Abstract
Nitric oxide (NO) is a short-lived, diatomic, lipophilic gas that plays an integral role in defending against pathogens. Among its many functions are involvement in immune cell signaling and in the biochemical reactions by which immune cells defend against bacteria, fungi, viruses and parasites. NO signaling directs a broad spectrum of processes, including the differentiation, proliferation, and apoptosis of immune cells. When secreted by activated immune cells, NO diffuses across cellular membranes and exacts nitrosative and oxidative damage on invading pathogens. These observations led to the development of NO delivery systems that can harness the antimicrobial properties of this evanescent gas. The innate microbicidal properties of NO, as well as the antimicrobial activity of the various NO delivery systems, are reviewed.
Introduction
Nitric oxide (NO) is an endogenously produced molecule critical in defending against infection.Citation1 Depending on its concentration, NO exerts antimicrobial effects in two ways. At low concentrations, NO acts as a signaling molecule that promotes the growth and activity of immune cells.Citation2 At high concentrations, such as during the respiratory burst of a neutrophil, NO covalently binds DNA, proteins and lipids, thereby inhibiting or killing target pathogens. Considering that NO is an integral and highly conserved part of the host immune response, it is not surprising that few bacteria are able to escape the antimicrobial effect of NO.Citation3-Citation5
Exogenous NO delivery systems capitalize on NO's immunoregulatory and antimicrobial properties to treat infectious disease. Multiple NO delivery systems have been developed, each attempting to provide NO in a safe, effective and convenient manner. Available technologies range from simple gaseous NO (gNO) stored in a tankCitation6 to complex (NO molecules packed within nanoparticlesCitation7). Although only a small subset of NO delivery systems have been evaluated for antimicrobial efficacy, those that have been evaluated are bacteriostatic or bactericidal. The success of NO delivery systems in treating laboratory models of infection spurs continued development of this promising technology.
This article summarizes the immunoregulatory actions of NO as they pertain to the mammalian immune system. The biochemistry underlying the antimicrobial actions of NO is also covered. Finally, the mechanism of delivery, antimicrobial activity and strengths and limitations of the various NO delivery systems are reviewed in depth.
The Role of Nitric Oxide in Immunity
NO production is a key feature of immune cells. NO is principally synthesized by one of three NO synthase (NOS) enzymes: neuronal NOS (nNOS), inducible NOS (iNOS) and endothelial NOS (eNOS). The isoforms differ in respect to regulation, amplitude and duration of NO production, as well as cellular and tissue distribution.Citation8 Both eNOS and nNOS act as constitutively expressed proteins, and their expression is not limited to endothelial cells or neurons.Citation9 In fact, NO is produced from both nNOS and eNOS during infection and autoimmunity.Citation10-Citation12 Cell types that contain eNOS and nNOS generate low fluxes of NO for short periods of time. NO at these low concentrations (< 1 µM) acts as an intracellular signal, activating or inhibiting different proteins.Citation13,Citation14 The third isoform, iNOS, which was originally described in activated macrophages, predominantly functions as a component of the innate immune system. Cytokines and microbial products, often acting synergistically, stimulate iNOS expression.Citation8 iNOS or eNOS are expressed in dendritic cells, natural killer (NK) cells, mast cells, monocytes, macrophages, microglia, Kupffer cells, eosinophils and neutrophils, as well as other cells involved in immune reactions.Citation9
Unlike nNOS and eNOS, which are tightly regulated and dependent on calcium entry into the cell, iNOS produces high amounts of NO when induced. When NO is produced at high concentrations (> 1 µM), it is able to perform nitrosation, nitration, and oxidation reactions. iNOS is regulated at multiple levels ranging from its transcription, to its synthesis, stability, activity and degradation.Citation12 Relative to nNOS or eNOS, iNOS is less susceptible to feedback inhibition by NO.Citation9,Citation15 This allows iNOS to continually produce NO as a means of defense against microbes when activated. The use of exogenous NO for antimicrobial therapy is similar to action of iNOS in that both are designed to produce high amounts of NO for longer periods of time, to fight microbes.
Biochemistry of NO
NO is a lipophilic and hydrophilic natural gas, with a small Stokes radius that allows it to cross membranes readily.Citation1 NO is a radical gas, and is therefore unstable in an oxygen environment. Reactions of NO with oxygen or superoxide spontaneously produce reactive nitrogen and oxygen intermediates that lead to the formation of a variety of antimicrobial species. The formation of these intermediates becomes biologically significant when the concentration of NO is greater than 1 µM. At these concentrations, reactive nitrogen oxide species (RNOS) causes oxidative and nitrosative damage by altering DNA, inhibiting enzyme function, and inducing lipid peroxidation, which account for the majority of NO’s antimicrobial properties.Citation13
NO derivative molecules such as peroxynitrite (OONO-), S-nitrosothiols (RSNO), nitrogen dioxide (NO2), dinitrogen trioxide and dinitrosyl-iron complexes are generated.Citation16 Other NO-related species may be formed within target microbes if oxygen radicals are also present. Each of these species is distinct and has its own molecular stability and reactivity. Peroxynitrite, which has greater cytotoxic potential than NO or O2- alone is an example of the synergy between ROS and RNOS interactions.Citation1,Citation17 Like NO, peroxynitrite is also capable of passing through cell membranes; however its greater reactivity with lipids and proteins may limit its ability to diffuse into target cells relative to NO. Nitrogen dioxide can be formed from the autoxidation of NO, or by the oxidation of NO2- by myeloperoxidase and H2O2. Potent nitrosating agents can arise from the auto-oxidation of NO• with thiols and nonheme iron.Citation1,Citation17,Citation18 Reactive nitrogen intermediates can also react with proteins through heme groups, iron sulfur clusters, phenol or aromatic amino acid residues or amines.Citation19,Citation20
Inherent Antimicrobial Properties of NO
Chemical alteration of DNA by RNOS is one of the main mechanisms of NO mediated antimicrobial action. NO damages DNA by three mechanisms: direct reaction of RNOS with DNA structure, inhibition of DNA repair and increased generation of alkylating agents and hydrogen peroxide, which are genotoxic. NO itself does not chemically alter DNA, but rather damage is caused by RNOS formed from the autoxidation of NO.Citation21 N-nitrosating intermediates (N2O3 has been proposed) deaminate cytosine, adenine and guanine. Peroxynitrite and NO2• in particular induce DNA strand breaks, abasic sites and other DNA alterations.Citation1,Citation22 NO related DNA damage has also been shown in intact bacteria.Citation21,Citation23
NO inhibits DNA repair enzymes associated with the repair of alkylation to DNA.Citation24,Citation25 DNA alkyl transferases have cysteine residues whose -SH group reacts with NO to form S-NO adducts. These adducts inhibit the transfer of the alkyl group from guanine to the protein. RNOS are capable of reacting with and modifying proteins at cysteine, methionine, tyrosine, phenylalanine and tryptophan residues.Citation18-Citation20
NO-associated lipid damage has been demonstrated with peroxynitrite and nitrogen dioxide. Peroxynitrite has been shown to mediate lipid peroxidation of liposomes.Citation1,Citation26 Lipid peroxidation has been shown to contribute to the antimicrobial activities of NO.Citation27
The cytotoxic actions of NO are enhanced by the production of acid, glutathione, and reactive oxygen species (ROS) by macrophages.Citation8 S-nitrosylation (addition of NO+ to sulfhydryl groups) of thiols is an important mechanism for NO mediated toxicity against microbes. S-nitrosothiols (RSNO), N2O3 and dinitrosyl-thiol-iron complexes are potent nitrosylators. GSNO can be actively taken up and processed by microbial systems which typically function to import glutathione and other short peptides.Citation28 Modification of thiols can alter protein function, and the alteration of surface thiols has been implicated in S-nitrosothiol mediated inhibition of Bacillus cereus spores.Citation29
NO readily reacts with proteins that contain heme moieties. These include guanylate cyclase, cytochrome P450 and NOS. At low concentrations, NO binds reversibly to the Fe(II) moiety in the guanylate cyclase, ultimately resulting in activation of the protein. At high concentrations, RNOS irreversibly binds to heme proteins, resulting in heme removal from the protein. The release of iron from metalloenzymes causes bacterial iron depletion.Citation1,Citation13 NO• has been shown to reduce Fe(III) complexes, allowing for the enhancement of Fe(II) catalyzed formation of hydroxyl radicals.Citation30
Gastric NO also plays a role in host defense. Enteropathogens are capable of surviving for long periods of time in acid alone, but the combination of acid and nitrite is bactericidal.Citation31,Citation32 The role of acidified nitrate is also relevant to combating common cutaneous pathogens. Nitric oxide can be generated from sweat nitrates in the acidic environment of the skin surface, and this is thought to protect against potential invasion by pathogens.Citation33,Citation34
Interestingly, while NO can boost the antimicrobial function of the respiratory burst, it can also protect host cells from oxidative injury. NO• antagonizes oxidant membrane injury by terminating lipid peroxidation reactions.Citation26 Though NO can protect against hydrogen peroxide mediated cytotoxicity in mammalian cells, it dramatically potentiates the cytotoxicity of hydrogen peroxide in E. coli.Citation35 Prokaryotes are more sensitive to NO/H2O2 treatment because bacteria depend on iron sulfur clusters to a greater extent than mammalian cells. The subsequent degradation of these proteins by NO or RNOS allows the released Fe to bind DNA. This free iron is then capable of catalyzing the formation of highly reactive free radicals that damage membranes and DNA.Citation13,Citation36
As microbial resistance to antibiotic therapy is a growing threat to our ability to combat infection, it is important to determine if there are any mechanisms that would confer resistance of NO to bacteria. To date the ability of bacteria to develop resistance to exogenous NO treatments has yet to be demonstrated. In a recent study, the potential for Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), Staphylococcus epidermidis, E. coli and Pseudomonas aeruginosa to develop NO resistance was evaluated using spontaneous and serial passage mutagenesis assays.Citation5 No significant increase in MIC for any of the bacterial species was observed. The lack of bacterial resistance is attributed to the multiple mechanisms of NO generated bacterial toxicity.Citation5,Citation6,Citation37-Citation40 The versatility of NO allows it to quickly penetrate microbial cell membranes, where various nitrosative and oxidative reactions can proceed, as discussed above. In order for microbial resistance to occur, multiple bacterial mutations would have to evolve concurrently. There are properties that can confer some protection from NO to bacteria. Several studies show that bacterial exposure to NO• leads to increased expression of bacterial enzymes that have evolved to detoxify this radical. Flavohemaglobin analogs are known to be protective in cells that are exposed to NO•, including S. aureus and S. Typhimurium.Citation41,Citation42 The induction of lactate dehydrogenase (LDH) by NO• observed in methicillin-sensitive (MSSA) and methicillin-resistant S. aureus protects the bacteria from physiologic concentrations of NO. NO induced LDH allows S. aureus to maintain redox homeostasis during nitrosative stress.Citation43 These mechanisms are specific for allowing microbes to survive host defenses. However, these compensatory mechanisms are not capable of protecting pathogens from the high levels of NO exposure seen with the use of NO donor drugs. These drugs are microbicidal for a wide array of pathogens, including S. aureus.Citation38-Citation40,Citation44,Citation45 The inherent broad spectrum antimicrobial activity of NO make it a worthy target for development and clinical use.
Nitric Oxide Delivery Platforms
In order to harness the potential benefits of NO as an antimicrobial agent, this evanescent gas needs to be delivered to target cells at precise concentrations for significant periods of time in a convenient and non-toxic manner. Only a handful of NO delivery platforms have been evaluated for their antimicrobial properties. The NO delivery platforms evaluated include: gaseous NO from a tank, NO generation from a probiotic patch, NO released or donated from a pro-drug, and release and generation from nanoparticles.
Gaseous NO
The antimicrobial properties of NO were initially investigated by exposing pathogens to gaseous NO (gNO). Simple application of gNO was effective against bacteria, fungi, mycobacteria, parasites and viruses.Citation46 In order to deliver gNO to an infected tissue a specialized chamber is required. The chamber allows for controlled delivery of NO and prevents oxygen species from reacting with the NO to produce toxic NO2. Continuous exposure to 80 ppm gNO in the controlled chamber inhibited growth of P. aeruginosa and S. aureus.Citation47 When levels are raised to 160 ppm, gNO is bactericidal. Interestingly, intermittent exposure for 30 min every four hours is also effective, but requires twice the concentration of gNO to be bactericidal.Citation48 At 200 ppm, gaseous NO reduced S. aureus burden in a rabbit wound model but was not cytotoxic to human fibroblast, keratinocyte, endothelial, monocyte and macrophage cells in culture.Citation48,Citation49 Although effective, the delivery device is cumbersome as it includes pressure regulators, heaters, humidifiers and multiple tanks of gas. Furthermore, in order to achieve the reported results, three 8 h treatment sessions were required. Gaseous NO is already approved for the treatment of pulmonary hypertension of the newborn where it has proven to be a safe and cost-effective alternative to extracorporeal membrane oxygenation.Citation50 Clinical experience with gNO shows that it is a viable option for the hospital setting where patients are immobile and there is access to specialized equipment, but it is too costly (at $125 per hour of treatment) and impractical for other settings.Citation51
NO probiotic patch
A probiotic patch that releases gNO has also been developed.Citation52 In this system, immobilized Lactobacilli convert glucose into lactic acid. The lactic acid reacts with nitrite salt () to produce gNO that then diffuses through the mesh lining of the patch to reach the target tissue. The production of lactic acid by bacteria limits the rate at which NO is formed and allows for continued production of variable levels of NO for over a 24 h period.Citation53 Because the rate of NO production is dependent on the activity of the lactobacilli, there is patch to patch variability in peak gNO production. Some patches produce in excess of 400 ppm gNO while others do not surpass 150 ppm.Citation53 Despite the variability in NO production, in vitro tests have shown the patch to be cidal against E. coli, S. aureus, P. aeruginosa, MRSA, T. mentagrophytes, T. rubrum and have demonstrated some activity against A. baumannii.Citation52 In vivo results were less favorable—in an S. aureus-infected open wound model the patch reduced bacterial burden, but this impact did not reach statistical significance.Citation53 The probiotic patch may be a viable alternative to gNO from a tank as it is capable of producing similar concentrations of gNO, but at this time the patch requires additional modifications to achieve consistent release rates.
Acidified nitrite
Acidified nitrite creams also produce NO through the reaction of nitrite with an acid. Unlike the probiotic patch that relies on lactic acid, acidified nitrite creams use ascorbic acid to reduce nitrite to NO. These two part cream preparations are easy to transport and use but must be mixed together immediately before being applied. Another advantage of the cream is it penetrates the skin and produces NO within the follicular ostia.Citation54 The potency of the cream can be regulated by varying the concentration of nitrite and ascorbic acid. The standard preparation produces a quick burst of NO (12 ppm) that drops rapidly over one hour.Citation54 Comparatively weak to gNO delivery devices, daily treatment with acidified nitrite cream is still effective against tinea pedis,Citation55 molluscum contagiosum,Citation56 mycobacterium ulceransCitation57 and MRSA abscessesCitation58 in human trials. The low levels of NO may still be effective because of the cream's ability to penetrate the skin as well as to stimulate the host immune system.Citation54 Leishmania in culture is also sensitive to acidified nitrite, but when applied to human subjects, only 28% showed clinical improvement.Citation59 Although effective against a variety of cutaneous infections, the use of acidified nitrite cream is limited by skin irritation. When applied to normal skin, the resulting erythema and inflammation are equivalent to that of psoriasis.Citation54 Given the variety of non-irritating antimicrobial treatments available, the irritating effects of acidified nitrite are likely to be a barrier to use of the drug.
Organic nitrates and sodium nitroprusside
NO donor drugs are the largest and most well understood of the NO delivery systems. Sodium nitroprusside and organic nitrates (nitroglycerin and isosorbide mononitrate) are not used as antibiotics, but are commonly used as blood pressure lowering medications to treat cardiovascular disease. Sodium nitroprusside spontaneously releases NO, but gives off carbon monoxide as a byproduct. Organic nitrates only release NO in tissues expressing the enzyme mitochondrial aldehyde dehydrogenase 2, but this enzyme is irreversibly inactivated with use and causes tachyphylaxis (also known as nitrite/nitrate tolerance).Citation60 These drugs have limited antibacterial and biofilm disrupting propertiesCitation61,Citation62 and are unlikely to be used as antibacterial agents given their potent cardiovascular effects.
Diazeniumdiolate (NONOates)
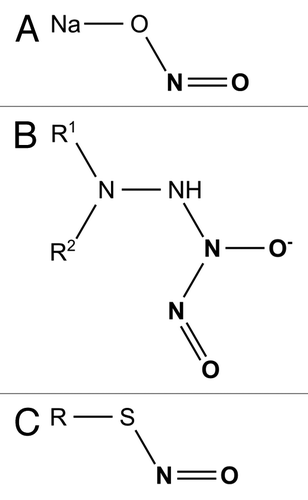
Diazeniumdiolates, also known as NONOates, consists of a diolate group [-N(O-)n = O] bound to a nucleophile adduct ().Citation63 Frequently, the nitrogen of an amine group is used as the nucleophile. A diolate group can be added to nitrogen by exposing the base compound to gNO in the absence of oxygen. The simplicity of diazeniumdiolate synthesis has allowed for a variety of NONOates to be created. In the same way that NO molecules are added to the base compound, NONOates spontaneously release up to two molecules of NO. The rate of release is dependent on the stability of bond between the nucleophile and the diazeniumdiolate.Citation63 Side groups joined to the nucleophile can alter the rate of NO release from seconds to hours or even prevent release of NO until the side group is enzymatically cleaved.Citation64,Citation65 Not all NONOates are clinically useful because the parent compound may become toxic when the diazeniumdiolate group is converted to a reactive N-nitroso group.Citation66 For example, O(2)-vinyl 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate (V-PYRRO/NO) is an NONOate that is activated by hepatic cells. The N-nitroso byproduct of V-PYRRO/NO metabolism is the potent hepatocarcinogen N-nitrosopyrrolidine.Citation67 Clearly, toxic or carcinogenic NONOates should not be used to treat infections when many effective and non-toxic alternatives exist. Two major strategies have been adopted in an effort to mitigate toxicity: using an established drug or a ubiquitous molecule already present in cells as the parent molecule or incorporating the parent compound into a large, insoluble structure.
Although there is a large number of NONOates being investigated for use as vasodilators or as chemotherapeutics, only a few have been evaluated for their antimicrobial activity. Diethylenetriamine, a ubiquitous polyamine found in both eukaryotic and prokaryotic cells,Citation68 can be used to make the NONOate DETA-NO. DETA-NO is effective against gram negative and gram positive bacteria.Citation69,Citation70 Furthermore DETA-NO inhibits candidal growth and acts synergistically with azole antifungals.Citation71 In an effort to further reduce the potential toxicity of the diazeniumdioate, a hybrid of ketoconazole diazeniumdioate was synthesized and shown to be more potent than ketoconazole alone.Citation72 NONOates, especially those based on established medications, are a promising source of NO releasing antimicrobials and would benefit from further study.
In order to reduce the risk of the toxic metabolites, several groups have created NONOates bound to large immobile structures and nanoparticles.Citation73-Citation77 Sol-gel based coatings which integrate N-(6-aminohexyl) aminopropyltrimethoxysilane (AHAP3) can be loaded with diazeniumdiolates.Citation78 When the NO molecules are subsequently released, the metabolites remain bound to the sol-gel coating. However, even with the application of a surface coating, the toxic N-nitroso groups are still cytotoxic to adjacent fibroblast cells at antimicrobial concentrations (40%).Citation78 Atomic force microscopy of E. coli and P. aeruginosa exposed to NONOate coatings showed disorganized adhesion and increased surface roughness similar to the changes caused by amoxicillin.Citation27 These findings suggest that NONOate coatings are as effective in perturbing cell wall structure as a traditional antibiotic that targets cell wall synthesis. In in vitro studies of silicone,Citation73 steelCitation74 and polypropylene implants,Citation75 NONOate coatings decreased bacterial adhesion of S. aureus, E. coli and P. aeruginosa. The results of the two available in vivo studies are mixed. In the first study, NONOate-coated silicone implants decreased the rate of infection by 82%.Citation73 The second study found an increased number of bacteria surrounding the NONOate coated polypropylene implants, but the coating on the polypropylene implant only released 1/6 as much NO as the coating used in the first study.Citation75 Clearly, proper NO dosing is critical to the antimicrobial activity of NO delivery systems. A NO releasing wound dressing made from nanofibers has also been developed. The dressing inhibited S. aureus growth in vitro and, in a small human case series, accelerated healing of cutaneous leishmaniasis ulcers.Citation79 The major limitation of NONOate coatings are the cytotoxicity and the limited number of NO molecules that can be released by the coating in comparison to the lifetime of the device. Nonetheless, the initial release of NO during the first 24 h after implantation is sufficient to decrease the rate of infection, making the currently available coatings clinically valuable.Citation73
S-nitrosothiols
S-nitrosothiols are molecules that contain NO bound to a thiol (sulfhydryl) group (R-SH; ). NO is released when the bond between the two is cleaved. Although this process occurs in the physiologic environment, it does not occur spontaneously. Three mechanisms of release have been identified: copper ion-mediated decomposition, direct reaction with ascorbate and homolytic cleavage by 550–600 nm wavelength light.Citation80-Citation82 Additionally, S-nitrosothiols can transfer bound NO to other thiol groups through a process called transnitrosylation.Citation83 Multiple modes of action may give S-nitrosothiols unique properties compared with gNO and other NO donors. A disadvantage of S-nitrosothiols is that target pathogens have clearance mechanisms that rapidly degrade the active form of the drug.Citation84 S-nitrosoglutathione and S-nitroso-N-acetylcysteine are bactericidal against common Gram-negative and Gram-positive pathogens.Citation85,Citation86 GSNO is bactericidal at concentrations safe to human cells—about twice the concentration of GSNO in human serum.Citation87,Citation88S-nitrosothiols like GSNO, SNAC and S-nitroso-N-acetyl-DL-penicillamine (SNAP) have antimicrobial activity against parasites including Leishmania species,Citation89T. cruzi,Citation90P. falciparumCitation91 and A. castellaniiCitation92 in vitro. Cystine residues found in proteins can also be S-nitrosylated. This technique has led to S-nitrosylation of albumin which is effective against S. Typhimurium in vitro.Citation93 Human studies of S-nitrosothilos are limited to case reports and one small study of 16 patients with cutaneous leishmaniasis. All patients treated with SNAP had complete healing of their ulcers while those treated with vehicle alone showed no clinical improvement after one month.Citation94 The utility of S-nitrosothiols is limited because thiols spontaneously form disulfide bonds in the presence of water and heat. Thus most S-nitrosothiols must be kept refrigerated as dry powder until they are mixed and administered.
Zeolites
Zeolites are nanoporous materials composed of a metal-organic framework. Their large surface area and metallic components allow zeolites to bind large amounts of NO relative to their size. Although stable when dry, when exposed to moisture the water molecules force NO from the zeolite.Citation95 The rate of NO release can be adjusted by altering the composition of the metal-organic framework. Although NO releasing zeolites are a recent discovery, they were shown to have antibacterial properties against P. aeruginosa, MRSA, C. difficile,Citation96 E. coli and B. subtilis.Citation97 For the zeolites examined, NO release is rapid (peaking after 10 min) and short lived (complete release after 1 h). Fast releasing zeolites could be used as disinfectants or as a coating for medical devices. Further work may lead to slow releasing zeolites more suitable for treating infections.
Nanoparticle Platforms
Nanoparticle platforms have unique properties conferred upon them by their small size. In regards to NO delivery, the design of the nanoparticle can influence release rates and sites of delivery.Citation7,Citation98-Citation100 Furthermore, certain nanoparticles have intrinsic antimicrobial activity or can deliver multiple drugs simultaneously. Because these platforms are versatile and customizable, they are an optimal system for delivering NO.
Silica-based nanoparticles can be fashioned from the same materials used to make NONOate implant coatings.Citation99,Citation100 One technique developed by Carpenter, Slomberg, Rao and SchoenfischCitation99 replaces the steel surface of an implant with a silica-based AHAP doped sol-gel. These NONOate covered nanoparticles are effective against P. aeruginosa in vitro. As the size of the particles shrinks, more nanoparticles are able to associate with the surface of the pathogen and the antimicrobial activity rises. Remarkably, the toxicity to human fibroblast in culture did not increase with the smaller-sized particles. In a different study, a NONOate coated silica nanoparticles synthesized without micelles were more effective than a small molecule NONOate in killing P. aeruginosa. The same nanoparticles were bactericidal when applied to P. aeruginosa, E. coli, S. aureus, S. epidermidis and C. albicans biofilms.Citation100 At concentrations sufficient to kill over 99% of the bacteria in a biofilm, the nitric oxide releasing nanoparticles were no more toxic to fibroblasts than the antiseptics povidone iodine and chlorhexidine.
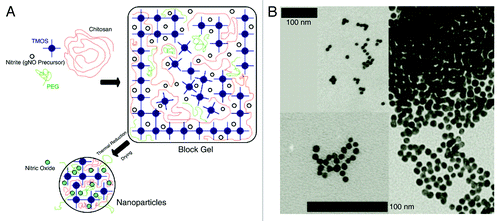
In reviewing the various platforms and their shortcomings, a platform that does not rely on NO-donating chemicals or external reducing agents to generate and release NO would be ideal. Recently, a sol-gel based NO releasing nanoparticle (NO-np) was described that can generate NO through the thermal reduction of nitrite and release it in a slow, sustained manner.Citation7 The core of the nanoparticle is composed of a tetramethoxysilane derived silica network augmented with polyethylene glycol (PEG), chitosan and a glass forming disaccharide (). During synthesis, nitrite is encapsulated within the composite matrix of the nanoparticle and then reduced to NO. Production of NO from nitrite is accomplished by a thermal reduction process requiring the long range transport of electrons from the sugar glass throughout the composites extensive hydrogen bonding network.Citation7,Citation101,Citation102 Chitosan is a positively charged natural polymer that contributes to the structure of the nanoparticle but also has antimicrobial properties of its own.Citation103 After the sol-gel is lyophilized it spontaneously breaks into nanoparticles. The final product is shelf stable at room temperature until it is exposed to moisture. The dry nanoparticles are 10 nm in diameter and form aggregates approximately 130 nm across.Citation7 Once exposed to water, the nanoparticles expand and steadily release NO over 24 h. The rate and total NO released can be modified by changing the amount of nitrite or the molecular weight and the concentration of PEG incorporated into the molecule. The nanoparticle platform shows minimal toxicity to human fibroblasts in culture,Citation7 applied topically in various murine models, or when administered intravenously in a hamster model.Citation104 NO-np has clinical potential because it is an inexpensive, simple to synthesize, shelf stable and nontoxic method for the sustained delivery of NO.
Figure 2. (A) Schematic of NO-np synthesis. When combined the components of the silica hydrogel form a gel. As the gel dries it spontaneously breaks into nanoparticles. The heat from the drying process converts NO from nitrite. (B) TEM of NO-np. The scale bars represent 100 nm. The top scale bar represents the top right inset and the bottom scale bar represents the remaining insets. Reproduced with permission from Cabrales et al.Citation104
In culture, both Gram-positive (MRSA, S. pyogenes and E. faecalis) and Gram-negative (E. coli, K. pneumoniae and P. aeruginosa) bacteria are killed by NO-np doses corresponding to 1.25–5 μM NO.Citation105 Bacteriostatic concentration of NO-np inhibited growth for 12 to 24 h dependent on bacterial species. When NO-np was co-administered with glutathione (GSH), GSNO was generated from the nitrosylation of GSH. This combination treatment even more potently inhibited the growth of MRSA, E. coli, P. aeruginosa and K. pneumonia in culture.Citation106 In murine models, topically applied NO nanoparticles were also effective in decreasing bacterial burden in MRSA-infected wounds by 99.9%,Citation107 MRSA-infected dermal abscesses by 81%Citation108 and A. baumannii-infected wounds by approximately 90%.Citation109 In a MRSA intramuscular abscess model, topical and intralesional NO-np were superior to vancomycin. While vancomycin decreased wound burden by 94%, topical NO-np and intralesional NO-np reduced bacterial burden by 98% and 99%, respectively.Citation110 NO-np is also effective against fungal infections caused by C. albicans (Nacherla et. al, in press) and T. metagrophytes (unpublished findings). An additional benefit of NO-np is their proven ability to accelerate wound healing through recruitment of macrophages, upregulation of collagen gene expression, and acceleration of neoangiogenesis as compared with wounds treated with an empty nanoparticle.Citation111
While other NO delivery systems are bulky, expensive, toxic or unstable, NO-np is a potent and locally acting antimicrobial that is low cost, shelf stable and easy to apply. The combination of broad spectrum antimicrobial activity and the ability to accelerate healing makes NO-np an ideal candidate for treating contaminated wounds. Future work with NO-np is directed at further characterizing its antimicrobial properties in animal models of infection and eventually in human trials.
Conclusion
NO is an important cellular signaling molecule and potent antimicrobial. Its role in physiologic function as well as its potential as a therapeutic agent won it the title of Molecule of the Year in 1992. Over the ensuing 20 years, a plethora of delivery systems have been developed to harness NO for clinical use. Only a few of the available systems have been assessed for their efficacy as an antimicrobial. However, the NO delivery systems assessed thus far exhibit a broad spectrum of antimicrobial activity in vitro and in vivo. The limited work in humans shows that topically delivered NO is clinically useful and no more toxic than currently available antimicrobials. Presently, research into the use of NO as an antimicrobial is focused on characterizing and optimizing delivery systems using in vitro and animal studies. In the future, studies need to investigate the safety, efficacy and feasibility of use in humans.
Declaration of Conflict of Interests
Drs Joshua Nosanchuk and Adam Friedman are on the Advisory Board of Makefield Therapeutics.
References
- Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest 1997; 99:2818 - 25; http://dx.doi.org/10.1172/JCI119473; PMID: 9185502
- Tripathi P, Tripathi P, Kashyap L, Singh V. The role of nitric oxide in inflammatory reactions. FEMS Immunol Med Microbiol 2007; 51:443 - 52; http://dx.doi.org/10.1111/j.1574-695X.2007.00329.x; PMID: 17903207
- Andreakis N, D’Aniello S, Albalat R, Patti FP, Garcia-Fernàndez J, Procaccini G, et al. Evolution of the nitric oxide synthase family in metazoans. Mol Biol Evol 2011; 28:163 - 79; http://dx.doi.org/10.1093/molbev/msq179; PMID: 20639231
- Joerink M, Savelkoul HFJ, Wiegertjes GF. Evolutionary conservation of alternative activation of macrophages: structural and functional characterization of arginase 1 and 2 in carp (Cyprinus carpio L.). Mol Immunol 2006; 43:1116 - 28; http://dx.doi.org/10.1016/j.molimm.2005.07.022; PMID: 16257446
- Privett BJ, Broadnax AD, Bauman SJ, Riccio DA, Schoenfisch MH. Examination of bacterial resistance to exogenous nitric oxide. Nitric Oxide 2012; 26:169 - 73; http://dx.doi.org/10.1016/j.niox.2012.02.002; PMID: 22349019
- Ghaffari A, Miller CC, McMullin B, Ghahary A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide 2006; 14:21 - 9; http://dx.doi.org/10.1016/j.niox.2005.08.003; PMID: 16188471
- Friedman AJ, Han G, Navati MS, Chacko M, Gunther L, Alfieri A, et al. Sustained release nitric oxide releasing nanoparticles: characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide 2008; 19:12 - 20; http://dx.doi.org/10.1016/j.niox.2008.04.003; PMID: 18457680
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol 1997; 15:323 - 50; http://dx.doi.org/10.1146/annurev.immunol.15.1.323; PMID: 9143691
- Bogdan C. Nitric oxide and the immune response. Nat Immunol 2001; 2:907 - 16; http://dx.doi.org/10.1038/ni1001-907; PMID: 11577346
- Durand JL, Mukherjee S, Commodari F, De Souza AP, Zhao D, Machado FS, et al. Role of NO synthase in the development of Trypanosoma cruzi-induced cardiomyopathy in mice. Am J Trop Med Hyg 2009; 80:782 - 7; PMID: 19407124
- Cui X, Besch V, Khaibullina A, Hergen A, Quezado M, Eichacker P, et al. Neuronal nitric oxide synthase deficiency decreases survival in bacterial peritonitis and sepsis. Intensive Care Med 2007; 33:1993 - 2003; http://dx.doi.org/10.1007/s00134-007-0814-9; PMID: 17684724
- Bogdan C. Regulation of lymphocytes by nitric oxide. Methods Mol Biol 2011; 677:375 - 93; http://dx.doi.org/10.1007/978-1-60761-869-0_24; PMID: 20941622
- Wink DA, Mitchell JB. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 1998; 25:434 - 56; http://dx.doi.org/10.1016/S0891-5849(98)00092-6; PMID: 9741580
- Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med 2008; 45:18 - 31; http://dx.doi.org/10.1016/j.freeradbiomed.2008.03.020; PMID: 18439435
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol 1997; 15:323 - 50; http://dx.doi.org/10.1146/annurev.immunol.15.1.323; PMID: 9143691
- Jones ML, Ganopolsky JG, Labbé A, Wahl C, Prakash S. Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl Microbiol Biotechnol 2010; 88:401 - 7; http://dx.doi.org/10.1007/s00253-010-2733-x; PMID: 20680266
- Wink DA, Grisham MB, Mitchell JB, Ford PC. Direct and indirect effects of nitric oxide in chemical reactions relevant to biology. In: Lester P, ed. Methods in Enzymology. Vol Volume 268: Academic Press; 1996:12-31.
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2004; 2:820 - 32; http://dx.doi.org/10.1038/nrmicro1004; PMID: 15378046
- Gow AJ, Buerk DG, Ischiropoulos H. A novel reaction mechanism for the formation of S-nitrosothiol in vivo. J Biol Chem 1997; 272:2841 - 5; http://dx.doi.org/10.1074/jbc.272.5.2841; PMID: 9006926
- Ischiropoulos H, al-Mehdi AB. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett 1995; 364:279 - 82; http://dx.doi.org/10.1016/0014-5793(95)00307-U; PMID: 7758583
- Wink DA, Kasprzak KS, Maragos CM, Elespuru RK, Misra M, Dunams TM, et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science 1991; 254:1001 - 3; http://dx.doi.org/10.1126/science.1948068; PMID: 1948068
- Juedes MJ, Wogan GN. Peroxynitrite-induced mutation spectra of pSP189 following replication in bacteria and in human cells. Mutat Res 1996; 349:51 - 61; http://dx.doi.org/10.1016/0027-5107(95)00152-2; PMID: 8569792
- Maragos CM, Andrews AW, Keefer LK, Elespuru RK. Mutagenicity of glyceryl trinitrate (nitroglycerin) in Salmonella typhimurium. Mutat Res 1993; 298:187 - 95; http://dx.doi.org/10.1016/0165-1218(93)90040-K; PMID: 7678153
- Laval F, Wink DA, Laval J. A discussion of mechanisms of NO genotoxicity: implication of inhibition of DNA repair proteins. Rev Physiol Biochem Pharmacol 1997; 131:175 - 91; PMID: 9204692
- Laval F, Wink DA. Inhibition by nitric oxide of the repair protein, O6-methylguanine-DNA-methyltransferase. Carcinogenesis 1994; 15:443 - 7; http://dx.doi.org/10.1093/carcin/15.3.443; PMID: 8118926
- Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem 1994; 269:26066 - 75; PMID: 7929318
- Deupree SM, Schoenfisch MH. Morphological analysis of the antimicrobial action of nitric oxide on gram-negative pathogens using atomic force microscopy. Acta Biomater 2009; 5:1405 - 15; http://dx.doi.org/10.1016/j.actbio.2009.01.025; PMID: 19250890
- Abouhamad WN, Manson M, Gibson MM, Higgins CF. Peptide transport and chemotaxis in Escherichia coli and Salmonella typhimurium: characterization of the dipeptide permease (Dpp) and the dipeptide-binding protein. Mol Microbiol 1991; 5:1035 - 47; http://dx.doi.org/10.1111/j.1365-2958.1991.tb01876.x; PMID: 1956284
- Morris SL, Hansen JN. Inhibition of Bacillus cereus spore outgrowth by covalent modification of a sulfhydryl group by nitrosothiol and iodoacetate. J Bacteriol 1981; 148:465 - 71; PMID: 6795179
- Kim YM, Bergonia HA, Müller C, Pitt BR, Watkins WD, Lancaster JR Jr. Nitric oxide and intracellular heme. Adv Pharmacol 1995; 34:277 - 91; http://dx.doi.org/10.1016/S1054-3589(08)61092-3; PMID: 8562440
- Björne H, Weitzberg E, Lundberg JO. Intragastric generation of antimicrobial nitrogen oxides from saliva--physiological and therapeutic considerations. Free Radic Biol Med 2006; 41:1404 - 12; http://dx.doi.org/10.1016/j.freeradbiomed.2006.07.020; PMID: 17023267
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 2008; 7:156 - 67; http://dx.doi.org/10.1038/nrd2466; PMID: 18167491
- Weller R, Price RJ, Ormerod AD, Benjamin N, Leifert C. Antimicrobial effect of acidified nitrite on dermatophyte fungi, Candida and bacterial skin pathogens. J Appl Microbiol 2001; 90:648 - 52; http://dx.doi.org/10.1046/j.1365-2672.2001.01291.x; PMID: 11309079
- Weller R, Finnen MJ. The effects of topical treatment with acidified nitrite on wound healing in normal and diabetic mice. Nitric Oxide 2006; 15:395 - 9; http://dx.doi.org/10.1016/j.niox.2006.04.002; PMID: 16731016
- Pacelli R, Wink DA, Cook JA, Krishna MC, DeGraff W, Friedman N, et al. Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. J Exp Med 1995; 182:1469 - 79; http://dx.doi.org/10.1084/jem.182.5.1469; PMID: 7595217
- Fang FC. Antimicrobial actions of reactive oxygen species. MBio 2011; 2:e00141 - 11; http://dx.doi.org/10.1128/mBio.00141-11; PMID: 21896680
- Bogdan C. Nitric oxide and the immune response. Nat Immunol 2001; 2:907 - 16; http://dx.doi.org/10.1038/ni1001-907; PMID: 11577346
- Ghaffari A, Jalili R, Ghaffari M, Miller C, Ghahary A. Efficacy of gaseous nitric oxide in the treatment of skin and soft tissue infections. Wound Repair Regen 2007; 15:368 - 77; http://dx.doi.org/10.1111/j.1524-475X.2007.00239.x; PMID: 17537124
- Friedman AJ, Blecher K, Schairer D, Tuckman-Vernon C, Nacharaju P, Sanchez D, et al. Improved antimicrobial efficacy with nitric oxide releasing nanoparticle generated S-nitrosoglutathione. Nitric Oxide 2011; 25:381 - 6; http://dx.doi.org/10.1016/j.niox.2011.09.001; PMID: 21946032
- Englander L, Friedman A. Nitric oxide nanoparticle technology: a novel antimicrobial agent in the context of current treatment of skin and soft tissue infection. J Clin Aesthet Dermatol 2010; 3:45 - 50; PMID: 20725551
- Christman MF, Morgan RW, Jacobson FS, Ames BN. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell 1985; 41:753 - 62; http://dx.doi.org/10.1016/S0092-8674(85)80056-8; PMID: 2988786
- Richardson AR, Dunman PM, Fang FC. The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol Microbiol 2006; 61:927 - 39; http://dx.doi.org/10.1111/j.1365-2958.2006.05290.x; PMID: 16859493
- Richardson AR, Libby SJ, Fang FC. A nitric oxide-inducible lactate dehydrogenase enables Staphylococcus aureus to resist innate immunity. Science 2008; 319:1672 - 6; http://dx.doi.org/10.1126/science.1155207; PMID: 18356528
- Hetrick EM. Antimicrobial and wound healing properties of nitric oxide-releasing xerogels and silica nanoparticles. University of North Carolina; 2008.
- Hetrick EM, Shin JH, Paul HS, Schoenfisch MH. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009; 30:2782 - 9; http://dx.doi.org/10.1016/j.biomaterials.2009.01.052; PMID: 19233464
- De Groote MA, Fang FC. NO inhibitions: antimicrobial properties of nitric oxide. Clin Infect Dis 1995; 21:Suppl 2 S162 - 5; http://dx.doi.org/10.1093/clinids/21.Supplement_2.S162; PMID: 8845445
- Ghaffari A, Neil DH, Ardakani A, Road J, Ghahary A, Miller CC. A direct nitric oxide gas delivery system for bacterial and mammalian cell cultures. Nitric Oxide 2005; 12:129 - 40; http://dx.doi.org/10.1016/j.niox.2005.01.006; PMID: 15797841
- Miller C, McMullin B, Ghaffari A, Stenzler A, Pick N, Roscoe D, et al. Gaseous nitric oxide bactericidal activity retained during intermittent high-dose short duration exposure. Nitric Oxide 2009; 20:16 - 23; http://dx.doi.org/10.1016/j.niox.2008.08.002; PMID: 18789393
- Ghaffari A, Jalili R, Ghaffari M, Miller C, Ghahary A. Efficacy of gaseous nitric oxide in the treatment of skin and soft tissue infections. Wound Repair Regen 2007; 15:368 - 77; http://dx.doi.org/10.1111/j.1524-475X.2007.00239.x; PMID: 17537124
- Ichinose F, Roberts JD Jr., Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation 2004; 109:3106 - 11; http://dx.doi.org/10.1161/01.CIR.0000134595.80170.62; PMID: 15226227
- Angus DC, Clermont G, Watson RS, Linde-Zwirble WT, Clark RH, Roberts MS. Cost-effectiveness of inhaled nitric oxide in the treatment of neonatal respiratory failure in the United States. Pediatrics 2003; 112:1351 - 60; http://dx.doi.org/10.1542/peds.112.6.1351; PMID: 14654609
- Jones ML, Ganopolsky JG, Labbé A, Prakash S. A novel nitric oxide producing probiotic patch and its antimicrobial efficacy: preparation and in vitro analysis. Appl Microbiol Biotechnol 2010; 87:509 - 16; http://dx.doi.org/10.1007/s00253-010-2490-x; PMID: 20300748
- Jones M, Ganopolsky JG, Labbé A, Gilardino M, Wahl C, Martoni C, et al. Novel nitric oxide producing probiotic wound healing patch: preparation and in vivo analysis in a New Zealand white rabbit model of ischaemic and infected wounds. Int Wound J 2012; no - , no; http://dx.doi.org/10.1111/j.1742-481X.2011.00889.x; PMID: 22221913
- Ormerod AD, Copeland P, Hay I, Husain A, Ewen SW. The inflammatory and cytotoxic effects of a nitric oxide releasing cream on normal skin. J Invest Dermatol 1999; 113:392 - 7; http://dx.doi.org/10.1046/j.1523-1747.1999.00692.x; PMID: 10469339
- Weller R, Ormerod AD, Hobson RP, Benjamin NJ. A randomized trial of acidified nitrite cream in the treatment of tinea pedis. J Am Acad Dermatol 1998; 38:559 - 63; http://dx.doi.org/10.1016/S0190-9622(98)70117-3; PMID: 9555794
- Ormerod AD, White MI, Shah SAA, Benjamin N. Molluscum contagiosum effectively treated with a topical acidified nitrite, nitric oxide liberating cream. Br J Dermatol 1999; 141:1051 - 3; http://dx.doi.org/10.1046/j.1365-2133.1999.03204.x; PMID: 10606851
- Phillips R, Adjei O, Lucas S, Benjamin N, Wansbrough-Jones M. Pilot randomized double-blind trial of treatment of Mycobacterium ulcerans disease (Buruli ulcer) with topical nitrogen oxides. Antimicrob Agents Chemother 2004; 48:2866 - 70; http://dx.doi.org/10.1128/AAC.48.8.2866-2870.2004; PMID: 15273093
- Ormerod AD, Shah AA, Li H, Benjamin NB, Ferguson GP, Leifert C. An observational prospective study of topical acidified nitrite for killing methicillin-resistant Staphylococcus aureus (MRSA) in contaminated wounds. BMC Res Notes 2011; 4:458; http://dx.doi.org/10.1186/1756-0500-4-458; PMID: 22032298
- Davidson RN, Yardley V, Croft SL, Konecny P, Benjamin N. A topical nitric oxide-generating therapy for cutaneous leishmaniasis. Trans R Soc Trop Med Hyg 2000; 94:319 - 22; http://dx.doi.org/10.1016/S0035-9203(00)90341-9; PMID: 10975011
- Daiber A, Wenzel P, Oelze M, Münzel T. New insights into bioactivation of organic nitrates, nitrate tolerance and cross-tolerance. Clin Res Cardiol 2008; 97:12 - 20; http://dx.doi.org/10.1007/s00392-007-0588-7; PMID: 17938848
- Bátai I, Kerényi M, Tekeres M. The growth of bacteria in intravenous glyceryl trinitrate and in sodium nitroprusside. Anesth Analg 1999; 89:1570 - 2; http://dx.doi.org/10.1213/00000539-199912000-00049; PMID: 10589651
- Barraud N, Storey MV, Moore ZP, Webb JS, Rice SA, Kjelleberg S. Nitric oxide-mediated dispersal in single- and multi-species biofilms of clinically and industrially relevant microorganisms. Microb Biotechnol 2009; 2:370 - 8; http://dx.doi.org/10.1111/j.1751-7915.2009.00098.x; PMID: 21261931
- Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, et al. Complexes of. NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J Med Chem 1991; 34:3242 - 7; http://dx.doi.org/10.1021/jm00115a013; PMID: 1956043
- Miller MR, Megson IL. Recent developments in nitric oxide donor drugs. Br J Pharmacol 2007; 151:305 - 21; http://dx.doi.org/10.1038/sj.bjp.0707224; PMID: 17401442
- Keefer LK. Nitric oxide (NO)- and nitroxyl (HNO)-generating diazeniumdiolates (NONOates): emerging commercial opportunities. Curr Top Med Chem 2005; 5:625 - 36; http://dx.doi.org/10.2174/1568026054679380; PMID: 16101424
- Kröncke K-D, Suschek CV. Adulterated effects of nitric oxide-generating donors. J Invest Dermatol 2008; 128:258 - 60; http://dx.doi.org/10.1038/sj.jid.5701162; PMID: 18195740
- Keefer LK. Progress toward clinical application of the nitric oxide-releasing diazeniumdiolates. Annu Rev Pharmacol Toxicol 2003; 43:585 - 607; http://dx.doi.org/10.1146/annurev.pharmtox.43.100901.135831; PMID: 12415121
- Tabor H, Tabor CW. Biosynthesis and Metabolism of 1,4-diaminobutane, Spermidine, Spermine, and Related Amines. Advances in Enzymology and Related Areas of Molecular Biology: John Wiley & Sons, Inc.; 2006:203-268.
- Raulli R, McElhaney-Feser G, Hrabie J, Cihlar R. Antimicrobial properties of nitric oxide using diazeniumdiolates as the nitric oxide donor. Rec Res Devel Microbiol. 2002; 6:177 - 83
- Dukelow AM, Weicker S, Karachi TA, Razavi HM, McCormack DG, Joseph MG, et al. Effects of nebulized diethylenetetraamine-NONOate in a mouse model of acute Pseudomonas aeruginosa pneumonia. Chest 2002; 122:2127 - 36; http://dx.doi.org/10.1378/chest.122.6.2127; PMID: 12475857
- McElhaney-Feser GE, Raulli RE, Cihlar RL. Synergy of nitric oxide and azoles against Candida species in vitro. Antimicrob Agents Chemother 1998; 42:2342 - 6; PMID: 9736560
- Konter J, Möllmann U, Lehmann J. NO-donors. Part 17: Synthesis and antimicrobial activity of novel ketoconazole-NO-donor hybrid compounds. Bioorg Med Chem 2008; 16:8294 - 300; http://dx.doi.org/10.1016/j.bmc.2008.05.008; PMID: 18710813
- Nablo BJ, Prichard HL, Butler RD, Klitzman B, Schoenfisch MH. Inhibition of implant-associated infections via nitric oxide release. Biomaterials 2005; 26:6984 - 90; http://dx.doi.org/10.1016/j.biomaterials.2005.05.017; PMID: 15978663
- Nablo BJ, Rothrock AR, Schoenfisch MH. Nitric oxide-releasing sol-gels as antibacterial coatings for orthopedic implants. Biomaterials 2005; 26:917 - 24; http://dx.doi.org/10.1016/j.biomaterials.2004.03.031; PMID: 15353203
- Engelsman AF, Krom BP, Busscher HJ, van Dam GM, Ploeg RJ, van der Mei HC. Antimicrobial effects of an NO-releasing poly(ethylene vinylacetate) coating on soft-tissue implants in vitro and in a murine model. Acta Biomater 2009; 5:1905 - 10; http://dx.doi.org/10.1016/j.actbio.2009.01.041; PMID: 19251498
- Shin JH, Metzger SK, Schoenfisch MH. Synthesis of nitric oxide-releasing silica nanoparticles. J Am Chem Soc 2007; 129:4612 - 9; http://dx.doi.org/10.1021/ja0674338; PMID: 17375919
- Charville GW, Hetrick EM, Geer CB, Schoenfisch MH. Reduced bacterial adhesion to fibrinogen-coated substrates via nitric oxide release. Biomaterials 2008; 29:4039 - 44; http://dx.doi.org/10.1016/j.biomaterials.2008.07.005; PMID: 18657857
- Nablo BJ, Schoenfisch MH. In vitro cytotoxicity of nitric oxide-releasing sol-gel derived materials. Biomaterials 2005; 26:4405 - 15; http://dx.doi.org/10.1016/j.biomaterials.2004.11.015; PMID: 15701369
- Bhide M. Nitric oxide delivery from polymeric wound dressings. The University Of Akron; 2006.
- Dicks AP, Swift HR, Williams DLH, Butler AR, Al-Sa'doni HH, Cox BG. Identification of Cu+ as the effective reagent in nitric oxide formation from S-nitrosothiols (RSNO). Journal of the Chemical Society, Perkin Transactions 2 1996; 4
- Holmes AJ, Williams DLH. Reaction of ascorbic acid with S-nitrosothiols: clear evidence for two distinct reaction pathways. Journal of the Chemical Society, Perkin Transactions 2 2000; 8
- Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. J Biol Chem 1996; 271:18596 - 603; http://dx.doi.org/10.1074/jbc.271.31.18596; PMID: 8702510
- Hogg N. Biological chemistry and clinical potential of S-nitrosothiols. Free Radic Biol Med 2000; 28:1478 - 86; http://dx.doi.org/10.1016/S0891-5849(00)00248-3; PMID: 10927172
- Laver JR, Stevanin TM, Messenger SL, Lunn AD, Lee ME, Moir JW, et al. Bacterial nitric oxide detoxification prevents host cell S-nitrosothiol formation: a novel mechanism of bacterial pathogenesis. FASEB J 2010; 24:286 - 95; http://dx.doi.org/10.1096/fj.08-128330; PMID: 19720623
- Souza-Lima RA Sr, Cariello AJ, Bispo M Sr, et al. Bactericidal Effect Of Nitric Oxide Donors Against Clinical Isolates From Keratitis. Invest Ophthalmol Vis Sci 2011; 52:5839 - 41
- Marcinkiewicz J. Nitric oxide and antimicrobial activity of reactive oxygen intermediates. Immunopharmacology 1997; 37:35 - 41; http://dx.doi.org/10.1016/S0162-3109(96)00168-3; PMID: 9285242
- Richie JP Jr., Skowronski L, Abraham P, Leutzinger Y. Blood glutathione concentrations in a large-scale human study. Clin Chem 1996; 42:64 - 70; PMID: 8565235
- Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, et al. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci U S A 2004; 101:4308 - 13; http://dx.doi.org/10.1073/pnas.0306706101; PMID: 15014175
- de Souza GFP, Yokoyama-Yasunaka JKU, Seabra AB, Miguel DC, de Oliveira MG, Uliana SRB. Leishmanicidal activity of primary S-nitrosothiols against Leishmania major and Leishmania amazonensis: implications for the treatment of cutaneous leishmaniasis. Nitric Oxide 2006; 15:209 - 16; http://dx.doi.org/10.1016/j.niox.2006.01.011; PMID: 16527502
- Vespa GN, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun 1994; 62:5177 - 82; PMID: 7523307
- Rockett KA, Awburn MM, Cowden WB, Clark IA. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun 1991; 59:3280 - 3; PMID: 1879941
- Cariello AJ, de Souza GFP, Foronda AS, Yu MCZ, Hofling-Lima AL, de Oliveira MG. In vitro amoebicidal activity of S-nitrosoglutathione and S-nitroso-N-acetylcysteine against trophozoites of Acanthamoeba castellanii. J Antimicrob Chemother 2010; 65:588 - 91; http://dx.doi.org/10.1093/jac/dkp485; PMID: 20085996
- Ishima Y, Sawa T, Kragh-Hansen U, Miyamoto Y, Matsushita S, Akaike T, et al. S-Nitrosylation of human variant albumin Liprizzi (R410C) confers potent antibacterial and cytoprotective properties. J Pharmacol Exp Ther 2007; 320:969 - 77; http://dx.doi.org/10.1124/jpet.106.114959; PMID: 17135341
- López-Jaramillo P, Ruano C, Rivera J, Terán E, Salazar-Irigoyen R, Esplugues JV, et al. Treatment of cutaneous leishmaniasis with nitric-oxide donor. Lancet 1998; 351:1176 - 7; http://dx.doi.org/10.1016/S0140-6736(05)79119-4; PMID: 9643692
- Wheatley PS, Butler AR, Crane MS, Fox S, Xiao B, Rossi AG, et al. NO-releasing zeolites and their antithrombotic properties. J Am Chem Soc 2006; 128:502 - 9; http://dx.doi.org/10.1021/ja0503579; PMID: 16402837
- Fox S, Wilkinson TS, Wheatley PS, Xiao B, Morris RE, Sutherland A, et al. NO-loaded Zn(2+)-exchanged zeolite materials: a potential bifunctional anti-bacterial strategy. Acta Biomater 2010; 6:1515 - 21; http://dx.doi.org/10.1016/j.actbio.2009.10.038; PMID: 19861185
- Narin G, Albayrak ÇB, Ülkü S. Antibacterial and bactericidal activity of nitric oxide-releasing natural zeolite. Appl Clay Sci 2010; 50:560 - 8; http://dx.doi.org/10.1016/j.clay.2010.10.013
- Jin Y, Lohstreter S, Pierce DT, et al. Silica Nanoparticles with Continuously Tunable Sizes: Synthesis and Size Effects on Cellular Contrast Imaging. Chemistry of Materials. 2008; 20:4411 - 9; http://dx.doi.org/10.1021/cm8007478
- Carpenter AW, Slomberg DL, Rao KS, Schoenfisch MH. Influence of scaffold size on bactericidal activity of nitric oxide-releasing silica nanoparticles. ACS Nano 2011; 5:7235 - 44; http://dx.doi.org/10.1021/nn202054f; PMID: 21842899
- Hetrick EM, Shin JH, Paul HS, Schoenfisch MH. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009; 30:2782 - 9; http://dx.doi.org/10.1016/j.biomaterials.2009.01.052; PMID: 19233464
- Ray A, Friedman BA, Friedman JM. Trehalose glass-facilitated thermal reduction of metmyoglobin and methemoglobin. J Am Chem Soc 2002; 124:7270 - 1; http://dx.doi.org/10.1021/ja0201348; PMID: 12071726
- Navati MS, Friedman JM. Sugar-derived glasses support thermal and photo-initiated electron transfer processes over macroscopic distances. J Biol Chem 2006; 281:36021 - 8; http://dx.doi.org/10.1074/jbc.M606866200; PMID: 17005567
- Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 2003; 4:1457 - 65; http://dx.doi.org/10.1021/bm034130m; PMID: 14606868
- Cabrales P, Han G, Roche C, Nacharaju P, Friedman AJ, Friedman JM. Sustained release nitric oxide from long-lived circulating nanoparticles. Free Radic Biol Med 2010; 49:530 - 8; http://dx.doi.org/10.1016/j.freeradbiomed.2010.04.034; PMID: 20460149
- Friedman A, Blecher K, Sanchez D, Tuckman-Vernon C, Gialanella P, Friedman JM, et al. Susceptibility of Gram-positive and -negative bacteria to novel nitric oxide-releasing nanoparticle technology. Virulence 2011; 2:217 - 21; http://dx.doi.org/10.4161/viru.2.3.16161; PMID: 21577055
- Friedman AJ, Blecher K, Schairer D, Tuckman-Vernon C, Nacharaju P, Sanchez D, et al. Improved antimicrobial efficacy with nitric oxide releasing nanoparticle generated S-nitrosoglutathione. Nitric Oxide 2011; 25:381 - 6; http://dx.doi.org/10.1016/j.niox.2011.09.001; PMID: 21946032
- Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, et al. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J Invest Dermatol 2009; 129:2463 - 9; http://dx.doi.org/10.1038/jid.2009.95; PMID: 19387479
- Han G, Martinez LR, Mihu MR, Friedman AJ, Friedman JM, Nosanchuk JD. Nitric oxide releasing nanoparticles are therapeutic for Staphylococcus aureus abscesses in a murine model of infection. PLoS One 2009; 4:e7804; http://dx.doi.org/10.1371/journal.pone.0007804; PMID: 19915659
- Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, Martinez LR. The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence 2010; 1:62 - 7; http://dx.doi.org/10.4161/viru.1.2.10038; PMID: 21178416
- Schairer D, Martinez LR, Blecher K, Chouake J, Nacharaju P, Gialanella P, et al. Nitric oxide nanoparticles: Pre-clinical utility as a therapeutic for intramuscular abscesses. Virulence 2012; 3:62 - 7; http://dx.doi.org/10.4161/viru.3.1.18816; PMID: 22286699
- Han G, Nguyen LN, Macherla C, Chi Y, Friedman JM, Nosanchuk JD, et al. Nitric oxide-releasing nanoparticles accelerate wound healing by promoting fibroblast migration and collagen deposition. Am J Pathol 2012; 180:1465 - 73; http://dx.doi.org/10.1016/j.ajpath.2011.12.013; PMID: 22306734