Abstract
Larvae of Galleria mellonella are widely used to study the virulence of microbial pathogens and for assessing the potency of antimicrobial agents. This work examined the effect of nutritional deprivation on the ability of larvae to withstand infection in order to establish standardized conditions for the treatment of larvae for in vivo testing. Larvae deprived of food for seven days demonstrated an increased susceptibility to infection by the yeast Candida albicans. These larvae displayed a lower density of hemocytes compared with controls but hemocytes from starved and control larvae demonstrated the same ability to kill yeast cells. Hemolymph from starved larvae demonstrated reduced expression of a range of antimicrobial peptides (e.g., lipocalin) and immune proteins (e.g., apolipophorin and arylphorin). Deprivation of G. mellonella larvae of food leads to a reduction in the cellular and immune responses and an increased susceptibility to infection. Researchers utilizing these larvae should ensure adequate food is provided to larvae in order to allow valid comparisons to be made between results from different laboratories.
Introduction
The immune system of insects and the innate immune response of mammals display many structural and functional similaritiesCitation1,Citation2 and, as a result, insects have been employed for routine screening of microbial mutantsCitation3 and for assessing the efficacy of antimicrobial drugsCitation4 and give results that are comparable to those that may be obtained with mammals.Citation5,Citation6 A wide range of insect species are currently employed as model organismsCitation7 and larvae of the greater wax moth, Galleria mellonella, have been used to assess the virulence of bacteria,Citation3 yeastsCitation8, filamentous fungiCitation9 and to quantify the relative potency of antimicrobial compounds.Citation10 G. mellonella larvae are useful as model organisms due to their low cost, ease of purchase and give results within 24–48 h.
The use of G. mellonella larvae to study a wide range of pathogensCitation9 has made it essential to identify parameters that need to be constant for the larvae to be utilized correctly and to give reproducible results. It has previously been documented that G. mellonella larvae display the ability to alter their immune response when exposed to microbial cell wall components,Citation11 fungal cells,Citation12 physical stressCitation13 or thermal variation.Citation14 It was postulated that a reduction or elimination of food could also alter the immune response of insects since food provides energy for maintaining homeostasis and immune function. Immune priming in the absence of compensatory feeding in bumblebees has been shown to be fatalCitation15 indicating an important link between nutritional status and survival. Starvation is a stress that may be counteracted by the metabolism of the lipid content of the fat body or by the release of amino acids from storage proteins in the hemolymph. This has previously been shown in D. melanogasterCitation16 where starvation negatively affected the metabolic rate. If metabolism slows down, the production of proteins such as antimicrobial peptides may be reduced.Citation17
The aim of the work presented here was to establish whether nutritional deprivation affected the ability of G. mellonella larvae to withstand infection with the yeast C. albicans and to determine how this was manifested. Some researchers incubate larvae with food (e.g., wood shavings or filter paper) during experiments while others do not thus raising the possibility that the presence or absence of food may affect the outcome of an experiment and thus make inter-laboratory comparisons difficult.
Results
Effect of lack of nutrition on susceptibility of G. mellonella larvae to infection with Candida albicans
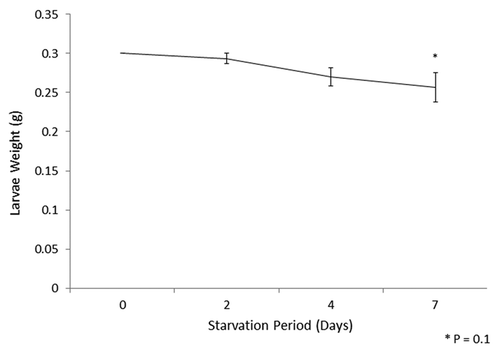
Larvae (0.3 g, 10 larvae per treatment and performed on three independent occasions) were maintained at 30°C in the absence of food for 0, 2, 4 or 7 d and their weights determined at each time interval. The results () reveal that there is a small (p = 0.1) reduction in average weight following deprivation of food for 7 d.
Figure 1. Average weight of G. mellonella larvae incubated in the absence of food for 7 d. Ten larvae each weighing 0.3 g, on three separate occasions, were incubated in the absence of food and weighed after 2, 4 and 7 d. The reduction in weight was calculated.
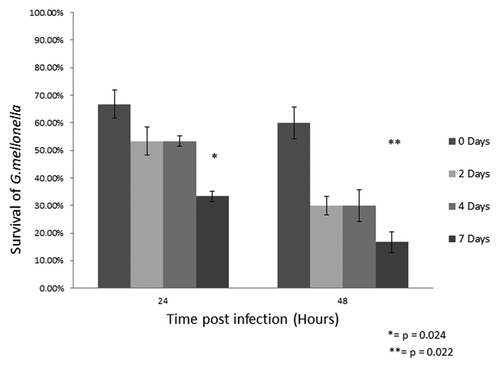
Larvae of G. mellonella were incubated at 30°C in the absence of any food source for periods of 0, 2, 4 or 7 d prior to infection with C. albicans cells as described below. Larvae were subsequently incubated at 30°C and viability was assessed at 24 and 48 h post infection. The results () indicate that those larvae incubated in the absence of food for 7 d prior to infection with C. albicans were the most susceptible to infection and showed 33.3 ± 1.9% survival at 24 h compared with 66.6 ± 5.0% survival for those larvae that did not have a restricted diet (p < 0.05). Survival was reduced to 16.67 ± 3.85% at 48 h for the larvae that had been incubated in the absence of food for 7 d compared with 60 ± 5.87% at 48 h for control larvae (p < 0.05). It was also demonstrated that the larvae that had been starved for 2 or 4 d showed reduced survival particularly at 48 h.
Figure 2. Survival of G. mellonella larvae deprived of food for 0, 2, 4 and 7 d prior to inoculation with 1 × 106/20 µL C. albicans cells. For assessment of larval death, larvae were probed with a needle and if no response was observed the larvae were deemed to be dead. The statistics were based on the values obtained from the unstarved larvae (*p < 0.05).
Effect of nutrition depletion on the density and function of hemocytes in G. mellonella larvae
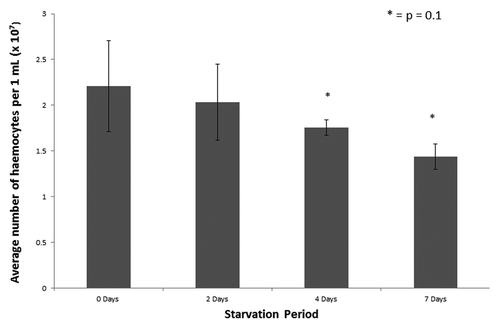
Hemocytes are an essential part of the cellular immune response of insects and fluctuation in hemocyte density can be indicative of the immune response.Citation18 The hemocyte density of larvae incubated in the absence of food for 0, 2, 4 or 7 d was assessed and the results demonstrate a slight decrease in the hemocyte density of larvae deprived of food for 7 d but this was not statistically significant ().
Figure 3. Hemocyte density in G. mellonella larvae that were starved for 0, 2, 4 and 7 d. Hemocytes were extracted as described and enumerated. The difference in hemocyte density was measured relative to the density in the unstarved larvae at day 0.
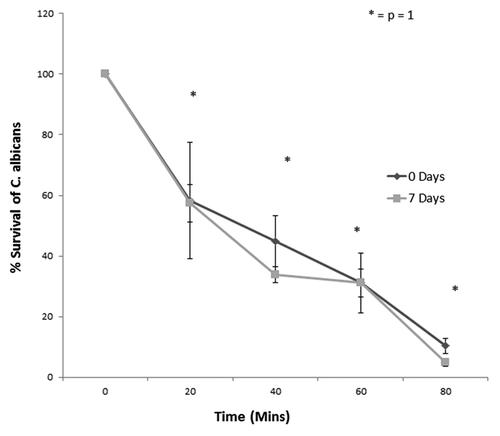
The killing ability of hemocytes from control larvae or larvae starved for 7 d was assessed in order to determine whether they displayed similar killing kinetics. The hemocyte-mediated killing assay was performed as described below. The results indicate that hemocytes from both sets of larvae displayed a killing rate that is statistically identical (). Twenty minutes subsequent to incubation the survival of C. albicans incubated with hemocytes from control larvae was at 58.2 ± 19.1% while C. albicans survival when incubated with hemocytes from the 7 d starved larvae was 57.4 ± 6.2% (no significant difference). Following an 80 min incubation period with the opsonized C. albicans the rate of survival of the yeast cells with hemocytes from unstarved G. mellonella was 10.2 ± 2.5%, this value was 8.3 ± 1.3% in hemocytes from larvae starved for 7 d (p = 1.0). These results demonstrate that incubation of larvae in the absence of food leads to a slightly reduced hemocyte density but that hemocytes demonstrate the same killing kinetics as those from control larvae.
Analysis of the proteome of larvae starved for 7 d
The hemolymph of larvae that were starved for 0 or 7 d was extracted and prepared for 2D SDS-PAGE, as described. Examination of the resulting gels indicated a downregulation in the expression of proteins in the serum from starved larvae (). In particular, a number of proteins with known immune function were reduced in expression (). Spot 3 showed homology to apolipophorin, which functions in the hemolymph to transport lipids and aids in the commencement of the innate immune response,Citation19 and its expression was reduced by approximately 50% in hemolymph from starved larvae. Arylphorin (spot number 4) was downregulated by 37% in the hemolymph of starved larvae. Arylphorin functions in the storage of the aromatic amino acids but it also plays a central role in insect immunity.Citation20
Figure 5. Proteomic profiles from hemolymph of unstarved larvae (A) and larvae starved for 7 d (B). Protein was extracted from larval hemolymph as described and resolved by 2D SDS-PAGE (300 µg/250 µL was loaded onto each Immobiline Drystrip). Peptide spots showing alterations in expression were extracted and identified.
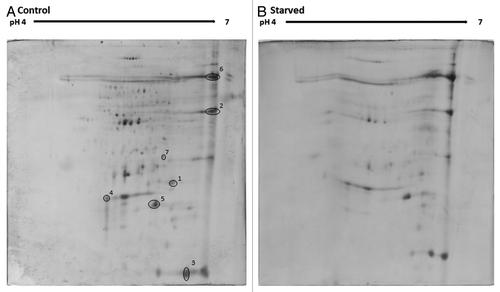
Table 1. Relative expression of Galleria proteins in hemolymph of larvae deprived of food for 7 d
Spot 5 showed homology to lipocalin, which functions in the immune response by binding to toxins and by complexing invading pathogensCitation21 and its expression was reduced by approximately 14% in starved larvae. Ferritin (spot 1) and transferrin precursor (spot 6) were reduced in expression by approximately 50% in food-deprived larvae. Ferritin is important in iron binding and storage and maintaining cellular iron homeostasis along with playing a role in the immune response.Citation22,Citation23 Transferrin has the ability to remove essential iron ions from invading pathogens and so retard microbial colonization of the insect hemocoel.Citation24
Spot 2 showed homology to imaginal disc growth factor-like protein and its expression was reduced by 40% in starved larvae. The expression of juvenile hormone binding protein (spot 7) remained relatively constant at approximately 90% in starved larave and as such may be considered as a loading control. The expression of this protein was demonstrated to be maintained at a constant level when G. mellonella larvae were challenged with β-glucan.Citation11
Analysis of the proteome of starved larvae indicates a downregulation in the expression of a range of proteins associated with the immune response. Given the importance of the humoral immune response in defending insects from infection, this decrease in the expression of antimicrobial peptides could contribute to the increased susceptibility to infection of larvae.
Discussion
The insect immune response displays a number of structural and functional similarities to the innate immune response of mammals.Citation5 As a consequence of this, insects may be used in place of mammals for assessing the virulence of microbial cells or for screening the in vivo toxicity of antimicrobial drugs and yield results that are comparable to those that can be obtained using mammals.Citation6 A variety of insects are employed in in vivo tests but larvae of G. mellonella have been established as a good model for studying fungalCitation9 and bacterialCitation3 pathogens. Their utility in screening antimicrobial drugs has also been demonstrated.Citation10
Standardized conditions for the use of G. mellonella larvae have yet to be established. In previous work we demonstrated that physical stressCitation13 and thermal variationCitation14 can alter the susceptibility of larvae to infection with C. albicans. In the work presented here we sought to establish whether nutritional depletion would alter the susceptibility of larvae to infection since some workers incubate larvae with a food source during experimentsCitation25 while others do not.Citation26
Larvae deprived of nutrition for 7 d demonstrated increased susceptibility to infection with the fungal pathogen C. albicans. Starved larvae demonstrated a slight reduction in hemocyte density but the hemocytes from starved larvae were as effective at killing C. albicans cells as those from unstarved larvae. In addition, starved larvae demonstrated reduced expression of a variety of antimicrobial peptides and immune proteins.
The increased susceptibility of starved larvae to infection may be a result of the reduced hemocyte density and antimicrobial peptide expression. Immune priming in the absence of compensatory feeding in bumblebees has been shown to be fatalCitation15 indicating the link between nutritional status and survival. Deprivation of food leads to impaired learning in honey bees (Apis mellifera) demonstrating a clear link between feeding and neurological function.Citation27 Analysis of the changes in the proteome of larvae starved for 7 d indicated decreased expression of a number of proteins with known anti-microbial action (e.g., transferrin) and immune function (e.g., arylphorin).
The reduction of mass-specific metabolism in D. melanogasterCitation28 is evidence that stress has implications on lipid transport. Apolipophorin 3 (spot number 3) is involved in lipid transport and this may explain the decrease in apolipophorin 3 expression in the starved larvae.
The immune response requires a high degree of resources to be maintained at an optimum level to fight infection. The absence of food would adversely affect metabolism and consequently lead to a downregulation in immune function. A reduction in the expression of arylphorin and lipoprotein was recently demonstrated in Bombyx mori when a calorie restriction was applied to the insect’s diet.Citation29 In contrast, Tribolium casteneum deprived of food demonstrated increased expression of selected stressor specific microRNAs in a gender specific manner thus suggesting that different types of insect react differently to nutrient deprivation.Citation30
The results presented here demonstrate a clear correlation between access to nutrition and the ability of G. mellonella larvae to retard microbial infection. Unstarved larvae demonstrated a slightly higher level of hemocytes and antimicrobial peptide expression, which leads to significantly greater ability to curtail or eliminate the pathogen.
These results indicate that incubating larvae in the presence or absence of nutrition will significantly affect the ability of the larvae to mount an immune response. Consequently, researchers utilizing G. mellonella larvae for studying the virulence of fungal or bacterial pathogens should specify whether feeding is provided for larvae and the type of material supplied since this may affect the result and influence inter-laboratory comparisons.
Materials and Methods
Chemicals and reagents
All chemicals and reagents were of the highest purity and were purchased from Sigma Aldrich Chemical Company Ltd., unless stated otherwise.
Fungal strain and culture conditions
C. albicans MEN (originally isolated from an eye infection and a gift from Prof D. Kerridge, Cambridge) was cultured in YEPD broth [2% (w/v) glucose, 2% (w/v) Bactopeptone and 1% (w/v) yeast extract] at 30°C in an orbital shaker. Stocks were maintained on YEPD agar plates.
Insect larvae
Sixth instar larvae of Galleria mellonella (Lepidoptera: pyralidae, greater wax moth) were obtained from the Mealworm Company and stored in wood shavings in the dark at 15°C for 24 h. This incubation period did not adversely affect the viability or activity of larvae (data not presented). Larvae weighing 0.3 g were placed in 9 cm Petri dishes for 0, 2, 4 and 7 d without a food source (0.1 g of wood shavings with a 9 cm Whatman filter paper) and stored in the dark. At the end of the incubation period larvae were inoculated with 5 × 106 C. albicans cells per 20 µL through the last left pro-leg.Citation8 Controls consisted of untouched larvae or those that received a 20 µL PBS inoculation. Ten larvae were used per treatment, with all treatments being performed on three independent occasions.
Isolation of insect hemocytes
Hemocytes were extracted by bleeding 10 larvae into 10 mL of sterile anti-coagulant buffer, IPS (150 mM NaCl, 5 mM KCl, 0.1 M TRIS-HCl, 10 mM EDTA and 30 mM Trisodium citrate in dH2O, pH 6.9). Cells were centrifuged at 1,500× g, washed once and finally resuspended in 5 mM PBS-glucose containing 1 mg/ml Pepstatin A, 1 mg/ml Aprotitin, 1 mM PMSF and 1 mg/ml leupeptin. Hemocyte viability was approximately 95% as measured by Trypan blue staining at the time of isolation from each group of larvae.
The hemocyte density was calculated by piercing the head of three larvae with a sterile needle (gauge size of 23 G, Terumo) and collecting the hemolymph into a pre-chilled Eppendorf tube as described previously.Citation18 Hemolymph was diluted 1:10 in cold PBS containing 0.37% (v/v) 2-Mercaptoethanol to reduce clotting and melanization. Hemocytes were enumerated using a hemocytometer.
Measurement of killing ability of insect hemocytes
C. albicans cells were opsonised using cell free hemolymph diluted in IPS (1:10). Killing was measured by incubating 2 × 105 yeast cells with 1 × 105 hemocytes in a stirred chamber at 37°C, in a final volume of 2 ml. An aliquot was removed immediately after addition of the yeast cells (time zero) and after 20, 40, 60 and 80 min incubation, diluted in minimum essential medium (Sigma Aldrich) and plated onto YEPD plates supplemented with erythromycin (1 µg/ml). The viability of cells was assessed by counting colony number and the results were determined in triplicate for each sample. The viability data are expressed as percentage of viability at time zero.
2D SDS-PAGE separation of hemolymph proteins and image analysis
Hemolymph (100 μl) was collected from larvae that had been starved for 0 or 7 d by piercing the head of the insect and bleeding the hemolymph into a pre-chilled microcentrifuge tube. Isoelectric focusing of protein samples and 2D electrophoresis was performed as described previously.Citation11 Each 2D gel was scanned on a Hewlett Packard scanjet 5100c scanner and the images were analyzed using Progenesis SameSpot Software. The protein spots of interest on each gel were normalized, edited and manually matched to a reference gel. The intensity volume of each spot was processed by background subtraction and total spot volume normalization, and the resulting spot volume percentage was used for comparison. Mass spectrometric analysis of trypsin-digested proteins was performed using an Agilent ESI Trap LC/MS. Resulting spectra were analyzed using MASCOT with a score over 80 considered significant (www.matrixscience.com/search). The mass error tolerance was 1 Da, allowing for a maximum of no more than two missed cleavages. Verification of protein sequences was confirmed by blasting the protein sequence on the Uniprot (www.uniprot.org) and NCBI (www.ncbi.nlm.nih.gov) websites.
Statistical analysis
All values are the mean of at least three independent determination ± SD. Experimental data were tested for statistical significance at p < 0.05 using a Student’s t-test (Minitab 14 package). Larval viability data () were analyzed using the Log rank test.
Acknowledgments
The authors acknowledge funding for this work from the Higher Education Authority of Ireland under PRTLI 4. N.Browne is the recipient of a Hume Scholarship from NUI Maynooth.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Strand MR. The insect cellular immune response. Insect Sci 2008; 15:1 - 14; http://dx.doi.org/10.1111/j.1744-7917.2008.00183.x
- Hoffmann JA, Kafatos FC Jr., Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science 1999; 284:1313 - 8; http://dx.doi.org/10.1126/science.284.5418.1313; PMID: 10334979
- Mukherjee K, Altincicek B, Hain T, Domann E, Vilcinskas A, Chakraborty T. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl Environ Microbiol 2010; 76:310 - 7; http://dx.doi.org/10.1128/AEM.01301-09; PMID: 19897755
- Seed KD, Dennis JJ. Experimental bacteriophage therapy increases survival of Galleria mellonella larvae infected with clinically relevant strains of the Burkholderia cepacia complex. Antimicrob Agents Chemother 2009; 53:2205 - 8; http://dx.doi.org/10.1128/AAC.01166-08; PMID: 19223640
- Kavanagh K, Reeves EP. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev 2004; 28:101 - 12; http://dx.doi.org/10.1016/j.femsre.2003.09.002; PMID: 14975532
- Brennan M, Thomas DY, Whiteway M, Kavanagh K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol 2002; 34:153 - 7; http://dx.doi.org/10.1111/j.1574-695X.2002.tb00617.x; PMID: 12381467
- Kavanagh K, Fallon J. Galleria mellonella larvae as models for studying fungal virulence. Fungal Biol Rev 2010; 24:79 - 83; http://dx.doi.org/10.1016/j.fbr.2010.04.001
- Cotter G, Doyle S, Kavanagh K. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol Med Microbiol 2000; 27:163 - 9; http://dx.doi.org/10.1111/j.1574-695X.2000.tb01427.x; PMID: 10640612
- Fuchs BB, O’Brien E, Khoury JB, Mylonakis E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 2010; 1:475 - 82; http://dx.doi.org/10.4161/viru.1.6.12985; PMID: 21178491
- Rowan R, Moran C, McCann M, Kavanagh K. Use of Galleria mellonella larvae to evaluate the in vivo anti-fungal activity of [Ag2(mal)(phen)3]. [Ag2(mal)(phen)3] Biometals 2009; 22:461 - 7; http://dx.doi.org/10.1007/s10534-008-9182-3; PMID: 19082779
- Mowlds P, Coates C, Renwick J, Kavanagh K. Dose-dependent cellular and humoral responses in Galleria mellonella larvae following β-glucan inoculation. Microbes Infect 2010; 12:146 - 53; http://dx.doi.org/10.1016/j.micinf.2009.11.004; PMID: 19925881
- Bergin D, Murphy L, Keenan J, Clynes M, Kavanagh K. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microbes Infect 2006; 8:2105 - 12; http://dx.doi.org/10.1016/j.micinf.2006.03.005; PMID: 16782387
- Mowlds P, Barron A, Kavanagh K. Physical stress primes the immune response of Galleria mellonella larvae to infection by Candida albicans. Microbes Infect 2008; 10:628 - 34; http://dx.doi.org/10.1016/j.micinf.2008.02.011; PMID: 18457977
- Mowlds P, Kavanagh K. Effect of pre-incubation temperature on susceptibility of Galleria mellonella larvae to infection by Candida albicans. Mycopathologia 2008; 165:5 - 12; http://dx.doi.org/10.1007/s11046-007-9069-9; PMID: 17922218
- Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science 2000; 290:1166 - 8; http://dx.doi.org/10.1126/science.290.5494.1166; PMID: 11073456
- Gibbs AG, Gefen E. 2009. Physiological adaptations in laboratory environments. T. Garland and M. R. Rose, eds. Experimental evolution. Univ. of California Press, Berkeley.
- Gilchrist GW, Huey RB. The direct response of Drosophila melanogaster to selection on knockdown temperature. Heredity (Edinb) 1999; 83:15 - 29; http://dx.doi.org/10.1038/sj.hdy.6885330; PMID: 10447699
- Bergin D, Brennan M, Kavanagh K. Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella. Microbes Infect 2003; 5:1389 - 95; http://dx.doi.org/10.1016/j.micinf.2003.09.019; PMID: 14670452
- Gupta L, Noh JY, Jo YH, Oh SH, Kumar S, Noh MY, et al. Apolipophorin-III mediates antiplasmodial epithelial responses in Anopheles gambiae (G3) mosquitoes. PLoS One 2010; 5:e15410; http://dx.doi.org/10.1371/journal.pone.0015410; PMID: 21072214
- Beresford PJ, Basinski-Gray JM, Chiu JK, Chadwick JS, Aston WP. Characterization of hemolytic and cytotoxic Gallysins: a relationship with arylphorins. Dev Comp Immunol 1997; 21:253 - 66; http://dx.doi.org/10.1016/S0145-305X(97)00011-6; PMID: 9258607
- Pandian GN, Ishikawa T, Vaijayanthi T, Hossain DM, Yamamoto S, Nishiumi T, et al. Formation of macromolecule complex with Bacillus thuringiensis Cry1A toxins and chlorophyllide binding 252-kDa lipocalin-like protein locating on Bombyx mori midgut membrane. J Membr Biol 2010; 237:125 - 36; http://dx.doi.org/10.1007/s00232-010-9314-x; PMID: 21079938
- Levy F, Bulet P, Ehret-Sabatier L. Proteomic analysis of the systemic immune response of Drosophila. Mol Cell Proteomics 2004; 3:156 - 66; http://dx.doi.org/10.1074/mcp.M300114-MCP200; PMID: 14645501
- Choi CW, Seo DH, Yuk JE, Park JB, Hwang SY, Koh SK, et al. Up-regulation of hemolymph and tissue ferritin by dietary HgCl2 in the wax moth Galleria mellonella. Entomol Res 2006; 36:185 - 90; http://dx.doi.org/10.1111/j.1748-5967.2006.00025.x
- Seitz V, Clermont A, Wedde M, Hummel M, Vilcinskas A, Schlatterer K, et al. Identification of immunorelevant genes from greater wax moth (Galleria mellonella) by a subtractive hybridization approach. Dev Comp Immunol 2003; 27:207 - 15; http://dx.doi.org/10.1016/S0145-305X(02)00097-6; PMID: 12590972
- Schmolz E, Schulz O. Calorimetric investigations on thermoregulation and growth of wax moth larvae Galleria mellonella. Thermochim Acta 1995; 251:241 - 5; http://dx.doi.org/10.1016/0040-6031(94)02094-5
- Klingen I, Eilenberg J, Meadow R. Effects of farming system, field margins and bait insect on the occurrence of insect pathogenic fungi in soils. Agric Ecosyst Environ 2002; 91:191 - 8; http://dx.doi.org/10.1016/S0167-8809(01)00227-4
- Toth AL, Kantarovich S, Meisel AF, Robinson GE. Nutritional status influences socially regulated foraging ontogeny in honey bees. J Exp Biol 2005; 208:4641 - 9; http://dx.doi.org/10.1242/jeb.01956; PMID: 16326945
- Djawdan M, Rose MR, Bradley TJ. Does selection for stress resistance lower metabolic rate?. Ecology 1997; 78:828 - 37; http://dx.doi.org/10.1890/0012-9658(1997)078[0828:DSFSRL]2.0.CO;2
- Li JY, Chen X, Fan W, Moghaddam SH, Chen M, Zhou ZH, et al. Proteomic and bioinformatic analysis on endocrine organs of domesticated silkworm, Bombyx mori L. for a comprehensive understanding of their roles and relations. J Proteome Res 2009; 8:2620 - 32; http://dx.doi.org/10.1021/pr8006123; PMID: 19382758
- Freitak D, Knorr E, Vogel H, Vilcinskas A. Gender- and stressor-specific microRNA expression in Tribolium castaneum. Biol Lett 2012; 8:860 - 3; http://dx.doi.org/10.1098/rsbl.2012.0273; PMID: 22628099