Abstract
Bacterial infections are a major cause of morbidity and mortality worldwide and are increasingly problematic to treat due to the rise in antibiotic-resistant strains. It becomes more and more challenging to develop new antimicrobials that are able to withstand the ever-increasing repertoire of bacterial resistance mechanisms. This necessitates the development of alternative approaches to prevent and treat bacterial infections. One of the first steps during bacterial infection is adhesion of the pathogen to host cells. A pathogen’s ability to colonize and invade host tissues strictly depends on this process. Thus, interference with adhesion (anti-adhesion therapy) is an efficient way to prevent or treat bacterial infections. As a basis to present different strategies to interfere with pathogen adhesion, this review briefly introduces general concepts of bacterial attachment to host cells. We further discuss advantages and disadvantages of anti-adhesion treatments and issues that are in need of improvement so as to make anti-adhesion compounds a more broadly applicable alternative to conventional antimicrobials.
Concepts of Bacterial Adhesion
A bacterium’s ability to colonize its host highly depends on the mechanisms it has in place to withstand the host’s mechanical and immunological clearance mechanisms. To avoid being removed from the organism, bacteria have to be able to quickly and effectively attach to host cells. Adhesion is also a universal prerequisite for pathogens to efficiently deploy their repertoire of virulence factors and exert effects on host cells, no matter if they are effector-mediated or toxin-mediated. For example, a wide range of gram-negative pathogens employ type III, type IV, or type VI secretion systems to inject effector proteins into host cells where they biochemically tune the host’s cellular machinery to facilitate infection. Translocation of effector proteins from the bacterial cytoplasm into the host cell’s cytoplasm requires direct contact between bacterium and host.Citation1 Binding needs to be tight such that the interaction is long enough to allow the correct sequence of proteins to be injected over time. Several studies have shown that different effector proteins are not all injected simultaneously but follow a sequence depending on their initial concentrations and affinity for the translocation machinery.Citation2,Citation3 This makes sense since, in some cases, effectors injected by the same pathogen at different time points during infection can have opposing activities.Citation4,Citation5 Hence, if bacteria are removed from host cells prematurely, infection is not productive.Citation6 In the case of autotransporter-toxins, which are secreted by bacteria into the extracellular medium prior to entering host cells, local toxin concentration is critical to their activity.Citation7 For example, in the case of pore-forming toxins, subunits have to be co-localized to be able to form a pore in the host cell membrane. Hence, their mechanism of action also depends on close proximity between pathogen and host cell to avoid a dip in local protein concentration through diffusive loss. Because attachment is so crucial to the fate of infection, bacterial pathogens have devised a vast repertoire of attachment mechanisms for initial contact with host cells.
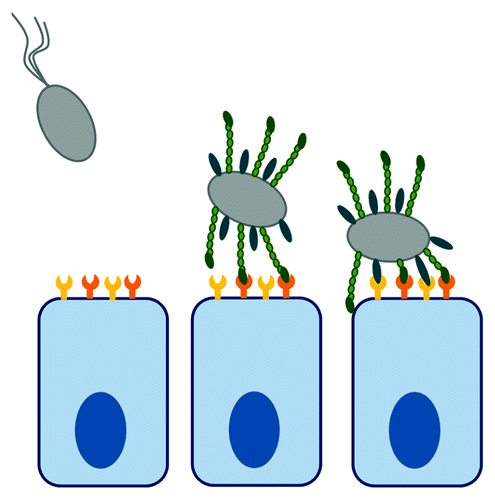
Upon encountering the host cell, bacteria first attach via weak non-specific interactions with the host cell surface. This is not mediated by specific adhesin-receptor pairing, but rather by overall physicochemical properties of the bacterial and host surfaces, such as charge and hydrophobicity.Citation8 This reversible adsorption process is followed by initial adhesion, which can be mediated by specific interactions, but still gives the bacteria enough freedom of movement to sample the host cell surface through a rolling or gliding motion.Citation9 These initial, transient interactions are then reinforced by high affinity bacterial–host cell interactions, which rely on specific interactions between bacterial surface molecules and host cell receptors (). The binding moiety on both the bacterial and host side can vary in terms of their chemical identity to be a sugar, a protein or a lipid. All these pair-wise combinations can be involved in mediating specific bacterial–host cell interactions.
Figure 1. Bacterial attachment to host cells. Upon encountering host cells, bacteria are attracted by weak, non-specific forces, which are driven by physicochemical properties of bacterial and host surface. Initial low-affinity attachment is driven by specific surface receptors but still allows the bacterium to sample the host cell surface. Initial interactions are reinforced by additional receptor pairing, leading to overall high affinity of binding.
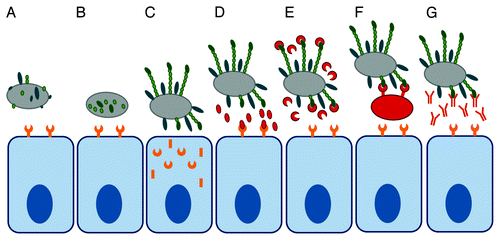
All steps of this multi-stage process can potentially be targeted in anti-adhesion therapy (). Changing the surface properties of either bacteria or host cell can discourage non-specific interactions. The biogenesis of bacterial adhesins or host receptors can be inhibited, either by interfering with biosynthesis of subunits or by blocking translocation and surface assembly. Specific interactions between bacterial adhesins and host cell receptors can be targeted in several ways: Anti-adhesion compounds can competitively inhibit attachment by mimicking bacterial or host cell binding partners and alternatively, antibodies recognizing bacterial surface epitopes can be used to either actively or passively immunize the host ().
Figure 2. Strategies for anti-adhesion therapy. Bacterial attachment can be inhibited by interfering with adhesin biosynthesis (A), adhesin assembly (B), or host receptor assembly (C). Binding can be inhibited by competitive replacement of the adhesin from the host (D) or of the host receptor from the adhesin (E) using soluble molecules or by using designer microbes (F). Antibodies against bacterial adhesins can block surface epitopes required for binding (G).
Disrupting Surface Receptor Biogenesis
Impairing pathogen receptor biogenesis
Several studies have described that sub-inhibitory concentrations of certain antibiotics; in particular the fluoroquinolone ciprofloxacine and the aminoglycoside amikacin, can lead to altered physicochemical properties of the bacterial surface and decreased bacterial adhesion to host cells (). This is thought to be caused by aberrant protein synthesis leading to the production of partially or incorrectly folded proteins and thus impaired surface display of outer membrane proteins and assembly of fimbrial adhesins. The resulting change in surface charge as well as inhibition of specific interactions with host receptors both act synergistically in preventing adhesion.Citation10-Citation12
Chaperone–usher (C/U) pili are large, multi-subunit organelles mediating host cell adhesion and are important virulence factors in a range of bacterial pathogens, including Escherichia coli and species of Salmonella, Yersinia, Pseudomonas, Klebsiella, and Haemophilus. Although Type 1 and P pili are the two most prominent examples of C/U pili, a further 17 putative chaperone–usher operons are encoded in the genomes of sequenced E. coli strains.Citation13 Consequently, inhibition of pilus assembly is a promising strategy for preventing infection. C/U pilus biogenesis is accomplished by translocation of pilin subunits via the Sec pathway and subsequent association with a periplasmic chaperone. The chaperone delivers subunits to an outer membrane usher complex, which secretes them and simultaneously acts as an assembly platform. The structure of the complex between the P pilus chaperone PapD and a synthetic peptide mimicking the C-terminus of the pilus protein PapG was solved and used as a basis to rationally design small molecule inhibitors to prevent pilus assembly (pilicides) by disrupting the chaperon–pilin complex.Citation14 Another study reported the design of small compounds interfering with association of the chaperone–pilin–usher complex.Citation15,Citation16 As key structural features responsible for mediating the chaperone–pilin–usher interactions are conserved, pilicides are effective against a range of chaperone–usher pili. More recent studies have aimed at improving the efficacy of pilicides by varying substituents on the main peptidomimetic pilicide fragment and at extending the approach to generate inhibitors of curli assembly (curlicides).Citation17,Citation18
Inhibition of host receptor biogenesis
Many bacterial adhesins and toxins rely on host glycosphingolipids (GSLs) for host cell binding and membrane translocationCitation19,Citation20 and depletion of GSLs from the host cell membrane has been proposed as an efficient strategy to prevent or treat infections ().Citation21 GSL depletion can be accomplished by administering inhibitors specific for enzymes in the GSL biosynthetic pathway. For example, inhibitors blocking the ceramide-specific glycosyltransferase which catalyzes the formation of glucosyl ceramide, the precursor for GSLs, have successfully been used to diminish bacterial colonization of cultivated human uroepithelial cells and in a murine model of urinary tract infection (UTI).Citation22 Glycosylation inhibitors have been shown to be safe and effective in patients with lipid storage diseases and thus their off-label use for treatment of bacterial infections may be a viable option.Citation23,Citation24 Alternatively, GSL depletion can be accomplished by enzyme replacement therapy with human glucosyl ceramide glucosidase, and this has been successfully used to treat a patient suffering from Gaucher disease and systemic salmonellosis.Citation25
Use of Receptor Analogs in Competition-Based Strategies
Sugar-based inhibitors and glycomimetics
Specific bacterial host interactions are frequently mediated by carbohydrates, which are present in large numbers both on the bacterial surface (in the form of capsules, lipopolysaccharides, and glycoproteins) and the host surface (as glycoproteins and glycosphingolipids) (). It is thus unsurprising that a large body of research has focused on the use of glycomimetics and synthetic glycosides that would act as anti-adhesives by competitively inhibiting pathogen binding. A number of excellent reviews have been published over the past few years discussing various aspects of carbohydrate-mediated adhesion and the use of sugar-based inhibitorsCitation26-Citation29 so we will only discuss key concepts and present recent developments here.
Some of the most promising anti-adhesive compounds made in recent years are targeted at preventing infections of the urogenital tract caused by fimbriated uropathogenic E. coli (UPEC). FimH, the adhesive subunit at the tip of type 1 pili, is a bacterial lectin recognizing mannosylated uroplakins and N-linked oligosaccharides on β1 and α3 integrins located on the luminal surface of the bladder. FimH is a key virulence factor in UTIs and is crucial for multiple stages of infection, such as colonization and invasion of bladder tissue as well as formation of intracellular bacterial communities which are responsible for disease recurrence. The interaction of FimH with host cells has thus long been a target for the development of anti-adhesives. The first study demonstrating the anti-adhesive effect of mannoside-based host receptor analogs in a murine model of UTI goes back to the 1970s.Citation30 However, monovalent mannose derivatives displayed comparatively weak inhibition and it proved difficult to maintain them at an effective dose over a prolonged period.Citation31 Since then, two strategies were pursued to improve the efficacy of FimH inhibitors: Synthesis of multivalent compounds with increased binding avidity and rational design of monovalent inhibitors with novel aglucan moieties to increase affinity. To generate multivalent inhibitors, monovalent FimH antagonists are coupled to a multivalent scaffold, such as a synthetic polymer, sugar core or peptide backbone.Citation32-Citation34 The resulting inhibitors are not only potent anti-adhesives, they also cause cross-linking of bacteria.Citation32 Structural studies of FimH bound to mannosides revealed that the key determinant for their interaction was a carbohydrate binding pocket with a hydrophobic entrance (tyrosine gate). It was rationalized that compounds containing a mannoside glucan moiety and large aglucan moieties that would be excluded from the pocket and engage in stacking interactions with tyrosine (out-docking mode) would display higher affinities for FimH than those with an aglucan entering the gate (in-docking mode).Citation35 Thus, the aglucan moiety can be systematically varied to achieve improved affinity, solubility and metabolic stability. The most recent generation of FimH antagonists are biphenyl mannosides, which are approximately 200 000-fold more potent than D-mannose and are orally bioavailable.Citation36 FimH antagonists have been demonstrated to have low cytotoxicity and despite being based on mannose, they are not cross-reactive with human mannose receptors.Citation37,Citation38 In murine models, they have proven to decrease bacterial colonization to levels similar to those achieved with the antibiotic ciprofloxacine, making them viable alternatives to antimicrobials.Citation39,Citation40
Many food components have long been known to have a protective effect against bacterial infection but in many cases this was based only on anecdotal evidence. More recently, the active compounds for some of these foods have been isolated and demonstrated to have anti-adhesive properties in vitro, underlining their effectiveness as anti-infective compounds. A good example for this is cranberry juice, which has long been described to protect against bacterial infections, in particular UTIs. This protective effect was later shown to be due to anti-adhesive properties of cranberry compounds, and eventually a family of high molecular weight polyphenols, proanthocyanidins, proved to be the bioactive compounds contained in cranberries.Citation41,Citation42 Proanthocyanidins inhibit the adhesion and co-aggregation of UPEC, Helicobacter pylori and the oral pathogen Porphyromonas gingivalis, among others.Citation42-Citation44 Their mechanism of action seems to be binding to flagella and pili, thus inhibiting bacterial surface attachment, swarming motility and aggregation into biofilms.Citation42,Citation45,Citation46 Clinical studies evaluating the effects of consumption of both cranberry juice and cranberry extracts on the incidence of UTIs had mixed outcomes, with some reporting no significant benefit and others reporting significant decreases in the rate of infection upon consumption.Citation47-Citation50 The outcome of these studies seems to strongly depend on whether or not patients had preexisting, recurring UTIs or received prophylaxis (in which case the effect was more significant), and on how much of and for how long the active compound was consumed. Many more foods such as plantains, tea, coffee, and wine, to name a few, contain compounds with anti-adhesive properties and this topic has recently been reviewed comprehensively.Citation51
Many of the body’s endogenous defense mechanisms against bacterial infection are based on sugars, which act as decoys for bacterial surface receptors. For example, mucus is secreted by the intestinal epithelium and acts as a physical barrier against colonization by enteropathogens. Mucus contains a variety of mucin glycoproteins and the glycosylation pattern of mucins mimics the pattern found in epithelial surface receptors. Mucins act by binding and immobilizing bacteria, which are subsequently cleared from the gastrointestinal tract by shedding of the mucus layer.Citation52-Citation54 This strategy has been adapted for therapeutic use, for example by using purified mucins as anti-adhesives. Purified bovine Muc1, a highly glycosylated mucin derived from cow milk, efficiently prevents bacterial infection of cultured intestinal epithelial cells. Muc1 selectively inhibits the attachment of gram-negative pathogens (E. coli and Salmonella Typhimurium) but is not effective in inhibiting attachment of gram-positive organisms such as Staphylococcus aureus or Bacillus subtilis. Muc1 has little effect on the detachment of pre-bound bacteria from host cells, restricting its use to prophylactic rather than therapeutic applications.Citation55
Inhibition of bacterial binding can also be based on sialic acid moieties present on mucins, which act as decoys by mimicking host sialylated receptors. Human breast milk also contains an abundancy of sialic acid-containing oligosaccharides, which are thought to protect the infant from colonization by bacterial pathogens, particularly of the intestinal tract.Citation27,Citation56,Citation57 However, the anti-adhesive properties of human milk oligosaccharides are intrinsically difficult to evaluate in vivo, due to the fact that they impact the host in many ways, for example by affecting the composition of the microbiota and modulating immunological development.Citation58 Soluble sialic acid-containing oligosaccharides isolated from milk or synthetic oligosaccharides mimicking the structure and multivalency of endogenous sialic acid-containing decoys show great promise as anti-adhesive compounds both in tissue culture models and animal studies.Citation59,Citation60 For example, the human milk derived oligosaccharide disialyllacto-N-tetraose was shown to prevent necrotizing enterocolitis, one of the most common and fatal infections in preterm infants, in a rat model of infection.Citation60 Sugar-based inhibitors are also being extensively investigated for use against respiratory pathogens, many of which are biothreat agents, and this has been recently reviewed.Citation61
Peptide-based inhibitors
Even though peptide-based inhibition of bacterial adhesion has been demonstrated extensively in vitro,Citation62-Citation64 its therapeutic potential has not been fully realized yet (). Thus, peptide-based anti-adhesives remain rather understudied compared with sugar-based inhibitors. Examples of peptide-based inhibitors demonstrating the potential of this approach are anti-caries (anti-cavity) peptides. Streptococcus mutans, one of the main causative agents of dental caries, expresses a surface protein streptococcal antigen (SA) I/II that binds to salivary receptors adsorbed on the hydroxyapatite matrix of the tooth surface. SA I/II is the key attachment factor of S. mutans and monoclonal antibodies raised against SA I//II can prevent tooth colonization and caries in nonhuman primates as well as colonization of the oral cavity in humans.Citation65,Citation66 Both full-length SA I/II as well as a recombinant 38 kDa peptide matching the proline-rich or P-region of SA I/II were tested for inhibition of S. mutans adhesion to salivary receptors and saliva-coated hydroxyapatite beads. The full-length protein and the peptide inhibited binding by 80% and 65%, respectively, when used at a concentration of 1 mg/ml. Since the peptide motif is well conserved across a range of oral streptococcal species, this approach promised to be useful in the prevention of caries as well as other streptococcal infections.Citation67 The same authors subsequently developed a shorter (22 residue) synthetic peptide, p1025, matching a smaller epitope of the same region that was able to inhibit binding to salivary receptors in vitro. In human volunteers, topical application of the peptide to teeth twice a week over three weeks prevented recolonization with S. mutans after its prior depletion from the oral flora using chlorhexidine. Even though the peptide was persistent in saliva and plaque for only a few hours, its protective effect extended over several days and repeated application prevented S. mutans recolonization over the entire period of the study (120 d) in most subjects. The initial exclusion of S. mutans probably allows sufficient time for other species to colonize the niche and to establish a competitive advantage.Citation68,Citation69 The minimal dosage required to prevent recolonization was not established, but is likely to be far lower than the 10mg/ml dosage used in the study.
Another promising candidate for the development of a peptide-based anti-adhesive is the bacterial surface protein multivalent adhesion molecule (MAM) 7. MAM7 is involved in the initial host attachment in a range of species, including enteropathogenic E. coli (EPEC), Yersinia pseudotuberculosis, Vibrio cholerae, and Vibrio parahaemolyticus and uses two host surface receptors, fibronectin and the membrane lipid phosphatidic acid.Citation6,Citation70 MAM7-coupled polymer beads were successfully used to decrease surface attachment and infection by a range of multidrug-resistant bacteria isolated from patient wounds in tissue culture models.Citation71,Citation72 The broad-spectrum efficacy of MAM7-based inhibitors holds promise for the prevention of a range of gram-negative infections but their development is still at an early stage. It has yet to be investigated if smaller peptide epitopes could be used for inhibition instead of full-length protein and whether variants with improved affinity could be engineered.
A few factors have to be considered in the design and use of peptide-based anti-adhesives, such as their stability and persistence in the host environment and their binding avidity. Peptide stability can be improved by using tailored delivery systems, allowing sustained and targeted release of the inhibitor at the treatment site.Citation73,Citation74 Moving from full-length proteins toward identifying minimal binding epitopes and using synthetic peptides will allow us to improve inhibitors with respect to their stability and affinity by introducing chemical modifications unnatural or D-amino acids. Peptides may also be used as design templates for peptide mimetics.Citation75,Citation76 Lastly, it is important to note that some adhesins, by binding to host receptors, may trigger signaling pathways which favor infection. In a therapeutic setting, this may cause unwanted side-effects and therefore should be the main consideration and focus of pre-clinical studies on peptide-based inhibitors.Citation77 Although peptide inhibitors may seem like challenging targets for the pharmaceutical industry, their large-scale synthesis is feasible and such drugs can be successful. This has been exemplified by the drug Fuzeon, a peptide-based HIV fusion inhibitor. This drug consists of a 36-residue peptide corresponding to part of the envelope glycoprotein gp140 and competitively blocks the binding and fusion of viral particles to host cells. Fuzeon has shown efficacy in phase III clinical trials and has been approved by the FDA for treatment of drug-resistant HIV infections.Citation78
Anti-Adhesion Antibodies and Vaccines
Several studies have reported the development and use of antibodies or antisera directed against bacterial adhesins as an anti-adhesive strategy (). In principle, several approaches are possible. The host can be directly or passively immunized using a bacterial adhesin, an adhesin subunit (as in the case of multi-subunit adhesive organelles, such as fimbriae), or an immunogenic peptide fragment based either on an individual adhesin or on the consensus derived from a group of adhesins. Lastly, the host can be immunized using a DNA vaccine encoding the adhesin or part thereof, and we will describe examples of these strategies below.
The treatment for Salmonella enterica serovar Typhi, the causative agent of typhoid fever, is increasingly complicated due to the emergence of multidrug resistant strains. The S. Typhi adhesin T2544 is a major contributor to bacterial host interaction and disease pathogenesis and a potential target for development of an anti-adhesion vaccine. Deletion of T2544 results in reduced systemic invasion and a 10-fold increase in LD50 in a murine model. T2544 is highly immunogenic and elicits elevated titers of serum IgG and intestinal secretory IgA in immunized mice. T2544 antiserum enhances both the uptake and clearance of bacteria by macrophages as well as complement-mediated lysis. Mice either immunized with T2544 or passively immunized with anti-T2544 antiserum were protected against subsequent bacterial challenge and showed increased bacterial shedding. As T2455 is widely distributed in clinical isolates of S. Typhi and S. Paratyphi and shows very limited variation, it is potentially a good candidate for vaccine development.Citation79 However, T2455-based immunization did not completely inhibit disease and this is likely due to other structures promoting cellular invasion, such as type IV pili.Citation79-Citation81 With S. Typhimurium, the effect of multifactorial adhesion on the outcome of immunization attempts is equally problematic. SadA, a trimeric autotransporter involved in biofilm formation, autoaggregation and host binding by S. Typhimurium, was tested as a vaccine candidate. Although purified SadA itself triggered an immune response, which was even more pronounced when administered together with an adjuvant, it provided only limited protection against subsequent bacterial challenge.Citation82
Subunit-based vaccines are often used in the context of fimbrial adhesins and several of them have been described for therapeutic use against pathogenic E. coli. Enterotoxigenic E. coli (ETEC) are a major cause of diarrheal disease in humans and other animals and pathogenicity is to a large extent caused by enterotoxins. A fusion protein consisting of FaeG, the major subunit of E. coli K88ac fimbriae, an epitope from the B subunit of heat-labile (LT) toxin and the A subunit of shiga toxin (STa) was used to immunize rabbits. Animals generated anti-K88ac, anti-LT and anti-STa antibodies, which inhibited adhesion of fimbrial E. coli to small intestinal enterocytes and neutralized both shiga toxin and cholera toxin.Citation83 A similar strategy was pursued to generate antibodies against enterohemorrhagic E. coli (EHEC). EHEC attachment to the host is primarily due to intimin and the subsequent pathology is caused by shiga toxin. A fusion protein containing two different toxin antigens as well as an intimin antigen fragment, was used to immunize mice. The SSI fusion protein induced a strong humoral immune response, with both toxin neutralizing and anti-adhesion antibodies being generated. Subsequent bacterial challenge of twice-immunized mice with an otherwise lethal dose of EHEC strain O157:H7 did not cause any pathology.Citation84 The use of preventive vaccines against recurring UTIs based on fimbrial subunits FimCH has been investigated quite extensively both in animal models and clinical trials. Immunization with candidate FimH vaccines reduced in vivo colonization of the bladder mucosa by more than 99% in a murine model.Citation85 Immunization of monkeys with FimCH adhesin–chaperone complex in combination with an adjuvant elicited a strong IgG antibody response and protected 3/4 of the animals against subsequent UPEC infection.Citation86 A recent clinical study comparing the efficacy of preventive vaccination to prophylactic treatment with antibiotics concluded that vaccination was a more effective strategy to reduce frequency, duration and severity of recurring UTIs.Citation87
An example for a consensus-based vaccine developed against Pseudomonas aeruginosa was described by Cachia and Hodges.Citation88 P. aeruginosa is an opportunistic pathogen causing a broad spectrum of diseases, such as urinary and respiratory tract infections, skin infections, and systemic infections, particularly in immunocompromised patients. P. aeruginosa strains are increasingly resistant to traditional antimicrobials and only a few groups of antibiotics are left to treat P. aeruginosa infections especially if they persist over an extended period, for example in cystic fibrosis patients. Cachia and Hodges developed a synthetic peptide consensus sequence anti-adhesin vaccine and a related therapeutic monoclonal antibody to be used in prevention and treatment of P. aeruginosa infections. They identified a small peptide structural element found in P. aeruginosa strain K (PAK) bacterial pili that binds host epithelial cells. Since heterologous peptides were found in all sequenced P. aeruginosa strains, they used a peptide based on the consensus sequence to raise anti-adhesin antibodies, which were effective against multiple strains. Another study utilizing P. aeruginosa strain K pili as an immunogenic target described the generation of monoclonal antibodies which were also cross-protective against P. aeruginosa PAO and PAK strains when used for active immunization in a murine model. The authors noted that the generation of an effective antibody relied on the appropriate presentation of the immunogenic peptide, which was achieved through conformational restriction of the peptide by both C- and N-terminal coupling to a carrier.Citation89
To improve surface display of bacterial antigens and trigger both humoral and cellular immune responses, several approaches have been taken to ensure the epitope is displayed in a physiological conformation. For example, this can be achieved by associating adhesin antigens with outer membrane vesicles and this strategy was used to create vaccines against Neisseria meningitides, a causative agent of meningitis and septicemia.Citation90-Citation92 Alternatively, antigens can be displayed on the surface of live, attenuated bacterial strains which can be used as oral vaccines.Citation93 This is a low cost approach and thus particularly suitable for the prevention of zoonotic infectious diseases as it could be used to immunize large herds of animals. For example, a live attenuated Salmonella Typhimurium strain expressing a combination of E. coli fimbrial antigens (K88ab, K88ac, FedA, and FedF) prevented post-weaning diarrhea in piglets when used to immunize pregnant pigs. No environmental exposure was reported because no live bacteria from the vaccine strain were shed by the animals following immunization.Citation94,Citation95 However, caution must be paid to evaluate risks associated with environmental exposure to genetically modified organisms and the potential of the attenuated vector to revert to a virulent strain.Citation96
DNA vaccines contain DNA encoding pathogen-derived antigens, which upon their expression in the host are able to elicit protective immunity. Theoretically, this strategy is advantageous because it improves antigen processing and presentation and induces both humoral and cellular immune responses.Citation97 DNA vaccines have been generated and tested as a tool to prevent S. aureus infections. S. aureus causes a broad spectrum of diseases, ranging from wound infections to life-threatening conditions such as endocarditis, osteomyelitis and septicemia. Treatment of infections becomes increasingly complicated by the high level of multidrug resistance seen with S. aureus. S. aureus binding to host cells is mediated by a number of surface proteins binding to extracellular matrix components with extremely high affinity. A DNA vaccine based on collagen-binding protein (CNA), a major S. aureus adhesin, was used to immunize Balb/c mice. Mice injected with three doses of the eukaryotic expression vector pCNA, expressing the collagen-binding domain of CNA, showed evidence of both antibody- and cell-mediated immune response against CNA. Even though the antibodies recognized intact bacteria and inhibited binding to collagen in vitro, they failed to protect mice against intra-peritoneal infection by S. aureus.Citation98 A polyprotein DNA vaccine against S. aureus, consisting of a series of plasmids expressing clumping factor A(ClfA), fibronectin binding protein A (FnBPA), and the enzyme sortase (Srt), triggered both antibody production and T-cell response and provided partial protection against S. aureus isolate Sa042 and full protection against reactive arthritis after challenge with S. aureus strain Newman.Citation99
One of the major drawbacks of anti-adhesion therapy is the high degree of redundancy in bacterial adhesive strategies that in many cases interfere with effective treatment. The use of anti-adhesion antibodies or vaccines may still be effective in such cases as antibody opsonization can increase bacterial uptake and clearance by macrophages and antibodies may trigger complement-mediated bacteriolysis, even if they are unable to fully inhibit bacterial adhesion.Citation79 Although one could argue that antigenic variability of bacterial adhesins can potentially impair the efficacy of anti-adhesion antibodies, the fact is that many adhesins show a remarkable degree of conservation, making them good vaccine candidates.Citation100,Citation101
Advantages of Anti-Adhesion Therapy
One of the major reasons why anti-adhesion strategies are being considered as an alternative approach to conventional antimicrobials is that their mechanism of action does not give rise to bacterial resistance. Because anti-adhesive compounds only inhibit bacterial binding without affecting microbial viability, there is no selective pressure upon the pathogen that would affect the balance between wild-type and treatment-resistant mutants in the population. Although in principle it is possible that mutations affecting the efficacy of anti-adhesion compounds could occur, these would also directly affect the pathogen’s ability to bind the host receptor. As a result, resistance against anti-adhesion treatment would negatively impact the pathogen’s fitness and likely be naturally selected against. It has been shown that individual point mutations in bacterial adhesins can change tissue tropism and even distinguish commensal from pathogenic strains.Citation102 Knowledge of such variations opens up the potential to design species-specific and even strain-specific anti-adhesive compounds, thus avoiding side effects caused by changes in the microbiota.Citation103 An additional advantage of anti-adhesion compounds is their stability under physiological conditions. Both bacterial and host receptor molecules are evolutionary adapted to withstand the physiological conditions encountered upon extracellular exposure. As such, anti-adhesion compounds designed to closely mimic bacterial or host surface structures are likely to be more resistant against degradation than conventional antimicrobial compounds, which are artificially introduced into the host system and have to be specifically designed to be both bio-available and yet able to cross the bacterial outer membrane and be stable under physiological conditions. Because anti-adhesion compounds are not bactericidal, they circumvent problems associated with the therapeutic use of certain bactericidal drugs, such as the release of bacterial toxins and endotoxins, which have detrimental effects on patients’ health.Citation104,Citation105 Instead, they leave the host exposed to intact but non-functional bacteria, enabling the host to elicit protective immunity that protects against re-infection and speeds up immunological clearance of bacteria that have not been removed mechanically.Citation79
Improving Efficacy by Exploiting Multivalency
Despite the many beneficial attributes associated with anti-adhesion therapy, there are downsides to this approach and we will have to overcome these before anti-adhesion approaches can gain broad validity in the treatment of a wide range of infections. One of the main practical problems in the use of competition-based anti-adhesion inhibitors is to achieve high enough avidity to efficiently compete with bacteria, which often carry hundreds of adhesion molecules on their surface. A variety of clever ways have been thought out to deal with this challenge, and most of them rely on tethering monovalent ligands to functionalized scaffolds, such as polymers,Citation32 dendrimers,Citation106 nanoparticles,Citation107 or even fullerenes.Citation108 By introducing multivalency, inhibitors can be used at lower concentrations than monovalent compounds to achieve the same extent of inhibition. Methods for introducing multivalency into inhibitors have been reviewed extensively,Citation109-Citation111 as has the possibility of using engineered bacteria to produce soluble oligosaccharides, thus preventing problems attached to the large-scale organo-synthesis of such molecules.Citation112 In the following, we will describe two approaches to multivalency of perhaps more general relevance.
Use of dynamic scaffolds for ligand clustering
Tethering of functional ligands to scaffolds often restricts their freedom of movement due to the rigidity of the backbone structure, which may render them less able to adapt to the conformation of bacterial surface receptors and thus make them less effective in treatment. An alternative approach is the use of supramolecular dynamic scaffolds, which may give the tethered epitopes more freedom of movement thereby allowing them to maximize their interactions with surface receptors. One such example is pseudopolyrotaxanes, “beads on a string” structures consisting of “wheels” or “beads” of clustered mannoside ligands arranged on a polymer “string”, allowing the ligands to freely rotate around and move along the backbone. This way, these scaffolds provide much more scope for adjustments in affinity, ligand density and mobility than rigid scaffolds.Citation113 Several such assemblies showed inhibition of UPEC adhesion to uroepithelial cells in a tissue culture model of infection.Citation114 Lipid-based nanostructures, such as functionalized liposomes, supported colloidal bilayers, or protocells, which allow the display of embedded receptors or receptor analogs in an optimal conformation and density, are another approach to the dynamic presentation of inhibitors.Citation115 Proof-of-principle in vitro studies have demonstrated that such lipid membrane assemblies can function efficiently in inhibiting pathogen-mediated effects.Citation116,Citation117
Designer probiotics
A cheap and efficient way to achieve the necessary multivalency of inhibitive epitopes is their heterologous expression on the surface of probiotic bacteria.Citation118 The protective effects of probiotic bacteria against infections has long been appreciated, and has been systematically demonstrated, at least in some settings, in a range of trials (for reviews of some of these, see refs. Citation119 and Citation120). More recently, probiotic strains have also been used to reduce pathogen colonization of animals raised for human consumption. For example, treatment of broiler chicken with a multi-species probiotic consisting of bacteria isolated from the chicken gut prevented their colonization by Campylobacter jejuni.Citation121 The beneficial effects of probiotics are, to some extent, due to competitive exclusion of pathogenic bacteria from host binding sites,Citation122-Citation124 although this is challenging to demonstrate in vivo because of the complexity of the probiotics’ mechanisms of action. Over recent years, probiotics have been specifically engineered to mimic sugars on host receptors, thereby blocking the host cell binding of toxins released by pathogenic bacteria including ETEC, shiga toxin-producing E. coli (STEC) and V. cholerae.Citation125-Citation127 As a basis for these probiotics, non-pathogenic E. coli strains expressing a truncated lipopolysaccharide (LPS) core terminating in a glucose residue were used. Transformation of these strains with constructs encoding heterologous glycosyltransferases from Neisseria gonorrhoeae and C. jejuni resulted in the production of chimeric LPS where the terminal glucose is conjugated to oligosaccharides mimicking the functionalities of host cell receptors. For example, a strain expressing LPS terminating in Galα(1→4)Galβ(1→4)Glc was used to mimic globotriaosyl ceramide (Gb3), a glycolipid receptor of the structure Galα(1→4)Galβ(1→4)Glc-ceramide recognized by shiga toxins. The recombinant strain efficiently bound free shiga toxin and protected mice against an otherwise lethal dose of STEC after oral administration.Citation128 The use of probiotics for host receptor mimicry has been reviewed in more detail elsewhereCitation118,Citation126,Citation129 but the message should be that such agents could be a viable and cheap way to achieve efficient presentation of multivalent epitopes for anti-adhesion therapies.
Outlook and Future Prospects
Despite the advances made in recent years, which have brought many anti-adhesion therapies within the realms of possibility, there is still much progress to be made to make these approaches applicable on a large scale. Further advancements will be achieved by improving the stability and affinity of currently available compounds and by developing combinatorial approaches to therapy (e.g., improving the efficacy of conventional antimicrobials against biofilms by combining it with an anti-adhesive). Discovery of novel leads will rely on the use of high-throughput screening methods for bacterial adhesion or for evaluation of the impact of bacterial adhesion on tissues.Citation130,Citation131 Where possible, efforts should be made to test more inhibitors in in vivo settings or at least under physiologically relevant conditions. Parameters not usually present in ex vivo settings, such as fluid dynamics and shear stress, can have a large impact and even reverse the outcome of inhibition studies and their influence on the experimental outcome can be counterintuitive.Citation132
Finally, we would like to point out that the repertoire of anti-adhesive strategies is by far not exploited. For example, a recent study tested the concept of interfering with the mechanical compliance of pili to decrease bacterial adhesion. Bacteria can withstand high amounts of fluid shear and this is in part due to their ability to redistribute external forces among a large number of adhesive surface structures such as pili. When exposed to force, pili can reversibly extend by the uncoiling and recoiling of their quaternary structure. Compounds interfering with the pilis’ structural dynamics (coilicides) could potentially enable bacterial surface detachment. In a proof-of-principle experiment, the purified pilin PapD was shown to impair recoiling of P pili fibers, thus leaving them unable to withstand flow.Citation133 This just goes to show that new and unconventional approaches targeting bacterial adhesion may be conceived, revealing new targets for anti-adhesion therapy.
| Abbreviations: | ||
| ClfA | = | clumping factor A |
| CAN | = | collagen-binding protein |
| EHEC | = | enterohemorrhagic E. coli |
| EPEC | = | enteropathogenic E. coli |
| ETEC | = | enterotoxigenic E. coli |
| FnbpA | = | fibronectin binding protein A |
| Gal | = | galactose |
| Glc | = | glucose |
| GSL | = | glycosphingolipid |
| LPS | = | lipopolysaccharide |
| LT | = | heat-labile toxin |
| MAM | = | multivalent adhesion molecule |
| PAK | = | P. aeruginosa strain K |
| SA | = | streptococcal antigen |
| Srt | = | Sortase |
| STa | = | shiga toxin subunit A |
| STEC | = | shiga toxin producing E. coli |
| UPEC | = | uropathogenic E. coli |
| UTI | = | urinary tract infection |
Acknowledgments
We thank the Orth and Krachler labs for critical reading and comments on the manuscript. A.M.K is supported by an EMBO Fellowship (ALTF 938-2011) and is a Birmingham Fellow. K.O. is supported by grants from NIH-Allergy and Infectious Disease (R01-AI056404 and R01-AI087808) and the Welch Foundation (I-1561). K.O. is a Burroughs Wellcome Investigator in Pathogenesis of Infectious Disease and a W.W. Caruth Jr. Biomedical Scholar.
Note
We would like to remark that this review is far from being comprehensive and can only act in exemplifying selected recent studies underlining emerging concepts of anti-adhesion strategies. We would like to apologize for any omissions we had to make due to space limitations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hayes CS, Aoki SK, Low DA. Bacterial contact-dependent delivery systems. Annu Rev Genet 2010; 44:71 - 90; http://dx.doi.org/10.1146/annurev.genet.42.110807.091449; PMID: 21047256
- Winnen B, Schlumberger MC, Sturm A, Schüpbach K, Siebenmann S, Jenny P, et al. Hierarchical effector protein transport by the Salmonella Typhimurium SPI-1 type III secretion system. PLoS One 2008; 3:e2178; http://dx.doi.org/10.1371/journal.pone.0002178; PMID: 18478101
- Schlumberger MC, Müller AJ, Ehrbar K, Winnen B, Duss I, Stecher B, et al. Real-time imaging of type III secretion: Salmonella SipA injection into host cells. Proc Natl Acad Sci U S A 2005; 102:12548 - 53; http://dx.doi.org/10.1073/pnas.0503407102; PMID: 16107539
- Zhou D, Galán J. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect 2001; 3:1293 - 8; http://dx.doi.org/10.1016/S1286-4579(01)01489-7; PMID: 11755417
- Neunuebel MR, Chen Y, Gaspar AH, Backlund PS Jr., Yergey A, Machner MP. De-AMPylation of the small GTPase Rab1 by the pathogen Legionella pneumophila. Science 2011; 333:453 - 6; http://dx.doi.org/10.1126/science.1207193; PMID: 21680813
- Krachler AM, Ham H, Orth K. Outer membrane adhesion factor multivalent adhesion molecule 7 initiates host cell binding during infection by gram-negative pathogens. Proc Natl Acad Sci U S A 2011; 108:11614 - 9; http://dx.doi.org/10.1073/pnas.1102360108; PMID: 21709226
- Kim YR, Lee SE, Kook H, Yeom JA, Na HS, Kim SY, et al. Vibrio vulnificus RTX toxin kills host cells only after contact of the bacteria with host cells. Cell Microbiol 2008; 10:848 - 62; http://dx.doi.org/10.1111/j.1462-5822.2007.01088.x; PMID: 18005241
- Yuehuei H. An, Richard B. Dickinson, Doyle RJ. Molecular Basis of Bacterial Adhesion. In: Yuehuei H. An, Friedman RJ, eds. Handbook of Bacterial Adhesion Principles, Methods, and Applications: Springer, 2000:29-41.
- Anderson BN, Ding AM, Nilsson LM, Kusuma K, Tchesnokova V, Vogel V, et al. Weak rolling adhesion enhances bacterial surface colonization. J Bacteriol 2007; 189:1794 - 802; http://dx.doi.org/10.1128/JB.00899-06; PMID: 17189376
- Breines DM, Burnham JC. Modulation of Escherichia coli type 1 fimbrial expression and adherence to uroepithelial cells following exposure of logarithmic phase cells to quinolones at subinhibitory concentrations. J Antimicrob Chemother 1994; 34:205 - 21; http://dx.doi.org/10.1093/jac/34.2.205; PMID: 7814281
- Dal SM, Bovio C, Culici M, Braga PC. The combination of the SH metabolite of erdosteine (a mucoactive drug) and ciprofloxacin increases the inhibition of bacterial adhesiveness achieved by ciprofloxacin alone. Drugs Exp Clin Res 2002; 28:75 - 82; PMID: 12224380
- Wojnicz D, Jankowski S. Effects of subinhibitory concentrations of amikacin and ciprofloxacin on the hydrophobicity and adherence to epithelial cells of uropathogenic Escherichia coli strains. Int J Antimicrob Agents 2007; 29:700 - 4; http://dx.doi.org/10.1016/j.ijantimicag.2007.01.007; PMID: 17382520
- Chen SL, Hung CS, Xu J, Reigstad CS, Magrini V, Sabo A, et al. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc Natl Acad Sci U S A 2006; 103:5977 - 82; http://dx.doi.org/10.1073/pnas.0600938103; PMID: 16585510
- Svensson A, Larsson A, Emtenäs H, Hedenström M, Fex T, Hultgren SJ, et al. Design and evaluation of pilicides: potential novel antibacterial agents directed against uropathogenic Escherichia coli. Chembiochem 2001; 2:915 - 8; http://dx.doi.org/10.1002/1439-7633(20011203)2:12<915::AID-CBIC915>3.0.CO;2-M; PMID: 11948880
- Pinkner JS, Remaut H, Buelens F, Miller E, Aberg V, Pemberton N, et al. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc Natl Acad Sci U S A 2006; 103:17897 - 902; http://dx.doi.org/10.1073/pnas.0606795103; PMID: 17098869
- Chorell E, Pinkner JS, Phan G, Edvinsson S, Buelens F, Remaut H, et al. Design and synthesis of C-2 substituted thiazolo and dihydrothiazolo ring-fused 2-pyridones: pilicides with increased antivirulence activity. J Med Chem 2010; 53:5690 - 5; http://dx.doi.org/10.1021/jm100470t; PMID: 20586493
- Chorell E, Pinkner JS, Bengtsson C, Banchelin TS, Edvinsson S, Linusson A, et al. Mapping pilicide anti-virulence effect in Escherichia coli, a comprehensive structure-activity study. Bioorg Med Chem 2012; 20:3128 - 42; http://dx.doi.org/10.1016/j.bmc.2012.01.048; PMID: 22464688
- Chorell E, Pinkner JS, Bengtsson C, Edvinsson S, Cusumano CK, Rosenbaum E, et al. Design and synthesis of fluorescent pilicides and curlicides: bioactive tools to study bacterial virulence mechanisms. Chemistry 2012; 18:4522 - 32; http://dx.doi.org/10.1002/chem.201103936; PMID: 22431310
- Cho JA, Chinnapen DJ, Aamar E, Te Welscher YM, Lencer WI, Massol R. Insights on the trafficking and retro-translocation of glycosphingolipid-binding bacterial toxins. Front Cell Infect Microbiol 2012; 2:51; http://dx.doi.org/10.3389/fcimb.2012.00051; PMID: 22919642
- Hartlova A, Cerveny L, Hubalek M, Krocova Z, Stulik J. Membrane rafts: a potential gateway for bacterial entry into host cells. Microbiol Immunol 2010; 54:237 - 45; http://dx.doi.org/10.1111/j.1348-0421.2010.00198.x; PMID: 20377752
- Radin NS. Preventing the binding of pathogens to the host by controlling sphingolipid metabolism. Microbes Infect 2006; 8:938 - 45; http://dx.doi.org/10.1016/j.micinf.2005.09.005; PMID: 16460984
- Svensson M, Frendeus B, Butters T, Platt F, Dwek R, Svanborg C. Glycolipid depletion in antimicrobial therapy. Mol Microbiol 2003; 47:453 - 61; http://dx.doi.org/10.1046/j.1365-2958.2003.03306.x; PMID: 12519195
- Svensson M, Platt FM, Svanborg C. Glycolipid receptor depletion as an approach to specific antimicrobial therapy. FEMS Microbiol Lett 2006; 258:1 - 8; http://dx.doi.org/10.1111/j.1574-6968.2006.00175.x; PMID: 16630247
- Pastores GM, Barnett NL, Kolodny EH. An open-label, noncomparative study of miglustat in type I Gaucher disease: efficacy and tolerability over 24 months of treatment. Clin Ther 2005; 27:1215 - 27; http://dx.doi.org/10.1016/j.clinthera.2005.08.004; PMID: 16199246
- Margalit M, Ash N, Zimran A, Halkin H. Enzyme replacement therapy in the management of longstanding skeletal and soft tissue salmonella infection in a patient with Gaucher’s disease. Postgrad Med J 2002; 78:564 - 5; http://dx.doi.org/10.1136/pmj.78.923.564; PMID: 12357022
- Sharon N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim Biophys Acta 2006; 1760:527 - 37; http://dx.doi.org/10.1016/j.bbagen.2005.12.008; PMID: 16564136
- Sharon N, Ofek I. Safe as mother’s milk: carbohydrates as future anti-adhesion drugs for bacterial diseases. Glycoconj J 2000; 17:659 - 64; http://dx.doi.org/10.1023/A:1011091029973; PMID: 11421356
- Shoaf-Sweeney KD, Hutkins RW. Adherence, anti-adherence, and oligosaccharides preventing pathogens from sticking to the host. Adv Food Nutr Res 2009; 55:101 - 61; PMID: 18772103
- Mulvey G, Kitov PI, Marcato P, Bundle DR, Armstrong GD. Glycan mimicry as a basis for novel anti-infective drugs. Biochimie 2001; 83:841 - 7; http://dx.doi.org/10.1016/S0300-9084(01)01291-3; PMID: 11530217
- Aronson M, Medalia O, Schori L, Mirelman D, Sharon N, Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis 1979; 139:329 - 32; http://dx.doi.org/10.1093/infdis/139.3.329; PMID: 376757
- Firon N, Ashkenazi S, Mirelman D, Ofek I, Sharon N. Aromatic alpha-glycosides of mannose are powerful inhibitors of the adherence of type 1 fimbriated Escherichia coli to yeast and intestinal epithelial cells. Infect Immun 1987; 55:472 - 6; PMID: 3542836
- Almant M, Moreau V, Kovensky J, Bouckaert J, Gouin SG. Clustering of Escherichia coli type-1 fimbrial adhesins by using multimeric heptyl α-D-mannoside probes with a carbohydrate core. Chemistry 2011; 17:10029 - 38; http://dx.doi.org/10.1002/chem.201100515; PMID: 21774001
- Schierholt A, Hartmann M, Lindhorst TK. Bi- and trivalent glycopeptide mannopyranosides as inhibitors of type 1 fimbriae-mediated bacterial adhesion: variation of valency, aglycon and scaffolding. Carbohydr Res 2011; 346:1519 - 26; http://dx.doi.org/10.1016/j.carres.2011.04.023; PMID: 21645881
- Richards SJ, Jones MW, Hunaban M, Haddleton DM, Gibson MI. Probing bacterial-toxin inhibition with synthetic glycopolymers prepared by tandem post-polymerization modification: role of linker length and carbohydrate density. Angew Chem Int Ed Engl 2012; 51:7812 - 6; http://dx.doi.org/10.1002/anie.201202945; PMID: 22715146
- Han Z, Pinkner JS, Ford B, Obermann R, Nolan W, Wildman SA, et al. Structure-based drug design and optimization of mannoside bacterial FimH antagonists. J Med Chem 2010; 53:4779 - 92; http://dx.doi.org/10.1021/jm100438s; PMID: 20507142
- Han Z, Pinkner JS, Ford B, Chorell E, Crowley JM, Cusumano CK, et al. Lead optimization studies on FimH antagonists: discovery of potent and orally bioavailable ortho-substituted biphenyl mannosides. J Med Chem 2012; 55:3945 - 59; http://dx.doi.org/10.1021/jm300165m; PMID: 22449031
- Scharenberg M, Schwardt O, Rabbani S, Ernst B. Target Selectivity of FimH Antagonists. J Med Chem 2012; 55:9810 - 6; http://dx.doi.org/10.1021/jm3010338; PMID: 23088608
- Hartmann M, Papavlassopoulos H, Chandrasekaran V, Grabosch C, Beiroth F, Lindhorst TK, et al. Inhibition of bacterial adhesion to live human cells: activity and cytotoxicity of synthetic mannosides. FEBS Lett 2012; 586:1459 - 65; http://dx.doi.org/10.1016/j.febslet.2012.03.059; PMID: 22673511
- Klein T, Abgottspon D, Wittwer M, Rabbani S, Herold J, Jiang X, et al. FimH antagonists for the oral treatment of urinary tract infections: from design and synthesis to in vitro and in vivo evaluation. J Med Chem 2010; 53:8627 - 41; http://dx.doi.org/10.1021/jm101011y; PMID: 21105658
- Jiang X, Abgottspon D, Kleeb S, Rabbani S, Scharenberg M, Wittwer M, et al. Antiadhesion therapy for urinary tract infections--a balanced PK/PD profile proved to be key for success. J Med Chem 2012; 55:4700 - 13; http://dx.doi.org/10.1021/jm300192x; PMID: 22519985
- Shmuely H, Ofek I, Weiss EI, Rones Z, Houri-Haddad Y. Cranberry components for the therapy of infectious disease. Curr Opin Biotechnol 2012; 23:148 - 52; http://dx.doi.org/10.1016/j.copbio.2011.10.009; PMID: 22088310
- Labrecque J, Bodet C, Chandad F, Grenier D. Effects of a high-molecular-weight cranberry fraction on growth, biofilm formation and adherence of Porphyromonas gingivalis. J Antimicrob Chemother 2006; 58:439 - 43; http://dx.doi.org/10.1093/jac/dkl220; PMID: 16735419
- Burger O, Ofek I, Tabak M, Weiss EI, Sharon N, Neeman I. A high molecular mass constituent of cranberry juice inhibits helicobacter pylori adhesion to human gastric mucus. FEMS Immunol Med Microbiol 2000; 29:295 - 301; http://dx.doi.org/10.1111/j.1574-695X.2000.tb01537.x; PMID: 11118911
- Burger O, Weiss E, Sharon N, Tabak M, Neeman I, Ofek I. Inhibition of Helicobacter pylori adhesion to human gastric mucus by a high-molecular-weight constituent of cranberry juice. Crit Rev Food Sci Nutr 2002; 42:Suppl 279 - 84; http://dx.doi.org/10.1080/10408390209351916; PMID: 12058986
- Toivanen M, Ryynänen A, Huttunen S, Duricová J, Riihinen K, Törrönen R, et al. Binding of Neisseria meningitidis pili to berry polyphenolic fractions. J Agric Food Chem 2009; 57:3120 - 7; http://dx.doi.org/10.1021/jf803488s; PMID: 19281178
- O’May C, Tufenkji N. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl Environ Microbiol 2011; 77:3061 - 7; http://dx.doi.org/10.1128/AEM.02677-10; PMID: 21378043
- Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol 2002; 9:1558 - 62; PMID: 12121581
- Stapleton AE, Dziura J, Hooton TM, Cox ME, Yarova-Yarovaya Y, Chen S, et al. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc 2012; 87:143 - 50; http://dx.doi.org/10.1016/j.mayocp.2011.10.006; PMID: 22305026
- Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Koskela M, Uhari M. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ 2001; 322:1571; http://dx.doi.org/10.1136/bmj.322.7302.1571; PMID: 11431298
- Bonetta A, Di Pierro F. Enteric-coated, highly standardized cranberry extract reduces risk of UTIs and urinary symptoms during radiotherapy for prostate carcinoma. Cancer Manag Res 2012; 4:281 - 6; PMID: 22977312
- Signoretto C, Canepari P, Stauder M, Vezzulli L, Pruzzo C. Functional foods and strategies contrasting bacterial adhesion. Curr Opin Biotechnol 2012; 23:160 - 7; http://dx.doi.org/10.1016/j.copbio.2011.08.006; PMID: 21906930
- Van Klinken BJ, Dekker J, Büller HA, Einerhand AW. Mucin gene structure and expression: protection vs. adhesion. Am J Physiol 1995; 269:G613 - 27; PMID: 7491952
- Lillehoj EP, Kim BT, Kim KC. Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. Am J Physiol Lung Cell Mol Physiol 2002; 282:L751 - 6; PMID: 11880301
- Lindén SK, Sheng YH, Every AL, Miles KM, Skoog EC, Florin TH, et al. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog 2009; 5:e1000617; http://dx.doi.org/10.1371/journal.ppat.1000617; PMID: 19816567
- Parker P, Sando L, Pearson R, Kongsuwan K, Tellam RL, Smith S. Bovine Muc1 inhibits binding of enteric bacteria to Caco-2 cells. Glycoconj J 2010; 27:89 - 97; http://dx.doi.org/10.1007/s10719-009-9269-2; PMID: 19936918
- Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 2012; 22:1147 - 62; http://dx.doi.org/10.1093/glycob/cws074; PMID: 22513036
- Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr 2005; 25:37 - 58; http://dx.doi.org/10.1146/annurev.nutr.25.050304.092553; PMID: 16011458
- Donovan SM, Wang M, Li M, Friedberg I, Schwartz SL, Chapkin RS. Host-microbe interactions in the neonatal intestine: role of human milk oligosaccharides. Adv Nutr 2012; 3:450S - 5S; http://dx.doi.org/10.3945/an.112.001859; PMID: 22585924
- Simon PM, Goode PL, Mobasseri A, Zopf D. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect Immun 1997; 65:750 - 7; PMID: 9009338
- Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, et al. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012; 61:1417 - 25; http://dx.doi.org/10.1136/gutjnl-2011-301404; PMID: 22138535
- Thomas RJ. Receptor mimicry as novel therapeutic treatment for biothreat agents. Bioeng Bugs 2010; 1:17 - 30; http://dx.doi.org/10.4161/bbug.1.1.10049; PMID: 21327124
- Lee KK, Wong WY, Sheth HB, Hodges RS, Paranchych W, Irvin RT. Use of synthetic peptides in characterization of microbial adhesins. Methods Enzymol 1995; 253:115 - 31; http://dx.doi.org/10.1016/S0076-6879(95)53013-4; PMID: 7476380
- Yu L, Lee KK, Paranchych W, Hodges RS, Irvin RT. Use of synthetic peptides to confirm that the Pseudomonas aeruginosa PAK pilus adhesin and the Candida albicans fimbrial adhesin possess a homologous receptor-binding domain. Mol Microbiol 1996; 19:1107 - 16; http://dx.doi.org/10.1046/j.1365-2958.1996.454982.x; PMID: 8830267
- Relman D, Tuomanen E, Falkow S, Golenbock DT, Saukkonen K, Wright SD. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell 1990; 61:1375 - 82; http://dx.doi.org/10.1016/0092-8674(90)90701-F; PMID: 2364431
- Lehner T, Caldwell J, Smith R. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect Immun 1985; 50:796 - 9; PMID: 4066030
- Ma JK, Hunjan M, Smith R, Lehner T. Specificity of monoclonal antibodies in local passive immunization against Streptococcus mutans. Clin Exp Immunol 1989; 77:331 - 7; PMID: 2478321
- Munro GH, Evans P, Todryk S, Buckett P, Kelly CG, Lehner T. A protein fragment of streptococcal cell surface antigen I/II which prevents adhesion of Streptococcus mutans. Infect Immun 1993; 61:4590 - 8; PMID: 7691754
- Kelly CG, Younson JS, Hikmat BY, Todryk SM, Czisch M, Haris PI, et al. A synthetic peptide adhesion epitope as a novel antimicrobial agent. Nat Biotechnol 1999; 17:42 - 7; http://dx.doi.org/10.1038/5213; PMID: 9920267
- Younson J, Kelly C. The rational design of an anti-caries peptide against Streptococcus mutans. Mol Divers 2004; 8:121 - 6; http://dx.doi.org/10.1023/B:MODI.0000025655.93643.fa; PMID: 15209163
- Krachler AM, Orth K. Functional characterization of the interaction between bacterial adhesin multivalent adhesion molecule 7 (MAM7) protein and its host cell ligands. J Biol Chem 2011; 286:38939 - 47; http://dx.doi.org/10.1074/jbc.M111.291377; PMID: 21937438
- Krachler AM, Ham H, Orth K. Turnabout is fair play: use of the bacterial Multivalent Adhesion Molecule 7 as an antimicrobial agent. Virulence 2012; 3:68 - 71; http://dx.doi.org/10.4161/viru.3.1.18172; PMID: 22086133
- Krachler AM, Mende K, Murray C, Orth K. In vitro characterization of multivalent adhesion molecule 7-based inhibition of multidrug-resistant bacteria isolated from wounded military personnel. Virulence 2012; 3:389 - 99; http://dx.doi.org/10.4161/viru.20816; PMID: 22722243
- Putney SD, Burke PA. Improving protein therapeutics with sustained-release formulations. Nat Biotechnol 1998; 16:153 - 7; http://dx.doi.org/10.1038/nbt0298-153; PMID: 9487521
- Malik DK, Baboota S, Ahuja A, Hasan S, Ali J. Recent advances in protein and peptide drug delivery systems. Curr Drug Deliv 2007; 4:141 - 51; http://dx.doi.org/10.2174/156720107780362339; PMID: 17456033
- Saraogi I, Hamilton AD. Recent advances in the development of aryl-based foldamers. Chem Soc Rev 2009; 38:1726 - 43; http://dx.doi.org/10.1039/b819597h; PMID: 19587965
- Liskamp RM, Rijkers DT, Kruijtzer JA, Kemmink J. Peptides and proteins as a continuing exciting source of inspiration for peptidomimetics. Chembiochem 2011; 12:1626 - 53; http://dx.doi.org/10.1002/cbic.201000717; PMID: 21751324
- Eucker TP, Konkel ME. The cooperative action of bacterial fibronectin-binding proteins and secreted proteins promote maximal Campylobacter jejuni invasion of host cells by stimulating membrane ruffling. Cell Microbiol 2012; 14:226 - 38; http://dx.doi.org/10.1111/j.1462-5822.2011.01714.x; PMID: 21999233
- Lalezari JP, Henry K, O’Hearn M, Montaner JS, Piliero PJ, Trottier B, et al, TORO 1 Study Group. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med 2003; 348:2175 - 85; http://dx.doi.org/10.1056/NEJMoa035026; PMID: 12637625
- Ghosh S, Chakraborty K, Nagaraja T, Basak S, Koley H, Dutta S, et al. An adhesion protein of Salmonella enterica serovar Typhi is required for pathogenesis and potential target for vaccine development. Proc Natl Acad Sci U S A 2011; 108:3348 - 53; http://dx.doi.org/10.1073/pnas.1016180108; PMID: 21300870
- Wagner C, Hensel M. Adhesive mechanisms of Salmonella enterica. Adv Exp Med Biol 2011; 715:17 - 34; http://dx.doi.org/10.1007/978-94-007-0940-9_2; PMID: 21557055
- Bravo D, Blondel CJ, Hoare A, Leyton L, Valvano MA, Contreras I. Type IV(B) pili are required for invasion but not for adhesion of Salmonella enterica serovar Typhi into BHK epithelial cells in a cystic fibrosis transmembrane conductance regulator-independent manner. Microb Pathog 2011; 51:373 - 7; http://dx.doi.org/10.1016/j.micpath.2011.07.005; PMID: 21782926
- Raghunathan D, Wells TJ, Morris FC, Shaw RK, Bobat S, Peters SE, et al. SadA, a trimeric autotransporter from Salmonella enterica serovar Typhimurium, can promote biofilm formation and provides limited protection against infection. Infect Immun 2011; 79:4342 - 52; http://dx.doi.org/10.1128/IAI.05592-11; PMID: 21859856
- Zhang C, Zhang W. Escherichia coli K88ac fimbriae expressing heat-labile and heat-stable (STa) toxin epitopes elicit antibodies that neutralize cholera toxin and STa toxin and inhibit adherence of K88ac fimbrial E. coli. Clin Vaccine Immunol 2010; 17:1859 - 67; http://dx.doi.org/10.1128/CVI.00251-10; PMID: 20980482
- Gao X, Cai K, Li T, Wang Q, Hou X, Tian R, et al. Novel fusion protein protects against adherence and toxicity of enterohemorrhagic Escherichia coli O157:H7 in mice. Vaccine 2011; 29:6656 - 63; http://dx.doi.org/10.1016/j.vaccine.2011.06.106; PMID: 21742003
- Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner JS, Burlein J, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 1997; 276:607 - 11; http://dx.doi.org/10.1126/science.276.5312.607; PMID: 9110982
- Langermann S, Möllby R, Burlein JE, Palaszynski SR, Auguste CG, DeFusco A, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis 2000; 181:774 - 8; http://dx.doi.org/10.1086/315258; PMID: 10669375
- Lorenzo-Gómez MF, Padilla-Fernández B, García-Criado FJ, Mirón-Canelo JA, Gil-Vicente A, Nieto-Huertos A, et al. Evaluation of a therapeutic vaccine for the prevention of recurrent urinary tract infections versus prophylactic treatment with antibiotics. Int Urogynecol J 2013; 24:127 - 34; http://dx.doi.org/10.1007/s00192-012-1853-5; PMID: 22806485
- Cachia PJ, Hodges RS. Synthetic peptide vaccine and antibody therapeutic development: prevention and treatment of Pseudomonas aeruginosa. Biopolymers 2003; 71:141 - 68; http://dx.doi.org/10.1002/bip.10395; PMID: 12767116
- Sheth HB, Glasier LM, Ellert NW, Cachia P, Kohn W, Lee KK, et al. Development of an anti-adhesive vaccine for Pseudomonas aeruginosa targeting the C-terminal region of the pilin structural protein. Biomed Pept Proteins Nucleic Acids 1995; 1:141 - 8; PMID: 9346845
- Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 2012; 30:Suppl 2 B87 - 97; http://dx.doi.org/10.1016/j.vaccine.2012.01.033; PMID: 22607904
- Su EL, Snape MD. A combination recombinant protein and outer membrane vesicle vaccine against serogroup B meningococcal disease. Expert Rev Vaccines 2011; 10:575 - 88; http://dx.doi.org/10.1586/erv.11.32; PMID: 21604979
- Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant Meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis 2010; 51:1127 - 37; http://dx.doi.org/10.1086/656741; PMID: 20954968
- Carleton HA. Pathogenic bacteria as vaccine vectors: teaching old bugs new tricks. Yale J Biol Med 2010; 83:217 - 22; PMID: 21165341
- Hur J, Stein BD, Lee JH. A vaccine candidate for post-weaning diarrhea in swine constructed with a live attenuated Salmonella delivering Escherichia coli K88ab, K88ac, FedA, and FedF fimbrial antigens and its immune responses in a murine model. Can J Vet Res 2012; 76:186 - 94; PMID: 23277697
- Hur J, Lee JH. Development of a novel live vaccine delivering enterotoxigenic Escherichia coli fimbrial antigens to prevent post-weaning diarrhea in piglets. Vet Immunol Immunopathol 2012; 146:283 - 8; http://dx.doi.org/10.1016/j.vetimm.2012.02.002; PMID: 22417986
- Frey J. Biological safety concepts of genetically modified live bacterial vaccines. Vaccine 2007; 25:5598 - 605; http://dx.doi.org/10.1016/j.vaccine.2006.11.058; PMID: 17239999
- Arciola CR, Speziale P, Montanaro L. Perspectives on DNA vaccines. Targeting staphylococcal adhesins to prevent implant infections. Int J Artif Organs 2009; 32:635 - 41; PMID: 19882551
- Therrien R, Lacasse P, Grondin G, Talbot BG. Lack of protection of mice against Staphylococcus aureus despite a significant immune response to immunization with a DNA vaccine encoding collagen-binding protein. Vaccine 2007; 25:5053 - 61; http://dx.doi.org/10.1016/j.vaccine.2007.04.067; PMID: 17532546
- Gaudreau MC, Lacasse P, Talbot BG. Protective immune responses to a multi-gene DNA vaccine against Staphylococcus aureus. Vaccine 2007; 25:814 - 24; http://dx.doi.org/10.1016/j.vaccine.2006.09.043; PMID: 17027124
- Ofek I, Hasty DL, Sharon N. Anti-adhesion therapy of bacterial diseases: prospects and problems. FEMS Immunol Med Microbiol 2003; 38:181 - 91; http://dx.doi.org/10.1016/S0928-8244(03)00228-1; PMID: 14522453
- Wizemann TM, Adamou JE, Langermann S. Adhesins as targets for vaccine development. Emerg Infect Dis 1999; 5:395 - 403; http://dx.doi.org/10.3201/eid0503.990310; PMID: 10341176
- Sokurenko EV, Chesnokova V, Dykhuizen DE, Ofek I, Wu XR, Krogfelt KA, et al. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc Natl Acad Sci U S A 1998; 95:8922 - 6; http://dx.doi.org/10.1073/pnas.95.15.8922; PMID: 9671780
- Blondeau JM. What have we learned about antimicrobial use and the risks for Clostridium difficile-associated diarrhoea?. J Antimicrob Chemother 2009; 63:238 - 42; http://dx.doi.org/10.1093/jac/dkn477; PMID: 19028718
- Holzheimer RG. Antibiotic induced endotoxin release and clinical sepsis: a review. J Chemother 2001; 13:Spec No 1 159 - 72; PMID: 11936361
- Rogers TJ, Paton JC. Therapeutic strategies for Shiga toxin-producing Escherichia coli infections. Expert Rev Anti Infect Ther 2009; 7:683 - 6; http://dx.doi.org/10.1586/eri.09.51; PMID: 19681694
- Mintzer MA, Dane EL, O’Toole GA, Grinstaff MW. Exploiting dendrimer multivalency to combat emerging and re-emerging infectious diseases. Mol Pharm 2012; 9:342 - 54; http://dx.doi.org/10.1021/mp2005033; PMID: 22126461
- Tseng YT, Chang HT, Chen CT, Chen CH, Huang CC. Preparation of highly luminescent mannose-gold nanodots for detection and inhibition of growth of Escherichia coli. Biosens Bioelectron 2011; 27:95 - 100; http://dx.doi.org/10.1016/j.bios.2011.06.021; PMID: 21757332
- Durka M, Buffet K, Iehl J, Holler M, Nierengarten JF, Taganna J, et al. The functional valency of dodecamannosylated fullerenes with Escherichia coli FimH--towards novel bacterial antiadhesives. Chem Commun (Camb) 2011; 47:1321 - 3; http://dx.doi.org/10.1039/c0cc04468g; PMID: 21103505
- Schengrund CL. “Multivalent” saccharides: development of new approaches for inhibiting the effects of glycosphingolipid-binding pathogens. Biochem Pharmacol 2003; 65:699 - 707; http://dx.doi.org/10.1016/S0006-2952(02)01553-8; PMID: 12628483
- Pieters RJ. Intervention with bacterial adhesion by multivalent carbohydrates. Med Res Rev 2007; 27:796 - 816; http://dx.doi.org/10.1002/med.20089; PMID: 17022032
- Pieters RJ. Maximising multivalency effects in protein-carbohydrate interactions. Org Biomol Chem 2009; 7:2013 - 25; http://dx.doi.org/10.1039/b901828j; PMID: 19421435
- Endo T, Koizumi S. Large-scale production of oligosaccharides using engineered bacteria. Curr Opin Struct Biol 2000; 10:536 - 41; http://dx.doi.org/10.1016/S0959-440X(00)00127-5; PMID: 11042450
- Hyun H, Yui N. Ligand accessibility to receptor binding sites enhanced by movable polyrotaxanes. Macromol Biosci 2011; 11:765 - 71; http://dx.doi.org/10.1002/mabi.201000507; PMID: 21384556
- Kim J, Ahn Y, Park KM, Lee DW, Kim K. Glyco-pseudopolyrotaxanes: carbohydrate wheels threaded on a polymer string and their inhibition of bacterial adhesion. Chemistry 2010; 16:12168 - 73; http://dx.doi.org/10.1002/chem.201001538; PMID: 20859967
- Bricarello DA, Patel MA, Parikh AN. Inhibiting host-pathogen interactions using membrane-based nanostructures. Trends Biotechnol 2012; 30:323 - 30; http://dx.doi.org/10.1016/j.tibtech.2012.03.002; PMID: 22464596
- Bricarello DA, Mills EJ, Petrlova J, Voss JC, Parikh AN. Ganglioside embedded in reconstituted lipoprotein binds cholera toxin with elevated affinity. J Lipid Res 2010; 51:2731 - 8; http://dx.doi.org/10.1194/jlr.M007401; PMID: 20472870
- Shi J, Yang T, Kataoka S, Zhang Y, Diaz AJ, Cremer PS. GM1 clustering inhibits cholera toxin binding in supported phospholipid membranes. J Am Chem Soc 2007; 129:5954 - 61; http://dx.doi.org/10.1021/ja069375w; PMID: 17429973
- Paton AW, Morona R, Paton JC. Designer probiotics for prevention of enteric infections. Nat Rev Microbiol 2006; 4:193 - 200; http://dx.doi.org/10.1038/nrmicro1349; PMID: 16462752
- Khani S, Hosseini HM, Taheri M, Nourani MR, Imani Fooladi AA. Probiotics as an alternative strategy for prevention and treatment of human diseases: a review. Inflamm Allergy Drug Targets 2012; 11:79 - 89; http://dx.doi.org/10.2174/187152812800392832; PMID: 22280243
- Jeppsson B, Mangell P, Thorlacius H. Use of probiotics as prophylaxis for postoperative infections. Nutrients 2011; 3:604 - 12; http://dx.doi.org/10.3390/nu3050604; PMID: 22254113
- Ghareeb K, Awad WA, Mohnl M, Porta R, Biarnés M, Böhm J, et al. Evaluating the efficacy of an avian-specific probiotic to reduce the colonization of Campylobacter jejuni in broiler chickens. Poult Sci 2012; 91:1825 - 32; http://dx.doi.org/10.3382/ps.2012-02168; PMID: 22802174
- Mukai T, Asasaka T, Sato E, Mori K, Matsumoto M, Ohori H. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol Med Microbiol 2002; 32:105 - 10; http://dx.doi.org/10.1111/j.1574-695X.2002.tb00541.x; PMID: 11821231
- Servin AL, Coconnier MH. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol 2003; 17:741 - 54; http://dx.doi.org/10.1016/S1521-6918(03)00052-0; PMID: 14507585
- Campana R, Federici S, Ciandrini E, Baffone W. Antagonistic activity of Lactobacillus acidophilus ATCC 4356 on the growth and adhesion/invasion characteristics of human Campylobacter jejuni. Curr Microbiol 2012; 64:371 - 8; http://dx.doi.org/10.1007/s00284-012-0080-0; PMID: 22271268
- Paton AW, Morona R, Paton JC. A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nat Med 2000; 6:265 - 70; http://dx.doi.org/10.1038/73111; PMID: 10700227
- Paton AW, Jennings MP, Morona R, Wang H, Focareta A, Roddam LF, et al. Recombinant probiotics for treatment and prevention of enterotoxigenic Escherichia coli diarrhea. Gastroenterology 2005; 128:1219 - 28; http://dx.doi.org/10.1053/j.gastro.2005.01.050; PMID: 15887106
- Focareta A, Paton JC, Morona R, Cook J, Paton AW. A recombinant probiotic for treatment and prevention of cholera. Gastroenterology 2006; 130:1688 - 95; http://dx.doi.org/10.1053/j.gastro.2006.02.005; PMID: 16697733
- Pinyon RA, Paton JC, Paton AW, Botten JA, Morona R. Refinement of a therapeutic Shiga toxin-binding probiotic for human trials. J Infect Dis 2004; 189:1547 - 55; http://dx.doi.org/10.1086/383417; PMID: 15116289
- Paton AW, Morona R, Paton JC. Bioengineered bugs expressing oligosaccharide receptor mimics: toxin-binding probiotics for treatment and prevention of enteric infections. Bioeng Bugs 2010; 1:172 - 7; http://dx.doi.org/10.4161/bbug.1.3.10665; PMID: 21326923
- Gustke H, Kleene R, Loers G, Nehmann N, Jaehne M, Bartels KM, et al. Inhibition of the bacterial lectins of Pseudomonas aeruginosa with monosaccharides and peptides. Eur J Clin Microbiol Infect Dis 2012; 31:207 - 15; http://dx.doi.org/10.1007/s10096-011-1295-x; PMID: 21604096
- Otto K. Biophysical approaches to study the dynamic process of bacterial adhesion. Res Microbiol 2008; 159:415 - 22; http://dx.doi.org/10.1016/j.resmic.2008.04.007; PMID: 18550342
- Mascari L, Ymele-Leki P, Eggleton CD, Speziale P, Ross JM. Fluid shear contributions to bacteria cell detachment initiated by a monoclonal antibody. Biotechnol Bioeng 2003; 83:65 - 74; http://dx.doi.org/10.1002/bit.10650; PMID: 12740934
- Klinth JE, Pinkner JS, Hultgren SJ, Almqvist F, Uhlin BE, Axner O. Impairment of the biomechanical compliance of P pili: a novel means of inhibiting uropathogenic bacterial infections?. Eur Biophys J 2012; 41:285 - 95; http://dx.doi.org/10.1007/s00249-011-0784-2; PMID: 22237603