Abstract
A naturally occurring gastrointestinal disease, primarily of ruminants (Johne disease), is a chronic debilitating disease that is caused by Mycobacterium avium subspecies paratuberculosis (MAP). MAP infection occurs primarily in utero and in newborns. Outside our Dietzia probiotic treatment, there are no preventive/curative therapies for bovine paratuberculosis. Interestingly, MAP is at the center of controversy as to its role in (cause of) Crohn disease (CD) and more recently, its role in diabetes, ulcerative colitis, and irritable bowel syndrome (IBS); the latter two, like CD, are considered to be a result of chronic intestinal inflammation. Treatments, both conventional and biologic agents, which induce and maintain remission are directed at curtailing processes that are an intricate part of inflammation. Most possess side effects of varying severity, lose therapeutic value, and more importantly, none routinely result in prevention and/or cures. Based on (a) similarities of Johne disease and Crohn disease, (b) a report that Dietzia inhibited growth of MAP under specific culture conditions, and (c) findings that Dietzia when used as a probiotic, (i) was therapeutic for adult bovine paratuberculosis, and (ii) prevented development of disease in MAP-infected calves, the goal of the present investigations was to design protocols that have applicability for IBD patients. Dietzia was found safe for cattle of all ages and for normal and immunodeficient mice. The results strongly warrant clinical evaluation as a probiotic, in combination with/without dexamethasone..
Introduction
Crohn disease (CD), ulcerative colitis (UC), and irritable bowel syndrome (IBS), sub-phenotypes of inflammatory bowel disease (IBD), are generally considered a result of chronic gastrointestinal inflammation. Based primarily on histopathology, genetic predisposition, and effective prophylactic treatments, this inflammation has been postulated to be a consequence of an unregulated immune response due to lack of regulator cell function, deletion of antigen-reactive cells (oral mucosal tolerance), loss of epithelial barrier architecture, and the most recent model, innate immune deficiency/dysfunction.Citation1–Citation3 Selection of an optimum treatment depends on several factors, including disease severity and location, co-morbidities, previous response to specific therapeutics, and the presence of surgical resection. Conventional treatments are directed at achieving symptomatic relief and preventing relapses via interference with immune processes. They include anti-inflammatory drugs (azathioprine, 6-mercaptopurine), steroidsCitation4,Citation5 and more recently, biologic agents; infliximab,Citation6,Citation7 adalimumab,Citation8,Citation9 certoilizumab pegolCitation10 and natailizumab.Citation11 Major drawbacks of the older modalities are: many fail over prolonged periods; serious adverse events; discontinued use; and some, like steroids, are associated with an increased risk of lymphomaCitation12 and/or death.Citation13 In population-based studies, approximately 75% of individuals relapse over five years and the most effective agents are only 25–40% effective in maintaining remission over one year.Citation14,Citation15 Many of the monoclonal antibody biologics also have short-term side effects (with specific differences associated with each), in many cases lose effectiveness with extended use, and long-term effects have yet to be determined.Citation16–Citation23 However, based on the number of ongoing investigative-trials, they are considered the most promising. Even though these newer drugs have improved control of IBD considerably, it cannot be over emphasized, prevention, permanent remission, and more importantly, cures still remain unmet medical needs.
Nonconventional interventions not at the forefront of research include diet,Citation24–Citation26 antibiotics,Citation27–Citation32,Citation131 helminthesCitation33,Citation34 and probiotics. Of these, the benefit of helminthes is impressive in that they are efficacious, presumably via increased regulatory T cell activity,Citation35 for symptomatic control and for preventing relapses in patients without serious accompanying side effects. Presently, the largest obstacle impeding more extensive use appears to be a limited supply relative to the number of patients. Diet modification and antibiotics have also been found beneficial, but not yet sufficiently to warrant extensive use.
The greatest benefits of probiotic treatments are seen in patients with less severe disease and for preventing relapses. Proposed mechanisms that underlie probiotic activity include: increased mucosal epithelial barrier, competitive exclusion of pathogens, immune-modulation (regulatory T cells), stimulation of epithelial innate immunity, and alteration of nutrients that interfere with growth of pathogens.Citation36–Citation40 Continuous administration of probiotics appears to be required because luminal or fecal concentrations of these organisms are increased only transiently; they return to undetectable levels within weeks after discontinuation. The importance of probiotic viability for beneficial effects remains questionable.Citation41 All probiotics do not appear to be equally therapeutic, likely because of different models in which they are studied and unresolved questions regarding dose, strain, and duration of treatment.Citation42–Citation45 Indigenous probiotic bacteria have beneficial effects for: (a) necrotizing enterocolitis and other maladies in preterm infants,Citation46–Citation49 (b) Clostridium difficile diarrhea and other infectious (viral) and antibiotic related diarrheas,Citation50–Citation54 (c) prevention and maintenance of UC/IBS remission,Citation55–Citation59 (d) recurrent UC-associated pouchitis,Citation60–Citation62 and (e) minimal hepatic encephalopathy.Citation63
The present investigations are extensions of previous reportsCitation81–Citation83 on the therapeutic value of Dietzia as a probiotic for an IBD-like disease of ruminants, called Johne disease.Citation64–Citation66 This disease, found predominantly in cattle, sheep, and goats has many manifestations in common with Crohn diseaseCitation66,Citation67 including debilitating diarrhea. As is the situation for IBD patients, outside Dietzia therapy, there are no preventive/curative treatments for animals with Johne disease. The etiologic agent of Johne disease is Mycobacterium avium subspecies paratuberculosis (MAP). Infection of cattle with MAP that results in clinical disease, usually at >2 years of age, occurs primarily in utero and in newborns (reviewed in ref. Citation68). For disease to be manifested in cattle, infection with MAP and immune incited intestinal inflammation are required.Citation65,Citation69 Interestingly, MAP is at the center of controversy regarding its role in CD, as well as sarcoidosis,Citation70 and more recently, in type-1 diabetes mellitus,Citation71–Citation73 Blau syndrome,Citation74 ulcerative colitisCitation75 and irritable bowel syndrome;Citation75 the latter two, like CD, are considered to be a result of chronic gastrointestinal inflammation. Although association of MAP with Crohn disease appears to be specific, and all the evidence taken together strongly indicate a causative role, such a role remains to be unequivalently established.Citation75–Citation79
A rarely studied genus of bacteria, Dietzia was found to (a) inhibit growth of MAP (hypothesized to be due to competition for iron) under specific in vitro culture conditions,Citation80 (b) effectively prevent/delay clinical manifestations of Johne disease in asymptomatic adult cattleCitation81,Citation82 and (c) eradicate MAP from calves that are infected in utero and/or as neonates (Click, unpublished). Based on these findings, plus the many similarities of Johne and Crohn diseases, the study herein was undertaken to assess whether a therapeutic protocol defined for cattle at different stages of infection (see Materials and Methods for definition of the four different stages) might have value for resolution of diarrhea, as well as inflammation, in Crohn patients, irrespective of whether MAP is/is not the etiologic agent. Since bovine biologic agents similar to those used for treating humans are not readily available or characterized, the steroid, dexamethasone was chosen as a means to mimic the human counterpart for controlling/reducing inflammation.
Results
Control of diarrhea in end-stage IV diseased cows.
The first experiments were to determine if Dietzia would have any therapeutic value for cows with daily and persistent “pipestream” diarrheic manifestations (at each bowel movement, independent of the number/day); this parameter, being the most easily monitored, defines (and is unique for) end-stage Johne disease (Stage IV), irrespective of in vitro test-parameters (ELISA, AGID, and/or fecal shedding values), weight loss and/or depressed appetite. Six clinically end-stage animals were fed 1×1011 colony forming units (cfu) of freshly prepared, non-frozen, viable Dietzia. For these initial investigations, Dietzia was grown in agar plates, which imposed severe limitations on quantities available. Consequently, many lapses occurred in daily treatment, which resulted in diarrheic relapse. In addition, inactivation of Dietzia by gamma-radiation also resulted in loss of its clinical benefit. Resuming treatment with viable Dietzia reversed clinical manifestations. In contrast, the 10 Stage IV control animals that were not treated with Dietzia continued with unabated diarrhea and never went into remission. Thus, oral treatment with viable Dietzia effectively controlled diarrhea in Stage IV animals although daily treatment was required to maintain this status.
An uninterrupted, daily Dietzia treatment became possible once protocols were developed to grow them in large biofermenters. To balance the abundance of material that would have to be stored frozen (viability decreased one log in six months at −20°C) versus costs, it was predetermined that the treatment-dose would be adjusted based on changes in clinical status and not on changes in any specific sero- or fecal-value. Pictured in (left panel) is an emaciated, Stage IV diseased cow, referred to as “Green-4,” prior to treatment. The consensus of three local veterinarians, plus my own assessment based on previous experience with untreated Stage IV animals, was that the odds of her surviving two weeks was zero. (right panel) shows her in remission after four months of daily treatment. Changes in body mass, milk production, ELISA and fecal MAP values are shown at different doses of Dietzia in . As shown in , once started on Dietzia, body mass and milk production both increased. Upon attaining remission, the Dietzia dose was tapered (), after which she clinically relapsed, lost weight and produced less milk. She also had a left side displaced abomasum (LDA)—one of the four stomachs became twisted—which was corrected non-surgically on 2/10/02. Because of the relapse and LDA, the dose of Dietzia was increased, and again an increase in body mass occurred. Being within months of calving, milking was discontinued (dried off) at this time. After recovering, Dietzia was again lowered; she calved with twins and later succumbed with end-stage IV clinical disease. At autopsy she was confirmed to harbor MAP in multiple tissues and the intestinal pathology was characteristic of paratuberculosis. As shown in , ELISA values remained essentially unchanged over the entire treatment period. She became AGID-positive (a serology test that correlates with a more advanced stage of disease than ELISA values) at the second test-date and remained so for the remainder of her life. In contrast, the extent of fecal shedding was extremely variable, appearing to be associated with the dose of Dietzia; the higher the dose, the lower the shedding. Changes in body mass appeared to lag changes in fecal shedding.
Dietzia treatment of animals with Stage II or III disease.
The second goal was to further refine a Dietzia treatment for animals with Johne disease that could be tested in humans with chronic diarrheal diseases. Because of variable ELISA values of animals with low to moderate initial values (ODs ≤3), determination of the longitudinally best-linear fit of values was the most informative way to assess effectiveness of treatment.Citation83 Such analysis also reduced the impact of each individual value. Employing this type analysis, it was previously reported that approximately half of Dietzia-treated Johne diseased cows had decreasing longitudinal ELISA changes, whereas all non-treated cows had increasing values.Citation83 shows such decreasing ELISA values for seven cows considered cured by treatment with Dietzia only; however, as previously reported, not all animals with decreasing values were cured. For the cured cows, treatment was discontinued after all paratuberculosis test-parameters became negative. Only one animal succumbed early (cow 13) due to complications from milk fever post-calving; all others were terminated at the end of the study with no evidence of clinical disease [milk fever is due to inadequate Ca++ absorption from the intestines, compensated by calcium depletion in muscle that causes an inability to stand and/or move (potentially causing permanent nerve damage in limbs and if sufficiently severe, cardiac failure and death)]. The median treatment time was 15 months and the median survival from the time they were detected paratuberculosis positive was >50 months (), a value not significantly different from that of paratuberculosis-free animals.Citation83 Survival times with a positive symbol (+) indicate the animal was terminated for reasons other than Johne disease; i.e., survival would have been longer than shown. Because some animals never fecally shed MAP and others shed only once or twice, shedding values are shown only in the table. None were shedding when terminated and none ever became AGID-positive. Moreover, none showed any clinical symptoms of Johne disease at any time, and at autopsy, cows #13 and #52 were completely devoid of any intestinal inflammation characteristic of Johne disease and lacked culture/PCR detectable MAP in all tissues.
In contrast, the median survival of animals with increasing longitudinal ELISA values was, (a) 9 months for untreated animals (all but 33 and 3056 succumbed with end stage disease and only one survived to became AGID-positive) and (b) 21 months for those treated [again “months number” with a positive symbol (+) indicate the animal was terminated for reasons other than Johne disease]. Median treatment time was again 15 months; however, unlike the animals considered cured, 12 of 16 eventually succumbed with end-stage clinical disease. All 12 were AGID-positive; three of the four that remained AGID-negative had very short survival times and the fourth one was terminated for reproductive reasons with Stage II disease. Thus, even though animals with increasing ELISA values were not cured, their survival time was significantly extended.
The initial, maximum, and final ELISA and fecal shedding values are also presented in . The mean initial ELISA values of the three groups (not treated, treated with a declining ELISA, and treated with an increasing ELISA) were not significantly different (p > 0.35), suggesting that each group, on average, was at a similar stage of disease when treatment was initiated. The final, mean ELISA value of the cured group (declining ELISA) was significantly less than the final means of the other two (p < 0.0001); the latter two were not significantly different from each other (p > 0.05). Also fecal shedding (final) was absent in the cured group, but was detected in most animals in the other groups.
One animal (cow #198) had longitudinal ELISA changes that were biphasic. As shown in , during the initial 18 months of treatment, the best-linear-fit curve declined to negative values similar to that found for animals considered cured. However, after discontinuing treatment for nine months, the longitudinal best-linear-fit curve increased similar to that of non-treated paratuberculosis animalsCitation81,Citation82 and she succumbed seven months later with end-stage disease. Changes in the dose of Dietzia are also shown. She was AGID-negative throughout her treatment, shed MAP only once, 60 cfu on her final test, and had intestinal pathology consistent with paratuberculosis at autopsy.
Combination treatment of animals with Stage II , III , or IV disease with Dietzia plus dexamethasone.
The finding that a Dietzia probiotic treatment was extremely effective for some Stage II (asymptomatic) animals, but not for all, raised an important question as to why. The observed preferential curtailment (or maintenance of low to undetectable levels) of fecal MAP at high doses of Dietzia prior to any measurable loss in serum ELISA or AGID antibodies (presumed to be a response to MAP antigens and to be relevant for intestinal mucosal damage), suggested an exploratory trial in which immune function was curtailed independently of Dietzia. Therefore, Dietzia treatment was combined with dexamethasone, a glucocorticoid shown have immunosuppressive activity in cattle.Citation84 The rationale was to inhibit immune activity independently of that which followed declining MAP levels after treatments with high doses of Dietzia. Results for such dual treatments are shown for individual animals in and –.
As shown in , April (weighing 800 pounds) relapsed from Stage II (asymptomatic) to Stage IV (severe clinical disease) on 11/25/05 while being treated with Dietzia only. At this time she was given dexamethasone (2 mg/day IM) for seven days, which reversed the clinical symptoms. She was then maintained on 0.4 mg every other day for two months followed by 0.2 mg every other day for another two months; at which time dexamethasone was discontinued (Dietzia was continued). She remained asymptomatic (Stage II) for an additional seven months at which time she again relapsed (Stage III). Based on previous experience, it was deemed unlikely further treatment would be beneficial and she was terminated. As shown, her ELISA values increased or remained unchanged up until dexamethasone was initiated, at which time, antibodies detected by ELISA declined. AGID antibodies detected at the first five test points became undetectable by the last two, further supporting a curtailment of humoral immune activity to MAP. Fecal shedding increased to a stable level and only declined after dexamethasone was discontinued and Dietzia was increased to >9 × 1011 cfu.
shows the effects of Dietzia only at Stage II, and later (at Stage III) in combination with dexamethasone for cow #232, a Holstein weighing 1200 pounds. ELISA values increased steadily during early Dietzia treatment, reached a plateau, and declined after dexamethasone treatment was initiated. AGID became positive and eventually returned to negative after extended treatment with dexamethasone. The induction dose of dexamethasone (5 mg/day) that resulted in remission was higher than that found for April, most likely due to body weight differences. Fecal shedding was low during Dietzia treatment, but dramatically higher while on dexamethasone. She was also terminated when clinical Stage III disease, presumed irresolvable, reoccurred.
shows the effect of treating Trixie, a Jersey weighing 1000 pounds with lifetime (Stage III) intermittent diarrhea, with Dietzia daily, and dexamethasone only during relapses. As shown, fecal shedding was not controlled by the dual treatment. She was AGID-positive at the first test-point and was negative at all subsequent times. Her ELISA values steadily declined, and two weeks prior to her unexpected death, was 1.4, the cut-off value to be classified as paratuberculosis sero-negative. In contrast to all other cows that succumbed with Johne disease, she showed no signs of clinical disease prior to death (no weight loss or watery manure), but was constipated some 2–3 weeks prior to succumbing. At autopsy, she was found to have severe granulomatous colitis with ulceration, which led to subacute fibrinosupporative peritonitis.
In an effort to circumvent the apparent counter-effect of dexamethasone on Dietzia's curtailment of MAP shedding, two cows (#255 and Monica), Jerseys weighing 850 pounds each and showing clinical symptoms (255 with Stage III and Monica with Stage IV), were treated with dexamethasone prior to initiation of Dietzia treatment. Cow 255 was treated with dexamethasone, 6 mg every other day, for three weeks and then discontinued. Three weeks later, she was started on a high daily dose of Dietzia and continued for five months at which time Dietzia treatment was discontinued. As shown in , the ELISA values fell to 0.93 and fecal shedding was undetectable on multiple tests prior to her untimely accidental death five months post-treatment. Monica, with Stage IV clinical disease, was given 5 mg dexamethasone daily for two weeks, which alleviated pipestream diarrhea and depressed appetite, and then discontinued. Thereafter, a daily dose of 1012 cfu Dietzia was initiated. She succumbed two weeks later with normal manure consistency and appetite, suggesting that intestinal damage was so advanced that treatment was ineffective. Unfortunately, an autopsy was not an option because she died on a summer Saturday when the university diagnostic laboratory was closed.
Safety of Dietzia.
Both paratuberculosis-free and -positive adult cattle reported herein and previouslyCitation81–Citation83 showed no adverse effects, some for over 3½ years, that could be attributed to longterm Dietzia treatment; moreover, five paratuberculosis-free cows fed Dietzia for up to a year never became sero-positive for Dietzia.Citation81 In addition, none of 46 newborn calves (36 from paratuberculosis-negative and 10 from -positive dams) fed daily with Dietzia for 60 days became positive for any parameter (including antibody) associated with Johne disease or showed any adverse long-term side-effects (Click, unpublished). All calves gained weight and matured indistinguishably from untreated calves. During their lives of up to 70 months, there were no signs of disease or sickness related to treatment. Further evidence of safety was demonstrated by IP injection of viable Dietzia into normal C.B20 and immunoincompetent C.B17 scid/scid mice. All of the parameters monitored (weight, diarrhea, reproduction) in the treated mice were indistinguishable from those of untreated mice.
Any impact of oral consumption of Dietzia related to food chain safety was also examined. The amount of viable Dietzia in LTLT pasteurized milk was <1 cfu/L, compared to the unpasteurized, spiked original, which still possessed 1×108 cfu/ml. Further, Dietzia was undetectable in milk from three Dietziatreated cows.
Discussion
Based on similarities of Crohn and Johne diseases, the investigations presented herein with adult cattle were undertaken to evaluate whether a protocol employing Dietzia, a bacterium with MAP growth inhibitory activity in culture, and one found therapeutic when used as a probiotic, short- and long-term, for cattle, could be adapted for use in IBD (especially CD) patients. The ultimate goal was two-fold: (1) ameliorate diarrhea in the short-term, and (2) define a protocol with potential long-term therapeutic (curative) value. Findings with end-stage (Stage IV) Johne diseased animals indicated that diarrhea was ameliorated by a daily treatment of viable Dietzia, but not by gamma-radiated Dietzia or by intermittent treatment; suggesting viable Dietzia functioned as a conventional probiotic. Clinical remission of Stage IV diseased animals (example, cow Green-4) was achieved with uninterrupted high doses of Dietzia, whereas at lower doses or intermittent dosing, relapses occurred. This oscillation was most likely due to both a probiotic effect and a reduction (killing?) of the etiologic agent, MAP per se. The findings suggest that a consistently high dose of Dietzia (>1012 cfu) over the entire duration of treatment may have resulted in even better therapeutic outcomes; however, at the time it was deemed more important to investigate effectiveness of specific doses at different stages of disease.
To be an effective, long-term treatment for Johne disease, two processes must be curtailed: (a) inflammation of the intestine, presumed to be due to an immune response to MAP, and (b) eradication of MAP, the etiologic agent stimulating immune reactivity. Previous and present results indicate that the clinical efficacy of Dietzia treatment was directly associated with longitudinal changes of ELISA values; maximum survival and cures were associated with decreasing ELISA values. Treated animals with increasing values were not cured, but median survival was extended relative to those not treated.
The results obtained with cow #198, in which the ELISA best-fit analysis showed a biphasic curve, raise a question regarding animals defined as cured. Initial treatment of 198 resulted in declining best-fit ELISA values similar to those of animals considered cured. The ELISA-negative values were maintained for nine months post-treatment. At this time, the ELISA values began to increase at a rate similar to that of non-treated animalsCitation82,Citation83 and she eventually succumbed with end stage clinical disease, which was confirmed at autopsy. Thus, was disappearance of disease-associated test-parameters actually a consequence of MAP elimination (cured) or was the undetected level of fecal MAP simply insufficient to induce antibody synthesis? Or would have positive parameters reappeared in the eight animals considered cured had they survived longer? Irrespective of the answer, a number of cows (#s 228, 229, 36, 1734) survived more than 50 months after initiation of treatment favoring the absence of MAP. The probability of recurrence cannot be ruled out for those with shorter survival times, although cows #13 and #52 were devoid of any evidence suggestive of paratuberculosis at autopsy. To distinguish between the alternatives is likely untestable because of limited lifespans; indeed, is it even relevant for dairy cattle because of their short productive lifetimes (average of 4.5 years)? More importantly, cures obtained with Dietzia were unlike the palliative benefits described for paratuberculosis goats and cattle that were treated with anti-mycobacterials.Citation85–Citation87
Other unanswered questions are: Would continued treatment of cow #198, after becoming negative for paratuberculosis parameters, prevented recurrence? Why was a daily dose required to achieve beneficial results and why were all cows treated with Dietzia not cured. Possible explanations include: (a) the bovine intestine is not an optimal environment for Dietzia since it grows best in vitro at 29°C (bovine body temperature is 39°C), (b) Dietzia requires fructose as its carbon source and it has a rather slow generation time of 3–4 h, (c) different bovine resistance/susceptibility genetics,Citation88–Citation92 (d) genetics of different subtypes of MAP present on the farms the cows came from, (e) use of insufficient amounts of Dietzia, and/or (f) differences in intestinal integrity present at the start of treatment.
As a means to (a) enhance cures/survival times, (b) uncouple Dietzia's reduction of MAP fecal excretion from subsequent antibody decline, and (c) more closely mimic protocols presently employed to treat IBD patients, a pilot investigation was undertaken with dexamethasone, a glucocorticoid shown to be immunosuppressive in cattle,Citation84 in combination with Dietzia. As expected, dexamethasone effectively reduced both serum ELISA and/or AGID detectable antibodies specific for MAP in all animals that were dually treated (). However, such impairment was accompanied by an exacerbation of the reduced and/or maintained low levels of fecal shedding associated with high doses of Dietzia; a reduction that normally occurred prior to any curtailment of specific antibodies.Citation83 In addition, the one animal treated extensively with dexamethasone developed an ulcer, similar to fistulas present in Crohn patients, which led to her death.
Even though the mechanism by which Dietzia curtailed MAP remains unknown, because it, unlike indigenous probiotics, prevented growth of the etiologic agent (MAP) in culture, hypotheses being considered are; (a) close intracellular (macrophage?) encounters of MAP and Dietzia could easily be enhanced by crossreactive antibodies specific for epitopes they share (Richards, personal communication and Click, unpublished), thus allowing enhanced opsonization of Dietzia/MAP by phagocytes possessing MAP/Dietzia, (b) Dietzia, when in close proximity to MAP, either inhibits its growth via competition for a nutrient(s), or directly kills it, (c) steroid disruption of normal phagocytic cellular activity/integrity could impair potential MAP killing, and (d) in the absence of co-infection or macrophage impairment, MAP levels would not be curtailed. The most intriguing aspect of these postulates is that for Dietzia to effectively reduce and eliminate MAP, immune reactivity (with an emphasis on humoral) to MAP is essential, even though such activity in the absence of Dietzia is not beneficial/curative. Thus, the perplexing problem is: How can inflammation at the mucosal barrier be curtailed sufficiently without impacting the immunological reactivity necessary to eliminate MAP? In many cases, curtailment could take a very long time, during which damage to the intestines may become so extensive that recovery is improbable, as appears to be the case for the Jersey cow, Monica (she was shedding an astonishingly actual count of 9,200 cfu/2 gms fecal material). Such a scenario may be extremely relevant for immunosuppressive biologic regimens presently used for IBD patients; i.e., can cures ever be attained using only immune modulating protocols? A short-term immunosuppressive treatment prior to treating with Dietzia (#255), might be an alternative.
In summary, the results presented herein define:
a successful treatment and eradication of asymptomatic MAP infection with Dietzia, when used as a probiotic;
a successful Dietzia treatment for symptomatic and otherwise terminal MAP infection;
that the Dietzia treatment for cows with symptomatic disease is enhanced by short treatment-intervals with dexamethasone;
in cows with asymptomatic MAP infection, daily probiotic therapy can be discontinued once in vitro test-parameters of MAP infection become negative, while animals with end-stage clinical disease manifested by severe diarrhea, must be continued on daily therapy for their lifetime;
that daily administration of viable Dietzia to cows for up to three years and injection into normal or immunodeficient mice was safe, with no apparent lifetime side effects.
Similarity of Human and Bovine Gastrointestinal Diseases
Crohn disease, ulcerative colitis, and IBS are generally accepted to be a result of chronic gastrointestinal inflammation; the present discussion will, however, focus primarily on Crohn disease. Environmental factors play a role in the development, and prevalence varies with time, geography, socioeconomic conditions, and occupation. The incidence is more common in urban than in rural areas and in highly developed industrialized countries than in less developed tropical countries.Citation33,Citation93 CD is a chronic debilitating disease characterized by an unpredictable disease course, potential complications such as fistulas, and the frequent need for surgery. Ultimately, alterations in the composition of the intestinal flora may promote bacterial invasion of the mucosa and predispose patients to chronic inflammation. Inflammation is characterized by an inductive or initiation phase followed by a sustained response that ends when there is resolution of the process(es). This abnormal response may have two origins:Citation94 “an increased proinflammatory response to a bacterial component or a decreased regulatory response, which may lead to an excessive effector immune response.” As a consequence, an unregulated immune response develops to, at least in part, microbial antigens. Although the genetic make-up of an individual confers risk to develop CD, specific susceptibility genes (some in common with those present in cattleCitation91,Citation130) are neither required nor sufficient.
Factors that influence the choice of therapy in Crohn disease are multifactorial. Conventional treatments are directed primarily at achieving symptomatic relief, preventing relapses, and steroid sparing; i.e., treatments are directed at controlling inflammation directly. They are not directed at the elusive undefined etiologic agent(s). Immune modulation is most efficacious if implemented during early disease and tends to be less effective for late stages.Citation95–Citation100 At present there are no conventional treatments that result in cures or permanent remissions. It is postulated that microbial species in non-inflamed tissue, prior to detection of inflammation, hold clues to microbial pathogenesis, primarily because initiation of disease process(es) precedes clinical manifestations.Citation76,Citation79,Citation101,Citation102 Microorganisms implicated in CD (some for UC), besides MAP, include Candida,Citation103 enteroadherent-invasive E. coli strains,Citation104–Citation106 Bacteroides and Fusobacterium speciesCitation107 and even normal commensal nonpathogenic species.Citation108–Citation111 Perhaps the most important question to ask regarding a role for any of these organisms is: Are they etiologic agents? Are they merely perpetuators of disease once events leading to disease are initiated?Citation112 Or are they merely opportunistic bystanders? Presently, association of these different organisms with disease best fit the latter two alternatives, and not etiologically initiators, primarily because the time of initial insult remains unknown.Citation79,Citation113–Citation116 If the initiation event, relative to detectable parameters associated with disease, is similar to one (in utero) ascribed for Johne disease of cattle, it very well may occur years prior to onset of detectable disease, clearly way before identification of intestinal microbes present at the time of diagnosis of clinical disease.
It has been proposed that a therapy directed against both a bacterial etiology agent and against inflammation may be a more fruitful approach to controlling Crohn disease than conventional mono-therapies.Citation27,Citation117,Citation118 Therapeutic strategies aimed at restoring the host microbe balance at the intestinal mucosa by fecal bacteriotherapyCitation132 or with probiotics may prove superior to, with far less side-effects, treatments that broadly suppress inflammation and/or innate immunityCitation119 or may prove to be an exceedingly beneficial adjunct therapy. An excellent example of this is that combination therapy (probiotics as one agent) improved cure rates for Helicobacter pylori.Citation120
Proposed Adaption of Dietzia Treatment to Crohn Patients
Treatments for Crohn and Johne diseases are most effective when started at early stages of disease. Based on findings that human gastrointestinal,Citation46–Citation62 hepaticCitation63 and other diseasesCitation121 were successfully treated with organisms functioning as probiotics, it is surprising that probiotics are not effective for CD;Citation58,Citation122,Citation123 in fact, Lactobacilli were completely ineffective.Citation124,Citation125 However, because this group of IBD patients has the highest incidence of MAP infection, MAP could be a useful target for mitigating disease, irrespective of whether it is/is not the causative/perpetuitive etiology agent. This fact, in conjunction with the presumed dual activity—probiotic function (ameliorate diarrhea) plus direct inhibition of MAP growth (in culture)—of the non-indigenous probiotic, Dietzia, is sufficiently compelling to warrant undertaking a clinical Dietzia trial with Crohn patients, especially those documented to harbor MAP. Because viable Dietzia had no adverse side effects and was nonpathogenic when administered orally to adult cows or newborn calves (Click, unpublished), as well as when injected IP into mice, it is anticipated that it will also be found safe for oral consumption by humans. Following is a proposed adaption of the cattle protocol for testing in a clinical trial.
A standard, constant (not variable as used herein for cattle) Dietzia dose of 1011 viable cfu per 100 lbs. body weight would be orally administered daily. Pass-through would be monitored.
Two groups of patients would be enrolled—those documented to be infected with MAP and those that are MAP-test-negative (even though they may also be infected). Appropriate randomization of non-treated and treated patients would be done to meet statistical criteria.
Parameters to be monitoredCitation126,Citation127 include serum antibody, quantitative determination of MAP in blood, and standard clinical assessment. Side effects would also be monitored.
Daily treatment would continue until all in vitro test parameters become negative and then each group would be divided into treatment terminated, or treatment continued (undefined time).
Materials and Methods
Experimental design.
The primary goal was to refine a previously published therapeutic protocol used to treat adult paratuberculosis cows for testing in humans. To this end, a roughly 4:1 ratio of paratuberculosis-free and -positive animals (see below for definition of each), under St. Croix Valley Farm (SCVF) ownership and management, were housed together in a tie-stall facility (each animal tied in their own space) as a single dairy herd. All aspects of the research were conducted using standard operating conditions of a normal dairy farm. At any given time, the herd was comprised of 50–60 females. Once a paratuberculosis parameter was detected positive, treatment was or was not initiated and then various paratuberculosis parameters were monitored over the animal's remaining lifetime. It was predetermined for this study, based on cost considerations and as a means to define effective doses for different stages of disease, that the dose of Dietzia would be adjusted for each animal based only on changes in clinical status and not on changes in any paratuberculosis-specific test parameter. Since many animals showed clinical relapse, the dose was empirically varied based on body mass and severity of clinical disease throughout the study. Emaciated animals were defined as having end-stage clinical Johne disease based on the presence of both “pipestream” diarrhea and depressed appetite. Local veterinarians humanely euthanized recumbent, emaciated and/or cachectic end-stage animals by intravenous injection of a sodium pentobarbital solution (Fatal Plus, 6 gm/ml, Vortech Pharmaceuticals, Dearborn, MI) at 1 ml/4.5 kg body weight when they no longer could get up and stand on their own. Animals that showed potential life-threatening non-Johne disease ramifications were sent to slaughter. All other aspects of the project were handled by normal dairy procedures.
Animals.
Most animals in the study were adult dairy cows in their second through fourth lactation. Severity of disease was defined by the following classification:Citation128 animals that were asymptomatic, and test-parameter negative were classified at Stage I, those asymptomatic, and either ELISA and/or fecal positive were classified as being at Stage II. Stage III animals showed signs of early clinical disease and were either ELISA and/or fecal positive, whereas, Stage IV animals were severe, end-stage clinically, irrespective of whether they were ELISA and/or fecal negative/positive (almost all were fecal shedding). Of the original Stage II and III diseased cows,Citation83 detected positive at dry-off (two months prior to their predicted calving date, milking was discontinued) by paratuberculosis-specific ELISAs (determined by the owners and their veterinarians), purchased from seven local, well-managed, moderately high-prevalence herds over a five-year period, 32, plus an additional 18 Stage IV and 3 Stage III cows not in the original group, are included in the present study. The final classification of an ELISA-positive animal as having Johne disease was based on whether it eventually (a) tested positive for a different parameter (fecal shedding or serum AGID), (b) developed end-stage (Stage IV) clinical disease, (c) was determined to have paratuberculosis via complete autopsy, and/or (d) only as a last resort, tested ELISA-positive multiple times. As reported previouslyCitation83 when an ELISA was positive (OD > 1.4), it was considered an accurate indicator of paratuberculosis. For the present experiments, animals were divided into two distinct groups; those with Stage II or III disease with ELISA values equal to or less than 3.0, and those with Stage IV disease (end-stage clinical disease). As the purpose was to assess the effect of intervention rather than confirm the findings of others that non-treated paratuberculosis animals eventually succumb with clinical disease, more Stage II and III animals were in the treated group (n = 26) than in the non-treated group (n = 9). Stage IV animals were either treated (n = 8) or not treated (n = 10).
Dietzia.
Dietzia (partially characterized and originally misclassified as Mycobacterium gardonae) was isolated from fecal material of a paratuberculosis sero- and fecal-positive cow.Citation80 It was reclassified as Dietzia based on its 16S rRNA sequence (performed by MIDI Labs, Inc., Newark, DE), which is considered the gold standard for bacterial identification.Citation129 Growth requirements were defined in agar plates. Ultimately, Dietzia was grown for 3–4 days at 29°C under contract in a 75-liter fermenter at the University of Minnesota Biotechnology Institute (St. Paul, MN) in fructose-supplemented tryptic soy broth. Batches were centrifuged, washed and concentrated 20-fold prior to storage in 45 ml aliquots at −80°C (long term) or −20°C (short term). New lots were prepared as needed, approximately every 2–3 months. The number of colony forming units/ml was determined prior to use. Once thawed, aliquots were maintained at 4°C for up to 10 days only. Dietzia treatment was always initiated after an animal was detected ELISA-positive. Based on preliminary dosage experiments, small cows (Jerseys and Jersey × Holstein crosses), and large cows (Holsteins and Guernseys) were initially treated by supplementing their morning feed with Dietzia at a minimally effective daily dose of 2–3×1011 and 4–5×1011 cfu, respectively. The dose was increased if an animal showed clinical signs of disease and then lowered if remission was achieved (see above reason). For a few specific treatments, Dietzia was inactivated by 10 Gy gamma-radiation from a cesium source. Based on the ineffectiveness of gamma-radiated Dietzia, non-treated animals were not given any placebo-type growth medium or other inert material.
The sensitivity of Dietzia to LTLT (Low Temperature, Long Time, 145°C for 30 min) pasteurization was determined to assess the possibility of viable Dietzia getting into the food chain through the milk supply. Milk from non-treated paratuberculosis-free animals was spiked with viable Dietzia to achieve 1x108 cfu/ml. One half of this milk was pasteurized and the other half served as the control. The amount of viable Dietzia in each was then determined. In addition, milk from three Dietzia-treated cows was cultured for Dietzia. The Dietzia culture assays were performed by Encore, LLC (Minneapolis, MN).
Body and milk weights, serum and fecal protocols.
Monthly changes in body weights and daily production of milk (official monthly DHIA milk weights) were initially used to monitor the progression of disease; but were discontinued because changes in each paralleled one another and because fecal composition and reduced feed intake were found to better and earlier define onset of impending clinical disease. Fecal material collected directly from the rectum using individual disposable gloves and blood obtained aseptically from the tail vein were transferred to sterile containers, coded, and sent chilled on the day of collection to Allied Monitor, Inc. (Fayette, MO). Allied Monitor is a USDA- and NVSL-approved laboratory that specializes in assays for Johne disease. The majority, but not all, fecal and serum samples were obtained concurrently. All serum ELISA and AGID assays and fecal MAP cultures were performed upon receipt. Assessment of the validity, sensitivity and specificity of assays (ELISA, AGID, fecal culture) was reported previously.Citation83 The ELISA for antibodies specific for MAP was performed using a crude, soluble, MAP protoplasmic antigen prepared by Allied. Test sera were preabsorbed with Mycobacterium phlei. Split-sample repeatability, as well as duplicate samples, varied less than 5 percent of the mean. The content of each well was read at a single wavelength (405 nm). ELISA values were calculated by dividing the test-sample OD by a value equivalent to ¼ the OD of a standard reference positive serum (range 0.13–0.14), and interpreted as follows: Negative ≤1.4 OD and Positive >1.4. Allied's classification of ELISAs as Negative (≤1.4 OD), Suspect (1.5 to 2.0 OD) and Positive (>2.0 OD) was reinterpretedCitation83 based on the fact that 11 of 14 animals with initial “suspect” serum ELISA values eventually became either fecal shedders and/or succumbed with end-stage clinical disease. Of the remaining three, one had multiple ELISAs >2.0, one was Dietzia-treated and became negative for all parameters, and one, not treated, was terminated for unresponsive mastitis three months after initiation of treatment. Therefore, the “suspect” category was not used and all animals with serum ELISAs >1.4 were classified paratuberculosis-positive.Citation83
Postmortem analysis.
As an additional means to document Johne disease status, pathological postmortem analysis and culture-determination of MAP in tissues was done on randomly chosen cows at the University of Minnesota Veterinary School Diagnostic Laboratory (St. Paul, MN). Summation of the University's standard basic necropsy, tissue histopathology of multiple organs, culture/PCR, bacteriology, parasitology, serology and molecular diagnostics was used to confirm positive/negative status only; it was not intended to define specific aspects, category of disease, or be compared to ante-mortem parameters.
Safety studies.
In addition to adult cattle that were treated daily with Dietzia, some for over 3½ years, the safety of Dietzia as a probiotic was tested on 46 newborn calves—10 from paratuberculosis-positive dams and 36 from -negative dams. A single daily oral dose of 1×1011 cfu of viable Dietzia was administered, for their first 60 days, in milk replacer (Click, unpublished). Males were slaughtered at 23–26 months and females, raised as replacements for the milking herd, were monitored over their lifetime, some over 70 months, for any adverse effects related to the Dietzia treatment.
The safety of Dietzia was also assessed using inbred, conventional CB.20 mice and immuno-incompetent CB.17-scid/scid mice. They were housed at the UWRF accredited facilities and were under the supervision of the Local University Staff Veterinarian. Four male and four females (one of each sex per cage) were injected IP with 1×108 cfu viable Dietzia. Four of each sex were not injected and served as controls. The mice were monitored for 6–12 months for any signs of disease (weight loss, diarrhea and reproductive problems). No discomfort, distress, pain or injury was observed outside the initial IP injection of Dietzia.
Statistical methods.
Survival time was defined as the number of months from the first detected positive ELISA value (or initial ELISA test for negative animals) until their demise. Linear longitudinal best-fit analysis was used to estimate trends in ELISA values for each animal. The student's t-test was used to assess differences in mean ELISA values. For all comparisons, p-values less than 0.05 were considered statistically significant.
Conflict of Interest
The author is a partner in Altick Associates, in SCVF, as well as a member of Paralab, LLC, which is the assignee of a patent application covering the Dietzia technology presented in this paper.
Figures and Tables
Figure 1 Photographs of Stage IV, end-stage, diseased cow, Green-4, before and after Dietzia treatment. Photo on the left is prior to treatment and photo on the right is four months post-treatment: note increase in body mass and improved coat appearance.
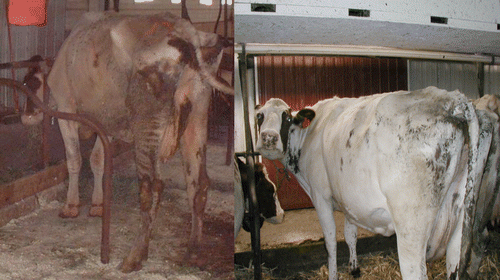
Figure 2 Longitudinal changes in body weight, milk production, ELISA values and fecal MAP for Stage IV cow, Green-4. (A) Dashed line is body weight and solid line is official DHIA weight of milk produced/day. (B) Solid line is ELISA OD405 nm values and dashed line is fecal MAP. Symbol (+) signifies a positive AGID. (C) Dose (viable colony forming units, cfu) of Dietzia.
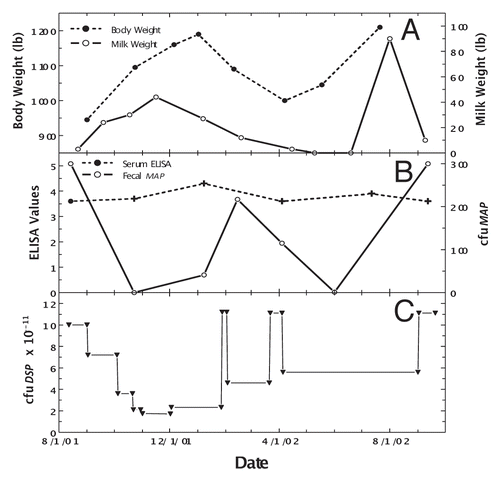
Figure 3 Longitudinal ELISA values for seven individual Stage II or III cows considered cured after Dietzia treatment. Vertical lines with animal's identification number indicate the time at which Dietzia treatment was terminated. Thin dashed lines denote best-fit of ELISA OD405 nm values. BL is 1.4 ELISA-positive/negative cutoff. As discussed in the text, during the initial months of treatment, the best-linear-fit curves declined to negative values (<1.4), suggesting the absence of systemic MAP (animal was cured)
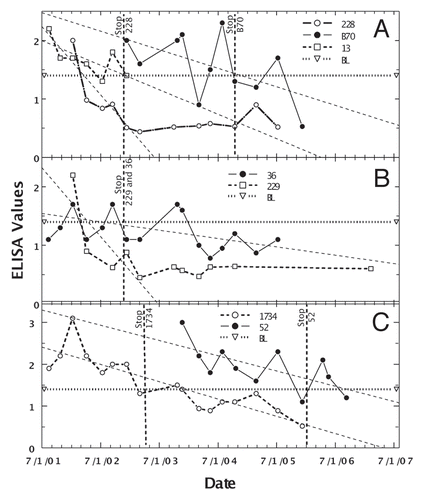
Figure 4 Longitudinal ELISA values for cow with Stage II, then Stage I and finally Stage IV disease (biphasic ELISA changes) at different doses of Dietzia. Thin dashed lines are best-fit for ELISA OD405 nm values. BL is 1.4 ELISA-positive/negative cutoff.
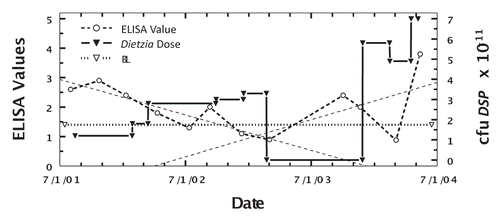
Figure 5 Longitudinal ELISA and fecal values for cow, April, with Stage II or IV disease at different doses of Dietzia and dexamethasone. Upper panel. Solid line is ELISA OD405 nm values and dashed line is fecal MAP. Symbol (+) signifies a positive AGID. BL is 1.4 ELISA-positive/negative cutoff. Lower panel. Dose of Dietzia and dexamethasone.
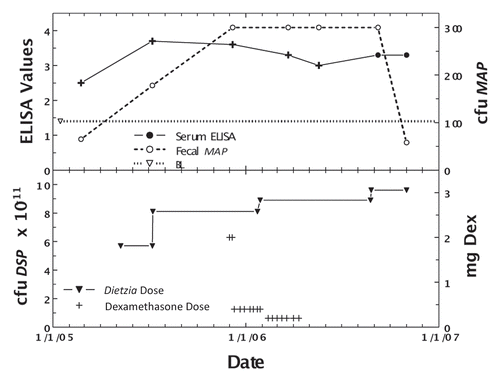
Figure 6 Longitudinal ELISA and fecal values for cow, 232, with initial Stage II and then Stage III disease at different doses of Dietzia and dexamethasone. Upper panel, solid line is ELISA OD405 nm values and dashed line is fecal MAP. Symbol (+) signifies a positive AGID. Thin dashed lines denote best-fit of ELISA values. BL is 1.4 ELISA-positive/negative cutoff. Lower panel, dose of Dietzia and dexamethasone.
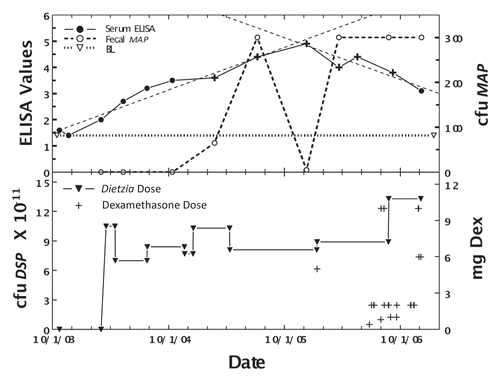
Figure 7 Longitudinal ELISA and fecal values for cow, Trixie, with lifetime intermittent Stage II or III disease at different doses of Dietzia and dexamethasone. Upper panel. Solid line is ELISA OD405 nm values and dashed line is fecal MAP. Symbol (+) signifies a positive AGID. Thin dashed lines denote best-fit of ELISA values. BL is 1.4 ELISA-positive/negative cutoff. Lower panel. Dose of Dietzia and dexamethasone.
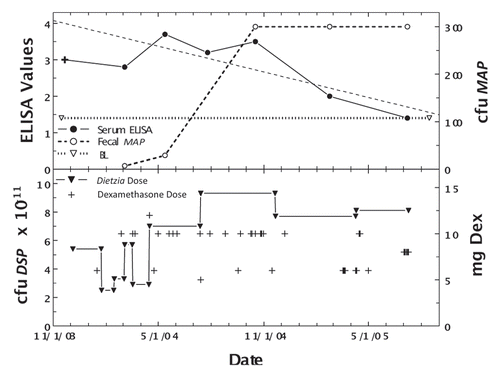
Figure 8 Longitudinal ELISA and fecal values for cow, 255, with initial Stage III disease at different doses of Dietzia and dexamethasone. Solid line is ELISA OD405 nm values and dashed line is fecal MAP. Symbol (+) signifies a positive AGID. BL is 1.4 ELISA-positive/negative cutoff. Start and stop Dietzia shown by vertical lines.
Table 1 Parameters of Dietzia-treated and non-treated Stage II or III paratuberculosis cows
Table 2 Dietzia plus dexamethasone treatment of Stage III or IV paratuberculosis cows
Acknowledgements
This research was funded, in part, by NIH Grant R01AI027331 (prior to retirement from Univ. WI-RF) and by Altick Associates, River Falls, WI. I thank William D. Richards for the initial ICON 6 isolate (Dietzia) and Dr. Craig Van Kampen for statistical analysis and editorial assistance. The sponsors, outside their funding, had no involvement in any part of the study.
References
- Hayee B, Rahman FZ, Sewel G, Smith AM, Segal AW. Crohn's disease as an immunodeficiency. Exper Rev Clin Immunol 2010; 6:585 - 596
- Lalande JD, Behr MA. Mycobacteria in Crohn's disease: how innate immune deficiency may result in chronic inflammation. Exper Rev Clin Immunol 2010; 6:633 - 641
- Beisner J, Stange EF, Wehkamp J. Innate antimicrobial immunity in inflammatory bowel diseases. Exper Rev Clin Immunol 2010; 6:809 - 818
- Siegel CA, Sands BE. Review article: Practical management of inflammatory bowel disease patients taking immunomodulators. Ali Pharm Ther 2005; 22:1 - 16
- Akobeng AK. Review article: The evidence base for interventions used to maintain remission in Crohn's disease. Ali Pharm Ther 2008; 27:11 - 18
- Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. New Engl J Med 1997; 337:1029 - 1035
- Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. New Engl J Med 1999; 340:1398 - 1405
- Sandborn WJ, Hanauer S, Loftus EV Jr, Tremaine WJ, Kane S, Cohen R, et al. An open-label study of the human anti-TNF monoclonal antibody adalimumab in subjects with prior loss of response or intolerance to infliximab for Crohn's disease. Am J Gastro 2004; 99:1984 - 1989
- Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-1 trial. Gastro 2006; 130:323 - 333
- Schreiber S, Rutgeerts P, Fedorak RN, Khaliq-Kareemi M, Kamm MA, Bolvin M, et al. CDP870 Crohn's Disease Study Group. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastro 2005; 129:807 - 818
- Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, et al. Natalizumab Pan-European Study Group. Natalizumab for active Crohn's disease. New Engl J Med 2003; 348:24 - 32
- Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut 2005; 54:1121 - 1125
- Lewis JD, Gelfand JM, Troxel AB, Forde KA, Newcomb C, Kim H, et al. Immunosuppressant medications and mortality in inflammatory bowel disease. Am J Gastro 2008; 103:1428 - 1435
- Valentine JF, Sninsky CA. Prevention and treatment of osteoporosis in patients with inflammatory bowel disease. Am J Gastro 1994; 94:878 - 883
- Yang YX, Lichtenstein GR. Corticosteroids in Crohn's disease. Am J Gastro 2002; 97:803 - 823
- Imperato AK, Smiles S, Abramson SB. Long-term risks associated with biologic response modifiers used in rheumatic diseases. Curr Opin Gastro 2004; 16:199 - 205
- Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. New Engl J Med 2005; 353:362 - 368
- Reddy JG, Loftus EV Jr. Safety of infliximab and other biologic agents in the inflammatory bowel diseases. Gastro Clin N Am 2006; 35:837 - 855
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arth Rheum 2007; 56:2886 - 2895
- Blonski W, Lichtenstein GR. Safety of biologic therapy. Inflam Bowel Dis 2007; 13:769 - 796
- Colombel JF, Sandborn WJ, Panaccione R, Robinson AM, Lau W, Li J, et al. Adalimumab safety in global clinical trials of patients with Crohn's disease. Inflam Bowel Dis 2009; 15:1308 - 1319
- Marehbian J, Arrighi HM, Hass S, Tian H, Sandborn WJ. Adverse events associated with common therapy regimens for moderate-to-severe Crohn's disease. Am J Gastro 2010; 16:1443 - 1444
- Singh JA, Wells GA, Christensen R, Tanjong GE, Maxwell L, MacDonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev 2011; 2:CD008794
- Krok KL, Lichtenstein GR. Nutrition in Crohn's disease. Curr Opin Gastro 2003; 19:148 - 153
- Drisko J, Bischoff B, Hall M, McCallum R. Treating irritable bowel syndrome with a food elimination diet followed by food challenge and probiotics. J Am Col Nut 2006; 25:514 - 522
- Roberts CL, Keita AV, Duncan SH, O'Kennedy N, Söderholm JD, Rhodes JM, Campbell BJ. Translocation of Crohn's disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut 2010; 59:1331 - 1339
- Borody TJ, Leis S, Warren EF, Surace R. Treatment of severe Crohn's disease using antimycobacterial triple therapy—approaching a cure?. Dig Liver Dis 2002; 34:29 - 38
- Sartor RB. Targeting enteric bacteria in treatment of inflammatory bowel diseases: Why, How, and When. Curr Opin Gastro 2003; 19:358 - 365
- Chamberlin W, Ghobrial G, Chehtane M, Naser SA. Successful treatment of a Crohn's disease patient infected with bacteremic Mycobacterium paratuberculosis. Am J Gastro 2007; 102:1572 - 1575
- Frissora CL, Cash BD. Review article: The role of antibiotics vs. conventional pharmaco-therapy in treating symptoms of irritable bowel syndrome. Ali Pharm Ther 2007; 25:1271 - 1281
- Feller M, Huwiler K, Schoepfer A, Shang A, Furrer H, Egger M. Long-term antibiotic treatment for Crohn's disease: systematic review and meta-analysis of placebo-controlled trials. Clin Infect Dis 2010; 50:473 - 480
- Lochs H. Antibiotic induces remission of Crohn's disease in phase 2 trial. Presented at 18th Annual United European Gastroenterology Week 2010; Abstract 3075
- Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune-mediated inflammation. Int J Parasitology 2007; 37:457 - 464
- Elliott DE, Weinstock JV. Helminthic therapy: using worms to treat immune-mediated disease. Adv Exp Med Biol 2009; 666:157 - 166
- Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-b pathway. J Exp Med 2010; 207:2331 - 2341
- O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastro 2005; 128:541 - 551
- Andresen V, Baumgart DC. Role of probiotics in the treatment of irritable bowel syndrome: Potential mechanisms and current clinical evidence. Intl J Probiot Prebiot 2006; 1:11 - 18
- Boirivant M, Strober W. Mechanism of action of probiotics. Curr Opin Gastro 2007; 23:679 - 692
- Guarino A, Lo Vecchio A, Canani RB. Probiotics as prevention and treatment for diarrhea. Curr Opin Gastro 2009; 25:18 - 23
- Pagnini C, Saced R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci 2010; 107:454 - 459
- Kirjavainen PV, Salminen SJ, Isolauri E. Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. J Ped Gastro Nutr 2003; 36:223 - 227
- Rioux KP, Fedorak RN. Probiotics in the treatment of inflammatory bowel disease. J Clin Gastro 2006; 40:260 - 263
- Quigley EM. Probiotics in the management of colonic disorders. Curr Gasto Rep 2007; 9:434 - 440
- Quigley EM. Probiotics in functional gastrointestinal disorders: what are the facts?. Curr Opin Gastro 2008; 8:704 - 708
- Ligaarden SC, Axelsson L, Naterstad K, Lydersen S, Farup PG. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomized controlled trial. BMC Gastroenterol 2010; 10:16 - 19
- Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, et al. Oral probiotics prevent necrotizing in very low birth weight neonates. J Ped 2005; 147:192 - 196
- Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, et al. Oral probiotics reduce incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005; 115:1 - 4
- Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotizing enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet 2007; 369:1614 - 1620
- Thomas DW, Greer FR. Probiotics and prebiotics in pediatrics. Pediatrics 2010; 126:1217 - 1231
- Plummer S, Weaver MA, Harris JC, Dee P, Hunter J. Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhea. Int Micro 2004; 7:59 - 62
- Hickson M, D'Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, et al. Use of probiotic Lactobacillus preparation to prevent diarrhea associated with antibiotics: randomized double blind placebo controlled trial. Brit Me J 2007; 335:80
- Guarino A, Lo Vecchio A, Canani RB. Probiotics as prevention and treatment for diarrhea. Curr Opin Gastro 2009; 25:18 - 23
- Grandy G, Medina M, Soria R, Teran C, Araya M. Probiotics in the treatment of acute rotavirus diarrhea. A randomized, double-blind controlled trial using two different probiotic preparations in Bolivian children. BMC Infect Dis 2010; 10:253 - 257
- Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhea. Cochrane Database Syst Rev 2010; 11:CD003048
- Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomized trial. Lancet 1999; 354:635 - 639
- Kajander K, Hatakka K, Poussa T, Farkkila M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: A controlled 6-month intervention. Ali Pharm Therap 2005; 22:387 - 394
- Borowiec AM, Fedorak RN. The role of probiotics in management of irritable bowel syndrome. Curr Gastro Rep 2007; 9:393 - 400
- Hedin C, Whelan K, Lindsay JO. Evidence for the use of probiotics and prebiotics in inflammatory bowel disease: a review of clinical trials. Proc Nutr Soc 2007; 66:307 - 315
- Quigley EM. What is the evidence for the use of probiotics in functional disorders?. Curr Gastro Rep 2008; 10:379 - 384
- Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, et al. Daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 2004; 53:108 - 114
- O'Sullivan GC, Kelly P, O'Halloran S, Collins C, Collins JK, Dunne C, et al. Probiotics: an Emerging therapy. Curr Pharm Des 2005; 11:3 - 10
- Gionchetti P, Rizzello F, Morselli C, Poggioli G, Tambasco R, Calabrese C, et al. High dose probiotics for the treatment of active pouchitis. Dis Colon &; Rectum 2007; 50:2075 - 2078
- Bajaj JS, Saeian K, Christensen KM, Hafeezullah M, Varma RR, Franco J, et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol 2008; 103:1707 - 1715
- Clarke CJ. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp Path 1997; 116:217 - 261
- Chiodini RJ, Van Kruiningen HJ, Merkal RS. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet 1984; 74:218 - 262
- Chiodini RJ. Crohn's disease and the mycobacterioses: A review and comparison of two disease entities. Clin Micro Rev 1989; 2:90 - 117
- Scanu AM, Bull TJ, Cannas S, Sanderson JD, Sechi LA, Dettori G, et al. Mycobacterium avium subsp. paratuberculosis infection in cases of irritable bowel syndrome and comparison with Crohn's disease and Johne's disease: common neural and immune pathogenicities. J Clin Micro 2007; 45:3883 - 3890
- Whittington RJ, Windsor PA. In utero infection of cattle with Mycobacterium avium subsp. paratuberculosis: A critical review and meta-analysis. Vet J 2009; 179:60 - 69
- Chiodini RJ. Immunology: resistance to paratuberculosis. Vet Clin N Am Food Anim Pract 1996; 12:313 - 343
- El-Zaatari FA, Naser SA, Markesich DC, Kalter DC, Engstand L, Graham DY. Identification of Mycobacterium avium complex in sarcoidosis. J Clin Microbiol 1996; 34:2240 - 2245
- Sechi LA, Rosu V, Pacifico A, Fadda G, Ahmed N, Zanetti S. Humoral immune responses of Type 1 Diabetes patients to Mycobacterium avium subspecies paratuberculosis lend support to the infectious trigger hypothesis. Clin Vaccine Immunol 2008; 15:320 - 326
- Paccagnini D, Sieswerda L, Rosu V, Masala S, Pacifico A, Gazouli M, et al. Linking chronic infection and autoimmune diseases: Mycobacterium avium subspecies paratuberculosis, SLC11A1 polymorphisms and type-1 diabetes mellitus. PloS One 2009; 9:e7109
- Rani PS, Sechi LA, Ahmed N. Mycobacterium avium subspecies paratuberculosis as a trigger of type-1 diabetes: destination Sardinia, or beyond?. Gut Path 2010; 2:1 - 6
- Dow CT, Ellingson JLE. Detection of Mycobacterium avium ss. paratuberculosis in Blau Syndrome tissues. Autoimmun Dis 2010; http://dx.doi.org/10.4061/2010/127692-697
- Pierce ES. Ulcerative colitis and Crohn's disease: is Mycobacterium avium subspecies paratuberculosis the common villain?. Gut Path 2010; 2:21
- Chamberlin WM, Naser SA. Integrating theories of the etiology of Crohn's disease. On the etiology of Crohn's disease; questioning the hypotheses. Med Sci Monit 2006; 12:RA27 - RA33
- Feller M, Huwiler K, Stephan R, Altpeter E, Shang A, Furrer H, et al. Mycobacterium avium subspecies paratuberculosis and Crohn's disease: a systematic review and meta-analysis. Lancet Infect Dis 2007; 7:607 - 613
- Behr MA, Kapur V. The evidence for Mycobacterium paratuberculosis in Crohn's disease. Curr Opin Gastro 2008; 24:17 - 21
- Hermon-Taylor J. Mycobacterium avium subspecies paratuberculosis Crohn's disease and the Doomsday scenario. Gut Patho 2009; 1:1 - 15
- Richards WD. Milner A R, Wood P R. In vitro and in vivo inhibition of Mycobacterium paratuberculosis by iron deprivation: A hypothesis. Proceedings Conference on Johne's Disease in Australia 1988; MA Int Assoc Paratub Inc Rehoboth 87 - 94
- Click RE, Van Kampen CL. Progression of Johne's disease curtailed by a probiotic. J Dairy Sci 2009; 92:4846 - 4851
- Click RE, Van Kampen CL. Assessment of Dietzia subsp. C79793-74 for treatment of cattle with evidence of paratuberculosis. Virulence 2010; 1:145 - 155
- Click RE, Van Kampen CL. Comparison of antemortem assays to assess progression-regression of paratuberculosis in individual dairy animals. Virulence 2010; 1:134 - 144
- Lomborg SR, Agerholm JS, Jensen AL, Nielsen LR. Effects of experimental immune-suppression in cattle with persistently high antibody levels to Salmonella Dublin lipopolysaccharide O-antigens. BMC Vet Res 2007; 3:17 - 24
- Slocombe RF. Combined streptomycin-isoniazidrifampin therapy in the treatment of Johne's disease in a goat. Can Vet J 1982; 23:160 - 163
- St.-Jean G, Jernigan AD. Treatment of infection in ruminates. Vet Clin N Am Food Anim Pract 1991; 7:793 - 804
- St.-Jean G. Treatment of clinical paratuberculosis in cattle. Vet Clin N Am Food Anim Pract 1996; 12:417 - 430
- Koets AP, Adugna G, Janss LL, van Weering HJ, Kalis CH, Wentink GH, et al. Genetic variation of susceptibility to Mycobacterium avium subspecies paratuberculosis infection in dairy cattle. J Dairy Sci 2000; 83:2702 - 2708
- Mortensen H, Nielsen SS, Berg P. Genetic variation and heritability of the antibody response to Mycobacterium avium subspecies paratuberculosis in Danish Holstein cows. J Dairy Sci 2004; 87:2108 - 2113
- Gonda MG, Chang YM, Shook GE, Collins MT, Kirkpatrick BW. Genetic variation of Mycobacterium avium ssp. paratuberculosis infection in US Holsteins. J Dairy Sci 2006; 89:1804 - 1812
- Pinedo PJ, Buergelt CD, Donovan GA, Melendez P, Morel L, Wu R, et al. Association between CARD15/NOD2 gene polymorphisms and paratuberculosis infection in cattle. Vet Micro 2009; 134:346 - 352
- Pinedo PJ, Buergelt CD, Donovan GA, Melendez P, Morel L, Wu R, et al. Candidate gene polymorphisms (BoIFNG, TLR4, SLC11A1) as risk factors for paratuberculosis infection in cattle. Pre Vet Med 2009; 91:189 - 196
- Gunesh S, Thomas GAO, Williams GT, Roberts A, Hawthorne AB. The incidence of Crohn's disease in Cardiff over the last 75 years: An update for 1996–2005. Ali Pharma Ther 2008; 27:211 - 219
- Strober W. Inside the microbial and immune labyrinth: Gut microbes: friends or fiends?. Nature Med 2010; 16:1195 - 1197
- Kugathasan S, Werlin SL, Martinez A, Rivera MT, Heikenen JB, Binion DG. Prolonged duration of response in early but not late pediatric Crohn's disease. Am J Gast 2000; 95:3189 - 3194
- Lionetti P, Bronzini F, Salvestrini C, Bascietto C, Berni Canani R, De Angelis GL, et al. Response to infliximab is related to disease duration in pediatric Crohn's disease. Ali Pharma Ther 2003; 18:425 - 432
- Schreiber S, Colombel JF, Panes J. Recent onset Crohn's disease shows higher remission rates and durability of response to treatment with subcutaneous monthly certolizumab pegol: Results from the analysis of the PRECISE 2 Phase III study. Gut 2006; 55:A131
- Panaccione R. Top-down vs. Step-up therapy in Crohn's Disease: What does the evidence say?. Medscape Gastro 2007; view article/560005
- Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastro 2007; 132:863 - 873
- Schreiber S, Reinisch W, Colombel JF. Early Crohn's disease shows high levels of remission to therapy with adalimumab: Sub-analysis of CHARM. Gastro 2007; 132:suppl 2 A985
- Sepehri S, Kotlowski R, Bernstein CN, Krause DO. Microbial diversity of inflamed and non-inflamed gut biopsy tissues in inflammatory bowel disease. Inflam Bowel Dis 2007; 13:675 - 683
- Behr MA. The path to Crohn's disease : is mucosal pathology a secondary event?. Inflamm Bowel Dis 2010; 16:896 - 902
- Pineton de Chambrun G, Colombel JF, Poulain D, Darfeuille-Michaud A. Pathogenic agents in inflammatory bowel diseases. Curr Opin Gastro 2008; 24:440 - 447
- Barnich N, Darfeuille-Michaud A. Adherent-invasive Escherichia coli and Crohn's disease. Curr Opin Gastro 2007; 23:16 - 20
- Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. ISME Journal 2007; 1:403 - 418
- Kotlowski R, Bernstein CN, Sepehri S, Krause DO. High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut 2007; 56:669 - 675
- Ohkusa T, Sato N, Ogihara T, Morita K, Ogawa M, Okayasu I. Fusobacterium varium localized in the colonic mucosa of patients with ulcerative colitis stimulates species-specific antibody. J Gastro Hepatol 2002; 17:849 - 853
- Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, et al. Mucosal flora in inflammatory bowel disease. Gastro 2002; 122:44 - 54
- Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, et al. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastro 2004; 127:80 - 93
- Posserud I, Stotzer PO, Bjornsson ES, Abrahamsson H, Simren M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 2007; 56:802 - 808
- Othman M, Aguero R, Lin HC. Alterations in intestinal microbial flora and human disease. Curr Opin Gastro 2008; 24:11 - 16
- Sartor RB. Bacteria in Crohn's disease: mechanisms of inflammation and therapeutic implications. J Clin Gastroenterol 2007; 41:S37 - S43
- Thompson NP, Montgomery SM, Wadsworth ME, Pounder RE, Wakefield AJ. Early determinants of inflammatory bowel disease: use of two national longitudinal birth cohorts. Eur J Gastro Hepatol 2000; 12:25 - 30
- Baron S, Turck D, Leplat C, Merle V, Gower-Rousseau C, Marti R, et al. Environmental risk factors in paediatric inflammatory bowel diseases: a population based case control study. Gut 2005; 54:357 - 363
- Asakura H, Suzuki K, Kitahora T, Morizane T. Is there a link between food and intestinal microbes and the occurrence of Crohn's disease and ulcerative colitis?. J Gastro Hepatol 2008; 23:1794 - 1801
- Markowitz J, Kugathasan S, Dubinsky M, Mei L, Crandall W, LeLeiko N, et al. Age of diagnosis influences serologic response in children with Crohn's disease: a possible clue to etiology?. Inflam Bowel Dis 2009; 15:714 - 719
- Chamberlin W, Graham DY, Hulten K, El-Zimaity HMT, Schwartz MR, Naser S, et al. Review article: Mycobacterium avium subsp. paratuberculosis as one cause of Crohn's disease. Ali Pharm Ther 2001; 15:337 - 346
- Chamberlin WM, Shafran I. Anti-mycobacterials and Crohn's disease. Ali Pharm Ther 2008; 28:373 - 374
- Wehkamp J, Stange EF. A new look at Crohn's disease: breakdown of the mucosal antibacterial defense. Ann N Y Acad Sci 2006; 1072:321 - 331
- De Bortoli N, Leonardi G, Ciancia E, Merlo A, Bellini M, Costa F, et al. Helicobacter pylori eradication: a randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am J Gastro 2007; 102:951 - 956
- Baron M. A patented strain of Bacillus coagulans increased immune response to viral challenge. Postgrad Med 2009; 121:114 - 118
- Sartor RB. Probiotic therapy of intestinal inflammation and infections. Curr Opin Gastro 2005; 21:44 - 50
- Rolfe VE, Fortun PJ, Hawkey CJ, Bath-Hextall F. Probiotics for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev 2006; Art. No. CD004826
- Prantera C, Scribano ML, Falasco G, Andreoli A, Luzi C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn's disease: a randomized controlled trial with Lactobacillus GG. Gut 2002; 51:405 - 409
- Marteau P, Lemann M, Seksik P, Laharie D, Colombel JF, Bouhnik Y, et al. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn's disease: a randomized, double blind, placebo controlled GETAID trial. Gut 2006; 55:842 - 847
- Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet 2004; 364:1039 - 1044
- Juste RA, Elguezabal N, Pavon A, Garrido JM, Geijo M, Sevilla I, et al. Association between Mycobacterium avium subspecies paratuberculosis DNA in blood and cellular and humoral immune response in inflammatory bowel disease patients and controls. Intl J Infect Dis 2009; 13:247 - 254
- Whitlock RH, Buergelt C. Preclinical and clinical manifestations of paratuberculosis (including pathology). Vet Clinic N Am Food Anim Pract 1996; 12:345 - 356
- Woese CR. Bacterial evolution. Microbiol Rev 1987; 51:221 - 271
- Cukovic-Cavka S, Vermeirem S, Hrstic I, Claessens G, Kolacek S, Jakic-Cazumovic J, et al. NOD2/CARD15 mutations in Croatian patients with Crohn's disease: prevalence and genotype-phenotype relationship. Eur J Gastro Hep 2006; 18:895 - 899
- Gui GPH, Thomas PRS, Tizard MLV, Lake J, Sanderson JD, Hermon-Taylor J. Two-year Outcomes: analysis of Crohn's disease treated with rifabutin and macrolide antibiotics. J Antimicro Chemother 1997; 39:393 - 400
- Grehan MJ, Borody TJ, Leis SM, Campbell J, Mitchell H, Wettstein A. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J Clin Gastrol 2010; 44:551 - 561