Abstract
Dynamic changes in microvascular endothelial structure and function are pivotal in the acute inflammatory response, the body’s rapid, coordinated effort to localize, sequester, and eliminate microbial invaders at their portal of entry. To achieve this, the endothelium becomes leaky and inflamed, providing innate immune cells and humoral effector molecules access to the site of infection. During sepsis this locally adaptive response becomes manifest throughout the body, leading to dangerous host consequences. Increased leakiness in the pulmonary circulation contributes to acute respiratory distress syndrome (ARDS), a complication of sepsis associated with 40% mortality. Understanding the molecular governance of vascular leak and inflammation has major diagnostic, prognostic, and potentially therapeutic implications for this common and pernicious disease. This review summarizes results from cell-based experiments, animal models, and observational human studies; together, these studies suggest that an endothelial receptor called Tie2 and its ligands, called angiopoietins, form a signaling axis key to the vascular dyshomeostasis that underlies sepsis.
Introduction
The vascular endothelium constitutes the innermost lining of the body’s circulatory system and the largest tissue in the body. In capillaries, this cell is the primary barrier between elements in the blood and the parenchymal cells that rely on perfusion to deliver nutrients and remove wastes. Rather than serving as a static structure, microvascular endothelium performs several essential functions: maintenance of a semipermeable barrier to water and biomolecules, regulation of leukocyte diapedesis through the expression of apical adhesion molecules, modulation of vascular tone, and fine-tuning of hemostasis. The manifestations of tissue inflammation readily apparent to the novice physical examiner—rubor, dolor, calor, and tumor—attest to the centrality and prominence of microvascular changes in inflammation. Yet, in the field of sepsis, the molecular pathogenesis of microvascular disruption has been relatively under-studied, with the focus instead being the initial interactions of innate immune effectors vs. invasive microbes.
Why the Cytokine Theory and the “Vasculo-Centric View” of Sepsis Are Not Mutually Exclusive
In the 1980s, investigations led by Beutler, Cerami, Dinarello, Tracey, and others identified the first host molecules responsible for the fever and shock response to bacteria: tumor necrosis factor-α (TNFα), interleukin-1 (IL-1), and interferon-gamma (IFNγ).Citation1-Citation3 In one landmark paper, Tracey and colleagues used three baboons intravenously injected with E. coli to demonstrate that neutralization of TNFα was sufficient to prevent death, without the administration of antibiotics!Citation4 This startling finding spawned a revolution in our understanding of sepsis, focusing the research community’s attention on an expanding list of cytokine mediators of the septic phenotype that were potentially targetable with recombinant drugs.
Against this backdrop of excitement, the first negative clinical trials of TNFα neutralization in sepsis were a grave disappointment. While follow-up pre-clinical studies suggested that better animal models of sepsis would have revealed the ineffectiveness of TNFα neutralization,Citation5 only 4% of subjects in the highest drug arm of one of these trials had detectable circulating levels of TNFα at the time of enrollment.Citation6 More recently, a large Phase 3 trial of a drug that inhibits the Toll-like receptor 4 (TLR4) failed to show benefit among patients with sepsis.Citation7 TLR4 is the body’s primary sensor of gram-negative endotoxin.Citation8 Its discovery was recognized by the 2011 Nobel Prize in Medicine because this research is considered foundational in immunology.
Do these examples imply that TNFα (or any other cytokine or bacterial product) does not contribute to sepsis pathogenesis in humans? Not at all. Rather, they illustrate the unique heterogeneity of sepsis as it comes through hospitals’ doors. Sufferers present to medical attention at different times in their illness with different pathogens and portals of entry, bearing genetic differences in their acute inflammatory response that are further compounded by age and comorbidities. By the time patients present for medical care, levels of early mediators like endotoxin or TNFα may well have waned.
The remarkable heterogeneity of early sepsis narrows down to a handful of near-stereotypical features as the patient approaches death: the development of shock and the progressive dysfunction of key organs. Working backward from this final pathway reveals a commonality—namely, that endothelial functions have become deranged. In the lungs, microvessel leak contributes to pulmonary edema. In the kidneys, leukocyte adhesion to glomerular and peritubular capillaries impairs perfusion and filtration. In the macrocirculation, inability to regulate vascular tone necessitates vasopressor drugs. Thus, based on empiricism alone, one could argue that patients with sepsis die not of the early induction of inflammatory cytokines, but rather, the late sequelae that impact the vasculature.
While such a distinction may be semantic, there are several practical implications. Focusing investigative effort on the septic vasculature may lead to the identification of novel molecular contributors to the disease. Measuring such molecules in patients may help segregate severe sepsis from milder forms or even identify individuals about to progress in disease severity. Intervening on such vascular molecular pathways may be more feasible than cytokine blockade simply because patients present hours to days after infection develops, not the timeframe of minutes to hours used in most pre-clinical models. By this “late” time point, levels of acute phase reactants such as TNFα have likely spiked and abated, setting off a biological chain reaction that culminates in clinical disease, but no longer present for neutralization in the bloodstream. Below, the author will summarize studies that implicate angiopoietin-1 and -2 (Angpt-1,-2) and their receptor, Tie-2, as an important candidate vascular pathway in sepsis.
Introduction to ANGPTs and TIEs
In 1992, Dumont and colleagues cloned a new transmembrane tyrosine kinase from endothelial DNA called tek.Citation9 The gene was later re-named Tie-2 after Sato et al. independently cloned both Tie-2 and the related receptor Tie-1 from a brain microvessel cDNA library.Citation10 Their PCR-based search for Tie-2 was in turn, based on a 1992 report from Alitalo’s group describing Tie-1.Citation11 Subsequent gene targeting experiments in mice revealed that Tie-2 expression was highly restricted to the endothelium and that its expression was essential to blood vessel maturation during embryonic development.Citation12 The homology of the extracellular domain to other receptor tyrosine kinases suggested the existence of peptide ligands. Through an innovative adaptation of expression cloning, scientists led by George Yancopoulos identified Angpt-1 and, soon thereafter, Angpt-2.Citation13,Citation14
Angpt-1 is largely made and secreted by peri-endothelial cells and platelets whereas Angpt-2 is synthesized in endothelium where pre-formed protein is stored for rapid release in granules called Weibel–Palade bodies.Citation15 To a lesser extent, Angpt-2 is also made by macrophages. Both bind Tie-2 with nanomolar affinity, and excess Angpt-2 competes Angpt-1 off the receptor, suggesting that the latter is a competitive antagonist of the former on endothelial cells.Citation13 Crystallographic results show that the C-terminal fibrinogen domain common to Angpt-1 and Angpt-2 binds to an “arrowhead” structure within the ectodomain of Tie-2 composed of two immunoglobulin folds and three epidermal growth factor domains.Citation16,Citation17 While the difference in downstream signaling achieved by these ligands is not completely explained, biochemical studies suggest that an N-terminal region unique to Angpt-1 favors its multimerization into large aggregates, leading to more intense Tie-2 clustering and greater cross-phosphorylation.Citation18 Consistent with this agonist-antagonist framework, the Angpt-1 knockout mouse and the Angpt-2 transgenic mouse phenocopy the vascular defects of the Tie-2 knockout mouse. Results described in the next section strongly suggest that Angpt-1 and Angpt-2 also have opposing functions in the setting of inflammation ().
Figure 1. The angiopoietin–Tie-2 axis in sepsis and acute respiratory distress syndrome. In quiescence, clusters of angiopoietin-1 (Angpt-1) aggregate and activate the transmembrane receptor tyrosine kinase, Tie-2, which is highly specifically expressed on endothelial cells. Tie-2 signals into the cell to favor phenotypes such as fortification of barrier function. In sepsis, angiopoietin-2 (Angpt-2) is upregulated and is believed to antagonize Angpt-1. The tonic homeostatic signaling through Tie-2 (pTie-2, phosphorylated Tie-2) is attenuated, contributing to the vascular leak and inflammation observed in sepsis and related conditions.
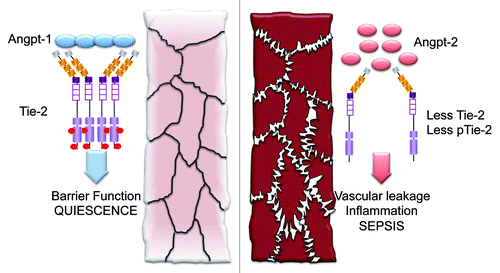
Other molecules in the Angpt–Tie pathway include a paralog of Angpt-1 called Angpt-3/4 that also activates Tie-2Citation19 and a paralog of Tie-2 called Tie-1 that has no agreed-upon ligand and is thought to inhibit Tie-2 signaling by heterodimerizing with it.Citation11,Citation20-Citation22 A naturally occurring extracellular cleavage product of Tie-2 may exert dominant-negative effects,Citation23 and a transmembrane tyrosine phosphatase called VE-PTP also attenuates Tie-2 signaling by removing phosphate from key tyrosines in Tie-2’s intracellular domain.Citation24,Citation25 Specific integrins appear to be alternative receptors for Angpts.Citation26,Citation27 Finally, a growing number of Angpt-like proteins have been cloned, but they do not appear to act on endothelial cells per se or signal through Tie-2. To summarize the most rigorously tested hypotheses in a straightforward fashion, the rest of this review will focus on Angpt-1, Angpt-2, and Tie-2.
Tie-2 Signaling and Functional Consequences in Experimental Inflammation
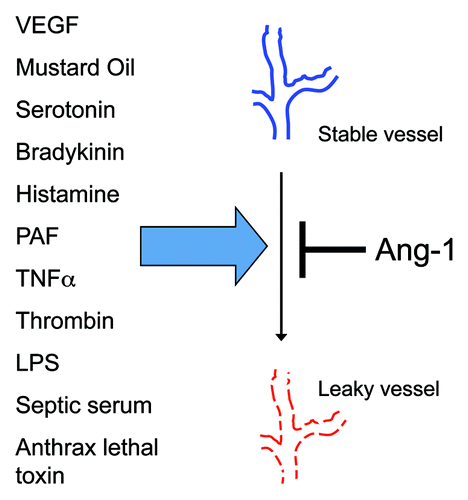
In 1997, Wong, et al. showed that Tie-2 was not only expressed in the mature, non-angiogenic adult vasculature, but was also substantially phosphorylated.Citation28 This important description suggested that Tie-2 signaling aided one or more maintenance functions in the mature endothelium. Based on the fact that Tie-2 expression was necessary for nascent blood vessels to develop into mature vessels during embryogenesis, collaborative studies led by Yancopoulos and McDonald hypothesized that Tie-2 signaling may similarly “stabilize” non-angiogenic blood vessels. Using Angpt-1 transgenic mice and adenoviral Angpt-1 gene transfer, they showed that excess Angpt-1 prevented vascular leak induced by disparate stimuli, including vascular endothelial growth factor (VEGF), mustard oil, and serotonin.Citation29,Citation30
The list of permeability mediators against which Angpt-1 defends barrier function was soon extended to gram-negative endotoxin. Witzenbichler et al. demonstrated that excess Angpt-1 confers a survival benefit in murine endotoxemia associated with less vascular leakage and less cellular inflammation.Citation31 Mammoto et al. showed that Angpt-1 prevents endotoxin-induced leak and inflammation by signaling through phosphatidylinositol-3-kinase (PI3-K) and Akt to regulators of the endothelial actin cytoskeleton called Rac1 and RhoA.Citation32 This work provided the mechanistic complement to a live microscopy study of rat tracheal microvessels performed by Baffert et al. that strongly implicated junctional and cytoskeletal remodeling in Angpt-1-mediated barrier defense.Citation33 Finally, Brindle’s group showed that Angpt-1 application to endothelial cells induced an inhibitor of the canonical inflammatory transcription factor NFκB.Citation34 Together, these results described a novel phenomenon—vascular barrier defense against diverse ligands mediated by Angpt-1—and a molecular mechanism to account for this remarkable effect (). More recent studies from the laboratories of Vestweber, Deutsch, Koh, Olsen, Mochizuki and Alitalo present compelling evidence for important phenotypic differences arising from Tie-2’s localization within the cell membrane and its downstream signaling partners.Citation35-Citation38
Figure 2. Angiopoietin-1 ameliorates endothelial barrier dysfunction induced by diverse ligands. The ability of excess angiopoietin-1 (Angpt-1) to prevent vascular leakage induced by diverse mediators of permeability, all of which act through unique cell surface receptors or have incompletely known mechanisms of action, suggests that Angpt-1-induced Tie-2 activation impacts a final common pathway for permeability, such as the remodeling of intercellular junctions and the actin cytoskeleton. PAF, platelet activating factor; TNFα, tumor necrosis factor α; LPS, lipopolysaccharides.
During this period, a converse set of findings for Angpt-2 was emerging from several laboratories. In 2006, Parikh et al. reported that circulating Angpt-2 was elevated in humans with severe sepsis and that acute disruption of Tie-2 weakened endothelial barrier function.Citation39 Their findings suggested a positive association with sepsis-associated ARDS and a mechanism whereby Tie-2 inhibition forced the contraction of endothelial cells through remodeling of the cytoskeleton. Shortly thereafter, a group led by Augustin used knockout mice and siRNA to show that Angpt-2 sensitizes the endothelium to inflammation by dose-dependently inducing vascular cell adhesion molecule-1 (VCAM-1),Citation40 and an independent study from teams led by Elias and Matthay used knockout mice, siRNA, and bronchoalveolar lavage samples from people with ARDS to implicate Angpt-2 in inflammatory acute lung injury.Citation41 Augustin’s group also showed that Angpt-2 synthesized by endothelial cells is stored in Weibel–Palade bodies and rapidly exocytosed upon stimulation with different inflammatory mediators such as thrombin.Citation15 Since sepsis in humans has been associated with a sustained elevation in Angpt-2, there is likely ongoing transcription and translation to generate Angpt-2 protein de novo, but the mechanisms are not well-understood. Finally, two recent reports suggest that Angpt-2 may actually be beneficial in acute infection-related inflammation,Citation42,Citation43 perhaps through an agonistic effect on Tie-2,Citation44,Citation45 but additional data may be needed to understand why these results refute the larger literature.Citation46
Relatively few experimental studies in sepsis have focused directly on Tie-2, where the prediction would be that reduced Tie-2 signaling is associated with adverse outcomes. Stewart’s group applied intratracheal endotoxin to show that Tie-2 heterozygous mice develop worse lung injury and earlier mortality than wild-type littermates.Citation47 They also reported that levels of total and phosphorylated Tie-2 were depressed in whole lung homogenates of endotoxin-challenged mice. Using systemic endotoxin to model sepsis, David et al. also observed a decrease in total Tie-2 expression and a fall in the phosphorylated fraction of the receptor, arguing for a “two-hit” model of impaired Tie-2 signaling that results from a combination of receptor antagonism and reduced expression.Citation48
David and colleagues also applied a peptide mimetic of Angpt-1 (identified by phage display experiments and bearing no sequence homology to Angpt-1) called vasculotideCitation49 to endotoxemic mice and showed an improvement in Tie-2 expression, Tie-2 phosphorylation, endothelial barrier function, vascular permeability, and survival in endotoxemia. This same group also applied cecal-ligation-puncture to mice and observed similar beneficial effects of vasculotide, even demonstrating a rescue effect after sepsis induction.Citation50 While the therapeutic potential of vasculotide may one day be realized, the mechanistic implication of these experiments is clear: by showing that a completely non-homologous Tie-2 activator achieves similar molecular and physiological effects to Angpt-1 in septic mice, the vasculotide data independently corroborate the importance of Tie-2 signaling (vs. non-canonical angiopoietin receptors such as integrins) in septic vascular phenotypes.
Proof-of-Concept Studies in Humans
No targeted therapies and few biomarkers inspired by pre-clinical studies have successfully translated to advances in the care of patients with sepsis or ARDS. There are many reasons for the chasm between mouse studies and human disease.Citation51 As proposed above, the focus on translating innate immune effectors may be one factor in this divide: (1) the circulating leukocyte pool in mice is shifted toward lymphocytes whereas human WBC counts are dominated by neutrophils, (2) molecular aspects of acute inflammation may be different as well,Citation52 and finally, (3) the temporo-regional complexity and redundancy of this highly evolved response may be impossible to summate into a single measurement or target. Early human testing of pre-clinical observations may streamline the process of molecular discovery and application in critical illness. The availability of commercial ELISAs for Angpt-1 and Angpt-2 has facilitated efforts to validate the involvement of this pathway in human sepsis and ARDS ().
Table 1. Human findings in the Angpt–Tie2 axis related to sepsis and ARDS
Intriguing genetic and biochemical evidence suggests that tonic Tie-2 activation in the mature quiescent vasculature could be a ligand-independent phenomenon.Citation53,Citation54 Nonetheless, Angpt-1 is poised to mediate this effect since it is made and secreted by platelets and by cells adjacent to the endothelium. The N-terminal region of Angpt-1 may even promote local adherence to the extracellular matrix,Citation29 leading to a high tissue concentration despite low circulating levels. In sepsis, ARDS, and related conditions, circulating Angpt-1 appears to be suppressed (),Citation55,Citation56 consistent with the experimental observation that Tie-2 phosphorylation falls. The mechanisms driving Angpt-1 suppression in these settings are not known. However, the magnitude of Angpt-1’s decline tends to be 2- to 3-fold or less, compared with ~5- to 20-fold increase in circulating Angpt-2 observed under similar conditions. As shown in , an Angpt-2/Angpt-1 ratio may outperform Angpt-1 alone in clinical correlations. In addition to non-covalent interactions favoring Angpt-1’s adherence to the matrix, some studies of circulating Angpt-1 may also be confounded by its measurement in serum, where ex vivo platelet aggregation may release Angpt-1 and lead to artifactual elevation.Citation57
Circulating Angpt-2 concentrations have a much broader dynamic range than Angpt-1. In 2006, Parikh et al. reported 10- to 20-fold elevation in circulating Angpt-2 among individuals with severe sepsis at the time of ICU admission compared with those with uncomplicated sepsis and hospitalized controls. The authors noted that subjects with severe sepsis developed higher peak Angpt-2 concentrations than those with uncomplicated sepsis and further observed that individuals with impaired lung gas exchange had higher peak Angpt-2 values than those with normal gas exchange.Citation39 Combined with animal and cellular data, the authors speculated that Angpt-2 may be both a marker and mediator of vascular leakage during sepsis. Studies led by Bhandari and van der Heijden independently corroborated these concepts by showing accumulation of Angpt-2 in the alveolar fluid of patients with acute lung injury (ALI) and a positive correlation between Angpt-2 and quantitative measures of fluid extravasation in the lungs.Citation41,Citation58
The association between high Angpt-2 and ALI/ARDS has been borne out in surgical populations,Citation59 non-infection-associated ALI,Citation60 and in primary graft dysfunction following lung transplantation.Citation61 The link between Angpt-2 and pulmonary vascular leak may be coincidental or a product of the ease with which hyperpermeability in this vascular bed can be detected. It could also relate to the fact that the lung contains such an extensive capillary network that endothelial cells—i.e., cells expressing the Tie-2 receptor as well the primary source of Angpt-2—constitute nearly 10% of the total cell population.
Further evidence linking Angpt-2 to acute vascular leakage throughout the body has come from studies of patients receiving the immune stimulator IL-2 for cancer therapy—whose dose-limiting toxicity is shock from vascular hyperpermeability—and from careful correlations of fluid balance in the ICU to serial Angpt-2 values.Citation60,Citation62,Citation63 Finally, induction of circulating Angpt-2 has also been reported in other conditions associated with acute vascular leakage, including severe malaria,Citation64,Citation65 systemic anthrax (in baboons, not yet studied in humans),Citation66 acute pancreatitis,Citation67 polytrauma,Citation68,Citation69 and bacterial toxic shock syndrome.Citation70
Possibilities for Applying ANGPTs to Improve Patient Care
Is Angiopoietin-2 or Angpt-2/Angpt-1 a biomarker of sepsis or ARDS? Measurement of these proteins could be used for diagnosis and/or prognosis in afflicted or at-risk individuals for critical illness. This kind of information could be particularly useful in resource-limited settings, where a quantitative, operator-independent tool could be deployed for triaging incoming patients to more intensive care and monitoring, such as battlefields. They could help risk-stratify patients in future ICU-based clinical trials to segregate patients into pathophysiological groups, even for interventions that do not per se intersect with the Angpt–Tie-2 axis. Criteria identified by Sir Austin Bradford Hill, an epidemiologist famous for linking cigarette smoke to lung cancer, may be instructive for considering the Angpts in a clinical application. These criteria are bolded below.Citation71
The experimental results outlined above highlight the biological plausibility of Angpt-2-driven features in sepsis and ARDS. Independent studies consistently report a strong association between higher Angpt-2 concentrations and disease severity measured variously by a clinical score of organ impairment, duration of ICU care, development of shock, or inpatient mortality. This association is proportional to sepsis severity in studies from different investigators.Citation55,Citation72,Citation73 The marked induction of circulating Angpt-2 appears to be specific to conditions of acute vascular injury and leakage ranging from sepsis to anthrax as noted above. Finally, the induction of Angpt-2 clearly precedes adverse outcomes, a point strongly illustrated in an emergency-ward based study of 270 adults suspected of infection in whom circulating Angpt-2 measured within the 1st hour of hospitalization predicted inpatient mortality with a receiver-operator characteristics (ROC) area under the curve of 0.91.Citation72 Larger studies will be needed to evaluate the utility of Angpt-2 cut-off values. In further support of temporal precedence, targeted genetic scans suggest that common variants in the ANGPT-2 locus that may affect gene expression are associated with ALI/ARDS.Citation74,Citation75
Since the initial elevation in Angpt-2 may be sustained for several days in severely ill patients,Citation63,Citation72 Angpt-2 may be more easily targeted for antibody-mediated neutralization compared with cytokines such as TNFα. Partial genetic deletion of Angpt-2 appears to be sufficient to attenuate inflammation, improve organ function, reduce vascular leak, and improve survival.Citation72 Antibodies specific for Angpt-2 have also been shown to prevent septic plasma-induced microvascular endothelial barrier dysfunction and to attenuate vascular remodeling and inflammation in a chronic lung infection model.Citation72,Citation76 Theoretically, Angpt-2 neutralization should restore basal Tie-2 phosphorylation, though this remains to be formally demonstrated. Other therapeutic approaches could drive Tie-2 activation to supra-physiological levels—e.g., recombinant Angpt-1 itself, a derivative thereof such as COMP-Angpt-1,Citation77 an unrelated agonist such as vasculotide,Citation49 administration of cells that express Angpt-1,Citation47 or even inhibition of the tyrosine phosphatase VE-PTP.Citation25
Summary
The cloning of Tie-2 was reported just over 20 years ago, and its major ligands were identified in the late 1990s. Since then, an accelerating body of work has demonstrated fascinating biology related to this pathway in cancer, vascular patterning, angiogenesis, lymphangiogenesis, inflammation, and vascular permeability. Drugs based on the Angpt–Tie-2 pathway have already been developed and are matriculating through clinical trials. The breathtaking pace at which clinical applications have been sought attests to the intense interest these proteins have generated in the biomedical community. In the field of sepsis, the traditional focus on early innate immune aspects of the host response is gradually broadening to consider the penultimate vascular changes that directly lead to the most damaging clinical manifestations of this disease. The Angpt–Tie-2 axis is a particularly strong candidate vascular pathway based on the remarkable convergence of experimental and human observational data. Tie-2 signaling impairment—via Angpt-2 induction and other potential mechanisms—may potentiate the vascular leak and inflammation induced by the early cytokine wave of sepsis. The ultimate proof of these concepts will require carefully designed clinical trials.
Disclosure of Potential Conflicts of Interest
Dr Parikh is listed as an inventor on disclosures regarding angiopoietins filed with Beth Israel Deaconess Medical Center.
Acknowledgments
The author would like to thank Drs Ananth Karumanchi, Sascha David, and Chandra Ghosh for critical feedback. Dr Parikh is supported by NIH grants R01-HL093234 and R01-DK095072 and by the American Diabetes Association (1-13-BS-141).
References
- Baracos V, Rodemann HP, Dinarello CA, Goldberg AL. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med 1983; 308:553 - 8; http://dx.doi.org/10.1056/NEJM198303103081002; PMID: 6402699
- Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 1985; 229:869 - 71; http://dx.doi.org/10.1126/science.3895437; PMID: 3895437
- Tracey KJ, Lowry SF, Cerami A. Cachectin/TNF mediates the pathophysiological effects of bacterial endotoxin/lipopolysaccharide (LPS). Prog Clin Biol Res 1988; 272:77 - 88; PMID: 3293084
- Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 1987; 330:662 - 4; http://dx.doi.org/10.1038/330662a0; PMID: 3317066
- Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol 1992; 148:2724 - 30; PMID: 1315357
- Fisher CJ Jr., Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, et al, The Soluble TNF Receptor Sepsis Study Group. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. N Engl J Med 1996; 334:1697 - 702; http://dx.doi.org/10.1056/NEJM199606273342603; PMID: 8637514
- Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, et al, ACCESS Study Group. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 2013; 309:1154 - 62; PMID: 23512062
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998; 282:2085 - 8; http://dx.doi.org/10.1126/science.282.5396.2085; PMID: 9851930
- Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML. tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene 1992; 7:1471 - 80; PMID: 1630810
- Sato TN, Qin Y, Kozak CA, Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci U S A 1993; 90:9355 - 8; http://dx.doi.org/10.1073/pnas.90.20.9355; PMID: 8415706
- Partanen J, Armstrong E, Mäkelä TP, Korhonen J, Sandberg M, Renkonen R, et al. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol 1992; 12:1698 - 707; PMID: 1312667
- Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 1994; 8:1897 - 909; http://dx.doi.org/10.1101/gad.8.16.1897; PMID: 7958865
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997; 277:55 - 60; http://dx.doi.org/10.1126/science.277.5322.55; PMID: 9204896
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996; 87:1171 - 80; http://dx.doi.org/10.1016/S0092-8674(00)81813-9; PMID: 8980224
- Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 2004; 103:4150 - 6; http://dx.doi.org/10.1182/blood-2003-10-3685; PMID: 14976056
- Barton WA, Tzvetkova D, Nikolov DB. Structure of the angiopoietin-2 receptor binding domain and identification of surfaces involved in Tie2 recognition. Structure 2005; 13:825 - 32; http://dx.doi.org/10.1016/j.str.2005.03.009; PMID: 15893672
- Barton WA, Tzvetkova-Robev D, Miranda EP, Kolev MV, Rajashankar KR, Himanen JP, et al. Crystal structures of the Tie2 receptor ectodomain and the angiopoietin-2-Tie2 complex. Nat Struct Mol Biol 2006; 13:524 - 32; http://dx.doi.org/10.1038/nsmb1101; PMID: 16732286
- Kim KT, Choi HH, Steinmetz MO, Maco B, Kammerer RA, Ahn SY, et al. Oligomerization and multimerization are critical for angiopoietin-1 to bind and phosphorylate Tie2. J Biol Chem 2005; 280:20126 - 31; http://dx.doi.org/10.1074/jbc.M500292200; PMID: 15769741
- Kim I, Kwak HJ, Ahn JE, So JN, Liu M, Koh KN, et al. Molecular cloning and characterization of a novel angiopoietin family protein, angiopoietin-3. FEBS Lett 1999; 443:353 - 6; http://dx.doi.org/10.1016/S0014-5793(99)00008-3; PMID: 10025962
- Maisonpierre PC, Goldfarb M, Yancopoulos GD, Gao G. Distinct rat genes with related profiles of expression define a TIE receptor tyrosine kinase family. Oncogene 1993; 8:1631 - 7; PMID: 7684830
- Seegar TC, Eller B, Tzvetkova-Robev D, Kolev MV, Henderson SC, Nikolov DB, et al. Tie1-Tie2 interactions mediate functional differences between angiopoietin ligands. Mol Cell 2010; 37:643 - 55; http://dx.doi.org/10.1016/j.molcel.2010.02.007; PMID: 20227369
- Yuan HT, Venkatesha S, Chan B, Deutsch U, Mammoto T, Sukhatme VP, et al. Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival. FASEB J 2007; 21:3171 - 83; http://dx.doi.org/10.1096/fj.07-8487com; PMID: 17504972
- Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peters K. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest 1997; 100:2072 - 8; http://dx.doi.org/10.1172/JCI119740; PMID: 9329972
- Bäumer S, Keller L, Holtmann A, Funke R, August B, Gamp A, et al. Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) activity is required for blood vessel development. Blood 2006; 107:4754 - 62; http://dx.doi.org/10.1182/blood-2006-01-0141; PMID: 16514057
- Winderlich M, Keller L, Cagna G, Broermann A, Kamenyeva O, Kiefer F, et al. VE-PTP controls blood vessel development by balancing Tie-2 activity. J Cell Biol 2009; 185:657 - 71; http://dx.doi.org/10.1083/jcb.200811159; PMID: 19451274
- Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol 2005; 170:993 - 1004; http://dx.doi.org/10.1083/jcb.200507082; PMID: 16157706
- Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest 2012; 122:1991 - 2005; http://dx.doi.org/10.1172/JCI58832; PMID: 22585576
- Wong AL, Haroon ZA, Werner S, Dewhirst MW, Greenberg CS, Peters KG. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res 1997; 81:567 - 74; http://dx.doi.org/10.1161/01.RES.81.4.567; PMID: 9314838
- Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 2000; 6:460 - 3; http://dx.doi.org/10.1038/74725; PMID: 10742156
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 1999; 286:2511 - 4; http://dx.doi.org/10.1126/science.286.5449.2511; PMID: 10617467
- Witzenbichler B, Westermann D, Knueppel S, Schultheiss HP, Tschope C. Protective role of angiopoietin-1 in endotoxic shock. Circulation 2005; 111:97 - 105; http://dx.doi.org/10.1161/01.CIR.0000151287.08202.8E; PMID: 15611372
- Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, et al. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem 2007; 282:23910 - 8; http://dx.doi.org/10.1074/jbc.M702169200; PMID: 17562701
- Baffert F, Le T, Thurston G, McDonald DM. Angiopoietin-1 decreases plasma leakage by reducing number and size of endothelial gaps in venules. Am J Physiol Heart Circ Physiol 2006; 290:H107 - 18; http://dx.doi.org/10.1152/ajpheart.00542.2005; PMID: 16126815
- Tadros A, Hughes DP, Dunmore BJ, Brindle NP. ABIN-2 protects endothelial cells from death and has a role in the antiapoptotic effect of angiopoietin-1. Blood 2003; 102:4407 - 9; http://dx.doi.org/10.1182/blood-2003-05-1602; PMID: 12933576
- Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol 2008; 10:527 - 37; http://dx.doi.org/10.1038/ncb1715; PMID: 18425119
- Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, et al. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol 2008; 10:513 - 26; http://dx.doi.org/10.1038/ncb1714; PMID: 18425120
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 2009; 10:165 - 77; http://dx.doi.org/10.1038/nrm2639; PMID: 19234476
- Zhang J, Fukuhara S, Sako K, Takenouchi T, Kitani H, Kume T, et al. Angiopoietin-1/Tie2 signal augments basal Notch signal controlling vascular quiescence by inducing delta-like 4 expression through AKT-mediated activation of beta-catenin. J Biol Chem 2011; 286:8055 - 66; http://dx.doi.org/10.1074/jbc.M110.192641; PMID: 21212269
- Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 2006; 3:e46; http://dx.doi.org/10.1371/journal.pmed.0030046; PMID: 16417407
- Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 2006; 12:235 - 9; http://dx.doi.org/10.1038/nm1351; PMID: 16462802
- Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med 2006; 12:1286 - 93; http://dx.doi.org/10.1038/nm1494; PMID: 17086189
- Kurniati NF, van Meurs M, Vom Hagen F, Jongman RM, Moser J, Zwiers PJ, et al. Pleiotropic effects of angiopoietin-2 deficiency do not protect mice against endotoxin-induced acute kidney injury. Nephrol Dial Transplant 2013; 28:567 - 75; http://dx.doi.org/10.1093/ndt/gfs336; PMID: 22872727
- Tzepi IM, Giamarellos-Bourboulis EJ, Carrer DP, Tsaganos T, Claus RA, Vaki I, et al. Angiopoietin-2 enhances survival in experimental sepsis induced by multidrug-resistant Pseudomonas aeruginosa. J Pharmacol Exp Ther 2012; 343:278 - 87; http://dx.doi.org/10.1124/jpet.112.195180; PMID: 22859861
- Daly C, Pasnikowski E, Burova E, Wong V, Aldrich TH, Griffiths J, et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci U S A 2006; 103:15491 - 6; http://dx.doi.org/10.1073/pnas.0607538103; PMID: 17030814
- Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol 2009; 29:2011 - 22; http://dx.doi.org/10.1128/MCB.01472-08; PMID: 19223473
- David S, Kümpers P, van Slyke P, Parikh SM. Mending leaky blood vessels: the angiopoietin-Tie2 pathway in sepsis. J Pharmacol Exp Ther 2013; 345:2 - 6; http://dx.doi.org/10.1124/jpet.112.201061; PMID: 23378191
- McCarter SD, Mei SH, Lai PF, Zhang QW, Parker CH, Suen RS, et al. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med 2007; 175:1014 - 26; http://dx.doi.org/10.1164/rccm.200609-1370OC; PMID: 17322110
- David S, Ghosh CC, Kümpers P, Shushakova N, Van Slyke P, Khankin EV, et al. Effects of a synthetic PEG-ylated Tie-2 agonist peptide on endotoxemic lung injury and mortality. Am J Physiol Lung Cell Mol Physiol 2011; 300:L851 - 62; http://dx.doi.org/10.1152/ajplung.00459.2010; PMID: 21421750
- Van Slyke P, Alami J, Martin D, Kuliszewski M, Leong-Poi H, Sefton MV, et al. Acceleration of diabetic wound healing by an angiopoietin peptide mimetic. Tissue Eng Part A 2009; 15:1269 - 80; http://dx.doi.org/10.1089/ten.tea.2007.0400; PMID: 18939935
- Kumpers P, Gueler F, David S, Slyke PV, Dumont DJ, Park JK, et al. The synthetic tie2 agonist peptide vasculotide protects against vascular leakage and reduces mortality in murine abdominal sepsis. Crit Care 2011; 15:R261; http://dx.doi.org/10.1186/cc10523; PMID: 22040774
- Marshall JC, Deitch E, Moldawer LL, Opal S, Redl H, van der Poll T. Preclinical models of shock and sepsis: what can they tell us?. Shock 2005; 24:Suppl 1 1 - 6; http://dx.doi.org/10.1097/01.shk.0000191383.34066.4b; PMID: 16374365
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al, Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013; 110:3507 - 12; http://dx.doi.org/10.1073/pnas.1222878110; PMID: 23401516
- Yamakawa D, Kidoya H, Sakimoto S, Jia W, Naito H, Takakura N. Ligand-independent Tie2 dimers mediate kinase activity stimulated by high dose Angiopoietin-1. J Biol Chem 2013; 288:12469 - 77; http://dx.doi.org/10.1074/jbc.M112.433979; PMID: 23504320
- Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, et al. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 2011; 121:2278 - 89; http://dx.doi.org/10.1172/JCI46322; PMID: 21606590
- Giuliano JS Jr., Lahni PM, Harmon K, Wong HR, Doughty LA, Carcillo JA, et al. Admission angiopoietin levels in children with septic shock. Shock 2007; 28:650 - 4; PMID: 18092380
- Ricciuto DR, dos Santos CC, Hawkes M, Toltl LJ, Conroy AL, Rajwans N, et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med 2011; 39:702 - 10; http://dx.doi.org/10.1097/CCM.0b013e318206d285; PMID: 21242795
- David S, van Meurs M, Kümpers P. Does low angiopoietin-1 predict adverse outcome in sepsis?. Crit Care 2010; 14:180; http://dx.doi.org/10.1186/cc9090; PMID: 20727223
- van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, van Hinsbergh VW, Groeneveld AB. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax 2008; 63:903 - 9; http://dx.doi.org/10.1136/thx.2007.087387; PMID: 18559364
- Gallagher DC, Parikh SM, Balonov K, Miller A, Gautam S, Talmor D, et al. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock 2008; 29:656 - 61; PMID: 18091573
- Calfee CS, Gallagher D, Abbott J, Thompson BT, Matthay MA, NHLBI ARDS Network. Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med 2012; 40:1731 - 7; http://dx.doi.org/10.1097/CCM.0b013e3182451c87; PMID: 22610178
- Diamond JM, Porteous MK, Cantu E, Meyer NJ, Shah RJ, Lederer DJ, et al, Lung Transplant Outcomes Group. Elevated plasma angiopoietin-2 levels and primary graft dysfunction after lung transplantation. PLoS One 2012; 7:e51932; http://dx.doi.org/10.1371/journal.pone.0051932; PMID: 23284823
- Gallagher DC, Bhatt RS, Parikh SM, Patel P, Seery V, McDermott DF, et al. Angiopoietin 2 is a potential mediator of high-dose interleukin 2-induced vascular leak. Clin Cancer Res 2007; 13:2115 - 20; http://dx.doi.org/10.1158/1078-0432.CCR-06-2509; PMID: 17404094
- van der Heijden M, Pickkers P, van Nieuw Amerongen GP, van Hinsbergh VW, Bouw MP, van der Hoeven JG, et al. Circulating angiopoietin-2 levels in the course of septic shock: relation with fluid balance, pulmonary dysfunction and mortality. Intensive Care Med 2009; 35:1567 - 74; http://dx.doi.org/10.1007/s00134-009-1560-y; PMID: 19551369
- Lovegrove FE, Tangpukdee N, Opoka RO, Lafferty EI, Rajwans N, Hawkes M, et al. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 2009; 4:e4912; http://dx.doi.org/10.1371/journal.pone.0004912; PMID: 19300530
- Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, Piera K, et al. Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci U S A 2008; 105:17097 - 102; http://dx.doi.org/10.1073/pnas.0805782105; PMID: 18957536
- Ghosh CC, Mukherjee A, David S, Knaus UG, Stearns-Kurosawa DJ, Kurosawa S, et al. Impaired function of the Tie-2 receptor contributes to vascular leakage and lethality in anthrax. Proc Natl Acad Sci U S A 2012; 109:10024 - 9; http://dx.doi.org/10.1073/pnas.1120755109; PMID: 22665799
- Whitcomb DC, Muddana V, Langmead CJ, Houghton FD Jr., Guenther A, Eagon PK, et al. Angiopoietin-2, a regulator of vascular permeability in inflammation, is associated with persistent organ failure in patients with acute pancreatitis from the United States and Germany. Am J Gastroenterol 2010; 105:2287 - 92; http://dx.doi.org/10.1038/ajg.2010.183; PMID: 20461065
- Ganter MT, Cohen MJ, Brohi K, Chesebro BB, Staudenmayer KL, Rahn P, et al. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma?. Ann Surg 2008; 247:320 - 6; http://dx.doi.org/10.1097/SLA.0b013e318162d616; PMID: 18216540
- Giamarellos-Bourboulis EJ, Kanellakopoulou K, Pelekanou A, Tsaganos T, Kotzampassi K. Kinetics of angiopoietin-2 in serum of multi-trauma patients: correlation with patient severity. Cytokine 2008; 44:310 - 3; http://dx.doi.org/10.1016/j.cyto.2008.09.003; PMID: 18952457
- Page AV, Kotb M, McGeer A, Low DE, Kain KC, Liles WC. Systemic dysregulation of angiopoietin-1/2 in streptococcal toxic shock syndrome. Clin Infect Dis 2011; 52:e157 - 61; http://dx.doi.org/10.1093/cid/cir125; PMID: 21460306
- Hill AB. The environment and disease: association or causation?. Proc R Soc Med 1965; 58:295 - 300; PMID: 14283879
- David S, Mukherjee A, Ghosh CC, Yano M, Khankin EV, Wenger JB, et al. Angiopoietin-2 may contribute to multiple organ dysfunction and death in sepsis*. Crit Care Med 2012; 40:3034 - 41; http://dx.doi.org/10.1097/CCM.0b013e31825fdc31; PMID: 22890252
- Kümpers P, Lukasz A, David S, Horn R, Hafer C, Faulhaber-Walter R, et al. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care 2008; 12:R147; http://dx.doi.org/10.1186/cc7130; PMID: 19025590
- Meyer NJ, Li M, Feng R, Bradfield J, Gallop R, Bellamy S, et al. ANGPT2 genetic variant is associated with trauma-associated acute lung injury and altered plasma angiopoietin-2 isoform ratio. Am J Respir Crit Care Med 2011; 183:1344 - 53; http://dx.doi.org/10.1164/rccm.201005-0701OC; PMID: 21257790
- Su L, Zhai R, Sheu CC, Gallagher DC, Gong MN, Tejera P, et al. Genetic variants in the angiopoietin-2 gene are associated with increased risk of ARDS. Intensive Care Med 2009; 35:1024 - 30; http://dx.doi.org/10.1007/s00134-009-1413-8; PMID: 19271210
- Tabruyn SP, Colton K, Morisada T, Fuxe J, Wiegand SJ, Thurston G, et al. Angiopoietin-2-driven vascular remodeling in airway inflammation. Am J Pathol 2010; 177:3233 - 43; http://dx.doi.org/10.2353/ajpath.2010.100059; PMID: 20952594
- Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, et al. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci U S A 2004; 101:5547 - 52; http://dx.doi.org/10.1073/pnas.0307574101; PMID: 15060279
- Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, et al. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med 2007; 35:199 - 206; http://dx.doi.org/10.1097/01.CCM.0000251640.77679.D7; PMID: 17110873
- Siner JM, Bhandari V, Engle KM, Elias JA, Siegel MD. Elevated serum angiopoietin 2 levels are associated with increased mortality in sepsis. Shock 2009; 31:348 - 53; http://dx.doi.org/10.1097/SHK.0b013e318188bd06; PMID: 18791490
- Kümpers P, van Meurs M, David S, Molema G, Bijzet J, Lukasz A, et al. Time course of angiopoietin-2 release during experimental human endotoxemia and sepsis. Crit Care 2009; 13:R64; http://dx.doi.org/10.1186/cc7866; PMID: 19416526
- Ebihara I, Hirayama K, Nagai K, Kakita T, Miyamoto Y, Nagai M, et al. Angiopoietin balance in septic shock patients treated by direct hemoperfusion with polymyxin b-immobilized fiber. Ther Apher Dial 2009; 13:520 - 7; http://dx.doi.org/10.1111/j.1744-9987.2009.00777.x; PMID: 19954476
- Kümpers P, Hafer C, David S, Hecker H, Lukasz A, Fliser D, et al. Angiopoietin-2 in patients requiring renal replacement therapy in the ICU: relation to acute kidney injury, multiple organ dysfunction syndrome and outcome. Intensive Care Med 2010; 36:462 - 70; http://dx.doi.org/10.1007/s00134-009-1726-7; PMID: 19956923
- Davis JS, Yeo TW, Piera KA, Woodberry T, Celermajer DS, Stephens DP, et al. Angiopoietin-2 is increased in sepsis and inversely associated with nitric oxide-dependent microvascular reactivity. Crit Care 2010; 14:R89; http://dx.doi.org/10.1186/cc9020; PMID: 20482750
- Mankhambo LA, Banda DL, Jeffers G, White SA, Balmer P, Nkhoma S, et al, IPD Study Group. The role of angiogenic factors in predicting clinical outcome in severe bacterial infection in Malawian children. Crit Care 2010; 14:R91; http://dx.doi.org/10.1186/cc9025; PMID: 20492647
- Alves BE, Montalvao SA, Aranha FJ, Siegl TF, Souza CA, Lorand-Metze I, et al. Imbalances in serum angiopoietin concentrations are early predictors of septic shock development in patients with post chemotherapy febrile neutropenia. BMC Infect Dis 2010; 10:143; http://dx.doi.org/10.1186/1471-2334-10-143; PMID: 20509945