Abstract
Non-mammalian models have been used to investigate fungal virulence. In this work we have explored the use of Galleria mellonella as an infection model for the pathogenic dimorphic fungi Histoplasma capsulatum and Paracoccidioides lutzii. In mammalian models these fungi cause similar infections, and disease outcomes are influenced by the quantity of the infective inocula. We describe a similar aspect in a G. mellonella model and characterize the pathogenesis features in this system. Infection with P. lutzii or H. capsulatum, in all inoculum used, killed larvae at 25 and 37°C. However, there was a lack of correlation between the number of yeast cells used for infection and the time to larvae death, which may indicate that the fungi induce protective responses in a dynamic manner as the lowest concentrations of fungi induced the most rapid death. For both fungi, the degree of larvae melanization was directly proportional to the inocula size, and this effect was visibly more apparent at 37°C. Histological evaluation of the larvae showed a correlation between the inoculum and granuloma-like formation. Our results suggest that G. mellonella is a potentially useful model to study virulence of dimorphic fungi.
Introduction
Invertebrates have increasingly been viewed as a valid model for studying the virulence of human pathogens because of the similarities in the basic innate immune systems between these non-vertebrate hosts and mammals.Citation1-Citation3 Furthermore, microbial virulence mechanisms, especially of environmental opportunistic pathogens, are likely conserved among different pathogens as these microbes often have evolved and maintained their virulence through interactions with a diverse range of environmental predators such as amoeba or nematodes.Citation4-Citation6 However, some fungi are found free-living in soil and do not require hosts for replication and survival, and it has been proposed that their virulence factors required for mammalian infection emerged as a result of selection in the soil by interactions with environmental competitors.Citation7 Popular non-vertebrate hosts for virulence studies of human pathogenic fungi include the nematode Caenorhabditis elegans,Citation8-Citation11 the fruit fly Drosophila melanogasterCitation12-Citation14 and the greater wax moth Galleria mellonella.Citation15-Citation18
Larvae of G. mellonella are inexpensive to keep, easy to manipulate and their use may reduce the need for pathogenicity testing in mammals, with a concomitant reduction in potential mammalian suffering.Citation15,Citation19 The immune response of insects such as G. mellonella is similar to that of mammals, which consists of structural and passive barriers, and generates cellular responses via hemocytes within the hemolymph. Antimicrobial peptides play a crucial role in fighting against pathogens in insects because they lack adaptative immune system.Citation20
The greater wax moth has previously been used to examine traits associated with the pathogenicity of diverse bacterial species, including wild-type and lipopolysaccharide deficient mutants of Pseudomonas aeruginosaCitation21 as well as strains of Proteus mirabilis,Citation22 Escherichia coli and Bacillus cereus.Citation21,Citation23 Moreover, G. mellonella is an effective host model to study fungal pathogenesis. For example, G. mellonella has been used to investigate the role of filamentationCitation24 and β-glucansCitation25 in Candida albicans. This is especially important as C. albicans virulence in G. mellonella has been shown to correlated with disease in mice.Citation18 G. mellonella has also been used to study the pathogenicity of Cryptococcus neoformans,Citation16 Microsporum gypseum and Trichophyton rubrum,Citation26 Aspergillus flavusCitation27 and A. fumigatus.Citation15 Moreover, C. neoformans morphological changes during infection in mice correlate with those found in G. mellonella; hence, G. mellonella has been validated as an alternative model host for the study of C. neoformans virulence and pathogenicity.Citation28
Endemic deep or systemic mycoses are common in specific geographical areas of the world. Paracoccidioidomycosis (PCM) and histoplasmosis are prevailing examples in tropical regions. Paracoccidioides brasiliensis and Paracoccidioides lutzii (formerly P. brasiliensis isolate 01 and recently designated as a separate species based on phylogenetic differencesCitation29) are thermally dimorphic fungi that cause PCM, the most prevalent systemic mycosis in several countries of Latin America, including Brazil, Argentina, Venezuela and Colombia. PCM represents the major cause of disability and death among young adult rural workers during their most productive stage of life. PCM is the tenth most prevalent fatal chronic infectious diseases in Brazil, and is the systemic mycosis with highest mortality rate in Brazil.Citation30 In fact, a survey of records from 1996–2006 shows that paracoccidiodomycosis was the main cause of death among systemic mycoses in Brazil, followed by cryptococcosis, candidiasis and histoplasmosisCitation31 Histoplasmosis is caused by the dimorphic fungus Histoplasma capsulatum. Although it is highly endemic in tropical climate zones in Central America, South America and in the Ohio and Mississippi River valleys in the USA, histoplasmosis is a global problem with more than 60 countries reporting the disease. Histoplasmosis is the most prevalent cause of fungal respiratory infections in the USA, with an estimated 500,000 individuals acquiring the fungus annually. A national survey of hospital discharge diagnoses from 2002 identified 3,370 patients hospitalized for histoplasmosis in the USA with a crude mortality rate of 8%.Citation32
In the present study, we have investigated the capacity of G. mellonella to serve as a model host to assess the virulence of P. lutzii and H. capsulatum at environmental (25°C) and physiological (37°C) temperatures. We evaluated the survival of the larvae when infected with different inocula and we verified the presence of granulomas in the tissue of the larvae by histopathology. Our results demonstrate that these fungal pathogens can cause significant disease in G. mellonella. Our findings indicate that G. mellonella can be used as a host model to study virulence of dimorphic fungal pathogens.
Results
Survival of G. mellonella after infection with P. lutzii and H. capsulatum
The dimorphic fungi, P. lutzii strain Pb01 and H. capsulatum strain G184AR, killed G. mellonella larvae at 25 and 37°C. However, there was a lack of correlation between the inocula size and the time to death. Each concentration of P. lutzii and of H. capsulatum G184AR tested significantly reduced the survival of G. mellonella compared with sham or PBS infected larvae ( and ).
Figure 1. Log-Rank plots of the survival of G. mellonella after infection with different concentrations of P. lutzii yeast cells. G. mellonella infected and incubated at (A) 25°C or (B) 37°C. Controls included uninfected larva (Sham) and larva injected with PBS. n = 40 larvae per group.
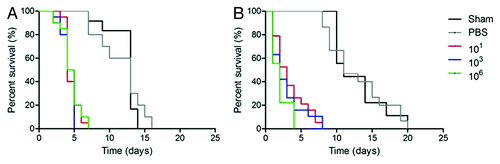
Figure 2. Log-Rank plots of the survival of G. mellonella after infection with different concentrations of H. capsulatum G184AR yeast cells. G. mellonella infected and incubated at (A) 25°C or (B) 37°C. Controls included uninfected larva (Sham) and larva injected with PBS. n = 60 larvae per group.
For P. lutzii at 25°C or 37°C, all inocula were lethal (p ˂ 0.0001 compared with PBS and Sham) with median survival of 3 and 2 d, respectively (). shows that challenges with 103 P. lutzii yeast cells resulted in the most rapid death, although this was not a significant increase relative to the other inoculums tested (p > 0.05). depict one concentration for each group (101, 103 and 106) in one representative experiment. For all groups tested at 25°C, the lowest (101 and 102), the middle (103 and 104) and the highest (105 and 106) number of yeast cells uniformly killed all larvae by day 5–7. For P. lutzii at 37°C (), infection the higher inocula groups (105 and 106) was more rapidly lethal compared in the lowest (101 and 102) and middle doses (103 and 104) (p < 0.05). At 37°C, each inocula of P. lutzii killed all the larvae by day 4–8.
Cultivation of the larvae at 37°C did not enhanced the lethality of 101, 102, 103, 104 or 105 cells of P. lutzii per larvae relative to 25°C (p > 0.05). However the inoculum of 106 at 37°C () was more lethal than at 25°C (p = 0.0001). For both temperatures, the most rapid rate of death occurred in the middle inocula groups, and the concentration 103 was more lethal at 25°C (p = 0.327). Nevertheless, the median survival time in aggregate for the infected larvae at both 25°C and 37°C was 6 d.
Similar to the infections with P. lutzii at 25°C, all concentrations of H. capsulatum G184AR killed the larvae more rapidly relative to controls (p ˂ 0.001 compared with PBS and Sham) with a median survival of 7 d (). The most rapid lethality occurred with 101 and 102 H. capsulatum and these inocula were significantly more virulent than the other challenge doses (p ˂ 0.0001). There was a trend for the highest challenges (105 and 106) to be the second most lethal. At 37°C, there were fewer differences between the infection groups (). The lower inocula groups died more slowly at 37°C compared with 25°C (p < 0.05). The higher temperature was significantly more lethal at the inoculum of 103 (p = 0.0014) when compared with incubation at 25°C. show the results for one concentration of inoculum for each group (101, 103 and 106).
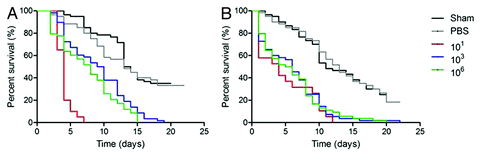
To examine whether lethality was similar across strains, we also tested survival of G. mellonella with H. capsulatum ATCC G217B (). Interestingly, G217B was less virulent than G184AR in our model system. At 25°C, there were no differences between any of the infected groups relative to controls (p > 0.05). Survival was significantly reduced for each inocula incubated at 37°C compared with the same challenged dose at 25°C (p < 0.001). The median survival time for the infected larvae at 37°C was 11 d and the infected groups were significantly different from insects that received PBS (p < 0.05).
Figure 3. Log-Rank plots of the survival of G. mellonella after infection with different concentrations of H. capsulatum ATCC G217B yeast cells. G. mellonella infected and incubated at (A) 25°C or (B) 37°C. Controls included uninfected larva (Sham) and larva injected with PBS. n = 32 larvae per group.
Assessment of melanization of G. mellonella in response to infection
Since melanization is a key step in the antimicrobial response of G. mellonella, we assessed the pigmentation of the larvae after fungal challenge. Infected larvae from all groups developed varying degrees of melanization, whereas the control (PBS and Sham) larvae did not. Melanization in the infected larvae was induced within hours of challenge after infection with either P. lutzii or H. capsulatum. Responses of G. mellonella to H. capsulatum yeast cells at 6 h after infection at 25°C and 37°C are shown in and , respectively. The melanization was more pronounced at 37°C. At both temperatures, the intensity of pigment formation correlated with the number of inoculated yeast cells. Similar results occurred after infection with P. lutzii (data not shown).
Figure 4. Infection of G. mellonella with H. capsulatum G184AR yeast cells induces melanization of the larva in a dose dependent manner. Larvae were injected with (A) PBS, (B) 101, (C) 102, (D) 103, (E) 104, (F) 105 or (G) 106 colonies of H. capsulatum/larvae. The images were taken 6 h after infection at 25°C.
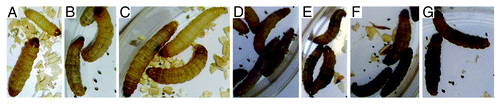
Figure 5. Infection of G. mellonella with H. capsulatum G184AR yeast cells induces melanization of the larva in a dose dependent manner. Larvae were injected with (A) PBS, (B) 101, (C) 102, (D) 103, (E) 104, (F) 105, or (G) 106 colonies of H. capsulatum/larvae. The images were taken 6 h after infection at 37°C.

G. mellonella histopathological evaluation
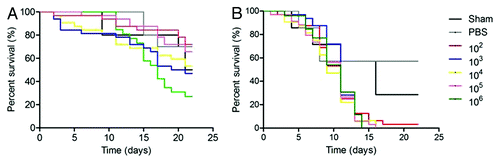
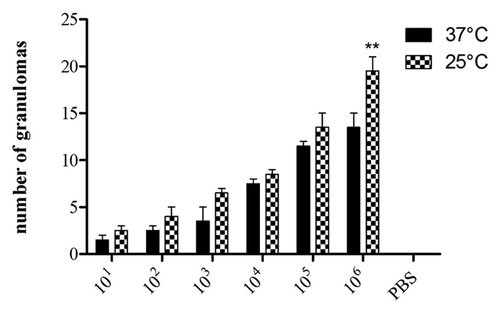
To further understand the fate of P. lutzii inoculated into G. mellonella, infected larvae were fixed in formalin and processed for histopathology. and show PAS-stained sections of uninfected and infected larvae incubated at 25°C and 37°C. P. lutzii induced histopathological changes in G. mellonella, and yeast cells were observed in sections from infected larvae. There was evidence of tissue integrity damage in the infected larvae and pigmented nodules and granulomas were associated with yeast cells. Microscopically, we observed more yeast cells at 25°C () compared with those at 37°C (). The presence of granulomas-like containing yeast in the tissue of the larva was also confirmed by counting the nodules using Image-Pro-Plus, at 25°C and 37°C (). Additionally, the number of granulomas-like paralleled the inoculum density at both temperatures. More yeast cells were visualized in the insects that received higher inocula (105 and 106) at 25°C relative to 37°C (p = 0.0027). In the other inocula, the lower and intermediate, there was no statistical difference. Although P. lutzii can undergo morphogenesis at room temperature, no hyphae or mycelia structures were detected in infected larvae incubated at 25°C over the 3 d incubation point examined. No nodules or granulomas-like were detected in the uninfected control larvae.
Figure 6. PAS-stained sections of G. mellonella. (A) Uninfected control larva inoculated with 0.1 M PBS. Larva infected with P. lutzii at concentrations of (B) 101, (C) 102, (D) 103, (E) 104 and (F) 105 colony forming units. All larva were incubated at 25°C. Structures are annotated as follows: a, adipose bodies; b, fungal cells; c, cuticle; h, hemolymph.
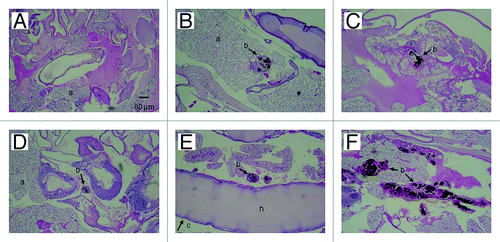
Figure 7. PAS-stained sections of G. mellonella. (A) Uninfected control larva inoculated with 0.1 M PBS. Larva infected with P. lutzii at concentrations of (B) 101, (C) 102, (D) 103, (E) 104 and (F) 105 colony forming units. All larva were incubated at 37°C. Structures are annotated as follows: a, adipose bodies; b, fungal cells; c, cuticle; h, hemolymph.
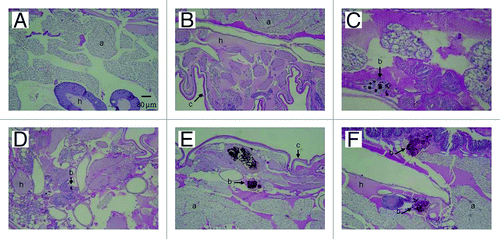
Figure 8. Number of granulomas containing yeast cells of P. lutzii as visualized microscopically on histology slides. PAS-stained sections of G. mellonella were counted using Image-Pro-Plus. The graphs represent the infected insects incubated at 25°C or 37°C. The control shown is insects injected with PBS. **p = 0.0027 comparing two temperatures.
Colony forming units (CFU)
Despite plating onto antibiotic agar, no P. lutzii were recovered from the plates. In all experiments, non-P. lutzii molds contaminated all plates after 2 weeks.
Discussion
We assessed the virulence of the dimorphic fungus P. lutzii and H. capsulatum in a G. mellonella model using different inocula at two different temperatures. At 25°C in the laboratory and in their environmental niches, these fungi exist in mycelium forms, and undergo a morphogenic transition to yeast forms at 37°C, which is the characteristic pathological forms in mammalian hosts. Survival analysis of G. mellonella larvae challenged with different inoculums of both yeast showed that P. lutzii and H. capsulatum G184AR strain are lethal to the insect. In the first 4–7 d after infection half of the all insects died. However, there was not a positive correlation between increased densities of the yeast cells used in infection with a more rapid time to death, except for P. lutzii at 37°C at which the highest doses killed more rapidly.
Melanization in G. mellonella is due to activation of phenoloxidase in the hemolymph.Citation33 In our experiments, we detected significant melanization in groups of larvae within 6 h of infection. The process of melanization is key to the insect’s defense against a wide range of pathogens, such as Aspergillus spp,Citation34 C. albicansCitation35 and C. neoformans.Citation10 The melanization process is intended to encapsulate the invading pathogen within the pigment. The visual observation of the melanization of the G. mellonella body in all concentrations used showed that the higher inoculums induced more rapid melanization ( and ). It is even possible that infection with lower amounts of the fungi avoided early triggering of this response, allowing the pathogens to more readily proliferate. This is consistent with a dynamic response by the insectCitation36 to the quantity of these fungal pathogens. However, it is clear that triggering of the melanization response is insufficient to combat these fungi, especially given the observation that the heavily melanized larva infected with H. capsulatum G184AR at 37°C succumbed to infection. Interestingly, a melanin-deficient A. fumigatus mutants that triggered enhanced G. mellonella melanization displayed increased virulence in the insect.Citation34 Hence, the activation of melanization may be either insufficient to combat infection or even potentiate host damage, perhaps by blocking cellular processes or increasing the rigidity of the insect.
The immune response of the G. mellonella may differ when the larvae are incubated at 25°C and 37°C. In and we show differences between the formations of the granulomas-like. Notably, the histopathologic analyses reveal that G. mellonella granulomas-like have similarities with the granulomas associated with paracoccidiodomycosis infection in mammals.Citation37,Citation38 The amount of melanin detected correlated with the degree of melanization of the insects, which also corresponded to the increased numbers of yeast cells observed in the insects challenged with the highest density inocula.
We compared the virulence of two wild-type strains of H. capsulatum. G184AR is RFLP class III, while G217B is RFLP class II.Citation39 Interestingly, G184AR was more virulent at both 25°C and 37°C, while G217B was severely attenuated at 25°C. G. mellonella has a pathogen recognition protein that recognizes β-glucan.Citation40 G184AR blocks the interaction of cell surface β-glucan with mammalian dectin-1 by displaying an α-glucan layer over the β-glucans.Citation40 In contrast, G217B lacks α-glucans on the fungal cell surface, which unmasks β-glucans for interaction with G. mellonella recognition proteins. Future studies using wild-type G184AR and yeast cells in which α-glucan has either been disrupted or silenced may provide further information regarding this process.
Hyphal transformation of either yeast species over the course infection at 25°C was not visualized. This was not surprising as transformation of Paracoccidioides sp in medium specific for transformation, such as MMcM, requires several weeks to achieve switching of yeast cells into mycelia growth. Future studies will investigate whether infection with conidia results in mycelia growth or the transformation to yeast forms at either temperature. We also tried to isolate viable yeast of P. lutzii and H. capsulatum after 24, 48 and 72 h of the infection, using the protocol described in reference Citation41; however, the plates were contaminated despite harvesting the larvae in a laminar flow hood. Notably, plating of homogenates from control uninfected G. mellonella also resulted in similar contaminants, suggesting that these insects have fungi as a component of their normal flora.Citation42,Citation43
Our findings with H. capsulatum and P. lutzii are consistent with the growing literature on the use of insect models to study fungal pathogenesis. The dynamic of lethality that we observed with our high, middle and low doses suggests that dimorphic fungi may be useful in characterizing new information about the response of G. mellonella to pathogenic challenge. The results using two different strains of H. capsulatum indicate that this model may be an effective system for screening isolates for virulence as well as for assessing changes in virulence after disruption of or interference with fungal processes. In summary, G. mellonella is potentially useful model to study and compare virulence of dimorphic fungi. Moreover, these organisms may serve as a useful platform for further studies to dissect the relative role of melanin in defense vs. injury in this insect species.
Materials and Methods
Strain and growth conditions
H. capsulatum strain G184ARAR (ATCC 26027) and ATCC G217B (ATCC 26032; virulent strain lacking α-glucan on the yeast cell surface) and P. lutzii strain Pb01 (from Dr. George Deepe) yeast cells were grown in Sabouraud broth (BD Difco Bacto) at 37°C for 3 d. Cells were collected and washed the concentration determined by counting using a hemocytometer and the viability assessed by 0.4% Trypan Blue exclusion, to increase reproducibility. Galleria mellonella larvae wax-moth in the final instar larval stage (Vanderhorst Wholesale, Inc.) were selected that were similar in size and weighed between 0.10–0.15 g. Prior to use, the insects were without any external gray markings. The larvae were incubated at 4°C for 30 min before the infection to impede movement in order to facilitate infection. Twenty larvae per group were inoculated by injecting 10 µl containing different inoculums of H. capsulatum or P. lutzii (101,102, 103, 104, 105 and 106) diluted in sterile PBS into the hemocoel through the last proleg as described previously.Citation44 The inoculations were performed with a Hamilton syringe with a needle diameter of 0.75 mm. And the needles were cleaned after all injections and between fungal strains. The same number of caterpillars was inoculated with PBS in each experiment to monitor killing due to physical injury and another group of caterpillars without any manipulation was followed in parallel as an untreated (sham) control. All larvae were placed in sterile Petri dishes and maintained in the dark at 25 or 37°C in a stationary incubator. Mortality was monitored twice daily. Larvae death was assessed by the lack of movement of larvae in response to stimulation together with discoloration of the cuticle. Different PBS and untreated controls were used for each experiment at each temperature. Killing curves were plotted and statistical analyses were performed using the Log-rank (Mantel-Cox) test survival Graph Pad Prism 5.
Assessment of melanization of G. mellonella after infection
After 6 h of the infection with different inocula of P. lutzii and H. capsulatum G184ARAR, pictures were taken with Olympus camera X-845, to show the darker areas as a result of the melanization process.
Histopathology
P. lutzii infected and uninfected larvae (three per group) were fixed by immersion in phosphate buffered 4% formalin after 3 d of infection. The larvae were cut into two symmetric pieces, and immersed in 70% ethanol. Sections were then embedded in paraffin wax, sectioned and stained with Periodic acid-Schiff (PAS) for microscopic examination at 10x magnification. The granuloma-like structures containing yeast were counted using Image Pro Plus 4.0 (1.45 Wayne Rasband NIH).
Determination of fungal burder in G. mellonella
Five days after the infection, four inoculated larvae were homogenized in 4 ml of PBS and 100 µl aliquots of this solution were plated on Brain Heart Infusion (BHI) agar (BD Difco Bacto) containing 0.1% and 1% penicillin/streptomycin (Gibco-BRL/Life Technologies) to prevent bacterial growth. This experiment was performed using different inocula of P. lutzii and H. capsulatum. The plates were incubated at 37°C.
Statistical analysis
The data using P. lutzii were generated from two independent experiments, whereas the experiments with H. capsulatum G184AR (ATCC 26027) and G217B (ATCC 26032) were performed three and two times, respectively. Statistical analyses utilized Kaplan-Meyer curves, Log-rank (Mantel-Cox) test survival, Gehan-Breslow-Wilcoxon and one way ANOVA, depending on the data using Graph Pad Prism 5 and Microsoft Excel. Statistical comparisons were made between treatments performed in parallel on the same day.
Acknowledgments
L.T. was supported by a grant from FAPESP No. 2007/53175-1, Brazil. The work was supported in part by an Interhemispheric Research Training Grant in Infectious Diseases, Fogarty International Center (NIH D43-TW007129) J.D.N. is supported in part by NIH AI52733. Arturo Casadevall and Monica Garcia provided the larvae.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kavanagh K, Reeves EP. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev 2004; 28:101 - 12; http://dx.doi.org/10.1016/j.femsre.2003.09.002; PMID: 14975532
- Bergin D, Reeves EP, Renwick J, Wientjes FB, Kavanagh K. Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect Immun 2005; 73:4161 - 70; http://dx.doi.org/10.1128/IAI.73.7.4161-4170.2005; PMID: 15972506
- Brennan CA, Anderson KV. Drosophila: the genetics of innate immune recognition and response. Annu Rev Immunol 2004; 22:457 - 83; http://dx.doi.org/10.1146/annurev.immunol.22.012703.104626; PMID: 15032585
- Swanson MS, Hammer BK. Legionella pneumophila pathogesesis: a fateful journey from amoebae to macrophages. Annu Rev Microbiol 2000; 54:567 - 613; http://dx.doi.org/10.1146/annurev.micro.54.1.567; PMID: 11018138
- Casadevall A, Steenbergen JN, Nosanchuk JD. ‘Ready made’ virulence and ‘dual use’ virulence factors in pathogenic environmental fungi--the Cryptococcus neoformans paradigm. Curr Opin Microbiol 2003; 6:332 - 7; http://dx.doi.org/10.1016/S1369-5274(03)00082-1; PMID: 12941400
- Casadevall A. Host as the variable: model hosts approach the immunological asymptote. Infect Immun 2005; 73:3829 - 32; http://dx.doi.org/10.1128/IAI.73.7.3829-3832.2005; PMID: 15972467
- Steenbergen JN, Casadevall A. The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans.. Microbes Infect 2003; 5:667 - 75; http://dx.doi.org/10.1016/S1286-4579(03)00092-3; PMID: 12787743
- Mylonakis E, Ausubel FM, Perfect JR, Heitman J, Calderwood SB. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc Natl Acad Sci U S A 2002; 99:15675 - 80; http://dx.doi.org/10.1073/pnas.232568599; PMID: 12438649
- Mylonakis E, Ausubel FM, Tang RJ, Calderwood SB. The art of serendipity: killing of Caenorhabditis elegans by human pathogens as a model of bacterial and fungal pathogenesis. Expert Rev Anti Infect Ther 2003; 1:167 - 73; http://dx.doi.org/10.1586/14787210.1.1.167; PMID: 15482109
- London R, Orozco BS, Mylonakis E. The pursuit of cryptococcal pathogenesis: heterologous hosts and the study of cryptococcal host-pathogen interactions. FEMS Yeast Res 2006; 6:567 - 73; http://dx.doi.org/10.1111/j.1567-1364.2006.00056.x; PMID: 16696652
- Tang RJ, Breger J, Idnurm A, Gerik KJ, Lodge JK, Heitman J, et al. Cryptococcus neoformans gene involved in mammalian pathogenesis identified by a Caenorhabditis elegans progeny-based approach. Infect Immun 2005; 73:8219 - 25; http://dx.doi.org/10.1128/IAI.73.12.8219-8225.2005; PMID: 16299318
- Fuchs BB, Mylonakis E. Using non-mammalian hosts to study fungal virulence and host defense. Curr Opin Microbiol 2006; 9:346 - 51; http://dx.doi.org/10.1016/j.mib.2006.06.004; PMID: 16814595
- Tournu H, Serneels J, Van Dijck P. Fungal pathogens research: novel and improved molecular approaches for the discovery of antifungal drug targets. Curr Drug Targets 2005; 6:909 - 22; http://dx.doi.org/10.2174/138945005774912690; PMID: 16375674
- Lionakis MS, Kontoyiannis DP. Fruit flies as a minihost model for studying drug activity and virulence in Aspergillus.. Med Mycol 2005; 43:Suppl 1 S111 - 4; http://dx.doi.org/10.1080/13693780400020030; PMID: 16110801
- Reeves EP, Messina CG, Doyle S, Kavanagh K. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella.. Mycopathologia 2004; 158:73 - 9; http://dx.doi.org/10.1023/B:MYCO.0000038434.55764.16; PMID: 15487324
- Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, et al. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun 2005; 73:3842 - 50; http://dx.doi.org/10.1128/IAI.73.7.3842-3850.2005; PMID: 15972469
- Dunphy GB, Oberholzer U, Whiteway M, Zakarian RJ, Boomer I. Virulence of Candida albicans mutants toward larval Galleria mellonella (Insecta, Lepidoptera, Galleridae). Can J Microbiol 2003; 49:514 - 24; http://dx.doi.org/10.1139/w03-064; PMID: 14608387
- Brennan M, Thomas DY, Whiteway M, Kavanagh K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol 2002; 34:153 - 7; http://dx.doi.org/10.1111/j.1574-695X.2002.tb00617.x; PMID: 12381467
- Renwick J, Daly P, Reeves EP, Kavanagh K. Susceptibility of larvae of Galleria mellonella to infection by Aspergillus fumigatus is dependent upon stage of conidial germination. Mycopathologia 2006; 161:377 - 84; http://dx.doi.org/10.1007/s11046-006-0021-1; PMID: 16761185
- Cytryńska M, Mak P, Zdybicka-Barabas A, Suder P, Jakubowicz T. Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides 2007; 28:533 - 46; http://dx.doi.org/10.1016/j.peptides.2006.11.010; PMID: 17194500
- Jarrell KF, Kropinski AM. The virulence of protease and cell surface mutants of Pseudomonas aeruginosa for the larvae of Galleria mellonella.. J Invertebr Pathol 1982; 39:395 - 400; http://dx.doi.org/10.1016/0022-2011(82)90065-9; PMID: 6123536
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996; 86:973 - 83; http://dx.doi.org/10.1016/S0092-8674(00)80172-5; PMID: 8808632
- Apidianakis Y, Rahme LG, Heitman J, Ausubel FM, Calderwood SB, Mylonakis E. Challenge of Drosophila melanogaster with Cryptococcus neoformans and role of the innate immune response. Eukaryot Cell 2004; 3:413 - 9; http://dx.doi.org/10.1128/EC.3.2.413-419.2004; PMID: 15075271
- Fuchs BB, Eby J, Nobile CJ, El Khoury JB, Mitchell AP, Mylonakis E. Role of filamentation in Galleria mellonella killing by Candida albicans.. Microbes Infect 2010; 12:488 - 96; http://dx.doi.org/10.1016/j.micinf.2010.03.001; PMID: 20223293
- Mowlds P, Coates C, Renwick J, Kavanagh K. Dose-dependent cellular and humoral responses in Galleria mellonella larvae following beta-glucan inoculation. Microbes Infect 2010; 12:146 - 53; http://dx.doi.org/10.1016/j.micinf.2009.11.004; PMID: 19925881
- Achterman RR, Smith AR, Oliver BG, White TC. Sequenced dermatophyte strains: growth rate, conidiation, drug susceptibilities, and virulence in an invertebrate model. Fungal Genet Biol 2011; 48:335 - 41; http://dx.doi.org/10.1016/j.fgb.2010.11.010; PMID: 21145410
- St Leger RJ, Screen SE, Shams-Pirzadeh B. Lack of host specialization in Aspergillus flavus.. Appl Environ Microbiol 2000; 66:320 - 4; http://dx.doi.org/10.1128/AEM.66.1.320-324.2000; PMID: 10618242
- García-Rodas R, Casadevall A, Rodríguez-Tudela JL, Cuenca-Estrella M, Zaragoza O. Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLoS One 2011; 6:e24485; http://dx.doi.org/10.1371/journal.pone.0024485; PMID: 21915338
- Teixeira MM, Theodoro RC, de Carvalho MJ, Fernandes L, Paes HC, Hahn RC, et al. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol Phylogenet Evol 2009; 52:273 - 83; http://dx.doi.org/10.1016/j.ympev.2009.04.005; PMID: 19376249
- Coutinho ZF, Silva D, Lazera M, Petri V, Oliveira RM, Sabroza PC, et al. Paracoccidioidomycosis mortality in Brazil (1980-1995). Cad Saude Publica 2002; 18:1441 - 54; http://dx.doi.org/10.1590/S0102-311X2002000500037; PMID: 12244377
- Prado M, Silva MB, Laurenti R, Travassos LR, Taborda CP. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: a review from 1996 to 2006. Mem Inst Oswaldo Cruz 2009; 104:513 - 21; http://dx.doi.org/10.1590/S0074-02762009000300019; PMID: 19547881
- Chu JH, Feudtner C, Heydon K, Walsh TJ, Zaoutis TE. Hospitalizations for endemic mycoses: a population-based national study. Clin Infect Dis 2006; 42:822 - 5; http://dx.doi.org/10.1086/500405; PMID: 16477560
- Ratcliffe NA. Invertebrate immunity--a primer for the non-specialist. Immunol Lett 1985; 10:253 - 70; http://dx.doi.org/10.1016/0165-2478(85)90100-2; PMID: 3930392
- Jackson JC, Higgins LA, Lin X. Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella.. PLoS One 2009; 4:e4224; http://dx.doi.org/10.1371/journal.pone.0004224; PMID: 19156203
- Cotter G, Doyle S, Kavanagh K. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol Med Microbiol 2000; 27:163 - 9; http://dx.doi.org/10.1111/j.1574-695X.2000.tb01427.x; PMID: 10640612
- Bidla G, Hauling T, Dushay MS, Theopold U. Activation of insect phenoloxidase after injury: endogenous versus foreign elicitors. J Innate Immun 2009; 1:301 - 8; http://dx.doi.org/10.1159/000168009; PMID: 20375588
- Thomaz L, Apitz-Castro R, Marques AF, Travassos LR, Taborda CP. Experimental paracoccidioidomycosis: alternative therapy with ajoene, compound from Allium sativum, associated with sulfamethoxazole/trimethoprim. Med Mycol 2008; 46:113 - 8; http://dx.doi.org/10.1080/13693780701651681; PMID: 18324489
- Buissa-Filho R, Puccia R, Marques AF, Pinto FA, Muñoz JE, Nosanchuk JD, et al. The monoclonal antibody against the major diagnostic antigen of Paracoccidioides brasiliensis mediates immune protection in infected BALB/c mice challenged intratracheally with the fungus. Infect Immun 2008; 76:3321 - 8; http://dx.doi.org/10.1128/IAI.00349-08; PMID: 18458072
- Spitzer ED, Lasker BA, Travis SJ, Kobayashi GS, Medoff G. Use of mitochondrial and ribosomal DNA polymorphisms to classify clinical and soil isolates of Histoplasma capsulatum.. Infect Immun 1989; 57:1409 - 12; PMID: 2565290
- Kim CH, Shin YP, Noh MY, Jo YH, Han YS, Seong YS, et al. An insect multiligand recognition protein functions as an opsonin for the phagocytosis of microorganisms. J Biol Chem 2010; 285:25243 - 50; http://dx.doi.org/10.1074/jbc.M110.134940; PMID: 20519517
- Castaneda E, Brummer E, Perlman AM, McEwen JG, Stevens DA. A culture medium for Paracoccidioides brasiliensis with high plating efficiency, and the effect of siderophores. J Med Vet Mycol 1988; 26:351 - 8; http://dx.doi.org/10.1080/02681218880000501; PMID: 2977619
- Jarosz J. Gut flora of Galleria mellonella suppressing ingested bacteria. J Invertebr Pathol 1979; 34:192 - 8; http://dx.doi.org/10.1016/0022-2011(79)90101-0; PMID: 119813
- Bucher GE, Williams R. The microbial flora of laboratory cultures of the greater wax moth and its effect rearing parasites. J Invertebr Pathol 1967; 9:467 - 73; http://dx.doi.org/10.1016/0022-2011(67)90125-5
- Fuchs BB, O’Brien E, Khoury JB, Mylonakis E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 2010; 1:475 - 82; http://dx.doi.org/10.4161/viru.1.6.12985; PMID: 21178491