Abstract
Shiga toxin-producing E. coli represents a significant global health concern, especially as hypervirulent pathogens surface amidst outbreaks of hemolytic uremic syndrome (HUS). Shiga toxin (Stx) is key in the microangiopathic events underlying the disease and its central role is underscored by the unprecedented HUS outbreak in Germany in 2011. The mechanisms of Stx-mediated endothelial dysfunction have been a major focus of research that has contributed to the current understanding of the pathogenic changes in endothelial phenotype leading to HUS. Among the newer concepts are Stx-mediated gene regulation in the absence of protein synthesis inhibition, a potential role for complement activation, and accumulating evidence for detectable serum markers before the onset of the classic clinical features of HUS. Further investigation of newer therapeutic targets and potential prognostic markers is essential to assess their utility in mitigating disease and/or predicting outcomes and will provide an improved overall understanding of HUS pathogenesis.
Introduction
Hemolytic uremic syndrome (HUS) is a thrombotic microangiopathy that is clinically characterized by thrombocytopenia, non-immune hemolytic anemia, and acute renal failure. Approximately 10% of cases are categorized as atypical HUS, a disorder associated with genetic or acquired deficiencies in complement regulation, among others.Citation1,Citation2 Onset of atypical HUS in predisposed patients is often triggered by non-enteric infection, pregnancy, cancer, organ transplant, or drugs.Citation3 These patients unfortunately have a poor prognosis, with progression to end-stage renal disease in half of the cases and a mortality rate of 25%.Citation3 Typical HUS, also known as diarrhea-associated HUS (D+HUS), accounts for the majority of cases and is the most serious, life-threatening complication following gastrointestinal infection with Shiga toxin-producing E. coli (STEC).Citation1,Citation3 Shiga toxin (Stx)-mediated endothelial dysfunction is thought to be a primary event underlying the microangiopathic changes that occur in HUS.Citation4-Citation6 This review will cover the main events in STEC-mediated HUS pathogenesis, with a focus on Stx-induced endothelial dysfunction. The recent, unprecedented STEC outbreak in Germany will be discussed and emerging concepts in HUS pathogenesis and their implications for patient therapy will also be addressed.
Overview of STEC-Mediated HUS
Karmali and colleagues’ seminal reports 30 years ago made the association between STEC infection and sporadic cases of HUS.Citation7,Citation8 Because Stx is also widely referred to as verotoxin, the terms STEC and verotoxigenic E. coli (VTEC) are used interchangeably. Enterohemorrhagic E. coli (EHEC) are a sub-group of STEC that have the ability to form attaching and effacing lesions (A/E lesions) that facilitate intimate adhesion to the intestinal mucosa.Citation9 Since Karmali’s initial reports, Stx-producing E. coli O157:H7 has become recognized as the leading cause of HUS worldwide.Citation10 The principal route of infection is consumption of contaminated food and drinking water, although infection may also occur via direct contact with animals, person-to-person contact, and environmental exposure.Citation11,Citation12 STEC-HUS is most prevalent in infants and young children and is, in fact, a leading cause of acute renal failure in the pediatric population.Citation13 STEC causes a spectrum of diseases, from uncomplicated infection to hemorrhagic colitis and HUS. For reasons not yet well understood, 5–15% of STEC cases progress to HUS.Citation10,Citation14 The inability to predict which patients will recover spontaneously and which will develop HUS remains a significant dilemma for clinicians. The presence of STEC, especially Stx2-producing E. coli, in patient stools should immediately alert the physicians involved.
Histopathological manifestations of Stx-associated HUS include fibrin-rich microvascular thrombi primarily in the renal glomeruli, although pre-glomerular arterioles and medium-sized vessels may also be affected.Citation6 Endothelial swelling and detachment from the underlying basement membrane and subendothelial deposits accompanied by vascular edema and narrowing of the vessel lumen are also observed.Citation10,Citation15 The classical view argues that thrombocytopenia results from platelet consumption in thrombi and that red blood cells become fragmented as they flow through vessels with marked edema, fibrin deposits, and intravascular thrombosis, though newer evidence challenges these long-held beliefs.Citation16
Although the kidneys are the prime targets, extra-renal complications may develop. Neurological involvement occurs in 10% to 25% of patients and is the most common cause of mortality during the acute phase of disease.Citation10,Citation17 Although infrequent, cardiac complications are associated with a high risk of mortality in HUS patients (for a review, see ref. Citation18). Involvement of other organs such as the gastrointestinal tract, lungs, and pancreas is likely under-appreciated. The risk of mortality or end-stage renal disease associated with STEC-HUS is 12% and 25% of survivors experience long-term renal sequelae, including decreased glomerular filtration rate, proteinuria, hypertension, chronic kidney disease, and end-stage renal disease.Citation19-Citation21
Patients currently receive supportive care to manage symptoms, as no specific treatment exists for D+HUS. Antibiotic treatment is generally discouraged because of the potential to enhance the synthesis and release of Stx from EHEC and worsen disease severity.Citation22,Citation23 Recent in vitro studies suggest that, at least for E. coli O104:H4, ciprofloxacin increases Stx2 production while other antibiotics either suppress or do not affect toxin expression.Citation24 The use of first-line plasma therapy in STEC-HUS is controversialCitation25,Citation26 and a recent prospective study of 619 children with HUS identified an association between plasma therapy and poor long-term outcome.Citation21
Volume resuscitation and re-expansion of extracellular fluid volume is also a key component of clinical management.Citation27,Citation28 Ironically, the pathophysiology of the vascular leak and increased vascular permeability is poorly understood.
Unusual Stx-Producing Pathotype Emerges
E. coli O157:H7 is recognized as the leading cause of HUS globally.Citation10,Citation29 From May to July 2011, Germany was at the center of an unprecedented outbreak of STEC-HUS that quickly garnered attention for its unusual epidemiologic characteristics, the organism’s unexpected genetic composition and startlingly high virulence. Among 3816 people who fell ill in Germany, 845 developed HUS and 54 people died from HUS or gastroenteritis.Citation30 The higher than normal rate of progression to HUS (22%), together with the unusually high incidence in adult patients (88%) made this an extraordinary outbreak that required immediate action. Other atypical features of the disease included a longer incubation period and disproportionately greater frequency in women.Citation6,Citation30 The clinical parameters are argued to reflect the implicated food source, salad sprouts, associated with infections during this epidemic.Citation31
The pathogen was quickly identified as E. coli O104:H4, an enteroaggregative E. coli (EAEC) that colonizes the bowel in a characteristic “stacked brick” pattern. It normally causes watery diarrhea in children, travelers’ diarrhea, and persistent diarrhea in HIV patients and had previously been associated with only a handful of sporadic cases of HUS.Citation32-Citation34 Rapid genomic sequencing of the outbreak strain revealed it carried many common EAEC features but surprisingly, had acquired the stx2 gene and the ability to produce extended-spectrum β-lactamase via horizontal gene transfer.Citation22,Citation35-Citation37 The dramatic pathology that accompanied the newly acquired ability of this organism to produce Stx2 underscores the fundamental role Stx plays in the pathogenesis of HUS.
Following the German outbreak, comparative genomic analyses were performed on O104:H4 isolates from historic cases, 2011 outbreak cases, and cases identified after the 2011 outbreak.Citation38 The authors concluded that, while the isolates were closely related and shared a common ancestor, the post-outbreak isolates were distinct from the outbreak strain. While all expressed Stx2, each isolate had a unique combination of virulence factors encoded on genomic islands, prophages, and plasmids, suggesting that O104:H4 isolates are widespread and have the potential to cause foodborne outbreaks in the future.Citation38
Shiga Toxins
Shiga toxins, also commonly referred to as verotoxins, are implicated as key virulence factors in STEC-induced disease. The Stx family, comprising Stx1, Stx2, and their variants (Stx1c, Stx1d, Stx2b, Stx2c, Stx2d, Stx2e, Stx2f, and Stx2g),Citation39 belongs to a larger family of prokaryotic and plant A:B toxins that includes cholera, pertussis, diptheria, and ricin. Stx1 and Stx2 are encoded by distinct genes present in the late regions of lambdoid prophages and consist of a pentameric ring of receptor-binding B subunits linked to a single enzymatic A subunit.Citation40-Citation42 E. coli strains expressing Stx2, including O157:H7, have historically been associated with more severe human disease.Citation30,Citation40,Citation43
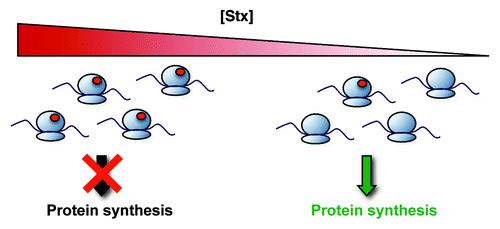
STEC release Stx in the gastrointestinal tract, after which the toxin translocates across the intestinal epithelium into the systemic circulation. The major Stx carrier in the blood has yet to be unequivocally identified. The toxin gains entry into susceptible cells via binding to cell-surface globotriaosylceramide (Gb3).Citation44 The presence of Gb3 targets Stx to the glomerular endothelium, where it causes major vascular injury. Endothelial cells from different vascular beds display varying degrees of sensitivity to Stx due, in large part, to the level of Gb3 expression.Citation45 Once inside the cell, the Stx A subunit specifically removes an adenine residue within the highly conserved α-sarcin/ricin loop on eukaryotic 28S rRNACitation46,Citation47 and, in doing so, inhibits protein translation ().Citation46,Citation48,Citation49 In vitro and in vivo studies have shown that Stx-mediated cell damage may be accompanied by apoptosis.Citation50-Citation53
Figure 1. Molecular mechanisms of Stx pathobiology. Stx inactivates host ribosomes by removing a specific adenine residue from the 28S rRNA, a lesion depicted by the red circles in the figure. Overall protein synthesis is therefore inhibited. However, at lower concentrations that have only minor effects on global protein synthesis, Stx induces changes in gene expression that alter the endothelial phenotype. Adapted with permission from Lippincott Williams and Wilkins/Wolters Kluwer Health: Current Opinion in Nephrology and Hypertension,Citation6 copyright 2012.
Shiga Toxin-Mediated Vascular Injury
While toxin-mediated ribosomal damage inhibits protein synthesis and can lead to cell death, Stx has profound effects on endothelial gene expression, effects that occur with little to no translation inhibition ().Citation5,Citation16,Citation43 Under normal conditions, the endothelium has a vasodilatory, thromboresistant, anti-adhesive, and anti-inflammatory phenotype.Citation54,Citation55 Recent findings have indicated that Stx elicits a very unique change in endothelial phenotype, in part through changes in patterns of expression of specific endothelial cell mRNAs.Citation16,Citation56
In an effort to understand the endothelial dysfunction mediated by Stx, several groups have studied the effects of Stx on vascular endothelial cells. Louise and Obrig have shown that Stx induces an increase in the ratio of plasminogen activator inhibitor-1 (PAI-1) to tissue plasminogen activator (tPA), suggesting that toxin treatment induced an endothelial phenotype favoring stabilization of fibrin clots.Citation57 Endothelial-derived tissue factor increases in the presence of Stx, likely as a result of increased expression of tissue factor, itself, and decreased activity of tissue factor pathway inhibitor.Citation16,Citation58
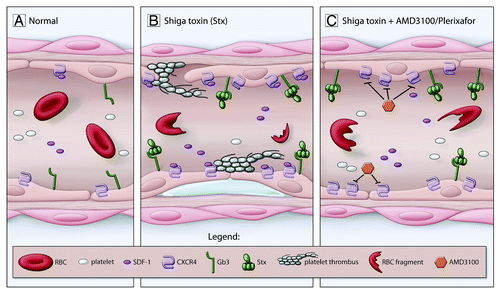
Recently, Stx was shown to upregulate the chemokine stromal cell-derived factor-1 (SDF-1) in cultured endothelial cells and in children with STEC-HUS ().Citation16 SDF-1 was initially cloned from a bone marrow stromal cell line but is constitutively expressed in the kidney, spleen, liver, lung, heart, brain, and muscle.Citation59,Citation60 CXCR4, the receptor for SDF-1, is expressed on a wide variety of cells, including cells within the hematopoietic lineage, endothelium, platelets, and neurons.Citation61-Citation64 This chemokine pathway plays critical roles in the vasculature both during development and post-natal angiogenesis and repair.Citation65-Citation68 SDF-1 has also been shown to enhance platelet activation induced by thrombin, resulting in increased platelet aggregation.Citation64,Citation69 Antagonism of the SDF-1/CXCR4 pathway using AMD3100 (plerixafor), a small molecule antagonist of CXCR4, significantly improved kidney function and overall survival of mice injected with Stx, indicating that activation of this pathway contributed to Stx pathogenesis.Citation16 Furthermore, inhibition of CXCR4/SDF-1 normalized platelet levels in vivo and prevented formation of platelet strings on a Stx-treated endothelial monolayer in vitro ().Citation16
Figure 2. Contribution of the CXCR4/SDF-1 pathway to Stx pathophysiology. (A) Normal blood vessel. (B) Underlying HUS pathophysiology is detachment of the endothelium and exposure of the underlying basement membrane, subendothelial edema, increased platelet adhesion accompanied by thrombocytopenia, and red blood cell (RBC) fragmentation. Gb3 on the surface of the endothelium binds Stx. Among the changes stimulated by Stx is upregulation of endothelial CXCR4 and increased blood SDF-1 levels. (C) Inhibition of CXCR4/SDF-1 interaction using AMD3100 (plerixafor) reduces Stx-mediated platelet adhesion to the endothelium in vitro and improves thrombocytopenia in vivo. Adapted with permission from Lippincott Williams and Wilkins/Wolters Kluwer Health: Current Opinion in Nephrology and Hypertension,Citation6 copyright 2012.
Children with E. coli O157:H7-mediated HUS exhibit significant pro-coagulant changes, including evidence for increased thrombin generation and fibrin accumulation, prior to onset of HUS.Citation70 Stx was also shown to induce rapid release of ultra-large vWF multimers from endothelial cells in vitro and inhibited multimer cleavage by the metalloprotease ADAMTS13.Citation71 The sum of these data indicate that toxin-mediated endothelial dysfunction results in a pro-thrombogenic intravascular environment.
Stx also modulates the production of an important mediator of vascular tone, namely the vasoconstrictive peptide, endothelin-1 (ET-1).Citation43 In other studies, endothelial cells treated with Stx1 and cultured under flow demonstrated enhanced leukocyte adhesion as a result of upregulation of endothelial adhesion molecules E-selectin, ICAM-1, and VCAM-1.Citation72,Citation73 Induction of chemokines, such as IL8 and MCP1, may also play a role in leukocyte adhesion.Citation74 In addition to the gene-specific studies described above, Matussek et al. reported the gene expression profiles of human umbilical vein endothelial cells exposed to Stx1 and Stx2.Citation56 These studies demonstrated that Stx stimulated mRNA and protein production of a variety of chemokines and cytokines, among other genes, which may serve to exacerbate Stx-induced endothelial damage. Importantly, Stx-mediated cytokine regulation alters cell sensitivity to the toxin as Gb3 itself may be upregulated by cytokines.Citation45,Citation75 The studies performed by Matussek et al. also demonstrated key differences in gene regulation by Stx1 compared with Stx2 that may, at least in part, explain the differences in virulence associated with each toxin.
Regulation of Endothelial Gene Expression by Shiga Toxin
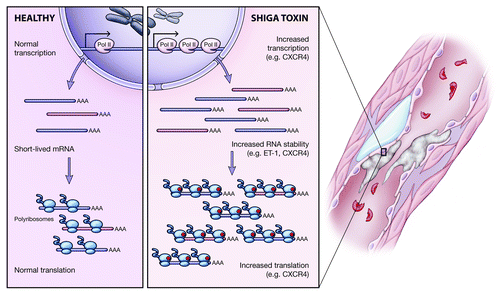
Large-scale, unbiased gene expression profiling studies demonstrated that Stx profoundly affects endothelial gene expression and phenotype at concentrations that have only minor effects on protein synthesis.Citation16,Citation56 Importantly however, the molecular mechanisms responsible for changes in endothelial phenotype in response to Stx remain largely unknown. There is growing evidence that Stx significantly alters RNA metabolism within the cell. Using endothelin-1 as a model, Bitzan et al. conducted nuclear run-on and actinomycin D experiments to study transcription and mRNA stability, respectively, and found that Stx increased ET-1 mRNA levels by stabilization of its mRNA transcript but not via transcription.Citation43 Recently, we demonstrated Stx-dependent upregulation of CXCR4 mRNA in endothelial cells.Citation16 Further investigation revealed a combination of enhanced transcription and mRNA stability contributed to the dramatic increase in CXCR4 mRNA levels.Citation16
Because Stx is a potent ribosomal inhibitor, it is important to consider its effects on translation. Ribosome profiling demonstrated higher association of CXCR4 transcripts with polyribosomes following Stx treatment, suggesting enhanced translation.Citation16 This effect was specific as the housekeeping gene cyclophilin A did not show the same pattern. These findings suggest that Stx-mediated gene expression is a complex process that affects many aspects of gene regulation. The various regulatory mechanisms are summarized in .
Figure 3. Mechanisms of endothelial gene regulation by Stx. Investigation into the mechanisms by which Stx affects gene expression in the endothelium revealed multi-level regulation. Stx may increase expression of select transcripts by upregulating transcription but also by enhancing the stability of short-lived mRNAs. Additionally, despite its ribosome inactivating properties, Stx has transcript-specific effects on translation, whereby it increases association of target mRNAs with polyribosomes.
Complement Activation in STEC-HUS
Mutations in complement regulatory genes are associated with atypical HUS and are found in 50% of patients.Citation76 Evidence for excessive complement activation in STEC-HUS is now also gaining attention. In vitro studies showed that relatively high concentrations of purified Stx incubated with normal serum induced formation of terminal complement complex (TCC) and that Stx bound factor H, resulting in delayed cell surface factor H activity.Citation77 Studies in 17 children with STEC-HUS showed that levels of the TCC were increased during the acute phase of disease and normalized within a month of patient discharge.Citation78 An independent analysis of 12 HUS patients in Sweden revealed elevated plasma levels of C3a and TCC during HUS compared with levels at recovery and pediatric controls.Citation79 Additional in vitro studies demonstrated C3 deposition on microvascular endothelial cells and subsequently an enhanced thrombogenic state as a result of Stx-mediated upregulation of P-selectin.Citation80 P-selectin inhibitory antibodies and P-selectin soluble ligand (PSGL-1) each reduced C3 deposition and thrombus formation.Citation80 These data support a role for complement dysregulation in STEC-HUS pathogenesis.
Eculizumab (Soliris®, Alexion Pharmaceuticals), a humanized monoclonal antibody that targets C5 to prevent formation of the membrane attack complex, was approved by the US FDA in September 2011 for treatment of atypical HUS. Publication of a study on the use of eculizumab in three 3-year-old patients suffering from severe STEC-HUS coincided with the 2011 HUS outbreak in GermanyCitation81 and prompted off-label, compassionate use of the drug to treat severe cases of HUS.Citation1 While preliminary results appeared promising,Citation82 two groups have since reported no benefit of eculizumab treatment on short-term outcome.Citation83,Citation84 These findings were based on retrospective analyses of cases where treatment strategy was dictated by disease severity and therefore, bias in patient outcome cannot be excluded. A prospective, controlled study with randomized treatment and control groups is essential in order to determine the efficacy of eculizumab therapy in STEC-HUS patients.
Evidence of Early Vascular Dysfunction in Predicting Patient Outcome
One of the major obstacles facing clinicians is the inability to predict which patients will develop life-threatening complications from STEC infection. Recent advances in understanding the underlying pathophysiology of HUS have brought to light several significant concepts that may provide insight on the matter. In a prospective study of children with E. coli O157:H7 infection, Chandler et al. reported evidence of elevated thrombin generation and diminished fibrinolysis when patient hematocrit, platelet, and serum creatinine levels were still normal.Citation70 These studies are particularly important because they indicate that prothrombotic coagulation abnormalities precede onset of HUS and these changes are detectable in a patient’s bloodstream before the classical features of the disease are clinically evident.
Other studies demonstrated that additional key mediators of vascular homeostasis and function exhibit important changes prior to the onset of the hallmark clinical features. Plasma levels of the chemokine stromal cell-derived factor-1 (SDF-1) exhibited a 4-fold increase in children who were later diagnosed with HUS compared with children with uncomplicated infection.Citation16 More recently, Page et al. described the disruption of angiopoietin-1 and angiopoietin-2 homeostasis in E. coli O157:H7-infected children.Citation85 Dysregulation was evident before the onset of HUS and became more pronounced with disease progression. The increased Ang2:Ang-1 ratio observed in HUS may represent an important mechanism of microvascular barrier disruption.Citation85 Taken together, the above findings not only identify potential targets for therapeutic development, but also may represent key, early warning signs that a patient is at higher risk of developing complications from STEC infection and should be monitored closely in order to mitigate severity of disease. Large-scale studies are required to determine the suitability of these molecules as biomarkers, but further investigation is warranted.
Conclusion
Recent events involving an alarmingly virulent pathogen underscore the important public health threat posed by STEC. Advances in our understanding of the pathogenic mechanisms involved are imperative for better patient treatment strategies. Studies focused on the molecular mechanisms of endothelial dysfunction continue to provide valuable insight into the thrombotic microangiopathy at the heart of Stx-mediated HUS. The accumulating evidence of uncontrolled complement activation is intriguing and supports the need for a multi-center, controlled trial of complement inhibition in STEC-HUS patients. A novel concept emerging from recent literature indicates that detectable changes in vital mediators of endothelial function occur in patients prior to the onset of HUS. While it may be difficult to discriminate conclusively between uncomplicated infection and infection that will progress to HUS using a single biomarker, it would be worthwhile to explore the suitability of combinations of markers as a more definitive predictive test. Together, these recent advances in our understanding of Stx-mediated disease provide attractive potential prognostic and therapeutic avenues worth exploring.
| Abbreviations: | ||
| HUS | = | hemolytic uremic syndrome |
| Stx | = | Shiga toxin |
| STEC | = | Shiga toxin-producing E. coli |
| SDF-1 | = | Stromal cell-derived factor-1 |
| TCC | = | terminal complement complex |
| VTEC | = | verotoxigenic E. coli |
| EHEC | = | enterohemorrhagic E. coli |
Acknowledgments
This work was supported by the grants from the Canadian Institutes of Health, especially MOP 79475, and grant PO1 HL076540-06A1 from NHLBI/NIH to PAM. PAM holds the Keenan Chair in Medical Research at St. Michael’s Hospital and the University of Toronto.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Johnson S, Waters A. Is complement a culprit in infection-induced forms of haemolytic uraemic syndrome?. Immunobiology 2012; 217:235 - 43; http://dx.doi.org/10.1016/j.imbio.2011.07.022; PMID: 21852019
- Lemaire M, Frémeaux-Bacchi V, Schaefer F, Choi M, Tang WH, Le Quintrec M, Fakhouri F, Taque S, Nobili F, Martinez F, et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet 2013; 45:531 - 6; http://dx.doi.org/10.1038/ng.2590; PMID: 23542698
- Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med 2009; 361:1676 - 87; http://dx.doi.org/10.1056/NEJMra0902814; PMID: 19846853
- Karmali MA. Host and pathogen determinants of verocytotoxin-producing Escherichia coli-associated hemolytic uremic syndrome. Kidney Int Suppl 2009; Suppl S4 - 7; http://dx.doi.org/10.1038/ki.2008.608; PMID: 19180132
- Petruzziello TN, Mawji IA, Khan M, Marsden PA. Verotoxin biology: molecular events in vascular endothelial injury. Kidney Int Suppl 2009; Suppl S17 - 9; http://dx.doi.org/10.1038/ki.2008.612; PMID: 19180125
- Petruzziello-Pellegrini TN, Marsden PA. Shiga toxin-associated hemolytic uremic syndrome: advances in pathogenesis and therapeutics. Curr Opin Nephrol Hypertens 2012; 21:433 - 40; http://dx.doi.org/10.1097/MNH.0b013e328354a62e; PMID: 22660553
- Karmali MA, Steele BT, Petric M, Lim C. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet 1983; 1:619 - 20; http://dx.doi.org/10.1016/S0140-6736(83)91795-6; PMID: 6131302
- Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis 1985; 151:775 - 82; http://dx.doi.org/10.1093/infdis/151.5.775; PMID: 3886804
- Jerse AE, Yu J, Tall BD, Kaper JB. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A 1990; 87:7839 - 43; http://dx.doi.org/10.1073/pnas.87.20.7839; PMID: 2172966
- Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005; 365:1073 - 86; PMID: 15781103
- Karmali MA, Gannon V, Sargeant JM. Verocytotoxin-producing Escherichia coli (VTEC). Vet Microbiol 2010; 140:360 - 70; http://dx.doi.org/10.1016/j.vetmic.2009.04.011; PMID: 19410388
- Warshawsky B, Gutmanis I, Henry B, Dow J, Reffle J, Pollett G, Ahmed R, Aldom J, Alves D, Chagla A, et al. Outbreak of Escherichia coli 0157:H7 related to animal contact at a petting zoo. Can J Infect Dis 2002; 13:175 - 81; PMID: 18159389
- Scheiring J, Andreoli SP, Zimmerhackl LB. Treatment and outcome of Shiga-toxin-associated hemolytic uremic syndrome (HUS). Pediatr Nephrol 2008; 23:1749 - 60; http://dx.doi.org/10.1007/s00467-008-0935-6; PMID: 18704506
- Obrig TG. Escherichia coli Shiga Toxin Mechanisms of Action in Renal Disease. Toxins (Basel) 2010; 2:2769 - 94; http://dx.doi.org/10.3390/toxins2122769; PMID: 21297888
- Jansen A, Kielstein JT. The new face of enterohaemorrhagic Escherichia coli infections. Euro Surveill 2011; 16:19898; PMID: 21722615
- Petruzziello-Pellegrini TN, Yuen DA, Page AV, Patel S, Soltyk AM, Matouk CC, Wong DK, Turgeon PJ, Fish JE, Ho JJ, et al. The CXCR4/CXCR7/SDF-1 pathway contributes to the pathogenesis of Shiga toxin-associated hemolytic uremic syndrome in humans and mice. J Clin Invest 2012; 122:759 - 76; http://dx.doi.org/10.1172/JCI57313; PMID: 22232208
- Trachtman H, Austin C, Lewinski M, Stahl RA. Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat Rev Nephrol 2012; 8:658 - 69; http://dx.doi.org/10.1038/nrneph.2012.196; PMID: 22986362
- Siegler RL. Spectrum of extrarenal involvement in postdiarrheal hemolytic-uremic syndrome. J Pediatr 1994; 125:511 - 8; http://dx.doi.org/10.1016/S0022-3476(94)70001-X; PMID: 7931868
- Zoja C, Buelli S, Morigi M. Shiga toxin-associated hemolytic uremic syndrome: pathophysiology of endothelial dysfunction. Pediatr Nephrol 2010; 25:2231 - 40; http://dx.doi.org/10.1007/s00467-010-1522-1; PMID: 20424866
- Spinale JM, Ruebner RL, Copelovitch L, Kaplan BS. Long-term outcomes of Shiga toxin hemolytic uremic syndrome. Pediatr Nephrol 2013; In press http://dx.doi.org/10.1007/s00467-012-2383-6; PMID: 23288350
- Rosales A, Hofer J, Zimmerhackl LB, Jungraithmayr TC, Riedl M, Giner T, Strasak A, Orth-Höller D, Würzner R, Karch H, German-Austrian HUS Study Group. Need for long-term follow-up in enterohemorrhagic Escherichia coli-associated hemolytic uremic syndrome due to late-emerging sequelae. Clin Infect Dis 2012; 54:1413 - 21; http://dx.doi.org/10.1093/cid/cis196; PMID: 22412065
- Rasko DA, Webster DR, Sahl JW, Bashir A, Boisen N, Scheutz F, Paxinos EE, Sebra R, Chin CS, Iliopoulos D, et al. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med 2011; 365:709 - 17; http://dx.doi.org/10.1056/NEJMoa1106920; PMID: 21793740
- Wong CS, Mooney JC, Brandt JR, Staples AO, Jelacic S, Boster DR, Watkins SL, Tarr PI. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: a multivariable analysis. Clin Infect Dis 2012; 55:33 - 41; http://dx.doi.org/10.1093/cid/cis299; PMID: 22431799
- Bielaszewska M, Idelevich EA, Zhang W, Bauwens A, Schaumburg F, Mellmann A, Peters G, Karch H. Effects of antibiotics on Shiga toxin 2 production and bacteriophage induction by epidemic Escherichia coli O104:H4 strain. Antimicrob Agents Chemother 2012; 56:3277 - 82; http://dx.doi.org/10.1128/AAC.06315-11; PMID: 22391549
- Tarr PI, Karpman D. Editorial commentary: Escherichia coli O104:H4 and hemolytic uremic syndrome: the analysis begins. Clin Infect Dis 2012; 55:760 - 3; http://dx.doi.org/10.1093/cid/cis533; PMID: 22670035
- Colic E, Dieperink H, Titlestad K, Tepel M. Management of an acute outbreak of diarrhoea-associated haemolytic uraemic syndrome with early plasma exchange in adults from southern Denmark: an observational study. Lancet 2011; 378:1089 - 93; http://dx.doi.org/10.1016/S0140-6736(11)61145-8; PMID: 21871657
- Ake JA, Jelacic S, Ciol MA, Watkins SL, Murray KF, Christie DL, Klein EJ, Tarr PI. Relative nephroprotection during Escherichia coli O157:H7 infections: association with intravenous volume expansion. Pediatrics 2005; 115:e673 - 80; http://dx.doi.org/10.1542/peds.2004-2236; PMID: 15930195
- Ullrich S, Bremer P, Neumann-Grutzeck C, Otto H, Rüther C, von Seydewitz CU, Meyer GP, Ahmadi-Simab K, Röther J, Hogan B, et al. Symptoms and clinical course of EHEC O104 infection in hospitalized patients: a prospective single center study. PLoS One 2013; 8:e55278; http://dx.doi.org/10.1371/journal.pone.0055278; PMID: 23460784
- Johannes L, Römer W. Shiga toxins--from cell biology to biomedical applications. Nat Rev Microbiol 2010; 8:105 - 16; PMID: 20023663
- Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, et al, HUS Investigation Team. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med 2011; 365:1771 - 80; http://dx.doi.org/10.1056/NEJMoa1106483; PMID: 21696328
- Buchholz U, Bernard H, Werber D, Böhmer MM, Remschmidt C, Wilking H, Deleré Y, an der Heiden M, Adlhoch C, Dreesman J, et al. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med 2011; 365:1763 - 70; http://dx.doi.org/10.1056/NEJMoa1106482; PMID: 22029753
- Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol 2010; 8:26 - 38; PMID: 19966814
- Scheutz F, Nielsen EM, Frimodt-Møller J, Boisen N, Morabito S, Tozzoli R, Nataro JP, Caprioli A. Characteristics of the enteroaggregative Shiga toxin/verotoxin-producing Escherichia coli O104:H4 strain causing the outbreak of haemolytic uraemic syndrome in Germany, May to June 2011. Euro Surveill 2011; 16:19889; PMID: 21699770
- European Centre for Disease Prevention and Control and European Food Safety Authority. Shiga toxin/verotoxin-producing Escherichia coli in humans, food and animals in the EU/EEA, with special reference to the German outbreak strain STEC O104. Stockholm: ECDC, 2011.
- Bielaszewska M, Mellmann A, Zhang W, Köck R, Fruth A, Bauwens A, Peters G, Karch H. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect Dis 2011; 11:671 - 6; PMID: 21703928
- Mellmann A, Harmsen D, Cummings CA, Zentz EB, Leopold SR, Rico A, Prior K, Szczepanowski R, Ji Y, Zhang W, et al. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS One 2011; 6:e22751; http://dx.doi.org/10.1371/journal.pone.0022751; PMID: 21799941
- Rohde H, Qin J, Cui Y, Li D, Loman NJ, Hentschke M, Chen W, Pu F, Peng Y, Li J, et al, E. coli O104:H4 Genome Analysis Crowd-Sourcing Consortium. Open-source genomic analysis of Shiga-toxin-producing E. coli O104:H4. N Engl J Med 2011; 365:718 - 24; http://dx.doi.org/10.1056/NEJMoa1107643; PMID: 21793736
- Grad YH, Godfrey P, Cerquiera GC, Mariani-Kurkdjian P, Gouali M, Bingen E, Shea TP, Haas BJ, Griggs A, Young S, et al. Comparative genomics of recent Shiga toxin-producing Escherichia coli O104:H4: short-term evolution of an emerging pathogen. MBio 2013; 4:e00452 - 12; http://dx.doi.org/10.1128/mBio.00452-12; PMID: 23341549
- Scheutz F, Teel LD, Beutin L, Piérard D, Buvens G, Karch H, Mellmann A, Caprioli A, Tozzoli R, Morabito S, et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol 2012; 50:2951 - 63; http://dx.doi.org/10.1128/JCM.00860-12; PMID: 22760050
- Scotland SM, Smith HR, Willshaw GA, Rowe B. Vero cytotoxin production in strain of Escherichia coli is determined by genes carried on bacteriophage. Lancet 1983; 2:216; http://dx.doi.org/10.1016/S0140-6736(83)90192-7; PMID: 6135046
- O’Brien AD, Newland JW, Miller SF, Holmes RK, Smith HW, Formal SB. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 1984; 226:694 - 6; http://dx.doi.org/10.1126/science.6387911; PMID: 6387911
- Newland JW, Strockbine NA, Miller SF, O’Brien AD, Holmes RK. Cloning of Shiga-like toxin structural genes from a toxin converting phage of Escherichia coli. Science 1985; 230:179 - 81; http://dx.doi.org/10.1126/science.2994228; PMID: 2994228
- Bitzan MM, Wang Y, Lin J, Marsden PA. Verotoxin and ricin have novel effects on preproendothelin-1 expression but fail to modify nitric oxide synthase (ecNOS) expression and NO production in vascular endothelium. J Clin Invest 1998; 101:372 - 82; http://dx.doi.org/10.1172/JCI522; PMID: 9435309
- Lingwood CA, Binnington B, Manis A, Branch DR. Globotriaosyl ceramide receptor function - where membrane structure and pathology intersect. FEBS Lett 2010; 584:1879 - 86; http://dx.doi.org/10.1016/j.febslet.2009.11.089; PMID: 19948172
- Obrig TG, Louise CB, Lingwood CA, Boyd B, Barley-Maloney L, Daniel TO. Endothelial heterogeneity in Shiga toxin receptors and responses. J Biol Chem 1993; 268:15484 - 8; PMID: 8340376
- Endo Y, Tsurugi K, Yutsudo T, Takeda Y, Ogasawara T, Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem 1988; 171:45 - 50; http://dx.doi.org/10.1111/j.1432-1033.1988.tb13756.x; PMID: 3276522
- Saxena SK, O’Brien AD, Ackerman EJ. Shiga toxin, Shiga-like toxin II variant, and ricin are all single-site RNA N-glycosidases of 28 S RNA when microinjected into Xenopus oocytes. J Biol Chem 1989; 264:596 - 601; PMID: 2642481
- Furutani M, Kashiwagi K, Ito K, Endo Y, Igarashi K. Comparison of the modes of action of a Vero toxin (a Shiga-like toxin) from Escherichia coli, of ricin, and of alpha-sarcin. Arch Biochem Biophys 1992; 293:140 - 6; http://dx.doi.org/10.1016/0003-9861(92)90376-8; PMID: 1731630
- Obrig TG, Moran TP, Brown JE. The mode of action of Shiga toxin on peptide elongation of eukaryotic protein synthesis. Biochem J 1987; 244:287 - 94; PMID: 3663122
- Karpman D, Håkansson A, Perez MT, Isaksson C, Carlemalm E, Caprioli A, Svanborg C. Apoptosis of renal cortical cells in the hemolytic-uremic syndrome: in vivo and in vitro studies. Infect Immun 1998; 66:636 - 44; PMID: 9453620
- Jones NL, Islur A, Haq R, Mascarenhas M, Karmali MA, Perdue MH, Zanke BW, Sherman PM. Escherichia coli Shiga toxins induce apoptosis in epithelial cells that is regulated by the Bcl-2 family. Am J Physiol Gastrointest Liver Physiol 2000; 278:G811 - 9; PMID: 10801274
- Suzuki A, Doi H, Matsuzawa F, Aikawa S, Takiguchi K, Kawano H, Hayashida M, Ohno S. Bcl-2 antiapoptotic protein mediates verotoxin II-induced cell death: possible association between bcl-2 and tissue failure by E. coli O157:H7. Genes Dev 2000; 14:1734 - 40; PMID: 10898788
- Tesh VL. The induction of apoptosis by Shiga toxins and ricin. Curr Top Microbiol Immunol 2012; 357:137 - 78; http://dx.doi.org/10.1007/82_2011_155; PMID: 22130961
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 2007; 100:158 - 73; http://dx.doi.org/10.1161/01.RES.0000255691.76142.4a; PMID: 17272818
- Aird WC. Endothelium in health and disease. Pharmacol Rep 2008; 60:139 - 43; PMID: 18276995
- Matussek A, Lauber J, Bergau A, Hansen W, Rohde M, Dittmar KE, Gunzer M, Mengel M, Gatzlaff P, Hartmann M, et al. Molecular and functional analysis of Shiga toxin-induced response patterns in human vascular endothelial cells. Blood 2003; 102:1323 - 32; http://dx.doi.org/10.1182/blood-2002-10-3301; PMID: 12702508
- Louise CB, Obrig TG. Human renal microvascular endothelial cells as a potential target in the development of the hemolytic uremic syndrome as related to fibrinolysis factor expression, in vitro. Microvasc Res 1994; 47:377 - 87; http://dx.doi.org/10.1006/mvre.1994.1030; PMID: 8084301
- Nestoridi E, Tsukurov O, Kushak RI, Ingelfinger JR, Grabowski EF. Shiga toxin enhances functional tissue factor on human glomerular endothelial cells: implications for the pathophysiology of hemolytic uremic syndrome. J Thromb Haemost 2005; 3:752 - 62; http://dx.doi.org/10.1111/j.1538-7836.2005.01205.x; PMID: 15842359
- Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science 1993; 261:600 - 3; http://dx.doi.org/10.1126/science.8342023; PMID: 8342023
- Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, Honjo T. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics 1995; 28:495 - 500; http://dx.doi.org/10.1006/geno.1995.1180; PMID: 7490086
- Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, Koni PA, Nagasawa Y, Hamano T, Matsui I, Kawada N, et al. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol 2009; 20:1714 - 23; http://dx.doi.org/10.1681/ASN.2008060640; PMID: 19443644
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 1998; 393:591 - 4; http://dx.doi.org/10.1038/31261; PMID: 9634237
- Lévesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest 2003; 111:187 - 96; PMID: 12531874
- Panicot-Dubois L, Thomas GM, Furie BC, Furie B, Lombardo D, Dubois C. Bile salt-dependent lipase interacts with platelet CXCR4 and modulates thrombus formation in mice and humans. J Clin Invest 2007; 117:3708 - 19; http://dx.doi.org/10.1172/JCI32655; PMID: 18037996
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004; 10:858 - 64; http://dx.doi.org/10.1038/nm1075; PMID: 15235597
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med 2006; 12:557 - 67; http://dx.doi.org/10.1038/nm1400; PMID: 16648859
- Strasser GA, Kaminker JS, Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood 2010; 115:5102 - 10; http://dx.doi.org/10.1182/blood-2009-07-230284; PMID: 20154215
- Ruiz de Almodovar C, Luttun A, Carmeliet P. An SDF-1 trap for myeloid cells stimulates angiogenesis. Cell 2006; 124:18 - 21; http://dx.doi.org/10.1016/j.cell.2005.12.023; PMID: 16413476
- Gear AR, Suttitanamongkol S, Viisoreanu D, Polanowska-Grabowska RK, Raha S, Camerini D. Adenosine diphosphate strongly potentiates the ability of the chemokines MDC, TARC, and SDF-1 to stimulate platelet function. Blood 2001; 97:937 - 45; http://dx.doi.org/10.1182/blood.V97.4.937; PMID: 11159520
- Chandler WL, Jelacic S, Boster DR, Ciol MA, Williams GD, Watkins SL, Igarashi T, Tarr PI. Prothrombotic coagulation abnormalities preceding the hemolytic-uremic syndrome. N Engl J Med 2002; 346:23 - 32; http://dx.doi.org/10.1056/NEJMoa011033; PMID: 11777999
- Nolasco LH, Turner NA, Bernardo A, Tao Z, Cleary TG, Dong JF, Moake JL. Hemolytic uremic syndrome-associated Shiga toxins promote endothelial-cell secretion and impair ADAMTS13 cleavage of unusually large von Willebrand factor multimers. Blood 2005; 106:4199 - 209; http://dx.doi.org/10.1182/blood-2005-05-2111; PMID: 16131569
- Morigi M, Zoja C, Figliuzzi M, Foppolo M, Micheletti G, Bontempelli M, Saronni M, Remuzzi G, Remuzzi A. Fluid shear stress modulates surface expression of adhesion molecules by endothelial cells. Blood 1995; 85:1696 - 703; PMID: 7535583
- Morigi M, Galbusera M, Binda E, Imberti B, Gastoldi S, Remuzzi A, Zoja C, Remuzzi G. Verotoxin-1-induced up-regulation of adhesive molecules renders microvascular endothelial cells thrombogenic at high shear stress. Blood 2001; 98:1828 - 35; http://dx.doi.org/10.1182/blood.V98.6.1828; PMID: 11535517
- Zoja C, Angioletti S, Donadelli R, Zanchi C, Tomasoni S, Binda E, Imberti B, te Loo M, Monnens L, Remuzzi G, et al. Shiga toxin-2 triggers endothelial leukocyte adhesion and transmigration via NF-kappaB dependent up-regulation of IL-8 and MCP-1. Kidney Int 2002; 62:846 - 56; http://dx.doi.org/10.1046/j.1523-1755.2002.00503.x; PMID: 12164866
- Kaye SA, Louise CB, Boyd B, Lingwood CA, Obrig TG. Shiga toxin-associated hemolytic uremic syndrome: interleukin-1 beta enhancement of Shiga toxin cytotoxicity toward human vascular endothelial cells in vitro. Infect Immun 1993; 61:3886 - 91; PMID: 8359910
- Benz K, Amann K. Thrombotic microangiopathy: new insights. Curr Opin Nephrol Hypertens 2010; 19:242 - 7; http://dx.doi.org/10.1097/MNH.0b013e3283378f25; PMID: 20186056
- Orth D, Khan AB, Naim A, Grif K, Brockmeyer J, Karch H, Joannidis M, Clark SJ, Day AJ, Fidanzi S, et al. Shiga toxin activates complement and binds factor H: evidence for an active role of complement in hemolytic uremic syndrome. J Immunol 2009; 182:6394 - 400; http://dx.doi.org/10.4049/jimmunol.0900151; PMID: 19414792
- Thurman JM, Marians R, Emlen W, Wood S, Smith C, Akana H, Holers VM, Lesser M, Kline M, Hoffman C, et al. Alternative pathway of complement in children with diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol 2009; 4:1920 - 4; http://dx.doi.org/10.2215/CJN.02730409; PMID: 19820137
- Ståhl AL, Sartz L, Karpman D. Complement activation on platelet-leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood 2011; 117:5503 - 13; http://dx.doi.org/10.1182/blood-2010-09-309161; PMID: 21447825
- Morigi M, Galbusera M, Gastoldi S, Locatelli M, Buelli S, Pezzotta A, Pagani C, Noris M, Gobbi M, Stravalaci M, et al. Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J Immunol 2011; 187:172 - 80; http://dx.doi.org/10.4049/jimmunol.1100491; PMID: 21642543
- Lapeyraque AL, Malina M, Fremeaux-Bacchi V, Boppel T, Kirschfink M, Oualha M, Proulx F, Clermont MJ, Le Deist F, Niaudet P, et al. Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med 2011; 364:2561 - 3; http://dx.doi.org/10.1056/NEJMc1100859; PMID: 21612462
- Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol 2012; 8:622 - 33; http://dx.doi.org/10.1038/nrneph.2012.195; PMID: 22986360
- Menne J, Nitschke M, Stingele R, Abu-Tair M, Beneke J, Bramstedt J, Bremer JP, Brunkhorst R, Busch V, Dengler R, et al, EHEC-HUS consortium. Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study. BMJ 2012; 345:e4565; http://dx.doi.org/10.1136/bmj.e4565; PMID: 22815429
- Kielstein JT, Beutel G, Fleig S, Steinhoff J, Meyer TN, Hafer C, Kuhlmann U, Bramstedt J, Panzer U, Vischedyk M, et al, Collaborators of the DGfN STEC-HUS registry. Best supportive care and therapeutic plasma exchange with or without eculizumab in Shiga-toxin-producing E. coli O104:H4 induced haemolytic-uraemic syndrome: an analysis of the German STEC-HUS registry. Nephrol Dial Transplant 2012; 27:3807 - 15; http://dx.doi.org/10.1093/ndt/gfs394; PMID: 23114903
- Page AV, Tarr PI, Watkins SL, Rajwans N, Petruzziello-Pellegrini TN, Marsden PA, Kain KC, Liles WC. Dysregulation of Angiopoietin 1 and 2 in Escherichia coli O157:H7 Infection and the Hemolytic-Uremic Syndrome. J Infect Dis 2013; 208:929 - 33; http://dx.doi.org/10.1093/infdis/jit268; PMID: 23801605