Abstract
Our laboratory previously described the binding characteristics of the murine IgG3 monoclonal antibody (MuAb) F26G3. This antibody binds the poly-glutamic acid capsule (PGA) of Bacillus anthracis, an essential virulence factor in the progression of anthrax. F26G3 IgG3 MuAb binds PGA with a relatively high functional affinity (10 nM), produces a distinct “rim” quellung reaction, and is protective in a murine model of pulmonary anthrax. This study engineered an IgG subclass family of F26G3 mouse–human chimeric antibodies (ChAb). The F26G3 ChAbs displayed 9- to 20-fold decreases in functional affinity, as compared with the parent IgG3 MuAb. Additionally, the quellung reactions that were produced by the ChAbs all differed from the parent IgG3 MuAb in that they appeared “puffy” in nature. This study demonstrates that human constant domains may influence multiple facets of antibody binding to microbial capsular antigens despite their spatial separation from the traditional antigen-binding site.
Introduction
Bacillus anthracis, the etiologic agent of anthrax, may cause life-threatening illness in large mammals including humans.Citation1,Citation2 Pathogenic strains of B. anthracis possess two extrachromosomal plasmids that encode for the tripartite toxin (pXO1) and capsule (pXO2). Together, these factors contribute significantly to virulence.Citation3 The capABCDE operon is responsible for the synthesis, anchorage, and release of a polypeptide capsule that is composed of γ-linked d-glutamic acid (PGA).Citation4,Citation5 Like many microbial capsules, PGA has a high molecular weight, is resistant to degradation, and presents a significant barrier to the mammalian immune system.Citation6-Citation9 Previous studies identified the capsule as a potential therapeutic target in both active and passive immunization strategies.Citation10-Citation12 Capsule-binding antibodies increase opsonization and phagocytosis of vegetative bacteria.Citation13
Our laboratory has previously reported isolation of the murine monoclonal antibody (MuAb) F26G3 IgG3 that binds to PGA.Citation11 More recently, we reported the construction of a murine variable-domain identical IgG subclass-switch family of F26G3 (IgG3, IgG1, IgG2b, and IgG2a).Citation14 These antibodies had a diverse array of heavy chain (CH)-dependent immunochemical and protective activities. For example, the IgG3 had comparatively high functional affinity (10 nM), whereas the subclass-switch variants (IgG1, IgG2b, and IgG2a) all showed comparatively low functional affinities (85–110 nM). Additionally, quellung reactions found that the IgG3 remodels the intact capsule upon binding, creating a pattern that was distinct from subclass-switch MuAbs. Finally, the IgG3 was protective in a murine model of pulmonary anthrax, whereas the subclass-switch MuAbs were not.
Given these unexpected results, it was of interest to determine the immunochemical properties of an IgG subclass family of mouse-human chimeric antibodies (ChAbs) (IgG1, IgG2, IgG3, and IgG4) that contain the F26G3 variable domain. The results of this study found a remarkable similarity between the immunochemical activities of the F26G3 IgG1, IgG2b, and IgG2a MuAbs and the F26G3 IgG1, IgG2, IgG3, and IgG4 ChAbs. Our findings add to the current body of work, which suggests that antibody CH may influence antigen binding, despite their spatial separation from the traditional antigen-binding site.
Results
Recombinant chimeric IgGs
The genes encoding the F26G3 immunoglobulin heavy- and light-chain variable domains (VH and VL) were isolated from the IgG3 MuAb hybridoma cell line. The ChAbs were engineered by cloning the VH into separate plasmids that encoded human IgG1, IgG2, IgG3, and IgG4 constant domains. Similarly, the VL was cloned into a human Igκ expression plasmid. The recombinant F26G3 ChAbs were expressed in the DG44 Chinese hamster ovary (CHO) cell line. The F26G3 ChAbs were purified from the supernatant fluids of stably transfected cells. The purity of each F26G3 ChAb was assessed by ELISA and SDS-PAGE (reducing and non-reducing). The results show that the F26G3 ChAbs were of the correct molecular weight, had the correct stoichiometry of heavy and light chains, and that each preparation carried the expected human subclass (data not shown).
Functional affinity
Surface plasmon resonance (SPR) was used to determine the functional affinity of each antibody. Functional affinity is often referred to when describing bivalent binding of an antibody to a multivalent antigen.Citation15 Here, the antigen was a synthesized oligomer of 25 PGA residues (25-mer). Previous reports found that the binding pocket of F26G3 accommodates 5 PGA residues; thus, we predict that inter- and intra-antigen binding may collectively contribute to the overall strength of the interaction.Citation11,Citation16
To evaluate the functional affinity of each antibody, 100 response units (RU) of the PGA 25-mer were immobilized to a sensor surface of a BIAcore X100 SPR instrument. In the SPR platform, RU is directly proportional to the mass on the sensor surface (1 RU = 1 pg).Citation17 Therefore, at 100 RU, we calculate the total number of 25-mers that are present on the sensor surface as 2.6 × 1010.
Fluid-phase antibody of the F26G3 IgG3 MuAb or the F26G3 IgG1, IgG2, IgG3, and IgG4 ChAbs was individually injected over the immobilized PGA 25-mer surface. The total RU that was generated after the injection of each antibody, at several concentrations (5–333 nM), was recorded (). These RU values were extrapolated to infinite antibody concentration, which subsequently allows for the calculation of the maximum response at antibody saturation (RUmax) and the dissociation constant (KD) (). In a previous study, the KD of the F26G3 IgG3 MuAb was calculated at 13 nM, furthermore an average of two antibody molecules per PGA 25-mer were bound at saturation.Citation16 The current study confirmed these past results ( and ).
Figure 1. Binding of anti-capsular antibodies to PGA-coated sensor surface. The total RU that was achieved following a 180 sec pulse is given as a function of antibody concentration. The KD of each antibody was determined using the steady-state equilibrium model in BIAevaluation software, where the theoretical RUmax is determined and the apparent KD is equal to the antibody concentration that is necessary to reach ½ RUmax.
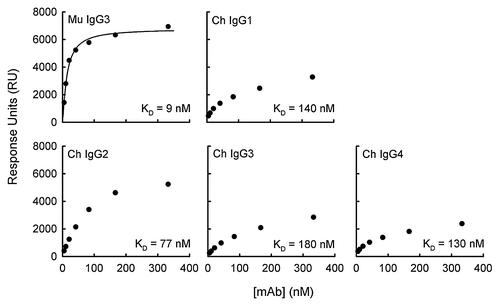
Table 1. Immunochemical activities of murine and mouse–human anti-PGA F26G3 antibodies
An analysis of the F26G3 IgG1, IgG2, IgG3, and IgG4 ChAbs found that the binding characteristics differed from the parental F26G3 IgG3 MuAb and, overall, were similar to previous reports of the F26G3 subclass-switch IgG1, IgG2b, and IgG2a MuAbs.Citation14 These differences included larger KD and smaller RUmax values. The functional affinity of each antibody examined in this study falls into the following ranked-order: F26G3 MuAb IgG3 (9 nM) > ChAb IgG2 (77 nM) > ChAb IgG4 (130 nM) > ChAb IgG1 (140 nM) > ChAb IgG3 (180 nM). The greatest difference in functional affinity between any two antibodies that share an identical variable domain, but differ in their CH was 9:180 or 20-fold (ChAb IgG3 vs. MuAb IgG3) ( and ). Likewise, the greatest difference in the number of antibody molecules that bound a single PGA 25-mer at RUmax was 0.73:2.1 or 3-fold (ChAb IgG4 vs. MuAb IgG3).
Influence of electrostatic forces
The PGA antigen has a uniform and highly negative charge. Electrostatic forces occur over fairly long distances (1/r2) and may contribute to non-specific binding. This phenomenon may be important when considering the specificity of molecular recognition between a negatively charged antigen and positively charged antibodies. Moreover, it is conceivable that the differences in positive charge among the constant domains of the various mouse and human subclasses may influence functional affinity, possibly through the formation of contacts that are made with the antigen outside of the traditional antigen-binding pocket.
A series of comparisons was used to assess the potential influence of electrostatic forces on the functional affinity of the F26G3 antibodies. The functional affinity of each antibody, as determined by SPR, was used to calculate the free energy of dissociation (ΔGo) using ΔGo = −RTlnKD where R is the gas constant (1.985 calK−1mol−1), and T is the temperature (298 K). The total electrostatic charge was determined for each antibody (). A plot of the electrostatic charge of each antibody against their respective ΔGo values resulted in a poor correlation coefficient (r = 0.29; P = 0.64) (data not shown).
Quellung-type reaction
Antibodies can bind to intact microbial capsules to produce a variety of visible phenotypes. Previous examples of this phenomenon have been reported for the opportunist yeast Cryptococcus neoformans as well as B. anthracis.Citation11,Citation14,Citation18 Two phenotypes make up the most commonly observed patterns; these are categorized as either “rim”-type or “puffy”-type, depending on the distribution of the antibody over and through the intact capsule.
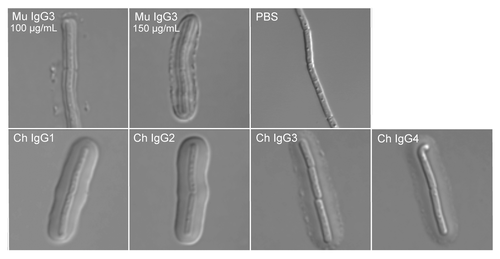
As noted above, we have observed that the F26G3 MuAb IgG subclass-switch family binds the intact capsule of B. anthracis with either a “rim”- or “puffy”-phenotype, and a correlation exists between phenotype and antibody CH. Similarly, for this study each F26G3 ChAb was assayed to determine if human CH influences capsule quellung reactions (). As with F26G3 IgG1, IgG2b, and IgG2a MuAbs, we found that transfer of the F26G3 variable domain to the human IgG1, IgG2, IgG3, and IgG4 CH resulted in an alteration of the binding phenotype from the parental “rim-type” to the “puffy-type”. Binding of F26G3 IgG1 and IgG2 ChAbs created puffy-type patterns that were identical to those created by the F26G3 IgG1, IgG2b, and IgG2a MuAbs.Citation14 The binding of F26G3 IgG3 and IgG4 ChAbs to B. anthracis cells was also puffy but with one minor exception; punctate depositions of antibody occurred interspersed along the capsular edge. This pattern was clearly distinct from the rim-type pattern observed after binding of the F26G3 IgG3 MuAb in two ways. First, IgG3 MuAb binding results in an inner layer structure that is characterized by a sharp change in refractive index proximal to the bacterial cell wall that is immediately followed by another sharp change in refractive index (, Mu IgG3 100 μg/mL). This structure is absent in both the IgG3 ChAb and the IgG4 ChAb. Second, the punctate depositions that were caused by the F26G3 Ch IgG3 and Ch IgG4 occurred at both low and high concentrations of antibody (not shown). In contrast, the punctate depositions produced by F26G3 IgG3 MuAb coalesce at high antibody concentrations to form a continuous rim (, Mu IgG3 150 μg/mL).
Figure 2. Capsule reaction produced by antibody binding. Top panel: Visualization of capsular polypeptide following the addition of F26G3 MuAbs IgG3 or PBS. Addition of the F26G3 MuAb IgG3 (100–150 μg/mL) produces a rim-type reaction with deposition both proximal to the cell wall and at the capsular edge. The capsule is not visible in the absence of specific antibody (PBS). Bottom panel: Visualization of capsular polypeptide following the addition of F26G3 ChAbs IgG1–4 (100 μg/mL). The addition of F26G3 ChAbs produces a puffy-type reaction that occurs uniformly throughout the capsule.
Discussion
The immunochemical activities of several variable-domain identical antibodies were examined to study the influence of human CH on antibody–antigen interactions. We found multiple differences in the binding characteristics of variable-domain identical antibodies following the conversion of a murine IgG3 antibody to murine–human chimeric antibodies. The influence of antibody CH on the affinity of variable-domain identical human antibodies has been described for protein antigens such as hepatitis B surface antigen, keyhole limpet hemocyanin, and others.Citation19-Citation22 Our knowledge of the influence of human CH on antibody binding to multivalent capsular antigens, such as PGA, is less extensive. Current reports largely describe ChAbs that react with the glucuronoxylomannan (GXM) capsule of C. neoformans.Citation23,Citation24
SPR was used to measure the functional affinity of antibody binding. This platform allows for the measurement of antibody-antigen interactions in real time and without chemical reporters. Furthermore, the binding response is directly proportional to the total mass located on the sensor surface. Thus, it is possible to estimate the total number of antibody-antigen complexes that are formed at RUmax, along with the total strength of the interaction (KD).Citation25
The RUmax values were different for each antibody used in this study, indicating that human, as well as murine, CH of each IgG subclass differentially influence the total number of antibody-antigen complexes that form on the sensor surface. These results are in agreement with studies performed by McCloskey et al., which identified differences in RU values with ChAbs to tumor-associated glycoprotein 72 (TAG72), and our previous work with F26G3 subclass-switch MuAbs.Citation14,Citation26 We found that F26G3 IgG1, IgG3, and IgG4 ChAbs each produced RUmax values that were markedly less than the parental F26G3 IgG3 MuAb. The binding of F26G3 IgG2 ChAb resulted in similar RUmax values as the IgG3 MuAb; however, the strength of the interaction was reduced.
A range of antibody densities was present on the sensor surface at antibody saturation (0.68 to 2.1 antibodies per PGA 25-mer). For the F26G3 IgG3 MuAb and the IgG2 ChAb, the binding of multiple antibodies to a single PGA 25-mer is consistent with the multivalent nature of the antigen, and suggests that there are a number of overlapping epitopes on each oligomer. Previous work determined that the binding pocket of F26G3 is optimally filled by an oligomer of five PGA residues. The data was derived experimentally and in silico.Citation11,Citation16 Therefore, it is likely that the RUmax values that were determined in this study do not represent a total saturation of antigen binding sites. It may be the point at which the repulsive forces between antibodies become greater than the attractive forces of antigen binding, or another unknown cause. However, it is clear that antibody CH influence the ability of the antibody to orient itself on the PGA sensor surface to optimally fill the antigen-binding sites. The same phenomenon may occur when antibody interacts with the intact bacterial capsule.
Further measurements of the functional affinity of each antibody found that transfer of the F26G3 variable domain from the IgG3 MuAb CH to the IgG1–4 ChAb CH always resulted in lower functional-affinity binding. The KD values of the ChAbs were similar to the KD values of F26G3 IgG1, IgG2b, and IgG2a MuAbs from our previous study. It is not readily apparent why the increase of KD occurs following transfer of the variable domain. The PGA antigen is highly anionic, leading to concerns over the influence of non-directional ionic forces; however, when we examine the total charges of these antibodies at physiologic pH, we found no correlation between electrostatic potential and ΔGo. Importantly the IgG3 MuAb, IgG1 ChAb, and IgG3 ChAb have an overall charge that is nearly identical; however, the KD and RUmax of IgG1 ChAb and IgG3 ChAb are poor in comparison to the IgG3 MuAb.
Considerable work has evaluated differences in segmental flexibility of antibodies and the potential effect on antibody-antigen interactions. For example, Morelock et al. found that differences in the KD of a family of ChAbs reflected the flexibility of the hinge region (IgG1 > IgG4 > IgG2).Citation27,Citation28 However, the KD of anti-PGA ChAbs did not reflect the hierarchy in segmental flexibility (IgG2 > IgG4 > IgG1 > IgG3), nor did the theoretical RUmax (IgG2 > IgG1 > IgG3 > IgG4).
Quellung reactions allow for the study of microbial capsules by microscopy. These reactions show that antibody binding to capsular structures may produce a variety of patterns based on the capsule architecture, epitope accessibility, and sometimes antibody isotype/subclass.Citation11,Citation18 Our current understanding of these patterns largely comes from studying antibodies that react with the GXM capsule of C. neoformans. These studies separate antibody-capsule reactions into two distinct classes. In the present study, we found that transfer of the F26G3 variable domain from the parental IgG3 MuAb to IgG1, IgG2, IgG3, and IgG4 ChAbs always resulted in changes to the pattern of binding to the intact capsule of B. anthracis. Although the quellung reactions that were produced by F26G3 IgG3 and IgG4 ChAbs contained minor deviations from the traditional “puffy” pattern, this binding is clearly not a “rim” pattern; it is possible that such differences may be an artifact of the antibody preparation itself or another unknown cause.
Our previous findings indicated that the protective activity of the murine IgG subclass-switch antibodies was influenced by CH-dependent affects. Due to the multiplicity of variables between the binding activities of the murine IgG3 and other subclass-switch antibodies it is difficult to say with certainty what the true determinants of protection are. Immunochemical properties such as binding affinity and capsular reactivity may be important; however, it is also likely that the physiologic effector functions of each IgG subclass, such as engagement of Fc receptors and activation of the complement cascade, may contribute to protection as well. This study did not analyze the protective activity of the F26G3 ChAbs. Their immunochemical properties share remarkable similarity to those of the F26G3 IgG1, IgG2b, and IgG2a MuAbs, which suggests their protective activities may be similar. Nevertheless, further studies would be needed to more fully address this relationship.
In conclusion, our study found a remarkable congruency between the results that were previously obtained with the F26G3 MuAb IgG subclass-switch family and the newly engineered F26G3 ChAb IgG subclass family. Like murine IgG CH, human IgG CH clearly influences multiple facets of antibody activity when binding to the capsule of B. anthracis. Importantly, these studies offer further evidence that elements that lie outside of the traditional antigen-binding site may significantly contribute to the antibody-antigen interaction.
Materials and Methods
Bacillus anthracis strains and isolation of PGA
Formalin-killed B. anthracis Ames bacilli were provided by Drs Julie Lovchik and C Rick Lyons at the University of New Mexico Health Sciences Center. The strain was originally obtained from the US Army Medical Research Institute of Infectious Diseases (USAMRIID). Bacillus licheniformis strain 9945 was obtained from the American Type Culture Collection. PGA was isolated from culture filtrates of B. licheniformis as described.Citation11 B. licheniformis was grown on Medium E that contained 2 mM MnCl2•4 H2O to stimulate maximal production of PGA in the d isoform. An acid hydrolysate of the purified PGA had a specific optical rotation (−25.2°) indicating that ≈84% of the glutamic acid was in the d isomer. The PGA 25-mer oligomers were synthesized by the Nevada Proteomics Center at the University of Nevada, Reno.Citation11
Construction of F26G2 ChAbs
First-strand cDNA was prepared from total mRNA isolated from the F26G3 IgG3 MuAb hybridoma cell line as described.Citation11 The complete sequences are available on the GenBank database: mAb F26G3 EF030731 (VH) and EF030737 (VL). The F26G3 VH and VL were analyzed by the Nevada Genomics center to ensure they were identical to previously reported sequences. The F26G3 VH and VL were combined with plasmids encoding the human IgG1, IgG2, IgG3, IgG4, and Igκ by overlap PCR (plasmids were provided by Dr Sherie Morrison, UCLA). All of the reagents for subcloning and expression of the ChAbs were provided in the DG44 Freedom Kit (Invitrogen). Restriction sites XbaI (5′) and NotI (3′) were used for subsequent cloning of the complete CH constructs into the mammalian expression vector pOptivec that was also linearized with XbaI and NotI (New England Biolabs). The complete Igκ construct was prepared with XbaI restriction sites on both the 5′ and 3′ ends. The digested construct was ligated with the mammalian expression vector pcDNA3.3 that was similarly linearized with XbaI restriction sites. The pOptivec and pcDNA3.3 plasmids were introduced to DH5α E. coli by heat shock (New England Biolabs). Single bacterial colonies were recovered from LB agar plates containing 100 μg/mL ampicillin. DNA sequencing at the Nevada Genomics Center confirmed the presence of the antibody-encoding constructs in the expression plasmids.
Expression of recombinant cAbs
Stable expression of recombinant F26G3 ChAbs was done by co-transfection of DG44 Chinese hamster ovary (CHO) cells with F26G3 heavy or light chain-encoding plasmids. Prior to transfection, each construct was linearized by digestion with the endonuclease PvuI (New England Biolabs), as both the pOptivec and pcDNA3.3 plasmids contain a single PvuI restriction site in the ampicillin resistance gene. The DG44 CHO cells were transfected with 10 μg of both heavy- and light-chain plasmids (total 20 μg) with Freestyle transfection reagent, according to the manufacturer’s suggested protocol. The transfected cells were grown at 37 °C in 8% CO2. Stable cell lines of each ChAb were selected by growth on HT-deficient media to select for presence of the antibody heavy chain. G418 was subsequently added to ensure the presence of the antibody light chain. The expression and secretion of each ChAb into the culture supernatant fluids was verified by enzyme-linked immunosorbent assay (ELISA). The ELISA was performed as previously described, with the exception of the HRP-conjugate secondary antibodies being used (goat anti-human IgG and goat anti-human Igκ).Citation11 The F26G3 ChAbs were purified by affinity chromatography on recombinant protein A sepharose (GE Healthcare) or protein G sepharose (GE Healthcare). The concentration of each purified ChAb in solution was verified by spectrophotometry (A280) using an optical density of 1.43 = 1 mg/ml.
Surface plasmon resonance
Binding experiments were performed using a BIAcore X100 instrument (GE Healthcare). The running and sample buffer used for all experiments was 1× HBS-EP+ (GE Healthcare): 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.05% surfactant P20, pH 7.4. For ligand preparation, 2 mg of PGA 25-mer was biotinylated by standard amine coupling chemistry (Pierce) and purified over a desalting column (Pierce).
Biotinylated PGA 25-mer was immobilized onto the surface of a streptavidin (SA) sensor chip (GE Healthcare) until 100 response units (RU) were reached. A flow cell was left unmodified for reference subtraction. To evaluate antibody affinities, the samples were diluted in HBS-EP+ at several concentrations (5–333 nM). At each specified concentration, antibody was injected over the chip surface at 30 μl/min for 180 sec. The chip surface was regenerated between runs with a 60 sec pulse of 10 mM Glycine pH 1.5. The binding evaluations for all of the antibodies in this study were done in triplicate. BIAevaluation software (GE Healthcare) was used to determine the dissociation constants (KD), and maximum binding density (RUmax) using the steady-state model.
Quellung-type reaction
B. anthracis Ames cells were used to evaluate capsular “quellung” type reactions. The cells were washed with sterile phosphate-buffered saline (PBS) and stored as a suspension at 4 °C. Capsular reactions were evaluated by co-incubation of bacterial cells with 100–150 μg/mL of each antibody for 10 min at 37 °C. Differential interference contrast (DIC) microscopy images were taken using a Zeiss LSM 700 microscope (Zeiss Inc.).
| Abbreviations: | ||
| PGA | = | γ-linked d-glutamic acid |
| MuAb | = | murine monoclonal antibody |
| ChAb | = | chimeric monoclonal antibody |
| SPR | = | surface plasmon resonance |
Acknowledgments
This work was supported by Public Health Service grants R01SAI059348 and R01AI093365 from the National Institutes of Allergy and Infectious Diseases (TRK).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Inglesby TV, O’Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al, Working Group on Civilian Biodefense. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 2002; 287:2236 - 52; http://dx.doi.org/10.1001/jama.287.17.2236; PMID: 11980524
- Sweeney DA, Hicks CW, Cui X, Li Y, Eichacker PQ. Anthrax infection. Am J Respir Crit Care Med 2011; 184:1333 - 41; http://dx.doi.org/10.1164/rccm.201102-0209CI; PMID: 21852539
- Kaspar RL, Robertson DL. Purification and physical analysis of Bacillus anthracis plasmids pXO1 and pXO2. Biochem Biophys Res Commun 1987; 149:362 - 8; http://dx.doi.org/10.1016/0006-291X(87)90375-5; PMID: 3122734
- Makino S, Uchida I, Terakado N, Sasakawa C, Yoshikawa M. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J Bacteriol 1989; 171:722 - 30; PMID: 2536679
- Candela T, Fouet A. Bacillus anthracis CapD, belonging to the gamma-glutamyltranspeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Mol Microbiol 2005; 57:717 - 26; http://dx.doi.org/10.1111/j.1365-2958.2005.04718.x; PMID: 16045616
- Hanby WE, Rydon HN. The capsular substance of Bacillus anthracis.. Biochem J 1946; 40:297 - 309; PMID: 20989452
- Zwartouw HT, Smith H. Polyglutamic acid from Bacillus anthracis grown in vivo; structure and aggressin activity. Biochem J 1956; 63:437 - 42; PMID: 13341899
- Wang TT, Lucas AH. The capsule of Bacillus anthracis behaves as a thymus-independent type 2 antigen. Infect Immun 2004; 72:5460 - 3; http://dx.doi.org/10.1128/IAI.72.9.5460-5463.2004; PMID: 15322045
- Drysdale M, Heninger S, Hutt J, Chen Y, Lyons CR, Koehler TM. Capsule synthesis by Bacillus anthracis is required for dissemination in murine inhalation anthrax. EMBO J 2005; 24:221 - 7; http://dx.doi.org/10.1038/sj.emboj.7600495; PMID: 15616593
- Chabot DJ, Scorpio A, Tobery SA, Little SF, Norris SL, Friedlander AM. Anthrax capsule vaccine protects against experimental infection. Vaccine 2004; 23:43 - 7; http://dx.doi.org/10.1016/j.vaccine.2004.05.029; PMID: 15519706
- Kozel TR, Thorkildson P, Brandt S, Welch WH, Lovchik JA, AuCoin DP, et al. Protective and immunochemical activities of monoclonal antibodies reactive with the Bacillus anthracis polypeptide capsule. Infect Immun 2007; 75:152 - 63; http://dx.doi.org/10.1128/IAI.01133-06; PMID: 17060470
- Chen Z, Schneerson R, Lovchik J, Lyons CR, Zhao H, Dai Z, et al. Pre- and postexposure protection against virulent anthrax infection in mice by humanized monoclonal antibodies to Bacillus anthracis capsule. Proc Natl Acad Sci U S A 2011; 108:739 - 44; http://dx.doi.org/10.1073/pnas.1017677108; PMID: 21187383
- Sanford JE, Lupan DM, Schlageter AM, Kozel TR. Passive immunization against Cryptococcus neoformans with an isotype-switch family of monoclonal antibodies reactive with cryptococcal polysaccharide. Infect Immun 1990; 58:1919 - 23; PMID: 2341184
- Hovenden M, Hubbard MA, Aucoin DP, Thorkildson P, Reed DE, Welch WH, et al. IgG subclass and heavy chain domains contribute to binding and protection by mAbs to the poly γ-D-glutamic acid capsular antigen of Bacillus anthracis. PLoS Pathog 2013; 9:e1003306; http://dx.doi.org/10.1371/journal.ppat.1003306; PMID: 23637599
- Hornick CL, Karuch F. Antibody affinity. 3. The role of multivalance. Immunochemistry 1972; 9:325 - 40; http://dx.doi.org/10.1016/0019-2791(72)90096-1; PMID: 4556115
- Hubbard MA, Thorkildson P, Welch WH, Kozel TR. Stereo-selective binding of monoclonal antibodies to the poly-gamma-D-glutamic acid capsular antigen of Bacillus anthracis. Mol Immunol 2013; 55:337 - 44; http://dx.doi.org/10.1016/j.molimm.2013.03.010; PMID: 23602451
- Richard RB, Schasfoort BM, Tudos AJ. Handbook of surface plasmon resonance. Royal Society of Chemistry. 2008; 134-135.
- MacGill TC, MacGill RS, Casadevall A, Kozel TR. Biological correlates of capsular (quellung) reactions of Cryptococcus neoformans.. J Immunol 2000; 164:4835 - 42; PMID: 10779792
- Persson MA, Brown SE, Steward MW, Hammarström L, Smith CI, Howard CR, et al. IgG subclass-associated affinity differences of specific antibodies in humans. J Immunol 1988; 140:3875 - 9; PMID: 3131419
- Devey ME, Bleasdale-Barr KM, Bird P, Amlot PL. Antibodies of different human IgG subclasses show distinct patterns of affinity maturation after immunization with keyhole limpet haemocyanin. Immunology 1990; 70:168 - 74; PMID: 2373517
- Devey ME, Bleasdale KM, French MA, Harrison G. The IgG4 subclass is associated with a low affinity antibody response to tetanus toxoid in man. Immunology 1985; 55:565 - 7; PMID: 4018840
- Devey ME, Lee SR, Le Page S, Feldman R, Isenberg DA. Serial studies of the IgG subclass and functional affinity of DNA antibodies in systemic lupus erythematosus. J Autoimmun 1988; 1:483 - 94; http://dx.doi.org/10.1016/0896-8411(88)90069-8; PMID: 3075912
- Torres M, Fernandez-Fuentes N, Fiser A, Casadevall A. Exchanging murine and human immunoglobulin constant chains affects the kinetics and thermodynamics of antigen binding and chimeric antibody autoreactivity. PLoS One 2007; 2:e1310; http://dx.doi.org/10.1371/journal.pone.0001310; PMID: 18074033
- Beenhouwer DO, Yoo EM, Lai CW, Rocha MA, Morrison SL. Human immunoglobulin G2 (IgG2) and IgG4, but not IgG1 or IgG3, protect mice against Cryptococcus neoformans infection. Infect Immun 2007; 75:1424 - 35; http://dx.doi.org/10.1128/IAI.01161-06; PMID: 17220317
- Sagawa T, Oda M, Morii H, Takizawa H, Kozono H, Azuma T. Conformational changes in the antibody constant domains upon hapten-binding. Mol Immunol 2005; 42:9 - 18; http://dx.doi.org/10.1016/j.molimm.2004.07.004; PMID: 15488939
- McCloskey N, Turner MW, Steffner P, Owens R, Goldblatt D. Human constant regions influence the antibody binding characteristics of mouse-human chimeric IgG subclasses. Immunology 1996; 88:169 - 73; http://dx.doi.org/10.1111/j.1365-2567.1996.tb00001.x; PMID: 8690447
- Morelock MM, Rothlein R, Bright SM, Robinson MK, Graham ET, Sabo JP, et al. Isotype choice for chimeric antibodies affects binding properties. J Biol Chem 1994; 269:13048 - 55; PMID: 7909805
- Dangl JL, Wensel TG, Morrison SL, Stryer L, Herzenberg LA, Oi VT. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J 1988; 7:1989 - 94; PMID: 3138110