There is controversy about the usefulness of granulocyte transfusions (GTX) for the treatment of invasive pulmonary aspergillosis (IPA). Moreover, this strategy is associated with frequently severe respiratory complications. As antifungal combinations with a cell wall acting echinocandins is common scenario for treatment of breakthrough IPA to Aspergillus-active azole prophylaxis, we hypothesize that respiratory complications following GTX would be expected to be increasingly frequent. Echinocandins uncover immunogenic epitopes in Aspergillus cell wall, and influx of neutrophils into tissue harboring echinocandin-exposed Aspergillus could result in diffuse inflammation. If this hypothesis is correct, immune reconstitution inflammatory syndrome (IRIS) associated with neutrophil recovery should be also more common in cases of IPA treated with echinocandins compared with other agents (triazoles, liposomal amphoteric B products) that do not induce immune activation of damaged Aspergillus.
In the treatment of profoundly neutropenic patients with severe bacterial or fungal infections, few areas have generated as much controversy as the use of granulocyte transfusions (GTX).Citation1 This strategy was conceived following the success of platelet transfusions for reducing bleeding complications in severely thrombocytopenic cancer patients in the early 1960s.Citation2 However, despite its theoretical appeal, conclusive evidence of a benefit with adjunctive GTX in the treatment of invasive fungal infections, particularly for invasive molds such as aspergillosis,Citation1 remain limited in the current era of improved diagnostics and therapeutic options.Citation3 Even contemporary trials that utilized improved technologies for WBC procurement, storage, and mobilization (i.e., G-CSF elicited) and higher granulocyte doses have failed to show a survival benefit.Citation4
GTX transfusions can unequivocally improve neutrophil counts, but a magic ANC dosing threshold associated with improved clinical response to antifungal therapy remains elusive.Citation1 Furthermore, concerns about various adverse reactions persist. Perhaps the most worrisome is the issue of severe pulmonary complications following GTX. The frequency of such complication has ranged broadly from 10%Citation5 to 53%Citation6 in recent studies. The wide range in reported pulmonary events can be explained, in part, by different GTX administration practices (frequency, dose, pre-medications), how pulmonary complications were diagnosed or documented (always difficult retrospectively), and differences in indication for GTX.Citation1,Citation5,Citation6 For example, compared with febrile neutropenia population as a whole, patients with established IPA are often present at later stages of relapsing underlying malignancy, frequently have received more transfusions of blood products, thereby increasing the risk for alloimmunization and volume overload. Also, these patients could have a greater probability of undiagnosed pulmonary co-infections that “flare” with the GTX.
We would like to offer another possible explanation on pulmonary complications remain problematic with GTX. In preclinical experiments, exposure of Aspergillus to echinocandins alters the fungal cell wall and uncovers immunogenic epitopes, resulting in increased inflammatory responses.Citation7-Citation10 If this is also true in patients, influx of neutrophils into tissue harboring echinocandin-exposed Aspergillus could theoretically result in diffuse inflammation and tissue damage () and severe bleeding from necrotizing IRIS reactions surrounding proximal pulmonary vessels. Although there is no experimental data, there is little theoretical reason to believe that differences exist among the echinocandins in regards to increased pro-inflammatory responses in the setting of neutrophil influx in fungal lung lesions. Because combination therapy with echinocandins and liposomal amphotericin B are frequently considered in severe cases of aspergillosis that have failed triazole prophylaxis, the combination of two “inflammation” eliciting drugs may intensify this phenomenon in select patients. Amphotericin B, directly activate immune cells through microbial pattern recognition receptors (Toll-like receptor 2, CD14), causing the release of proinflammatory cytokines (TNF-α, IL-6, IL-1Ra, IL-1β), chemokines (IL-8, MCP-1, MIP-1β), nitric oxide, and prostaglandins.Citation11 Older studies have suggested that co-administration of amphotericin B–deoxycholate with GTX results in high rates of severe pulmonary reactions.Citation12 This phenomenon has not been replicated nor observed as frequently, however, with the lipid amphotericin B formulations, perhaps due to immunomodulatory properties of lipid formulations of amphotericin B.Citation13 In addition, antifungal-induced immune activation of damaged Aspergillus could potentiate the vasculopathy, endothelial, and platelet activation associated with fungal invasion of the lungsCitation14-Citation16 ().
Figure 1. A depiction of the mechanism of lung injury in the setting of GTX in genetically predisposed patients with established breakthrough aspergillosis treated with cell-wall damaging agent or IRIS.
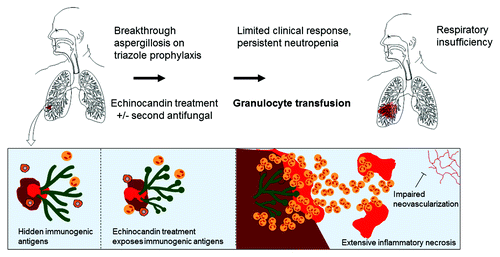
The recently completed phase III NHLBI-sponsored randomized controlled study (RING trial, NCT00627393) hopefully will address some of the controversies of the impact of GTX in established pulmonary aspergillosis in neutropenic patients to make some meaningful comparisons. Should that be the case, we would like to suggest that carefully factoring the influence of prior or concomitant antifungals, particularly echinocandin therapy would be important for the analysis. This information could complement the careful plan to evaluate alloimmunization (anti-HLA antibodies) and other GTX-related serious adverse effects. Finally, the “outliers” with severe lung reactions secondary to GTX in the setting of an established lung infection could have an immunogenetic component for their susceptibility. Emerging research suggests a tissue specificity of organ damage and clearance of fungi and other pathogens can be influenced by genetic lesions in specific chemokines or cytokines.Citation17,Citation18 We believe that a tissue (blood, bronchoalveolar lavage) registry along with compilation of well documented cases of severe GTX reactions in the setting of pulmonary aspergillosis in the context of different antifungal treatments, especially the echinocandins, would be of interest. A carefully conducted comparative study on whether the IRIS associated with neutrophil recoveryCitation19 is more common in cases of pulmonary aspergillosis treated with echinocandins compared with other agents (triazoles, liposomal amphoteric B products) could be of interest and strengthen the current hypothesis (). Finally, our hypothesis should be testable experimentally in the mouse model of invasive pulmonary aspergillosis. Specifically, one could compare (survival, fungal burden, immune profiling in serum and BAL, immunopathology) in neutropenic mice infected with Aspergillus that were given subtherapeutic doses of an echinocandin vs. subtherapeutic doses of a triazole (as a control) vs. no treatment, both during neutropenia and at the time of neutrophil recovery.
Disclosure of Potential Conflicts of Interest
D.P.K. acknowledges the Frances King Black Endowed Professorship. D.P.K. serves on the advisory board of Merck and received research support from Astellas and Merck and honoraria from Gilead.
Acknowledgments
I thank Russel E Lewis, PharmD for assistance with the figure.
References
- Marfin AA, Price TH. Granulocyte Transfusion Therapy. J Intensive Care Med 2013; Forthcoming http://dx.doi.org/10.1177/0885066613498045; PMID: 23920161
- Freireich EJ. Leukocyte transfusion and the development of the continuous-flow blood cell separator. Transfus Med Rev 2011; 25:344 - 50; http://dx.doi.org/10.1016/j.tmrv.2011.04.004; PMID: 21632206
- Wingard JR, Ribaud P, Schlamm HT, Herbrecht R. Changes in causes of death over time after treatment for invasive aspergillosis. Cancer 2008; 112:2309 - 12; http://dx.doi.org/10.1002/cncr.23441; PMID: 18338758
- Seidel MG, Peters C, Wacker A, Northoff H, Moog R, Boehme A, Silling G, Grimminger W, Einsele H. Randomized phase III study of granulocyte transfusions in neutropenic patients. Bone Marrow Transplant 2008; 42:679 - 84; http://dx.doi.org/10.1038/bmt.2008.237; PMID: 18695660
- Kim KH, Lim HJ, Kim JS, Kim BS, Bang SM, Kim I, Han KS, Kim BK, Lee SM, Yoon SS. Therapeutic granulocyte transfusions for the treatment of febrile neutropenia in patients with hematologic diseases: a 10-year experience at a single institute. Cytotherapy 2011; 13:490 - 8; http://dx.doi.org/10.3109/14653249.2010.529889; PMID: 21090917
- Raad II, Chaftari AM, Al Shuaibi MM, Jiang Y, Shomali W, Cortes JE, Lichtiger B, Hachem RY. Granulocyte transfusions in hematologic malignancy patients with invasive pulmonary aspergillosis: outcomes and complications. Ann Oncol 2013; 24:1873 - 9; http://dx.doi.org/10.1093/annonc/mdt110; PMID: 23519997
- Hohl TM, Feldmesser M, Perlin DS, Pamer EG. Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal beta-glucan exposure. J Infect Dis 2008; 198:176 - 85; http://dx.doi.org/10.1086/589304; PMID: 18500928
- Lamaris GA, Lewis RE, Chamilos G, May GS, Safdar A, Walsh TJ, Raad II, Kontoyiannis DP. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J Infect Dis 2008; 198:186 - 92; http://dx.doi.org/10.1086/589305; PMID: 18500936
- Lewis RE, Liao G, Young K, Douglas C, Kontoyiannis DP. Macrophage Reporter Cell Assay for Screening Immunopharmacological Activity of Cell-Wall Active Antifungals. Antimicrob Agents Chemother 2014; Forthcoming http://dx.doi.org/10.1128/AAC.02408-13; PMID: 24395226
- Ben-Ami R, Lewis RE, Kontoyiannis DP. Immunocompromised hosts: immunopharmacology of modern antifungals. Clin Infect Dis 2008; 47:226 - 35; http://dx.doi.org/10.1086/589290; PMID: 18540822
- Sau K, Mambula SS, Latz E, Henneke P, Golenbock DT, Levitz SM. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J Biol Chem 2003; 278:37561 - 8; http://dx.doi.org/10.1074/jbc.M306137200; PMID: 12860979
- Wright DG, Robichaud KJ, Pizzo PA, Deisseroth AB. Lethal pulmonary reactions associated with the combined use of amphotericin B and leukocyte transfusions. N Engl J Med 1981; 304:1185 - 9; http://dx.doi.org/10.1056/NEJM198105143042001; PMID: 7219459
- Lewis RE, Chamilos G, Prince RA, Kontoyiannis DP. Pretreatment with empty liposomes attenuates the immunopathology of invasive pulmonary aspergillosis in corticosteroid-immunosuppressed mice. Antimicrob Agents Chemother 2007; 51:1078 - 81; http://dx.doi.org/10.1128/AAC.01268-06; PMID: 17194825
- Kamai Y, Lossinsky AS, Liu H, Sheppard DC, Filler SG. Polarized response of endothelial cells to invasion by Aspergillus fumigatus. Cell Microbiol 2009; 11:170 - 82; http://dx.doi.org/10.1111/j.1462-5822.2008.01247.x; PMID: 19016788
- Kontoyiannis DP. Manipulation of host angioneogenesis: A critical link for understanding the pathogenesis of invasive mold infections?. Virulence 2010; 1:192 - 6; http://dx.doi.org/10.4161/viru.1.3.11380; PMID: 21178441
- Speth C, Hagleitner M, Ott HW, Würzner R, Lass-Flörl C, Rambach G. Aspergillus fumigatus activates thrombocytes by secretion of soluble compounds. J Infect Dis 2013; 207:823 - 33; http://dx.doi.org/10.1093/infdis/jis743; PMID: 23225903
- Lionakis MS, Fischer BG, Lim JK, Swamydas M, Wan W, Richard Lee CC, Cohen JI, Scheinberg P, Gao JL, Murphy PM. Chemokine receptor Ccr1 drives neutrophil-mediated kidney immunopathology and mortality in invasive candidiasis. PLoS Pathog 2012; 8:e1002865; http://dx.doi.org/10.1371/journal.ppat.1002865; PMID: 22916017
- Nouailles G, Dorhoi A, Koch M, Zerrahn J, Weiner J 3rd, Faé KC, Arrey F, Kuhlmann S, Bandermann S, Loewe D, et al. CXCL5-secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis. J Clin Invest 2014; Forthcoming http://dx.doi.org/10.1172/JCI72030; PMID: 24509076
- Miceli MH, Maertens J, Buvé K, Grazziutti M, Woods G, Rahman M, Barlogie B, Anaissie EJ. Immune reconstitution inflammatory syndrome in cancer patients with pulmonary aspergillosis recovering from neutropenia: Proof of principle, description, and clinical and research implications. Cancer 2007; 110:112 - 20; http://dx.doi.org/10.1002/cncr.22738; PMID: 17525971
