Abstract
Avian influenza viruses (AIV) of H5 and H7 subtypes exhibit two different pathotypes in poultry: infection with low pathogenic (LP) strains results in minimal, if any, health disturbances, whereas highly pathogenic (HP) strains cause severe morbidity and mortality. LPAIV of H5 and H7 subtypes can spontaneously mutate into HPAIV. Ten outbreaks caused by HPAIV are known to have been preceded by circulation of a predecessor LPAIV in poultry. Three of them were caused by H5N2 subtype and seven involved H7 subtype in combination with N1, N3, or N7. Here, we review those outbreaks and summarize the genetic changes which resulted in the transformation of LPAIV to HPAIV under natural conditions. Mutations that were found directly in those outbreaks are more likely to be linked to virulence, pathogenesis, and early adaptation of AIV.
Introduction
Avian influenza viruses (AIV) belong to the genus Influenzavirus A within the family Orthomyxoviridae, which contains five genera: Influenzavirus A, B, C, Thogotovirus, and Isavirus.Citation1 AIV, the only orthomyxovirus known to infect birds, are enveloped viruses which contain a negative-sense, single-stranded ribonucleic acid (RNA) multipartite genome of eight separate gene segments encoding at least 11 viral proteins.Citation2,Citation3 The viral proteins have been allocated into three main categories: envelope proteins (hemagglutinin [HA], neuraminidase [NA], and matrix protein-2 [M2]), internal proteins (polymerase subunits [PB2, PB1, PA], nucleoprotein [NP], matrix protein 1 [M1], and nuclear export protein [NEP]) and non-structural proteins (NS1 and PB1-F2).Citation3-Citation5 Of the two envelope glycoproteins HA and NA 16 HA (H1–H16) and 9 NA (N1–N9) subtypes have been distinguished in AIV,Citation1,Citation6 whereas recently a novel H17N10 virus has been identified in bats in Guatemala.Citation7 All AIV subtypes are present in wild aquatic birds, the natural reservoir, and transmit sporadically to poultry and/or mammals.Citation4 Host range of AIV is primarily determined by receptor binding preference. Avian viruses attach to α2-3 sialic acid linked galactose (SA) which is abundant in the alimentary tract of avian species, whereas mammalian including human influenza viruses prefer binding to α2-6 SA abundant in the human respiratory tract.Citation8,Citation9 Three HA (H1, H2, and H3) and two NA (N1 and N2) subtypes resulted in pandemic infections in humans, but avian H5, H7, and H9 viruses also infected humans in several countries.Citation10
Due to the viral error-prone RNA polymerase constant antigenic variation of AIV is an intriguing feature for continuous evolution of the virus in nature to evade the host’s immune response and allow reinfection.Citation5 Gradual antigenic variation via incremental acquisition of point mutations is defined as “antigenic drift” which is commonly regarded as the driving mechanism for annual influenza virus epidemics. “Antigenic shift” of influenza virus occurs by exchange (“reassortment”) of genes from different influenza subtypes leading to a complete change in the antigenic structure and emergence of new virusesCitation5,Citation11,Citation12 that may result in severe pandemics.Citation13,Citation14 According to their pathogenicity in poultry, AIV is classified into two pathotypes. Low pathogenic (LP) strains result in mild or asymptomatic infections, whereas highly pathogenic (HP) strains cause up to 100% morbidity and mortality.Citation15 Any AIV that exhibits an intravenous pathogenicity index (IVPI) in 6-week-old chickens greater than 1.2 or kills at least 75% of 4- to 8-week-old chickens during a 10-day-observation period is defined as a highly pathogenic strain.Citation16-Citation18 Moreover, all influenza A viruses are proteolytically cleaved within their HA protein at an amino acid sequence designated as the proteolytic cleavage site (PCS). This consists of an arginine (R) residue adjacent to a conserved glycine (G) which becomes the N-terminus of the HA2 subunit.Citation19 There is a strong positive correlation between the number of basic amino acids in the PCS and an increase of virulence of AIV.Citation20-Citation24 LPAIV usually exhibit a single basic amino acid, arginine (R) or lysine (K), at the cleavage site, while multiple basic amino acids with a minimal −4R-X-K/R-R−1 motif are characteristic for HPAIV.Citation20,Citation25 Two different classes of proteases are responsible for cleavage-activation of the hemagglutinin of influenza viruses and the distribution of these proteases in the host appears to be a prime determinant of AIV tissue tropism and pathogenicity. The trypsin-like proteases that cleave LPAIV are present only in a limited number of cells or tissues, so that these viruses normally cause localized infections in, for example, the respiratory tract of mammals or intestinal tract of birds. In contrast, furin and subtilisin-like proteases that activate HPAIV are ubiquitously expressed causing systemic spread of the virus.Citation11,Citation26-Citation29 To date, several strains of H5 or H7 subtypes fulfill the criteria of high pathogenicity. They emerge at irregular intervals and can devastate poultry flocks within a few days.Citation11,Citation30-Citation32 No avian or mammalian reservoirs for HPAIV have been identified yet but there are several well recorded examples of how they evolved from LPAIV.Citation16,Citation33,Citation34 Within the last three decades, the development of reverse genetic techniques helped researchers to correlate many genetic markers with virulence of AIV.Citation33,Citation35-Citation41 However, the steps by which LPAIV becomes HPAIV in nature remain poorly understood.
To date, ten outbreaks of HPAIV in poultry are documented in which the HPAIV isolated from birds in these outbreaks is thought to originate from an LPAIV previously circulating in the poultry population (). Three outbreaks were caused by H5N2 subtype and seven by H7 in combination with N1, N3, or N7 in 6 countries on 4 different continents. Genetic changes in HPAIV that occurred during acquisition of virulence under field conditions () can give a molecular clue for better understanding the mechanism of AIV pathogenicity. Therefore, this review aims to provide an insight into the epidemiology and molecular aspects which accompanied the shift in virulence of AIV in natural outbreaks in poultry.
Figure 1. Major genetic changes found in HPAIV that have directly evolved from LPAIV. Shown are the genetic changes found in the hemagglutinin (particularly proteolytic cleavage site; denoted as a red square) that accompanied shifts of low pathogenic avian influenza viruses from wild birds reservoir toward higher pathogenicity in land based poultry. The transition periods ranged from few days to several years. All outbreaks were eradicated while the exclamation mark refers to the on-going H7N3 outbreak in domesticated poultry in Mexico since June 2012.
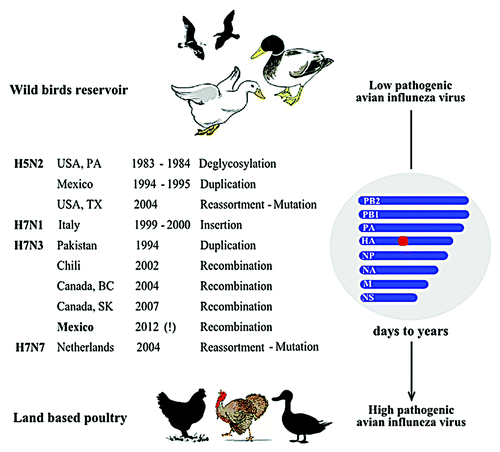
Table 1. Epidemiology and genetic changes of outbreaks caused by HPAIV that have directly evolved from LPAIV
Epidemic of H5N2 in Pennsylvania, USA
LPAIV A/Chicken/Pennsylvania/1/83 (H5N2), which belongs to the North American lineage of wild bird AIV, was first reported in Pennsylvania in April 1983 at a chicken farm as the causative agent of an acute respiratory disease, causing 2.6% mortality and 31% drop in egg production.Citation42-Citation44 During in vivo pathogenicity test, the virus did not produce clinical signs or mortality in experimentally infected chickens and replicated in vitro only in the presence of exogenous trypsin. Therefore, it was classified as LPAIV. Epidemiological investigations indicated that the virus was probably transmitted from wild waterfowl to the gallinaceous poultry.Citation42,Citation45-Citation47 In October 1983, an H5N2 virus was isolated from commercial chickens after complete cessation of egg production, exhibiting nervous signs and 50–89% mortality within few days. All experimentally infected white leghorn chickens died and the virus replicated well in the absence of trypsin, prompting its classification as HPAIV.Citation34,Citation43,Citation44 Thereafter, experimental in vitro, in ovo, and in vivo passages of the parental LPAIV H5N2 generated several different HPAIV variants.Citation23,Citation31,Citation48,Citation49 The HPAIV H5N2 produced mild transient, if any, clinical signs after infection of pheasants, gulls, quails, and Pekin ducks.Citation45 The HPAIV H5N2 had also been isolated from pigs without further pig-to-pig transmissionCitation34,Citation43,Citation44 and flies caught in the chicken houses.Citation34,Citation43,Citation44 It was also isolated from 2 out of 40 investigated workers involved in culling the infected chickens when samples were collected immediately within 12 h after exposure to infected birds.Citation34,Citation43,Citation44
Surprisingly, the PCS motifs of the low and highly pathogenic viruses were identical, specifying PQKKKR/GLF. Using reverse genetics, pathogenicity of the Pennsylvanian H5N2 viruses was linked to the HA gene, since a reassortant virus with the HA gene of the virulent strain and other gene segments from the avirulent strain killed all experimentally infected chickens.Citation50 It was subsequently detected that a mutation (T13K) in the HA of the virulent virus led to loss of an N-glycosylation consensus motif (asparagine) at position 11 which enhanced the cleavability of PCS by furin-proteases.Citation42,Citation47,Citation50,Citation51 Interestingly, in vitro studies aimed to increase the number of basic amino acids in the PCS of the avirulent virus abolished the masking effect of glycosylation at position 11 and induced shift in the pathogenicity of the avirulent virus.Citation48 Furthermore, reassortant LPAI H5N1 and H5N9 viruses of shorebird origin containing the HA and M gene segments of the Pennsylvanian HPAIV exhibited increased virulence in chickens.Citation52 In addition, a 20 amino acid deletion in the NA stalk region (residues 63 to 82) was reported which is common for chicken-adapted AIV.Citation53
Epidemic of H5N2 in Mexico
Early serologic evidence of circulation of an H5N2 influenza virus among Mexican chickens was reported in October 1993.Citation54 In May 1994, the LPAIV was isolated for the first time from several flocks nationwide suffering from mild respiratory disorders. Gradual increase in virulence of the H5N2 virus was observed in November 1994 in Puebla, Mexico. However, the virus still exhibited only mild virulence in chickens, since zero to 4 out of 8 intravenously infected chickens died.Citation54,Citation55 However, by January 1995, the virus had become highly lethal for poultry and killed all experimentally infected chickens.Citation55 Based on the sequence and phylogenetic analysis of the HA it was stated that the Mexican virus belonged to the North American lineage introduced by migrating waterfowl which evolved thereafter in the Mexican chicken population and that the HPAIV was derived directly from the LPAIV isolated approximately one month earlier.Citation54,Citation56,Citation57
The PCS motif of the early LPAIV was PQRETR/G (or more rarely PQKETR/G), a feature of avirulent viruses.Citation54,Citation58 However, substitutions by and insertions of basic amino acids, PQRKRKTR/G (one isolate had PQRKRKRKTR/GCitation58), due to strand slippage and a duplication in the PCS region were found in both mildly (isolated in November 1994) and highly pathogenic viruses (isolated in January 1995).Citation54,Citation56,Citation57 These changes enhanced HA cleavability. Existence of intermediate PCS (PQRKTR/G or PQRKRETR/G) has been suggested.Citation54 Although the PCS was identical in mildly and highly pathogenic viruses, there was no remarkable difference in the cleavability of HA suggesting that virulence of these viruses was determined by factors beyond the PCS, which remain to be investigated.Citation54,Citation55,Citation57 Interestingly, after infection of chickens with mildly pathogenic viruses, lesions were restricted to few organs and birds probably died due to respiratory and/or renal failure. In contrast, HPAIV was pantropic in replication and death occurred due to failure of multiple vital organs particularly the heart and pancreas. This different tropism may be governed by other, yet undetermined genomic changes rather than the PCS.Citation34
This HPAIV H5N2 epidemic in Mexico had been controlled by inactivated vaccines containing local seed virus and has not been reported since 1996, but LPAIV H5N2 remains endemic in Mexico and has spread to poultry in two other adjacent countries, Guatemala and El Salvador.Citation58
Epidemic of H5N2 in Texas, USA
Presence of an LPAIV H5N3 in commercial chickens in Texas connected with live bird markets was first reported in May 2002.Citation59 It has been suggested that the virus was introduced by wild birds.Citation59-Citation61 The Texas/H5N3 virus specified the PCS sequence PQREKR/G,Citation59,Citation62 but was classified as LPAIV based on the pathogenicity in chickens.Citation59 Experimental infections demonstrated that LPAIV Texas/H5N3 replicated efficiently in turkeys followed by chickens and ducks.Citation59,Citation61,Citation62 The virus induced signs of illness and spread to several organs in chickens beyond the trachea and intestine including the cerebrum.Citation59 It was assumed that this virus had been circulating in domestic chickens for some period of time before it was isolated from diseased commercial poultry. The Texas/H5N3 LPAIV had a 28 amino acid deletion in the NA stalk and all 6 potential glycosylation sites in the HA1 protein but lacked the glycosylation at position 158, which is considered a genetic marker for adaptation to poultry.Citation9 Moreover, no HPAI evolved from this virus after six serial passages in experimentally infected chickens.Citation62
On February 2004, an HPAIV H5N2 was confirmed in a broiler flock and 2 of 5 investigated live bird markets.Citation60,Citation63,Citation64 The affected birds showed only mild clinical signs resembling LPAIV infection. However, the deduced amino acid sequence in the PCS of Texas/H5N2 revealed that the virus had acquired one additional basic amino acid at the PCS (PQRKKR/GLF) complying with the definition of HPAIV. Nonetheless, the virus was not virulent for experimentally infected chickens (IVPI = 0.0).Citation63 It was found that Texas/H5N2 acquired HA, NP, M, and NS genes from Texas/H5N3, but the polymerase and NA genes were donated by another unknown virus.Citation63 Although investigation failed to elucidate why a virus with multibasic PCS remained mildly virulent for chickens, insertion of one or two additional basic amino acids at the PCS increased virulence both in vitro and in vivo.Citation63 It is worth pointing out that both Texas/H5N2 and its precursor had the same HA glycosylation pattern and conserved receptor binding sites. In contrast, Texas/H5N2 virus had only a 13 amino acid deletion (residues 51 to 63) at the NA stalk region.Citation63
Epidemic of H7N1 in Italy
On 29 March 1999, LPAIV H7N1 was diagnosed and subsequently detected in 199 outbreaks in northern Italy to be marked as the largest epidemic of LPAIV reported in a Western country. Surveillance in wild ducks, gulls, and coot revealed absence of circulation of LPAIV H7N1 among tested wild birds prior to or immediately after emergence of the virus in domestic poultry.Citation65,Citation66 However, other hosts might be implicated in its introductionCitation65 since the LPAIV clustered phylogenetically within a group of Eurasian and South African viruses of wild-bird origin.Citation67 After 9 mo, on December 13, an HPAIV of the same subtype emerged in multiple poultry species in a densely populated areaCitation68,Citation69 causing 413 reported outbreaks in turkeys, chickens, guinea-fowl, quail, pheasants, ostriches, ducks, geese, sparrows, doves, and Saker falconsCitation68-Citation74 and, after experimental infection, killed a red-legged partridge.Citation75 The disease resulted in culling of more than 13 million birds, disrupted the poultry marketing system, and was eradicated within 5 mo.Citation32,Citation68
The LPAIV H7N1 had the monobasic PCS motif PKGR/GLF, while the subsequent HPAIV specified the multibasic PCS motif PKGSRVRR/GLF.Citation68,Citation69 The progenitor LPAIV grew well in embryonated eggs obtained from mallard ducks, but not in chicken, turkey, and Muscovy duck embryos, whereas the HPAIV replicated well in all embryos.Citation71,Citation76 All Italian AIV had a deletion of 22 amino acids (position 54 to 75) in the NA stalk region.Citation67 Recently, using reverse genetics, an Italian LPAI with a short-stalk-NA was generated and found to be more virulent for chicken than the long-stalk-NA mutant. In contrast, short-stalk-NA was found to be disadvantageous for a sustained virus replication and transmission in ducks.Citation77 The NS1 gene of the HPAIV showed a progressive C-terminal truncation resulting in a 6 amino acid deletion in the NS1 protein in addition to V136I and D139N substitutions. Truncation of the NS1 gene increased the lethality of the HPAIV for embryonated chicken eggs, whereas V136I and D139N substitutions increased the virulence of the LPAIV in mice.Citation78 Recently, LPAIV H7N1 with a six-amino-acid truncation within NS1 was created which did not influence virus replication in duck or chicken cells.Citation79 However, truncation of NS1 to 99 amino acids in length converted an Italian LPAIV into a strong interferon inducer in duck cells.Citation80 An Italian LPAIV H7N1 with a human C-terminal RSKV motif in NS1 increased viral replication in ducks and induced high levels of the interferon-stimulated Mx gene, whereas the NS1 with the avian C-terminal ESEV motif increased virulence in mice causing severe pneumonia and a high level of type I interferon.Citation81
Interestingly, infection was not recorded in any non-avian species including humans throughout the duration of the H7N1 outbreak in Italy.Citation82 Serological examination of 798 serum samples collected from persons with close contact with infected poultry yielded no evidence of infection, and viral RNA could not be detected in clinical samples tested by RT-PCR.Citation32,Citation83 Yet, the Italian HPAIV H7N1 had the potential of interspecies transmission to mammals under experimental conditions. One ostrich-origin virus with 627K in the PB2 was lethal to experimentally infected mice and the virus has been recovered from the nervous and respiratory systems.Citation84,Citation85 Moreover, chicken-origin virus which specified 627E in PB2 protein was able to mutate to 627K after only one passage in mice.Citation84,Citation85 Different vaccines developed from Italian HPAIV H7N1 protected mice and ferrets against experimental infections.Citation86-Citation88
Epidemic of H7N3 in Pakistan
1994–1995 epidemic
First outbreaks of H7N3 were reported in broiler-breeder and broiler flocks in northern Pakistan from December 1994 to April 1995.Citation89 The virus was classified as LPAIVCitation89-Citation91 and no vaccination had been implemented to control the disease at that time.Citation89 However, in 1995, after few months, the LPAIV mutated to HPAIVCitation92 which has been controlled within four months by enforcing biosecurity measures and vaccination with inactivated local-strain seeded vaccine.Citation89,Citation90
The precursor LPAIV H7N3 had the monobasic PCS motif PEIPKGR/R while multibasic PCS with PETPKRKRKR/GLF or PETPKRRKR/GLF (very rarely PETPKRRNR/GLF) was characteristic for the HPAIV.Citation91,Citation93 These mutations occurred most likely by duplication in the PCS.Citation57 Interestingly, this LPAIV H7N3 had 89.5% nucleotides (nt) identity with the HA genes of the Pakistani HPAIV H7N3, whereas it showed 99.7% identity with the historic A/Parrot/North Ireland/YF73-67/1973 (H7N1) and up to 93% with recent viruses from poultry in China and Italy during 1997–2003 indicative of a probable common ancestor.Citation91 These extensive alterations, particularly in the HA1 gene in Pakistani HPAIV H7N3 are surprising. A possible explanation for this anomaly could be a reassortment with another unidentified strain(s).Citation89 Moreover, NS1 and NS2 were truncated to respectively 216–217 and 120–121 amino acids, whereas the other genes of the Pakistani LPAIV and HPAIV H7N3 were identical.
2003–2004 epidemic
Isolation of LPAIV H7N3 from commercial layer flocks was reported from April to October 2003 in the coastal southern town of Karachi, where the majority of the Pakistani table-egg layer flocks were raised.Citation90 In November 2003 a devastating HPAIV H7N3 suddenly emerged and widely spread within few weeks to the broiler-breeder stocks in the Northern part of the country.Citation90 This outbreak lasted until June 2004 and was finally controlled by vaccination and enforcement of biosecurity measures.Citation94 Although the Pakistani H7N3 viruses had so far some capacity for replication in mice and ferrets, their poor transmissibility suggests that they pose less risk of pandemic potential in comparison to other H7 viruses.Citation89 The Pakistani H7N3 viruses are closely related to AIV from Europe, Asia, and Australia. Between 1998 and 2000, isolation of LPAIV H7N3 from commercial chickens has been sporadically reported. Therefore, it has been suggested that the introduction of the H7N3 virus occurred in 1994 followed by undetected virus circulation in unknown reservoirs until 2003, possibly in unvaccinated backyard poultry, which have an epidemiological link with the commercial poultry sectors.Citation91 Although the Pakistani H7N3 lineage diversified into two genetic clusters (1995 and 2003), their antigenicity remained highly conservedCitation89,Citation95 which could be explained by the failure of those viruses to transmit to other hostsCitation89 or due to rapid eradication of the virus before emergence of dominant vaccine-escape lineage(s).Citation95 Genetic analysis showed that HPAIV H7N3 originated from mutation of the progenitor LPAIV (PEIPKGR/GLF) by insertion of multiple basic amino acids PETPKRRKR/GLF in the PCS.Citation90,Citation91 The Pakistani HPAIV H7N3 had a full length NA stalk. Unfortunately, a full genome sequence of the LPAIV H7N3 reported in April–October 2003 is not available.Citation91
Epidemic of H7N3 in Chile
In April 2002, an LPAIV H7N3 with the monobasic PKTR/GLF cleavage motif was isolated from broiler-breeder chickens. One month later HPAIV emerged.Citation96 Both viruses co-circulated for some period in the index premise.Citation97 Insertion of ten amino acids (PKTCSPLSRCRETR/GLF) in the HA cleavage site of HPAIV was attributed to recombination between the NP and HA genes.Citation98 Thereafter, viruses with a PKTCSPLSRCRKTR/GLF PCS motif evolved.Citation98 Although both variants of the PCS did not comply with the minimum PCS motif (R-X-R/K-R) of HPAIV,Citation23,Citation98 the viruses grew well in tissue culture with or without addition of trypsin, whereas the LPAIV grew only in presence of trypsin.Citation98 Furthermore, a strong tropism of HPAIV H7N3 for the cardiovascular system was demonstratedCitation99 resembling other HPAIV. In contrast to other LPAIV, the Chilean LPAIV H7N3 replicated also in the cardiac myocytes after intravenous inoculation, which could support that this virus was in the transition stage from low to high pathogenicity.Citation99 Phylogenetic analysis indicated that NP and PA were most closely related to H7N7 equine influenza viruses but other gene segments were closely related to the North American H7 lineage.Citation98
Epidemic of H7N3 in British Columbia (BC), Canada
LPAIV H7N3 was reported from the Fraser Valley (BC) in 52-week-old broiler–breeder chickens on 6 February 2004. The virus was related to LPAIV A/TU/Ontario/2000 (H7N3) which suggested that the latter may have been silently circulated among poultry species and was not detected due to limited active surveillance.Citation100 Suddenly, an HPAIV H7N3 outbreak was reported in a nearby 24-week-old chicken flock at 17 February.Citation101 The virus spread to 40 commercial poultry facilities before massive depopulation efforts resulted in complete eradication on 21 May 2004.Citation100,Citation101 HPAIV H7N3 was isolated from 2 workers 3 days after direct exposure to the infected poultry, both involved in the depopulation of infected birds, suffered from conjunctivitis, headache, and coryza.Citation101 Increased affinity of this Canadian HPAIV H7N3 toward α2-6 SA linkage predominant in human tracheal epithelial cells was reported.Citation102 The virus was also capable of using the eye as a portal of entry to mount a productive and lethal infection in the mouse model.Citation103
Sequence analysis of the HA genes of both low and high pathogenicity viruses isolated from the index farm, as well as from the 2 infected workers, revealed an insertion of 21 nt at the HA cleavage site of the HPAIV. Non-homologous recombination between the HA gene and seven amino acids of the M1 gene of the same virus was the most probable scenario for the sudden emergence of this HPAIV.Citation104 The original PCS of LPAIV was PKTR/GLF, while insertion resulted in alteration to PKQAYQKRMTR/GLF which subsequently mutated to different forms including PKQAYRKRMTR/GLF or PKQAYKKRMTR/GLF, PKQAYHKRMTR/GLF, PKQAHQKRMTR/GLF, PKQACQKRMTR/GLF and PKQAYQKQMTR/GLF without adverse effect on the pathogenicity.Citation104,Citation105 The insertion of seven amino acid in the PCS formed an enlarged exposed loop in the HA0 protein which likely enhanced the accessibility of furin-like proteases to the cleavage site and consequently increased the pathogenicity of those viruses.Citation104,Citation105 No deletion within the NA-stalk domain was observed.
Epidemic of H7N3 in Saskatchewan (SK), Canada
In September 2007, 55-week-old broiler–breeders were found with high H7 antibody titers in sera without showing clinical signs indicating exposure to LPAIV H7N3. A few days later, HPAIV H7N3 emerged. The isolated HPAIV had an insertion of six amino acids within the PCS, PKTTKPRPRR/GLF.Citation106,Citation107 The potential origin of this TKPRPR insert has been assumed to be a recombination with a hypothetical eukaryotic protein of domestic chicken Gallus gallus. This limited outbreak was successfully contained quickly without evidence of further spillover to nearby birds.Citation108 The HPAIV had close phylogenetic relationship with other contemporary LPAIV of wild waterfowl origin in North America suggesting a possible evolutionary relationship.Citation106 Sequence of this virus revealed full NS1 (230 amino acids), full PB1-F2 (90 amino acids), and no deletion within the NA-stalk domain.
Epidemic of H7N3 in Mexico
On 13 June 2012, several outbreaks caused by HPAIV H7N3 in commercial layer chicken farms in Jalisco, Mexico were reported,Citation109 resulting in the culling of 4.5 million birds.Citation110 Until now, the origin of the virus is unknown. However, wild birds and/or poultry trade were suspected to be the source of infection.Citation110 Vaccination in addition to enforcement of biosecurity measures and test-and-slaughter strategy were statutory control measures.Citation110 Two farm workers acquired the infection through direct contact with infected chickens. Patients suffered from conjunctivitis without showing any other symptoms and both recovered.Citation111,Citation112
Genetically the Mexican H7N3 isolates from poultry and humans are closely related to each other. Apart from the cleavage site, they showed high similarity to LPAI H7N7 viruses in the North American lineage recently isolated from wild birds.Citation112 The closest H7N3 viruses were chicken and human isolates from Canada.Citation110 The virus had an IVPI of 2.9. Analysis of the HA indicated an insertion of eight amino acids in the cleavage site PKDRKSRHRRTR/GLF which presumably occurred due to recombination of low virulent precursor with a part of the chicken genome. No deletion has been observed in the NA, PB1-F2, or NS1 genes.
Epidemic of H7N7 in the Netherlands
From February to May 2003, a devastating HPAIV H7N7 epidemic occurred in poultry in the Netherlands which also spread to Germany and Belgium.Citation113-Citation115 Serological screening of Dutch poultry suggested that the HPAI H7N7 epidemic was not preceded by circulation of an LPAIV H7N7 but occurred by reassortment after two separate introductions of AIV from wild mallard into commercial poultry sectors was the likely scenario.Citation113 It has been stated that the HPAIV A/Chicken/Netherlands/1/2003 (H7N7) acquired the HA gene from A/Mallard/Netherlands/12/2000 (H7N3) and the NA gene from the A/Mallard/Netherlands/2/2000 (H10N7).Citation113,Citation115,Citation116 The precursor LPAI mallard virus had the monobasic PCS “PKGR/GLF”, while the HPAIV H7N7 had the multibasic PCS motif “PKRRRR/GLF”Citation115 and no deletion in the NA-stalk region.
Unexpectedly, HPAIV H7N7 infected 89 humans in the largest documented AIV-related outbreak.Citation117 The virus infected 86 poultry workers and was further transmitted to three of their family members.Citation117 Although prophylactic use of oseltamivir reduced virus transmission to and between professionals exposed to infected poultry as shown by decreased seroprevalence of H7 antibodies in those persons,Citation118 a veterinarian died of pneumonia due to handling of infected poultry.Citation114,Citation119,Citation120 The majority of infected humans suffered from conjunctivitis rather than respiratory illness,Citation117,Citation119,Citation121 and H7N7 was mostly detected from eye swabs compared with throat swabs.Citation119 Interestingly, serological surveillance indicated that at least one thousand people contracted H7N7 virus, most of them with no symptoms. It was suggested that the virus transmitted from the primary cases (i.e., cullers, workers, veterinarians, etc.) to approximately half of their household contacts.Citation121 Interestingly, Belser and associatesCitation102 found that the H7N7 virus retained binding-preferences for the avian α2-3 SA receptor.Citation122
Genome sequence of the index HPAIV H7N7 isolated from poultry A/Chicken/Netherlands/1/03 was almost identical to the virus isolated from human A/Netherlands/33/03 who suffered from conjunctivitis; only two silent mutations in the PB2 gene and one amino acid substitution in NS1 protein were uncovered.Citation115 Furthermore, virus isolates obtained from a family cluster, the wife and daughter of a poultry worker, had only one silent mutation in the HA gene.Citation115 However, the genome of the virus isolated from the fatal case had novel 12 synonymous and 14 non-synonymous substitutions in the PB2, NA, HA, PA, and NS1 that did not exist in the virus from the conjunctivitis case nor the index virus isolated from poultry.Citation115,Citation120 However, these mutations, except PB2 E627K and HA K416R, were also found in viruses from other poultry houses including birds in the farm where the veterinarian contracted the fatal infection,Citation115 which indicate that deadly viruses with efficient replication in mammals could be generated in poultry.Citation122 The PB2 E627K and HA K416R substitutions potentially resulted from virus adaptation to the new host as evidenced nine days after the onset of symptoms.Citation115
Based on reverse genetics and animal models, the HA, PB2, PA, and NA genes increased replication of the fatal case virus in humansCitation122 and the HA and PB2 genes were responsible for increased pathogenicity.Citation120 In a mouse model, the E627K substitution in PB2 was the main determinant of pathogenicity,Citation120,Citation123 while A143T in the HA introduced a potential N-linked glycosylation site at position 141 near the RBD increased virus titers in the lower respiratory tract and enhanced dissemination of the virus into different organs.Citation120,Citation122,Citation123 Likewise, the virus isolated from fatal case was also highly virulent for ferrets but did not transmit to contact ferrets while virus isolated from the conjunctivitis case was less virulent and transmitted to 2 out of 3 contact animals.Citation103
Interspecies transmission of the virus to other mammals has been recorded. Pigs kept in the same farm with infected poultry were seropositive for H7 antibodies. However, neither clinical signs nor virus shedding was seen and there was no evidence of pig-to-pig transmission.Citation114 Moreover, van Riel et al.Citation124 showed that HPAIV H7N7 isolated from the fatal human case was also able to infect cats experimentally and cause respiratory illness but was unable to spread beyond the respiratory tract.
Other Examples Linking Possible Evolution of HPAIV from LPAIV in Nature
Epidemic of H5N1 in Norfolk, England
In December 1991, 7129 out of 8000 18-week-old turkeys died within 8 days in a commercial farm in Norfolk, England.Citation125 The outbreak was limited and did not spread due to enforced biosecurity and stamping out policy. Since the farm was close to lakes and rivers, possible wild-bird involvement was assumed for the first introduction.Citation125 Isolation of two H5N1 viruses displaying variable phenotypic characters from a dead frozen carcass was recorded. The virulent A/Turkey/England/50-92/91 virus isolated from a swab sample of the respiratory tract had an IVPI of 3.0, while the avirulent A/Turkey/England/87-92/91 virus isolated from the brain tissue had an IVPI of 0.0.Citation24,Citation125,Citation126 Interestingly, the avirulent virus could not be recovered from other organs of the same carcass beyond the brain.Citation24
Sequence comparison of the HA protein of both LPAIV and HPAIV revealed the identical PCS PQRKRTR/GLFCitation24 and three amino acid substitutions, K205E, R348G, and G435V.Citation126 In addition, seven amino acids alterations have been reported in PB2, PB1, PA, and NP.Citation126 Interestingly, pathogenicity of the LPAIV could not be increased either by serial passages in specific pathogen-free (SPF) chicken eggs or by intravenous passages in 6-week-old chickens,Citation127 but succeeded after intracerebral passage in 1- to 2-day-old SPF chicks.Citation126 Intriguingly, after the first passage of the LPAIV, mutations of the HPAI pathotype (substitution in the NP was retained) were induced but the virus remained LPAIV, IVPI = 0.4, but after the second passage the IVPI amounted to 1.9.Citation126 Several amino acid changes had been assumed to explain the difference between both pathotypes; namely K123E in PB2, G656E in PB1, and Y74H in PA, which could influence the folding of the polymerase subunits. Nevertheless, there are, so far, no available in vitro reverse-genetic investigations on virulence determinants of this virus.Citation126
H5N1 in Guangdong province, China
During the summer and early fall of 1996, geese in a commercial farm in Guangdong province, China suffered from respiratory disorders and up to 40% mortality. Two different H5N1 pathotypes were isolated from this outbreak: A/Goose/Guangdong/1/96 displayed a high pathogenic phenotype for chickens with an IVPI of 2.1 while A/Goose/Guangdong/2/96 replicated poorly in chickens with an IVPI of 0.0.Citation128-Citation131 Furthermore, the avirulent virus was also detected in several organs (except brain) of experimentally infected geese.Citation128 The avirulent A/Goose/Guangdong/2/1996 virus was assumed to be the ancestor of the virulent virus and the asymptomatically infected goose were considered to be the ideal reservoir host for spread of the virus to chickens.Citation128
Sequence comparison of those two viruses revealed identical multiple basic PCS motif PQRERRRKKR/GLF and only eight nucleotide differences; three synonymous mutations in PB1 and NP and five non-synonymous mutations in PA, NP, M1, and NS1.Citation128 Using reverse genetics Li and colleaguesCitation128 found that the NS1 gene modulated the virulence of the two viruses in chickens. Thus, the V149A substitution of the NS1 protein was responsible for the difference in virulence observed in chickens since it was able to diminish the ability of the NS1 protein to antagonize the induction of IFN-α and β in chicken embryo fibroblast cells and thus increase virulence and lethality. In geese, it was postulated that NS1 did not play a role in the replication and variation but changes in the NP may be responsible for that.Citation128 It is worth pointing out that A/Goose/Guangdong/1/96 was probably the immediate precursor of the Asian lineage “Z genotype” of HPAIV H5N1 that spread globally since 2003 resulted in destruction of tens of billions of birds and killed 375 out of 630 (~59.5% fatality rate) infected humans as of 4 June 2013.Citation131-Citation133 More details on the virulence determinants of the contemporary HPAIV H5N1 in poultry and humans have been previously published.Citation134-Citation140
H5N9 in Wisconsin, USA
In February 1968, an outbreak of A/Turkey/Wisconsin/68 (H5N9) caused severe losses in turkeys in Wisconsin, USA.Citation141 Coexistence of two closely related competitive and distinct virus populations within the original virus isolate was reported.Citation142,Citation143 The virulent strain induced rapid embryo lethality of SPF chicken embryonated eggs and mortality in four-week-old chicks while the avirulent pathotype induced slowly embryo lethality (more than 72 h) and was nonlethal to chicks. Both virus populations were stable through 12 serial passages in SPF eggs.Citation144 Two amino acid differences in the NS gene were foundCitation145: valine and phenylalanine (in the nonlethal phenotype) or leucine and serine (in the virulent phenotype) at position 119 in NS1 and 48 in NS2, respectively. Nevertheless, these mutations did not affect the rates of viral replication neither in cell culture nor in embryonated chicken eggs.Citation145 The avirulent strain had monobasic PCS PQRETR/GLF.Citation146 However, so far no genomic data are available for the virulent virus. Therefore, further investigation is required to determine the virulence markers of those two viral populations mentioned in the earlier studies.Citation142-Citation145
Epidemic of H7N7 in Victoria, Australia
In January 1976, a limited outbreak of AIV occurred on a combined layer and broiler farm in Victoria, Australia.Citation147 Recorded mortality was up to 25%Citation148 and the disease was eradicated by culling.Citation149 The source of the virus was not traced.Citation148 Isolation of H7N7 viruses displaying different virulence was recorded. A/Chicken/Victoria/76 caused classical AIV signs with variable pathogenicity indices ranging from 0.97 to 1.98,Citation149-Citation152 whereas A/Duck/Victoria/76 isolated from asymptomatic commercial ducks had an IVPI of 0.00 and induced, contrary to the chicken isolate, no clinical signs in experimentally infected chickens, turkeys, and ducks.Citation150,Citation152 Cleavage site of the virulent chicken virus specified PKKREKR/GLF while the avirulent duck virus had PKKR/GLF.Citation149,Citation153 Although the two viruses showed different pathogenicity and the chicken virus was isolated first,Citation152 it remains speculative which virus altered its phenotype.
General Remarks and Outlook
Molecular changes found in the naturally evolving HPAIV are thought to be a major contributing factor for the appearance of devastating epidemics besides ecological niches, the host’s immune system, the intracellular machinery of the host and their complexity. From this comprehensive review, it could be concluded that: (1) wild birds were confirmed or claimed as a source of first introduction of LPAIV to the terrestrial poultry. (2) The elapsed time between first introduction of LPAIV and emergence of HPAIV in poultry is extremely variable from few days to several months and even years. (3) Molecular details of evolution of highly virulent viruses from LP precursor(s) seem to be virus-dependent. (4) Acquisition of cleavage site alterations occurred due to insertion of basic amino acids or conformational changes that make the PCS more accessible to ubiquitous proteases. (5) Existence of multibasic amino acids in the PCS was not the sole genetic change; concurrent mutations in the HA adjacent to or apart from the PCS were also observed. (6) Compatible genetic constellation to generate a virulent virus was essential, hence some viruses (from Mexico, Canada, Chile, Pennsylvania, and Italy) required only few point mutations while others (from Texas, the Netherlands, and Pakistan) probably evolved by a multistep process of reassortment and mutations. (7) Several changes have been observed in all gene segments particularly the polymerase subunits, in particular the PA gene (). The NEP had very few, if any, differences between both pathotypes which may exclude any direct role in shift of pathogenicity of LPAIV. (8) Deletion of the NA stalk region was observed in all H5N2 outbreaks. In contrast, only the Italian H7N1 had a 22 amino acid deletion while neither H7N3 nor N7N7 subtypes specified an NA-deletionCitation154 indicating that this deletion is NA-subtype-specific. Whether internal gene segments can influence this HA-NA meticulous balance needs further investigation. (9) Extensive pangenomic non-synonymous mutations were observed in viruses isolated from all outbreaks and their role in pathogenicity or adaptation has not been elucidated yet. (10) Evolution of influenza viruses by recombination has been previously reported,Citation155-Citation160 but in this review it has been recorded in four outbreaks which should be a hot-spot for future research. (11) Some viruses crossed species barriers and infected a wide host spectrum while others circulate among much narrower range of hosts (i.e., Pakistani H7N3 viruses). (12) Effective control of these outbreaks was successful mainly by elimination of the infected birds and sometimes with adoption of inactivated vaccines developed from local LPAI strains (i.e., Mexico and Pakistan). (13) Genetic markers mentioned here should be considered in studies of virulence, pathogenesis, adaptation and transmissibility of AIV using reverse genetics. Although data in this area of research is substantially growing, different facets of virulence determination exist and further investigation is highly required. Our increased understanding of the genetic changes accompanying the shift from LPAIV to HPAIV under natural conditions may help to assess the risk posed by any LPAIV after introduction into domestic poultry to become HPAIV. It may also assist in the development of new attenuated vaccines specifically targeted at the most relevant LPAIV circulating in nature.
| Abbreviations: | ||
| AIV | = | avian influenza viruses |
| BC | = | British Columbia |
| HA | = | hemagglutinin |
| HP | = | highly pathogenic |
| IVPI | = | intravenous pathogenicity index |
| LBM | = | live bird markets |
| LP | = | low pathogenic |
| M | = | matrix |
| NA | = | neuraminidase |
| NEP | = | nuclear export protein |
| NP | = | nucleoprotein |
| nt | = | nucleotide(s) |
| NS | = | non-structural |
| PA | = | acidic polymerase |
| PB | = | basic polymerase |
| PCS | = | proteolytic cleavage site |
| RBD | = | receptor binding domain |
| SA | = | sialic acid |
| SK | = | Saskatchewan |
Submitted
05/02/13
Revised
07/09/13
Accepted
07/10/13
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- ICTVdB. International Committee on Taxonomy of Viruses Index of Viruses, Index of Viruses: Orthomyxoviridae. In: Büchen-Osmond CE, ed. ICTVdB - The Universal Virus Database, version 4: Columbia University, New York, USA., 2006.
- Tollis M, Di Trani L. Recent developments in avian influenza research: epidemiology and immunoprophylaxis. Vet J 2002; 164:202 - 15; http://dx.doi.org/10.1053/tvjl.2002.0716; PMID: 12505393
- Cheung TK, Poon LL. Biology of influenza a virus. Ann N Y Acad Sci 2007; 1102:1 - 25; http://dx.doi.org/10.1196/annals.1408.001; PMID: 17470908
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev 1992; 56:152 - 79; PMID: 1579108
- Brown EG. Influenza virus genetics. Biomed Pharmacother 2000; 54:196 - 209; http://dx.doi.org/10.1016/S0753-3322(00)89026-5; PMID: 10872718
- Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, et al. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol 2005; 79:2814 - 22; http://dx.doi.org/10.1128/JVI.79.5.2814-2822.2005; PMID: 15709000
- Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, et al. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A 2012; 109:4269 - 74; PMID: 22371588
- Webby RJ, Perez DR, Coleman JS, Guan Y, Knight JH, Govorkova EA, et al. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet 2004; 363:1099 - 103; http://dx.doi.org/10.1016/S0140-6736(04)15892-3; PMID: 15064027
- Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol 1999; 73:1146 - 55; PMID: 9882316
- Perdue ML, Swayne DE. Public health risk from avian influenza viruses. Avian Dis 2005; 49:317 - 27; http://dx.doi.org/10.1637/7390-060305R.1; PMID: 16252482
- Rott R. The pathogenic determinant of influenza virus. Vet Microbiol 1992; 33:303 - 10; http://dx.doi.org/10.1016/0378-1135(92)90058-2; PMID: 1481363
- Webster RG, Wright SM, Castrucci MR, Bean WJ, Kawaoka Y. Influenza--a model of an emerging virus disease. Intervirology 1993; 35:16 - 25; PMID: 8407243
- Ferguson NM, Galvani AP, Bush RM. Ecological and immunological determinants of influenza evolution. Nature 2003; 422:428 - 33; http://dx.doi.org/10.1038/nature01509; PMID: 12660783
- Peiris JS, de Jong MD, Guan Y. Avian influenza virus (H5N1): a threat to human health. Clin Microbiol Rev 2007; 20:243 - 67; http://dx.doi.org/10.1128/CMR.00037-06; PMID: 17428885
- Swayne DE. Avian influenza vaccines and therapies for poultry. Comp Immunol Microbiol Infect Dis 2009; 32:351 - 63; http://dx.doi.org/10.1016/j.cimid.2008.01.006; PMID: 18442853
- Alexander DJ. A review of avian influenza in different bird species. Vet Microbiol 2000; 74:3 - 13; http://dx.doi.org/10.1016/S0378-1135(00)00160-7; PMID: 10799774
- Halvorson DA. The control of H5 or H7 mildly pathogenic avian influenza: a role for inactivated vaccine. Avian Pathol 2002; 31:5 - 12; http://dx.doi.org/10.1080/03079450120106570; PMID: 12430550
- Alexander DJ. Avian influenza.Chapter 2.3.4. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2010. World Organisation for Animal Health, Paris, France 2009:http://www.oie.int/eng/normes/mmanual/2008/pdf/2.03.04 AI.pdf.
- Garten W, Klenk HD. Characterization of the carboxypeptidase involved in the proteolytic cleavage of the influenza haemagglutinin. J Gen Virol 1983; 64:2127 - 37; http://dx.doi.org/10.1099/0022-1317-64-10-2127; PMID: 6619800
- Bosch FX, Garten W, Klenk HD, Rott R. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of Avian influenza viruses. Virology 1981; 113:725 - 35; http://dx.doi.org/10.1016/0042-6822(81)90201-4; PMID: 7023022
- Kawaoka Y, Nestorowicz A, Alexander DJ, Webster RG. Molecular analyses of the hemagglutinin genes of H5 influenza viruses: origin of a virulent turkey strain. Virology 1987; 158:218 - 27; http://dx.doi.org/10.1016/0042-6822(87)90256-X; PMID: 3576972
- Klenk HD, Rott R. The molecular biology of influenza virus pathogenicity. Adv Virus Res 1988; 34:247 - 81; http://dx.doi.org/10.1016/S0065-3527(08)60520-5; PMID: 3046255
- Horimoto T, Kawaoka Y. Reverse genetics provides direct evidence for a correlation of hemagglutinin cleavability and virulence of an avian influenza A virus. J Virol 1994; 68:3120 - 8; PMID: 8151777
- Wood GW, Banks J, McCauley JW, Alexander DJ. Deduced amino acid sequences of the haemagglutinin of H5N1 avian influenza virus isolates from an outbreak in turkeys in Norfolk, England. Arch Virol 1994; 134:185 - 94; http://dx.doi.org/10.1007/BF01379117; PMID: 7506519
- Garten W, Bosch FX, Linder D, Rott R, Klenk HD. Proteolytic activation of the influenza virus hemagglutinin: The structure of the cleavage site and the enzymes involved in cleavage. Virology 1981; 115:361 - 74; http://dx.doi.org/10.1016/0042-6822(81)90117-3; PMID: 7032055
- Steinhauer DA. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 1999; 258:1 - 20; http://dx.doi.org/10.1006/viro.1999.9716; PMID: 10329563
- Munch M, Nielsen LP, Handberg KJ, Jørgensen PH. Detection and subtyping (H5 and H7) of avian type A influenza virus by reverse transcription-PCR and PCR-ELISA. Arch Virol 2001; 146:87 - 97; http://dx.doi.org/10.1007/s007050170193; PMID: 11266220
- Kido H, Okumura Y, Takahashi E, Pan HY, Wang S, Chida J, et al. Host envelope glycoprotein processing proteases are indispensable for entry into human cells by seasonal and highly pathogenic avian influenza viruses. J Mol Genet Med 2008; 3:167 - 75; PMID: 19565019
- Bertram S, Glowacka I, Steffen I, Kühl A, Pöhlmann S. Novel insights into proteolytic cleavage of influenza virus hemagglutinin. Rev Med Virol 2010; 20:298 - 310; http://dx.doi.org/10.1002/rmv.657; PMID: 20629046
- Chambers TM, Hinshaw VS, Kawaoka Y, Easterday BC, Webster RG. Influenza viral infection of swine in the United States 1988-1989. Arch Virol 1991; 116:261 - 5; http://dx.doi.org/10.1007/BF01319247; PMID: 1848066
- Horimoto T, Kawaoka Y. Molecular changes in virulent mutants arising from avirulent avian influenza viruses during replication in 14-day-old embryonated eggs. Virology 1995; 206:755 - 9; http://dx.doi.org/10.1016/S0042-6822(95)80004-2; PMID: 7831837
- Capua I, Mutinelli F, Pozza MD, Donatelli I, Puzelli S, Cancellotti FM. The 1999-2000 avian influenza (H7N1) epidemic in Italy: veterinary and human health implications. Acta Trop 2002; 83:7 - 11; http://dx.doi.org/10.1016/S0001-706X(02)00057-8; PMID: 12062787
- Röhm C, Horimoto T, Kawaoka Y, Süss J, Webster RG. Do hemagglutinin genes of highly pathogenic avian influenza viruses constitute unique phylogenetic lineages?. Virology 1995; 209:664 - 70; http://dx.doi.org/10.1006/viro.1995.1301; PMID: 7778300
- Swayne DE, Perdue ML, Garcia M, Rivera-Cruz E, Brugh M. Pathogenicity and diagnosis of H5N2 Mexican avian influenza viruses in chickens. Avian Dis 1997; 41:335 - 46; http://dx.doi.org/10.2307/1592187; PMID: 9201397
- Goto H, Kawaoka Y. A novel mechanism for the acquisition of virulence by a human influenza A virus. Proc Natl Acad Sci U S A 1998; 95:10224 - 8; http://dx.doi.org/10.1073/pnas.95.17.10224; PMID: 9707628
- Munier S, Larcher T, Cormier-Aline F, Soubieux D, Su B, Guigand L, et al. A genetically engineered waterfowl influenza virus with a deletion in the stalk of the neuraminidase has increased virulence for chickens. J Virol 2010; 84:940 - 52; http://dx.doi.org/10.1128/JVI.01581-09; PMID: 19889765
- Ozawa M, Kawaoka Y. Taming influenza viruses. Virus Res 2011; 162:8 - 11; http://dx.doi.org/10.1016/j.virusres.2011.09.035; PMID: 21968297
- Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A 2000; 97:6108 - 13; http://dx.doi.org/10.1073/pnas.100133697; PMID: 10801978
- Stech J, Stech O, Herwig A, Altmeppen H, Hundt J, Gohrbandt S, et al. Rapid and reliable universal cloning of influenza A virus genes by target-primed plasmid amplification. Nucleic Acids Res 2008; 36:e139; http://dx.doi.org/10.1093/nar/gkn646; PMID: 18832366
- Gohrbandt S, Veits J, Hundt J, Bogs J, Breithaupt A, Teifke JP, et al. Amino acids adjacent to the haemagglutinin cleavage site are relevant for virulence of avian influenza viruses of subtype H5. J Gen Virol 2011; 92:51 - 9; http://dx.doi.org/10.1099/vir.0.023887-0; PMID: 20881092
- Tada T, Suzuki K, Sakurai Y, Kubo M, Okada H, Itoh T, et al. Emergence of avian influenza viruses with enhanced transcription activity by a single amino acid substitution in the nucleoprotein during replication in chicken brains. J Virol 2011; 85:10354 - 63; http://dx.doi.org/10.1128/JVI.00605-11; PMID: 21795332
- Kawaoka Y, Naeve CW, Webster RG. Is virulence of H5N2 influenza viruses in chickens associated with loss of carbohydrate from the hemagglutinin?. Virology 1984; 139:303 - 16; http://dx.doi.org/10.1016/0042-6822(84)90376-3; PMID: 6516214
- Kawaoka Y, Webster RG. Evolution of the A/Chicken/Pennsylvania/83 (H5N2) influenza virus. Virology 1985; 146:130 - 7; http://dx.doi.org/10.1016/0042-6822(85)90059-5; PMID: 4036005
- Bean WJ, Kawaoka Y, Wood JM, Pearson JE, Webster RG. Characterization of virulent and avirulent A/chicken/Pennsylvania/83 influenza A viruses: potential role of defective interfering RNAs in nature. J Virol 1985; 54:151 - 60; PMID: 3973976
- Wood JM, Webster RG, Nettles VF. Host range of A/Chicken/Pennsylvania/83 (H5N2) influenza virus. Avian Dis 1985; 29:198 - 207; http://dx.doi.org/10.2307/1590708; PMID: 3985875
- Hinshaw VS, Wood JM, Webster RG, Deibel R, Turner B. Circulation of influenza viruses and paramyxoviruses in waterfowl originating from two different areas of North America. Bull World Health Organ 1985; 63:711 - 9; PMID: 3878741
- Kawaoka Y, Webster RG. Molecular mechanism of acquisition of virulence in influenza virus in nature. Microb Pathog 1988; 5:311 - 8; http://dx.doi.org/10.1016/0882-4010(88)90032-0; PMID: 3070264
- Ohuchi M, Orlich M, Ohuchi R, Simpson BE, Garten W, Klenk HD, et al. Mutations at the cleavage site of the hemagglutinin after the pathogenicity of influenza virus A/chick/Penn/83 (H5N2). Virology 1989; 168:274 - 80; http://dx.doi.org/10.1016/0042-6822(89)90267-5; PMID: 2916326
- Brugh M, Perdue ML. Emergence of highly pathogenic virus during selective chicken passage of the prototype mildly pathogenic chicken/Pennsylvania/83 (H5N2) influenza virus. Avian Dis 1991; 35:824 - 33; http://dx.doi.org/10.2307/1591616; PMID: 1838476
- Webster RG, Kawaoka Y, Bean WJ Jr.. Molecular changes in A/Chicken/Pennsylvania/83 (H5N2) influenza virus associated with acquisition of virulence. Virology 1986; 149:165 - 73; http://dx.doi.org/10.1016/0042-6822(86)90118-2; PMID: 3946082
- Deshpande KL, Fried VA, Ando M, Webster RG. Glycosylation affects cleavage of an H5N2 influenza virus hemagglutinin and regulates virulence. Proc Natl Acad Sci U S A 1987; 84:36 - 40; http://dx.doi.org/10.1073/pnas.84.1.36; PMID: 3467357
- Webster RG, Kawaoka Y, Bean WJ. What is the potential of avirulent influenza viruses to complement a cleavable hemagglutinin and generate virulent strains?. Virology 1989; 171:484 - 92; http://dx.doi.org/10.1016/0042-6822(89)90618-1; PMID: 2763464
- Deshpande KL, Naeve CW, Webster RG. The neuraminidases of the virulent and avirulent A/Chicken/Pennsylvania/83 (H5N2) influenza A viruses: sequence and antigenic analyses. Virology 1985; 147:49 - 60; http://dx.doi.org/10.1016/0042-6822(85)90226-0; PMID: 2414922
- Horimoto T, Rivera E, Pearson J, Senne D, Krauss S, Kawaoka Y, et al. Origin and molecular changes associated with emergence of a highly pathogenic H5N2 influenza virus in Mexico. Virology 1995; 213:223 - 30; http://dx.doi.org/10.1006/viro.1995.1562; PMID: 7483266
- Senne DA, Panigrahy B, Kawaoka Y, Pearson JE, Süss J, Lipkind M, et al. Survey of the hemagglutinin (HA) cleavage site sequence of H5 and H7 avian influenza viruses: amino acid sequence at the HA cleavage site as a marker of pathogenicity potential. Avian Dis 1996; 40:425 - 37; http://dx.doi.org/10.2307/1592241; PMID: 8790895
- García M, Crawford JM, Latimer JW, Rivera-Cruz E, Perdue ML. Heterogeneity in the haemagglutinin gene and emergence of the highly pathogenic phenotype among recent H5N2 avian influenza viruses from Mexico. J Gen Virol 1996; 77:1493 - 504; http://dx.doi.org/10.1099/0022-1317-77-7-1493; PMID: 8757992
- Perdue ML, García M, Senne D, Fraire M. Virulence-associated sequence duplication at the hemagglutinin cleavage site of avian influenza viruses. Virus Res 1997; 49:173 - 86; http://dx.doi.org/10.1016/S0168-1702(97)01468-8; PMID: 9213392
- Lee CW, Senne DA, Suarez DL. Effect of vaccine use in the evolution of Mexican lineage H5N2 avian influenza virus. J Virol 2004; 78:8372 - 81; http://dx.doi.org/10.1128/JVI.78.15.8372-8381.2004; PMID: 15254209
- Lee CW, Senne DA, Linares JA, Woolcock PR, Stallknecht DE, Spackman E, et al. Characterization of recent H5 subtype avian influenza viruses from US poultry. Avian Pathol 2004; 33:288 - 97; http://dx.doi.org/10.1080/0307945042000203407; PMID: 15223555
- Senne DA. Avian influenza in North and South America, 2002-2005. Avian Dis 2007; 51:Suppl 167 - 73; http://dx.doi.org/10.1637/7621-042606R1.1; PMID: 17494549
- Pillai SP, Pantin-Jackwood M, Suarez DL, Saif YM, Lee CW. Pathobiological characterization of low-pathogenicity H5 avian influenza viruses of diverse origins in chickens, ducks and turkeys. Arch Virol 2010; 155:1439 - 51; http://dx.doi.org/10.1007/s00705-010-0727-8; PMID: 20577770
- Dlugolenski D, Jones L, Saavedra G, Tompkins SM, Tripp RA, Mundt E. Passage of low-pathogenic avian influenza (LPAI) viruses mediates rapid genetic adaptation of a wild-bird isolate in poultry. Arch Virol 2011; 156:565 - 76; http://dx.doi.org/10.1007/s00705-010-0891-x; PMID: 21197555
- Lee CW, Swayne DE, Linares JA, Senne DA, Suarez DL. H5N2 avian influenza outbreak in Texas in 2004: the first highly pathogenic strain in the United States in 20 years?. J Virol 2005; 79:11412 - 21; http://dx.doi.org/10.1128/JVI.79.17.11412-11421.2005; PMID: 16103192
- Pelzel AM, McCluskey BJ, Scott AE. Review of the highly pathogenic avian influenza outbreak in Texas, 2004. J Am Vet Med Assoc 2006; 228:1869 - 75; http://dx.doi.org/10.2460/javma.228.12.1869; PMID: 16784375
- De Marco MA, Foni E, Campitelli L, Delogu M, Raffini E, Chiapponi C, et al. Influenza virus circulation in wild aquatic birds in Italy during H5N2 and H7N1 poultry epidemic periods (1998 to 2000). Avian Pathol 2005; 34:480 - 5; http://dx.doi.org/10.1080/03079450500368185; PMID: 16537162
- De Marco MA, Campitelli L, Foni E, Raffini E, Barigazzi G, Delogu M, et al. Influenza surveillance in birds in Italian wetlands (1992-1998): is there a host restricted circulation of influenza viruses in sympatric ducks and coots?. Vet Microbiol 2004; 98:197 - 208; http://dx.doi.org/10.1016/j.vetmic.2003.10.018; PMID: 15036528
- Banks J, Speidel ES, Moore E, Plowright L, Piccirillo A, Capua I, et al. Changes in the haemagglutinin and the neuraminidase genes prior to the emergence of highly pathogenic H7N1 avian influenza viruses in Italy. Arch Virol 2001; 146:963 - 73; http://dx.doi.org/10.1007/s007050170128; PMID: 11448033
- Capua I, Marangon S. The avian influenza epidemic in Italy, 1999-2000: a review. Avian Pathol 2000; 29:289 - 94; http://dx.doi.org/10.1080/03079450050118403; PMID: 19184817
- Capua I, Mutinelli F, Marangon S, Alexander DJ. H7N1 avian influenza in Italy (1999 to 2000) in intensively reared chickens and turkeys. Avian Pathol 2000; 29:537 - 43; http://dx.doi.org/10.1080/03079450020016779; PMID: 19184849
- Capua I, Mutinelli F, Terregino C, Cattoli G, Manvell RJ, Burlini F. Highly pathogenic avian influenza (H7N1) in ostriches farmed in Italy. Vet Rec 2000; 146:356; PMID: 10777051
- Capua I, Mutinelli F, Hablovarid MH. Avian embryo susceptibility to Italian H7N1 avian influenza viruses belonging to different genetic lineages. Arch Virol 2002; 147:1611 - 21; http://dx.doi.org/10.1007/s00705-002-0837-z; PMID: 12181679
- Capua I, Mutinelli F. Mortality in Muscovy ducks (Cairina moschata) and domestic geese (Anser anser var. domestica) associated with natural infection with a highly pathogenic avian influenza virus of H7N1 subtype. Avian Pathol 2001; 30:179 - 83; http://dx.doi.org/10.1080/03079450120044597; PMID: 19184894
- Zanella A, Dall’Ara P, Martino PA. Avian influenza epidemic in Italy due to serovar H7N1. Avian Dis 2001; 45:257 - 61; http://dx.doi.org/10.2307/1593038; PMID: 11332492
- Magnino S, Fabbi M, Moreno A, Sala G, Lavazza A, Ghelfi E, et al. Avian influenza virus (H7 serotype) in a saker falcon in Italy. Vet Rec 2000; 146:740; PMID: 10901223
- Bertran K, Pérez-Ramírez E, Busquets N, Dolz R, Ramis A, Darji A, et al. Pathogenesis and transmissibility of highly (H7N1) and low (H7N9) pathogenic avian influenza virus infection in red-legged partridge (Alectoris rufa). Vet Res 2011; 42:24; http://dx.doi.org/10.1186/1297-9716-42-24; PMID: 21314907
- Mutinelli F, Hablovarid H, Capua I. Avian embryo susceptibility to Italian H7N1 avian influenza viruses belonging to different lineages. Avian Dis 2003; 47:Suppl 1145 - 9; http://dx.doi.org/10.1637/0005-2086-47.s3.1145; PMID: 14575131
- Hoffmann TW, Munier S, Larcher T, Soubieux D, Ledevin M, Esnault E, et al. Length variations in the NA stalk of an H7N1 influenza virus have opposite effects on viral excretion in chickens and ducks. J Virol 2012; 86:584 - 8; http://dx.doi.org/10.1128/JVI.05474-11; PMID: 22013034
- Keiner B, Maenz B, Wagner R, Cattoli G, Capua I, Klenk HD. Intracellular distribution of NS1 correlates with the infectivity and interferon antagonism of an avian influenza virus (H7N1). J Virol 2010; 84:11858 - 65; http://dx.doi.org/10.1128/JVI.01011-10; PMID: 20844052
- Soubies SM, Hoffmann TW, Croville G, Larcher T, Ledevin M, Soubieux D, et al. Deletion of the C-terminal ESEV domain of NS1 does not affect the replication of a low-pathogenic avian influenza virus H7N1 in ducks and chickens. J Gen Virol 2013; 94:50 - 8; http://dx.doi.org/10.1099/vir.0.045153-0; PMID: 23052391
- Soubies SM, Volmer C, Guérin JL, Volmer R. Truncation of the NS1 protein converts a low pathogenic avian influenza virus into a strong interferon inducer in duck cells. Avian Dis 2010; 54:Suppl 527 - 31; http://dx.doi.org/10.1637/8707-031709-Reg.1; PMID: 20521689
- Soubies SM, Volmer C, Croville G, Loupias J, Peralta B, Costes P, et al. Species-specific contribution of the four C-terminal amino acids of influenza A virus NS1 protein to virulence. J Virol 2010; 84:6733 - 47; http://dx.doi.org/10.1128/JVI.02427-09; PMID: 20410267
- Capua I. Avian influenza in the EU. Zoonoses Public Health 2008; 55:1; http://dx.doi.org/10.1111/j.1863-2378.2007.01089.x; PMID: 18201320
- Puzelli S, Di Trani L, Fabiani C, Campitelli L, De Marco MA, Capua I, et al. Serological analysis of serum samples from humans exposed to avian H7 influenza viruses in Italy between 1999 and 2003. J Infect Dis 2005; 192:1318 - 22; http://dx.doi.org/10.1086/444390; PMID: 16170747
- Rigoni M, Shinya K, Toffan A, Milani A, Bettini F, Kawaoka Y, et al. Pneumo- and neurotropism of avian origin Italian highly pathogenic avian influenza H7N1 isolates in experimentally infected mice. Virology 2007; 364:28 - 35; http://dx.doi.org/10.1016/j.virol.2007.02.031; PMID: 17408714
- Rigoni M, Toffan A, Viale E, Mancin M, Cilloni F, Bertoli E, et al. The mouse model is suitable for the study of viral factors governing transmission and pathogenesis of highly pathogenic avian influenza (HPAI) viruses in mammals. Vet Res 2010; 41:66; http://dx.doi.org/10.1051/vetres/2010038; PMID: 20546698
- Whiteley A, Major D, Legastelois I, Campitelli L, Donatelli I, Thompson CI, et al. Generation of candidate human influenza vaccine strains in cell culture - rehearsing the European response to an H7N1 pandemic threat. Influenza Other Respi Viruses 2007; 1:157 - 66; http://dx.doi.org/10.1111/j.1750-2659.2007.00022.x; PMID: 19432631
- Cox RJ, Major D, Hauge S, Madhun AS, Brokstad KA, Kuhne M, et al. A cell-based H7N1 split influenza virion vaccine confers protection in mouse and ferret challenge models. Influenza Other Respi Viruses 2009; 3:107 - 17; http://dx.doi.org/10.1111/j.1750-2659.2009.00082.x; PMID: 19453487
- Hovden AO, Brokstad KA, Major D, Wood J, Haaheim LR, Cox RJ. A pilot study of the immune response to whole inactivated avian influenza H7N1 virus vaccine in mice. Influenza Other Respi Viruses 2009; 3:21 - 8; http://dx.doi.org/10.1111/j.1750-2659.2009.00075.x; PMID: 19453438
- Aamir UB, Naeem K, Ahmed Z, Obert CA, Franks J, Krauss S, et al. Zoonotic potential of highly pathogenic avian H7N3 influenza viruses from Pakistan. Virology 2009; 390:212 - 20; http://dx.doi.org/10.1016/j.virol.2009.05.008; PMID: 19535120
- Naeem K, Siddique N, Ayaz M, Jalalee MA. Avian influenza in Pakistan: outbreaks of low- and high-pathogenicity avian influenza in Pakistan during 2003-2006. Avian Dis 2007; 51:Suppl 189 - 93; http://dx.doi.org/10.1637/7617-042506R.1; PMID: 17494552
- Abbas MA, Spackman E, Swayne DE, Ahmed Z, Sarmento L, Siddique N, et al. Sequence and phylogenetic analysis of H7N3 avian influenza viruses isolated from poultry in Pakistan 1995-2004. Virol J 2010; 7:137; http://dx.doi.org/10.1186/1743-422X-7-137; PMID: 20576101
- Naeem K, Hussain M. An outbreak of avian influenza in poultry in Pakistan. Vet Rec 1995; 137:439; http://dx.doi.org/10.1136/vr.137.17.439; PMID: 8560706
- Banks J, Speidel EC, McCauley JW, Alexander DJ. Phylogenetic analysis of H7 haemagglutinin subtype influenza A viruses. Arch Virol 2000; 145:1047 - 58; http://dx.doi.org/10.1007/s007050050695; PMID: 10881690
- Naeem K, Siddique N. Use of strategic vaccination for the control of avian influenza in Pakistan. Dev Biol (Basel) 2006; 124:145 - 50; PMID: 16447505
- Abbas MA, Spackman E, Fouchier R, Smith D, Ahmed Z, Siddique N, et al. H7 avian influenza virus vaccines protect chickens against challenge with antigenically diverse isolates. Vaccine 2011; 29:7424 - 9; http://dx.doi.org/10.1016/j.vaccine.2011.07.064; PMID: 21803098
- Rojas H, Moreira R, Avalos P, Capua I, Marangon S. Avian influenza in poultry in Chile. Vet Rec 2002; 151:188; PMID: 12201269
- Max V, Herrera J, Moreira R, Rojas H. Avian influenza in Chile: a successful experience. Avian Dis 2007; 51:Suppl 363 - 5; http://dx.doi.org/10.1637/7631-042806R1.1; PMID: 17494584
- Suarez DL, Senne DA, Banks J, Brown IH, Essen SC, Lee CW, et al. Recombination resulting in virulence shift in avian influenza outbreak, Chile. Emerg Infect Dis 2004; 10:693 - 9; http://dx.doi.org/10.3201/eid1004.030396; PMID: 15200862
- Jones YL, Swayne DE. Comparative pathobiology of low and high pathogenicity H7N3 Chilean avian influenza viruses in chickens. Avian Dis 2004; 48:119 - 28; http://dx.doi.org/10.1637/7080; PMID: 15077805
- Bowes VA, Ritchie SJ, Byrne S, Sojonky K, Bidulka JJ, Robinson JH. Virus characterization, clinical presentation, and pathology associated with H7N3 avian influenza in British Columbia broiler breeder chickens in 2004. Avian Dis 2004; 48:928 - 34; http://dx.doi.org/10.1637/7218-060304R; PMID: 15666877
- Tweed SA, Skowronski DM, David ST, Larder A, Petric M, Lees W, et al. Human illness from avian influenza H7N3, British Columbia. Emerg Infect Dis 2004; 10:2196 - 9; http://dx.doi.org/10.3201/eid1012.040961; PMID: 15663860
- Belser JA, Blixt O, Chen LM, Pappas C, Maines TR, Van Hoeven N, et al. Contemporary North American influenza H7 viruses possess human receptor specificity: Implications for virus transmissibility. Proc Natl Acad Sci U S A 2008; 105:7558 - 63; http://dx.doi.org/10.1073/pnas.0801259105; PMID: 18508975
- Belser JA, Wadford DA, Xu J, Katz JM, Tumpey TM. Ocular infection of mice with influenza A (H7) viruses: a site of primary replication and spread to the respiratory tract. J Virol 2009; 83:7075 - 84; http://dx.doi.org/10.1128/JVI.00535-09; PMID: 19458003
- Pasick J, Handel K, Robinson J, Copps J, Ridd D, Hills K, et al. Intersegmental recombination between the haemagglutinin and matrix genes was responsible for the emergence of a highly pathogenic H7N3 avian influenza virus in British Columbia. J Gen Virol 2005; 86:727 - 31; http://dx.doi.org/10.1099/vir.0.80478-0; PMID: 15722533
- Hirst M, Astell CR, Griffith M, Coughlin SM, Moksa M, Zeng T, et al. Novel avian influenza H7N3 strain outbreak, British Columbia. Emerg Infect Dis 2004; 10:2192 - 5; http://dx.doi.org/10.3201/eid1012.040743; PMID: 15663859
- Pasick J, Berhane Y, Hisanaga T, Kehler H, Hooper-McGrevy K, Handel K, et al. Diagnostic test results and pathology associated with the 2007 Canadian H7N3 highly pathogenic avian influenza outbreak. Avian Dis 2010; 54:Suppl 213 - 9; http://dx.doi.org/10.1637/8822-040209-Reg.1; PMID: 20521634
- Berhane Y, Hisanaga T, Kehler H, Neufeld J, Manning L, Argue C, et al. Highly pathogenic avian influenza virus A (H7N3) in domestic poultry, Saskatchewan, Canada, 2007. Emerg Infect Dis 2009; 15:1492 - 5; http://dx.doi.org/10.3201/eid1509.080231; PMID: 19788823
- Pasick J, Berhane Y, Hooper-McGrevy K. Avian influenza: the Canadian experience. Rev Sci Tech 2009; 28:349 - 58; PMID: 19618638
- OIE. Immediate notification report: HPAI in Mexico report reference OIE 12067; Report date 21/06/2012. Avaialbel online at: http://web.oie.int/wahis/reports/en_imm_0000012067_20120622_132313.pdf. 2012.
- FAO. Highly pathogenic avian influenza in Mexico (H7N3): A signifcant threat to poultry production not be underestimated. EMPRES WATCH. Rome, Italy, 2012.
- Centers for Disease Control and Prevention (CDC). Notes from the field: Highly pathogenic avian influenza A (H7N3) virus infection in two poultry workers--Jalisco, Mexico, July 2012. MMWR Morb Mortal Wkly Rep 2012; 61:726 - 7; PMID: 22971746
- WHO. Antigenic and genetic characteristics of zoonotic influenza viruses and development of candidate vaccine viruses for pandemic preparedness. Wkly Epidemiol Rec 2012; 87:401 - 12; PMID: 23113329
- de Wit JJ, Koch G, Fabri TH, Elbers AR. A cross-sectional serological survey of the Dutch commercial poultry population for the presence of low pathogenic avian influenza virus infections. Avian Pathol 2004; 33:565 - 70; http://dx.doi.org/10.1080/03079450400013196; PMID: 15763723
- de Jong MC, Stegeman A, van der Goot J, Koch G. Intra- and interspecies transmission of H7N7 highly pathogenic avian influenza virus during the avian influenza epidemic in The Netherlands in 2003. Rev Sci Tech 2009; 28:333 - 40; PMID: 19618636
- Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A 2004; 101:1356 - 61; http://dx.doi.org/10.1073/pnas.0308352100; PMID: 14745020
- Metreveli G, Zohari S, Ejdersund A, Berg M. Phylogenetic analysis of the hemagglutinin gene of low pathogenic avian influenza virus H7N7 strains in mallards in Northern Europe. Avian Dis 2010; 54:Suppl 453 - 6; http://dx.doi.org/10.1637/8691-031309-ResNote.1; PMID: 20521678
- Meijer A, Bosman A, van de Kamp EE, Wilbrink B, Du Ry van Beest Holle M, Koopmans M. Measurement of antibodies to avian influenza virus A(H7N7) in humans by hemagglutination inhibition test. J Virol Methods 2006; 132:113 - 20; http://dx.doi.org/10.1016/j.jviromet.2005.10.001; PMID: 16271401
- Du Ry van Beest Holle M, Meijer A, Koopmans M, de Jager CM. Human-to-human transmission of avian influenza A/H7N7, The Netherlands, 2003. Euro Surveill 2005; 10:264 - 8; PMID: 16371696
- Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H, Vennema H, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 2004; 363:587 - 93; http://dx.doi.org/10.1016/S0140-6736(04)15589-X; PMID: 14987882
- Munster VJ, de Wit E, van Riel D, Beyer WE, Rimmelzwaan GF, Osterhaus AD, et al. The molecular basis of the pathogenicity of the Dutch highly pathogenic human influenza A H7N7 viruses. J Infect Dis 2007; 196:258 - 65; http://dx.doi.org/10.1086/518792; PMID: 17570113
- Olofsson S, Kumlin U, Dimock K, Arnberg N. Avian influenza and sialic acid receptors: more than meets the eye?. Lancet Infect Dis 2005; 5:184 - 8; PMID: 15766653
- de Wit E, Munster VJ, van Riel D, Beyer WE, Rimmelzwaan GF, Kuiken T, et al. Molecular determinants of adaptation of highly pathogenic avian influenza H7N7 viruses to efficient replication in the human host. J Virol 2010; 84:1597 - 606; http://dx.doi.org/10.1128/JVI.01783-09; PMID: 19939933
- de Wit E, Fouchier RA. Emerging influenza. J Clin Virol 2008; 41:1 - 6; http://dx.doi.org/10.1016/j.jcv.2007.10.017; PMID: 18340670
- van Riel D, Rimmelzwaan GF, van Amerongen G, Osterhaus AD, Kuiken T. Highly pathogenic avian influenza virus H7N7 isolated from a fatal human case causes respiratory disease in cats but does not spread systemically. Am J Pathol 2010; 177:2185 - 90; http://dx.doi.org/10.2353/ajpath.2010.100401; PMID: 20847292
- Alexander DJ, Lister SA, Johnson MJ, Randall CJ, Thomas PJ. An outbreak of highly pathogenic avian influenza in turkeys in Great Britain in 1991. Vet Rec 1993; 132:535 - 6; http://dx.doi.org/10.1136/vr.132.21.535; PMID: 8322444
- Löndt BZ, Banks J, Gardner R, Cox WJ, Brown IH. Induced increase in virulence of low virulence highly [corrected] pathogenic avian influenza by serial intracerebral passage in chickens. Avian Dis 2007; 51:Suppl 396 - 400; http://dx.doi.org/10.1637/7665-061206R.1; PMID: 17494593
- Banks J, Plowright L. Additional glycosylation at the receptor binding site of the hemagglutinin (HA) for H5 and H7 viruses may be an adaptation to poultry hosts, but does it influence pathogenicity?. Avian Dis 2003; 47:Suppl 942 - 50; http://dx.doi.org/10.1637/0005-2086-47.s3.942; PMID: 14575092
- Li Z, Jiang Y, Jiao P, Wang A, Zhao F, Tian G, et al. The NS1 gene contributes to the virulence of H5N1 avian influenza viruses. J Virol 2006; 80:11115 - 23; http://dx.doi.org/10.1128/JVI.00993-06; PMID: 16971424
- Guo Y, Wang M, Xu X. [Nucleotide sequence of A/Goose/Guangdong/2/96 (H5N1) virus M and NS RNA]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 1999; 13:305 - 8; PMID: 12759964
- Guo Y, Wang M, Guo J. [The complete nucleotide sequences of A/Goose/Guangdong/2/96(H5N1) virus RNA segment 1-3 and 5]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 1999; 13:205 - 8; PMID: 12569746
- Xu X, Subbarao, Cox NJ, Guo Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 1999; 261:15 - 9; http://dx.doi.org/10.1006/viro.1999.9820; PMID: 10484749
- Sims LD, Domenech J, Benigno C, Kahn S, Kamata A, Lubroth J, et al. Origin and evolution of highly pathogenic H5N1 avian influenza in Asia. Vet Rec 2005; 157:159 - 64; PMID: 16085721
- WHO. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2013. Avilable online at: http://www.who.int/influenza/human_animal_interface/EN_GIP_20130604CumulativeNumberH5N1cases.pdf (Accessed 03.July.2013). 2013.
- Schat KA, Bingham J, Butler JM, Chen LM, Lowther S, Crowley TM, et al. Role of position 627 of PB2 and the multibasic cleavage site of the hemagglutinin in the virulence of H5N1 avian influenza virus in chickens and ducks. PLoS One 2012; 7:e30960; http://dx.doi.org/10.1371/journal.pone.0030960; PMID: 22363523
- Tada T, Suzuki K, Sakurai Y, Kubo M, Okada H, Itoh T, et al. NP body domain and PB2 contribute to increased virulence of H5N1 highly pathogenic avian influenza viruses in chickens. J Virol 2011; 85:1834 - 46; http://dx.doi.org/10.1128/JVI.01648-10; PMID: 21123376
- Wasilenko JL, Sarmento L, Pantin-Jackwood MJ. A single substitution in amino acid 184 of the NP protein alters the replication and pathogenicity of H5N1 avian influenza viruses in chickens. Arch Virol 2009; 154:969 - 79; http://dx.doi.org/10.1007/s00705-009-0399-4; PMID: 19475480
- Korteweg C, Gu J. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am J Pathol 2008; 172:1155 - 70; http://dx.doi.org/10.2353/ajpath.2008.070791; PMID: 18403604
- Cinatl J Jr., Michaelis M, Doerr HW. The threat of avian influenza A (H5N1). Part I: Epidemiologic concerns and virulence determinants. Med Microbiol Immunol 2007; 196:181 - 90; http://dx.doi.org/10.1007/s00430-007-0042-5; PMID: 17492465
- Lycett SJ, Ward MJ, Lewis FI, Poon AF, Kosakovsky Pond SL, Brown AJ. Detection of mammalian virulence determinants in highly pathogenic avian influenza H5N1 viruses: multivariate analysis of published data. J Virol 2009; 83:9901 - 10; http://dx.doi.org/10.1128/JVI.00608-09; PMID: 19625397
- Wasilenko JL, Lee CW, Sarmento L, Spackman E, Kapczynski DR, Suarez DL, et al. NP, PB1, and PB2 viral genes contribute to altered replication of H5N1 avian influenza viruses in chickens. J Virol 2008; 82:4544 - 53; http://dx.doi.org/10.1128/JVI.02642-07; PMID: 18305037
- Homme PJ, Easterday BC. Avian influenza virus infections. I. Characteristics of influenza A-turkey-Wisconsin-1966 virus. Avian Dis 1970; 14:66 - 74; http://dx.doi.org/10.2307/1588557; PMID: 4314007
- Smithies LK, Emerson FG, Robertson SM, Ruedy DD. Two different type A influenza virus infections in turkeys in Wisconsin. II. 1968 outbreak. Avian Dis 1969; 13:606 - 10; http://dx.doi.org/10.2307/1588535; PMID: 5818061
- Smithies LK, Radloff DB, Friedell RW, Albright GW, Misner VE, Easterday BC. Two different type A influenza virus infections in turkeys in Wisconsin. I. 1965-66 outbreak. Avian Dis 1969; 13:603 - 6; http://dx.doi.org/10.2307/1588534; PMID: 5818060
- Perdue ML, Wainright P, Palmieri S, Brugh M. In ovo competition between distinct virus populations in an avian influenza isolate. Avian Dis 1989; 33:695 - 706; http://dx.doi.org/10.2307/1591147; PMID: 2619663
- Perdue ML. Naturally occurring NS gene variants in an avian influenza virus isolate. Virus Res 1992; 23:223 - 40; http://dx.doi.org/10.1016/0168-1702(92)90110-U; PMID: 1320795
- García M, Suarez DL, Crawford JM, Latimer JW, Slemons RD, Swayne DE, et al. Evolution of H5 subtype avian influenza A viruses in North America. Virus Res 1997; 51:115 - 24; http://dx.doi.org/10.1016/S0168-1702(97)00087-7; PMID: 9498610
- Turner AJ. The isolation of fowl plague virus in Victoria. Aust Vet J 1976; 52:384; http://dx.doi.org/10.1111/j.1751-0813.1976.tb09503.x; PMID: 985260
- Westbury HA. History of Highly Pathogenic Avian Influenza in Australia. Proceedings of the Fourth International Symposium on Avian Influenza, 1997. Avian Dis 2003; 47:23 - 30
- Bashiruddin JB, Gould AR, Westbury HA. Molecular pathotyping of two avian influenza viruses isolated during the Victoria 1976 outbreak. Aust Vet J 1992; 69:140 - 2; http://dx.doi.org/10.1111/j.1751-0813.1992.tb07485.x; PMID: 1642597
- Westbury HA, Turner AJ, Amon L. Transmissibility of two avian influenza a viruses (H7 N6) between chickens. Avian Pathol 1981; 10:481 - 7; http://dx.doi.org/10.1080/03079458108418498; PMID: 18770163
- Alexander DJ, Allan WH, Parsons DG, Parsons G. The pathogenicity of four avian influenza viruses for fowls, turkeys and ducks. Res Vet Sci 1978; 24:242 - 7; PMID: 653122
- Westbury HA, Turner AJ, Kovesdy L. The pathogenicity of three Australian fowl plague viruses for chickens, turkeys and ducks. Vet Microbiol 1979; 4:223 - 34; http://dx.doi.org/10.1016/0378-1135(79)90058-0
- Perdue ML, Latimer JW, Crawford JM. A novel carbohydrate addition site on the hemagglutinin protein of a highly pathogenic H7 subtype avian influenza virus. Virology 1995; 213:276 - 81; http://dx.doi.org/10.1006/viro.1995.1571; PMID: 7483275
- Li J, Zu Dohna H, Cardona CJ, Miller J, Carpenter TE. Emergence and genetic variation of neuraminidase stalk deletions in avian influenza viruses. PLoS One 2011; 6:e14722; http://dx.doi.org/10.1371/journal.pone.0014722; PMID: 21373190
- Bergmann M, García-Sastre A, Palese P. Transfection-mediated recombination of influenza A virus. J Virol 1992; 66:7576 - 80; PMID: 1279208
- Orlich M, Gottwald H, Rott R. Nonhomologous recombination between the hemagglutinin gene and the nucleoprotein gene of an influenza virus. Virology 1994; 204:462 - 5; http://dx.doi.org/10.1006/viro.1994.1555; PMID: 8091680
- Gibbs MJ, Armstrong JS, Gibbs AJ. The haemagglutinin gene, but not the neuraminidase gene, of ‘Spanish flu’ was a recombinant. Philos Trans R Soc Lond B Biol Sci 2001; 356:1845 - 55; http://dx.doi.org/10.1098/rstb.2001.0998; PMID: 11779383
- Gibbs MJ, Armstrong JS, Gibbs AJ. Recombination in the hemagglutinin gene of the 1918 “Spanish flu”. Science 2001; 293:1842 - 5; http://dx.doi.org/10.1126/science.1061662; PMID: 11546876
- Worobey M, Rambaut A, Pybus OG, Robertson DL. Questioning the evidence for genetic recombination in the 1918 “Spanish flu” virus. Science 2002; 296:211 - , discussion 211; http://dx.doi.org/10.1126/science.296.5566.211a; PMID: 11951002
- Khatchikian D, Orlich M, Rott R. Increased viral pathogenicity after insertion of a 28S ribosomal RNA sequence into the haemagglutinin gene of an influenza virus. Nature 1989; 340:156 - 7; http://dx.doi.org/10.1038/340156a0; PMID: 2544809