We found that the in vitro susceptibility of C. albicans and C. tropicalis (three isolates each) to antifungals (amphotericin B, caspofungin, and fluconazole) and their virulence in a Toll-deficient fly model of invasive candidiasis were not altered by the previous in vitro exposure to cytarabine, daunorubicin or their combination.
The year of 2013 marks the 40th anniversary for the highly successful 7 + 3 remission-induction regimen (7 d of continuous cytarabine [Ara-C] and the first 3 d of intravenous daunorubicin [DNR]) in acute myeloid leukemia (AML).Citation1 However, infections, including candidiasis, are well known toxicities of that regimen.Citation2 The treatment of candidiasis in cancer and/or transplant patients is difficult, and frequently is a failure. Although Candida resistance, an emerging problem in the AML population,Citation3,Citation4 has been attributed to antifungal selection pressure,Citation5 an intriguing hypothesis is that antineoplastic themselves could contribute to resistance-encoding mutations, through their DNA damaging action.Citation6-Citation9 Doxorubicin, for example, has been reported to induce efflux pump gene expression in Candida albicans,Citation8 and the antineoplastic methotrexate is likely to contribute to reduce amphotericin B activity by increasing the yeast catalase activity.Citation7 In addition, such subtle mutagenic effects could affect fitness and virulence of the Candida species.
To that end, we tested whether pre-exposure of C. albicans and C. tropicalis clinical strains (three strains each) to Ara-C, DNR or their combination, affects: (1) subsequent in vitro susceptibility to antifungals and (2) their virulence in a Toll-deficient fly model of invasive candidiasis. The Candida species used in our study were isolated from cancer patients with invasive candidiasis at the University of Texas MD Anderson Cancer Center. The strains were grown overnight in the dark at 30°C on yeast nitrogen base (YNB) liquid medium (control), YNB and Ara-C (cytosine β-d-arabinofuranoside hydrochloride, Sigma; 0.5 μg/ml), YNB and DNR (daunorubicin hydrochloride, Sigma; 100 μg/ml), and YNB with Ara-C (0.5 μg/ml) plus DNR (100 μg/ml). The overnight cultures were inoculated onto fresh YNB with or without the drugs at the above concentrations, which were based on pharmacokinetic studies and simulate the therapeutic plasma concentrations of these drugs.Citation7,Citation9 Yeast cells were collected by centrifugation, washed three times in sterile saline and counted with a hemacytometer.
The susceptibility of the C. albicans and C. tropicalis strains to amphotericin B, caspofungin and fluconazole was evaluated according to the Clinical and Laboratory Standards Institute-approved document M27-A23 protocol.Citation10 We also assessed differences in growth curves in the presence or not of Ara-C, DNR and Ara-C plus DNR for Candida blastospores growing in liquid medium for 24 h with the use of a plate reader (PowerWave HT; BioTek) at 30°C.Citation11 For the virulence experiments, we used female OregonR Toll-deficient flies and followed standard procedures for feeding, housing and manipulation of flies.Citation12 The flies were exposed to a 12-h light/dark cycle. The injection method used 5 × 105 Candida yeast cells/mL (~1 × 102 yeast cells per fly) accordingly to previously described procedures.Citation12 Survival curves were plotted using Kaplan-Meier analysis and differences in survival rates were analyzed using the log-rank test in the GraphPad Prism software (version 5.0; GraphPad Software, Inc.). A p value of ≤ 0.05 was considered statistically significant. All experiments were performed in triplicate on different days.
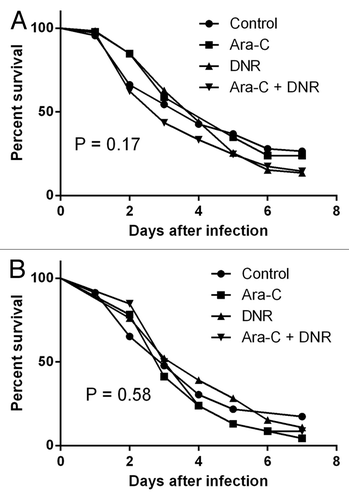
The growth rates of the C. albicans and C. tropicalis strains grown in the presence of Ara-C, DNR, and their combination were similar (). Likewise, the in vitro susceptibility to amphotericin B, caspofungin, and fluconazole of the strains pre-grown in the presence of Ara-C, DNR and both drugs in combination was not different to control (). Finally, the infection of Toll-deficient flies resulted in similar mortality rates among the antineoplastic pre-exposed C. albicans and C. tropicalis compared with control (p = 0.58 by the log-rank test; ). In this preliminary study, we found that the pre-exposure of C. albicans and C. tropicalis strains to Ara-C, DNR, or their combination had no apparent impact on their antifungal susceptibility or fitness. Further studies should focus on the effect of combined antineoplastic and antifungal/antibacterials to different Candida phenotypic traits, ideally in the context of complex microbial communities such is the gut microbiome.
Figure 1. Growth rates of one representative isolate of C. albicans (A) and C. tropicalis (B) previously grown in liquid medium in the presence or not of the antineoplastic drugs cytarabine (Ara-C) and daunorubicin (DNR). Drugs were washed 3× in PBS and 1 × 104 yeast cells were resuspended in YNB broth. Optical density curves were measured in triplicate in a plate reader. (C) Minimum inhibitory concentrations (MIC) of the C. albicans and C. tropicalis strains (three strains each) pre-exposure or not to the combination of Ara-C and DNR.
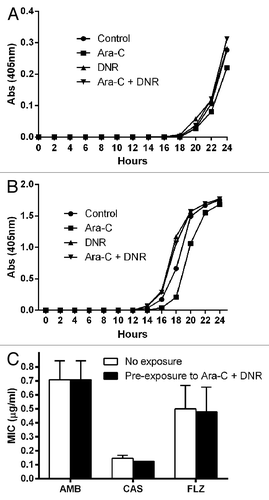
Figure 2. Survival rates of Toll-deficient flies infected with one representative isolate of C. albicans (A) and C. tropicalis (B) previously grown in liquid medium in the presence or not of the antineoplastic drugs cytarabine (Ara-C) and daunorubicin (DNR). Data shown are the means of three independent experiments (n = 23 flies/group).
Acknowledgments
D.P.K. acknowledges the MD Anderson Cancer Center core grant (CA 16672) and The Frances King Black Endowment. We thank Nathaniel D. Albert for invaluable technical support. This study was supported in part by the National Council of Scientific and Technological Development (CNPq, Brazil).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Lichtman MA. A historical perspective on the development of the cytarabine (7days) and daunorubicin (3days) treatment regimen for acute myelogenous leukemia: 2013 the 40th anniversary of 7+3. Blood Cells Mol Dis 2013; 50:119 - 30; http://dx.doi.org/10.1016/j.bcmd.2012.10.005; PMID: 23154039
- Madani TA. Clinical infections and bloodstream isolates associated with fever in patients undergoing chemotherapy for acute myeloid leukemia. Infection 2000; 28:367 - 73; http://dx.doi.org/10.1007/s150100070007; PMID: 11139156
- Kofteridis DP, Lewis RE, Kontoyiannis DP. Caspofungin-non-susceptible Candida isolates in cancer patients. J Antimicrob Chemother 2010; 65:293 - 5; http://dx.doi.org/10.1093/jac/dkp444; PMID: 20016020
- Garcia-Effron G, Kontoyiannis DP, Lewis RE, Perlin DS. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob Agents Chemother 2008; 52:4181 - 3; http://dx.doi.org/10.1128/AAC.00802-08; PMID: 18794386
- Lewis RE, Viale P, Kontoyiannis DP. The potential impact of antifungal drug resistance mechanisms on the host immune response to Candida.. Virulence 2012; 3:368 - 76; http://dx.doi.org/10.4161/viru.20746; PMID: 22722245
- Ahearn DG, McGlohn MS. In vitro susceptibilities of sucrose-negative Candida tropicalis, Candida lusitaniae, and Candida norvegensis to amphotericin B, 5-fluorocytosine, miconazole, and ketoconazole. J Clin Microbiol 1984; 19:412 - 6; PMID: 6325491
- Linares CEB, Griebeler D, Cargnelutti D, Alves SH, Morsch VM, Schetinger MRC. Catalase activity in Candida albicans exposed to antineoplastic drugs. J Med Microbiol 2006; 55:259 - 62; http://dx.doi.org/10.1099/jmm.0.46263-0; PMID: 16476788
- Kofla G, Turner V, Schulz B, Storch U, Froelich D, Rognon B, et al. Doxorubicin induces drug efflux pumps in Candida albicans. Med Mycol 2011; 49:132 - 42; http://dx.doi.org/10.3109/13693786.2010.512022; PMID: 20818920
- Rosen DB, Cordeiro JA, Cohen A, Lacayo N, Hogge D, Hawtin RE, et al. Assessing signaling pathways associated with in vitro resistance to cytotoxic agents in AML. Leuk Res 2012; 36:900 - 4; http://dx.doi.org/10.1016/j.leukres.2012.02.022; PMID: 22521550
- Clinical Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A3, 3rd ed. Wayne, PA: Clinical Laboratory Standards Institute, 2008.
- Ben-Ami R, Garcia-Effron G, Lewis RE, Gamarra S, Leventakos K, Perlin DS, et al. Fitness and virulence costs of Candida albicans FKS1 hot spot mutations associated with echinocandin resistance. J Infect Dis 2011; 204:626 - 35; http://dx.doi.org/10.1093/infdis/jir351; PMID: 21791665
- Chamilos G, Lionakis MS, Lewis RE, Lopez-Ribot JL, Saville SP, Albert ND, et al. Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J Infect Dis 2006; 193:1014 - 22; http://dx.doi.org/10.1086/500950; PMID: 16518764