Abstract
Acinetobacter baumannii (Ab) is a common cause of community-acquired pneumonia (CAP) in chronic alcoholics in tropical and sub-tropical climates and associated with a >50% mortality rate. We demonstrated that exposure of J774.16 macrophage-like cells to physiological alcohol (EtOH) concentrations decreased phagocytosis and killing of Ab. EtOH-mediated macrophage phagocytosis dysfunction may be associated with reduced expression of GTPase-RhoA, a key regulator of the actin polymerization signaling cascade. EtOH inhibited nitric oxide (NO) generation via inducible NO-synthase inactivation, which enhanced Ab survival within macrophages. Additionally, EtOH alters cytokine production resulting in a dysregulated immune response. This study is a proof of principle which establishes that EtOH might exacerbate Ab infection and be an important factor enhancing CAP in individuals at risk.
Introduction
Acinetobacter baumannii (Ab) is a common cause of nosocomial pneumonia primarily in immunodeficient patients as well as a primary agent for community-acquired pneumonia (CAP) in patients with alcoholism in whom Ab disease is associated with a >50% mortality rate in tropical and sub-tropical climates.Citation1 The source of infection may be carriage in the nasopharynx, which occurs in up to 10% of community residents with excessive alcohol (EtOH) consumption. In addition to the nasopharyngeal mucosa, Ab can colonize the skin of immunocompetent patients, creating asymptomatic carriers which aids in its spread worldwide as well making Ab an extremely successful opportunistic pathogen. Furthermore, Ab has an abundance of genomic resistance islands that can render single antibiotics useless in the treatment of infections.Citation2
EtOH is deleterious to the host’s innate and acquired immune responses. For instance, acute as well as chronic EtOH consumption lowers the ability of phagocytes to engulf, kill, and process diverse pathogens.Citation3,Citation4 It also impairs neutrophil adherence and chemotaxis to the site of infection. EtOH adversely regulates cell cycle and apoptosis genes in human alveolar macrophages.Citation5 EtOH ingestion alters the levels of pro-inflammatory cytokines mainly produced in the lungs by epithelial cells. Chronically EtOH-treated mice displayed impaired antigen-specific T-cell proliferation and delayed-type hypersensitivity response.Citation6 Moreover, EtOH exposure elevated serum IgG and IgA antibody levels indicative of B-cell dysfunction.Citation7
Macrophages play an important role in early modulation of immune response to multidrug-resistant Ab infection.Citation8 However, there is lack of information about EtOH abuse effects on specific effector functions of host cells in Ab infection. In this study, we hypothesized that physiological EtOH levels impair J774.16 macrophage-like cell microbicidal responses to Ab.
Results
EtOH inhibits J774.16 macrophage-like cells binding and phagocytosis
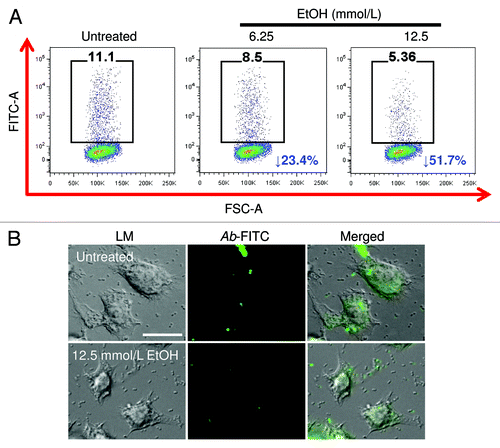
We analyzed the effects of physiological EtOH on Ab binding and phagocytosis by J774.16 macrophage‐like cells using FACS analysis. EtOH reduced binding and phagocytosis of Ab by macrophages, compared with the control (). Our results showed a 23.4% and 51.7% binding and phagocytosis inhibition in macrophage-like cells treated with 6.25 and 12.5 mmol/L EtOH, respectively, when compared with control cells. Interestingly, exposure of J774.16 cells to 25 mmol/L EtOH affected the cells’ viability (data not shown); therefore, most of the experiments were done with lower doses. Light and fluorescent microscopy images show the effect of EtOH on Ab phagocytosis by J774.16 cells (). The reduction in phagocytosis is apparent in EtOH‐treated (12.5 mmol/L) macrophages, compared with control macrophages.
Figure 1. Alcohol (EtOH) inhibits J774.16 macrophage-like cells binding and phagocytosis of Acinetobacter baumannii (Ab). J774.16 cells were untreated or exposed to EtOH for 2 h followed by incubation with Ab. (A) Binding and phagocytosis of FITC-labeled Ab by J774.16 cells was determined using fluorescent activated cell sorting (FACS) analysis. Representative plots of bound and internalized FITC-labeled bacteria by macrophage-like cells are shown. Each plot was generated after 10 000 events were analyzed. (B) Light (LM) and fluorescent microscopy images of J774.16 cells phagocytosis of FITC (green fluorescence)-labeled Ab after exposure to 12.5 mmol/L EtOH. Scale bar: 10 μm. The experiments were performed twice with similar results obtained.
EtOH interferes with complement receptor 3-mediated phagocytosis
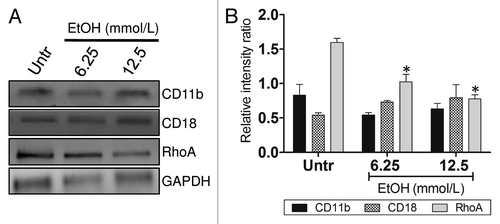
We investigated whether EtOH’s inhibition of macrophage phagocytic activity was in response to alterations in the complement receptor 3 (CR3; CD11b/CD18) expression levels. Macrophages recognize Ab via CR3 for phagocytosis and destruction of bacterial cell. Western blot analysis showed no differences in CD11b and CD18 expression between EtOH‐treated and control macrophages (). Furthermore, to test whether EtOH affected CR3-mediated phagocytosis protein expression cascade, we measured the expression of GTPase RhoA, a regulatory protein involved in the cascade for actin polymerization and formation of the phagocytosis pocket. We found that EtOH significantly reduces the expression of RhoA, therefore, decreasing bacterial internalization compared with untreated macrophages ().
Figure 2. EtOH impairs complement receptor 3-mediated phagocytosis. (A) Western blot (WB) analyses of CD11b, CD18, and RhoA of untreated and EtOH (6.25 or 12.5 mmol/L)-treated J774.16 cells. (B) Quantitative measurements of individual band intensity in WB in (A) for CD11b, CD18, and RhoA using Image J software. GAPDH was used as a loading control to determine the relative intensity ratio. Bars are the averages of three independent gel results, and error bars denote standard deviations. Asterisks denote a reduction (*P < 0.001) in the intensity of the band of RhoA compared with GAPDH. The experiments were performed thrice with similar results obtained.
EtOH reduces Ab killing by J774.16 cells
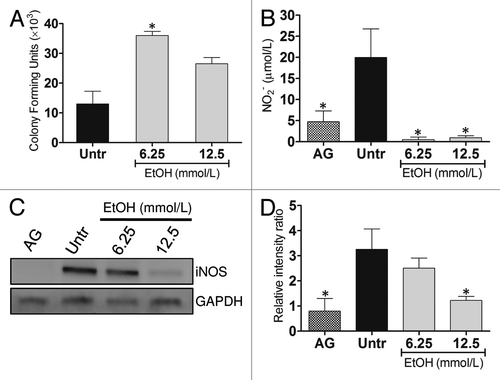
We examined whether EtOH interferes with macrophage-mediated killing of Ab cells. EtOH (6.25 mmol/L) significantly enhanced the proliferation of bacteria within macrophages (). There was a trend toward an increase in bacterial proliferation in macrophages exposed to 12.5 mmol/L EtOH, but it was not statistically significant. Consequently, we investigated the impact of EtOH on extracellular NO production by J774.16 cells after exposure to LPS. Our results showed that NO levels were significantly reduced in the supernatants of EtOH-treated cells when compared with controls (). The expression of iNOS was determined to confirm whether NO production by macrophages was affected by EtOH. We observed a dose-dependent reduction of iNOS expression when compared with untreated control cells (). AG, an iNOS inhibitor, was used as a control in both assays. The quantitative analysis is in .
Figure 3. EtOH reduces Ab killing by J774.16 cells. (A) Killing of Ab by J774.16 cells was determined using colony-forming units (CFU) analysis. (B) EtOH inhibits nitric oxide (NO) production by macrophage-like cells. NO production was quantified using the Griess method after J774.16 were untreated or EtOH-treated and co-incubated with LPS for 96 h. For (A and B), bars and error bars denote average of three measurements and standard deviations, respectively. Asterisks denote P value significance (*P < 0.001) calculated by analysis of variance and adjusted by use of the Bonferroni correction. (C) WB analysis of inducible nitric oxide synthase (iNOS) of untreated, aminoguanidine (AG; iNOS inhibitor)-, and EtOH-treated J774.16 cells. (D) Quantitative measurements of individual band intensity in WB in (C) for iNOS. GAPDH was used as a control to determine the relative intensity ratio. Bars are the averages of three independent gel results, and error bars denote standard deviations. Asterisks denote a reduction (*P < 0.001) in the intensity of the band of iNOS compared with GAPDH. The experiments were performed thrice with similar results obtained.
Alteration of macrophage cytokine expression by EtOH
We measured the cytokine response in the supernatants of macrophages incubated with Ab after exposure to EtOH (). Supernatants of J774.16 cells treated with EtOH contained significantly lower quantities of TNF-α, IL-1β, IL-4, and IL-12 than that of control macrophages. There was a significant increase in IL-6 production in 6.25 mmol/L EtOH-treated macrophages compared with controls but not in the 12.5 mmol/L EtOH group. There was no difference in the production of IL-10.
Table 1. Cytokine levels (pg/mL) in the supernatant of J774.16 macrophage-like cells after co-incubation with Ab
Discussion
Multidrug-resistant Ab associated CAP affects individuals with excess EtOH consumption resulting in considerable morbidity and mortality.Citation9 EtOH has deleterious consequences in the host’s innate and acquired immune responses. Previous work by Burnham et al. suggests that predisposition for CAP among EtOH users may be in part mediated through the effects of this substance of abuse on antimicrobial effector cells and molecules in the lungs.Citation10 In this study, we found that EtOH lowers the ability of J774.16 macrophage-like cells to attach, engulf, kill, and process Ab.
CR3 is a pattern recognition receptor in macophages, capable of recognizing and binding to many molecules found on the surfaces of invading bacteria. CR3 also recognizes iC3b when bound to the surface of microbial cells. Binding to the receptor causes phagocytosis and destruction of the foreign cell. We found that EtOH did not affect the expression of CR3 components, CD11b and CD18, suggesting that EtOH does not cause structural changes in the surface of macrophages and may not interfere with effector cells–Ab interaction. However, EtOH exposure of macrophages has negative effects in the expression of GTPase-RhoA, a key protein regulator of actin polymerization during the formation of the phagocytic pocket, thus, reducing bacterial engulfment.
After phagocytosis, bacteria are rapidly exposed to the microbicidal armamentarium of the macrophages, which consists of toxic reactive species, such as NO, and lysosomal hydrolases. Our data show that NO generation is significantly altered in J774.16 cells that are exposed to EtOH. To confirm this result, we found that EtOH-mediated inhibition of iNOS expression, affecting intracellular and extracellular production of NO. In this regard, reduction in TNF-α and IL-12 may account for the decreased expression of iNOS since TNF-α directly acts in the activation of the enzyme whereas IL-12 stimulates the production of IFN-γ by T cells. Impaired production of NO might create an ideal environment for microbial survival, facilitating intracellular replication and regulation of the phagolysosomal milieu. Furthermore, low levels of anti-inflammatory IL-4 might downregulate MHC II antigen presentation to the adaptive immune system.Citation11
Macrophages participate in the early inflammatory responses and host defense against Ab infection.Citation8 EtOH-treated macrophages displayed decreased production of pro-inflammatory TNF-α, IL-1β, and IL-12 and increased levels of IL-6. Low IL-12 production may inhibit the differentiation of naive T cells into Th1 cells and production of IFN-γ from T and natural killer (NK) cells. Low levels of TNF-α and IL-1β have been correlated with septic shock and increased risk of bacterial infections.Citation12-Citation15 High levels of circulating IL-6 are commonly implicated in inflammatory diseases, tissue damage, uncontrolled activation of macrophage cells, and increased rate of infections.Citation12,Citation13,Citation16 These observations may provide insight to the fulminant action of Ab-acquired pneumonia in which the patient typically presents with bacteremia and septic shock leading to death.
In conclusion, our study is a proof of principle that EtOH impairs macrophage effector functions which might be associated with enhanced severity of community-acquired Ab pneumonia in alcoholic patients resulting in high mortality. In order to reduce the incidence of this problematic infection, it is critical to understand the Ab pathogenesis, the bacterium interaction with the immune system and identify novel virulence factors. Finally, assessing the activity of alveolar macrophages in the environment of EtOH abuse in humans can help establish a definitive causation for the susceptibility of pulmonary infections seen in these individuals.
Materials and Methods
Bacteria
Ab 0057, a clinical isolate that was generously provided by Mark D Adams (Cleveland, OH), was chosen for this study because it has been sequenced and it is resistant to multiple antibiotics including carbapenem, tetracycline, ciprofloxacin, chloramphenicol, and penicillin which are commonly used to treat gram negative infections.Citation17,Citation18 The strain was collected from the bloodstream of a soldier in 2004 at Walter Reed Army Medical Center. The isolate was stored at −80 °C in Tryptic Soy Broth (TSB) with 50% glycerol. Frozen stocks were grown in TSB with rotary shaking at 150 rpm overnight at 37 °C. Optical density measurements were taken at 600 nm to monitor growth.
J774.16 macrophage-like cells
The J774.16 macrophage-cell line originated from a murine reticulum cell sarcoma and has been extensively used to study microbe-macrophage interactions. J774.16 cells were maintained at −80 °C prior to use. The J774.16 cells were suspended in feeding (Dulbecco’s modified Eagle) medium with 10% heat-inactivated fetal calf serum (Atlanta Biologicals), 10% NCTC-109 (Gibco Laboratories, Life Technologies, Inc.), and 1% nonessential amino acids (Cellgro; Mediatech), and grown as a confluent monolayer on tissue culture plates.
Phagocytosis assay
Binding and phagocytosis was determined by fluorescence‐activated cell sorting (FACS) analysis. J774.16 macrophages (106 cells) were incubated on 6 well plates with EtOH (6.25 or 12.5 mmol/L; Sigma), or phosphate buffer saline (PBS) for 2 h at 37 °C and 5% CO2. FITC (Molecular Probes)-labeled Ab cells were incubated with 25% mouse serum (Sigma) for 30 min to allow complement proteins to opsonize Ab. Bacterial cells were washed and then 107 bacterial cells were added to the 106 J774.16 cells for 2 h. Samples were processed and 10 000 events were recorded on a FACS Calibur flow cytometer (Becton Dickinson Biosciences) and analyzed using Cell Quest Pro software to generate representative plots.
Fluorescence microscopy
J774.16, with and without pretreatment with EtOH, and FITC-labeled, complement opsonized Ab cells were incubated at a 1:10 ratio in feeding medium for 30 min at 37 °C. The medium was aspirated, and the cells were fixed with 2.5% gluteraldehyde (Sigma) for 1 h and washed with PBS. A coverslip was mounted by using a solution of 50% glycerol (Sigma), 0.1 M n-propyl gallate (Sigma) in PBS. The slides were viewed with a FITC filter-equipped Zeiss Axiovert 200 M inverted microscope (Carl Zeiss). Fluorescent images were acquired utilizing Axio Vision 4.4 software in deconvolution mode (Carl Zeiss).
Ab killing assay
J774.16 and Ab cells interaction was performed as described in the phagocytosis assay protocol. Quantification of viable bacteria was determined at 18 h by measuring colony-forming units (cfu) after macrophages were lysed by forcibly pulling the culture through a 27-gauge needle 5–7 times.Citation19 Four microtiter wells per condition were used to ascertain cfu. For each well, serial dilutions were plated in triplicates onto TS agar plates, which were incubated at 37 °C for 24 h.
Western blot analysis
To further explore the impact of EtOH on J774.16 phagocytosis and microbial killing, we assessed macrophage CD11b, CD18, RhoA, and inducible nitric oxide synthase (iNOS) expression after incubation with Ab. GAPDH, a cytoplasmic housekeeping protein, was used as a control. Additionally, macrophages were treated with aminoguanidine (AG) as a control because it inhibits both constitutive and iNOS. Western blot analysis was conducted using cytoplasmic extracts made by NE-PER Nuclear and Cytoplasmic Extraction kit (Thermo Scientific) after co-incubation with complement-opsonized whole Ab cells (CD11b, CD18, and RhoA) or lipopolysaccharide (LPS; Sigma) alone (iNOS). The mixture was centrifuged at 10 000 g for 10 min at 4 °C and the resulting protein content of the supernatant was determined using the Bradford method employing a protein assay kit (BioRad Laboratories). Lysates were preserved in protease inhibitor cocktail (Complete Mini, Roche) and stored at −20 °C until use. The supernatant was added with a sample buffer containing 1.6% sodium dodecylsulphate, 5% glycerol, 0.1 M dithiothreitol, 0.002% bromphenol blue and 62.5 mM TRIS-HCl (pH 6.8). The mixture was then heated to 100 °C for 5 min. Fifty-five micrograms of protein was applied to each lane of a gradient gel (10%, BioRad Laboratories). After electrophoresis at a constant 130 V/gel, the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (BioRad Laboratories, 0.2 mm), and briefly stained with Ponceau S (Sigma) to verify efficient transfer of the protein. The PVDF membrane was incubated for 1 h at 37 °C in a blocking solution containing 5% non-fatty dry milk, 0.04 M TRIS-HCl, pH 7.6, 0.8% NaCl and 0.5% Tween 20, followed by an overnight incubation at 4 °C with a mouse monoclonal anti-CD11b (dilution 1:1000; Southern Biotech), CD18 (dilution 1:1000; BD Pharmigen), RhoA (dilution 1:1000; BD), or iNOS (dilution 1:3000; Abcam) antibodies and with subsequent addition of peroxidase linked anti-mouse secondary IgG antibody diluted (1:5000; Southern Biotech) in the blocking solution. Protein bands were detected and quantified with a lumino image analyzer (GE Typhoon 8600) after staining with chemiluminescence detection reagents (Thermo Scientific). Quantitative measurements of individual band intensity in western blot analyses for CD11b, CD18, RhoA, and iNOS were performed using Image J software (National Institutes of Health). GAPDH was used as a loading control to determine the relative intensity ratio.
Nitric oxide determinations (NO)
Nitric oxide (NO) produced in supernatants by untreated, AG-, or EtOH-treated macrophages was quantified 96 h after exposure to LPS using a Griess method kit (Promega).
Cytokine determinations
Supernatants were tested for TNF-α, IL-1β, IL-4, IL-6, IL-10, and IL-12 by ELISA (BD) after co-incubation of macrophages and bacteria for 18 h at 37 °C. Cell debris was removed from supernatants by centrifugation at 6000 g for 10 min. Samples were stored at −80 °C until tested in triplicates. The limits of detection were 7.8 pg/mL for IL-4, 15.6 pg/mL for TNF-α, IL-1β, and IL-6, 30 pg/mL for IL-10, and 62.5 pg/mL for 12p70r.
Statistical analysis
All data were subjected to statistical analysis using GraphPad Prism 5.0 (GraphPad Software). P values were calculated by analysis of variance and were adjusted by use of the Bonferroni correction. P values of <0.05 were considered significant.
Acknowledgments
LRM is supported by the NIH-NIAID 5K22A108781702 and Long Island University-Post Faculty Research Committee Awards. We thank Dr Joshua D Nosanchuk for his constructive suggestions.
Submitted
05/23/13
Revised
06/26/13
Accepted
07/05/13
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authorship
All authors contributed to the design of the experiments, analysis of the data, and writing of the manuscript. MBA performed the phagocytosis and killing assays, western blot, and cytokine analyses. CC and RJC performed the FACS and microscopy, respectively.
References
- Leung WS, Chu CM, Tsang KY, Lo FH, Lo KF, Ho PL. Fulminant community-acquired Acinetobacter baumannii pneumonia as a distinct clinical syndrome. Chest 2006; 129:102 - 9; http://dx.doi.org/10.1378/chest.129.1.102; PMID: 16424419
- Zarrilli R, Pournaras S, Giannouli M, Tsakris A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents 2013; 41:11 - 9; http://dx.doi.org/10.1016/j.ijantimicag.2012.09.008; PMID: 23127486
- Vander Top EA, Perry GA, Snitily MU, Gentry-Nielsen MJ. Smoke exposure and ethanol ingestion modulate intrapulmonary polymorphonuclear leukocyte killing, but not recruitment or phagocytosis. Alcohol Clin Exp Res 2006; 30:1599 - 607; http://dx.doi.org/10.1111/j.1530-0277.2006.00192.x; PMID: 16930223
- Karavitis J, Murdoch EL, Deburghgraeve C, Ramirez L, Kovacs EJ. Ethanol suppresses phagosomal adhesion maturation, Rac activation, and subsequent actin polymerization during FcγR-mediated phagocytosis. Cell Immunol 2012; 274:61 - 71; http://dx.doi.org/10.1016/j.cellimm.2012.02.002; PMID: 22381996
- Burnham EL, Phang TL, House R, Vandivier RW, Moss M, Gaydos J. Alveolar macrophage gene expression is altered in the setting of alcohol use disorders. Alcohol Clin Exp Res 2011; 35:284 - 94; http://dx.doi.org/10.1111/j.1530-0277.2010.01344.x; PMID: 21121937
- Dixon EP, King LM, Adams MD, Grønn P, Murphy PG, Brown CA, et al. Isolation of RNA from residual BD SurePath liquid-based cytology specimens and detection of HPV E6/E7 mRNA using the PreTectt HPV-Proofer assay. J Virol Methods 2008; 154:220 - 2; http://dx.doi.org/10.1016/j.jviromet.2008.08.002; PMID: 18761379
- Nickel GC, Tefft DL, Goglin K, Adams MD. An empirical test for branch-specific positive selection. Genetics 2008; 179:2183 - 93; http://dx.doi.org/10.1534/genetics.108.090548; PMID: 18689901
- Qiu H, KuoLee R, Harris G, Van Rooijen N, Patel GB, Chen W. Role of macrophages in early host resistance to respiratory Acinetobacter baumannii infection. PLoS One 2012; 7:e40019; http://dx.doi.org/10.1371/journal.pone.0040019; PMID: 22768201
- Falagas ME, Karveli EA, Kelesidis I, Kelesidis T. Community-acquired Acinetobacter infections. Eur J Clin Microbiol Infect Dis 2007; 26:857 - 68; http://dx.doi.org/10.1007/s10096-007-0365-6; PMID: 17701432
- Burnham EL, Gaydos J, Hess E, House R, Cooper J. Alcohol use disorders affect antimicrobial proteins and anti-pneumococcal activity in epithelial lining fluid obtained via bronchoalveolar lavage. Alcohol Alcohol 2010; 45:414 - 21; http://dx.doi.org/10.1093/alcalc/agq045; PMID: 20729531
- Woodward EA, Prêle CM, Nicholson SE, Kolesnik TB, Hart PH. The anti-inflammatory effects of interleukin-4 are not mediated by suppressor of cytokine signalling-1 (SOCS1). Immunology 2010; 131:118 - 27; PMID: 20406299
- Uematsu S, Engelberts D, Peltekova V, Otulakowski G, Post M, Kavanagh BP. Dissociation of inflammatory mediators and function: experimental lung injury in nonpulmonary sepsis. Crit Care Med 2013; 41:151 - 8; http://dx.doi.org/10.1097/CCM.0b013e318267606f; PMID: 23128385
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, et al. Cytokines and alcohol. Alcohol Clin Exp Res 2006; 30:720 - 30; http://dx.doi.org/10.1111/j.1530-0277.2006.00084.x; PMID: 16573591
- Arbabi S, Garcia I, Bauer GJ, Maier RV. Alcohol (ethanol) inhibits IL-8 and TNF: role of the p38 pathway. J Immunol 1999; 162:7441 - 5; PMID: 10358198
- Phumeetham S, Chat-Uthai N, Manavathongchai M, Viprakasit V. Genetic association study of tumor necrosis factor-alpha with sepsis and septic shock in Thai pediatric patients. J Pediatr (Rio J) 2012; 88:417 - 22; http://dx.doi.org/10.2223/JPED.2216; PMID: 23033000
- Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, et al. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans.. J Exp Med 1996; 183:1345 - 55; http://dx.doi.org/10.1084/jem.183.4.1345; PMID: 8666893
- Adams MD, Goglin K, Molyneaux N, Hujer KM, Lavender H, Jamison JJ, et al. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii.. J Bacteriol 2008; 190:8053 - 64; http://dx.doi.org/10.1128/JB.00834-08; PMID: 18931120
- Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa.. J Med Microbiol 2006; 55:1619 - 29; http://dx.doi.org/10.1099/jmm.0.46747-0; PMID: 17108263
- Moffat JF, Tompkins LS. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii.. Infect Immun 1992; 60:296 - 301; PMID: 1729191