Abstract
Dendritic cells (DCs) form an important link between innate and adaptive immunity. However, DCs are also deployed as vehicles for systemic spread of pathogens. Salmonella is an important gastrointestinal pathogen causing diseases ranging from gastroenteritis to typhoid fever. DCs play an important role in the immunity against Salmonella infection, but this pathogen has also evolved efficient mechanisms to persist after phagocytosis by DCs, to spread using DCs as vehicles and to interfere with the central function of DCs, the processing of antigens and presentation of antigen-derived peptides to T cells for the stimulation of adaptive immune responses. Here we review the routes used by Salmonella to breach intestinal barriers, the intracellular habitat of Salmonella in DCs, molecular mechanisms of Salmonella virulence factors for intracellular life and intracellular activities in DCs resulting in manipulation of DC functions.
Introduction
Dendritic cells (DCs) are myeloid or plasmacytoid phagocytes and have a key role in both the maintenance of T cell tolerance and the initiation and regulation of adaptive immune responses.Citation1 These functions require DC maturation by direct (pathogen-mediated) or indirect (cytokine-mediated) pathways. Maturation results in migration of DCs to defined lymphoid tissues and upregulation of major histocompatibility complex II (MHC II) and co-stimulatory molecules to optimize their antigen presentation capacityCitation2 (). These important properties also contribute to adaptive immune responses, allowing DCs to serve as a major link between innate and adaptive immunity.Citation3 By the use of murine models and infection with Salmonella enterica, researchers try to unravel the mechanisms of host immune responses required for the resistance against systemic infections.Citation4
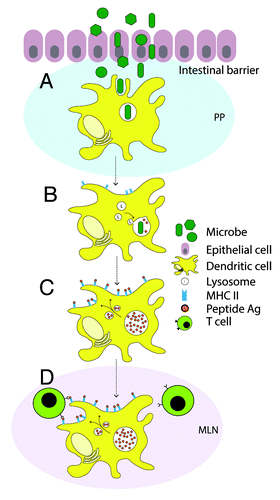
Figure 1. The role of dendritic cells (DCs) during bacterial infection in the mucosa. (A) Once microbes have overcome the intestinal barrier, they encounter DCs in underlying tissue or the Peyer’s patches (PP) at the site of infection. (B) Upon phagocytic internalization of bacteria, DC maturation is initiated, which is crucial for the initiation of immunity. During this maturation, DCs lose phagocytic properties, but surface expression of MHC class II is upregulated, concomitant with the ability to present antigens. At the same time, lysosomal compartments fuse with the pathogen-containing phagosome to ensure bacterial degradation into peptide antigens. (C) Peptide antigens derived from degraded microbial proteins are loaded on MHC II complexes, transported to the cell surface and displayed. (D) During maturation DCs migrate from peripheral locations at the intestine to mesenteric lymph nodes (MLN). Here, they present the antigens to CD4-expressing T cells to initiate adaptive immune responses.
Salmonella enterica is a major cause of bacterial food-borne infections with the ability to cause a variety of diseases in a range of hosts. The various S. enterica serovars remarkably differ with regard to their host range and degree of host adaptation.Citation5S. enterica serovar (ser) Typhi (S. Typhi) is the causative agent of the systemic infection typhoid fever, and has shown to be specific for humans and primates since it fails to cause a systemic disease in mice and most other species.Citation6 On the other hand, serovars such as Enteritidis or Typhimurium result in infections as gastroenteritis with milder disease outcome, and also show broader host specificity, including humans, livestock animals, and various wild animals.Citation7 Susceptible mouse strains infected with S. Typhimurium are mainly used to investigate host immunity against S. Typhi, since systemic infections are induced with characteristics of human typhoid fever.
Salmonella is a facultative intracellular pathogen and proliferates within eukaryotic host cells, where the bacteria reside in a specialized compartment, named the Salmonella-containing vacuole (SCV) (reviewed in refs. Citation7, Citation8 and Citation9). The capability of Salmonella to survive and eventually replicate intracellular is a key virulence trait and essential for its ability to cause systemic infection (reviewed in ref. Citation10). The pathogenesis of diseases caused by Salmonella depends on the coordinated function of various sets of virulence proteins encoded by so-called Salmonella pathogenicity islands (SPI). Salmonella pathogenicity island 1 (SPI1) and Salmonella pathogenicity island 2 (SPI2) each encode a complex protein translocation machinery, termed type III secretion system (T3SS). By the delivery of sets of effector proteins into the host cell, the SPI1-T3SS and SPI2-T3SS are essential for the invasion of non-phagocytic cells, and for the establishment and continued modification of the intracellular niche, respectively (reviewed in ref. Citation10). Additionally, effector proteins of T3SS can interfere with DC functions and have the capacity to prevent activation of adaptive immune responses to ensure bacterial survival in the intracellular space and the ability to cause systemic infection.
The intracellular activities of Salmonella in macrophages have been studied in detail, leading to the identification of several virulence factors essential for intracellular survival and proliferation in macrophages. However, only limited knowledge has been obtained concerning the activities of this intracellular pathogen within DCs. This review focuses on the interface between Salmonella and DCs: the initial interaction, bacterial internalization, intracellular activities and manipulation and evasion of DC-mediated immune responses. Additionally, questions that remained open so far will be addressed.
Virulence Factors of Salmonella enterica
Host cell entry
Salmonella is a facultative intracellular organism with the ability to survive and grow in the extracellular environment as well as inside a host cell. It can thrive inside a variety of professional and non-professional phagocytes, including macrophages, DCs, neutrophils, M cells and enterocytes.Citation8 The bacterium is able to induce a unique form of phagocytosis in non-phagocytic cells, like epithelial cells, through action of SPI1-T3SS. Initiation of diverse host signal transduction cascades leads to membrane ruffling and subsequently “triggers” internalization of Salmonella by the host cell. These events are regulated by a subset of SPI1-T3SS effectors (SipA, SipC, SopB, SopD, SopE and SopE2) that induce extensive rearrangements of both the plasma membrane and the underlying actin cytoskeleton, resulting in the formation of macropinosomes.Citation11 The more conventional route of uptake via phagocytosis does not need an active contribution of the bacteria. “Professional” phagocytes, such as macrophages and DCs possess various receptors that enable them to recognize the pathogen and trigger phagocytic uptake. SPI1-T3SS-mediated invasion of phagocytes is also possible, but this entry leads to a rapid form of apoptotic cell death, termed pyroptosis.Citation12 Invasion-induced cell death was also reported for murine DCs.Citation13
Intracellular life
Internalization by various host cells is followed by the intracellular phase of Salmonella pathogenesis. Despite of the host’s anti-microbial activities, Salmonella has developed the capability to survive inside host cells, and this trait is essential for the ability to cause systemic infections. The bacteria reside in the SCV and here the action of the SPI2-T3SS plays an important role in the evasion of host immune defenses through the delivery of a second set of effector proteins from the SCV into the host cell cytoplasm.Citation7,Citation14 Using these effector proteins, Salmonella actively directs the biogenesis of the SCV in order to segregate from the endosomal system, avoiding phagosome-lysosome fusion and degradation.Citation9,Citation15,Citation16 However, early reports suggest that Salmonella can survive within macrophages even after lysosomal compartments have fused with the SCV,Citation17,Citation18 and indicate that avoidance of phagolysosomal fusion is unlikely to be a major pathogenic strategy of Salmonella. Moreover, SPI2 function was found to allow Salmonella to survive exposure to lysosomal contents by inhibiting the delivery of reactive oxygen species (ROS) and reactive nitrogen species (RNS) generating activities to the SCV.Citation19-Citation21 Thereby Salmonella is able to avoid ROS- and RNS-dependent killing.
The SCV is maintained throughout intracellular life. During this lifetime, the SCV undergoes rapid modifications. The inchoate/early SCV is enriched in early endosome membrane markers, like EEA1, Rab5 and transferrin receptor,Citation22 whereas in the intermediate stage, these markers are replaced with late endosomal/lysosomal markers, including vacuolar H+ ATPase (vATPase) and lysosomal membrane glycoproteins (lgp) such as LAMPs. The transition between the early and the intermediate stage is accompanied by a decrease in the luminal pH to < 4.5, due to the activity of the vATPase.Citation15 The late stage and the intermediate stage can be distinguished by the formation of the characteristic, lgp-rich membrane tubules, termed Salmonella-induced filaments (SIF), emanating from the SCV and extended throughout the cell (reviewed in ref. Citation23). Recent work identified further Salmonella-induced tubular membrane compartments of distinct cellular origin.Citation23 Smith et al.Citation22 have shown that two independent, concurrent pathways, regulated by Syntaxin13 and Rab11, regulate recycling of cell surface proteins from the SCV. Interaction with these pathways is essential for efficient maturation of the SCV.
Although Salmonella mostly resides within the “safe” vacuole, a low percentage of wild-type S. Typhimurium succeeds to escape from the SCV shortly after invasion.Citation24-Citation26 The SPI1- T3SS is found to be responsible for damaging the SCV shortly following invasion and although a repair mechanism was proposed where calcium is released and lysosomes are recruited,Citation27 a small proportion of Salmonella is able to escape into the cytosol.
Cytosolic bacteria are recognized by the autophagy machinery. Here, the intracellular pathogen is selectively sequestered in compartments that enrich the autophagosome-specific membrane marker LC3 (review in ref. Citation28). Fusion of LC3-rich phagosomes with lysosomes is associated with a rapid acidification of the phagosomes, mainly mediated by the vacuolar vATPase.Citation29 Lysosomal proteases, which reach optimal proteolytic activity at a pH between 5.5 and 6.5, subsequently degrade the pathogen.Citation30
Birmingham et al.Citation31 demonstrated that autophagy restricts bacterial growth in the cytosol by targeting Salmonella in damaged SCVs during infection. In addition, Wild et al.Citation32 found that upon phosphorylation of the autophagy receptor optineurin, autophagy clearance of cytosolic Salmonella is promoted. This reveals a role for the autophagy machinery in the host immune responses against Salmonella.
Routes of Infection by Salmonella
Penetration of intestinal barriers
Several different routes are known for Salmonella to cross the intestinal barrier (). Salmonella is an invasive pathogen and the SPI1-T3SS triggers the uptake by non-phagocytic enterocytes. While the role of the SPI1-T3SS and its effector proteins in invasion have been impressively demonstrated by cell culture models, the role of enterocyte invasion during infection of host organisms is less clear. First, mutant strains deficient in SPI1-T3SS show only minor defects in eliciting typhoid fever-like diseases in a murine model, indicating alternative routes of entry. Second, a role of the SPI1-T3SS in eliciting intestinal inflammation has been reported that mainly affects the competing intestinal flora and promotes Salmonella growth in the intestine.Citation33
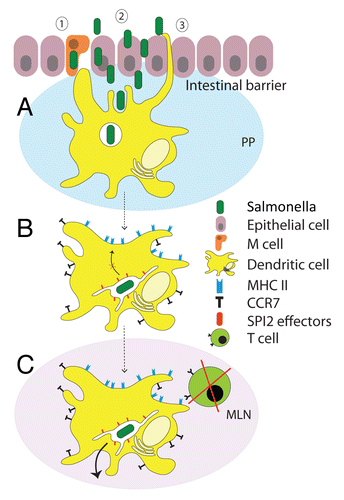
Figure 2. Interference of Salmonella with DCs functions. (A) Salmonella breaches the intestinal barrier using different mechanisms. The epithelium covering the Peyer’s Patches (PP) can be overcome through (1) uptake via M cells in PP, (2) invasion of enterocytes of the intestinal epithelium and (3) capture by DCs that sample antigens in the intestinal lumen with their dendrites which are extended through the epithelial cell layer. Once Salmonella has overcome the intestinal barrier, it encounters DCs, which internalize the bacteria through phagocytosis. (B) Upon phagocytosis, Salmonella induces the upregulation of CCR7 receptors on the DC surface, resulting in DC migration from PP to secondary lymphoid tissues such as mesenteric lymph nodes (MLN) and spleen, which both contain a high concentration of chemokines CCL19 and CCL21. At the same time, by means of specific effector proteins injected through the SPI2-encoded T3SS, Salmonella rapidly modifies the phagosome to form a unique Salmonella-containing vacuole (SCV) to segregate itself from the endolysosomal system, and so prevents bacterial degradation by the host cell. Additionally, by means of the SPI2-T3SS, Salmonella inhibits the presentation of antigens on the DC surface. (C) Arrived at MLN, infected DCs are reduced or unable in stimulation of T cells since they lack the presentation of antigens on the surface. Additionally, Salmonella disseminates into secondary lymphoid tissues, suggesting a role for DCs as vehicles exploited by Salmonella for systemic dissemination.
As various other pathogens, Salmonella is able to penetrate the intestinal epithelium via M cells.Citation34 M cells are a cell population residing in the follicle-associated epithelium (FAE) overlaying Peyer’s patches (PP), which sample antigens of the intestinal content and its normal flora. The M cells help transport the bacteria across the epithelial barrier, via transcytosis, into the subepithelial dome (SED), where they are delivered to the underlying lymphoid cells such as DCs in the lamina propria (LP) or Peyer’s patches (PP).
Moreover, Salmonella can be captured directly from the intestinal lumen by DCs. These DCs, located in the LP or the PP, sample intestinal antigens with their dendrites extending through the epithelial monolayers and interact with the intestinal microbiota in a CX3CR1-dependent process.Citation35 Yet, this DC subset has been recently found to be a non-migratory, gut resident populationCitation36 and is therefore very unlikely to participate in bacterial dissemination beyond the mucosa. However, the subset might serve as a first line barrier against invading pathogens by modulating immune responses directly in the mucosa.
DCs as “Trojan horses” for pathogen dissemination
Once Salmonella has overcome the intestinal barrier and is captured by DCs in PP or LP, DCs have to interact and trigger the activation of specific T lymphocytes. It was shown by Allenspach et al.Citation37 that antigen processing and presentation by both migratory and lymphoid-resident DCs is essential for the antigen-specific activation of T cells and to subsequently initiate adaptive immune responses. PP-resident CCR6+ DCs, recruited into the dome upon invasion of the FAE by pathogens, have been found to be responsible for rapid local activation of pathogen specific T cells.Citation38 This serves as a requisite step in T cell activation, since its function is to prime the T cells for subsequent interactions with migratory DCs.Citation37 CCR6+ DCs are not found in the LP.
Migratory DCs, on the other hand, do not induce local T cell activation. Upon pathogen recognition by pattern recognition receptors (PRRs), migratory DCs are activated to increase the expression of the CCR7, a receptor for the chemokines CCL19 and CCL21.Citation39 Up- and downregulation of this receptor enables DC migration along chemotactic gradients from the site of infection to lymphoid tissues such as lymph nodes and spleen.Citation40 Once in the lymph node, DCs that captured antigen will induce T cell differentiation by presenting pathogen-derived antigens and secreting cytokines, but this generic DC function is affected if virulent Salmonella is phagocytosed.
Salmonella-infected DCs from intestine, both LP- and PP-derived, migrate via afferent lymphatics to mesenteric lymph nodes (MLN) from where Salmonella can further disseminate to various organs. The route and vehicles for systemic spread are not fully understood. In order to extend the infection beyond the intestinal mucosa, Salmonella has to possess various factors allowing not only survival within macrophages, but also interference with the immunostimulatory capacity of DCs. This trait was found to be regulated by the multi-factorial PhoPQ regulatory system, which is responsible for the expression of proteins required for virulence and macrophage survival.Citation41 In this regard, the phoP locus has been shown to influence the processing of bacterial antigens by activated DCs.Citation42
However, besides specific protection strategies to ensure bacterial survival, Salmonella needs to have transport for its dissemination. In this regard, Cheminay et al.Citation40 discovered that Salmonella may exploit DC migration by showing that Salmonella induced upregulation of CCR7 expression, results in migration of DCs toward increased CCL19 and CCL211 concentrations in secondary lymphoid tissues. However, this did not require viable nor internalized Salmonella. Additionally, the bacteria co-localized with only one third of the recruited DCs.Citation40 These data combined with the discovery that intracellular Salmonella is able to interfere with antigen presentation by DCs (see below), strongly implies that Salmonella uses DCs as camouflaged vehicles for its dissemination, in other words, as “Trojan horses” for systemic dissemination ().
Recent research reveals a novel mechanism by which intracellular Salmonella interferes with host cell migration of phagocytes itself to evade bacterial clearance, whereby it is able to maintain a long-term chronic systemic infection in mice.Citation43 In this, the SPI2-T3SS effector protein SseI (alternative designation SrfH) plays two distinct roles: (1) regulating cell adherence during early stages of infection, causing early escape of Salmonella from the GI tract,Citation44 and (2) specific binding of the cell migration regulator, IQGAP1, during later stages, thereby blocking directed macrophage migration.Citation43 Interference with DC migration by SseI was found to correlate with reduced capacity of the host to clear Salmonella from systemic sites of infection.Citation43
Not only Salmonella, but also Mycobacterium tuberculosis,Citation45 pathogens associated with ileal Crohn disease and even viruses like HIVCitation46 use specialized mechanisms to interfere with, or exploit host cell migration to prevent the initiation of adaptive immune responses and to further their dissemination. In Crohn tissue, an increased recruitment and accumulation of immature CD83+ DCs was observed in SED after bacterial internalization.Citation47 The pathogen is able to downregulate CCR7 expression, confiscating the DC ability to migrate to secondary lymph node tissue, and thus prevent T cell stimulation. In the case of HIV-1, the virus exploits DC migration to aid the establishment and dissemination of infection by binding DC-SIGN on the surface of immature DCs via the glycoprotein gp120, and hence initiates maturation.Citation46 In this way, DCs carry HIV-1 to the T cell compartment in lymphoid tissues and promotes trans-infection of T cells in the absence of viral replication in the DCs themselves.
So, although DCs play an important role in the fight against pathogenic infections by initiating adaptive immunity, pathogens themselves, including Salmonella, pathogens causing Crohn disease and HIV-1, have developed mechanisms which enable interference with, or exploitation of DC migration. This way, pathogens are able to facilitate dissemination within the host and circumvent immune responses, through inhibition of T cell stimulation.
The Intracellular Habitat of Salmonella in DCs
Previous studies have indicated that the intracellular fate of Salmonella in DCs differs from that in macrophages.Citation48-Citation50 Studies using a DC-like cell line indicated that the pathogen-containing vacuole in DCs lacks the late endosomal/lysosomal membrane marker LAMP1;Citation48 however, work with murine bone-marrow derived DCs indicated LAMP1-positive SCV.Citation50
Compared with macrophages where the pH 5.0 is reached shortly after phagocytosis, phagosome acidification in DCs is delayed (reviewed in ref. Citation30). The maintenance of a slightly alkaline pH was shown to be important to prevent destruction of potential peptides for antigen presentation and recognition by T cells.Citation51 The NADPH oxidase or NOX2 is in charge of producing oxygen radicals and controlling phagosomal pH. The absence of NOX2 in DCs led to increased antigen degradation, resulting in impaired “cross-presentation” of phagocytosed antigens to CD8+ T cells and subsequently, decreased T cell activation.Citation51
Although both the SPI2-T3SS and PhoP/Q regulatory system are important for intracellular survival and replication in macrophages, both virulence factors are not essential for entry and survival of Salmonella in DCs.Citation49,Citation50 A further disparity between fates of Salmonella within macrophages and DCs is the intracellular proliferation. Inside murine macrophage cell line cells, replication initiates after an initial lag period of 3–4 h after internalization. The function of the SPI2-T3SS and its translocated effector proteins is required for intracellular replication. In contrast, in murine bone-marrow derived DCs, Salmonella is able to survive, but does not replicate.Citation50 Although the SPI2-T3SS is not essential for Salmonella survival in DCs, it does influence the maturation of the SCV.Citation50 This indicates that Salmonella is able to modify normal cellular processes in DCs. In search of factors required for the survival in DCs, Zenk et al.Citation52 observed that de novo protein biosynthesis by Salmonella inside DCs is not required, but that the O-antigen of the LPS appears be to a key factor for bacterial survival.
Interference of Salmonella with DC Functions
The capability of Salmonella to cause systemic diseases relies on the ability to survive and replicate inside the host cell.Citation53,Citation54 In addition, to achieve prolonged systemic persistence, Salmonella has developed mechanisms to alter normal host-cell functions by which it can evade host immune responses and subsequently avoid degradation. The SPI2-T3SS is activated upon host-cell invasion, and besides the modification and maintenance of the SCV, it has a key role in the escape of anti-microbial activities in macrophages.
Bueno et al.Citation55 have shown that, besides inducing phagocytosis in non-phagocytic cells, the SPI-1 is able to control the number of bacteria that enters DCs. Internalization via FcγRs receptors, accomplished by coating bacteria with Salmonella-specific IgG, has been shown to strongly enhance the efficiency of Ag uptake.Citation56 Here, DCs use a novel, PI3K/actin cytoskeleton/dynamin/Fcγ-receptor-independent mechanism to engulf IgG-coated Salmonella that remains yet to be elucidated.Citation57
Communication between host cells at the side of bacterial infection and cells that have to be recruited from the circulation is essential to combat pathogens. In response to Salmonella infection DCs produce cytokines which serve as messengers to activate resting immune cells, like natural killer (NK) cells, granulocytes, macrophages and T cells.Citation58,Citation59 Uchiya et al.Citation60 have shown three different, SPI2-dependent mechanisms for Salmonella to interfere with cytokine signaling, leading to the deactivation of macrophages: (1) through upregulation of interleukins, (2) through induction of the extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathway, leading to the expression of cyclooxygenase (COX) 2, and subsequently resulting in an increase in PGE2 and PGI2 production by macrophagesCitation61 and (3) through upregulation of SOCS-3, a protein from the suppressor of cytokine signaling (SOCS) family, which negatively regulate the Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathway, and thereby inhibit cytokine signaling.Citation62 These observations were made for interaction of Salmonella with macrophages, and it has to be shown if similar interference occurs for Salmonella-DC interaction.
The most studied evasion strategy of Salmonella is its ability to avoid initiation of adaptive immunity. In vitro experiments have shown that Salmonella is able to inhibit T-cell activation by interfering with the presentation of antigens on the DC surface. Since DCs are important APC linking innate and adaptive immunity, interfering with their capacity to stimulate naïve T cells may allow pathogens evading adaptive immunity. Such interference would promote pathogen survival and dissemination, i.e., crucial events in Salmonella pathogenesis. Cheminay et al.Citation63 showed that intracellular Salmonella causes, in a SPI2-dependent manner, the alteration of MHC-II-dependent antigen-presentation by DCs. Additionally, the SPI2-T3SS in combination with the induced production of NO synthase by DCs, was found to suppress Ag-dependent T cell proliferation. The suggested mechanism for the escape of Ag presentation is by the inhibition of lysosomal degradation;Citation56,Citation64 however, the exact mechanism remains unknown. The PhoP/PhoQ regulatory system, which controls the synthesis of many Salmonella proteins required for virulence and survival,Citation41 appears to play an important role in this escape mechanism, since Salmonella strains with mutations at the phoP/Q locus have been shown to fail to escape from lysososmal degradation and subsequently Ag processing and presentation.Citation49 Inhibition of lysososmal degradation can be overcome by, again, targeting Salmonella to FcγRs receptors on the DC surface.Citation56 Here, the entry route of Salmonella into the host cell appears to affect the initial phase of maturation of the SCV and its ability to avoid lysosomal degradation (). Subsequent work identified a subset of SPI2-T3SS effectors that are required for the inhibition of MHC-II-dependent antigen presentation in DCs.Citation65 The effector proteins SifA, SspH2, SlrP, PipB2 and SopD2 showed strong and SseF and SseG moderate contribution to suppression of Ag-dependent T-cell stimulation by Salmonella-infected DCs. Additionally, Salmonella can control the expression of MHC II on the DC surface through polyubiquitination, which subsequently may reduce the ability of DCs to present antigen to CD4 T cells.Citation66
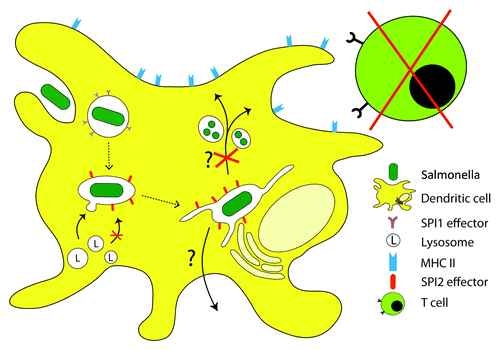
Figure 3. Manipulation of DC functions by intracellular Salmonella. Upon internalization into DCs, Salmonella remains in a membrane-bound compartment or SCV. The subsequent events of SCV biogenesis in DCs are not fully understood; however, fusion of lysosomal compartments with the SCV and killing of the bacterial is delayed or blocked. By functions of SPI2-T3SS effector proteins, intracellular Salmonella interferes with antigen processing and presentation. This in turn leads to a reduced stimulation of T cells and adaptive immune defense against Salmonella. Bacterial manipulation of DC function is an important factor for system spread and persistence of Salmonella. The exact mechanisms for the inhibition of Ag presentation, possible bacterial escape from the DCs, and the subsequent dissemination remain to be elucidated.
The impairment of DC functions by the activity of SPI2 gene products is crucial for Salmonella pathogenesis. However, the ability of Salmonella serovars to survive within DCs is host specific and is characterized by their capacity to interfere with the function of DCs and avoid host adaptive immune responses.Citation54 So is S. enterica ser Typhi specific for humans and primates and fails to cause systemic infection in mice and most other species, whereas S. enterica ser Typhimurium and Enteritidis have a broader host specificity and different disease outcome. Based on previous studies, the host’s immune response was suggested to be a key component in Salmonella host restriction.Citation54,Citation67 These observations suggest a role for DCs as cellular components influencing pathogen-host specificity. However, the exact molecular reasons for these differences are only partially understood and are probably multi-factorial.
Conclusions and Future Perspectives
DCs have the unique ability to link innate and adaptive immunity, which makes them attractive targets for manipulation by intracellular pathogens like Salmonella. Alteration of host cell functions allows Salmonella to evade immune responses, survive in the intracellular space and subsequently disseminate from the site of infection to internal tissues to cause systemic infections ().
Over the last two decades, several virulence mechanisms involved in the interaction between Salmonella and its host cells have been identified. Since most of the studies have been focused on the interaction with macrophages and epithelial cells, only limited information is available on the exact interface between Salmonella and DCs. The SCV within DCs, for example, has been reported to be unique compartment distinct from that in macrophages, since it lacks specific membrane markersCitation48 and does neither allow intracellular proliferation nor efficient killing of Salmonella.Citation50,Citation52 However, other phenotypes such as the induction of dynamic tubular membrane compartments appear shared in macrophages and DCs.Citation68 However, it remains unclear why the intracellular compartment of Salmonella in DCs differs from that in macrophages. Is there a specific set of Salmonella virulence factors for the modification of the SCV in DCs? How does Salmonella benefit from this difference? Despite the limited data available so far, in vitro experiments have revealed that the ability to interfere with the immune stimulatory capacity of DCs is most likely from high significance for Salmonella to be able to cause systemic disease in the host. By inhibiting processing and presentation of bacterial Ag on the DC surface, T cell activation and thus the initiation of adaptive immunity is prevented. Additionally this could allow Salmonella to exploit DCs as reservoirs and vehicles, for its dissemination to secondary lymphoid tissues and beyond. Nevertheless, numerous aspects remain unclear even in this regard (): What is the exact mechanism for interference with Ag presentation and how does Salmonella eventually escape from the DCs?
However, in contrast to in vitro experiments, in vivo studies have documented robust CD4 T cell activation within a few hours of oral Salmonella infection. Recent work has demonstrated that Salmonella is able to actively induce apoptosis of Ag-specific CD4 T cells in a SPI2-dependent manner.Citation69 This suggests that the inhibitory effect of SPI2 effectors may inhibit survival of Salmonella-specific T cells, rather than inhibit initial activation, in vivo. While a difference between in vitro and in vivo findings complicates the picture somewhat, it still supports the overall idea that SPI2 effectors are actively involved in the inhibition of T cell responses to Salmonella. Nevertheless, this emphasizes the importance of in vivo experiments to complement in vitro experiments. Additionally, host specificity of Salmonella is a complex phenomenon that remains yet unclear. Despite the fact that S. enterica subspecies share more than 90% for their genomes, these bacteria show differential abilities to survive and proliferate within DCs in line with their ability to avoid initiation of adaptive immunity. However, the genetic differences or other molecular reasons that could account for these variations are only partially understood and are most likely multi-factorial.
A major limitation for the research of Salmonella-DC interaction is the lack of an immortalized cell line that exhibits DC functions, and the heterogeneity of DCs obtained by differentiation from primary precursor cells. Furthermore, the correlation of in vitro studies for Salmonella-DC interaction to in vivo studies is difficult. A main obstacle is the identification and tracking of Salmonella-infected DCs in the setting of an animal infection. However, a number of recent studies in this directionCitation35,Citation70,Citation71 indicate that new imaging techniques and use of transgenic animals may enable to track Salmonella-DC interaction in vivo with high temporal and spatial resolution.
Besides being an attractive target for intracellular pathogens, DCs are being considered as valuable targets in the design of vaccines.Citation72 For example, by enhancing immunity using live attenuated vaccines based on Salmonella mutant strains, Salmonella infections can be prevented and treated.Citation73 Since these mutant strains would be unable to induce an inflammatory reaction, survive within macrophages and interfere with host cell functions, but would still be taken up by DCs in the PP, processing and presentation of Salmonella-derived antigens would occur more efficiently, even as the stimulation of naïve T cells. On the other hand, the targeting of genetically engineered microbial proteins to DCs would be an alternative strategy to boost immunogenesis.Citation72 Here, vaccine antigens would be directly delivered to specific receptors on the DC surface, accompanied by pathogen specific DC maturation and resulting in a > 100-fold enhancement of presentation efficiency. Although both strategies seem promising for the treatment and prevention of infections, still major gaps in the knowledge exits before this science can be taken into medicine and to solve this, more in vivo, but also patient-based research, is of great importance.
Further research is required to address the open questions concerning the interaction of Salmonella with DCs. Complete understanding of the interface between Salmonella and DCs would eventually provide valuable information for the development of new strategies to prevent systemic infection caused by this pathogen.
Acknowledgments
This work was supported by grants of the Deutsche Forschungsgemeinschaft (DFG).
References
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245 - 52; http://dx.doi.org/10.1038/32588; PMID: 9521319
- Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol 2006; 6:476 - 83; http://dx.doi.org/10.1038/nri1845; PMID: 16691244
- Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol 2006; 311:17 - 58; http://dx.doi.org/10.1007/3-540-32636-7_2; PMID: 17048704
- Tam MA, Rydström A, Sundquist M, Wick MJ. Early cellular responses to Salmonella infection: dendritic cells, monocytes, and more. Immunol Rev 2008; 225:140 - 62; http://dx.doi.org/10.1111/j.1600-065X.2008.00679.x; PMID: 18837781
- Fierer J, Guiney DG. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. J Clin Invest 2001; 107:775 - 80; http://dx.doi.org/10.1172/JCI12561; PMID: 11285291
- Ohl ME, Miller SI. Salmonella: a model for bacterial pathogenesis. Annu Rev Med 2001; 52:259 - 74; http://dx.doi.org/10.1146/annurev.med.52.1.259; PMID: 11160778
- Jantsch J, Chikkaballi D, Hensel M. Cellular aspects of immunity to intracellular Salmonella enterica. Immunol Rev 2011; 240:185 - 95; http://dx.doi.org/10.1111/j.1600-065X.2010.00981.x; PMID: 21349094
- Malik-Kale P, Jolly CE, Lathrop S, Winfree S, Luterbach C, Steele-Mortimer O. Salmonella - at home in the host cell. Front Microbiol 2011; 2:125; http://dx.doi.org/10.3389/fmicb.2011.00125; PMID: 21687432
- Bakowski MA, Braun V, Brumell JH. Salmonella-containing vacuoles: directing traffic and nesting to grow. Traffic 2008; 9:2022 - 31; http://dx.doi.org/10.1111/j.1600-0854.2008.00827.x; PMID: 18778407
- Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol 2008; 6:53 - 66; http://dx.doi.org/10.1038/nrmicro1788; PMID: 18026123
- Schlumberger MC, Hardt WD. Salmonella type III secretion effectors: pulling the host cell’s strings. Curr Opin Microbiol 2006; 9:46 - 54; http://dx.doi.org/10.1016/j.mib.2005.12.006; PMID: 16406778
- Fink SL, Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol 2007; 9:2562 - 70; http://dx.doi.org/10.1111/j.1462-5822.2007.01036.x; PMID: 17714514
- van der Velden AW, Velasquez M, Starnbach MN. Salmonella rapidly kill dendritic cells via a caspase-1-dependent mechanism. J Immunol 2003; 171:6742 - 9; PMID: 14662878
- Figueira R, Holden DW. Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology 2012; 158:1147 - 61; http://dx.doi.org/10.1099/mic.0.058115-0; PMID: 22422755
- Steele-Mortimer O. The Salmonella-containing vacuole: moving with the times. Curr Opin Microbiol 2008; 11:38 - 45; http://dx.doi.org/10.1016/j.mib.2008.01.002; PMID: 18304858
- Ramsden AE, Holden DW, Mota LJ. Membrane dynamics and spatial distribution of Salmonella-containing vacuoles. Trends Microbiol 2007; 15:516 - 24; http://dx.doi.org/10.1016/j.tim.2007.10.002; PMID: 17983751
- Ishibashi Y, Arai T. Salmonella typhi does not inhibit phagosome-lysosome fusion in human monocyte-derived macrophages. FEMS Immunol Med Microbiol 1995; 12:55 - 61; http://dx.doi.org/10.1111/j.1574-695X.1995.tb00175.x; PMID: 8580903
- Oh YK, Alpuche-Aranda C, Berthiaume E, Jinks T, Miller SI, Swanson JA. Rapid and complete fusion of macrophage lysosomes with phagosomes containing Salmonella typhimurium. Infect Immun 1996; 64:3877 - 83; PMID: 8751942
- Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM, Dinauer MC, et al. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 2000; 287:1655 - 8; http://dx.doi.org/10.1126/science.287.5458.1655; PMID: 10698741
- Vázquez-Torres A, Fantuzzi G, Edwards CK 3rd, Dinarello CA, Fang FC. Defective localization of the NADPH phagocyte oxidase to Salmonella-containing phagosomes in tumor necrosis factor p55 receptor-deficient macrophages. Proc Natl Acad Sci U S A 2001; 98:2561 - 5; http://dx.doi.org/10.1073/pnas.041618998; PMID: 11226278
- Chakravortty D, Hansen-Wester I, Hensel M. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J Exp Med 2002; 195:1155 - 66; http://dx.doi.org/10.1084/jem.20011547; PMID: 11994420
- Smith AC, Cirulis JT, Casanova JE, Scidmore MA, Brumell JH. Interaction of the Salmonella-containing vacuole with the endocytic recycling system. J Biol Chem 2005; 280:24634 - 41; http://dx.doi.org/10.1074/jbc.M500358200; PMID: 15886200
- Schroeder N, Mota LJ, Méresse S. Salmonella-induced tubular networks. Trends Microbiol 2011; 19:268 - 77; http://dx.doi.org/10.1016/j.tim.2011.01.006; PMID: 21353564
- Beuzón CR, Méresse S, Unsworth KE, Ruíz-Albert J, Garvis S, Waterman SR, et al. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J 2000; 19:3235 - 49; http://dx.doi.org/10.1093/emboj/19.13.3235; PMID: 10880437
- Brumell JH, Tang P, Zaharik ML, Finlay BB. Disruption of the Salmonella-containing vacuole leads to increased replication of Salmonella enterica serovar typhimurium in the cytosol of epithelial cells. Infect Immun 2002; 70:3264 - 70; http://dx.doi.org/10.1128/IAI.70.6.3264-3270.2002; PMID: 12011022
- Perrin AJ, Jiang X, Birmingham CL, So NS, Brumell JH. Recognition of bacteria in the cytosol of Mammalian cells by the ubiquitin system. Curr Biol 2004; 14:806 - 11; http://dx.doi.org/10.1016/j.cub.2004.04.033; PMID: 15120074
- Roy D, Liston DR, Idone VJ, Di A, Nelson DJ, Pujol C, et al. A process for controlling intracellular bacterial infections induced by membrane injury. Science 2004; 304:1515 - 8; http://dx.doi.org/10.1126/science.1098371; PMID: 15178804
- Deretic V. Autophagy in immunity and cell-autonomous defense against intracellular microbes. Immunol Rev 2011; 240:92 - 104; http://dx.doi.org/10.1111/j.1600-065X.2010.00995.x; PMID: 21349088
- Mijaljica D, Nazarko TY, Brumell JH, Huang WP, Komatsu M, Prescott M, et al. Receptor protein complexes are in control of autophagy. Autophagy 2012; 8:1701 - 5; http://dx.doi.org/10.4161/auto.21332; PMID: 22874568
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol 2005; 23:975 - 1028; http://dx.doi.org/10.1146/annurev.immunol.22.012703.104538; PMID: 15771591
- Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem 2006; 281:11374 - 83; http://dx.doi.org/10.1074/jbc.M509157200; PMID: 16495224
- Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 2011; 333:228 - 33; http://dx.doi.org/10.1126/science.1205405; PMID: 21617041
- Kaiser P, Diard M, Stecher B, Hardt WD. The streptomycin mouse model for Salmonella diarrhea: functional analysis of the microbiota, the pathogen’s virulence factors, and the host’s mucosal immune response. Immunol Rev 2012; 245:56 - 83; http://dx.doi.org/10.1111/j.1600-065X.2011.01070.x; PMID: 22168414
- Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med 1994; 180:15 - 23; http://dx.doi.org/10.1084/jem.180.1.15; PMID: 8006579
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005; 307:254 - 8; http://dx.doi.org/10.1126/science.1102901; PMID: 15653504
- Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 2009; 206:3101 - 14; http://dx.doi.org/10.1084/jem.20091925; PMID: 20008524
- Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity 2008; 29:795 - 806; http://dx.doi.org/10.1016/j.immuni.2008.08.013; PMID: 18951047
- Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, Maxwell JR, et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer’s patches. Immunity 2006; 24:623 - 32; http://dx.doi.org/10.1016/j.immuni.2006.02.015; PMID: 16713979
- Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol 2007; 25:787 - 820; http://dx.doi.org/10.1146/annurev.immunol.24.021605.090529; PMID: 17291188
- Cheminay C, Schoen M, Hensel M, Wandersee-Steinhäuser A, Ritter U, Körner H, et al. Migration of Salmonella typhimurium --harboring bone marrow--derived dendritic cells towards the chemokines CCL19 and CCL21. Microb Pathog 2002; 32:207 - 18; http://dx.doi.org/10.1006/mpat.2002.0497; PMID: 12071677
- Kato A, Groisman EA, Howard Hughes Medical Institute. The PhoQ/PhoP regulatory network of Salmonella enterica. Adv Exp Med Biol 2008; 631:7 - 21; http://dx.doi.org/10.1007/978-0-387-78885-2_2; PMID: 18792679
- Wick MJ, Harding CV, Twesten NJ, Normark SJ, Pfeifer JD. The phoP locus influences processing and presentation of Salmonella typhimurium antigens by activated macrophages. Mol Microbiol 1995; 16:465 - 76; http://dx.doi.org/10.1111/j.1365-2958.1995.tb02411.x; PMID: 7565107
- McLaughlin LM, Govoni GR, Gerke C, Gopinath S, Peng K, Laidlaw G, et al. The Salmonella SPI2 effector SseI mediates long-term systemic infection by modulating host cell migration. PLoS Pathog 2009; 5:e1000671; http://dx.doi.org/10.1371/journal.ppat.1000671; PMID: 19956712
- Worley MJ, Nieman GS, Geddes K, Heffron F. Salmonella typhimurium disseminates within its host by manipulating the motility of infected cells. Proc Natl Acad Sci U S A 2006; 103:17915 - 20; http://dx.doi.org/10.1073/pnas.0604054103; PMID: 17095609
- Humphreys IR, Stewart GR, Turner DJ, Patel J, Karamanou D, Snelgrove RJ, et al. A role for dendritic cells in the dissemination of mycobacterial infection. Microbes Infect 2006; 8:1339 - 46; http://dx.doi.org/10.1016/j.micinf.2005.12.023; PMID: 16697232
- van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol 2003; 3:697 - 709; http://dx.doi.org/10.1038/nri1182; PMID: 12949494
- Salim SY, Silva MA, Keita AV, Larsson M, Andersson P, Magnusson KE, et al. CD83+CCR7- dendritic cells accumulate in the subepithelial dome and internalize translocated Escherichia coli HB101 in the Peyer’s patches of ileal Crohn’s disease. Am J Pathol 2009; 174:82 - 90; http://dx.doi.org/10.2353/ajpath.2009.080273; PMID: 19095953
- García-Del Portillo F, Jungnitz H, Rohde M, Guzmán CA. Interaction of Salmonella enterica serotype Typhimurium with dendritic cells is defined by targeting to compartments lacking lysosomal membrane glycoproteins. Infect Immun 2000; 68:2985 - 91; http://dx.doi.org/10.1128/IAI.68.5.2985-2991.2000; PMID: 10768999
- Niedergang F, Sirard JC, Blanc CT, Kraehenbuhl JP. Entry and survival of Salmonella typhimurium in dendritic cells and presentation of recombinant antigens do not require macrophage-specific virulence factors. Proc Natl Acad Sci U S A 2000; 97:14650 - 5; http://dx.doi.org/10.1073/pnas.97.26.14650; PMID: 11121065
- Jantsch J, Cheminay C, Chakravortty D, Lindig T, Hein J, Hensel M. Intracellular activities of Salmonella enterica in murine dendritic cells. Cell Microbiol 2003; 5:933 - 45; http://dx.doi.org/10.1046/j.1462-5822.2003.00334.x; PMID: 14641178
- Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 2006; 126:205 - 18; http://dx.doi.org/10.1016/j.cell.2006.05.035; PMID: 16839887
- Zenk SF, Jantsch J, Hensel M. Role of Salmonella enterica lipopolysaccharide in activation of dendritic cell functions and bacterial containment. J Immunol 2009; 183:2697 - 707; http://dx.doi.org/10.4049/jimmunol.0900937; PMID: 19625639
- Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A 1986; 83:5189 - 93; http://dx.doi.org/10.1073/pnas.83.14.5189; PMID: 3523484
- Bueno SM, González PA, Carreño LJ, Tobar JA, Mora GC, Pereda CJ, et al. The capacity of Salmonella to survive inside dendritic cells and prevent antigen presentation to T cells is host specific. Immunology 2008; 124:522 - 33; http://dx.doi.org/10.1111/j.1365-2567.2008.02805.x; PMID: 18266715
- Bueno SM, Wozniak A, Leiva ED, Riquelme SA, Carreño LJ, Hardt WD, et al. Salmonella pathogenicity island 1 differentially modulates bacterial entry to dendritic and non-phagocytic cells. Immunology 2010; 130:273 - 87; http://dx.doi.org/10.1111/j.1365-2567.2009.03233.x; PMID: 20201987
- Tobar JA, González PA, Kalergis AM. Salmonella escape from antigen presentation can be overcome by targeting bacteria to Fc gamma receptors on dendritic cells. J Immunol 2004; 173:4058 - 65; PMID: 15356155
- Riquelme SA, Bueno SM, Kalergis AM. IgG keeps virulent Salmonella from evading dendritic cell uptake. Immunology 2012; 136:291 - 305; http://dx.doi.org/10.1111/j.1365-2567.2012.03578.x; PMID: 22352313
- Marriott I, Hammond TG, Thomas EK, Bost KL. Salmonella efficiently enter and survive within cultured CD11c+ dendritic cells initiating cytokine expression. Eur J Immunol 1999; 29:1107 - 15; http://dx.doi.org/10.1002/(SICI)1521-4141(199904)29:04<1107::AID-IMMU1107>3.0.CO;2-0; PMID: 10229077
- Yrlid U, Wick MJ. Antigen presentation capacity and cytokine production by murine splenic dendritic cell subsets upon Salmonella encounter. J Immunol 2002; 169:108 - 16; PMID: 12077235
- Uchiya K, Groisman EA, Nikai T. Involvement of Salmonella pathogenicity island 2 in the up-regulation of interleukin-10 expression in macrophages: role of protein kinase A signal pathway. Infect Immun 2004; 72:1964 - 73; http://dx.doi.org/10.1128/IAI.72.4.1964-1973.2004; PMID: 15039316
- Uchiya K, Nikai T. Salmonella enterica serovar Typhimurium infection induces cyclooxygenase 2 expression in macrophages: involvement of Salmonella pathogenicity island 2. Infect Immun 2004; 72:6860 - 9; http://dx.doi.org/10.1128/IAI.72.12.6860-6869.2004; PMID: 15557607
- Uchiya K, Nikai T. Salmonella pathogenicity island 2-dependent expression of suppressor of cytokine signaling 3 in macrophages. Infect Immun 2005; 73:5587 - 94; http://dx.doi.org/10.1128/IAI.73.9.5587-5594.2005; PMID: 16113275
- Cheminay C, Möhlenbrink A, Hensel M. Intracellular Salmonella inhibit antigen presentation by dendritic cells. J Immunol 2005; 174:2892 - 9; PMID: 15728500
- Tobar JA, Carreño LJ, Bueno SM, González PA, Mora JE, Quezada SA, et al. Virulent Salmonella enterica serovar typhimurium evades adaptive immunity by preventing dendritic cells from activating T cells. Infect Immun 2006; 74:6438 - 48; http://dx.doi.org/10.1128/IAI.00063-06; PMID: 17057096
- Halici S, Zenk SF, Jantsch J, Hensel M. Functional analysis of the Salmonella pathogenicity island 2-mediated inhibition of antigen presentation in dendritic cells. Infect Immun 2008; 76:4924 - 33; http://dx.doi.org/10.1128/IAI.00531-08; PMID: 18765734
- Lapaque N, Hutchinson JL, Jones DC, Méresse S, Holden DW, Trowsdale J, et al. Salmonella regulates polyubiquitination and surface expression of MHC class II antigens. Proc Natl Acad Sci U S A 2009; 106:14052 - 7; http://dx.doi.org/10.1073/pnas.0906735106; PMID: 19666567
- Raffatellu M, Chessa D, Wilson RP, Tükel C, Akçelik M, Bäumler AJ. Capsule-mediated immune evasion: a new hypothesis explaining aspects of typhoid fever pathogenesis. Infect Immun 2006; 74:19 - 27; http://dx.doi.org/10.1128/IAI.74.1.19-27.2006; PMID: 16368953
- Rajashekar R, Liebl D, Seitz A, Hensel M. Dynamic remodeling of the endosomal system during formation of Salmonella-induced filaments by intracellular Salmonella enterica. Traffic 2008; 9:2100 - 16; http://dx.doi.org/10.1111/j.1600-0854.2008.00821.x; PMID: 18817527
- Srinivasan A, Nanton M, Griffin A, McSorley SJ. Culling of activated CD4 T cells during typhoid is driven by Salmonella virulence genes. J Immunol 2009; 182:7838 - 45; http://dx.doi.org/10.4049/jimmunol.0900382; PMID: 19494308
- Hapfelmeier S, Müller AJ, Stecher B, Kaiser P, Barthel M, Endt K, et al. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J Exp Med 2008; 205:437 - 50; http://dx.doi.org/10.1084/jem.20070633; PMID: 18268033
- Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med 2006; 203:2841 - 52; http://dx.doi.org/10.1084/jem.20061884; PMID: 17145958
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007; 449:419 - 26; http://dx.doi.org/10.1038/nature06175; PMID: 17898760
- Biedzka-Sarek M, El Skurnik M. How to outwit the enemy: dendritic cells face Salmonella. APMIS 2006; 114:589 - 600; http://dx.doi.org/10.1111/j.1600-0463.2006.apm_465.x; PMID: 16948811