Abstract
The maintenance of endoplasmic reticulum (ER) homeostasis is critical for numerous aspects of cell physiology. Eukaryotic cells respond to the accumulation of misfolded proteins in the ER (ER stress) by activating the unfolded protein response (UPR), an intracellular signaling pathway that adjusts the folding capacity of the ER. Recent studies of several pathogenic fungi have revealed that the UPR is important for antifungal resistance and virulence; therefore, the pathway has attracted much attention as a potential therapeutic target. While the UPR is highly conserved among eukaryotes, our group recently discovered that the pathogenic yeast Candida glabrata lacks the typical fungal UPR, but possesses alternative mechanisms to cope with ER stress. This review summarizes how C. glabrata responds to ER stress and discusses the impacts of ER quality control systems on antifungal resistance and virulence.
Introduction
The endoplasmic reticulum (ER) is involved in important cellular functions, including the processing of secreted proteins, membrane synthesis, and calcium storage. The vast majority of secretory and transmembrane proteins are folded and assembled in the ER lumen before being delivered to the cell surface or outside the cell.Citation1 Increased demand of protein secretion overloads the ER with unfolded proteins, causing a condition termed ER stress. Unrelieved ER stress has detrimental consequences on eukaryotic cells. For instance, in humans, ER stress has been implicated in the pathology of various diseases, such as metabolic diseases, neurodegenerative disorders, and cancer.Citation2,Citation3
To maintain ER homeostasis, the cells activate a series of adaptive responses that are collectively termed the unfolded protein response (UPR). Many aspects of the UPR are broadly conserved across eukaryotes. The ER-resident transmembrane kinase/endoribonuclease Ire1 senses the accumulation of misfolded proteins in the ER and transmits a signal to the nucleus by splicing the mRNAs encoding basic-leucine zipper (bZIP) transcription factors (Hac1 in yeast, HacA in filamentous fungi, and Xbp1 in humans). After processing and translation of the mRNA, the transcription factor activates the UPR target genes, including those that function in the ER protein-folding machinery and ER-associated degradation.Citation4,Citation5 Recent studies on several pathogenic fungi have unveiled that the UPR plays important roles in fungal virulence and antifungal resistance; therefore, the pathway has been attracting attention as a potential target for antifungal therapy.Citation6-Citation11 While it is believed that the UPR is an evolutionarily conserved mechanism in eukaryotes, we recently discovered unexpected aspects of ER stress response mechanisms in the pathogenic yeast Candida glabrata.Citation12 In this review, we describe the unique evolution of ER quality control systems in C. glabrata and its impact on antifungal resistance and virulence, through comparisons with other pathogenic fungi.
Pathogenicity of C. glabrata
C. glabrata has emerged as an important fungal pathogen in clinical practice, partly because of its decreased susceptibility to azole antifungals such as fluconazole.Citation13,Citation14 A recent study on the epidemiology and outcomes of candidemia in 3648 patients reported that C. glabrata is the second most common cause of candidemia after Candida albicans (26.7% vs. 42.1%), with a lower 90-d post-diagnosis survival rate, compared with C. albicans infection (57.6% vs. 62.1%).Citation15 Echinocandin-class antifungals are the treatment of choice for candidemia with C. glabrata; however, recent surveillance data have revealed an increased number of C. glabrata clinical isolates that display resistance to not only azoles but also echinocandins.Citation16,Citation17
C. glabrata is phylogenetically close to Saccharomyces cerevisiae, yet it has evolved to survive within mammalian hosts. Several specific traits, including adhesion, high drug resistance, and enhanced ability to survive starvation, allow C. glabrata to exist as a human commensal and an opportunistic pathogen.Citation18 Despite having the components of the mating machinery, C. glabrata does not appear to undergo a sexual cycle, and all clinical isolates of C. glabrata identified to date are haploid.Citation19 Therefore, genomic plasticity seems to be important for the evolution of C. glabrata populations.Citation20
Adherence to host surfaces or medical devices is required for both initial colonization and persistence within the host. C. glabrata has gained a unique adhesion mechanism that is mediated largely by the EPA family genes located in subtelomeric regions and regulated by subtelomeric silencing.Citation21,Citation22 Although the C. glabrata genome contains orthologs of most S. cerevisiae genes and the two organisms have similar chromosomal structures in terms of gene order, whole genome sequencing of C. glabrata has revealed an increased rate of loss of genes from their common ancestor.Citation23 Specifically, genes involved in the metabolism of galactose, phosphate, nitrogen, and sulfur have been lost, and such reductive evolutionary processes make C. glabrata rely on its host to overcome critical auxotrophies.Citation18 Some Candida species were found to release hydrolytic enzymes, such as proteinases, phospholipases, lipases, and hemolysins, in infected hosts,Citation24 which facilitates not only the acquisition of nutrients in the host environment but also escape from host defense, allowing the colonization and invasion of Candida cells. For instance, hemolysins play a role in degrading hemoglobin to acquire elemental iron, which is essential for Candida cells to grow in the host. It has been reported that C. glabrata was able to produce hemolysins in vitro;Citation25 however, unlike C. albicans,Citation26 C. glabrata has no known receptors for heme.Citation27 Siderophore-mediated iron acquisition through the sole siderophore-iron transporter Sit1 was shown to be critical for C. glabrata cells to survive macrophage killing,Citation27 indicating that distinct mechanisms of iron acquisition have been developed in C. glabrata. Another important strategy to survive starvation is autophagy, which is a mechanism of intracellular nutrient mobilization by recycling cellular components and even organelles.Citation28,Citation29 To date, while the secretion of hydrolytic enzymes has no known role in C. glabrata virulence, autophagy supports C. glabrata survival during phagocytosis.Citation30
While C. albicans and C. glabrata are ubiquitous commensals and are the two most common pathogenic yeasts of humans, they have different strategies to survive in the hosts. In C. albicans, the morphological flexibility between the yeast and filamentous forms plays an important role in escape from phagocytosis and the penetration into the host tissues; however extensive host cell damage concurrently elicits proinflammatory cytokine responses. On the other hand, C. glabrata does not form hyphae and seems to follow a strategy of stealth and evasion to persist in the hosts without causing any strong immune response.Citation31
Together, these representative studies indicate that C. glabrata has developed distinct strategies to become a successful commensal or pathogen of humans.
ER Stress and Cell Death
Tunicamycin and dithiothreitol are well-known ER-stressing agents that lead to the accumulation of unfolded protein in the ER thorough different mechanisms; tunicamycin inhibits N-linked glycosylation that is important for proper protein folding, and dithiothreitol unfolds ER-localized proteins by interfering with disulfide-bond formation. Cumulative evidence suggests that ER stress induces cell death. For instance, intense or prolonged ER stress induced by tunicamycin leads to the activation of apoptosis pathways in mammalian cells.Citation32,Citation33 However, because apoptotic pathways are not entirely conserved in fungi,Citation34 ER stress-associated cell death is likely to be different. One possibility is that it involves an apoptotic-like process, but it is also possible that ER stress-induced cell death is simply due to the toxic accumulation of unfolded proteins that interfere with cell survival. Recently, Cunningham’s group demonstrated that tunicamycin led to cell death accompanied by vacuolar membrane permeabilization, which requires functional vacuolar H+-ATPase, and that this dying process is not apoptotic in S. cerevisiae.Citation35,Citation36 In contrast, tunicamycin induces apoptosis-like cell death in the fission yeast Schizosaccharomyces pombe.Citation37 To date, the mechanistic features and pathophysiological importance of ER stress-induced cell death in any of the Candida species remain unclear.
ER Stress Response Mechanisms in C. glabrata
Unfolded protein response (UPR) and regulated Ire1-dependent decay (RIDD)
Theoretically, ER-stressed cells must either increase the protein folding capacity of the ER or decrease the load of proteins entering the ER to maintain ER homeostasis. The ER-resident transmembrane stress transducer Ire1, which contains an ER luminal sensing domain and cytosolic protein kinase/endoribonuclease domains, directly interacts with accumulated unfolded proteins, resulting in the oligomerization of its luminal domain and subsequent signal transduction to the cytosolic domains.Citation38,Citation39 The protein kinase domain is required for autophosphorylation and concomitant activation of the nuclease domain that provides the endoribonuclease activity.Citation40-Citation42 Active Ire1 then cleaves the HAC1 mRNA to excise the intron, allowing translation of the bZIP transcription factor Hac1 that subsequently induces transcription of its target genes, including ER chaperones.Citation43,Citation44 This cellular machinery is collectively called the UPR. It is believed that the UPR is an evolutionarily conserved mechanism and plays a central role in ER stress response in eukaryotes.
C. glabrata is highly tolerant to ER stress relative to other fungi, such as S. cerevisiae or Cryptococcus neoformans.Citation12 Intriguingly, our group recently uncovered that the canonical UPR mechanism regulated by Ire1-Hac1 signaling is not conserved in C. glabrata;Citation12 to our surprise, Ire1 was found to be required for the ER stress response independently of Hac1. C. glabrata retains a single ortholog of each of IRE1 and HAC1; however, C. glabrata Ire1 does not cleave mRNAs encoding Hac1 or other bZIP transcription factors identified in the C. glabrata genome, even under severe ER stress conditions. These findings have raised the question as to how Ire1 is involved in the ER stress response in C. glabrata.
In metazoans, ER stress triggers two distinct outputs of the nuclease activity from Ire1, namely XBP1 splicing and regulated Ire1-dependent decay (RIDD).Citation1,Citation45 The former activates the UPR pathway, whereas the latter is an Xbp1-independent pathway that selectively degrades a small subset of ER-associated mRNAs and remodels the repertoire of proteins translated in ER-stressed cells.Citation46,Citation47 Such a cellular response is predicted to reduce the ER load by limiting protein influx and unfolded protein load into the ER lumen. The RIDD pathway, first uncovered in Drosophila melanogasterCitation47 and later confirmed in mammalian cells,Citation46,Citation48 has not yet been fully determined in pathogenic fungi. Our recent studies revealed that C. glabrata has lost the canonical UPR, but instead possesses RIDD that occurs in an Ire1 nuclease-dependent fashion.Citation12 It is thought that RIDD targets are nicked by the Ire1 endoribonuclease at sites that do not display any identifiable consensus sequence, in contrast to Ire1-mediated splicing of HAC1/XBP1 mRNA at highly conserved splice junctions.Citation1 Since excessive RIDD activation seems to be detrimental to cell integrity,Citation48 reducing the load of proteins entering the ER must be balanced with the need to sustain the synthesis of essential proteins. It remains unclear as to how the RIDD activity is regulated in C. glabrata; however, unlike Ire1 in metazoans, C. glabrata Ire1 may not need to switch between specific and nonspecific modes of cleavage.
On the other hand, RIDD does not occur in S. cerevisiae;Citation45,Citation46 the classic Ire1-Hac1 UPR mediates the downregulation of some membrane protein-encoding mRNAs.Citation47,Citation49 Interestingly, a recent study by Kimmig et al.Citation50 revealed that S. pombe lacks both a HAC1 ortholog and a UPR-dependent transcriptional program, but instead relies on RIDD as the sole means of alleviating ER stress. This study also estimated that RIDD may reduce the total protein influx into the ER by only 15%, even under severe ER stress conditions. It is unclear as to how such a minor reduction of bulk protein flux into the ER has a major impact on resolving lethal ER stress. As the authors suggested, one possible explanation is that the folding of RIDD targets may be difficult, thereby resulting in a disproportionate impact on the protein-folding load in the ER.
Other signaling pathways involved in ER stress response in C. glabrata
In C. glabrata, the transcriptional response to ER stress is not mediated by Ire1, but instead is dependent largely on calcineurin signaling and partially on the Slt2 MAPK pathway.Citation12 The phenotypic analyses using single and double C. glabrata mutants of these signaling pathways have revealed that Ire1, calcineurin, and Slt2 function in parallel to cope with ER stress, similar to previous findings in S. cerevisiae.Citation51-Citation53 Calcineurin mediates the calcium cell survival pathway by regulating intracellular Ca2+ homeostasis. While tunicamycin increases Ca2+ uptake by stimulating the high-affinity Ca2+ channel Cch1–Mid1, calcineurin dephosphorylates the Cch1 subunit of the channel to inhibit Ca2+ influx and therefore prevents nonapoptotic cell death in S. cerevisiae.Citation35,Citation51 Crz1 is the only known transcription factor downstream of calcineurin in C. glabrata; however, compared with a calcineurin mutant, the phenotypes of the Δcrz1 mutant were found to be intermediate in terms of the sensitivity to ER stress-inducing agents and echinocandin antifungals, as well as virulence in mice, suggesting that calcineurin has an additional effector in C. glabrata.Citation12,Citation54
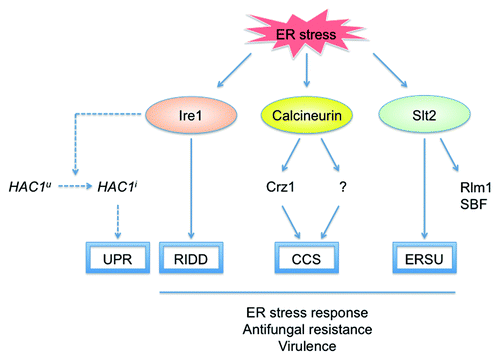
Slt2 plays a role in the ER stress surveillance (ERSU) pathway that ensures the transmission of only functional ER to daughter cells during cell division.Citation55 Upon ER stress, the ERSU pathway delays ER inheritance and cytokinesis to prevent the death of both mother and daughter cells. Indeed, many genes involved in the ribosome activity and cytoplasmic translation are downregulated in a Slt2-dependent manner during the late phase of the ER stress response in C. glabrata, in contrast to RIDD, which is a relatively rapid response.Citation12 Slt2 regulates two downstream transcription factors, Rlm1 and the SBF (Swi4/Swi6) complex. Contrary to the Δslt2 mutant, Δrlm1 and Δswi6 mutants did not display increased susceptibility to either tunicamycin or dithiothreitol (Miyazaki et al., unpublished data), suggesting that Slt2 plays a role in the ER stress response independently of Rlm1 and SBF. The signaling pathways implicated in the ER stress response in C. glabrata are schematically represented in .
Figure 1. Signaling pathways involved in the ER stress response in C. glabrata. The classic UPR pathway mediated by Ire1-Hac1 signaling (dotted lines) is not conserved in C. glabrata. In response to ER stress, Ire1 induces RIDD to degrade ER-localized mRNAs in an Ire1 nuclease-dependent fashion, whereas calcineurin regulates both Crz1-dependent and Crz1-independent pathways. In addition, Slt2 is required for the ER stress response independently of its downstream transcription factors Rlm1 and SBF (Swi4/Swi6). Ire1, calcineurin, and Slt2 have also been implicated indirectly in antifungal resistance and virulence in C. glabrata. HAC1u, uninduced form of HAC1; HAC1i, induced form of HAC1; SBF, Swi4/Swi6 cell cycle box-binding factor; UPR, unfolded protein response; RIDD, regulated Ire1-dependent decay; CCS, calcium cell survival; ERSU, ER stress surveillance.
Diversity in Ire1-Dependent Stress Response Mechanisms between C. glabrata and Other Fungi
The term UPR is originally derived from a cellular response to chemicals that interfere with proper protein folding (e.g., tunicamycin and dithiothreitol); to date, the UPR signaling pathway is known to be involved in various stress responses from yeast to humans.Citation56,Citation57 Indeed, in S. cerevisiae, downstream targets of the UPR include not only ER chaperones but also genes involved in diverse functions, including protein trafficking and quality control, lipid and sterol metabolism, heme biosynthesis, and cell wall biogenesis.Citation58
Importantly, the involvement of the UPR in antifungal resistance has been revealed in several pathogenic fungi. In Aspergillus fumigatus, the loss of HacA led to increased susceptibility to azole antifungals and an echinocandin-class antifungal caspofungin.Citation10 Increased susceptibilities to azoles and caspofungin have also been reported in C. neoformans Δire1 and C. albicans Δhac1 mutants.Citation6,Citation7 There seems to be no role for IRE1 or HAC1 in susceptibility of C. glabrata to echinocandins.Citation12 Concerning azoles, the deletion of IRE1 increases the susceptibility of calcineurin mutants, but not of wild-type cells. This possibly suggests redundancy of azole stress signaling or, alternatively, that azoles induce a more severe stress in calcineurin-defective C. glabrata mutants, and that Ire1 function is required to survive this severe stress.
Previous studies on several yeasts and filamentous fungi have demonstrated that the UPR plays a role in maintaining cell wall integrity and that mutants lacking Ire1 or Hac1 exhibited decreased tolerance to a variety of cell wall-perturbing agents.Citation6-Citation8,Citation10,Citation11,Citation59 Tunicamycin also induces cell wall stress, which may provide an explanation for why certain genes implicated in cell wall biogenesis were found to be upregulated during the UPR in tunicamycin-treated S. cerevisiae and C. albicans cells.Citation11,Citation58,Citation60 In contrast, the loss of Ire1 alone in C. glabrata did not confer a cell wall-defective phenotype, and the upregulation of some cell wall-related genes upon tunicamycin exposure was mainly dependent on calcineurin, but not Ire1.Citation12 In addition, the C. glabrata Δire1 mutant, unlike the A. fumigatus ΔireA mutant, was not sensitive to low-oxygen and low-iron conditions.Citation8,Citation12 Together, these findings provide evidence for diversities in Ire1-dependent stress response mechanisms between C. glabrata and other fungal species.
Impact of the ER Stress Response on C. glabrata Virulence
The importance of the UPR for the virulence of pathogenic fungi, including C. neoformans and A. fumigatus, is reviewed in this issue. Briefly, C. neoformans mutants lacking Ire1 and its downstream bZIP transcription factor encoded by HXL1 (HAC1 and XBP1-Like gene 1) are unable to grow at 37 °C and are avirulent in a mouse model of systemic cryptococcosis.Citation7 In A. fumigatus, attenuated virulence of the ΔhacA mutant has been demonstrated using murine models of invasive aspergillosis.Citation10 These observations may be explained by the fact that the UPR plays a role in the expression of several virulence-related traits, including protein secretion, rapid apical growth, and maintenance of both membrane and cell wall homeostasis.Citation61 Interestingly, the A. fumigatus ΔireA mutant is avirulent, in contrast to the partially attenuated ΔhacA mutant, indicating that Ire1 has hacA-independent functions that are important for the virulence of A. fumigatus. Consistently, several phenotypic differences have been observed between these mutants. For example, the ΔireA mutant is more sensitive to elevated growth temperature, hypoxia, and nutritional limitation such as iron starvation, all of which are conditions commonly encountered by pathogenic fungi in infected hosts.Citation8 In C. albicans, while the role of the UPR in the pathogenicity has not yet been directly investigated in vivo, Hac1 is known to be required for hyphal development, which is an important factor for biofilm formation and virulence.Citation11
In C. glabrata, Hac1 is dispensable for both the ER stress response and virulence, whereas loss of Ire1 resulted in hypovirulence in both immunocompromised and immunocompetent mice.Citation12 The phenotype of the C. glabrata Δire1 mutant is confined almost exclusively to impaired ER stress responses, in contrast to UPR-defective mutants of other fungi where the UPR is involved in various stress responses. These findings imply that C. glabrata cells experience ER stress during the process of infection. Inhibition of either calcineurin or Slt2 also resulted in attenuated virulence in C. glabrata,Citation54,Citation62 which may be attributed to multifactorial causes, since these signaling pathways have various physiological functions, including the maintenance of intracellular pH and cell wall homeostasis. However, it is very likely that attenuated virulence of the calcineurin and Slt2 mutants was caused at least in part by impaired ER homeostasis.
Concluding Remarks and Future Perspectives
While the basic aspects of the UPR are highly conserved throughout eukaryotes, diversities in the transcriptional induction system in response to ER stress have also been developed during evolution.Citation50,Citation63 For example, the Ire1–Hac1 signaling pathway is required for the upregulation of the ER-resident chaperone Kar2 in S. cerevisiae, whereas the pathway is dispensable for the induction of the Kar2 homolog GRP78/BiP in mammalian cells.Citation43,Citation64 In C. glabrata, the majority of ER stress-induced genes, including KAR2, are dependent on the calcineurin–Crz1 pathway, but not Ire1 signaling.Citation12 Although Ire1 seems to play a limited role in environmental stress responses, it is required for C. glabrata virulence. To our surprise, C. glabrata, unlike S. cerevisiae, lacks the canonical Ire1–Hac1 UPR and possesses a metazoan-like RIDD mechanism. Future studies should aim to focus on understanding how and why this unique property has been developed in C. glabrata, as well as which virulence trait is affected by such an evolutionary process. It is important to seek for a potential interaction between Ire1 and other signaling pathways, since whether the Ire1 kinase domain has another effector other than Ire1 itself remains a major unsolved question in this field. In addition, given the importance of the ER stress response to C. glabrata virulence, future targeting of Ire1 in conjunction with calcineurin might be a promising approach to improve the treatment options for C. glabrata infections.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Grant-in-Aid for Scientific Research (24791027 to T.M. and 21390305 to S.K.) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
References
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011; 334:1081 - 6; http://dx.doi.org/10.1126/science.1209038; PMID: 22116877
- Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest 2002; 110:1389 - 98; PMID: 12438434
- Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol 2012; 197:857 - 67; http://dx.doi.org/10.1083/jcb.201110131; PMID: 22733998
- Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol 2006; 22:487 - 508; http://dx.doi.org/10.1146/annurev.cellbio.21.122303.120200; PMID: 16822172
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8:519 - 29; http://dx.doi.org/10.1038/nrm2199; PMID: 17565364
- Blankenship JR, Fanning S, Hamaker JJ, Mitchell AP. An extensive circuitry for cell wall regulation in Candida albicans.. PLoS Pathog 2010; 6:e1000752; http://dx.doi.org/10.1371/journal.ppat.1000752; PMID: 20140194
- Cheon SA, Jung KW, Chen YL, Heitman J, Bahn YS, Kang HA. Unique evolution of the UPR pathway with a novel bZIP transcription factor, Hxl1, for controlling pathogenicity of Cryptococcus neoformans.. PLoS Pathog 2011; 7:e1002177; http://dx.doi.org/10.1371/journal.ppat.1002177; PMID: 21852949
- Feng X, Krishnan K, Richie DL, Aimanianda V, Hartl L, Grahl N, Powers-Fletcher MV, Zhang M, Fuller KK, Nierman WC, et al. HacA-independent functions of the ER stress sensor IreA synergize with the canonical UPR to influence virulence traits in Aspergillus fumigatus.. PLoS Pathog 2011; 7:e1002330; http://dx.doi.org/10.1371/journal.ppat.1002330; PMID: 22028661
- Richie DL, Feng X, Hartl L, Aimanianda V, Krishnan K, Powers-Fletcher MV, Watson DS, Galande AK, White SM, Willett T, et al. The virulence of the opportunistic fungal pathogen Aspergillus fumigatus requires cooperation between the endoplasmic reticulum-associated degradation pathway (ERAD) and the unfolded protein response (UPR). Virulence 2011; 2:12 - 21; http://dx.doi.org/10.4161/viru.2.1.13345; PMID: 21217201
- Richie DL, Hartl L, Aimanianda V, Winters MS, Fuller KK, Miley MD, White S, McCarthy JW, Latgé JP, Feldmesser M, et al. A role for the unfolded protein response (UPR) in virulence and antifungal susceptibility in Aspergillus fumigatus.. PLoS Pathog 2009; 5:e1000258; http://dx.doi.org/10.1371/journal.ppat.1000258; PMID: 19132084
- Wimalasena TT, Enjalbert B, Guillemette T, Plumridge A, Budge S, Yin Z, Brown AJ, Archer DB. Impact of the unfolded protein response upon genome-wide expression patterns, and the role of Hac1 in the polarized growth, of Candida albicans.. Fungal Genet Biol 2008; 45:1235 - 47; http://dx.doi.org/10.1016/j.fgb.2008.06.001; PMID: 18602013
- Miyazaki T, Nakayama H, Nagayoshi Y, Kakeya H, Kohno S. Dissection of Ire1 functions reveals stress response mechanisms uniquely evolved in Candida glabrata.. PLoS Pathog 2013; 9:e1003160; http://dx.doi.org/10.1371/journal.ppat.1003160; PMID: 23382685
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20:133 - 63; http://dx.doi.org/10.1128/CMR.00029-06; PMID: 17223626
- Li L, Redding S, Dongari-Bagtzoglou A. Candida glabrata: an emerging oral opportunistic pathogen. J Dent Res 2007; 86:204 - 15; http://dx.doi.org/10.1177/154405910708600304; PMID: 17314251
- Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, Horn D. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance®) registry, 2004-2008. Diagn Microbiol Infect Dis 2012; 74:323 - 31; http://dx.doi.org/10.1016/j.diagmicrobio.2012.10.003; PMID: 23102556
- Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 2012; 125:Suppl S3 - 13; http://dx.doi.org/10.1016/j.amjmed.2011.11.001; PMID: 22196207
- Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. Echinocandin and triazole antifungal susceptibility profiles for Candida spp., Cryptococcus neoformans, and Aspergillus fumigatus: application of new CLSI clinical breakpoints and epidemiologic cutoff values to characterize resistance in the SENTRY Antimicrobial Surveillance Program (2009). Diagn Microbiol Infect Dis 2011; 69:45 - 50; http://dx.doi.org/10.1016/j.diagmicrobio.2010.08.013; PMID: 21146713
- Kaur R, Domergue R, Zupancic ML, Cormack BP. A yeast by any other name: Candida glabrata and its interaction with the host. Curr Opin Microbiol 2005; 8:378 - 84; http://dx.doi.org/10.1016/j.mib.2005.06.012; PMID: 15996895
- Wong S, Fares MA, Zimmermann W, Butler G, Wolfe KH. Evidence from comparative genomics for a complete sexual cycle in the ‘asexual’ pathogenic yeast Candida glabrata.. Genome Biol 2003; 4:R10; http://dx.doi.org/10.1186/gb-2003-4-2-r10; PMID: 12620120
- Roetzer A, Gabaldón T, Schüller C. From Saccharomyces cerevisiae to Candida glabratain a few easy steps: important adaptations for an opportunistic pathogen. FEMS Microbiol Lett 2011; 314:1 - 9; http://dx.doi.org/10.1111/j.1574-6968.2010.02102.x; PMID: 20846362
- Castaño I, Pan SJ, Zupancic M, Hennequin C, Dujon B, Cormack BP. Telomere length control and transcriptional regulation of subtelomeric adhesins in Candida glabrata.. Mol Microbiol 2005; 55:1246 - 58; http://dx.doi.org/10.1111/j.1365-2958.2004.04465.x; PMID: 15686568
- Domergue R, Castaño I, De Las Peñas A, Zupancic M, Lockatell V, Hebel JR, Johnson D, Cormack BP. Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science 2005; 308:866 - 70; http://dx.doi.org/10.1126/science.1108640; PMID: 15774723
- Dujon B, Sherman D, Fischer G, Durrens P, Casaregola S, Lafontaine I, De Montigny J, Marck C, Neuvéglise C, Talla E, et al. Genome evolution in yeasts. Nature 2004; 430:35 - 44; http://dx.doi.org/10.1038/nature02579; PMID: 15229592
- Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev 2012; 36:288 - 305; http://dx.doi.org/10.1111/j.1574-6976.2011.00278.x; PMID: 21569057
- Luo G, Samaranayake LP, Yau JY. Candida species exhibit differential in vitro hemolytic activities. J Clin Microbiol 2001; 39:2971 - 4; http://dx.doi.org/10.1128/JCM.39.8.2971-2974.2001; PMID: 11474025
- Weissman Z, Shemer R, Conibear E, Kornitzer D. An endocytic mechanism for haemoglobin-iron acquisition in Candida albicans.. Mol Microbiol 2008; 69:201 - 17; http://dx.doi.org/10.1111/j.1365-2958.2008.06277.x; PMID: 18466294
- Nevitt T, Thiele DJ. Host iron withholding demands siderophore utilization for Candida glabrata to survive macrophage killing. PLoS Pathog 2011; 7:e1001322; http://dx.doi.org/10.1371/journal.ppat.1001322; PMID: 21445236
- Klionsky DJ. Autophagy. Curr Biol 2005; 15:R282 - 3; http://dx.doi.org/10.1016/j.cub.2005.04.013; PMID: 15854889
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol 2007; 9:1102 - 9; http://dx.doi.org/10.1038/ncb1007-1102; PMID: 17909521
- Roetzer A, Gratz N, Kovarik P, Schüller C. Autophagy supports Candida glabrata survival during phagocytosis. Cell Microbiol 2010; 12:199 - 216; http://dx.doi.org/10.1111/j.1462-5822.2009.01391.x; PMID: 19811500
- Brunke S, Hube B. Two unlike cousins: Candida albicans and C. glabrata infection strategies. Cell Microbiol 2013; 15:701 - 8; http://dx.doi.org/10.1111/cmi.12091; PMID: 23253282
- Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ 2006; 13:363 - 73; http://dx.doi.org/10.1038/sj.cdd.4401817; PMID: 16397583
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 2006; 7:880 - 5; http://dx.doi.org/10.1038/sj.embor.7400779; PMID: 16953201
- Sharon A, Finkelstein A, Shlezinger N, Hatam I. Fungal apoptosis: function, genes and gene function. FEMS Microbiol Rev 2009; 33:833 - 54; http://dx.doi.org/10.1111/j.1574-6976.2009.00180.x; PMID: 19416362
- Dudgeon DD, Zhang N, Ositelu OO, Kim H, Cunningham KW. Nonapoptotic death of Saccharomyces cerevisiae cells that is stimulated by Hsp90 and inhibited by calcineurin and Cmk2 in response to endoplasmic reticulum stresses. Eukaryot Cell 2008; 7:2037 - 51; http://dx.doi.org/10.1128/EC.00291-08; PMID: 18806210
- Kim H, Kim A, Cunningham KW. Vacuolar H+-ATPase (V-ATPase) promotes vacuolar membrane permeabilization and nonapoptotic death in stressed yeast. J Biol Chem 2012; 287:19029 - 39; http://dx.doi.org/10.1074/jbc.M112.363390; PMID: 22511765
- Guérin R, Arseneault G, Dumont S, Rokeach LA. Calnexin is involved in apoptosis induced by endoplasmic reticulum stress in the fission yeast. Mol Biol Cell 2008; 19:4404 - 20; http://dx.doi.org/10.1091/mbc.E08-02-0188; PMID: 18701708
- Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 1993; 73:1197 - 206; http://dx.doi.org/10.1016/0092-8674(93)90648-A; PMID: 8513503
- Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 1993; 74:743 - 56; http://dx.doi.org/10.1016/0092-8674(93)90521-Q; PMID: 8358794
- Papa FR, Zhang C, Shokat K, Walter P. Bypassing a kinase activity with an ATP-competitive drug. Science 2003; 302:1533 - 7; http://dx.doi.org/10.1126/science.1090031; PMID: 14564015
- Shamu CE, Walter P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J 1996; 15:3028 - 39; PMID: 8670804
- Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 1997; 90:1031 - 9; http://dx.doi.org/10.1016/S0092-8674(00)80369-4; PMID: 9323131
- Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 1996; 87:391 - 404; http://dx.doi.org/10.1016/S0092-8674(00)81360-4; PMID: 8898193
- Mori K, Kawahara T, Yoshida H, Yanagi H, Yura T. Signalling from endoplasmic reticulum to nucleus: transcription factor with a basic-leucine zipper motif is required for the unfolded protein-response pathway. Genes Cells 1996; 1:803 - 17; http://dx.doi.org/10.1046/j.1365-2443.1996.d01-274.x; PMID: 9077435
- Pavitt GD, Ron D. New insights into translational regulation in the endoplasmic reticulum unfolded protein response. Cold Spring Harb Perspect Biol 2012; 4; http://dx.doi.org/10.1101/cshperspect.a012278; PMID: 22535228
- Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 2009; 186:323 - 31; http://dx.doi.org/10.1083/jcb.200903014; PMID: 19651891
- Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 2006; 313:104 - 7; http://dx.doi.org/10.1126/science.1129631; PMID: 16825573
- Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 2009; 138:562 - 75; http://dx.doi.org/10.1016/j.cell.2009.07.017; PMID: 19665977
- Kimata Y, Ishiwata-Kimata Y, Yamada S, Kohno K. Yeast unfolded protein response pathway regulates expression of genes for anti-oxidative stress and for cell surface proteins. Genes Cells 2006; 11:59 - 69; http://dx.doi.org/10.1111/j.1365-2443.2005.00921.x; PMID: 16371132
- Kimmig P, Diaz M, Zheng J, Williams CC, Lang A, Aragón T, Li H, Walter P. The unfolded protein response in fission yeast modulates stability of select mRNAs to maintain protein homeostasis. Elife 2012; 1:e00048; http://dx.doi.org/10.7554/eLife.00048; PMID: 23066505
- Bonilla M, Cunningham KW. Mitogen-activated protein kinase stimulation of Ca(2+) signaling is required for survival of endoplasmic reticulum stress in yeast. Mol Biol Cell 2003; 14:4296 - 305; http://dx.doi.org/10.1091/mbc.E03-02-0113; PMID: 14517337
- Bonilla M, Nastase KK, Cunningham KW. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J 2002; 21:2343 - 53; http://dx.doi.org/10.1093/emboj/21.10.2343; PMID: 12006487
- Chen Y, Feldman DE, Deng C, Brown JA, De Giacomo AF, Gaw AF, Shi G, Le QT, Brown JM, Koong AC. Identification of mitogen-activated protein kinase signaling pathways that confer resistance to endoplasmic reticulum stress in Saccharomyces cerevisiae.. Mol Cancer Res 2005; 3:669 - 77; http://dx.doi.org/10.1158/1541-7786.MCR-05-0181; PMID: 16380504
- Miyazaki T, Yamauchi S, Inamine T, Nagayoshi Y, Saijo T, Izumikawa K, Seki M, Kakeya H, Yamamoto Y, Yanagihara K, et al. Roles of calcineurin and Crz1 in antifungal susceptibility and virulence of Candida glabrata.. Antimicrob Agents Chemother 2010; 54:1639 - 43; http://dx.doi.org/10.1128/AAC.01364-09; PMID: 20100876
- Babour A, Bicknell AA, Tourtellotte J, Niwa M. A surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance. Cell 2010; 142:256 - 69; http://dx.doi.org/10.1016/j.cell.2010.06.006; PMID: 20619447
- Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol 2010; 189:783 - 94; http://dx.doi.org/10.1083/jcb.201003138; PMID: 20513765
- Thibault G, Ismail N, Ng DT. The unfolded protein response supports cellular robustness as a broad-spectrum compensatory pathway. Proc Natl Acad Sci U S A 2011; 108:20597 - 602; http://dx.doi.org/10.1073/pnas.1117184109; PMID: 22143797
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 2000; 101:249 - 58; http://dx.doi.org/10.1016/S0092-8674(00)80835-1; PMID: 10847680
- Scrimale T, Didone L, de Mesy Bentley KL, Krysan DJ. The unfolded protein response is induced by the cell wall integrity mitogen-activated protein kinase signaling cascade and is required for cell wall integrity in Saccharomyces cerevisiae.. Mol Biol Cell 2009; 20:164 - 75; http://dx.doi.org/10.1091/mbc.E08-08-0809; PMID: 18971375
- Torres-Quiroz F, García-Marqués S, Coria R, Randez-Gil F, Prieto JA. The activity of yeast Hog1 MAPK is required during endoplasmic reticulum stress induced by tunicamycin exposure. J Biol Chem 2010; 285:20088 - 96; http://dx.doi.org/10.1074/jbc.M109.063578; PMID: 20430884
- Richie DL, Feng X, Krishnan K, Askew DS. Secretion stress and antifungal resistance: an Achilles’ heel of Aspergillus fumigatus?. Med Mycol 2011; 49:Suppl 1 S101 - 6; http://dx.doi.org/10.3109/13693786.2010.497504; PMID: 20608779
- Miyazaki T, Inamine T, Yamauchi S, Nagayoshi Y, Saijo T, Izumikawa K, Seki M, Kakeya H, Yamamoto Y, Yanagihara K, et al. Role of the Slt2 mitogen-activated protein kinase pathway in cell wall integrity and virulence in Candida glabrata.. FEMS Yeast Res 2010; 10:343 - 52; http://dx.doi.org/10.1111/j.1567-1364.2010.00611.x; PMID: 20214686
- Mori K. Signalling pathways in the unfolded protein response: development from yeast to mammals. J Biochem 2009; 146:743 - 50; http://dx.doi.org/10.1093/jb/mvp166; PMID: 19861400
- Oda Y, Okada T, Yoshida H, Kaufman RJ, Nagata K, Mori K. Derlin-2 and Derlin-3 are regulated by the mammalian unfolded protein response and are required for ER-associated degradation. J Cell Biol 2006; 172:383 - 93; http://dx.doi.org/10.1083/jcb.200507057; PMID: 16449189
