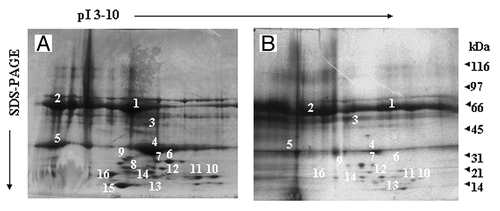
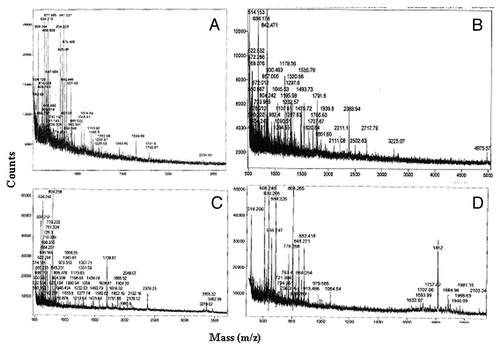
Figure 3. Mass spec analysis of excised proteins. Proteins of interest were excised, tryptic digested and analyzed by MALDI. Peptide profiles of albumin (A), LDL (B), Hemoglobin (C) and Apolipoprotein (D) are shown.
Control experiments were performed where human serum was passed through columns which had no LPS or LTA bound to the column matrix. Affinity chromatography and protein purification was performed as described above. No proteins were found to bind to the column matrix alone suggesting that there is no non-specific binding.
Role of serum proteins in LPS/LTA binding
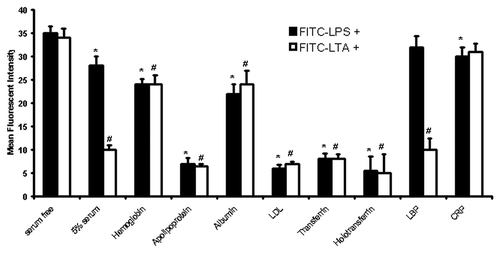
Once the LPS/LTA-binding serum proteins had been identified we proceeded to characterize the complexes formed with these proteins and LPS/LTA. In order to determine how the newly identified serum proteins modulate LPS/LTA bio-activity we labeled LPS and LTA with FITC and used it for binding studies. Monocytes were incubated with either FITC-LPS or FITC-LTA in the presence and absence of different concentrations of the different serum proteins. Flow cytometry was employed in order to determine the binding of LPS/LTA. It was shown that there was a significant (p < 0.05) inhibition of FITC-LPS and FITC-LTA binding when incubated with certain serum proteins, such as apolipoprotein AI, LDL, transferrin, holotransferrin (), which was concentration-dependent (data not shown). In particular LDL demonstrated the highest inhibition, thus suggesting that serum proteins can specifically modulate LPS or LTA interactions with the “microbial-sensing-apparatus” on cells. In order to test for non-specific binding of FITC-LLPS, the cells were also incubated with excess unlabeled LPS in the presence of FITC-LPS, which demonstrated that the binding could be out competed and thus was specific (data not shown).
Figure 4. LPS/LTA-binding on human monocytes in the presence and absence of serum proteins. Human monocytes were incubated with either 100ng/ml FITC-LPS or 10 μg/ml of FITC-LTA in the presence and absence of different serum proteins (1 mg/ml). Fluorescence was detected by flow cytometry using a FACSCalibur (Becton Dickinson), counting 10,000 cells, not gated. T-test was performed comparing results against serum free values for LPS+ and LTA+, respectively. * and # denote statistical significance (p < 0.05). The results are representative from three independent experiments.
Functional significance of LPS/LTA-serum proteins interactions
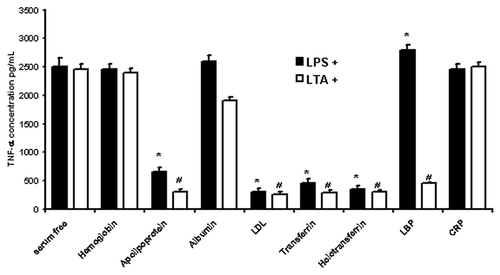
To determine the functional significance of LPS/LTA interaction with these serum proteins we proceeded to incubate monocytes isolated from healthy donors with different serum proteins prior to LPS/LTA stimulation. Following stimulation we measured TNF-α release in order to determine whether these proteins neutralize or enhance LPS- or LTA-induced cell activation (). It was shown that apolipoprotein AI and LDL as well as transferrin and holotransferrin were able to significantly (p < 0.05) neutralize the LPS- and LTA-induced cytokine response. In contrast, hemoglobin and albumin, although had shown to be able to bind LPS/LTA, were not able to neutralize LPS/LTA-induced responses.
Figure 5. LPS/LTA-induced cytokine secretion in the presence and absence of different serum proteins. Human monocytes were incubated with 1 mg/ml of different serum proteins prior to incubation with either 100 ng/ml LPS or 10 μg/ml of LTA. Supernatant was collected and analyzed for TNF-α using a flow cytometric (Th1/Th2) bead array system (Becton Dickinson). T-test was performed comparing results against serum free values for LPS+ and LTA+, respectively. * and # denote statistical significance (p < 0.05). The results are representative from three independent experiments.
Proteomic analysis of serum from septic patients
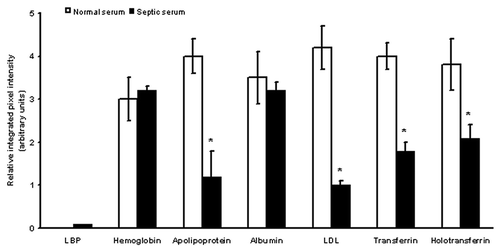
We investigated how the proteomic profile changes in septic patients. Serum from critically ill septic patients () was passed through LPS/LTA immunoaffinity columns. Bound proteins were eluted, concentrated and analyzed by two dimensional gel electrophoresis. The proteomic two-dimensional maps produced from septic serum were almost identical to the ones obtained with normal serum (), with the exception that the proteins of interest were less abundant in septic serum. This was obvious when two-dimensional gels from normal and septic serum were compared using densitometry. It was shown that key serum proteins such as apoliprotein, LDL, transferrin and holotransferrin, which had been shown to dampen the LPS/LTA-induced immune response, were less abundant in septic serum (). In contrast LBP and CRP were more abundant in septic serum and virtually non-existent in normal human serum.
Table 1. Septic patients: microbiological findings, APACHE and SOFA scores and outcome (Mean ± SD)
Figure 6. Two-dimensional gel electrophoresis of LPS/LTA-binding serum proteins from septic serum. LPS or LTA was covalently coupled to NHS-activated columns. Human serum obtained from septic patients was passed through the columns. Unbound proteins were washed away, bound proteins were eluted, concentrated and analyzed by two-dimensional gel electrophoresis. Proteins of interest were excised for tryptic digestion and mass spec analysis. Twenty five samples were analyzed; this is a representative gel.
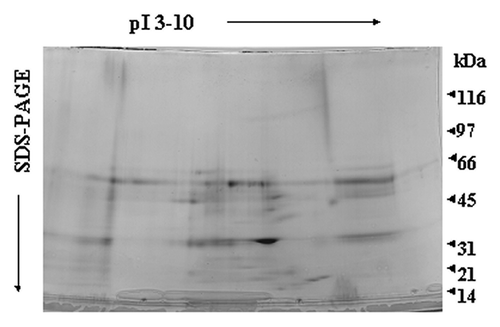
Figure 7. Differences in serum proteins from septic patients and normal controls. Relative integrated pixel intensity of each spot was quantitated by densitometry (Bio-Rad). The white bars represent mean protein quantitation from normal human serum, and the black bars are from septic serum. Asterisks denote significance (p < 0.05).
When we compared the abundance of these serum proteins in the proteomic profiles of sepsis survivors and non-survivors using densitometry, it was shown that sepsis survivors had significantly (p < 0.05) higher levels of these proteins than the non-survivors (). When we compared other characteristics such as APACHE and SOFA scores, age, CRP levels and WCC between sepsis surviving and non-surviving patients it was shown that none of the characteristics were significantly different ().
Figure 8. Comparison of proteomic profiles of sepsis survivors, non-survivors and healthy volunteers. Relative integrated pixel intensity of individual serum proteins from the proteomic profiles of sepsis survivors (white bar-charts), non-survivors (black bar-charts) or healthy volunteers (gray bar-charts) was quantitated by densitometry (Bio-Rad). **p < 0.001; *p < 0.05; n.s., not significant.
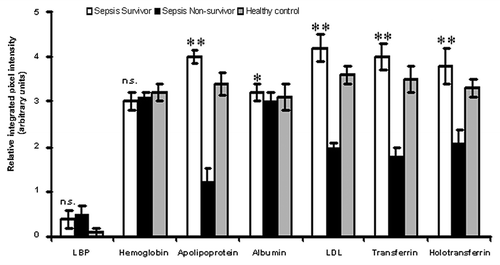
Table 2. Characteristics of sepsis surviving vs. non-surviving patients (mean ± SD)
Discussion
The results of this study define new functions for serum proteins that previously had been attributed to be completely independent of immune regulatory mechanisms. We showed that hemoglobin, apolipoprotein, LDL, albumin, transferrin and holotransferrin specifically bind to the toxic components of bacterial cell walls and contribute to their detoxification on the one hand and to the initiation of a tailored immune response on the other hand. In a clinical study we found that patients with reduced serum levels of apolipoprotein, albumin, LDL, transferrin and holotransferrin are more likely to die, even though APACHE and SOFA scores indicated similar severity of sepsis.
Most of the proteins that were isolated have been previously individually linked with LPS, but this is the first time that they have also been linked with LTA. Among the proteins isolated were several iron-binding and -containing proteins: transferrin, holotransferrin and hemoglobin. The interaction of iron-binding and -containing serum proteins with LPS has been previously reported. In particular, LPS has been previously linked to lactoferrin, which has been shown to exert a bactericidal activity.Citation9 Although in this study lactoferrin was not isolated another iron-binding serum protein, transferrin, was isolated. Transferrin has been shown to be an endotoxin binding protein that exhibits bacteriostatic action in vitro.Citation10,Citation11 These properties are thought to be limited to iron-free transferrin whereas iron-saturated or holo-transferrin is believed to lose its endotoxin binding capacity. This study has demonstrated that holotransferrin does in fact bind to LPS and LTA and interferes with their interaction with cellular targets. Restriction of bacterial access to serum iron is part of the defense strategy to limit bacterial replication, thus a dual role of proteins, which are involved in iron-regulation, to reduce serum iron and to deliver LPS and LTA to its membrane-bound target receptors seems to be feasible. Another iron-containing protein that was isolated was hemoglobin. Previous studies have demonstrated that hemoglobin binds LPS and this leads to oxidation of the heme-bound ferrous iron.Citation12,Citation13 The oxidation of hemoglobin by LPS seems to produce a synergic toxicity and thus it has been suggested that hemoglobin promotes LPS-induced activation.Citation13 This is in agreement with our study, since hemoglobin did not affect LPS- or LTA-induced TNF-α release. Iron-binding serum proteins as well as lipoproteins, were most effective in neutralizing bacterial LTA. This is in agreement with a recent study by Grunfled and coworkersCitation14 who investigated the role of LDL along with HDL in LTA-induced TNF-α secretion. It was shown that LDL and HDL could inhibit LTA-induced secretion of TNF-α.
In addition to iron-containing serum proteins, several lipoproteins were isolated. Early studies have demonstrated the interaction of LPS with lipoproteins, in particular HDL,Citation15,Citation16 VLDL and LDL have all been found to bind LPS and inactivate it.Citation17,Citation18 It has been previously shown that hypolipidemia is associated with the clinical outcome of critically ill surgical patients.Citation19 More recently, it has been demonstrated that administration of HDL can attenuate liver pro-inflammatory responses, thus demonstrating that HDL can neutralize LPS and LPS-induced activation.Citation20 In addition, apolipoproteins and in particular ApoA-I, which represents 75% of the HDL protein, have been shown to interact with LPS and to prevent LPS-induced cytokine release.Citation21-Citation25 In this study LDL was isolated as well as several apolipoproteins including mainly A-I as LPS and most importantly LTA-binding proteins. Apolipoprotein A-I was used as a representative of this class of molecules, which demonstrated a high affinity for the “microbial-sensing apparatus” and prevented the interaction of LPS and LTA with their sensing complex. Apolipoprotein A-I reduced the activation of monocytes by LPS and LTA in our study.
In addition to the already mentioned serum proteins, it has been suggested by a number of earlier studies that serum albumin may be a factor in the host defense system that facilitates interactions of LPS with protein components responsible for cell activation and clearance.Citation26,Citation27 An activating role of albumin on LPS activity in vitro and in vivo has been previously shown, which is in agreement with the findings in this study where albumin was found not to neutralize LPS- or LTA-induced cytokine responses.
As a control, two acute phase proteins, LBP and CRP, were utilized. It was shown that LBP could inhibit FITC-LTA binding, whereas CRP was not able to inhibit FITC-LTA binding. This is in agreement with the work of Mueller et al.Citation28 who have demonstrated that LBP can interfere with LTA-induced responses.
In order to investigate how this proteomic profile changes in septic patients, in this study we isolated LPS/LTA-binding serum proteins from septic serum. In septic patients these proteins are present in much lower amounts than in normal individuals. When we compared the individual proteomic profiles of sepsis survivors and non-survivors it was shown that the abundance of these serum proteins was statistically significant higher in patients surviving sepsis. When we compared other characteristics between survivors and non-survivors, such as APACHE and SOFA scores, age, CRP level or WCC (white blood cell count), none of them were found to be statistically significant, thus suggesting that serum proteins might play a critical role in the clinical outcome of sepsis patients.
It remains unclear why some individuals develop sepsis after a bacterial infection where others do not. This study adds an explanation and suggests that individuals that lack neutralizing (e.g., iron-binding proteins such as transferrin as well as lipoproteins) serum proteins could be more prone to develop sepsis. In this case, LPS/LTA enters the circulation and binds directly in high amounts to its cellular targets leading to abundant secretion of pro-inflammatory cytokines. In contrast, when the concentration of neutralizing serum proteins is high they will be able to bind LPS/LTA and inhibit the interaction with its cellular targets before it can stimulate the production of pro-inflammatory cytokines. The right balance among these serum proteins might contribute to the outcome of the patient. Additional research should focus on serum protein components since they seem to be the key modulators of the innate immune response against bacterial pathogens and could be used as biomarkers to predict clinical outcomes or even as a therapeutic intervention for sepsis.
Materials and Methods
Materials
E. coli LPS (0111:B4) from Quadratech was resuspended in endotoxin-free water containing 0.2% triethylamine followed by vortexing. LPS was repurified using a modified phenol-water extraction procedure followed by ethanol precipitation as described previously.Citation29-Citation31 Recovery of LPS was determined by 3-deoxy-D-manno-octulosonic acid assay. LTA from Staphylococcus aureus was prepared as previously described.Citation32 All fine chemicals, serum proteins and human pooled serum were purchased from Sigma Chemical Co. Purified serum proteins used in this study (such as LDL, transferrin, holotransferrin, etc.) were obtained from Genway Biotech and they were of the highest purity (99% purity). Serum proteins were checked for endotoxin contamination and any endotoxin was removed using EndoTrap columns prior to performing the cell activation assays.
FITC-LPS/LTA preparation
Repurified E. coli LPS (0111:B4) was labeled with FITC as previously described.Citation33,Citation34 LPS concentration was determined by 3-deoxy-D-manno-octulosonic acid assay. LTA from Staphylococcus aureus was prepared as previously describedCitation32 and labeled with FITC following the same protocol as with LPS. LTA concentration was determined by the phosphate molybdenum blue assay.
Cells
Monocytes were isolated from human A+ buffy coats. Adherent cell monolayers (1 × 105 to 2 × 105 monocytes/well) were cultured in 24-well plates in serum free medium (Invitrogen) supplemented with 0.01% L-glutamine and 40 μg of gentamicin/ml. This medium was used to maintain the cells and perform all stimulations.
FITC-LPS/LTA-binding on human monocytes
Human monocytes were incubated with either 100 ng/ml FITC-LPS or 10 μg/ml of FITC-LTA in the presence and absence of different serum proteins (1 mg/ml) for 30 min. After washing the unbound FITC-LPS/LTA with PBS, the cells were fixed for 10 min using 4% paraformaldehyde. FITC-LPS binding was assessed by flow cytometry using a FACSCalibur (Becton Dickinson), counting 10,000 cells.
Serum isolation from patients
Serum was collected from 25 septic patients () on admission to the ICU (close to the onset of sepsis). All patients fulfilled the sepsis criteria of the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference.Citation35 Patients who had blood transfusions, or were on lipid lowering drugs or iron were excluded. Blood tests were conducted according to the treating physician [e.g., white blood cell count or C-reactive protein (CRP)]. Participation was voluntary and written informed consent was obtained from each subject or legal representative upon entry into the study. The study was approved by the ethics committee of the University of Ulm (Ulm, Germany) and the West Sussex ethics committee (Brighton, UK).
Purification of LPS/LTA-binding serum proteins
LPS/LTA-binding serum proteins were purified by immunoaffinity chromatography. Normal human or septic serum was passed over immunoaffinity columns consisting of LPS or LTA covalently bound to NHS activated Hi-Trap columns (AmershamPharmacia). We prepared affinity columns using either LPS or LTA bound to the column matrix as previously describedCitation36, as well as control columns with no LPS/LTA bound to the column matrix. Normal human serum was injected onto the columns. Unbound proteins were washed away by passing at least six column volumes of PBS. Serum protein bound to the column were subsequently eluted using β-octyl-glucoside (β-OG) elution buffer (15 mM triethanolamine, pH 11.5, 140 mM NaCl, and 30 mM β-OG), neutralized with 1 M 2-(N-morpholino)ethanesulfonic acid pH 5, and dialysed against PBS. The eluted molecules were concentrated in a centrifuge microconcentrator (Centricon 10; Millipore) and analyzed by immobilized pH gradient electrophoresis and SDS-PAGE, and stained by Coomassie blue. The protein spots were excised. After in-gel digestion with trypsin the sequence of peptides was determined at the PAN-FACILITY (University of Stanford) and the Proteomic facility of the University of Southampton, by a MALDI mass spectrometer. Control experiments were performed where normal or septic serum was injected into NHS activated Hi-Trap columns, which had no LPS/LTA bound to the column matrix. No serum proteins were found to bind to the column matrix alone, demonstrating that the proteins isolated were specific.
TNF-α induction
Human monocytes (5 × 105) were mixed with either 100 ng/ml LPS or 10 μg/ml of LTA in serum free medium. After 5 h, the supernatant was collected and analyzed for TNF-α using a flow cytometric (Th1/Th2) bead array system (Becton Dickinson). For functional experiments using serum proteins, cells were pre-incubated with either 1 mg of individual serum proteins (saturation curves for each protein had been previously been performed), for 15 min prior to stimulation by LPS or LTA.
Statistical analysis
Statistical analyses were performed using SPSS software version 15.0 (SPSS Inc.). Comparison between two sets of patients or data was performed by the t-test or the Mann-Whitney U test.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC). We would like to thank Dick Winant at the PANFacility of the University of Stanford and Paul Skipp from the Proteomic facility of the University of Southampton for mass spectroscopy analysis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Poltorak A, Ricciardi-Castagnoli P, Citterio S, Beutler B. Physical contact between lipopolysaccharide and Toll-like receptor 4 revealed by genetic complementation. Proc Natl Acad Sci USA 2000; 97:2163 - 7; http://dx.doi.org/10.1073/pnas.040565397; PMID: 10681462
- Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, et al. Endotoxin-tolerant mice have mutations in toll-like receptor 4 (TLR4). J Exp Med 1999; 189:615 - 25; http://dx.doi.org/10.1084/jem.189.4.615; PMID: 9989976
- Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and Lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem 1999; 274:17406 - 9; http://dx.doi.org/10.1074/jbc.274.25.17406; PMID: 10364168
- Triantafilou M, Brandenburg K, Kusumoto S, Fukase K, Mackie A, Seydel U, et al. Combinational clustering of receptors following stimulation by bacterial products determines LPS responses. Biochem J 2004; 381:527 - 36; http://dx.doi.org/10.1042/BJ20040172; PMID: 15040785
- Triantafilou M, Lepper PM, Briault CD, Ahmed AM, Dmochowski JM, Schumann C, et al. Chemokine receptor 4 (CXCR4) is part of the lipopolysaccharide “sensing apparatus”. Eur J Immunol 2008; 38:192 - 203; http://dx.doi.org/10.1002/eji.200636821; PMID: 18081034
- Ulevitch RJ, Johnston AR. The modification of biophysical and endotoxin properties of bacterial lipopolysaccharides by serum. J Clin Invest 1978; 62:1313 - 24; http://dx.doi.org/10.1172/JCI109252; PMID: 372234
- Van Lenten BJ, Fogelman AM, Haberland ME, Edwards PA. The role of lipoproteins and receptor-mediated endocytosis in the transport of bacterial lipopolysaccharide. Proc Natl Acad Sci USA 1986; 83:2704 - 8; http://dx.doi.org/10.1073/pnas.83.8.2704; PMID: 3517876
- Harris HW, Grunfeld C, Feingold KR, Rapp JH. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J Clin Invest 1990; 86:696 - 702; http://dx.doi.org/10.1172/JCI114765; PMID: 2394827
- Miyazawa K, Mentel C, Lu L, Morrison DC, Broxmeyer HE. Lactoferrin-lipopolysaccharide interactions. J Immunol 1991; 146:723 - 9; PMID: 1702815
- Fletcher J. The effect of iron and transferrin on the killing of Escherichia coli in fresh medium. Immunology 1971; 20:493 - 500; PMID: 4994865
- Berger D, Berger H. Quantitation of the endotoxin-binding capacity of human transferrin. Bact Endotoxins 1988;115-124.
- Roth RI, Kaca W, Levin J. Hemoglobin, a newly recognized binding protein for bacterial endotoxins (LPS). Prog Clin Biol Res 1994; 388:161 - 72; PMID: 7831356
- Roth R, Kaca W. Toxicity of haemoglobin solutions: Haemoglobin is a lipopolysaccharide (LPS) binding protein which enhances LPS biological activity. Art Cells. Blood Subs and Immob Biotech 1994; 22:387 - 98; http://dx.doi.org/10.3109/10731199409117869
- Grunfeld C, Marshall M, Shigenaga JK, Moser AH, Tobias P, Feingold KR. Lipoproteins inhibit macrophage activation by lipoteichoic acid. J Lipid Res 1999; 40:245 - 52; PMID: 9925653
- Munford RS, Hall CL, Dietschy JM. Binding of Salmonella typhimurium lipopolysaccharide to rat high-density lipoproteins. Infect Immun 1981; 34:835 - 43; PMID: 7037642
- Ulevitch RJ, Johnston AR, Meinstein DB. New function of high density lipoproteins. J Clin Invest 1981; 67:827 - 37; http://dx.doi.org/10.1172/JCI110100; PMID: 7204557
- Kitchens RL, Wolfbauer G, Albers JJ, Munford RS. Plasma lipoproteins promote the release of bacterial lipopolysaccharide from the monocyte cell surface. J Biol Chem 1999; 274:34116 - 22; http://dx.doi.org/10.1074/jbc.274.48.34116; PMID: 10567381
- Sprong T, Netea MG, van der Ley PT, Verver-Jansen TJG, Jacobs LEH, Stalenhoef A, et al. Human lipoproteins have divergent neutralizing effects on E.Coli LPS, N. meningitidis LPS, and complete Gram-negative bacteria. J Lipid Res 2004; 45:742 - 9; http://dx.doi.org/10.1194/jlr.M300453-JLR200; PMID: 14754910
- Gordon BR, Parker TS, Levine DM, Saal SD, Wang JC, Sloan BJ, et al. Relationship of hypolipidemia to cytokine concentrations and outcomes in critically ill surgical patients. Crit Care Med 2001; 29:1563 - 8; http://dx.doi.org/10.1097/00003246-200108000-00011; PMID: 11505128
- Thabut D, Tazi KA, Bonnefont-Rousselot D, Aller M, Farges O, Guimont MC, et al. High-density lipoprotein administration attenuates liver proinflammatory response, restores liver endothelial nitric oxide synthase activity, and lowers portal pressure in cirrhotic rats. Hepatology 2007; 46:1893 - 906; http://dx.doi.org/10.1002/hep.21875; PMID: 17918268
- Flegel WA, Baumstark MW, Weinstock C, Berg A, Northoff H. Prevention of endotoxin-induced cytokine release by human low- and high-density lipoproteins and by apolipoprotein A-I. Infect Immun 1993; 61:5140 - 6; PMID: 8225591
- Hubsch AP, Powell FS, Lerch PG, Doran JE. A reconstituted, apolipoprotein A-1 containing lipoprotein reduces tumour necrosis factor release and attenuates shock in endotoxemic rabbits. Circ Shock 1993; 40:14 - 23; PMID: 8324886
- de Bont N, Netea MG, Demacker PNM, Verschueren I, Kullberg BJ, Van Dijk KW, et al. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. J Lipid Res 1999; 40:680 - 5; PMID: 10191292
- Recalde D, Ostos MA, Badell E, Garcia-Otin AL, Pidoux J, Castro G, et al. Human Apolipoprotein A-IV reduces secretion of pro-inflammatory cytokines and atherosclerotic effects of a chronic infection mimicked by lipopolysaccharide. Arterioscler Thromb Vasc Biol 2004; 24:756 - 61; http://dx.doi.org/10.1161/01.ATV.0000119353.03690.22; PMID: 14751811
- Berbée JF, Havekes LM, Rensen PC. Apolipoproteins modulate the inflammatory response to lipopolysaccharide. J Endotoxin Res 2005; 11:97 - 103; PMID: 15949136
- de Haas CJC, van Leeuwen HJ, Verhoef J, van Kessel KPM, van Strijp JAP. Analysis of lipopolysaccharide (LPS)-binding characteristics of serum components using gel filtration of FITC-labelled LPS. J Immunol Methods 2000; 242:79 - 89; http://dx.doi.org/10.1016/S0022-1759(00)00207-6; PMID: 10986391
- Dziarski R. Cell bound albumin is the 70 kDa peptidoglycan-, lipopolysaccharide, and lipoteichoic acid -binding protein on lymphocytes and macrophages. J Biol Chem 1994; 269:20431 - 6; PMID: 8051139
- Mueller M, Stamme C, Draing C, Hartung T, Seydel U, Schromm AB. Cell activation of human macrophages by lipoteichoic acid is strongly attenuated by lipopolysaccharide-binding protein. J Biol Chem 2006; 281:31448 - 56; http://dx.doi.org/10.1074/jbc.M605966200; PMID: 16928689
- Manthey CL, Vogel SN. Elimination of trace endotoxin protein from rough chemotype LPS. J Endotoxin Res 1994; 1:84 - 90
- Tapping RI, Akashi S, Miyake K, Godowski PJ, Tobias PS. Toll-like receptor 4, but not toll-like receptor 2, is a signalling receptor for Escherichia and Salmonella Lipopolysaccharides. J Immunol 2000; 165:5780 - 7; PMID: 11067937
- Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Repurification of lipopolysaccharide eliminates signalling through both human and murine toll-like receptor 2. J Immunol 2000; 165:618 - 22; PMID: 10878331
- Morath S, Geyer A, Spreitzer I, Hermann C, Hartung T. Structural decomposition and heterogeneity of commercial lipoteichoic acid preparations. Infect Immun 2002; 70:938 - 44; http://dx.doi.org/10.1128/IAI.70.2.938-944.2002; PMID: 11796629
- Troelstra A, Antal-Szalmas P, De Graaf-Miltenburg LA, Weersink AJ, Verhoef J, Van kessel KP, et al. Saturable CD14-dependent binding of fluorescein-labelled lipopolysaccharide to human monocytes. Infect Immun 1997; 65:2272 - 7; PMID: 9169763
- Triantafilou M, Triantafilou K, Fernandez N. Rough and smooth forms of fluorescein-labelled bacterial endotoxin exhibit CD14/LBP dependent and independent binding that is influenced by endotoxin concentartion. Eur J Biochem 2000; 267:2218 - 26; http://dx.doi.org/10.1046/j.1432-1327.2000.01222.x; PMID: 10759844
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31:1250 - 6; http://dx.doi.org/10.1097/01.CCM.0000050454.01978.3B; PMID: 12682500
- Triantafilou K, Triantafilou M, Dedrick RLA. CD14-independent LPS receptor cluster. Nat Immunol 2001; 2:338 - 45; http://dx.doi.org/10.1038/86342; PMID: 11276205