Abstract
Group A Streptococcus (GAS) causes human infections that range in severity from pharyngitis ("strep-throat") to necrotizing fasciitis ("flesh-eating disease"). To facilitate investigation of the molecular basis of host-pathogen interactions, infection models capable of rapidly screening for differences in GAS strain virulence are needed. To this end, we developed a Galleria mellonella larvae (wax worm) model of invasive GAS infection and used it to compare the virulence of serotype M3 GAS strains. We found that GAS causes severe tissue damage and kills wax worms in a dose-dependent manner. The virulence of genetically distinct GAS strains was compared by Kaplan-Meier survival analysis and determining 50% lethal doses (LD50). Host-pathogen interactions were further characterized using quantitative culture, histopathology and TaqMan assays. GAS strains known to be highly pathogenic in mice and monkeys caused significantly lower survival and had significantly lower LD50s in wax worms than GAS strains associated with attenuated virulence or asymptomatic carriage. Furthermore, isogenic inactivation of proven virulence factors resulted in a significantly increased LD50 and decreased lesion size compared to the wild-type strain, a finding that also strongly correlates with animal studies. Importantly, survival analysis and LD50 determination in wax worms supported our hypothesis that a newly emerged GAS subclone that is epidemiologically associated with more human necrotizing fasciitis cases than its progenitor lineage has significantly increased virulence. We conclude that GAS virulence in wax worms strongly correlates with the data obtained in vertebrate models, and thus, the Galleria mellonella larva is a useful host organism to study GAS pathogenesis.
Introduction
Advances in next-generation DNA sequencing technology and bioinformatics tools now make it possible to efficiently and economically perform genome-wide association studies on large bacterial strain collections.Citation1–Citation5 To this end, our laboratory has extensively used a comparative pathogenomic strategy to investigate the molecular genetic relationships between strain genotypes and disease phenotypes in human patients infected with serotype M3 strains of Group A Streptococcus (GAS, Streptococcus pyogenes).Citation6,Citation7 GAS is a human pathogen that causes infections ranging in severity from uncomplicated pharyngitis (“strep-throat”) and impetigo to life-threatening necrotizing fasciitis (“flesh-eating disease”) and necrotizing pneumonia.Citation8,Citation9 Serotype M3 strains of GAS are particularly interesting because they demonstrate epidemic behavior, undergo rapid shifts in disease frequency, and cause a disproportionate number of invasive and lethal infections compared to many other GAS serotypes.Citation10,Citation11
Our study of GAS strain genotype-human disease phenotype relationships has been made possible by the availability of a comprehensive 18 year prospective population-based sample of serotype M3 GAS strains collected from patients with invasive infections in Ontario, Canada.Citation3,Citation5,Citation12 To date, whole genome sequences have been determined for ∼100 serotype M3 GAS strains collected from well-described clinical episodes, resulting in new insight to the emergence of subclone populations and the evolution of strain virulence. For example, we recently discovered the significant role of the mtsR-prsA-SpeB virulence axis to GAS necrotizing fasciitis capacity.Citation5,Citation13 These studies have relied heavily on the testing of new hypotheses in mouse and monkey models; however, to keep pace with this quickly progressing line of investigation, new models capable of rapidly screening strain virulence are needed.Citation5,Citation13,Citation14 To this end, we sought to develop a Galleria mellonella model of invasive GAS infection.
Larvae of the greater wax moth (Galleria mellonella), colloquially termed “wax worms” due to their natural lifestyle of infesting beehives and consuming beeswax, have been recently used as an alternative to vertebrates as a model host for studying pathogenic microbes.Citation15 These include studies with Acinetobacter baumannii,Citation16 Aspergillus spp.,Citation17 Bacillus cereus,Citation18 Burkholderia cepacia,Citation19 Candida albicans,Citation20 Cryptococcus neoformans,Citation21 Francisella tularensis,Citation22 Listeria spp.Citation23 and Pseudomonas aeruginosa.Citation24 Wax worms have also been used to study the virulence of Staphylococcus aureus,Citation25 a Gram-positive bacterium that has many overlapping themes with GAS in the mechanism of its virulence factors.Citation7,Citation26 Both organisms effectively evade host immunity and destroy host tissue to cause severe necrotizing infections in humans.
Bearing directly on their suitability for studying human pathogens, and in contrast to other invertebrate model hosts such as Caenorhabditis elegans or Drosophila melanogaster, wax worms can be maintained at 37°C.Citation15 This trait is important because the GAS transcriptome markedly changes when cultures are grown at temperatures either above or below human body temperature.Citation27 Furthermore, Galleria mellonella larvae have an immune system with reasonable homology to vertebrates.Citation15 The hemocoel contains a digestive tract, loosely organized muscular system, biosynthetic fat body and hemolymph. These tissue types are similar to those encountered by GAS during invasive infections in humans. The hemolymph is analogous to blood in that it transports nutrients, hemocytes and immune molecules. At least two of the six subsets of hemocytes described in Galleria mellonella larvae are capable of phagocytosis.Citation28 Also, numerous enzymatic cascades akin to complement fixation and blood coagulation occur in the hemolymph.Citation15 These complex multi-component reactions result in hemolymph clotting and melanin production, key defense mechanisms against invading microbes.Citation29 As such, we hypothesized that the Galleria mellonella larva is a suitable host organism to study GAS pathogenesis. Herein, we describe studies designed to develop a Galleria mellonella larvae model of invasive GAS infection. This new model will be particularly useful as a rapid bioassay for screening differences in virulence among GAS isolates in large strain collections.
Results
GAS causes severe tissue destruction and disseminated infection in Galleria mellonella larvae.
GAS is a host-specific pathogen, causing natural disease only in humans. Several known and putative GAS virulence factors have only modest activity against their target molecules in other species.Citation30–Citation32 Thus, mouse and lower vertebrate infection models may have a somewhat limited capacity to test particular hypotheses bearing on GAS virulence. To begin to test the hypothesis that the Galleria mellonella larva is a suitable model host to study GAS pathogenesis, wax worms were infected with 107 CFU of representative serotype M3 strain MGAS315 and examined by visual and microscopic analysis (). This strain was selected because its genome has been sequenced, it is representative of highly virulent serotype M3 GAS strains causing severe invasive disease in humans, and it has been extensively studied in previous molecular pathogenesis experiments using mice and monkeys.Citation13,Citation14,Citation33,Citation34 Wax worm larvae that were sham-inoculated with sterile saline had no change in their appearance or activity. In contrast, all larvae infected with strain MGAS315 had distinct signs of invasive infection, including melanization (), rapid death () and formation of a destructive abscess-like lesion at the site of inoculation (). These abscesses were comprised of a dense central core of necrotic tissue and GAS organisms surrounded by a well-organized outer band of host hemoctyes, coagulated hemolymph and extracellular melanin pigment (). Most GAS present at the inoculation site were extracellular, suggesting they had overwhelmed the capacity of host phagocytes to contain the infection (). Also, many GAS organisms escaped from the melanin clot encapsulating the abscess to disseminate throughout the hemocoel (). These findings are similar to the histopathology that is commonly observed in mouse and monkey models of GAS necrotizing fasciitis and in humans with severe soft tissue infections. Thus, these results support the hypothesis that the Galleria mellonella larva is a suitable model host for studying GAS pathogenesis.
GAS causes dose-dependent killing in Galleria mellonella larvae.
To test the hypothesis that Galleria mellonella larvae are susceptible to dose-dependent killing by GAS, wax worms were inoculated with serial 10-fold dilutions of the highly virulent reference strain MGAS315, and survival was monitored for 96 h. Similarly, larvae were also inoculated with the same dilutions of strain MGAS12501, a GAS strain that was recovered from the oropharynx of an asymptomatic carrier and is significantly less virulent in mice and monkeys (Flores AR, Musser JM, unpublished data).Citation5 At the highest dose tested (107 CFU), both strains caused very high mortality (). However, at the 106 CFU dose, invasive strain MGAS315 killed significantly more wax worms than carrier strain MGAS12501 (). Importantly, both strains caused dose-dependent killing that was reproducible in independent assays (). Of note, larvae infected with the highly virulent invasive strain MGAS315 had rapidly progressive melanin accumulation and hemolymph coagulation, whereas larvae inoculated with the less virulent carrier strain MGAS12501 typically required 24–48 h to manifest an observable pathological effect (). No deaths or pathologic abnormalities occurred in larvae sham-inoculated with sterile saline ().
GAS grows in a typical logarithmic curve in Galleria mellonella larvae.
To monitor growth of GAS in infected larvae, wax worms were infected with a relatively low but potentially lethal dose (105 CFU) of highly virulent reference strain MGAS315, and bacterial burden was measured hourly in pools of larvae. After an initial decrease in CFUs recovered following the bolus inoculation, bacterial density increased exponentially with a doubling time of 38.0 minutes (). GAS reached a maximum concentration of 6.22 × 107 CFU/pool at 9 h and a plateau of ∼1.33 × 107 CFU/pool thereafter.
Virulence of wild-type GAS strains in Galleria mellonella larvae correlates with virulence in mice.
Previous work in our laboratory characterized the virulence of a panel of 11 serotype M3 GAS strains isolated from human patients, including mortality in a mouse model of bacteremia.Citation5 To test the hypothesis that GAS strain virulence in Galleria mellonella larvae correlates with virulence in mice, Kaplan-Meier survival curves () and the 50% lethal dose (LD50) () were determined for each strain in wax worms. Compared to the highly virulent reference strain MGAS315, which causes severe tissue destruction and high mortality in mice and monkeys, each GAS strain tested could be categorized as having either similarly high or significantly less virulence capacity in wax worms (). Importantly, the LD50 of each GAS strain tested in wax worms had a near-linear relationship with its LD50 in mice (). Taken together, these findings are consistent with the hypothesis that GAS strain virulence in Galleria mellonella larvae correlates with virulence in mice.
Virulence of isogenic mutant GAS strains in Galleria mellonella larvae correlates with virulence in mice.
We previously characterized the virulence of several isogenic mutant strains of GAS in mice and monkeys (Flores AR, Musser JM, unpublished data).Citation13,Citation34 These mutant strains of MGAS315 are deficient in the expression of the genes encoding metal transporter of Streptococcus regulator (mtsR), multiple gene activator of Streptococcus (mga), peptidyl-prolyl cis-trans isomerase A (prsA), streptococcal phospholipase A2 (slaA) or secreted streptococcal cysteine protease B (speB). Each isogenic mutant strain has significantly reduced virulence in models of necrotizing fasciitis, bacteremia and skin and soft tissue infection (Flores AR, Musser JM, unpublished data).Citation13,Citation34 To further test the hypothesis that GAS strain virulence in Galleria mellonella larvae correlates with virulence in commonly used vertebrate models, Kaplan-Meier survival curves () and the 50% lethal dose (LD50) () of each isogenic mutant strain were determined in wax worms. Compared to highly virulent reference strain MGAS315, the LD50 of all five gene-deficient strains was significantly higher, that is, they were significantly less virulent in wax worm larvae (). Wild-type virulence capacity was restored by gene complementation in all three strains tested ().
One potential explanation for the altered virulence capacity of GAS strains deficient in these virulence factors is decreased replication or survival in vivo. To test this hypothesis, GAS burden was measured in wax worm larvae infected with isogenic mutant strains. However, consistent with previous data from mouse and monkey models,Citation13 there was no significant difference in the CFUs recovered between the parental wild-type strain MGAS315 and the isogenic mutant strains (). An alternative hypothesis for the altered virulence capacity of the isogenic mutant GAS strains is a decreased ability to destroy host tissue in vivo. To test this hypothesis, tissue histopathology was examined in infected larvae. Consistent with this hypothesis, wild-type strain MGAS315 caused significantly larger lesions with more melanization than the less virulent gene-deficient strains ().
The mtsR-prsA-SpeB virulence axis is crucial to necrotizing fasciitis capacity in mice, monkeys and humans.Citation13 To confirm that these genes are also expressed in vivo in Galleria mellonella larvae infected with reference wild-type strain MGAS315, TaqMan real-time quantitative PCR assays were performed on hemolymph collected 18 h post-inoculation. Relative to the highly expressed reference gene tufA, transcripts for mtsR and prsA were abundant (). Consistent with recently published data bearing on the central role of SpeB-mediated tissue destruction in severe invasive infections,Citation13,Citation35,Citation36 speB transcripts were highly abundant in the infected tissue ().
Subclone 8 serotype M3 GAS strains are more virulent than subclone 5 strains in Galleria mellonella larvae.
We previously reported that a clonally related subset of serotype M3 GAS invasive infection isolates, designated subclone 5 strains, are epidemiologically associated with decreased necrotizing fasciitis cases in humans and have a significantly decreased capacity to cause necrotizing fasciitis in mice.Citation3,Citation5,Citation13 Ongoing epidemiological surveillance studies and genome sequencing have recently identified a new GAS strain lineage, designated subclone 8 strains, that are genetically descended from the necrotizing fasciitis-negative subclone 5 strains.Citation3 Importantly, subclone 8 strains have apparently regained necrotizing fasciitis capacity in humans.Citation3 However, this epidemiological association has not been investigated in animal models. To test the hypothesis that subclone 8 strains have increased virulence compared to their parental subclone 5 strains, Kaplan-Meier survival curves and the 50% lethal dose (LD50) of five representative strains from each subclone lineage were determined in wax worms. Results demonstrated that compared to subclone 5 strains, subclone 8 strains caused significantly lower survival over time () and have a significantly lower mean LD50 (). That is, subclone 8 strains are significantly more virulent than subclone 5 strains in wax worms. This finding is consistent with the human epidemiological data and suggests that further investigation of the molecular mechanism responsible for restoring necrotizing fasciitis capacity to subclone 8 stains is warranted.
Discussion
GAS is a human-specific pathogen with no known animal reservoir. As a consequence, we have extensively used non-human primates, the most human-relevant animal model possible, as the preferred host for testing hypotheses bearing on GAS molecular pathogenesis.Citation13,Citation14,Citation34,Citation37–Citation39 However, high cost and substantial experimental time may limit the use of monkeys in GAS research. Mice are also commonly used, but murine models suffer from some of the same limitations, plus they may be further hindered by a poor recapitulation of the complex host-pathogen interactions that underlie human infectious disease phenotypes. For example, the importance of streptokinase (ska) as a GAS virulence factor was not fully appreciated until studied in humanized mice,Citation30 and results of our recent investigation into the mtsR-prsA-SpeB virulence axis were confirmed in monkeys.Citation13 For this reason, any new GAS-host model must be thoroughly validated to appropriately interpret virulence assessments. As such, we carefully designed the studies reported herein to provide unambiguous evidence that the Galleria mellonella larva is a suitable model host for GAS.
Importantly, we documented a very high correlation of GAS strain virulence measured in wax worms and vertebrates (). Panels of well-characterized serotype M3 GAS strains isolated from humans and isogenic mutant GAS strains derived from a wild-type reference strain were tested. In nearly every case, the wax worm data were consistent with previously published results collected from mouse and monkey models. The one notable exception was carrier strain MGAS12502 (), which demonstrated unexpectedly high virulence in Galleria mellonella larvae. Whole-genome sequence analysis of this strain suggests that the probable genetic mechanism underlying its decreased virulence in mice, monkeys and humans is a 195 bp deletion that completely removes the amino-terminal region of the M-protein. As the major surface antigen of GAS, M-protein has been implicated in multiple virulence functions, including fibrinogen binding and complement inhibition.Citation9 Since insects do not express these specific host factors, the M-protein mutation may be less detrimental to GAS virulence in wax worms than vertebrates. In contrast, the two other carrier strains tested have mutations affecting major transcriptional regulators which may inactivate critical virulence factor pathways such as tissue destruction that are likely important to mortality in all models ().Citation40 Therefore, the carrier strain experiments illustrate an important example of the potentially imperfect recapitulation of GAS strain genotype-human disease phenotype relationships in Galleria mellonella larvae and emphasize the need to confirm all key results in higher animal models.
Tissue destruction has long been recognized as a characteristic feature of invasive GAS infection in humans,Citation41 and disseminated disease is associated with poor prognosis.Citation42,Citation43 The histopathology studies performed on GAS-infected wax worms indicate that host tissue destruction can be readily investigated in this model. Compared to reference strain MGAS315, the less virulent GAS carrier and isogenic mutant strains caused considerably smaller lesions and less melanization (). This finding in the wax worm model is consistent with previous results using the carrier and isogenic mutant strains in vertebrate models (Flores AR, Musser JM, unpublished data).Citation5,Citation13,Citation34 Of note, each of the genes targeted in the isogenic mutant strains either directly or indirectly inactivates a virulence pathway associated with tissue destruction.
An important trait of wax worms that directly bears on human microbial pathogenesis research is their tolerance for sustained incubation at 37°C.Citation15 Smoot et al. have previously shown that the GAS transcriptome significantly changes when cultures are not grown at human body temperature.Citation27 Growth at either 29°C or 40°C significantly altered the expression level of multiple proven and putative virulence factors, including genes implicated in carbohydrate and fatty acid metabolism, iron homeostasis and oxidative stress response, and cell wall and envelope biosynthesis.Citation27 Similar temperature-dependent transcriptome changes have also been reported for Streptococcus agalactiae and Streptococcus pneumoniae,Citation44,Citation45 emphasizing the importance of this trait. In contrast, alternative invertebrate model hosts such as Drosophila melanogaster and Caenorhabditis elegans cannot be maintained at 37°C.Citation27 Another beneficial feature of wax worms is the ease with which in vivo GAS gene expression studies can be performed. A growing body of evidence suggests an increased interest in performing GAS gene expression studies under human-relevant in vitro conditions or during in vivo infections.Citation14,Citation37,Citation46–Citation49 Infected hemolymph is easy to manipulate and enriched in GAS cells relative to host cells. This helps to overcome the challenges associated with extracting a sufficient quantity of high quality GAS RNA from infected animal tissues for TaqMan or expression microarray analyses.
This new wax worm model of invasive GAS infection is a simple but powerful tool to facilitate large-scale investigations. It is probably best used as a rapid in vivo bioassay to screen for significant differences in virulence among GAS isolates in sizable strain collections. For example, using a sample of ten different GAS isolates, we confirmed the hypothesis that subclone 8 strains are significantly more virulent than their parental lineage (). However, as noted above with carrier strain MGAS12502, this insect model may be sometimes limited by its imperfect recapitulation of GAS-human interactions. Thus, key findings from wax worm studies should be verified in higher animal models such as mice or monkeys. In the future, geneticists may be able to introduce specific genetic mutations or create humanized larvae, enabling the design of experiments that better incorporate host factors and co-morbidities that are known to significantly affect infection susceptibility and patient mortality.Citation42 Important to maintaining this line of investigation, the wax worm is a rapid, low cost, technically uncomplicated model for studying GAS pathogenesis in vivo.
Materials and Methods
Bacterial strains.
Serotype M3 GAS strains used in this study are described in . Wild-type reference strain MGAS315 (ATCC-BAA595) was recovered from a patient with streptococcal toxic shock syndrome, and its genome sequence has been reported.Citation33 Generation of isogenic mutant strains and complemented mutant strains in the MGAS315 genetic background was previously reported, and they are also described in .Citation13,Citation34 To prepare GAS stocks for wax worm infection, strains were grown in Todd Hewitt broth supplemented with 0.2% yeast extract (THY, Becton, Dickinson and Company, Sparks, MD) at 37°C with 5% CO2 to OD600 = 0.5. GAS were collected by centrifugation, washed twice in phosphate buffered saline (PBS), suspended in 80% PBS/20% glycerol, aliquoted in cryovials, and stored at −80° C until use. The concentration of each stock strain (CFU/mL) was determined by serial dilution and colony counting of at least three aliquots. At the time of inoculation, stocks were diluted to the desired concentration and the infecting dose was confirmed by quantitative culture.
Galleria mellonella larva infection.
Galleria mellonella larvae obtained from two vendors (Best Bet Inc., Blackduck, MN; and Knutson's Recreational Sales Inc., Brooklyn, MI) were evaluated and found to perform equivalently. Larvae from a single vendor and lot were used for each experiment. After receipt, larvae were allowed to equilibrate for at least 24 h by storage in the dark at 10–12°C and used within 10 days. Only larvae measuring 2.0–2.5 cm in length and having a cream colored cuticle with minimal speckling or discoloration were used. Larvae were injected with 10 µl GAS through the left hindmost proleg into the hemocoel using a Hamilton 100 µL 1710RN syringe fitted with a 29G needle. After inoculation, infected larvae were kept in vented 60 mm nematode petri dishes (Applied Scientific Products, San Francisco, CA) containing wood chips and incubated at 37°C and 0.5% CO2 without humidification.
Galleria mellonella survival assays.
For consistency, all survival assays were performed using larvae purchased from the same vendor (Best Bet Inc.). For survival and LD50 assays, cohorts of 10 larvae were infected with each GAS strain-dose, and mortality was monitored every 12 h for 96 h. In the first survival assay, larvae were infected with serial ten-fold dilutions (107-100 CFU in 10 µl PBS) of reference strain MGAS315. The 107-104 CFU dose treatments were shown to provide discriminatory power in virulence, so only these four doses were used in all subsequent assays. For each survival experiment, each strain was tested in at least three independent assays, with reference strain MGAS315 used as a comparator. As a negative control, the first and last cohort of injected larvae in every assay was sham infected with sterile PBS. No more than one control larva died in any single replicate, and for simplicity, control groups are not shown in all figures. Survival curves were plotted using the Kaplan-Meier method and differences in survival were calculated using the logrank test (Prism 4.03, GraphPad Software Inc., La Jolla, CA). LD50 was calculated using the Probit method (XLstat 2010, Addinsoft USA, New York, NY), and differences in strain virulence were compared using the Mann-Whitney test with p ≤ 0.05 considered to be statistically significant. The LD50 of each GAS strain in wax worms was compared to its LD50 in mice using Pearson's correlation (XLstat).
Quantitative GAS culture.
For the in vivo growth curve experiment, ∼500 larvae were infected with 105 CFU MGAS315 as described above. At each indicated time point, three pools of five surviving larvae each were randomly selected, transferred to a 5 ml Eppendorf tube containing 3 ml sterile PBS, homogenized, serially diluted and plated in duplicate on beta-select blood agar plates (Remel Products, Lenexa, KS). A subset of recovered colonies was confirmed as GAS using a latex agglutination kit (Becton, Dickinson and Company). Recovered CFUs/pool were plotted as mean ± standard error (Prism). For the CFU strain comparison experiment, larvae were infected with the indicated GAS strains, and four pools of five larvae each were harvested in the same manner. Differences in strain virulence were compared using the student's t-test with p ≤ 0.05 considered to be statistically significant (XLstat).
In vivo gene expression.
For in vivo GAS gene expression analysis, RNA was extracted from the hemolymph of infected larvae. Briefly, ∼200 larvae were infected with 107 CFU MGAS315 as described above. At 18 h post-inoculation, the hemolymph of ∼100 surviving larvae was harvested by sterilizing the cuticle with isopropanol, puncturing the rear proleg with a 18G needle and collecting the hemolymph into a sterile 15 ml conical tube that contained 10 ml RNAprotect (Qiagen, Valencia, CA). After incubation on ice for 5 min, the hemolymph was centrifuged, and the GAS/hemocyte cell pellet was flash frozen. Approximately 3 ml hemolymph was collected. RNA was isolated as described previously using a FastPrep Blue Kit (MP Biochemicals LLP, Solon, OH) and purified using QiaShredder and RNeasy kits (Qiagen). The concentration and quality of RNA were assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA), and cDNA was created using Superscript III (Invitrogen Corp., Carlsbad, CA) according to the manufacturer's instruction. TaqMan quantitative real time PCR was performed as previously described in reference Citation13.
Histopathology of GAS-infected larvae.
At the indicated time points, larvae were flash frozen and photographed using a Nikon inverted microscope (Nikon Inc., Melville, NY). For histopathology, larvae were fixed in formalin-aceto-alcohol for 24 hours, bisected and embedded in paraffin using standard techniques.Citation13 Hematoxylin and eosin, Wright's and Gram's stained sections were examined by a pathologist blinded to specimen type using a BX5 microscope (Olympus Inc., Center Valley, PA). Representative fields were photographed with a DP70 camera (Olympus).
Figures and Tables
Figure 1 GAS causes severe disseminated infection and tissue destruction in Galleria mellonella larvae. (A) Wax worm larvae were inoculated with 107 CFU of representative serotype M3 strain MGAS315 by injection through the left hindmost proleg using a 29 G needle. (B and C) Infection with MGAS315 resulted in rapid melanization (dark pigmentation) and high mortality (n = 10 larvae, red bar) by 24 h post-inoculation. No deaths occurred in larvae sham inoculated with sterile saline (PBS, gray bar). (D) Microscopic examination of infected wax worm larvae at 6 h post-inoculation shows an expanding abscess-like lesion at the GAS injection site. An area of extensive soft tissue necrosis and bacterial proliferation (dense blue-violet region in the center of the micrograph, demarcated with a white asterisk) is surrounded by melanized hemocytes (extracellular brown pigment and host immune cells walling off the expanding lesion, demarcated by black arrowheads). Extensive tissue destruction occurred in the thoracic and abdominal body segments (Gram's stain, 4x original magnification). (E) At the site of inoculation, a dense infiltrate of GAS organisms is seen in a background of anucleate agranular host hemocytes (Gram's stain, 40x original magnification). (F) By 6 h post-inoculation, chains of GAS have already disseminated throughout the hemolymph to begin infecting soft tissue near the first true leg (boxed region in A). The Galleria mellonella body cavity contains a variety of soft tissue types, including hemolymph with immune cells, fat, muscle and epithelium, that are similar to those encountered by GAS during invasive human infections such as necrotizing fasciitis (Gram's stain, 40x original magnification).
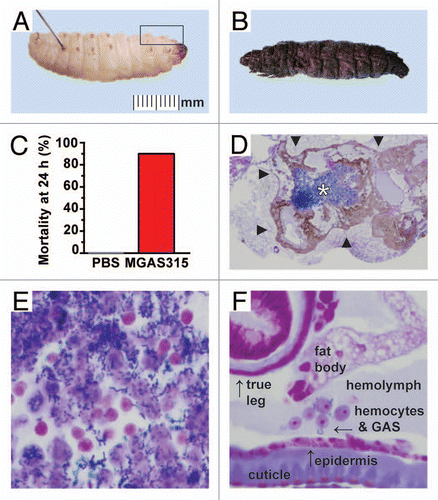
Figure 2 Virulence of GAS in the Galleria mellonella infection model. (A and B) Wax worm larvae were inoculated with serial dilutions (107, 106, 105 and 104 CFU) of strain MGAS315 (red squares), which is representative of highly virulent serotype M3 GAS strains causing severe invasive infections in humans or strain MGAS12501 (brown triangles), which was recovered from the oropharynx of an asymptomatic carrier. Survival was monitored for 96 h. Kaplan-Meier survival curves were determined for four or more independent experiments and showed a dose-dependent response with both strains (n = 10 larvae per dose per strain per experiment). The highest dose (107 CFU) of either strain resulted in significantly higher mortality than any of the three lower doses tested (p < 0.001 for either MGAS315 or MGAS12501, 107 CFU compared to 106 CFU, logrank test). Similarly, inoculation of 106 CFU resulted in significantly higher mortality than the lowest dose tested (p < 0.001 for MGAS315 and p = 0.033 for MGAS12501, 106 CFU compared to 104 CFU, logrank test). At the 106 CFU dose, invasive strain MGAS315 killed more wax worms than carrier strain MGAS12501 (p = 0.004, logrank test). No deaths occurred in larvae sham infected with sterile saline (PBS, gray circles). (C–D) Survival curves of wax worm larvae inoculated with either invasive strain MGAS315 (red squares) or carrier strain MGAS12501 (brown triangles) were highly reproducible. Results from multiple independent experiments using the highest dose tested (107, solid lines) and lowest dose tested (104 CFU, dotted lines) are shown as individual curves (n = 10 larvae per dose per strain per experiment). (E) Infected larvae demonstrated progressive melanization post-inoculation, indicating a robust host immune response to the invasive GAS infection. Compared to larvae infected with the highly virulent invasive strain MGAS315, wax worms infected with the less virulent carrier strain MGAS12501 developed pigmentation at a much slower rate. (F) In vivo growth of GAS organisms in wax worm larvae inoculated with 105 CFU of strain MGAS315 demonstrates a logarithmic curve. Bacterial burden was quantified from pools of five homogenized larvae at the time points indicated (n = 3 pools per time point).
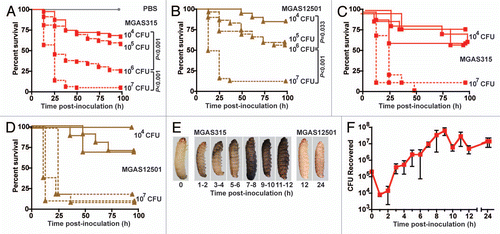
Figure 3 Virulence of serotype M3 GAS strains in the Galleria mellonella infection model correlates with virulence in mice. Wax worm larvae were infected with serial dilutions (107, 106, 105 and 104 CFU) of representative serotype M3 strain MGAS315 or one of 10 other GAS strains also recovered from human patients, and survival was monitored for 96 h. Results from three or more independent assays are shown (n = 10 larvae per strain per dose per experiment). (A) Kaplan-Meier survival curves were determined for wax worms inoculated with 106 CFU. Compared to all strains tested except MGAS3392 (pink circles), the highly virulent reference strain MGAS315 (red circles) results in a significantly lower survival over time (*indicates p < 0.05 compared to MGAS315, logrank test). (B) The 50% lethal dose (LD50) was determined by Probit analysis. The highly virulent reference strain MGAS315 (red bar) has a significantly lower LD50 (that is, it is more virulent) than other invasive strains known to be less virulent in mice (various blue bars) or strains recovered from the oropharynx of asymptomatic carriers (various brown bars) (* indicates p < 0.05 compared to MGAS315, Mann-Whitney test). (C) Overall, GAS strains such as MGAS315, MGAS3382 and MGAS3392 which are known to be highly virulent in mice and/or monkeys (red checkered bar) have a significantly lower mean LD50 in wax worm larvae (that is, they are more virulent) than GAS strains associated with lower virulence in animals (blue checkered bar) or asymptomatic carriage in humans (brown checkered bar) (* indicates p < 0.01 compared to MGAS315, Mann-Whitney test). (D) The virulence of these 11 serotype M3 GAS strains tested in Galleria mellonella larvae strongly correlates with their virulence in mice (Pearson's correlation r = 0.689 and p = 0.019).
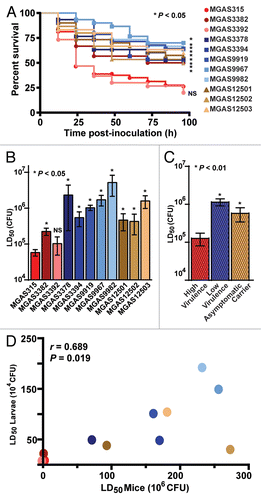
Figure 4 Virulence factors implicated in invasive human disease contribute to GAS virulence in the Galleria mellonella infection model. Wax worm larvae were infected with serial dilutions (107, 106, 105 and 104 CFU) of wild-type strain MGAS315 or one of five different isogenic mutant GAS strains lacking expression of the gene encoding a specific virulence factor, and survival was monitored for 96 hours. Results from three or more independent assays are shown (n = 10 larvae per strain per dose per experiment). (A) Kaplan-Meier survival curves were determined for wax worms inoculated with 106 CFU. Compared to all strains tested except MGAS315ΔslaA (yellow circles), the highly virulent reference strain MGAS315 (red circles) results in a significantly lower survival over time (logrank test). (B) The 50% lethal dose (LD50) was determined by Probit analysis. The highly virulent reference strain MGAS315 (red bar) has a significantly lower LD50 (that is, it is more virulent) than the isogenic mutant strain lacking expression of the gene encoding mtsR (MGAS315ΔmtsR), mga (MGAS315Δ12bpmga), prsA (MGAS315ΔprsA), slaA (MGAS315ΔslaA) or speB (MGAS315ΔspeB) (various green bars, p calculated relative to strain MGAS315, Mann-Whitney test). Gene complementation restored the wild-type virulence phenotype to strains MGAS315ΔmtsRcomp, MGAS315Δ12bpmgacomp and MGAS315ΔprsAcomp (various hatched green bars, p calculated relative to the comparator isogenic mutant strain, Mann-Whitney test). (C) Compared to wild-type reference strain MGAS315, the bacterial burden of the isogenic mutant strains was not significantly different in infected wax worm larvae (student's t-test). Bacterial burden was quantified from pools of five homogenized larvae (n = 4 pools per strain) inoculated with 105 CFU of the indicated strains and harvested at 18 h post-inoculation. (D) Microscopic examination of MGAS315-infected wax worm larvae at three hours post-inoculation shows multiple coalescing abscess-like lesions characterized by extensive melanization (boxed regions). In comparison, the SpeB-deficient strain causes considerably smaller lesions with less melanization (circled regions) (Hematoxylin and eosin stain, 4x original magnification). (E) In vivo expression of mtsR, prsA and speB by wild-type strain MGAS315 was confirmed by TaqMan quantitative real-time PC R analysis.
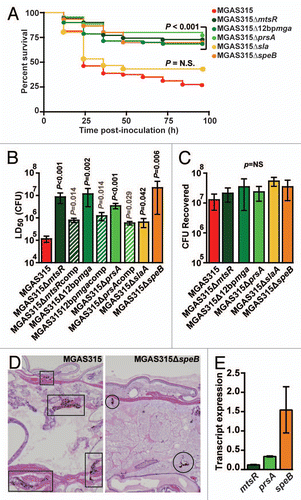
Figure 5 Subclone 8 GAS strains are significantly more virulent than subclone 5 GAS strains in the Galleria mellonella infection model. Subclone 5 strains of GAS were previously shown to lack necrotizing fasciitis capacity in mice, monkeys and humans.Citation13 Subclone 8 strains of GAS are newly emerged descendents of subclone 5 strains that epidemiologically have regained necrotizing fasciitis capacity in humans.Citation3 To test the hypothesis that subclone 8 strains are more virulent than subclone 5 strains, wax worm larvae were infected with serial dilutions (107, 106, 105 and 104 CFU) of five representative strains from each subclone lineage, and survival was monitored for 96 h. Results from five independent assays are shown (n = 10 larvae per strain per dose per experiment with five strains tested from each subclone lineage). (A) Kaplan-Meier survival curves were determined for wax worms inoculated with 107 CFU. Compared to subclone 5 strains (violet diamonds), subclone 8 strains (pink diamonds) result in significantly lower survival over time (logrank test). (B) The 50% lethal dose (LD50) was determined by Probit analysis. Subclone 8 strains (pink bars) have a significantly lower LD50 (that is, they are more virulent) than subclone 5 strains (violet bars) (Mann-Whitney test).
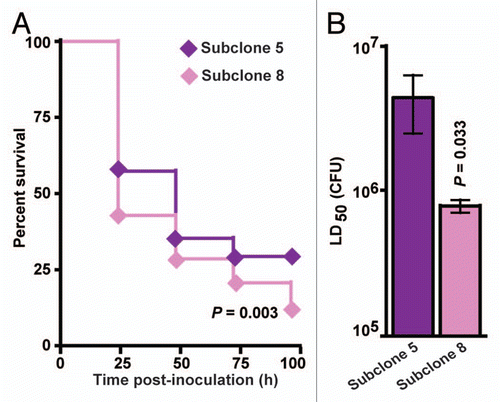
Table 1 Serotype M3 GAS strains used in this study
Acknowledgements
This work was supported in part by American Heart Association grant AHA0775045.
References
- Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D'Argenio DA, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA 2006; 103:8487 - 8492
- Mwangi MM, Wu SW, Zhou Y, Sieradzki K, de Lencastre H, Richardson P, et al. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci USA 2007; 104:9451 - 9456
- Beres SB, Carroll RK, Shea PR, Sitkiewicz I, Martinez-Gutierrez JC, Low DE, et al. Molecular complexity of successive bacterial epidemics deconvoluted by comparative pathogenomics. Proc Natl Acad Sci USA 2010; 107:4371 - 4376
- Kennedy AD, Otto M, Braughton KR, Whitney AR, Chen L, Mathema B, et al. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci USA 2008; 105:1327 - 1332
- Beres SB, Richter EW, Nagiec MJ, Sumby P, Porcella SF, DeLeo FR, et al. Molecular genetic anatomy of inter- and intraserotype variation in the human bacterial pathogen group A Streptococcus. Proc Natl Acad Sci USA 2006; 103:7059 - 7064
- Musser JM, DeLeo FR. Toward a genome-wide systems biology analysis of host-pathogen interactions in group A Streptococcus. Am J Pathol 2005; 167:1461 - 1472
- Olsen RJ, Musser JM. Molecular pathogenesis of necrotizing fasciitis. Annu Rev Pathol 2010; 5:1 - 31
- Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005; 5:685 - 694
- Olsen RJ, Shelburne SA, Musser JM. Molecular mechanisms underlying group A streptococcal pathogenesis. Cell Microbiol 2009; 11:1 - 12
- Davies HD, McGeer A, Schwartz B, Green K, Cann D, Simor AE, et al. Invasive group A streptococcal infections in Ontario, Canada. Ontario Group A Streptococcal Study Group. N Engl J Med 1996; 335:547 - 554
- Li Z, Sakota V, Jackson D, Franklin AR, Beall B. Array of M protein gene subtypes in 1,064 recent invasive group A Streptococcus isolates recovered from the active bacterial core surveillance. J Infect Dis 2003; 188:1587 - 1592
- Daneman N, Green KA, Low DE, Simor AE, Willey B, Schwartz B, et al. Surveillance for hospital outbreaks of invasive group A streptococcal infections in Ontario, Canada 1992 to 2000. Ann Intern Med 2007; 147:234 - 241
- Olsen RJ, Sitkiewicz I, Ayeras AA, Gonulal VE, Cantu C, Beres SB, et al. Decreased necrotizing fasciitis capacity caused by a single nucleotide mutation that alters a multiple gene virulence axis. Proc Natl Acad Sci USA 2010; 107:888 - 893
- Olsen RJ, Ashraf M, Gonulal VE, Ayeras AA, Cantu C, Shea PR, et al. Lower respiratory tract infection in cynomolgus macaques (Macaca fascicularis) infected with group A Streptococcus. Microb Pathog 2010; 49:336 - 347
- Kavanagh K, Reeves EP. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev 2004; 28:101 - 112
- Peleg AY, Jara S, Monga D, Eliopoulos GM, Moellering RC Jr, Mylonakis E. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob Agents Chemother 2009; 53:2605 - 2609
- Renwick J, Daly P, Reeves EP, Kavanagh K. Susceptibility of larvae of Galleria mellonella to infection by Aspergillus fumigatus is dependent upon stage of conidial germination. Mycopathologia 2006; 161:377 - 384
- Fedhila S, Daou N, Lereclus D, Nielsen-LeRoux C. Identification of Bacillus cereus internalin and other candidate virulence genes specifically induced during oral infection in insects. Mol Microbiol 2006; 62:339 - 355
- Seed KD, Dennis JJ. Development of Galleria mellonella as an alternative infection model for the Burkholderia cepacia complex. Infect Immun 2008; 76:1267 - 1275
- Cotter G, Doyle S, Kavanagh K. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol Med Microbiol 2000; 27:163 - 169
- Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, et al. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun 2005; 73:3842 - 3850
- Aperis G, Fuchs BB, Anderson CA, Warner JE, Calderwood SB, Mylonakis E. Galleria mellonella as a model host to study infection by the Francisella tularensis live vaccine strain. Microbes Infect 2007; 9:729 - 734
- Mukherjee K, Altincicek B, Hain T, Domann E, Vilcinskas A, Chakraborty T. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl Environ Microbiol 2010; 76:310 - 317
- Jander G, Rahme LG, Ausubel FM. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J Bacteriol 2000; 182:3843 - 3845
- Peleg AY, Monga D, Pillai S, Mylonakis E, Moellering RC Jr, Eliopoulos GM. Reduced susceptibility to vancomycin influences pathogenicity in Staphylococcus aureus infection. J Infect Dis 2009; 199:532 - 536
- Kobayashi SD, DeLeo FR. An update on community-associated MRSA virulence. Curr Opin Pharmacol 2009; 9:545 - 551
- Smoot LM, Smoot JC, Graham MR, Somerville GA, Sturdevant DE, Migliaccio CA, et al. Global differential gene expression in response to growth temperature alteration in group A Streptococcus. Proc Natl Acad Sci USA 2001; 98:10416 - 10421
- Tojo S, Naganuma F, Arakawa K, Yokoo S. Involvement of both granular cells and plasmatocytes in phagocytic reactions in the greater wax moth, Galleria mellonella. J Insect Physiol 2000; 46:1129 - 1135
- Soderhall K, Cerenius L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr Opin Immunol 1998; 10:23 - 28
- Sun H, Ringdahl U, Homeister JW, Fay WP, Engleberg NC, Yang AY, et al. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science 2004; 305:1283 - 1286
- Agniswamy J, Lei B, Musser JM, Sun PD. Insight of host immune evasion mediated by two variants of group A Streptococcus Mac protein. J Biol Chem 2004; 279:52789 - 52796
- Sumby P, Zhang S, Whitney AR, Falugi F, Grandi G, Graviss EA, et al. A chemokine-degrading extracellular protease made by group A Streptococcus alters pathogenesis by enhancing evasion of the innate immune response. Infect Immun 2008; 76:978 - 985
- Beres SB, Sylva GL, Barbian KD, Lei B, Hoff JS, Mammarella ND, et al. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype and clone emergence. Proc Natl Acad Sci USA 2002; 99:10078 - 10083
- Sitkiewicz I, Nagiec MJ, Sumby P, Butler SD, Cywes-Bentley C, Musser JM. Emergence of a bacterial clone with enhanced virulence by acquisition of a phage encoding a secreted phospholipase A2. Proc Natl Acad Sci USA 2006; 103:16009 - 16014
- Johansson L, Thulin P, Sendi P, Hertzen E, Linder A, Akesson P, et al. LL-37 in severe Streptococcus pyogenes soft tissue infections. Infect Immun 2008; 76:3399 - 3404
- Hollands A, Aziz RK, Kansal R, Kotb M, Nizet V, Walker MJ. A naturally occurring mutation in ropB suppresses SpeB expression and reduces M1T1 group A streptococcal systemic virulence. PLoS ONE 2008; 3:4102
- Shea PR, Virtaneva K, Kupko JJ 3rd, Porcella SF, Barry WT, Wright FA, et al. Interactome analysis of longitudinal pharyngeal infection of cynomolgus macaques by group A Streptococcus. Proc Natl Acad Sci USA 2010; 107:4693 - 4698
- Sumby P, Barbian KD, Gardner DJ, Whitney AR, Welty DM, Long RD, et al. Extracellular deoxyribo-nuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc Natl Acad Sci USA 2005; 102:1679 - 1684
- Sumby P, Tart AH, Musser JM. A non-human primate model of acute group A Streptococcus pharyngitis. Methods Mol Biol 2008; 431:255 - 267
- McIver KS. Stand-alone response regulators controlling global virulence networks in Streptococcus pyogenes. Contrib Microbiol 2009; 16:103 - 119
- Stevens DL, Tanner MH, Winship J, Swarts R, Ries KM, Schlievert PM, et al. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med 1989; 321:1 - 7
- Mehta S, McGeer A, Low DE, Hallett D, Bowman DJ, Grossman SL, et al. Morbidity and mortality of patients with invasive group A streptococcal infections admitted to the ICU. Chest 2006; 130:1679 - 1686
- Sharkawy A, Low DE, Saginur R, Gregson D, Schwartz B, Jessamine P, et al. Severe group A streptococcal soft-tissue infections in Ontario: 1992–1996. Clin Infect Dis 2002; 34:454 - 460
- Mereghetti L, Sitkiewicz I, Green NM, Musser JM. Remodeling of the Streptococcus agalactiae transcriptome in response to growth temperature. PLoS ONE 2008; 3:2785
- Pandya U, Allen CA, Watson DA, Niesel DW. Global profiling of Streptococcus pneumoniae gene expression at different growth temperatures. Gene 2005; 360:45 - 54
- Voyich JM, Musser JM, DeLeo FR. Streptococcus pyogenes and human neutrophils: a paradigm for evasion of innate host defense by bacterial pathogens. Microbes Infect 2004; 6:1117 - 1123
- Loughman JA, Caparon M. Regulation of SpeB in Streptococcus pyogenes by pH and NaCl: a model for in vivo gene expression. J Bacteriol 2006; 188:399 - 408
- Shelburne SA, Olsen RJ, Suber B, Sahasrabhojane P, Sumby P, Brennan RG, et al. A combination of independent transcriptional regulators shapes bacterial virulence gene expression during infection. PLoS Pathog 2010; 6:1000817
- Aziz RK, Kansal R, Aronow BJ, Taylor WL, Rowe SL, Kubal M, et al. Microevolution of group A streptococci in vivo: capturing regulatory networks engaged in sociomicrobiology, niche adaptation and hypervirulence. PLoS ONE 2010; 5:9798
- Hoe NP, Fullerton KE, Liu M, Peters JE, Gackstetter GD, Adams GJ, et al. Molecular genetic analysis of 675 Group A Streptococcus isolates collected in a carrier study at Lackland Air Force Base, San Antonio, Texas. J Infect Dis 2003; 188:818 - 827