Abstract
Mycobacterium bovis is the causative agent of bovine tuberculosis, a disease that affects approximately 5% of Argentine cattle. The aim of this research was to study if it is possible to infer the degree of virulence of different M. bovis genotypes based on scorified observations of tuberculosis lesions in cattle.
In this study, we performed association analyses between several parameters with tuberculosis lesions: M. bovis genotype, degree of progression of tuberculosis, and animal age. For this purpose, the genotype was determined by spoligotyping and the degree of bovine tuberculosis gross lesion was quantified with a score based on clinical observations (number, size, and location of granulomas along with histopathologic features). This study was performed with naturally infected cattle of slaughterhouses from three provinces in Argentina.
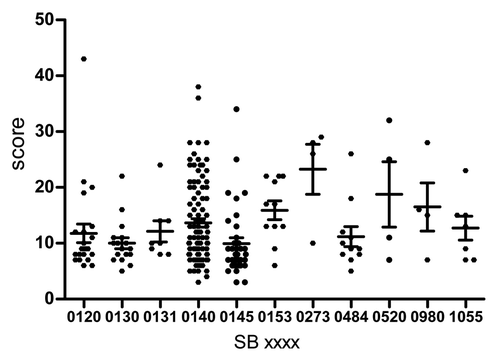
A total of 265 M. bovis isolates were obtained from 378 pathological lesion samples and 192 spoligotyping and VNTR (based on ETR sequences) typing patterns were obtained. SB0140 was the most predominant spoligotype, followed by SB0145. The spoligotype with the highest lesion score was SB0273 (median score of 27 ± 4.46), followed by SB0520 (18 ± 5.8). Furthermore, the most common spoligotype, SB0140, had a median score of 11 ± 0.74. Finally, the spoligotype with the lowest score was SB0145 (8 ± 1.0). ETR typing of SB0140, SB0145, SB0273, and SB0520 did not subdivide the lesion scores in those spoligotypes.
In conclusion, SB0273 and SB0520 were the spoligotypes with the strongest association with hypervirulence and both spoligotypes were only found in Río Cuarto at the south of Córdoba province. Interestingly, there is no other report of any of these spoligotyes in Latin America.
Introduction
Bovine tuberculosis (BTB) is an infectious disease that affects a wide range of mammals, including humans.Citation1 This disease is caused by Mycobacterium bovis, a member of the M. tuberculosis complex (MTBC) that also includes M. tuberculosis, M. africanum, M. canetti, M. microti, M. caprae, M. mungi, M. orygis, and M. pinnipedii. The population of cattle in Argentina has been estimated in 51 million heads.Citation2 In 2012, 9 258 541 bovines were slaughtered and 0.3% of these animals presented tuberculosis lesions.Citation3
Several methods, such as RFLPCitation3,Citation4 and VNTR,Citation5 have been described for genotyping M. bovis and other M. tuberculosis complex species. However, up to now, spoligotyping is the best option for large-scale screening studies on the distribution of M. bovis strains.Citation6 However, for a proper discrimination of the isolates other typing methods such as VNTR typing have to be performed additionally, especially in settings where one spoligotype is largely predominant.
In this study, we analyzed whether a relationship exists between the genotype of M. bovis and the degree of virulence that it causes in naturally infected cattle. While this topic has been already elucidated for other pathogenic bacteria, this question remains unclear for M. tuberculosis complex bacteria. For this purpose, we assessed possible associations between the genotype of M. bovis, the degree of tuberculosis progression and the age of the animals. For these analyses, the genotypes were determined based on the spoligotyping and VNTR typing and the degree of bovine tuberculosis lesion was quantified with a score based on clinical observations. These observations were based on the number and location of tuberculous granulomas as well as the histopathologic features. These data were subsequently stratified by the approximate age of the animal, within the following categories: calves, steers, heifers, etc. Six veterinary groups were involved in this study. They all performed the collection of the bovine tuberculosis lesions and studied the virulence in the infected cattle through a detailed analysis of the lesions found in the slaughterhouses.
Results
A total of 192 cultures of M. bovis were obtained from the 378 analyzed samples that were collected from individual animals. In addition, their spoligotyping data were also analyzed. These data showed that SB0140 is the most predominant spoligotype. This spoligotype grouped 33% of these isolates and displayed the highest prevalence (39%) in Santa Fe province and the lowest (26%) in Buenos Aires province. The second most prevalent spoligotype was SB0145 (18%).
Furthermore, clinical and epidemiological data from the 192 animals with typical TB lesions were analyzed, verifying the infection with M. bovis through bacteriological confirmation. The collected data were categorized according to sex, slaughterhouse location, and farm or fair origin, as well as macroscopical and microscopical score. No significant differences were observed in the macrocoscopical score distribution assigned by the different veterinary groups participating in this study (data not shown). For all animals, the visible lesions were photographed and macroscopical observation data were recorded on data entry sheets (data not shown). Representative lesions are shown in Figure S1. The macroscopical score was more informative () than the microscopical data, since most of the histopathological lesions had the top score 4. This score means advanced lesions with caseonecrotic granuloma, peripheral fibrosis and central mineralization. In contrast, the macroscopical scores went from 3 to 43, with 7 being the most frequent value (28 times) in the data set; which describes multifocal and small granulomas (1 cm diameter).
SB0273 showed the highest score with a median score of 27 ± 4.46 (several affected organs with granulomas and beaded forms) and was followed by SB0520 (18 ± 5.8). Additionally, SB0140, the most common spoligotype, had a median score of 11 ± 0.74. On the other hand, SB0145 displayed the lowest score (8 ± 1.0) (; ), even though it is predominant in south Buenos Aires province. The statistical comparison analysis performed through the Mann–Whitney test showed significant differences between the values of the first highest macro scores (which is the scores from SB0273 vs. those from SB140) (P < 0.05). Furthermore, the differences of SB0273 and SB0520 compared with the lowest score (SB0145) are also statistically significant (P < 0.01 and P < 0.05, respectively); thus both spoligotypes showed a correlation with virulence.
Table 1. Spoligotypes showing higher and lower macroscopic scores, and most predominant spoligotyes
In conclusion, the spoligotypes that showed the highest association with virulence were SB0273 and SB0520, suggesting a possible connection between the disseminated infection and disease activity. SB0273 and SB0520 were only found in the south of Córdoba province. There is no other report of any of these spoligotypes in other region of Latin America.
To obtain more recent data of the infection, we excluded the category “cow” in a new assessment, keeping younger animals for this particular analysis. In this case, not only did SB0273 show the highest macroscopical median score, but this score even increased without the older animals (28.5). SB520 (17) and SB0153 (17) followed those values.
Occurrence of SB0273, SB0520, and SB0145 at world level
There are reports of SB0273 in Ireland,Citation8 Argentina,Citation9,Citation10 Australia, and the UK (http://www.mbovis.org/spoligodatabase/returnsingleinfo.php and http://www.pasteur-guadeloupe.fr:8081/SITVITDemo/souchesParSpoligotype). SB0520 has been described only in Argentina.Citation11 SB0145 has been described as the most extensively distributed genotype, found in Argentina,Citation9,Citation10,Citation12,Citation13 Australia,Citation14 Brazil,Citation15 France,Citation5 Northern Ireland,Citation16,Citation17 and other countries (as seen at http://www.mbovis.org).
Subtyping of spoligotypes by VNTR
The highly predominant spoligotype SB0140 was divided in 25 VNTR types, where the profile 6–5–5–4–5 grouped 14 out of the 104 isolates. The 36 isolates belonging to SB0145 were divided in 7 VNTR types.
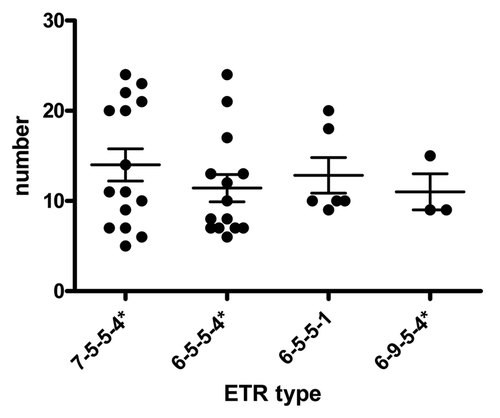
The ETR typing was applied to the most relevant spoligotypes: SB0140, the most predominant; SB0273 and SB0520, which seem to be associated with higher virulence; and SB0145, which is related to a lower virulence. With this typing, the four strains of SB0273 were grouped in three ETR types. One ETR type contained two strains with a high macroscopical pathology score. The four SB0520 isolates consistently had the 7–5–5–4* ETR type. However, the low number of SB0273 ETR-subtypes makes it impossible to validate any analysis of scores. SB0140 was divided into 56 types, but only 4 of these types grouped more than 2 isolates. The 4 major clusters did not show significant differences between them (; ). Besides, the SB0145 isolates were subdivided in three ETRAD clusters. A cluster (7–5–5–4*) of 10 isolates showed a median score of 13.1. This value was larger than that from SB0145 (9.9) and also of that from the cluster 7–9*–5,–4*, which showed low median scores (7.24). However, the differences between 7–9*–5,–4* and every other ETR clusters of SB0145 are not statistically significant (P = 0.10914).
Table 2. Macroscopical scores of VNTRs profiles among predominant spoligotypes
Discussion
The evaluation of field diseased animals for testing differential virulence of bacterial pathogens has advantages and disadvantages compared with the experimental inoculation approach. In experimental inoculation, the onset of infection is known. By contrast, this parameter is unclear in field screening. Special caution has to be taken when different strains are tested in experimental infection regarding the number of previous passages of the strains. For instance, the genes involved in the synthesis of phtiocerol-dimycocerosate (PDIM) are lost from M. tuberculosis when this bacterium is kept under culture,Citation19 which, in turn, reduces its virulence. Culture conditions may also alter the virulence.Citation20 While in an experimental infection, animals and pathogenic agents are controlled and uniform, in a field evaluation, the infection occurs by a natural route without any specific control. Thus, more animals have to be evaluated in field studies.
In their review, Nicoll and WilkinsonCitation21 claim that an important emerging area of research is the study of the outcome of an infection with Mycobacterium tuberculosis depending on the variety of the strain involved in the infection. The first report on virulence variability of M. tuberculosis isolates came from the pioneering works of Mitchinson on the lower virulence of M. tuberculosis isolates from South India.Citation22 This result was confirmed at cellular level by Rajashree and Das.Citation23 Additionaly, López et al. observed that M. tuberculosis isolates of the Beijing lineages were more virulent than other lineages in a mice model.Citation24 On the other hand, other non-Beijing strains were more virulent than Beijing lineage in human macrophages.Citation25 Other authors have also investigated the differential virulence of M. tuberculosis complex isolatesCitation26-Citation30 and we previously demonstrated this differential virulence with M. bovis isolates.Citation31
An essential aspect of this kind of study is that the scoring system has to be a reliable correlate of the pathogenesis. With this in mind, in the present study we used the macroscopical scores as a correlate of pathogenesis degree. The score was elaborated taking into account the number, size, and presentation of lesions. Other scoring scales have been proposed. For instance, a shorter scale score was proposed by Buddle.Citation32 This shorter score considers only the total number of lesions (lung lesion score: 0, no lesions; 1, 1 to 9 lesions; 2, 10 to 29 lesions; 3, 30 to 99 lesions; 4, 100 to 199 lesions; and 5, >200 lesions). Palmer et al.Citation33 assigned different scores for lymph nodes and lungs. In our study, lungs, lymph nodes, and other organs were subjected to semi-quantitative scoring of gross lesions adapted from previous studies.Citation34
Our scoring takes into account the size and pathological presentation of lesion and the total number because individual lesion score are added up. Also, this scoring method yields a larger linear rank. The specificity and sensitivity of scores is a matter of further research and analysis. The microscopical score was not considered in the analysis because it failed to provide more information to the global results (data not shown).
In this cohort, the slaughtered animals presented severe visible lesions with varying degrees of gross pathology scoring. The bovines with SB0273 and SB0980 isolates showed the highest macro scores and these values were significantly different from the others. Interestingly, these spoligotypes were present in the provinces with the highest prevalence of bovine tuberculosis. Furthermore, if individual scores are considered, 26, 28, and 29 are the most represented with a genotype associated to the largest number of macroscopic lesions observed in the inspected animals. Other high scores, such as 32, 34, 38, and 43, were obtained in only one case each. Therefore, they were exceptional findings among different genotypes with regular averages of score.
The spoligotype SB0273 was also described in IrelandCitation8; however it had been first identified in Australia. In the present study, this spoligotype was detected at south of Córdoba province. There are no other reports of SB0273 in Latin America.Citation10 Remarkably, the two spoligotypes with the highest score (SB0273 and SB0520) represent less than 0.7% of the total isolates of M. bovis from Argentina. The spoligotype SB0273 is a close relative of SB0140 showing difference in only one spacer (spacer #37). SB0520 is less related to SB0140 differing in various spacers. ETR types prevailing in SB0140 also appear in SB0273 but not in SB0520. These results suggest that SB0520 is more distantly related to SB0140 than SB0273. Furthermore, the largely prevalent SB0140 appears to have a moderate virulence. The ETR subtypes of SB0140 do not differ much in the macroscopical lesion media. The link between success in infection and transmission and a moderate virulence has been debated and analyzed and the trade-off hypothesis of virulence evolution has been proposed.Citation35
The molecular and cellular basis of the differential virulence of the isolates identified here is yet unknown. Krishnan et al.Citation36 observed that the M. tuberculosis strains that showed differential virulence in macrophages, dendritic cells, and mice had different compositions of cell envelope lipids. Because the molecular and cellular basis of the differential virulence of the isolates identified here are not known, we plan to sequence the genome of the strains with the highest and lowest virulence and look for differences in those genes related to lipids.
Materials and Methods
Animals, lesions, and M. bovis isolates
Around 35 000 animal carcasses were inspected in slaughterhouses between 2008 and 2011. The isolates came from bovines from Buenos Aires, Córdoba, and Santa Fe provinces. These three provinces hold 60% of the whole cattle population in the country. A convenience non-probabilistic sampling was performed at each slaughter plant with the assistance of the local animal health service staff (SENASA). The selected categories, which depend on the age of bovines and their weight, and the corresponding percentages were as follows: calves (<12 mo, <220 kg), 13%; young steers (12 to 18 mo, <350 kg), 5%; steers (>18 mo, >350 kg), 11%; heifers (12 to 30 mo), 8.3%; cows (>30 mo, >350 kg), 61%; and bulls, 0.5%.
The anatomical dissemination of the visible gross pathological lesions in organs and tissues were scored according to . The individual score of each animal was calculated based on the total score of all lesions. For example, if an animal has a granuloma with red halo (1), of yellow color (2), no calcification (0), multifocal (2), and with a size of 1–5 cm (3), it gives a score of 8 for this individual lesion, and so on up to complete all lesions. The microscopical score was calculated according to Wangoo et al.Citation37
Table 3. Macroscopical scoring for individual lesions
A total of 378 samples from individual animals were collected and submitted to histopathology and culture. A total of 192 M. bovis isolates coming from Buenos Aires (n = 63), Córdoba (n = 67), and Santa Fe (n = 62) provinces were included in this study.
Histopathology
Tissues with granulomatous macroscopic bovine tuberculosis-like lesions from 378 adult bovines obtained during the slaughtering were processed according to routine histopathological technique. The lesions consisted in different-sized granulomas, encapsulated, with caseous necrosis and calcification. After 24 h fixation, samples were embedded in paraffin, cut in 4-µm sections, and stained with hematoxylin–eosin (H&E) and Ziehl–Neelsen acid-fast stain. Microscopic lesions were observed and granulomas classified in stages 1 to 4 according to previously described criteria.Citation38 The presence of acid-fast bacilli was classified in scores 1 to 4 as suggested also by Wangoo et al.Citation38 All data were registered in individual ad hoc spreadsheets.
Spoligotypes
A total of 192 isolates were typed by spoligotyping, according to Kamerbeek et al.Citation6 The spoligotypes were collected in a binary format in an excel database and the scanned films were analyzed using BionumericsR (Version 3.5, Applied Maths, Sint-Martens-Latem, Belgium).
The spoligotypes of this study were compared with the M. bovis spoligotypes contained in the http://www.mbovis.org database from the University of Sussex, UK and were named according to a code composed by SB of four digits.
VNTR typing
The 192 isolates typed by spoligotyping were further analyzed by VNTR typing, using the six VNTR loci originally identified by Frothingham and Meeker-O’Connell.Citation37 The analysis was limited to the exact tandem repeat ETR-A to -D loci, because the ETR-E and -F loci were monomorphic within this data set. The VNTR genotype of a strain, representing the number of repeat elements at each locus, is presented as a series of four integers that ranges between 1 and 12 according to the different number of alleles and separated by hyphens. The variants of an integer were marked by an asterisk (*) as previously suggested.Citation18
Multiplex PCRs were used combining primer pairs of ETR-A/B and ETR-C/D (). The PCR mix was prepared in 96-well plates with the Hot Start Mastermix kit (Qiagen). Five nanograms of DNA were added to a final volume of 20 µL containing 0.4 µM of each primer. For each multiplex mixture, one primer of each oligonucleotide pair was tagged with a different fluorescent dye (). The thermocycler programs for the two multiplex reactions were identical. The PCRs were performed using an initial denaturation of 15 min at 94 °C followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 68 °C for 1 min and extension at 72 °C for 2 min, and with a final extension at 72 °C for 10 min.
Table 4. Primers sequences of VNTRs locus
Statistics
Lesion and histopathological scores were analyzed by the Mann–Whitney test and represented as median ± SEM. Statistical analyses were performed using GraphPad prism 5.03 software (GraphPad Software).
Additional material
Download Zip (627.5 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by National Agency of Research Promotion of Argentina Grant PICT 1114. We thank Dr Soledad Barandiaran for her help in VNTR analysis, Valeria Rocha, Pablo Huertas, and Liliana Rodríguez for their excellent technical help, and Dr Julia Sabio y García for her critical reading of this manuscript. K Caimi and A Cataldi are career members of CONICET, Argentina.
References
- Amanfu W. The situation of tuberculosis and tuberculosis control in animals of economic interest. Tuberculosis (Edinb) 2006; 86:330 - 5; http://dx.doi.org/10.1016/j.tube.2006.01.007; PMID: 16644282
- de Kantor IN, Ritacco V. An update on bovine tuberculosis programmes in Latin American and Caribbean countries. Vet Microbiol 2006; 112:111 - 8; http://dx.doi.org/10.1016/j.vetmic.2005.11.033; PMID: 16310980
- Collins DM, Erasmuson SK, Stephens DM, Yates GF, De Lisle GW. DNA fingerprinting of Mycobacterium bovis strains by restriction fragment analysis and hybridization with insertion elements IS1081 and IS6110. J Clin Microbiol 1993; 31:1143 - 7; PMID: 8099083
- Otal I, Martín C, Vincent-Lévy-Frebault V, Thierry D, Gicquel B. Restriction fragment length polymorphism analysis using IS6110 as an epidemiological marker in tuberculosis. J Clin Microbiol 1991; 29:1252 - 4; PMID: 1677943
- Haddad N, Ostyn A, Karoui C, Masselot M, Thorel MF, Hughes SL, Inwald J, Hewinson RG, Durand B. Spoligotype diversity of Mycobacterium bovis strains isolated in France from 1979 to 2000. J Clin Microbiol 2001; 39:3623 - 32; http://dx.doi.org/10.1128/JCM.39.10.3623-3632.2001; PMID: 11574583
- Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 1997; 35:907 - 14; PMID: 9157152
- Smith NH, Berg S, Dale J, Allen A, Rodriguez S, Romero B, Matos F, Ghebremichael S, Karoui C, Donati C, et al. European 1: a globally important clonal complex of Mycobacterium bovis. Infect Genet Evol 2011; 11:1340 - 51; http://dx.doi.org/10.1016/j.meegid.2011.04.027; PMID: 21571099
- Costello E, O’Grady D, Flynn O, O’Brien R, Rogers M, Quigley F, Egan J, Griffin J. Study of restriction fragment length polymorphism analysis and spoligotyping for epidemiological investigation of Mycobacterium bovis infection. J Clin Microbiol 1999; 37:3217 - 22; PMID: 10488180
- Zumárraga MJ, Martin C, Samper S, Alito A, Latini O, Bigi F, Roxo E, Cicuta ME, Errico F, Ramos MC, et al. Usefulness of spoligotyping in molecular epidemiology of Mycobacterium bovis-related infections in South America. J Clin Microbiol 1999; 37:296 - 303; PMID: 9889207
- Zumárraga MJ, Arriaga C, Barandiaran S, Cobos-Marín L, de Waard J, Estrada-Garcia I, Figueiredo T, Figueroa A, Giménez F, Gomes HM, et al. Understanding the relationship between Mycobacterium bovis spoligotypes from cattle in Latin American countries. Res Vet Sci 2013; 94:9 - 21; http://dx.doi.org/10.1016/j.rvsc.2012.07.012; PMID: 22884173
- Zumárraga MJ, Vivot MM, Marticorena D, Bernardelli A, Fasán R, Iachini R, Cataldi AA. Mycobacterium bovis in Argentina: isolates from cats typified by spoligotyping. Rev Argent Microbiol 2009; 41:215 - 7; PMID: 20085184
- Barandiaran S, Martínez Vivot M, Moras EV, Cataldi AA, Zumárraga MJ. Mycobacterium bovis in Swine: Spoligotyping of Isolates from Argentina. Vet Med Int 2011; 2011:979647; http://dx.doi.org/10.4061/2011/979647; PMID: 21547236
- Etchechoury I, Valencia GE, Morcillo N, Sequeira MD, Imperiale B, López M, Caimi K, Zumárraga MJ, Cataldi A, Romano MI. Molecular typing of Mycobacterium bovis isolates in Argentina: first description of a person-to-person transmission case. Zoonoses Public Health 2010; 57:375 - 81; http://dx.doi.org/10.1111/j.1863-2378.2009.01233.x; PMID: 19912616
- Cousins DV, Williams SN, Dawson DJ. Tuberculosis due to Mycobacterium bovis in the Australian population: DNA typing of isolates, 1970-1994. Int J Tuberc Lung Dis 1999; 3:722 - 31; PMID: 10460106
- Zanini MS, Moreira EC, Salas CE, Lopes MT, Barouni AS, Roxo E, Telles MA, Zumarraga MJ. Molecular typing of Mycobacterium bovis isolates from south-east Brazil by spoligotyping and RFLP. J Vet Med B Infect Dis Vet Public Health 2005; 52:129 - 33; http://dx.doi.org/10.1111/j.1439-0450.2005.00835.x; PMID: 15876225
- Skuce RA, McCorry TP, McCarroll JF, Roring SM, Scott AN, Brittain D, Hughes SL, Hewinson RG, Neill SD. Discrimination of Mycobacterium tuberculosis complex bacteria using novel VNTR-PCR targets. Microbiology 2002; 148:519 - 28; PMID: 11832515
- Roring S, Brittain D, Bunschoten AE, Hughes MS, Skuce RA, van Embden JD, Neill SD. Spacer oligotyping of Mycobacterium bovis isolates compared to typing by restriction fragment length polymorphism using PGRS, DR and IS6110 probes. Vet Microbiol 1998; 61:111 - 20; http://dx.doi.org/10.1016/S0378-1135(98)00178-3; PMID: 9646470
- Inwald J, Hinds J, Palmer S, Dale J, Butcher PD, Hewinson RG, Gordon SV. Genomic analysis of Mycobacterium tuberculosis complex strains used for production of purified protein derivative. J Clin Microbiol 2003; 41:3929 - 32; http://dx.doi.org/10.1128/JCM.41.8.3929-3932.2003; PMID: 12904421
- Domenech P, Reed MB. Rapid and spontaneous loss of phthiocerol dimycocerosate (PDIM) from Mycobacterium tuberculosis grown in vitro: implications for virulence studies. Microbiology 2009; 155:3532 - 43; http://dx.doi.org/10.1099/mic.0.029199-0; PMID: 19661177
- James BW, Williams A, Marsh PD. The physiology and pathogenicity of Mycobacterium tuberculosis grown under controlled conditions in a defined medium. J Appl Microbiol 2000; 88:669 - 77; http://dx.doi.org/10.1046/j.1365-2672.2000.01020.x; PMID: 10792526
- Nicol MP, Wilkinson RJ. The clinical consequences of strain diversity in Mycobacterium tuberculosis. Trans R Soc Trop Med Hyg 2008; 102:955 - 65; http://dx.doi.org/10.1016/j.trstmh.2008.03.025; PMID: 18513773
- Joseph S, Mitchison DA, Ramachandran K, Selkon JB, Subbaiah TV. Virulence in the Guinea-Pig and Sensitivity to Pas and Thiacetazone of Tubercle Bacilli from South Indian Patients with Pulmonary Tuberculosis. Tubercle 1964; 45:354 - 9; http://dx.doi.org/10.1016/S0041-3879(64)80049-0; PMID: 14239317
- Rajashree P, Das SD. Infection with prevalent clinical strains of Mycobacterium tuberculosis leads to differential maturation of monocyte derived dendritic cells. Immunol Lett 2008; 117:174 - 80; http://dx.doi.org/10.1016/j.imlet.2008.01.009; PMID: 18336920
- López B, Aguilar D, Orozco H, Burger M, Espitia C, Ritacco V, Barrera L, Kremer K, Hernandez-Pando R, Huygen K, et al. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol 2003; 133:30 - 7; PMID: 12823275
- Wong KC, Leong WM, Law HK, Ip KF, Lam JT, Yuen KY, Ho PL, Tse WS, Weng XH, Zhang WH, et al. Molecular characterization of clinical isolates of Mycobacterium tuberculosis and their association with phenotypic virulence in human macrophages. Clin Vaccine Immunol 2007; 14:1279 - 84; http://dx.doi.org/10.1128/CVI.00190-07; PMID: 17715326
- Palanisamy GS, Smith EE, Shanley CA, Ordway DJ, Orme IM, Basaraba RJ. Disseminated disease severity as a measure of virulence of Mycobacterium tuberculosis in the guinea pig model. Tuberculosis (Edinb) 2008; 88:295 - 306; http://dx.doi.org/10.1016/j.tube.2007.12.003; PMID: 18321783
- de Jong BC, Hill PC, Aiken A, Awine T, Antonio M, Adetifa IM, Jackson-Sillah DJ, Fox A, Deriemer K, Gagneux S, et al. Progression to active tuberculosis, but not transmission, varies by Mycobacterium tuberculosis lineage in The Gambia. J Infect Dis 2008; 198:1037 - 43; http://dx.doi.org/10.1086/591504; PMID: 18702608
- Chatterjee A, D’Souza D, Vira T, Bamne A, Ambe GT, Nicol MP, Wilkinson RJ, Mistry N. Strains of Mycobacterium tuberculosis from western Maharashtra, India, exhibit a high degree of diversity and strain-specific associations with drug resistance, cavitary disease, and treatment failure. J Clin Microbiol 2010; 48:3593 - 9; http://dx.doi.org/10.1128/JCM.00430-10; PMID: 20720028
- Be NA, Klinkenberg LG, Bishai WR, Karakousis PC, Jain SK. Strain-dependent CNS dissemination in guinea pigs after Mycobacterium tuberculosis aerosol challenge. Tuberculosis (Edinb) 2011; 91:386 - 9; http://dx.doi.org/10.1016/j.tube.2011.07.003; PMID: 21831713
- Faksri K, Drobniewski F, Nikolayevskyy V, Brown T, Prammananan T, Palittapongarnpim P, Prayoonwiwat N, Chaiprasert A. Epidemiological trends and clinical comparisons of Mycobacterium tuberculosis lineages in Thai TB meningitis. Tuberculosis (Edinb) 2011; 91:594 - 600; http://dx.doi.org/10.1016/j.tube.2011.08.005; PMID: 21920820
- Aguilar León D, Zumárraga MJ, Jiménez Oropeza R, Gioffré AK, Bernardelli A, Orozco Estévez H, Cataldi AA, Hernández Pando R. Mycobacterium bovis with different genotypes and from different hosts induce dissimilar immunopathological lesions in a mouse model of tuberculosis. Clin Exp Immunol 2009; 157:139 - 47; http://dx.doi.org/10.1111/j.1365-2249.2009.03923.x; PMID: 19659779
- Buddle BM, Denis M, Aldwell FE, Martin Vordermeier H, Glyn Hewinson R, Neil Wedlock D. Vaccination of cattle with Mycobacterium bovis BCG by a combination of systemic and oral routes. Tuberculosis (Edinb) 2008; 88:595 - 600; http://dx.doi.org/10.1016/j.tube.2008.01.005; PMID: 18439875
- Palmer MV, Waters WR, Thacker TC. Lesion development and immunohistochemical changes in granulomas from cattle experimentally infected with Mycobacterium bovis. Vet Pathol 2007; 44:863 - 74; http://dx.doi.org/10.1354/vp.44-6-863; PMID: 18039899
- Vordermeier HM, Chambers MA, Cockle PJ, Whelan AO, Simmons J, Hewinson RG. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect Immun 2002; 70:3026 - 32; http://dx.doi.org/10.1128/IAI.70.6.3026-3032.2002; PMID: 12010994
- Alizon S, Hurford A, Mideo N, Van Baalen M. Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J Evol Biol 2009; 22:245 - 59; http://dx.doi.org/10.1111/j.1420-9101.2008.01658.x; PMID: 19196383
- Krishnan N, Malaga W, Constant P, Caws M, Tran TH, Salmons J, Nguyen TN, Nguyen DB, Daffé M, Young DB, et al. Mycobacterium tuberculosis lineage influences innate immune response and virulence and is associated with distinct cell envelope lipid profiles. PLoS One 2011; 6:e23870; http://dx.doi.org/10.1371/journal.pone.0023870; PMID: 21931620
- Frothingham R, Meeker-O’Connell WA. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology 1998; 144:1189 - 96; http://dx.doi.org/10.1099/00221287-144-5-1189; PMID: 9611793
- Wangoo A, Johnson L, Gough J, Ackbar R, Inglut S, Hicks D, Spencer Y, Hewinson G, Vordermeier M. Advanced granulomatous lesions in Mycobacterium bovis-infected cattle are associated with increased expression of type I procollagen, gammadelta (WC1+) T cells and CD 68+ cells. J Comp Pathol 2005; 133:223 - 34; http://dx.doi.org/10.1016/j.jcpa.2005.05.001; PMID: 16154140