Abstract
Dendritic cells (DCs) are the bridge between the innate and adaptive immune system. DCs are responsible for sensing and patrolling the environment, initiating a host response and instructing the proper adaptive immune response against pathogens. Recent advances in medical treatments have led to increased use of immunosuppressive drugs, leading to the emergence of fungal species that cause life-threatening infections in humans. Three of these opportunistic fungal pathogens: Aspergillus fumigatus, Candida albicans and Cryptococcus neoformans pose the biggest concern for the immune-compromised host. Here we will review the interactions between DCs and these fungal pathogens, the receptors expressed on DCs that mediate these responses and the signaling mechanisms that shape the adaptive host response.
Introduction
Mycoses have emerged over the last three decades as life-threatening infectious diseases; establishing a pressing need for the development of new anti-fungal drugs to combat the increasing incidence of fungal infections and drug-resistant fungal species. Fungal infections are of great importance to immune-compromised hosts; particularly those infected with HIV or patients receiving immunosuppressive drugs to combat cancer or prevent organ rejection following transplantation.Citation1,Citation2 Fungi are among the most common microbes encountered by mammalian hosts, with the natural route of infection being the respiratory tract. To date, more than 100,000 fungal species ubiquitous to the environment have been described; however, only a handful cause disease in humans. Opportunistic fungal pathogens such as Candida albicans, Aspergillus fumigatus and Cryptococcus neoformans pose the biggest concern in immune-compromised hosts. Here we will review the interactions of these three pathogenic fungi with different subsets of dendritic cells (DCs).
The Big Three: Aspergillus fumigatus, Candida albicans and Cryptococcus neoformans
Increase in the incidence of fungal infections correlates with advances in medical treatment resulting in increased numbers of immune-compromised patients, particularly those suffering from AIDS, cancer patients receiving chemotherapy and those taking immunosuppressive drugs following solid organ transplantation.Citation3
Aspergillus fumigatus is the primary causative species of human aspergillosis. On a daily basis, humans inhale hundreds of microscopic conidia or non-motile spores of this mold. These 2–3 µm conidia are small enough to bypass the mucosal barriers and enter the lung alveoli. In immune-competent hosts the conidia are readily eliminated by the host innate immune system and, in most cases, the host remains asymptomatic. For many years A. fumigatus was viewed as a weak pathogen, causing mainly allergic forms of disease.Citation4 However, the prevalence of A. fumigatus infection and severity of disease has risen dramatically and correlates directly with the increased number of immunosuppressed patients. In these patients, the conidia are capable of bypassing mucocilliary clearance and establish infection in the lung. Once in the alveolar cavities, the conidia undergo a series of morphological changes, such as shedding of the hydrophobic protective layer, conidial swelling and the growth of branching filamentous structures or hyphae. Once A. fumigatus germinates into hyphae, it becomes the invasive form of the disease resulting in destruction of lung tissue. Therefore, it is not surprising that immune cells play a key role in regulating A. fumigatus infection.Citation5 For example, alveolar macrophages and DCs are responsible for phagocytosing conidia while neutrophils play a role in killing hyphae.
Cryptococcus neoformans is one of the etiological agents causing cryptococcosis. Similar to A. fumigatus, the incidence of C. neoformans infections has increased dramatically over the last three decades. This fungal pathogen is known to cause life-threatening disease in individuals with impaired T cell function, particularly AIDS patients and solid organ transplant patients.Citation6 It is estimated that C. neoformans causes approximately 1 million infections and over 600,000 deaths per year worldwide. Infection with C. neoformans occurs via inhalation of the yeast or spores into the lungs. In healthy individuals, once the organisms reaches the lungs, professional phagocytes such as macrophages, DCs and neutrophils are responsible for clearing the infection and inducing an adaptive immune response. However, in individuals with a compromised immune system, C. neoformans can disseminate from the lungs into the brain leading to meningoencephilitis.
The most common fungal pathogen, Candida albicans, is a commensal organism capable of colonizing mucosal surfaces and skin in healthy individuals. In contrast to C. neoformans and A. fumigatus, there is no environmental form of this yeast. This dimorphic fungus colonizes the skin, genital mucosa and/or gastrointestinal mucosa of approximately 50% of healthy individuals without causing severe disease. However, severe disease is observed in the absence of proper host-pathogen recognition.Citation7 In addition, perturbations in the distribution of competitive commensal bacteria may contribute to C. albicans infection. Similar to other fungal pathogens, the actions of phagocytes are required for controlling C. albicans infection and dissemination. One example is the prevalence of oropharyngeal candidiasis in AIDS patients, which results from the decreased numbers of CD4 T cells circulating in these patients.Citation8,Citation9
The role of phagocytes and antigen-presenting cells (APCs) are essential in host defense against pathogenic fungi. Advances in understanding the interactions between DCs and pathogenic fungi, by virtue of receptor-ligand interactions, have provided insights into the mechanisms employed by the immune system to provide protection against these pathogens. These advances could provide the key for developing vaccines against A. fumigatus, C. albicans and/or C. neoformans. Here we will review the role of DCs in the host response to these three major pathogenic fungi. In addition to “the big three,” there are other important fungal pathogens to be considered. Additional information on the role of DCs in response to other endemic fungal infections such as Blastomyces, Histoplasma and Coccidioides has been reviewed elsewhere and will not be covered in this review.Citation10-Citation14
Overview of Dendritic Cells
The activation of the innate immune system via pattern recognition receptors (PRRs) shapes the development of the adaptive immune response. DCs express a myriad of PRRs and provide the link between these two arms of the immune system. DCs are responsible for sampling antigenic material in the environment, shaping T cell responses through secretion of cytokines and priming T cell responses via antigen presentation.Citation15 Priming of T cells by DCs is mediated by pathogen-associated antigen on major histocompatibility complex (MHC) class I (MHC-I) or MHC class II (MHC-II) molecules for the priming of CD4+ or CD8+ T cells, respectively. In their immature state, DCs are highly phagocytic and are constantly patrolling the environment for the presence of pathogenic antigen. In order to perform these tasks, DCs express PRRs on their cell surface or in endosomal compartments, which serve to recognize an array of pathogen-associated molecular patterns (PAMPs) (). DC interactions with pathogens and the specific signals obtained from the PRRs, leads to the activation of a pathogen-specific T cell response. Moreover, cytokines secreted by DCs following engagement of PRRs lead to the activation and polarization of CD4 T cells into specific subsets characterized as T helper (Th)1, Th2, Th17 or T regulatory (Tregs). Therefore, DCs are essential players in balancing immune responses to fungal pathogens and prime candidates for vaccine development.
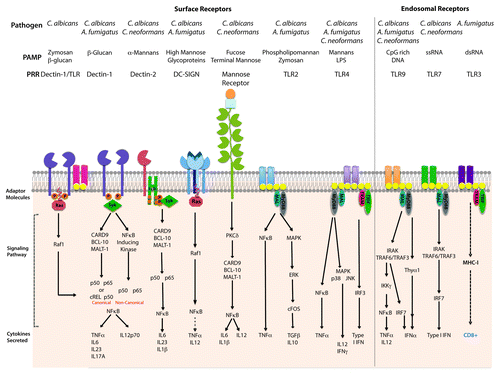
Figure 1. Pattern recognition receptors on DCs and signaling pathways that prime T cell differentiation. Recognition of C. neoformans, C. albicans and A. fumigatus mediates by detection of fungal pathogen-associated molecular patterns via Toll-like receptors (TLRs) in the cell surface or endosomes and C-type lectin receptors (CLRs). This graphic represents the TLRs and CLRs responsible for the detection of C. albicans, C. neoformans and A. fumigatus expressed on DCs and the signaling pathways involved in the antifungal response.
DC subsets are characterized by their expression of specific surface markers and functions. Although multiple subsets of DCs have been characterized, two main groups have been established: conventional DCs (cDCs) and plasmacytoid DCs (pDCs). pDCs express the surface markers CD123 (IL3R), PDCA-1, BDCA2, BDCA4, SiglecH and Bst2. In addition, pDCs are low (mouse) or negative (human) for CD11c, while expressing the B cell marker B220/CD45RA. Furthermore, human pDCs express endosomal TLR7 and TLR9, but lack expression of TLR4.Citation16 pDCs are characterized as IFNα (type I interferon)-producing cells, and have been primarily associated with antiviral responses.Citation16 Recently, pDCs were shown to play a non-redundant role in the antifungal response against A. fumigatus. The role of pDCs in the anti-Aspergillus response will be reviewed in a later section.
cDCs are typically characterized by their high-surface expression of CD11c and MHC-II (). cDCs, also known as resident DCs, exist in the lymphoid tissue as two distinct populations: CD8+ DCs and CD4+ DCs. These two populations are capable of cross-presenting antigen to T cells via MHC-I and MHC-II. In addition, both subsets of DCs express PRRs such as: the Toll-like receptors (TLRs), C-type Lectin receptors (CLRs) and Fc Receptors (FcR) that recognize PAMPs via opsonization-dependent and -independent mechanisms.Citation15,Citation17 Initially, pathogen engagement of PRRs on DCs results in the activation of a PAMP-specific signaling cascade and cytokine production. These secreted cytokines initiate an innate inflammatory response, which shapes the CD4 T cell response. Signaling via TLRs results in the activation of intracellular signaling cascades, which results in the nuclear translocation of the transcription factors activator protein-1 (AP-1) and nuclear factor-κB (NFκB), or the interferon regulatory factor 3 (IRF3) (). Transmission of PAMP recognition by TLRs is mediated by TIR-mediated engagement of myeloid differentiation factor 88 (MyD88); however, TLR3 signals via a MyD88-independent TIR-domain containing adaptor-inducing IFN-β (TRIF)-dependent signaling cascade. In addition, signaling of TLR4 is capable of signaling via MyD88 and TRIF-dependent mechanisms. MyD88 plays an instrumental role in the development of Th1 responses; which are required for a protective response against fungal pathogens.Citation18 In addition, engagement of the CLRs by carbohydrate moieties in the fungal cell wall also leads to the production of cytokines; an event that can be dependent or independent on the collaboration between the CLRs and TLRs. For example, interaction of Dectin-1 with the TLRs results to the canonical activation of NFκB (). Therefore, outcome of fungal infections is tightly connected to the interplay between the fungal pathogen and the host immune system. The fact that fungal pathogens, such as the mold A. fumigatus and the yeasts C. albicans and C. neoformans, affect mainly immune-compromised hosts indicate that our immune system has evolved mechanisms to prevent infection.Citation19 The fine interplay between the innate and adaptive arms of the immune system dictates the outcome of the disease.
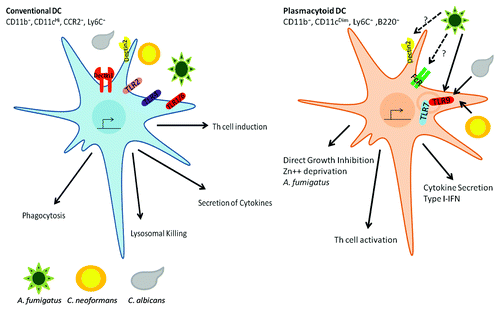
Figure 2. DC mediated responses to fungal pathogens. Conventional DCs are characterized by CD11b+, CD11cHI, Ly6CHi and CCR2+. These cDCs express the surface TLRs (TLR1, TLR2, TLR4 and TLR6) and the CLRs (Dectin1, Dectin2 and DC-SIGN). Upon recognition of the fungal pathogen, cDCs activate a series signaling cascades that result in: (1) phagocytosis of the fungal pathogen, (2) uptake to lysosomal compartments where the pathogen is killed and antigens can be loaded into MHC for presentation to T cells, (3) Secretion of cytokines and chemokines responsible for communicating with other cells to induce a host response and (4) induce the proper Th response. pDCs express the endosomal TLRs (TLR7 and TLR9), FcRγ and possibly the CLR Dectin2. pDCs are responsible for: (1) detection of exogenous DNA and RNA resulting in the induction of an inflammatory response, (2) orchestrating Th cell responses and (3) direct killing of A. fumigatus hyphae.
Conventional Dendritic Cells and PRR-Mediated Detection of Fungal Pathogens
Dendritic cells comprise a dense surveillance network in the lungs and periphery. In their immature or resting state, DCs are responsible for uptake, processing and presentation of antigen-following, cytokine-induced maturation signals. The complex process of antigen presentation is responsible for instructing the appropriate T cell response to the antigen. This process is of particular importance in the response to pathogenic fungi, as DCs are responsible for discriminating between different fungal morphotypes or growth stages. In order to discriminate between yeasts, conidia and hyphae, DCs express an array of cell surface receptors including TLRs, complement receptors (CRs), Fc Receptors (FcRs), Scavenger Receptors (SRs) and CLRs.Citation20 Depending on the receptor or combination of receptors engaged, DCs are responsible for priming and educating T cells toward the appropriate host response.
Host innate clearance of initial fungal infections is predominantly mediated by the complement system. Complement activation can take place via three pathways: the alternate pathway, the lectin pathway (MBL) and the classical pathway (CP). Even though target discrimination is different among these pathways, their activation leads to C3b binding to invading cells for opsonization and phagocyte killing. The MBL pathway is the most characterized in the antifungal response and has been reviewed elsewhere.Citation21 Recently, it was suggested that activation of the complment system by C. albicans is required for production of inflammatory cytokines via C5a-C5aR interactions; however, complement does not influence phagocytosis or killing of the yeast.Citation22 While fungal phagocytosis is most efficient following opsonization by serum opsonins, several non-opsonic PRRs have been described to mediate fungal uptake.Citation12,Citation19 Receptors such as Dectin-1, Dectin-2, dendritic cell-specific intracellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), macrophage mannose receptor (MMR) and Scavenger receptor class F member 1 (SCARF1) have been shown to mediate fungal uptake by recognition of cell wall components (i.e., β-glucans and mannans) ().
Dimorphic fungi, such as C. albicans and A. fumigatus, express different PAMPs during their morphological states. Therefore, it is not surprising that DCs have evolved to recognize and initiate a host response against these different morphotypes. Detection of fungal pathogens is highly dependent on detection of fungal cell wall components. The fungal cell wall is a complex and dynamic structure composed mainly of β-1,3 and β-1,6 glucans, chitin and mannoproteins.Citation19 The concentration and distribution of cell wall-associated glycoproteins differs greatly among the different fungal pathogens and their morphological state.Citation23,Citation24
For example, A. fumigatus conidia express a thick layer of a hydrophobic layer composed of small cysteine-rich proteins or hydrophobins, and a dense pigmented outer layer composed of the melanin. Following conidia swelling, the inner layer becomes translucent and exposes a network of carbohydrate polymers composed of the protein-associated glycoproteins: chitin, galactomannan, branched β-1,3/β-1,6 glucans and linear β-1,3/β-1,4 glucans also exposed in the germ tubes and hyphae. Thus, these PAMPs recognized by PRRs on DCs occur predominantly during A. fumigatus growth. In contrast, C. neoformans contains a hydrophobic capsule composed mainly of glucuronoxylomannan, galactoxylomannan and mannoproteins.Citation25 The C. neoformans capsule provides virulence to the yeast. The capsule functions as a physical barrier that interferes with normal phagocytosis, proinflammatory cytokine production and clearance by the immune system. Similarly, Candida yeast expresses a thick layer of N-linked and O-linked mannans in a network of glycoproteins associated with the fungal cell wall, including β-glucans and chitin.
It is well-established that the TLRs, mainly TLR2 and TLR4, and CLRs (such as Dectin-1) are responsible for the establishment and development of an effective host response against C. albicans, C. neoformans and A. fumigatus.Citation19 Both TLR2 and TLR4 have been shown to recognize cell wall components from pathogenic fungi; although these finding remain controversial and there exists contradictory findings between different TLR knockout mice. Perhaps some of this is due to the fact that TLR’s interactions with pathogenic fungi depend on the route of infection, morphotype of the fungi and strain of mice. During C. neoformans infection, TLR2 and TLR4 appear to play a minor role in the detection of yeast. Shoham et al. showed that GXM from the Cryptococcal capsule induces the translocation of NFκB in human PBMCs and RAW264 macrophages.Citation26 In vivo, TLR2−/− and TLR4−/− mice infected with C. neoformans produce IL1β, IL6, IL12p40 and TNFα, and show increased survival over their WT counterparts.Citation27 During C. albicans infection, TLR2−/− mice appear to be more susceptible to candidiasis as a result of a decreased proinflammatory response and reduced neutrophil recruitment.Citation28 Pietrella et al. suggested that mannoprotein (MP65) from C. albicans was responsible for stimulating DCs partially via TLR2, TLR4 and MyD88. Stimulation of DCs with MP65 results in the induction of TNF, IL6 and IL12; as well as DC maturation and increased expression of CD14 and FcRγ.Citation29
In addition to TLR2 and TLR4, other surface TLRs play a role in the development of fungal infectionsCitation30 (, adapted from Romani et al.Citation20). Kesh et al. analyzed the role of single nucleotide polymorphisms in TLR1, TLR4 and TLR6 in the development of invasive aspergillosis in 127 allogenic stem cell transplant recipients. The presence of TLR1 293 G > C or the presence of both TLR6 745C > T and TLR1 743A > G were associated with invasive aspergillosis.Citation31 In 2012, Rubino et al. analyzed the role of TLR1 and TLR6 in the recognition of A. fumigatus. Although the work was done in macrophages rather than DCs, the group analyzed cytokine production in WT, TLR1−/− and TLR6−/− mice following incubation with WT or A. fumigatus ΔrodA. In TLR1- and TLR6-deficient mice, there were lower amounts of IL12p40, CXCL2, IL6 and TNFα when compared with WT bone marrow-derived DCs (BMDCs). Lungs from TLR1−/−, TLR6−/− and WT mice infected intranasally (i.n.) with the mold revealed diminished CXCL1 and CXCL2 production in the TLR knockout mice. In addition, these mice showed higher fungal burden when compared with the WT counterparts. The observed response to the immunogenic A. fumigatus strain required the heterodimerization of murine TLR1/TLR2, TLR2/TLR6 and human TLR2/TLR1, but not human TLR2/TLR6.Citation32 These data suggest that TLR1 and TLR6 collaborate with TLR2, and possibly TLR4 in the host response against A. fumigatus. TLR1 and TLR6 also play a role in the host response against the yeast C. albicans.Citation33 In 2008, Netea et al. analyzed the role of these receptors in vivo. TLR1−/− and TLR6−/− mice were infected intravenously (i.v.) with C. albicans UC820 and followed for survival. In their model, TLR1 does not play a role in the recognition of C. albicans as mice showed comparable susceptibility to WT in the development of disseminated candidiasis.Citation34 However, TLR6 modulated the Th1/Th2 cytokine balance; as TLR6 mice displayed a defective production in IL10 and IFNγ. However, these mice displayed normal production of IL6, IL1β and TNF, as well as normal susceptibility to candidiasis; suggesting that TLR6 plays a minor role during Candida infection.Citation34 The extent of the role of TLR1 and TLR6 in host defense against fungal infections might be dependent on the species encountered, the morphology of the fungal pathogen and the association of TLR1 and/or TLR6 with other TLRs besides TLR2. Future studies should investigate the role of these receptors in association with other PRRs, such as other TLRs, CLRs and SRs.
Table 1. Polymorphisms associated with susceptibility to fungal infections
The contribution of TLR3, an endosomal dsRNA sensor, was recently analyzed in an A. fumigatus infection model. TLR3−/− mice were highly susceptible to A. fumigatus conidia following intranasal challenge and these mice failed to develop MHC-I-restricted CD8+ T cell responses. Moreover, CCR7+ DCs from TLR3−/− mice failed to migrate from the lungs to the lymph nodes and prime a proper T cell response to A. fumigatus. These results were confirmed by predisposition to invasive aspergillosis (IA) in hematopoietic-cell transplant (HSCT) recipients with SNPs in TLR3. In a cohort of 223 HSCT recipients and their corresponding donors, one mutation in TLR3 (95C/A) was found to significantly increase the risk of IA when present in the donors. Furthermore, functional analysis of this mutation demonstrated a defect in CD8+ T cell proliferation following incubation with DCs expressing this SNP.Citation35 To date, the role of TLR3 has been shown to be restricted to the recognition of viral RNA and synthetic poly I:C. However, Carvalho et al. recently showed for the first time that TLR3 in DCs mediates cross presentation of A. fumigatus RNA to CD8+ T cells.
The CLRs: Dectin-1
Dectin-1 was originally characterized on DCs, with high expression on the cell line on the DC cell line XS52, but not on the macrophage cell line J774.Citation36 In addition, Dectin-1 mRNA was predominantly expressed on spleen, thymus and skin-resident DCs.Citation36 In 2001, Brown et al. identified the ligand for Dectin-1.Citation37 The group screened RAW264 macrophages following treatment with zymosan, a β-glucan rich particle. They identified Dectin-1, a small type II membrane receptor on macrophages that binds β-1,3 glucans. In contrast to the original reports on Dectin-1, the receptor is expressed in all macrophage populations, with highest expression in the liver, lung and thymus.Citation37
The role of Dectin-1 expression on immature human DCs (iDCs) was further analyzed by microarray following challenge with A. fumigatus germ tubes.Citation38 Using short interference RNA, TLR2, TLR4, DC-SIGN, Pentraxin 3, Dectin 1 and CARD9 were silenced then knockdown iDCs were treated with A. fumigatus germ tubes. By RT-PCR, A. fumigatus germ tubes induced expression of cytokines, chemokines, costimulatory molecules and genes involved in fungal recognition and phagocytosis in iDCs. Silencing of Dectin-1 in these cells resulted in decreased expression of the proinflammatory cytokines TNFα and IL12. These data suggests that Dectin-1 might be the most important receptor in the detection of A. fumigatus germ tubes (). In contrast to previous reports, this group did not find alterations in the expression of TLR2 and/or TLR4 following treatment with A. fumigatus. This suggests that Dectin-1 plays an essential role in the recognition and immune response against mold, while TLR2 and TLR4 involvement plays a minor role in the detection of A. fumigatus.
Dectin-2 Expression on DCs
Another member of the CLR family, Dectin-2, binds highly mannosylated structures. Similar to Dectin-1, Dectin-2 is a glycosylated type II transmembrane protein with a carbohydrate recognition domain (CRD). However, Dectin-2 expresses a shorter cytoplasmic region with an arginine-based activation motif.Citation39 Binding of Dectin-2 to high-mannose structures, found in a wide range of fungal species, was shown using a glycan array ().Citation40 Furthermore, Dectin-2 was found to preferentially bind hyphal components of C. albicans in a soluble binding assay and in macrophages expressing Dectin-2. The adaptor molecule FcRγ interacts with Dectin-2 and after phosphorylation of the FcRγ chain, this interaction results in the activation of NFκB, internalization of ligand and the expression of TNFα and IL-1R.Citation41 Dectin-2 contributes to the activation of DCs by fungal particles via Syk and CARD9 pathway; however, the interaction with Syk and CARD9 is indirect through the association of the FcRγ chain (). In an infection model of C. albicans, blocking Dectin-2 with antibody did not affect the innate immune responses to the fungal pathogen. Instead specific T cell production of IL-17 was abrogated and, when combined with Dectin-1 deletion, decreased Th1 host responses.Citation42 Elucidations of the signaling mechanisms employed by Dectin-2 were further analyzed with the generation of Dectin-2-deficient mice. Following challenge with C. albicans mannans, Dectin-2−/− DCs did not produce any cytokines in response to α-mannans. In agreement with previous work, Dectin-2 signaling was dependent on Syk-CARD9-NFκB mechanism and independent of MAP kinases. In contrast to initial findings, Saijo et al. reported that both morphologies of C. albicans (yeast and hyphae) induce Th17 differentiation via a Dectin-2-dependent mechanism.Citation43 Thus Dectin-2 appears to play an important role in the induction of a Th17 host response, and in collaboration with Dectin-1 orchestrates a balanced CD4+ T cell response in the host. While the role of Dectin-2 has not been analyzed in other fungal infection models, it is possible that this receptor plays a similar role in the detection of other dimorphic fungi.
Mannose Receptors: MMR and DC-SIGN
The mannose receptors (MRs) are a subgroup of CLR family of PRRs responsible for detecting manosylated proteins. One characteristic of fungal glycoproteins (in contrast to mammalian) is their high degree of mannosylation or richness of mannose groups, which was shown to be critical for their immunogenicity. In humans, two receptors expressed on DCs and macrophages have been shown to play a role in mannose-binding: MMR/CD206 and the DC-SIGN/CD209.Citation44,Citation45 Furthermore, in the mouse, DC-SIGN has four isoforms (SIGN-R1, SIGN-R2, SIGN-R3 and SIGN-R4); but only SIGN-R1 and SIGN-R3 have been described to bind mannose moieties.Citation46
Membrane-bound CLRs, such as MMR, are characterized by the presence of a cytoplasmic sorting motif responsible for directing internalization into early endosomes via clathrin-coated vesicles.Citation47 Upon internalization and delivery of mannosylated antigen to the early endosome, MMR is recycled to the cell surface; while the antigen is processed and presented via MHC-II. Similarly, DC-SIGN also contains an internalization motif responsible for targeting mannosylated antigen for T cell presentation. However, DC-SIGN targets the antigen to the late endosomes and lysosomes.Citation47
The contribution of the membrane-bound CLRs was described by Mansour et al.Citation44 Initially, the group used stably transfected cells lines expressing human MMR or human DC-SIGN to determine whether these receptors bind and internalize C. neoformans mannoprotein (MP). Expression of human MMR and DC-SIGN in these genetically engineered cell lines results in avid binding and internalization of C. neoformans MP. However, parental cell lines (lacking MMR and DC-SIGN) were also capable of capturing small amounts of MP; which is not competitively inhibited by mannosylated ligands. Furthermore, DCs were capable of MHC-II presentation of MP and establish a T cell response. The uptake of MP by DCs was rapid and could be blocked by competitive inhibitors of MMR.Citation44 In 2005, Pietrella et al. demonstrated that MP activates and induces maturation of human DCs by a process that could be inhibited by blocking antibodies against MMR.Citation48 In 2008, Dan et al. further analyzed the importance of MMR in host response to C. neoformans.Citation49,Citation50 The group observed that MMR−/− mice challenged i.n. with C. neoformans were more susceptible to infection than WT mice; in addition, MMR−/− mice had increased fungal burden when compared with WT. Similar results were observed ex-vivo, where DCs from MMR−/− mice challenged with C. neoformans exhibited decreased CD4+ lymphoproliferation when compared with WT DCs.Citation50
In addition to C. neoformans, MMR has also been shown to play a role in the recognition of C. albicans.Citation51 The commensal yeast was shown to be internalized via MMR instead of Dectin-1 or another CLR. Donini et al. suggested that entry via MMR results in inhibition of the NADPH oxidative pathway, which, if activated, kills C. albicans.Citation51
DC-SIGN is another CLR responsible for the detection of fungal pathogens. While Mansour et al. demonstrated that cell lines stably transfected with human DC-SIGN could bind and internalize C. neoformans, there is no additional evidence of this receptor playing a role in host defense against the yeast.Citation47 However, DC-SIGN appears to play a role in the immune response to C. albicans and A. fumigatus conidia. In 2004, Serrano-Gomez et al. characterized the importance of DC-SIGN in the anti-Aspergillus response.Citation52 The group observed that DC-SIGN specifically interacts with clinical isolates of the mold.Citation52 Furthermore, the binding of A. fumigatus conidia was dependent on the expression of DC-SIGN; and uptake of the conidia directly correlated with the levels of expression of the receptor. In addition, analysis of hematological patients identified four SNPs in DC-SIGN allele that significantly increase the risk of IPA in these patients.Citation53 Similarly, Cambi et al. demonstrated that DC-SIGN binds C. albicans in a time and concentration-dependent manner.Citation54 In contrast to MMR, DC-SIGN expressed on immature DCs induces phagocytosis of the yeast. Uptake of C. albicans by DC-SIGN is mediated by exposure of N-linked mannans, rather than O-linked phosphomannans, in the cell wall of the yeast. Recognition of N-linked mannans on human DCs via DC-SIGN directly influences the production of the inflammatory cytokine IL-6.Citation55 Similarly, MMR also exhibits differential recognition of N-linked mannans vs O-linked mannans; however, there was no difference in the uptake of the different mannosylated glycoproteins.Citation56
Conventional DCs and T Cell Responses
Initiation of the proper adaptive immune response against A. fumigatus is dependent on the actions of DCs (). Phagocytosis of conidia by DCs leads to a protective Th1 response; however, interactions with hyphae result in a non-protective Th2 response and IL10-producing CD4 T cells.Citation57 Interaction of conidia with DCs, and subsequent phagocytosis, requires the shedding of the protective hydrophobic layer (rodA) from resting conidia. Incubation of DCs with swollen conidia results in DC maturation, CXCL8 secretion and the recruitment of neutrophils. The role of DCs in neutrophil migration was further characterized by Park et al. where, neutropenic mice showed a marked accumulation of DCs in the lungs of mice challenged with A. fumigatus. Phenotypically, these DCs were more immature in the neutropenic mice and in in vitro experiments of coincubation of iDCs with neutrophils resulted in enhanced expression of co-stimulatory molecules after exposure to A. fumigatus; a process dependent on cell contact and DC expression of the receptor DC-SIGN.Citation58
Activation of DCs, by engaging TLRs or non-opsonic receptors, leads to the activation of an adaptive immune response. Understanding the interactions between pathogenic fungi and DCs leads to the development of species/morphology-specific CD4-T cell responses, including Th1, Th2, Th17 and regulatory T cells (Tregs). Protection from fungal infections in human and mice are partially dependent on the development of fungal-specific CD4+ T cell responses. Depending on the PRR or combination of receptors engaged defines whether host response against the fungal pathogen will be Th1 (IFNγ secretion, phagocyte activation) vs. Th2 (IL4, IL5 and IL10 anti-inflammatory cytokine secretion). Activation of certain PRRs and the deregulation of the host response against any of the fungal pathogens can lead to an unfavorable Th2 response.Citation12 PRR engagement influences several steps in DC activation and, consequently, T cell differentiation.
Pulmonary infections with A. fumigatus induce concurrent Th1 and Th17 responses dependent on TLR/MyD88 activation.Citation59 Deletion of Dectin-1 leads to decreased secretion of IFNγ and IL12p40 during A. fumigatus infection, resulting in a decrease in Th17 differentiation and a Th1 non-protective response.Citation60 Recent experiments suggest that production of TNFα produced by Ly6C+CD11b+ DCs promotes IL17A secretion from CD4+ T cells.Citation61 In this infection model, C57Bl/6 mice and BalbC mice were sensitized with A. fumigatus via intratracheal (i.t.) infection and challenged eight additional times. When comparing these two mouse strains, BalbC mice exhibited a larger concentration of TNFα producing DCs in the lungs. Furthermore, CD11c-DTR, Dectin-1−/− and MyD88−/− BalbC mice exhibited decreased numbers of TNF-producing DCs and decreased IL17A production.Citation61 Production of TNFα by DCs, in this infection model, appears to play a role in orchestrating the events in DCs and CD4 T cells that lead to neutrophil airway inflammation.
Lysosomal Killing of Fungal Pathogens by DCs
Dendritic cells phagocytose and kill C. neoformans. Wozniak et al. examined the in vivo interactions of DCs with C. neoformans in the lungs using a murine infection model.Citation62 C57Bl/6 mice were infected i.n. with fluorescently labeled C. neoformans serotype A strain 145 and heat-killed C. neoformans. Within 2 h of infection, DCs in the lungs internalize the yeast. Furthermore, expression of the DC maturation markers, CD80, CD86 and MHC-II, are observed 7 d post-infection. Incubation of DCs from C. neoformans infected mice with antigen specific T cells resulted in an increase of IL2 production when compared with naïve mice, showing that DCs play a role in both the innate and adaptive anticryptococcal response. In 2008, Wozniak et al. analyzed the early events following C. neoformans phagocytosis by DCs.Citation63 Human and murine DCs were incubated with encapsulated C. neoformans in the presence of opsonizing antibody and, subsequently, intracellularly stained with EEA1 (endosome) and LAMP-1 (lysosome). In the murine DC model, live C. neoformans traffics to the endosome within 10 min and to the lysosome within 30 min post-infection. Similar results were observed in human DCs, with the yeast trafficking to the endosome within 20 min and to the lysosome within 60 min. The mechanism of phagocytosis follows traditional zipper phagocytosis and appears to be independent of the opsonization method. Furthermore, lysosomal components kill C. neoformans in a dose-dependent manner. Together, these data suggest that DCs play a key role in host defense against C. neoformans. DCs are responsible for the detection, phagocytosis, killing and cryptococcal antigen presentation following infection with the yeast.
Plasmacytoid Dendritic Cells (pDCs) and Endosomal TLRs
Plasmacytoid dendritic cells (pDCS) are a rare subtype (0.3–0.5% of PBMCs) of cells that develop in the bone marrow. In humans, pDCs can be found circulating in the blood where they can easily migrate to the lymph nodes. In the mouse, pDCs mainly reside in lymphoid organs. While the core gene expression program in pDCs is conserved between humans and mice, some differences in surface markers between human and murine pDCs have been established.Citation64 Surface expression of CD11c is low (mouse) or negative (human) in pDCs, but these cells express the B cell marker B220/CD45RA (). In addition, pDCs express specific markers such as BDCA2 and ILT7 in human and SiglecH and Bst2 in mice.
pDCs were initially identified as a very small subset of human leukocytes responsible for high-level IFNα production. In 2001, it was reported that pDCs produced type I IFN in response to unmethylated CpG DNA from viruses and bacteria.Citation65 Furthermore, pDCs express the endosomal nucleic acidTLR7 and TLR9, and can sense single stranded RNA and unmethylated CpG DNA, respectively (). While plenty of work has been performed to dissect the role of pDCs during viral and bacterial infection, the role these cells play in the antifungal host response has received scarce attention.
In the last decade, several advances have been made in understanding the role of endosomal TLRs in the detection of fungal PAMPs.Citation3 Initial observations suggesting a role for TLR9 in host defense against pathogenic fungi were performed in vivo following infection with C. albicans and A. fumigatus. In 2004, Bellochio et al. showed that mice deficient in TLR9 were susceptible to infection with C. albicans hyphae and A. fumigatus conidia.Citation28 Infection of TLR9−/− mice resulted in lower CFU in the kidneys (Candida yeast and hyphae), stomach (Candida yeast) and lung (Aspergillus conidia). Furthermore, TLR9−/− mice exhibited decreased cytokine secretion. These initial studies suggested that the endosomal nucleic acid sensor TLR9 might be involved in the inflammatory response to A. fumigatus and C. albicans.
Fungal DNA acts as a PAMP for TLR9. In 2008, two groups independently showed, for the first time, that A. fumigatus DNA was capable of stimulating a TLR9-dependent response.Citation66,Citation67 Ramirez-Ortiz et al. showed that A. fumigatus DNA contained unmethylated CpG motifs capable of inducing a TLR9-dependent response in mouse BMDCs and human pDCs. Stimulation with fungal DNA resulted in secretion of proinflammatory cytokines in a dose-dependent manner. Furthermore, the stimulatory activity of A. fumigatus DNA could be abolished by enzymatic methylation. An A. fumigatus genome wide analysis identified 23 murine and 87 human putative immunostimulatory motifs, as human TLR9 detects CpG-A motifs and murine TLR9 detects CpG-B motifs.Citation66 A second model introduced by Ramaprakash et al. investigated the role of TLR9 in a murine model of IA and fungal asthma. For the IA model, neutrophil-depleted WT and TLR9−/− mice were challenged with swollen or resting A. fumigatus conidia and monitored for lung inflammation. Following challenge with resting conidia TLR9−/− mice had less airway hyper-responsiveness when compared with WT. However, A. fumigatus-sensitized mice deficient in TLR9 showed an increase in fungal growth at 14 and 28 d post-challenge, which correlated with a decrease in Dectin-1.Citation67 In summary, A. fumigatus DNA has the immunostimulatory capacity to engage a TLR9-dependent response. Future studies are needed to determine whether TLR9 can collaborate with Dectin-1 and/or other CLRs in the establishment of the host response against pathogenic molds.
The role of pDCs in host response against A. fumigatus was initially analyzed by Romani et al.Citation68 They pulsed mDCs and pDCs with thymosin 1α, a naturally occurring thymus peptide, and analyzed antifungal response to A. fumigatus conidia. DCs treated with thymosin 1α induce DC maturation and IL12 production by A. fumigatus conidia pulsed DCs.Citation68 Thymosin 1α also increased the secretion of IL10 by human pDCs; however, secretion of IFNα was not detected following treatment with thymosin 1α in the presence or absence of A. fumigatus conidia. Furthermore, BM-transplanted mice treated with thymosin 1α peptide showed increased Th1-dependent antifungal immunity, accelerated myeloid cell recovery and protection against IA.Citation68 These data provide insights into one of possibly multiple molecules that play a role in modulating the host response of DCs against A. fumigatus. Identifying such molecules could be useful in the development of antifungal treatment. In 2008, Montagnoli et al. studied the ability of GM-CSF/IL4-derrived (cDCs) or Flt3-derrived (pDCs) DC subsets to induce T reg/Th1 cell priming against A. fumigatus. In addition, they analyzed the contribution of each murine DC subset to antifungal responses following adoptive transfer in hematopoietic transplanted mice. Flt3-DCs were found to prime antifungal Th1/Treg responses, induce tolerization against antigens and divert T cell responses from alloantigen-specific to antigen-specific responses in the presence of donor T lymphocytes. Furthermore, Treg development and tolerization involved thymosin 1α modulation of pDCs via TLR9, resulting in the activation of indoleamine 2,3-dioxygenase-dependent pathway ().Citation69
Recently, human pDCs were demonstrated to phagocytose A. fumigatus conidia and spread over these larger hyphae.Citation70 Human pDCs are capable of mounting an immune response to the fungal pathogen by release of proinflammatory cytokines, such as IFNα and TNFα. In addition, pDCs in close proximity to hyphae demonstrated high rates of cell death. The mechanism employed in the interaction between pDCs and A. fumigatus hyphae appears to be TLR9-independent, although A. fumigatus DNA can function as a PAMP for TLR9. In addition, pDCs showed increased cell death resulting in sequestering Zn2+ available in the environment and slowing down A. fumigatus growth. In a mouse infection model, pDCs migrated to the lungs following orotracheal (o.t.) challenge with A. fumigatus conidia. Furthermore, depletion of pDCs using the 120G8 antibody resulted in increased mortality.Citation70,Citation71 These data suggest a new non-redundant role for pDCs in the detection of pathogenic fungi.
While a direct role for pDCs in the detection of C. albicans has not been directly established to date, this yeast can induce a TLR7, TLR9 and IFNβ-dependent host response. Initial studies by Bellochio et al. suggested a role for TLR9 during C. albicans infection. Following challenge with C. albicans, TLR9−/− mice infected with the yeast morphotype showed decreased survival than mice infected with hyphae or when compared with their WT counterparts. However, TLR9−/− mice showed decreased CFU in the kidneys and stomach following challenge with either C. albicans hyphae or yeast.Citation28 In 2008, van de Veerdonk et al. suggested that TLR9 played a redundant role in the detection of C. albicans.Citation72 This group concluded that TLR9 was involved in cytokine production against the opportunistic yeast; however, TLR9 played a redundant role in the host response against C. albicans. A year later, Miyazato et al. showed that C. albicans DNA was sufficient to induce a TLR9-dependent response in DCs; however, the mechanism of nucleic acid detection was independent of DNA methylation.Citation73 In their model, Miyazato et al. observed that C. albicans DNA induced a stronger IL12p40 response in WT vs TLR9−/− mice and this response was not eliminated following treatment with a DNA methylase, but could be eliminated following DNase treatment.Citation73 Furthermore, Biondo et al. observed that cDCs, rather than pDCs and macrophages, are responsible for mounting an IFNβ response against the pathogenic yeast. In this model, the host response requires the adaptor molecule MyD88; however, it is only partially dependent on TLR7 and TLR9. Following i.v. challenge with C. albicans, IFNα/βR−/− mice showed decreased survival and increased CFU in the kidneys when compared with WT counterparts.Citation74
DCs are usually associated with the induction of protective and non-protective adaptive immune responses.Citation75 In 2000, Bauman et al. used a murine immunization model to analyze which DC subsets were involved in cell-mediated immunity to C. neoformans antigens.Citation76 In their model, mice were immunized s.c. with cryptococcal culture filtrate Ag-CFA (protective) or with heat-killed cryptococci-CFA (non-protective) and the infiltrating cells were characterized by flow cytometry. The protective response resulted in increased numbers of mDCs and Langerhans cells in the draining lymph nodes. However, mice immunized with the non-protective antigen showed no significant increase in the total number of cells in the draining lymph node, nor was there a change in the LDC (now pDCs):mDC ratio. These data suggest that mDCs are the primary APC during cryptococcal infection. However, DC-mediated responses to C. albicans have revealed an opposing mechanism. Using a murine model of vaginal candidiasis whereby female mice were inoculated with blastoconidia from stationary phase culture into the vagina, LeBlanc et al. examined the role of DCs in the initiation of immunoregulatory events.Citation77 This group found that following vaginal inoculation, DCs reorganize and become more concentrated in the T cell zones of the lymph nodes. Analysis of the draining lymph nodes of mice infected with Candida vs. control mice demonstrated that pDCs were the predominant DC subset pre- and post-vaginal infection. Interestingly, pDCs retained their predominant state throughout the infection indicating a tolerizing condition suggesting that pDCs are involved in the induction of Th1 responses to C. albicans vaginal infection.Citation77
Another fungal pathogen, C. neoformans, can induce a TLR9-dependent host response. Initial investigations suggested a role for TLR9 in mediating the Th1 vs. Th2-type response following during C. neoformans infection. Edwards et al. showed that immunization with CpG-DNA deviates the response from Th2 to Th1 type response following challenge with the yeast. This infection model resulted in increased IL12, TNF, MCP1 and macrophage nitric oxide production; as well as a decrease in pulmonary eosinophilia and fungal burden.Citation78 While initial studies engaged TLR9 activation prior to fungal infection, in 2008, Nakamura et al. analyzed the role of C. neoformans DNA as a PAMP.Citation79 Fungal DNA induced IL12p40 production and increased CD40 expression in BM-DCs, and C. neoformans DNA colocalized with CpG DNA to endosomal compartments. This response is abolished in TLR9−/− and MyD88−/− mice, and after treatment with DNase. In vivo, mice lacking TLR9 show increased susceptibility to C. neoformans infection, however TLR9 was required for the recruitment of effector cells such as macrophages and lymphocytes to the lungs during C. neoformans infection.Citation79,Citation80 Finally, Zhang et al. found that TLR9 did not affect the fungal burden at the innate immune response stage, but increased clearance of the yeast during the adaptive phase.Citation81
The role of the endosomal TLRs in the host defense against fungal pathogens has been well-characterized in the last decade. However, it is important to note that most of the work performed to dissect the role of TLR7 and TLR9 during infection utilizes mouse BM-DCs as their DC model. A basic difference between human and murine DCs is observed in the distribution of the PRRs among the different subsets of DCs. In humans, expression of the endosomal TLRs, TLR7 and TLR9 is restricted to pDCs while cDCs express all the surface TLRs. In mice, all DC subsets express all TLRs (with the exception of TLR3). Because of these differences in PRR, recognition of fungal PAMPs by human and murine DCs, it is important to be remindful that the results of fungal experiments performed in mice might not always be applicable to humans.
The importance of TLRs has also been shown during A. fumigatus disease. Polymorphisms in different TLRs, Dectin-1, CARD9 and chemokines have been shown to increase susceptibility to infection in an array of immunodeficiencies ().Citation10,Citation30,Citation31,Citation35,Citation82-Citation95 Studies performed with a cohort of 336 HSCT patients showed that polymorphisms in TLR4 can increase the risk of invasive Aspergillus infection.Citation10,Citation83 Furthermore, Bochud et al. showed that the haplotype was present in two single nucleotide polymorphisms (SNPs) that resulted in a strong linkage disequilibrium that affected TLR4 function. These results were confirmed by comparing 103 Aspergillus infected patients to control patients.Citation83 Another study examined the association between SNP in TLR1, TLR4 and TLR6 genes and development of IA from 22 patients with IA and 105 unaffected hematopoietic stem cell transplant patients. Kesh et al. found that the mutations in TLR1 239G > C (Arg80 > Thr) and TLR6 745C > T (Ser249 > Pro) were associated with IA.Citation31 The role of TLRs during infection was also assessed for susceptibility to non-invasive forms of pulmonary aspergillosis. Carvalho et al. found an association between the TLR4 mutation Asp299Gly and chronic cavitary pulmonary aspergillosis.Citation96 This group also found that pulmonary aspergillosis was associated with a mutation in TLR9 (T-1237C).Citation90
Concluding Remarks
In this review, we highlighted the indispensable role of DCs in the antifungal response. DCs are responsible for sensing the fungal pathogen via their PRRs, secreting cytokines and chemokines into the environment, capturing fungal particles by phagocytosis and presenting antigens to T cells to induce an adaptive immune response. Great advances have been done over the last three decades in identifying the receptors involved in host responses to medically important fungi, which have great morphological diversity. However, there is a pressing need for the development of new antifungal treatments to combat the increasing number of fungal infections and the increase in drug-resistant fungal species. Identifying additional receptor and signaling pathways in DCs involved in initiating and regulating T cell responses against pathogenic fungi will provide valuable information about disease development. One group of PRRs that have received scarce attention, the SRs, might collaborate with TLRs and/or CLRs to detect fungal PAMPs. Indeed, the SRs CD36 and SCARF1 were shown to recognize C. neoformans.Citation20 However, the work on these SRs was performed on macrophages. Further understanding the mechanisms employed by DCs to recognize and kill fungal pathogens as well as dissecting the pathways involved in the antifungal response could lead to the development of antifungal vaccines and immune-boosting biological agents.
References
- Perfect JR. The impact of the host on fungal infections. Am J Med 2012; 125:Suppl S39 - 51; http://dx.doi.org/10.1016/j.amjmed.2011.10.010; PMID: 22196208
- Perfect JR. Invasive mycoses: evolving challenges and opportunities in antifungal therapy. Introduction. Am J Med 2012; 125:Suppl S1 - 2; http://dx.doi.org/10.1016/j.amjmed.2011.10.007; PMID: 22196204
- Roy RM, Klein BS. Dendritic cells in antifungal immunity and vaccine design. Cell Host Microbe 2012; 11:436 - 46; http://dx.doi.org/10.1016/j.chom.2012.04.005; PMID: 22607797
- Latgé JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 1999; 12:310 - 50; PMID: 10194462
- Cramer RA, Rivera A, Hohl TM. Immune responses against Aspergillus fumigatus: what have we learned?. Curr Opin Infect Dis 2011; 24:315 - 22; http://dx.doi.org/10.1097/QCO.0b013e328348b159; PMID: 21666456
- Perfect JR, Casadevall A. Cryptococcosis. [v-vi.] Infect Dis Clin North Am 2002; 16:837 - 74, v-vi; http://dx.doi.org/10.1016/S0891-5520(02)00036-3; PMID: 12512184
- Vonk AG, Netea MG, van der Meer JW, Kullberg BJ. Host defence against disseminated Candida albicans infection and implications for antifungal immunotherapy. Expert Opin Biol Ther 2006; 6:891 - 903; http://dx.doi.org/10.1517/14712598.6.9.891; PMID: 16918256
- Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol 2012; 10:112 - 22; PMID: 22158429
- Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 2008; 6:67 - 78; http://dx.doi.org/10.1038/nrmicro1815; PMID: 18079743
- Hage CA, Knox KS, Wheat LJ. Endemic mycoses: overlooked causes of community acquired pneumonia. Respir Med 2012; 106:769 - 76; http://dx.doi.org/10.1016/j.rmed.2012.02.004; PMID: 22386326
- Bonifaz A, Vázquez-González D, Perusquía-Ortiz AM. Endemic systemic mycoses: coccidioidomycosis, histoplasmosis, paracoccidioidomycosis and blastomycosis. J Dtsch Dermatol Ges 2011; 9:705 - 14, quiz 715; http://dx.doi.org/10.1111/j.1610-0387.2011.07731.x; PMID: 21722309
- Wüthrich M, Deepe GS Jr., Klein B. Adaptive immunity to fungi. Annu Rev Immunol 2012; 30:115 - 48; http://dx.doi.org/10.1146/annurev-immunol-020711-074958; PMID: 22224780
- Borchers AT, Gershwin ME. The immune response in Coccidioidomycosis. Autoimmun Rev 2010; 10:94 - 102; http://dx.doi.org/10.1016/j.autrev.2010.08.010; PMID: 20728582
- Edwards JA, Rappleye CA. Histoplasma mechanisms of pathogenesis--one portfolio doesn’t fit all. FEMS Microbiol Lett 2011; 324:1 - 9; http://dx.doi.org/10.1111/j.1574-6968.2011.02363.x; PMID: 22092757
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 2012; 30:1 - 22; http://dx.doi.org/10.1146/annurev-immunol-100311-102839; PMID: 22136168
- Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol 2011; 29:163 - 83; http://dx.doi.org/10.1146/annurev-immunol-031210-101345; PMID: 21219184
- Benko S, Magyarics Z, Szabó A, Rajnavölgyi E. Dendritic cell subtypes as primary targets of vaccines: the emerging role and cross-talk of pattern recognition receptors. Biol Chem 2008; 389:469 - 85; http://dx.doi.org/10.1515/BC.2008.054; PMID: 18953714
- Rivera A, Ro G, Van Epps HL, Simpson T, Leiner I, Sant’Angelo DB, et al. Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity 2006; 25:665 - 75; http://dx.doi.org/10.1016/j.immuni.2006.08.016; PMID: 17027299
- Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol 2011; 29:1 - 21; http://dx.doi.org/10.1146/annurev-immunol-030409-101229; PMID: 20936972
- Romani L. Immunity to fungal infections. Nat Rev Immunol 2011; 11:275 - 88; http://dx.doi.org/10.1038/nri2939; PMID: 21394104
- Brummer E, Stevens DA. Collectins and fungal pathogens: roles of surfactant proteins and mannose binding lectin in host resistance. Med Mycol 2010; 48:16 - 28; http://dx.doi.org/10.3109/13693780903117473; PMID: 19639514
- Cheng SC, Sprong T, Joosten LA, van der Meer JW, Kullberg BJ, Hube B, et al. Complement plays a central role in Candida albicans-induced cytokine production by human PBMCs. Eur J Immunol 2012; 42:993 - 1004; http://dx.doi.org/10.1002/eji.201142057; PMID: 22531923
- Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol 2011; 9:737 - 48; http://dx.doi.org/10.1038/nrmicro2636; PMID: 21844880
- Bernard M, Latgé JP. Aspergillus fumigatus cell wall: composition and biosynthesis. Med Mycol 2001; 39:Suppl 1 9 - 17; PMID: 11800273
- Bose I, Reese AJ, Ory JJ, Janbon G, Doering TL. A yeast under cover: the capsule of Cryptococcus neoformans. Eukaryot Cell 2003; 2:655 - 63; http://dx.doi.org/10.1128/EC.2.4.655-663.2003; PMID: 12912884
- Shoham S, Huang C, Chen JM, Golenbock DT, Levitz SM. Toll-like receptor 4 mediates intracellular signaling without TNF-alpha release in response to Cryptococcus neoformans polysaccharide capsule. J Immunol 2001; 166:4620 - 6; PMID: 11254720
- Nakamura K, Miyagi K, Koguchi Y, Kinjo Y, Uezu K, Kinjo T, et al. Limited contribution of Toll-like receptor 2 and 4 to the host response to a fungal infectious pathogen, Cryptococcus neoformans. FEMS Immunol Med Microbiol 2006; 47:148 - 54; http://dx.doi.org/10.1111/j.1574-695X.2006.00078.x; PMID: 16706798
- Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, et al. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol 2004; 172:3059 - 69; PMID: 14978111
- Pietrella D, Bistoni G, Corbucci C, Perito S, Vecchiarelli A. Candida albicans mannoprotein influences the biological function of dendritic cells. Cell Microbiol 2006; 8:602 - 12; http://dx.doi.org/10.1111/j.1462-5822.2005.00651.x; PMID: 16548886
- Plantinga TS, Johnson MD, Scott WK, van de Vosse E, Velez Edwards DR, Smith PB, et al. Toll-like receptor 1 polymorphisms increase susceptibility to candidemia. J Infect Dis 2012; 205:934 - 43; http://dx.doi.org/10.1093/infdis/jir867; PMID: 22301633
- Kesh S, Mensah NY, Peterlongo P, Jaffe D, Hsu K, VAN DEN Brink M, et al. TLR1 and TLR6 polymorphisms are associated with susceptibility to invasive aspergillosis after allogeneic stem cell transplantation. Ann NY Acad Sci 2005; 1062:95 - 103; http://dx.doi.org/10.1196/annals.1358.012; PMID: 16461792
- Rubino I, Coste A, Le Roy D, Roger T, Jaton K, Boeckh M, et al. Species-specific recognition of Aspergillus fumigatus by Toll-like receptor 1 and Toll-like receptor 6. J Infect Dis 2012; 205:944 - 54; http://dx.doi.org/10.1093/infdis/jir882; PMID: 22315281
- Van der Graaf CA, Netea MG, Morré SA, Den Heijer M, Verweij PE, Van der Meer JW, et al. Toll-like receptor 4 Asp299Gly/Thr399Ile polymorphisms are a risk factor for Candida bloodstream infection. Eur Cytokine Netw 2006; 17:29 - 34; PMID: 16613760
- Netea MG, van de Veerdonk F, Verschueren I, van der Meer JW, Kullberg BJ. Role of TLR1 and TLR6 in the host defense against disseminated candidiasis. FEMS Immunol Med Microbiol 2008; 52:118 - 23; http://dx.doi.org/10.1111/j.1574-695X.2007.00353.x; PMID: 18036178
- Carvalho A, De Luca A, Bozza S, Cunha C, D’Angelo C, Moretti S, et al. TLR3 essentially promotes protective class I-restricted memory CD8⁺ T cell responses to Aspergillus fumigatus in hematopoietic transplanted patients. Blood 2012; 119:967 - 77; http://dx.doi.org/10.1182/blood-2011-06-362582; PMID: 22147891
- Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R 3rd, Kumamoto T, et al. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem 2000; 275:20157 - 67; http://dx.doi.org/10.1074/jbc.M909512199; PMID: 10779524
- Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature 2001; 413:36 - 7; http://dx.doi.org/10.1038/35092620; PMID: 11544516
- Mezger M, Kneitz S, Wozniok I, Kurzai O, Einsele H, Loeffler J. Proinflammatory response of immature human dendritic cells is mediated by dectin-1 after exposure to Aspergillus fumigatus germ tubes. J Infect Dis 2008; 197:924 - 31; http://dx.doi.org/10.1086/528694; PMID: 18279049
- Saijo S, Iwakura Y. Dectin-1 and Dectin-2 in innate immunity against fungi. Int Immunol 2011; 23:467 - 72; http://dx.doi.org/10.1093/intimm/dxr046; PMID: 21677049
- McGreal EP, Rosas M, Brown GD, Zamze S, Wong SY, Gordon S, et al. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 2006; 16:422 - 30; http://dx.doi.org/10.1093/glycob/cwj077; PMID: 16423983
- Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem 2006; 281:38854 - 66; http://dx.doi.org/10.1074/jbc.M606542200; PMID: 17050534
- Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med 2009; 206:2037 - 51; http://dx.doi.org/10.1084/jem.20082818; PMID: 19703985
- Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 2010; 32:681 - 91; http://dx.doi.org/10.1016/j.immuni.2010.05.001; PMID: 20493731
- Mansour MK, Schlesinger LS, Levitz SM. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J Immunol 2002; 168:2872 - 9; PMID: 11884457
- Mansour MK, Latz E, Levitz SM. Cryptococcus neoformans glycoantigens are captured by multiple lectin receptors and presented by dendritic cells. J Immunol 2006; 176:3053 - 61; PMID: 16493064
- Takahara K, Yashima Y, Omatsu Y, Yoshida H, Kimura Y, Kang YS, et al. Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int Immunol 2004; 16:819 - 29; http://dx.doi.org/10.1093/intimm/dxh084; PMID: 15096474
- Levitz SM, Specht CA. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res 2006; 6:513 - 24; http://dx.doi.org/10.1111/j.1567-1364.2006.00071.x; PMID: 16696647
- Pietrella D, Corbucci C, Perito S, Bistoni G, Vecchiarelli A. Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infect Immun 2005; 73:820 - 7; http://dx.doi.org/10.1128/IAI.73.2.820-827.2005; PMID: 15664921
- Dan JM, Wang JP, Lee CK, Levitz SM. Cooperative stimulation of dendritic cells by Cryptococcus neoformans mannoproteins and CpG oligodeoxynucleotides. PLoS One 2008; 3:e2046; http://dx.doi.org/10.1371/journal.pone.0002046; PMID: 18446192
- Dan JM, Kelly RM, Lee CK, Levitz SM. Role of the mannose receptor in a murine model of Cryptococcus neoformans infection. Infect Immun 2008; 76:2362 - 7; http://dx.doi.org/10.1128/IAI.00095-08; PMID: 18391001
- Donini M, Zenaro E, Tamassia N, Dusi S. NADPH oxidase of human dendritic cells: role in Candida albicans killing and regulation by interferons, dectin-1 and CD206. Eur J Immunol 2007; 37:1194 - 203; http://dx.doi.org/10.1002/eji.200636532; PMID: 17407098
- Serrano-Gómez D, Domínguez-Soto A, Ancochea J, Jimenez-Heffernan JA, Leal JA, Corbí AL. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin mediates binding and internalization of Aspergillus fumigatus conidia by dendritic cells and macrophages. J Immunol 2004; 173:5635 - 43; PMID: 15494514
- Sainz J, Lupíñez CB, Segura-Catena J, Vazquez L, Ríos R, Oyonarte S, et al. Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary Aspergillosis infection. PLoS One 2012; 7:e32273; http://dx.doi.org/10.1371/journal.pone.0032273; PMID: 22384201
- Cambi A, Gijzen K, de Vries JM, Torensma R, Joosten B, Adema GJ, et al. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol 2003; 33:532 - 8; http://dx.doi.org/10.1002/immu.200310029; PMID: 12645952
- Cambi A, Netea MG, Mora-Montes HM, Gow NA, Hato SV, Lowman DW, et al. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem 2008; 283:20590 - 9; http://dx.doi.org/10.1074/jbc.M709334200; PMID: 18482990
- Lam JS, Huang H, Levitz SM. Effect of differential N-linked and O-linked mannosylation on recognition of fungal antigens by dendritic cells. PLoS One 2007; 2:e1009; http://dx.doi.org/10.1371/journal.pone.0001009; PMID: 17925857
- Bozza S, Gaziano R, Spreca A, Bacci A, Montagnoli C, di Francesco P, et al. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J Immunol 2002; 168:1362 - 71; PMID: 11801677
- Park SJ, Burdick MD, Brix WK, Stoler MH, Askew DS, Strieter RM, et al. Neutropenia enhances lung dendritic cell recruitment in response to Aspergillus via a cytokine-to-chemokine amplification loop. J Immunol 2010; 185:6190 - 7; http://dx.doi.org/10.4049/jimmunol.1002064; PMID: 20926800
- Zelante T, Bozza S, De Luca A, D’Angelo C, Bonifazi P, Moretti S, et al. Th17 cells in the setting of Aspergillus infection and pathology. Med Mycol 2009; 47:Suppl 1 S162 - 9; http://dx.doi.org/10.1080/13693780802140766; PMID: 18608926
- Rivera A, Hohl TM, Collins N, Leiner I, Gallegos A, Saijo S, et al. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J Exp Med 2011; 208:369 - 81; http://dx.doi.org/10.1084/jem.20100906; PMID: 21242294
- Fei M, Bhatia S, Oriss TB, Yarlagadda M, Khare A, Akira S, et al. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc Natl Acad Sci USA 2011; 108:5360 - 5; http://dx.doi.org/10.1073/pnas.1015476108; PMID: 21402950
- Wozniak KL, Vyas JM, Levitz SM. In vivo role of dendritic cells in a murine model of pulmonary cryptococcosis. Infect Immun 2006; 74:3817 - 24; http://dx.doi.org/10.1128/IAI.00317-06; PMID: 16790753
- Wozniak KL, Levitz SM. Cryptococcus neoformans enters the endolysosomal pathway of dendritic cells and is killed by lysosomal components. Infect Immun 2008; 76:4764 - 71; http://dx.doi.org/10.1128/IAI.00660-08; PMID: 18678670
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol 2004; 5:1219 - 26; http://dx.doi.org/10.1038/ni1141; PMID: 15549123
- Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med 2001; 194:863 - 9; http://dx.doi.org/10.1084/jem.194.6.863; PMID: 11561001
- Ramirez-Ortiz ZG, Specht CA, Wang JP, Lee CK, Bartholomeu DC, Gazzinelli RT, et al. Toll-like receptor 9-dependent immune activation by unmethylated CpG motifs in Aspergillus fumigatus DNA. Infect Immun 2008; 76:2123 - 9; http://dx.doi.org/10.1128/IAI.00047-08; PMID: 18332208
- Ramaprakash H, Ito T, Standiford TJ, Kunkel SL, Hogaboam CM. Toll-like receptor 9 modulates immune responses to Aspergillus fumigatus conidia in immunodeficient and allergic mice. Infect Immun 2009; 77:108 - 19; http://dx.doi.org/10.1128/IAI.00998-08; PMID: 18936185
- Romani L, Bistoni F, Gaziano R, Bozza S, Montagnoli C, Perruccio K, et al. Thymosin alpha 1 activates dendritic cells for antifungal Th1 resistance through toll-like receptor signaling. Blood 2004; 103:4232 - 9; http://dx.doi.org/10.1182/blood-2003-11-4036; PMID: 14982877
- Montagnoli C, Perruccio K, Bozza S, Bonifazi P, Zelante T, De Luca A, et al. Provision of antifungal immunity and concomitant alloantigen tolerization by conditioned dendritic cells in experimental hematopoietic transplantation. Blood Cells Mol Dis 2008; 40:55 - 62; http://dx.doi.org/10.1016/j.bcmd.2007.06.016; PMID: 17827038
- Ramirez-Ortiz ZG, Lee CK, Wang JP, Boon L, Specht CA, Levitz SM. A nonredundant role for plasmacytoid dendritic cells in host defense against the human fungal pathogen Aspergillus fumigatus. Cell Host Microbe 2011; 9:415 - 24; http://dx.doi.org/10.1016/j.chom.2011.04.007; PMID: 21575912
- Ang DK, Oates CV, Schuelein R, Kelly M, Sansom FM, Bourges D, et al. Cutting edge: pulmonary Legionella pneumophila is controlled by plasmacytoid dendritic cells but not type I IFN. J Immunol 2010; 184:5429 - 33; http://dx.doi.org/10.4049/jimmunol.1000128; PMID: 20400697
- van de Veerdonk FL, Netea MG, Jansen TJ, Jacobs L, Verschueren I, van der Meer JW, et al. Redundant role of TLR9 for anti-Candida host defense. Immunobiology 2008; 213:613 - 20; http://dx.doi.org/10.1016/j.imbio.2008.05.002; PMID: 18950591
- Miyazato A, Nakamura K, Yamamoto N, Mora-Montes HM, Tanaka M, Abe Y, et al. Toll-like receptor 9-dependent activation of myeloid dendritic cells by Deoxynucleic acids from Candida albicans. Infect Immun 2009; 77:3056 - 64; http://dx.doi.org/10.1128/IAI.00840-08; PMID: 19433551
- Biondo C, Signorino G, Costa A, Midiri A, Gerace E, Galbo R, et al. Recognition of yeast nucleic acids triggers a host-protective type I interferon response. Eur J Immunol 2011; 41:1969 - 79; http://dx.doi.org/10.1002/eji.201141490; PMID: 21480215
- MacDonald AS, Straw AD, Bauman B, Pearce EJ. CD8- dendritic cell activation status plays an integral role in influencing Th2 response development. J Immunol 2001; 167:1982 - 8; PMID: 11489979
- Bauman SK, Nichols KL, Murphy JW. Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses. J Immunol 2000; 165:158 - 67; PMID: 10861048
- LeBlanc DM, Barousse MM, Fidel PL Jr.. Role for dendritic cells in immunoregulation during experimental vaginal candidiasis. Infect Immun 2006; 74:3213 - 21; http://dx.doi.org/10.1128/IAI.01824-05; PMID: 16714548
- Edwards L, Williams AE, Krieg AM, Rae AJ, Snelgrove RJ, Hussell T. Stimulation via Toll-like receptor 9 reduces Cryptococcus neoformans-induced pulmonary inflammation in an IL-12-dependent manner. Eur J Immunol 2005; 35:273 - 81; http://dx.doi.org/10.1002/eji.200425640; PMID: 15597328
- Nakamura K, Miyazato A, Xiao G, Hatta M, Inden K, Aoyagi T, et al. Deoxynucleic acids from Cryptococcus neoformans activate myeloid dendritic cells via a TLR9-dependent pathway. J Immunol 2008; 180:4067 - 74; PMID: 18322216
- Wang JP, Lee CK, Akalin A, Finberg RW, Levitz SM. Contributions of the MyD88-dependent receptors IL-18R, IL-1R, and TLR9 to host defenses following pulmonary challenge with Cryptococcus neoformans. PLoS One 2011; 6:e26232; http://dx.doi.org/10.1371/journal.pone.0026232; PMID: 22039448
- Zhang Y, Wang F, Bhan U, Huffnagle GB, Toews GB, Standiford TJ, et al. TLR9 signaling is required for generation of the adaptive immune protection in Cryptococcus neoformans-infected lungs. Am J Pathol 2010; 177:754 - 65; http://dx.doi.org/10.2353/ajpath.2010.091104; PMID: 20581055
- Pamer EG. TLR polymorphisms and the risk of invasive fungal infections. N Engl J Med 2008; 359:1836 - 8; http://dx.doi.org/10.1056/NEJMe0806412; PMID: 18946070
- Bochud PY, Chien JW, Marr KA, Leisenring WM, Upton A, Janer M, et al. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med 2008; 359:1766 - 77; http://dx.doi.org/10.1056/NEJMoa0802629; PMID: 18946062
- Van Der Veer J, Lewis RJ, Emtiazjoo AM, Allen SD, Wheat LJ, Hage CA. Cross-reactivity in the Platelia™ Aspergillus enzyme immunoassay caused by blastomycosis. Med Mycol 2012; 50:396 - 8; PMID: 21939346
- Tirado-Sánchez A, Bonifaz A, Ponce-Olivera RM. IgM in lesional skin is indicative of renal involvement in adults with Henoch-Schönlein purpura but not children. J Am Acad Dermatol 2011; 64:1183 - 4; http://dx.doi.org/10.1016/j.jaad.2010.12.044; PMID: 21571181
- Bonifaz A, Vázquez-González D. Tinea imbricata in the Americas. Curr Opin Infect Dis 2011; 24:106 - 11; http://dx.doi.org/10.1097/QCO.0b013e328342cbc1; PMID: 21169831
- Borchers AT, Naguwa SM, Shoenfeld Y, Gershwin ME. The geoepidemiology of systemic lupus erythematosus. Autoimmun Rev 2010; 9:A277 - 87; http://dx.doi.org/10.1016/j.autrev.2009.12.008; PMID: 20036343
- Mansour MK, Vyas JM, Levitz SM. Dynamic virulence: real-time assessment of intracellular pathogenesis links Cryptococcus neoformans phenotype with clinical outcome. MBio 2011; 2:e00217 - 11
- Lam JS, Mansour MK, Specht CA, Levitz SM. A model vaccine exploiting fungal mannosylation to increase antigen immunogenicity. J Immunol 2005; 175:7496 - 503; PMID: 16301657
- Carvalho A, Pasqualotto AC, Pitzurra L, Romani L, Denning DW, Rodrigues F. Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J Infect Dis 2008; 197:618 - 21; http://dx.doi.org/10.1086/526500; PMID: 18275280
- Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 2009; 361:1760 - 7; http://dx.doi.org/10.1056/NEJMoa0901053; PMID: 19864674
- Glocker EO, Hennigs A, Nabavi M, Schäffer AA, Woellner C, Salzer U, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 2009; 361:1727 - 35; http://dx.doi.org/10.1056/NEJMoa0810719; PMID: 19864672
- Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, et al. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood 2010; 116:5394 - 402; http://dx.doi.org/10.1182/blood-2010-04-279307; PMID: 20807886
- Mezger M, Steffens M, Beyer M, Manger C, Eberle J, Toliat MR, et al. Polymorphisms in the chemokine (C-X-C motif) ligand 10 are associated with invasive aspergillosis after allogeneic stem-cell transplantation and influence CXCL10 expression in monocyte-derived dendritic cells. Blood 2008; 111:534 - 6; http://dx.doi.org/10.1182/blood-2007-05-090928; PMID: 17957030
- Plantinga TS, Hamza OJ, Willment JA, Ferwerda B, van de Geer NM, Verweij PE, et al. Genetic variation of innate immune genes in HIV-infected african patients with or without oropharyngeal candidiasis. J Acquir Immune Defic Syndr 2010; 55:87 - 94; http://dx.doi.org/10.1097/QAI.0b013e3181e53c64; PMID: 20577092
- Carvalho A, Cunha C, Carotti A, Aloisi T, Guarrera O, Di Ianni M, et al. Polymorphisms in Toll-like receptor genes and susceptibility to infections in allogeneic stem cell transplantation. Exp Hematol 2009; 37:1022 - 9; http://dx.doi.org/10.1016/j.exphem.2009.06.004; PMID: 19539691