Many invasive intracellular bacterial pathogens such as Shigella and Listeria monocytogenes can spread from cell to cell during infection, and this spreading is an important pathogenic mechanism to expand their replicative niches. However, it is still unclear how motile bacteria are able to cross epithelial cell-cell junctions. We present evidence that Shigella-containing pseudopodia target tricellular junctions, and that tricellulin is functionally important to promote bacterial cell-cell spreading. We also showed that a Shigella cell-cell spreading depended on phosphoinositide 3-kinase, clathrin, Epsin-1 and Dynamin-2, which localized beneath the plasma membrane of the engulfing cells. Since knocking down tricelluin, Epsin-1, clathrin or Dynamin-2 expression reduced Shigella cell-cell spreading, whereas AP-2, Dab2 and Eps15 were not required for this process, it is likely that Shigella-containing pseudopodium that is fully engulfed by a neighboring epithelial cell undergoes non-canonical clathrin-dependent endocytosis.
In eukaryotic cells, the internalization of extracellular cargo via the endocytic machinery is an essential process for many cellular functions, including nutrient uptake, membrane trafficking, recycling extracellular receptors, cell migration, cell-cell communication and microbial infection. Intercellular bacterial movement, such as that mediated by Shigella, is a sequential process that partially resembles the intercellular trafficking of double-membrane vesicles such as during connexin and claudin trafficking. This process has been shown to require many bacterial and host factors, but the molecular basis remains still partly speculative.
The tight cell-cell junctions (TJs) in the intestinal epithelium are essential for maintaining epithelial integrity, which also act as an intrinsic barrier against microbial invasion as well as bacterial cell-cell spreading. Nevertheless some cytosolic invading bacterial pathogens such as Shigella and L. monocytogenes can move from one epithelial cell to another. This process consists of at least three distinctive stages. First, the motile bacterium attaches to the plasma membrane and impinges upon the membrane so that it protrudes as a pseudopodium. Second, the protruding pseudopodium is engulfed by a neighboring cell. Finally, the double plasma membranes are lysed, allowing the bacterium to disseminate into the cytoplasm of the neighboring cell. TJs are a network of transmembrane and peripheral proteins that form a semipermeable barrier to paracellular flux, and thus function as the main determinants of the epithelial and endothelial barriers. The distinct composition of different transmembrane proteins includes occludin and claudin family members. In addition, tricellulin, which is a basic element of tricellular tight junctions (tTJs) and discovered as the first tight junction protein and characterized by Shoichiro Tsukita’s group as distinct tetraspan transmembrane protein, mainly localizes to tricellular cell contacts and is present to a lesser extent in bicellular tight junctions (bTJs) (Ikenouchi et al., J Cell Biol 2005).
We thus undertook investigation of the mechanism that allows Shigella to move from one epithelial cell to neighboring epithelial cells. We used time-lapse imaging to monitor the fate of motile bacteria from the onset of bacteria-induced membrane protrusion until the bacteria disseminated into an adjacent cell using MK2 cells, which was formally termed as LLC-MK2 cells (rhesus monkey kidney epithelial cells). When MK2 cell monolayers were infected with Shigella, 80% of the bacteria moved into adjacent cells via tTJs, while 20% of bacteria spread via bTJs. The same tendency was observed with other epithelial cell lines, such as Caco2 (human colon carcinoma) and MDCK (Madin-Darby canine kidney) cells. Electron microscopic analysis confirmed that the Shigella-containing pseudopodium extended around the tTJ, which was then engulfed by a neighboring epithelial cell.
Of note this highly selective bacterial cell-cell movement at tTJs was not predominant when MK2 cell monolayers were infected with L. monocytogenes. Only 51% and 49% of L. monocytogenes cell-cell spreading occurred via bTJs and tTJs, respectively, suggesting that tTJs are not a preferential site of L. monocytogenes cell-cell spreading. Interestingly, Rajabian et al. (Nat Cell Biol 2009) reported that the L. monocytogenes virulence protein internalin C (InlC) plays an important role in cell-cell spreading. InlC inhibits Tuba, which perturbs the tension between the apical junctions and facilitates protrusion of Listeria-containing pseudopodia from bTJs. Indeed, the authors showed that ectopic expression of InlC in epithelial monolayers perturbed apical junctions via interactions with Tuba, which interfered with N-WASP binding and reduced tension at bTJs (Rajabian et al., Nat Cell Biol 2009). A previous study showed that Tuba is concentrated at bTJs via interactions with ZO-1, and that knocking down Tuba expression caused membrane curving and slack between cell-cell junctions (Otani et al., J Cell Biol 2006). We, therefore, speculate that InlC-mediated perturbation of bTJs allows L. monocytogenes to protrude from pseudopodia at both bTJs and tTJs.
Because tricellulin is highly expressed at tTJs and is an essential component of tTJs, we used polarized MDCK monolayers due to the feasibility of a plaque formation assay to examine Shigella cell-cell movement, and investigated whether tricellulin is functionary involved in Shigella cell-cell spreading. shRNA-mediated knockdown of tricellulin expression reduced the diameter of plaques in the cell monolayer that were due to bacterial cell-cell spreading compared with that of the control epithelial cells, and the fraction of bacteria-containing pseudopodia that protruded from tTJs was also reduced compared with the mock control. Since the number of bacteria-containing pseudopodia per epithelial cell was similar in the tricellulin knocked down and control cells, we concluded that Shigella dissemination into neighboring epithelial cells depends on the integrity of tTJs.
It has been shown that apical junctional complexes are markedly plastic under physiologic and pathophysiologic conditions. Tight junctions are frequently remodeled under physiologic conditions and change in response to such extracellular stimuli as tumor necrosis factor and interferon-γ in inflammatory diseases; these processes are characterized by the exchange of apical junctional complex proteins from junctional and cytoplasmic pools (Edelblum and Turner, Curr Opin Pharmacol 2009; McMahon and Boucrot, Nat Rev Mol Cell Biol 2011; Shen et al., J Cell Biol 2008; Shen et al., Neurochem Res 2009; Shen et al., Annu Rev Physiol 2011). In the intestinal epithelium, the plasticity of tight junctions is critically important for epithelial integrity, the intestinal barrier, and homeostasis, because bTJs and tTJs are constantly needed as dying cells are shed and the epithelium is rapidly sealed. Remodeling adhesive cell-cell contacts, including adjusting the junctional length and correctly localizing new epithelial cells, requires endocytosis and recycling of adhesion molecules (Madara, J Membr Biol 1990; Troyanovsky et al., Mol Biol Cell 2006). At present, the molecular mechanisms behind tTJs formation remains poorly understood, and how tricellulin per se contributes to the bacterial spreading also remains unclear. Since some studies indicated that tTJs are exploited as “window” for protrusions form epithelial cells to elongate into the lumen to sense the outer environment (Kubo et al., J Exp Med 2009; Shum et al., Cell 2008), it is likely that the plasma membrane around tTJs could be a frequent source and destination of endocytotic vesicles, which may facilitate protrusion and engulfment of pseudopodia during Shigella cell-cell movement.
Remarkably, it has been shown that a bacterium that is enclosed within a pseudopodium will not be released into the free space surrounding the host cell in the absence of neighboring cells, implying that a bacteria-containing pseudopodium must directly contact the neighboring cell to trigger pseudopodium engulfment by the neighboring cell membrane. Phosphoinositide (PI) 3-kinase activity is required to remodel the membrane surface architecture and regulate membrane trafficking, cytoskeletal dynamics, and signal transduction (Lindmo, J Cell Sci 2006). Hence, we first investigated the potential role of PI 3-kinase in the formation of Shigella-containing pseudopodia. To this end, we fused GFP to the pleckstrin homology (PH) domain of Akt (GFP-Akt-PH), which binds to PtdIns(3,4,5)P3 and PtdIns(3,4)P2 generated by PI 3-kinase activity. When a MK2 cell transiently expressing GFP-Akt-PH engulfed a Shigella-containing pseudopodium that was protruding from a neighboring MK2 cell that lacked GFP-Akt-PH expression, the GFP-Akt-PH signal was detected as early as 2 min after pseudopodium engulfment using time-lapse imagining. Indeed, when the PI 3-kinase activity in epithelial cells was blocked with LY294002 (PI 3-kinase inhibitor), the degree of bacterial intercellular spreading was markedly diminished, although the protrusion of Shigella-containing pseudopodium was not affected. Giemsa staining of a Shigella-infected Caco-2 cell monolayer confirmed the LY294002 treatment results and showed that Caco-2 cells were filled with bacteria that had replicated but failed to spread into adjacent cells. These data suggested that the PI 3-kinase activity of epithelial cells is essential to trigger the engulfment of Shigella-containing pseudopodia by adjacent epithelial cells but not for pseudopodium formation per se. Therefore, it appear that the mechanims underlying the internalization of bacteria-containing pseudopodia and clathrin-dependent recycling of transferin, epidermal growth factor (EGF) and low-density lipoporotein (LDL) appear to differ. Additionally, studies are needed to characterize this noncanonical endocytosis pathway during Shigella cell-cell spreading.
There are three major endocytic membrane trafficking pathways in mammalian cells, including clathrin-dependent endocytosis, caveolin-dependent endocytosis and macropinocytosis (McMahon and Boucrot, Nat Rev Mol Cell Biol 2011). Therefore, we wished to determine which of these trafficking pathways is involved in Shigella cell-cell spreading. To this end, we treated Caco2 monolayers with phenylarsine oxide (PAO), methyl-β-cyclodextrin (MβCD) and 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), which respectively inhibit clathrin, caveolin and macropinocytosis. Caco2 cells that were treated with PAO, MβCD or EIPA and infected with Shigella were examined for plaque formation as the consequence of Shigella cell-cell spreading. The plaque assay showed that Caco2 cells treated with PAO (and partially MβCD), but not EIPA, diminished Shigella cell-cell spreading. Indeed, the fraction of Shigella-positive plaques in the monolayer decreased to 21% of the untreated control, strongly suggesting that Shigella-containing pseudopodia are taken up by neighboring cells through a clathrin-mediated trafficking pathway. This was ensured by using shRNA to knock down clathrin expression in epithelial cells infected with Shigella in a plaque formation assay. Clathrin knockdown decreased the diameter of plaques to less than one-third of the mock control. The same was also true for dynamin-2 knockdown. The number of Shigella-containing pseudopodia in clathrin knocked down cells and dynamin-2 knocked down cells was 4.4 ± 0.2 and 4.2 ± 0.2, respectively, and pseudopodium formation was similar to that of mock control cells (4.4 ± 0.3), suggesting that the presence or absence of clathrin and dynamin-2 did not substantially affect pseudopodium formation by motile Shigella. Furthermore, when Caco-2 cell monolayers were infected with Shigella and stained with anti-human clathrin and anti-human dynamin-2 antibodies, both clathrin and dymanin-2 were detected around bacteria-containing pseudopodia. To further confirm that clathrin and dynamin-2 accumulate beneath the cell membrane of neighboring cells that engulfed bacteria-containing pseudopodia, MK2 cells transiently expressing clathrin-GFP (or dynamin-2-GFP) that had taken up Shigella-containing pseudopodia, were examined by immunofluorescence microscopy. When we analyzed a bacteria-containing pseudopodium that protruded from a clathrin-GFP-negative MK2 cell (or dynamin-2-GFP-negative) into a neighboring cell expressing clathrin-GFP (or dynamin-2-GFP), we detected GFP signals around the bacteria-containing pseudopodium. Importantly, time-laps movies further showed that clathrin accumulated around a long bacteria-containing pseudopodium that was engulfed by a clathrin-GFP-expressing epithelial cell. These GFP signals were detected after the 30 min time point, when clathrin-GFP was abundant at the tip of the elongated pseudopodium. In contrast, clathrin-GFP only minimally accumulated around the bacteria-containing pseudopodium at time points earlier than 30 min, which clearly indicated that clathrin-dependent engulfment of a Shigella-containing pseudopodium is the latest event during the engulfment by a neighboring cell. It was previously shown that the clathrin coat assembles within a few minutes during canonical clathrin-mediated endocytosis. Therefore, we examined the localization of early endosome markers on Shigella-containing pseudopodia since clathrin-coated pits ultimately pinch off from the endocytic membrane and translocate to early endosomes. We found that EEA1 (early endosome antigen 1) and the FYVE domain of EEA1, which binds to PtdIns(3)P, accumulated around the Shigella-containing pseudopodium. We also found that Rab5 accumulated as early as 2 min after the bacteria-containing pseudopodium entered a neighboring cell. However, shRNA-mediated knockdown of Rab5 did not impair Shigella cell-cell spreading. Based on this series of experiments, we speculated that neighboring cells use non-canonical clathrin-dependent endocytosis during the late stage of engulfment, followed by scission of the bacteria-containing pseudopodium.
We further characterized clathrin-dependent engulfment of Shigella-containing pseudopodia by neighboring cells in relation to the functional involvement of clathrin coat assembly. MDCK cells were treated with shRNA to knockdown AP-2, Eps15, Epsin-1 and Dab2, which are initiation adaptors of clathrin-coated pits. After knocking down these components, MDCK monolayers were infected with Shigella and then examined for Shigella plaque formation. Although Espin-1 knockdown had no effect on the subcellular localization of tricellulin, occludin and E-cadherin, the knockdown of Epsin-1, but not AP-2, Eps15 and Dab2, resulted in decreased plaque size. The number of bacteria-containing pseudopodia that protruded from Shigella-infected MDCK cells with or without Epsin-1 knockdown was approximately 4 pseudopodia per cell for 3 h post-infection, indicating that knocking down each of these adaptors had no effect on pseudopodium formation during Shigella cell-cell spreading. These results suggest that the mechanisms underlying internalization of bacteria-containing pseudopodia and clathrin-dependent recycling of transferrin, EGF and LDL, in which AP-2, Eps15 and Epsin-1 rapidly accumulate beneath the endocytosed plasma membrane, appear to somewhat differ. When the localization of GFP-Epsin-1 in Shigella-infected MK2 cells transiently expressing GFP-Epsin-1 was examined by time-lapse imaging, the GFP-Epsin-1 signals around the pseudopodium were detected after 25 min during the protrusion of a Shigella-containing pseudopodium. shRNA-mediated knockdown of Epsin-1 in MDCK cells resulted in less clathrin accumulation around the Shigella-containing pseudopodium compared with the mock control. However, AP-2 knockdown in epithelial cells did not alter the accumulation of clathrin around the pseudopodium. Together, these results indicate that Epsin-1 plays a functional role in recruiting clathrin to Shigella-containing pseudopodia.
To confirm that the Epsin-1-clathrin-dependent endocytic pathway is involved in the late stages of the engulfment of Shigella-containing pseudopodia, we identified the domain in Epsin-1 that is required for Shigella cell−cell spreading. Epsin-1 consists of the ENTH region [required to bind to PtdIns(4,5)P2 and PtdIns(3,4,5)P3 and induce membrane curvature], UIMs region (interacts with polyubiquitins and ubiquitinated cargo receptors for internalization), and COOH region (required to interact with clathrin, Eps15 and AP-2). We created in-frame deletions of Epsin-1 that lack the ENTH (ΔENTH), UIMs (ΔUIMs) or COOH (ΔCOOH) domains. We then infected MK2 cells expressing each of the Epsin-1 deletions with Shigella and examined the accumulation of Epsin-1 along bacteria-containing pseudopodia that were engulfed by neighboring cells. The results showed that MK2 cells expressing ΔENTH and ΔUIMs, but not ΔCOOH, failed to recruit Epsin-1 to the endocytosed pseudopodia. To ensure this, each of the Epsin-1 deletion derivatives were ectopically expressed in MDCK monolayer cells, and each of the MDCK cells infected with Shigella were investigated for the effect on the formation of plaques. The results showed that either of the deletions containing the ENTH, UIMs or COOH domains of Epsin-1 reduced the size of plaques formed by Shigella cell-cell spreading to less than half of the control level. Furthermore, we showed that all of the Epsin-1 deletion mutants prevented clathrin from accumulating around Shigella-containing pseudopodia. Together, these results suggest that Epsin-1 is functionally important in mediating the accumulation of clathrin around Shigella-containing pseudopodia and Shigella cell-cell spreading. In addition, when MK2 cells transiently expressing clathrin-GFP (or GFP-Epsin-1) were infected with Shigella and then treated with LY294002, the accumulation of clathrin or Epsin-1 around Shigella-containing pseudopodia was barely detected, assuring that PI 3-kinase activity is essential to recruit Epsin-1 and clathrin to the plasma membrane where the bacteria-containing pseudopodium was engulfed.
Aside from the well-documented role in vesicle endocytosis, clathrin has also been implicated in the internalization of large particles, such as bacteria, viruses and even double-membrane intercellular vesicles (Piehl et al., Mol Biol Cell 2007; Matsuda et al., J Cell Sci 2004). We discovered that the initial protrusion and subsequent penetration of Shigella-containing pseudopodia occur through a clathrin-independent pathway via tTJs, which may be directed by the bacteria-containing pseudopodium that protrudes due to the force of the motile bacterium (Cossart and Sansonetti, Science 2004; Ashida et al., Curr Top Microbiol Immunol 2009). Indeed, Shigella that lack the virG (icsA) gene, which is essential to mediate actin polymerization at one pole of the bacterium, are unable to induce pseudopodium protrusion. Thus, our results highlighted in the study that when an elongating pseudopodium is fully engulfed by a neighboring epithelial cell, this neighboring cell undergoes noncanonical clathrin-dependent endocytosis ().
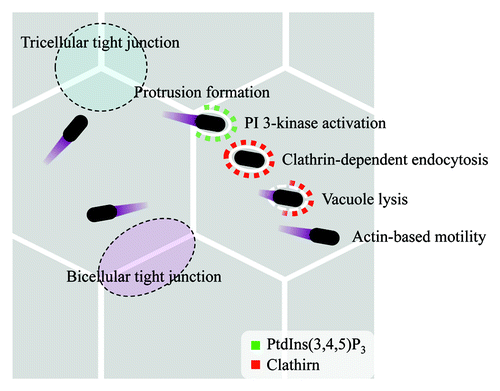
Figure 1. Proposed model for Shigella cell-cell spreading. When Shigella moves from one epithelial cell to neighboring epithelial cells, Shigella-containing pseudopodia target tricellular tight junctions. PI 3-kinase is activated upon formation of a Shigella-containing pseudopodium. PI 3-kinase activity is required to recruit clathrin to the plasma membrane where the bacteria-containing pseudopodium was engulfed. Finally, an elongating pseudopodium is fully engulfed and undergoes clathrin-dependent endocytosis by a neighboring cell. Then, Shigella lyses the double plasma membranes and obtains the actin-based motility. Shigella can spread cell-to-cell repeat these process.
Acknowledgments
We thank the members of the Sasakawa laboratory for their advice. This work was supported by Grant-in-Aid for Specially promoted Research [23000012 (C.S.)], a Grant-in-Aid for Young Scientists (A) [23689027 (M.K.)], a Grant-in-Aid for Young Scientists (B) [23790471 (M.O.)], a Grant-in-Aid for Scientific Research (B) [23390102 (H.M.)], a Grant-in-Aid for challenging Exploratory Research [23659220 (H.M.)], a Grant-in-Aid for Scientific Research on Priority Areas [18073003 (C.S.)] and Japan Initiative for Global Research Network on Infectious Diseases (C.S.). Part of this work was supported by grants from the Naito Foundation (H.M. and M.K.), the Waksman Foundation of Japan Inc. (M.O.), the Yakult Bio-Science Foundation (M.O.), the Yakult Central Institute (C.S.), the Hayashi Memorial Foundation for Female Natural Science (M.K.) and the Takeda Science Foundation (M.K.). The authors have no conflicting financial interests.