Abstract
In addition to phialidic conidia (PC), A. terreus produces accessory conidia (AC) both in vitro and in vivo. AC are distinct from PC in cell surface architecture, with the AC surfaces displaying more β-glucan, a molecule that can be a trigger for the induction of inflammatory responses. The present study follows β-glucan cell surface presentation throughout the course of germination of both types of conidia, and analyzes the differential capacity of AC and PC to elicit immune responses. Results show that AC display early, increased dectin-1 labeling on their cell surfaces compared to PC, and this differential dectin-1 labeling is sustained on the cell surface from the time of breaking dormancy through early germ tube emergence. Mouse alveolar macrophages showed a stronger inflammatory cytokine/chemokine response when challenged with AC than with PC in both ex vivo and in vivo experiments, correlating with the greater exposure of β-glucan exhibited by AC. Further, histopathologic staining of the lungs from mice challenged with AC demonstrated heightened cell recruitment and increased inflammatory response compared to the lungs of mice challenged with PC. Our study also demonstrates that AC are multinucleate structures with the ability to germinate rapidly, polarizing in multiple directions and producing several hyphal extensions. We present evidence that A. terreus AC are phenotypically distinct from PC and can be potent activators of the innate immune mechanism thus possibly playing a role in this organism's pathogenesis.
Introduction
Aspergillus terreus produces two types of asexual conidia: accessory conidia (AC, asexual conidia formed directly on hyphae) and phialidic conidia (PC, asexual conidia arising from conidiophores generated on hyphae).Citation1 Accessory conidia are produced both in vitro and in vivo, and a recent study from our laboratory indicated that AC were morphologically distinct from PC in that they lacked a pigment-like outer layer and were larger than PC.Citation1 We demonstrated other phenotypic differences between these two conidial forms including the ability of AC to germinate more rapidly, enhanced adherence of AC to microspheres, heightened AC metabolic activity, less cell membrane ergosterol and lower susceptibility of AC to the antifungal drug amphotericin B.Citation1,Citation27 Additionally, our study showed that dormant AC displayed uniform β-glucan staining, with enhanced staining in a ring pattern suggestive of a bud scar, in contrast to PC, for which β-glucan staining demonstrated a non-uniform staining pattern.Citation1
β-glucan recognition has been shown to be important in innate immune defenses against many pathogenic fungi, including Pneumocystis carinii, Coccidioides posadasii, Histoplasma capsulatum and Aspergillus fumigatus.Citation2–Citation13 In the yeast Candida albicans, exposure of β-glucan patches at budding and cell separation has been shown to elicit inflammatory responses in macrophages through Dectin-1 mediated β-glucan recognition.Citation14–Citation16 In A. fumigatus, the β-glucan moieties are concealed in dormant conidia by surface hydrophobins or rodlet layers.Citation17 Over the course of germination surface β-glucans are unmasked, becoming most evident during spore swelling and early germ tube formation, and are again veiled in the early stages of hyphal growth.Citation3,Citation5,Citation11,Citation18 Exposed β-glucans are recognized by Dectin-1 receptors on alveolar macrophages, and thus induce stage specific inflammatory responses against fungal morphological forms undergoing rapid cell cycle processes, indicative of the potential for invasion or early infection.Citation2,Citation5,Citation6,Citation11,Citation13 Blockage of Dectin-1 recognition of β-glucans substantially increases susceptibility to infection through downregulation of chemokine/cytokine production.Citation3
Phenotypic differences and unique germination and metabolic rates between Aspergillus species conidia may account for differences in invasiveness and dissemination.Citation19 The same may be true of AC compared to PC. Given our recent findings of differential labeling of β-glucan on AC compared to PC, the present study was designed with the following objectives: (1) to compare β-glucan labeling over time on A. fumigatus conidia and A. terreus AC and PC, (2) to measure cytokine/chemokine production by macrophages exposed to AC versus PC ex vivo and (3) to compare inflammatory responses to AC and PC in an in vivo pulmonary mouse model of aspergillosis. Additionally, we explored other phenotypic features of AC including number of nuclei and polarization of these structures.
Results
Species-specific differences in β-glucan display on phialidic conidia.
Staining with soluble dectin-1 was performed on A. fumigatus and A. terreus PC to demonstrate surface β-glucan display. As described previously in reference Citation20, no β-glucan label was observed on the surface of dormant A. fumigatus PC (). In contrast, dormant A. terreus PC exhibited irregular staining around the conidia. During germination, PC from both species displayed uniform binding of dectin-1 to the cell surface (). Early germlings of A. fumigatus displayed staining over the entire conidial and hyphal surface, with concentrated staining at the tips, whereas A. terreus germlings displayed uniform dectin-1 staining on both the conidia and the germ tube, without enhanced tip staining (). Dectin-1 labeling was apparent on mature A. fumigatus hyphae, but low staining was observed. Meanwhile, the A. terreus hyphae demonstrated intense punctuate (spotted) staining; the emergent AC also displayed Dectin-1 staining ().
Staining with soluble dectin-1 is heightened on Aspergillus terreus accessory conidia.
Given the differences observed in A. terreus PC and AC β-glucan labeling in our previous study,Citation1 we were also interested in looking at stage specific β-glucan display between these conidial forms. We therefore examined the ability of dectin-1 to recognize A. terreus AC at different stages of maturation (). Results showed that, in contrast to the irregular staining of A. terreus dormant PC (), the entire surface of freshly harvested AC bound s-dectin-mFc, with a heightened patch of detection evident on one side of the AC (). A. terreus PC exhibited uniform dectin-1 labeling as swelling occurred (), while the pattern of swollen AC continued to have uniform staining with enhanced “ring-shaped” staining (). Early germ tubes emerging from A. terreus PC stained homogenously (), whereas the AC germlings maintained very strong dectin-1 recognition on the originating conidial surface, with concentrated ring-shaped patch staining similar to that seen in the dormant and swollen stages, in addition to uniform staining of the entire germ tube (). During hyphal extension, dectin-1 labeling became more irregular for A. terreus PC () and AC () hyphae. At this stage, the two conidial forms looked identical, as both were producing AC on their hyphae, and the attached AC were clearly recognized and stained with soluble dectin-1. Accessory conidia that were attached to hyphae did not have the heightened ringshaped staining observed on harvested AC.
Aspergillus terreus conidia are multinucleate structures.
The DNA intercalating Hoechst dye was employed to ascertain nuclear content within freshly harvested AC () and PC (). Accessory conidia were found to be multinucleated, with the nuclear number varying considerably. On average, AC contained three to four nuclei, but as many as seven could be distinguished in some AC. Nuclear staining was absent in several AC, possibly due to inefficient incorporation of the Hoechst dye into the cells or nonviability of the conidia. Hoechst staining was also performed on AC still attached to the hyphae (). Again, the AC were found to contain multiple nuclei. Furthermore, AC were found to produce multiple germ tubes in early germ tube formation (). Dormant A. terreus PC were found to contain, on average, two, with as many as three nuclei (), whereas dormant A. fumigatus PC contained only one nucleus ().
Aspergillus terreus accessory conidia, but not phialidic conidia, elicit a heightened inflammatory response.
Given that β-glucan displayed different patterns of expression in AC and PC, we hypothesized that inflammatory responses to the two conidial forms would also differ. Initial examination of the inflammatory response utilized mouse alveolar macrophages exposed to either AC or PC ex vivo. The results showed that, consistent with the pattern of β-glucan display, co-culture of AC with alveolar macrophages elicited a heightened inflammatory response, with significantly increased production of MCP-1, IL-6, IL-1β, G-CSF, TNFα, CXCL2/MIP-2, KC, CCL4/MIP-1β, CCL3/MIP-1α and IL-1α compared to PC elicited responses after only 2 h (data not shown). Differences in chemokine/cytokine production proved more profound after 6 () and 20 () h. Production of chemokines and cytokines was not appreciably altered by heat killing the conidia (data not shown). Negligible levels of chemokine and cytokine production were observed for unstimulated alveolar macrophages (controls).
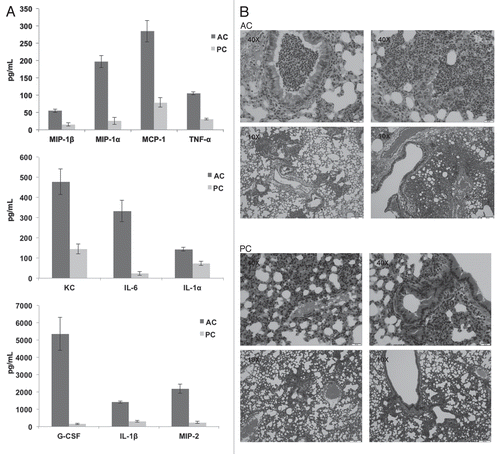
Further investigations assessed chemokine and cytokine production in the lungs after intratracheal challenge with A. terreus AC or PC. In vivo and ex vivo experiments demonstrated a similar trend of increased chemokine/cytokine production elicited by AC compared to PC, albeit with more G-CSF, MCP-1, IL-6 and IL-1β production observed in vivo (). Additionally, hematoxylin and eosin staining, also performed on the lungs of these intratracheally challenged mice, indicated infiltration of cells into the site of infection (), in support of the chemokine and cytokine data. Little inflammatory response was observed in PBS challenged mice (controls).
Discussion
The extent of differences inherent amongst the aspergilli is often underappreciated, but is evidenced by phenotypic features such as color, hydrophobicity, rate of germination, cell size and surface structure. For instance, although both A. fumigatus and A. nidulans conidia contain two hydrophobins, RodAp and RodBp (A. fumigatus), RodAp and DewAp (A. nidulans), and the rodA gene of A. fumigatus has been shown to complement homologous RodAp mutations in A. nidulans, the rodB gene is unable to complement A. nidulans DewAp mutations in spite of having the same molecular mass and similar signal sequence.Citation21 Analysis of physicochemical properties in the RodAp knockouts of the two species also suggests that there may be other differences in the composition of their outer cell walls, i.e., lipid and glycoprotein content, that are unmasked by disruption of the rodlets upon germination.Citation22
Similarly, the asexual conidia of A. fumigatus and A. terreus are distinct in color and cell size and recent studies in our laboratory indicate differential rates of germination and hydrophobicity between these conidia.Citation1 In this study, we further demonstrate that A. terreus PC have an average of two nuclei compared to A. fumigatus conidia, which often contains one nucleus. Additionally, PC of both species exhibit distinct cell surface characteristics including β-glucan expression. Specifically, there was no β-glucan staining on the surface of dormant A. fumigatus PC, in contrast to the irregular staining apparent on dormant A. terreus PC. Furthermore, early germlings of A. fumigatus displayed concentrated β-glucan staining at the tips while this was not observed on the tips of A. terreus germlings. Finally, in late polar growth, dectin-1 labeling was uniform throughout the surface but considerably diminished in A. fumigatus, while staining of A. terreus hyphae was punctuate and intense. No significant differences were observed between the clinical and environmental isolate tested. These species specific characteristics hold the potential to elicit differential immune responses to these organisms and will need to be tested in future experiments.
Our previous study indicated that A. terreus AC have enhanced β-glucan display compared to dormant A. terreus PC,Citation1 suggesting that these conidia differ markedly as well. In the present study, β-glucan display was continuous on the surface of AC throughout the stages of germination. In contrast, β-glucan display showed an irregular staining pattern on dormant A. terreus PC. β-glucan display was enhanced during swelling and early germ tube formation, and subsequently diminished. In A. fumigatus, conidia swell upon induction of germination, in the process losing a hydrophobin or rodlet layer, as well as pigments such as melanin. These surface molecules conceal β-glucans that serve as binding sites for Dectin-1, as well as other pathogen-associated molecular patterns (PAMPs) that can be recognized by immune cells, i.e., Toll-like receptor 4 (TLR4) and mannose receptors, thus modulating stage specific immune responses.Citation17,Citation20,Citation21,Citation23 Here, we show that the two different conidial types of A. terreus have differential β-glucan display and accordingly are recognized differently by immune cells. The influence of differential patterns of β-glucan presentation on pathogenicity for AC versus PC, and the consequences of altering these patterns, requires deeper investigation.
Phenotypically, A. terreus AC are lighter in color compared to PC. Previous SEM and TEM studies from our laboratory demonstrated that the cell surface of AC appear to be devoid of a yet to be characterized fungal pigment in contrast to the PC surface.Citation1 A. fumigatus conidia lacking the pigment melanin are white in color and a recent study demonstrated that albino A. fumigatus conidia induced significantly more proinflammatory cytokines in human peripheral blood mononuclear cells (PBMC), as compared to melanized wild-type conidia.Citation23 Similarly, in the present study, we found that production of cytokines/chemokines elicited by AC was greater than that elicited by PC, both ex vivo and in vivo in a mouse model. This heightened proinflammatory response could be attributed to “albino” AC (lacking pigment) that display strong cell surface β-glucan. Since we only inactivated the conidia by heat killing and did not perform additional blocking experiments with Laminarin (for β-glucan), we cannot at this time rule out the presence of other stimulatory PAMPs like mannan derivatives on the AC surface. However we did use A. fumigatus as a control, since staining of A. fumigatus with Dectin-1 has been well characterized in previous studies. An additional limitation of this study was that we did not elucidate fungal burden or survival and thus mice were sacrificed at 18 h post-challenge. Future studies, powered to understand survival after challenge with PC and AC need to be performed.
Our studies show that A. terreus AC are multinucleate and may contain as many as seven nuclei. To ensure that the AC were not breaking “dormancy” upon detachment from the hyphae, thus inducing cell cycle stages to progress within the conidial structures and the multiple nuclei observed, Hoechst staining was performed on AC still attached to the hyphae. These AC were also found to contain multiple nuclei, again varying in number. In A. oryzae, multi-nucleation of conidia conferred greater viability and resistance to UV radiation and freeze-thaw treatment, thus resulting in better adaptation to adverse environmental conditions and could ensure conidial preservation.Citation24 Although such viability experiments were not performed in the present study, there is some evidence that AC are at least more resistant to antifungal drugs than PC. If indeed multinucleation imparted viability benefits to AC, this would be a significant virulence factor for A. terreus as it would allow the organism to survive the harsh environment of the host during infection.
Alternatively, multinucleation could suggest a rudimentary form of conidia that have not yet evolved cell cycle control mechanisms, the evolutionary benefit of which might be rapid germ tube emergence. In fact, our previous studies demonstrated that AC germinated much more rapidly than either A. fumigatus or A. terreus PC, and this study found that the AC formed multiple germ tubes (). Similar to our study, in A. oryzae, multinucleate conidia had a higher germination efficiency than uninucleate conidia.Citation24 Additionally, late stage germination of both A. terreus PC and AC culminates in production of hyphae on which grow more AC, which in turn polarize in multiple directions to quickly form several more hyphal extensions. In spite of differences in germination potential between the two conidial forms, once both conidia achieved germination, the ensuing developmental stages appear to proceed comparably resulting in similar mycelial masses as assessed visually under a microscope (data not shown). The ability for each AC to produce multiple hyphae and subsequently more AC, thus ultimately more hyphae in a very short period of time perhaps sets the stage for rapid invasion and dissemination during infection in spite of inducing strong immune responses.
In summary, our study demonstrates phenotypic differences between A. fumigatus and A. terreus, specifically β-glucan display. Additionally, we demonstrate differences in cell surface β-glucan between the two A. terreus asexual conidia, elucidate the role of AC in inducing inflammatory responses in a mouse model of aspergillosis, and demonstrate multinucleation and hyperpolorization in these structures, all of which may contribute towards the pathogenicity of this organism.
Materials and Methods
Isolation of A. terreus phialidic and accessory conidia.
To assess surface β-glucan exposure and nuclear staining for both conidial forms, A. terreus AC and PC from isolates CLF 29 and CLF 52, recovered from an environmental and a clinical sample respectively, were harvested along with A. fumigatus (ATCC1022) conidia as control. Since no significant differences were observed between the two A. terreus isolates, all following ex vivo and in vivo studies were performed with A. terreus AC and PC from isolate CLF 52. A. terreus isolates were cultured on Sabouraud dextrose agar plates (SDA) and incubated at 37°C for three days. To prepare PC inocula, colonies were gently probed with a loop and the resultant conidia were suspended in Sabouraud dextrose broth (SDB) and counted on a hemocytometer. For AC harvest, PC were collected in phosphate-buffered saline (PBS), and 5 × 105 cells/ml transferred to SDB with 0.1% Tween 20 (SDB Tween) and incubated for seven days, after which AC were harvested as described previously in reference Citation25.
β-glucan exposure studies.
Conidia were adhered to cover slips and allowed to incubate at 0, 5, 10 and 20 h for A. fumigatus, 0, 8, 11 and 20 h for A. terreus PC and 0, 4, 7 and 12 h for A. terreus AC to induce swollen conidia, early and late germ tubes respectively. Conidia were incubated with s-dectin-mFc (Chad Steele),Citation26 on ice for 45 min, washed with 1x PBS (plus 0.01% Tween), resuspended in Alexa Fluor 594 conjugated chicken anti-mouse antibody (Molecular Probes) diluted 1:250 in 1x PBS/plus 0.01% Tween, and incubated on ice for 45 min. Conidia were washed with 1x PBS/0.01% Tween, resuspended in Fluoromount G (Electron Microscopy Sciences), mounted onto slides and examined under a fluorescent microscope (Zeiss Axiovert 25).
Animal experiments.
Male C57BL/6 mice, 6–8 weeks of age, were purchased from the National Cancer Institute, National Institutes of Health (Bethesda, Maryland United States). All mice were maintained in a specific pathogen free environment in microisolator cages within an American Association for Laboratory Animal Science certified animal facility at the University of Alabama at Birmingham. Animal studies were reviewed and approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee (IACUC).
For ex vivo studies, ten to fifteen healthy mice were anesthetized with isoflurane intraperitoneally and sacrificed by exsanguination. Lungs were subsequently lavaged with PBS, lavage fluids pooled and alveolar macrophages collected as previously described in reference Citation11. Harvested alveolar macrophages were co-cultured with live PC or AC at MOI of 1:1 ratio for 2, 6 or 20 h in a 96-well plate at 37°C, 5% CO2 in RPMI 1640 with 10% heat inactivated fetal calf serum. Controls included alveolar macrophages cultured in medium alone. Cytokine and chemokine levels were assessed in the supernatants using the Bio-Plex Protein Array System (Bio-Rad, Hercules, California United States) as per the manufacturer's instructions. MIP-2 concentrations were also determined using a commercially available ELISA kit (R&D Systems, Minneapolis, Minnesota, United States) as per manufacturer's instructions. In another set of experiments, harvested mouse alveolar macrophages were challenged with heat killed PC or AC at MOI of 1:1 for 6 h, and supernatants were assessed for cytokines/chemokin 10 min prior to co-culture with alveolar macrophages and efficacy of heat killing confirmed by plating on SDA.
For in vivo studies, eight mice were anesthetized and 7.5 × 106 conidia in 50 µl AC or PC administered intratracheally. At 18 h post-challenge, one lung was collected from each mouse for hematoxylin and eosin (H&E) and Gomori methenamine silver (GMS) staining, and the second lung homogenized and cytokine/chemokine levels measured as above.
Nuclear staining of PC and AC.
Nuclear staining of PC and AC was accomplished by fixing (3.7% formaldehyde, 0.2% Triton X-100, 50 mM phosphate buffer, pH 7) cells for 30 min, followed by incubation with Hoechst dye (1 mg/ml) for 5 min. Conidia were washed with distilled water, resuspended in Fluoromount G (Electron Microscopy Sciences), mounted onto slides and examined by UV detection under a fluorescent microscope (Zeiss Axiovert 25).
Disclaimer
The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the CDC.
Figures and Tables
Figure 1 Differential dectin-1 binding on Aspergillus fumigatus and Aspergillus terreus phialidic conidia. Soluble dectin-1 binding A. fumigatus and A. terreus phialidic conidia at various stages of germination (A) dormant conidia (B) swollen condia (C) early germ tube formation and (D) late germination. Differential interference microscopy (DIC) and fluorescence images were captured by microscopy at 40x (A) and 100x (B–D), and are representative of 3 experiments. Scale bars denote 5 µm and 10 µm for 40x and 100x magnification, respectively.
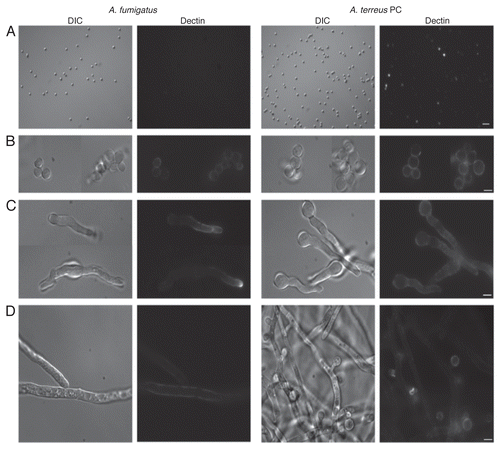
Figure 2 Dectin-1 binding on phialidic conidia and accessory conidia at different germination stages. Soluble dectin-1 binding on A. terreus phialidic and accessory conidia at (A) dormant conidia (B) swollen conidia, (C) early germ tube formation and (D) late germination. DIC and fluorescence images were captured by microscopy at 40x (A) and 100x (B–D), and are representative of 3 experiments. Within windows, arrows depict the ring-like staining pattern on AC at 100x (A). Scale bars denote 5 µm and 10 µm for 40x and 100x magnification, respectively.
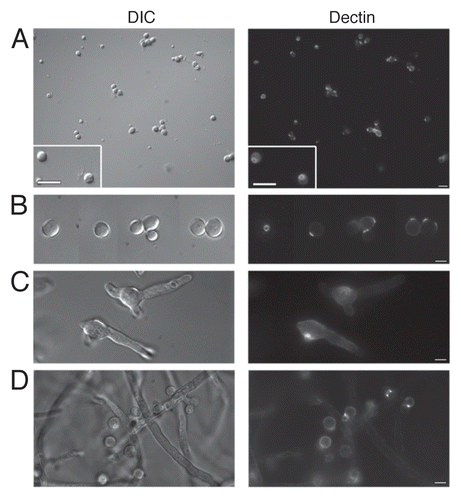
Figure 3 Accessory conidia are multinucleated prior to germination. Hoechst staining was performed on A. terreus accessory conidia, both attached (A) and detached (B) from the hyphae, and A. terreus PC (C) and A. fumigatus PC (D). DIC and UV images were captured by microscopy at 100x, and are representative of 3 experiments. Scale bars denote 10 µm.
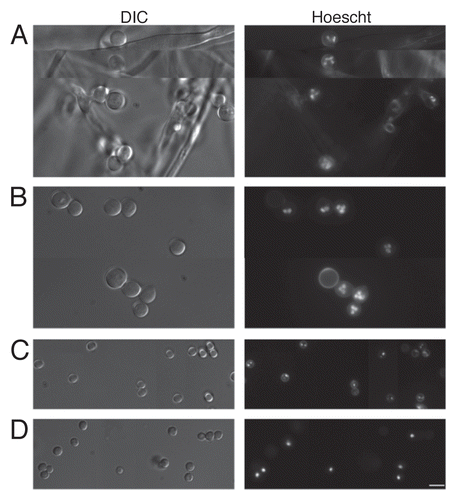
Figure 4 Accessory conidia undergo hyperpolarization during germination. Early germ tube formation and Hoechst nuclei staining was assessed for A. terreus accessory conidia. DIC and UV images were captured by microscopy at 100x, and are representative of 3 experiments. Scale bars denote 10 µm.
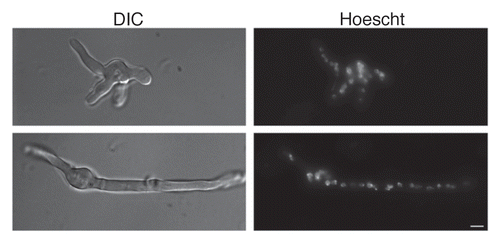
Figure 5 Aspergillus terreus accessory conidia elicit a heightened inflammatory response by alveolar macrophages. Alveolar macrophages were co-cultured with A. terreus AC or PC. Supernatants were collected at (A) 6 or (B) 20 h and assayed for chemokine/cytokine levels by Bio-Plex or ELISA. Experiments were performed three times.
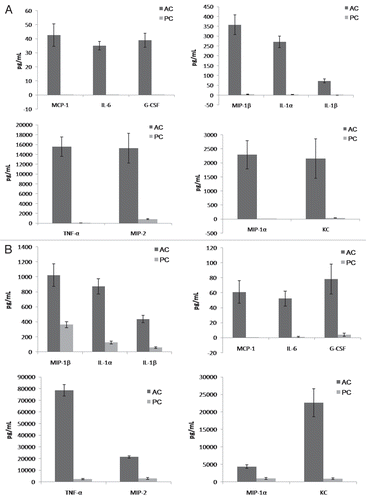
Figure 6 Mice intratracheally challenged with Aspergillus terreus accessory conidia exhibit enhanced chemokine/cytokine production and cell recruitment in lungs. C57BL/6 mice were intratracheally challenged with AC or PC. (A) Chemokine/cytokine levels in the supernatants of homogenized lungs collected at 18 h post-challenge with AC or PC (need to indicate from figure). (B) Hematoxylin and eosin staining of sections of lungs post-challenge with AC or PC (also denote figure labels). All experiments were performed 3 times.
References
- Deak E, Wilson SD, White E, Carr JH, Balajee SA. Aspergillus terreus accessory conidia are unique in surface architecture, cell wall composition and germination kinetics. PLoS One 2009; 4:7673
- Balloy V, Huerre M, Latge JP, Chignard M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect Immun 2005; 73:494 - 503
- Gersuk GM, Underhill DM, Zhu L, Marr KA. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J Immunol 2006; 176:3717 - 3724
- Gomez BL, Nosanchuk JD. Melanin and fungi. Curr Opin Infect Dis 2003; 16:91 - 96
- Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog 2005; 1:30
- Luther K, Torosantucci A, Brakhage AA, Heesemann J, Ebel F. Phagocytosis of Aspergillus fumigatus conidia by murine macrophages involves recognition by the dectin-1 beta-glucan receptor and Toll-like receptor 2. Cell Microbiol 2007; 9:368 - 381
- Overland G, Stuestol JF, Dahle MK, Myhre AE, Netea MG, Verweij P, et al. Cytokine responses to fungal pathogens in Kupffer Cells are Toll-like receptor 4 independent and mediated by tyrosine kinases. Scand J Immunol 2005; 62:148 - 154
- Rapaka RR, Goetzman ES, Zheng M, Vockley J, McKinley L, Kolls JK, et al. Enhanced defense against Pneumocystis carinii mediated by a novel dectin-1 receptor Fc fusion protein. J Immunol 2007; 178:3702 - 3712
- Rappleye CA, Eissenberg LG, Goldman WE. Histoplasma capsulatum alpha-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc Natl Acad Sci USA 2007; 104:1366 - 1370
- Segal BH. Role of macrophages in host defense against aspergillosis and strategies for immune augmentation. Oncologist 2007; 12:7 - 13
- Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, et al. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog 2005; 1:42
- Viriyakosol S, Fierer J, Brown GD, Kirkland TN. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect Immun 2005; 73:1553 - 1560
- Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, et al. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol 2009; 182:4938 - 4946
- Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J 2005; 24:1277 - 1286
- Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis 2007; 44:373 - 379
- Wheeler RT, Kombe D, Agarwala SD, Fink GR. Dynamic, morphotype-specific Candida albicans betaglucan exposure during infection and drug treatment. PLoS Pathog 2008; 4:1000227
- Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 2009; 460:1117 - 1121
- Hohl TM, Feldmesser M, Perlin DS, Pamer EG. Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal beta-glucan exposure. J Infect Dis 2008; 198:176 - 185
- Walsh TJ, Petraitis V, Petraitiene R, Field-Ridley A, Sutton D, Ghannoum M, et al. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J Infect Dis 2003; 188:305 - 319
- Paris S, Debeaupuis JP, Crameri R, Carey M, Charles F, Prevost MC, et al. Conidial hydrophobins of Aspergillus fumigatus. Appl Environ Microbiol 2003; 69:1581 - 1588
- Flori P, Bellete B, Durand F, Raberin H, Cazorla C, Hafid J, et al. Comparison between real-time PCR, conventional PCR and different staining techniques for diagnosing Pneumocystis jiroveci pneumonia from bronchoalveolar lavage specimens. J Med Microbiol 2004; 53:603 - 607
- Girardin H, Paris S, Rault J, Bellon-Fontaine MN, Latge JP. The role of the rodlet structure on the physicochemical properties of Aspergillus conidia. Lett Appl Microbiol 1999; 29:364 - 369
- Antonopoulou S, Aoun M, Alexopoulos EC, Baka S, Logothetis E, Kalambokas T, et al. Fenticonazole activity measured by the methods of the European Committee on Antimicrobial Susceptibility Testing and CLSI against 260 Candida vulvovaginitis isolates from two European regions and annotations on the prevalent genotypes. Antimicrob Agents Chemother 2009; 53:2181 - 2184
- Ishi K, Maruyama J, Juvvadi PR, Nakajima H, Kitamoto K. Visualizing nuclear migration during conidiophore development in Aspergillus nidulans and Aspergillus oryzae: multinucleation of conidia occurs through direct migration of plural nuclei from phialides and confers greater viability and early germination in Aspergillus oryzae. Biosci Biotechnol Biochem 2005; 69:747 - 754
- Dornbusch HJ, Buzina W, Summerbell RC, Lass-Florl C, Lackner H, Schwinger W, et al. Fusarium verticillioides abscess of the nasal septum in an immunosuppressed child: case report and identification of the morphologically atypical fungal strain. J Clin Microbiol 2005; 43:1998 - 2001
- Mattila PE, Metz AE, Rapaka RR, Bauer LD, Steele C. Dectin-1 Fc targeting of Aspergillus fumigatus betaglucans augments innate defense against invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2008; 52:1171 - 1172
- Lass-Florl C, Rief A, Leitner S, Speth C, Wurzner R, Dierich M. In vitro activities of amphotericin B and voriconazole against aleurioconidia from Aspergillus terreus. Antimicrob Agents Chemother 2005; 49:2539 - 2540