Abstract
Parents invest in their offspring by preparing them for defense against pathogens and parasites that only the parents have encountered, a phenomenon known as trans-generational immune priming. We investigated the underlying mechanism using the established lepidopteran model host Galleria mellonella. When larvae were fed with non-pathogenic bacteria, or the entomopathogenic species Pseudomonas entomophila and Serratia entomophila, the activity of lysozyme and phenoloxidase increased in the hemolymph, and immunity-related genes encoding antibacterial proteins such as gloverin were induced. Remarkably, the ingestion of bacteria by female larvae resulted in the differential expression of immunity-related genes in the eggs subsequently laid by the same females, providing evidence for trans-generational immune priming in G. mellonella. To determine the fate of these ingested microbes, the larval diet was supplemented with bacteria carrying a fluorescent label. We observed these bacteria crossing the midgut epithelium, their entrapment within nodules in the hemocoel, their accumulation within the ovary, and ultimately their deposition in the eggs. Therefore, we propose that trans-generational immune priming in Lepidoptera can be mediated by the maternal transfer of bacteria or bacterial fragments to the developing eggs.
Introduction
Insects can mount a more efficient immune response when exposed to a pathogen for the second time because the initial contact results in immune priming, although this varies in its specificity.Citation1-Citation3 Parents invest in their offspring by preparing them for defense against pathogens encountered only by the parent, a phenomenon known as specific trans-generational immune priming. This phenomenon has independently been discovered in the crustacean Daphnia magna,Citation4 and in insects such as the bumble bee.Citation5,Citation6 Later it was also shown to occur in beetles such as the mealworm beetle Tenebrio molitorCitation7 and the red flour beetle Tribolium castaneum.Citation8 Evidence for trans-generational immune priming among the Lepidoptera was first reported by Freitak et al.,Citation9,Citation10 who added bacteria to the diet of the cabbage looper larvae (Trichoplusia ni) resulting in the induction of immunity-related genes associated with trans-generational effects. Maternal effects have been well documented in the context of trans-generational priming, and are thought to play a central role e.g., in the induction of offspring immunocompetence, but significant paternal effects have only been observed in a few studies.Citation11
Despite the increasing evidence for trans-generational immune priming in insects, the mechanism underlying the transfer of information from the parental generation to the offspring is unknown. The direct transfer of immune-related factors (e.g., lysozymes or antimicrobial peptides) in the cytoplasm of the egg has been proposed.Citation3,Citation12 An additional possibility is genomic imprinting, in which parental experience causes heritable alterations in gene expression that prime the offspring, thus highlighting the potential contribution of epigenetic effects on the inheritance of immunity.Citation13 A third but until now theoretical strategy, is the direct transfer of microbial fragments derived from bacteria encountered by the parent generation.
Here we selected the greater wax moth Galleria mellonella to investigate the phenomenon of trans-generational immune priming. G. mellonella is an established and powerful model for the investigation of gut microbe homeostasis and foodborne diseases.Citation14-Citation16 This species is also being investigated as a source for novel anti-infectivesCitation17 and the availability of a comprehensive transcriptome allows the detailed molecular analysis of its immune response by transcriptional profiling and mass spectrometry.Citation18
We added bacteria to the G. mellonella larval diet to experimentally mimic the most common routes of natural exposure to microbes.Citation9,Citation10 In the first part of our investigation, we addressed whether specific trans-generational immune priming occurs in G. mellonella by supplementing the larval diet with either non-pathogenic or entomopathogenic bacteria followed by the analysis of immunity-related gene expression and protein expression in the exposed insects and the eggs of the next generation. Our results provide evidence for a bacterial strain dependent trans-generational immune response.
In the second part of our investigation, we considered how information concerning the presence of bacteria in the larval gut is translated into trans-generational immune priming. We found that expression of immunity-related genes in the eggs changed according to the ingested bacteria, inspiring us to test whether this could be caused by the translocation of bacteria or bacterial fragments from the gut to the eggs. We tested our hypothesis by feeding G. mellonella larvae with bacteria carrying a fluorescent label, and monitoring their fate after consumption by fluorescence microscopy. We discovered fluorescent bacteria crossing the midgut epithelium, their entrapment within nodules in the hemocoel, their accumulation within the ovary, and ultimately their deposition in the eggs. The observed maternal transfer of food-derived or injected bacteria into the developing eggs could therefore explain the reported strain-specific nature of trans-generational immune priming.
Results
Induction of immune responses
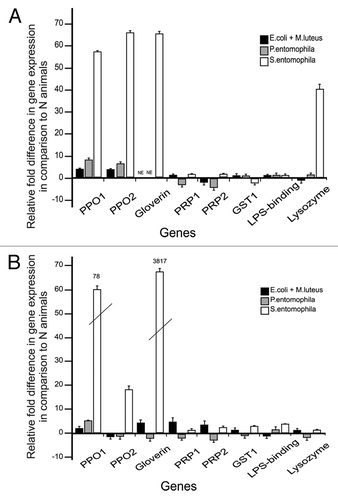
Contamination of the larval diet with either a mixture of gram-negative E. coli and gram-positive M. luteus (non-pathogenic), or with the entomopathogenic bacteria P. entomophila or S. entomophila, was sufficient to induce immune responses in the hemolymph, including enhanced lysozyme activity (Kruskall–Wallis ANOVA, H3.127 = 23.9, P < 0.000), enhanced phenoloxidase activity (Kruskall–Wallis ANOVA, H3.238 = 13.1, P = 0.004) as shown in . We also observed the induction of immunity-related genes such as those encoding lysozyme and the antibacterial protein gloverin (), the latter specific to the Lepidoptera.Citation19 Contamination of the larval diet with the pathogenic bacteria P. entomophila or S. entomophila induced stronger antibacterial activities than the non-pathogenic mixture, whereas phenoloxidase activity differed from the control group only in the larvae exposed to non-pathogenic bacteria (). We also found that the oral uptake of non-pathogenic bacteria by female larvae was sufficient to enhance the expression of gloverin in the eggs subsequently laid by the same individuals (). Strikingly, we observed the strong induction of prophenoloxidase and gloverin in eggs from the females that were fed with S. entomophila as larvae, whereas feeding with P. entomophila did have a much lower impact on immunity-related gene expression (). Both the immunity-related enzyme activities in exposed larvae and the gene expression profiles in eggs indicate species-dependent immune responses.
Figure 1. Immunity-related activities in the hemolymph of final-instar G. mellonella larvae (A) General antibacterial activity measured as the diameter of the lytic zone on agar plates and converted into lysozyme equivalents (μg/mL). Results represent medians with quartiles. (B) Phenoloxidase activity (slope at Vmax) measured from hemolymph samples. Results represent medians with quartiles.
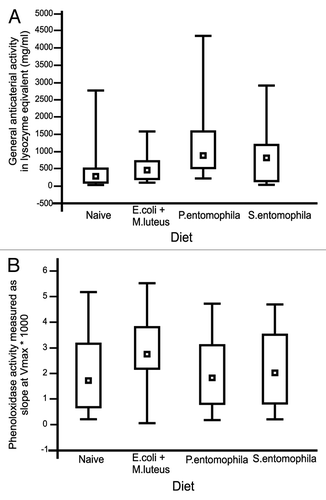
Figure 2. RT-qPCR results of differentially-expressed immunity-related genes in G. mellonella. PPO, prophenoloxidase; PRP, peptidoglycan recognition protein; GST, glutathione-S-transferase; LPS-binding, lipopolysaccharide binding protein. (A) Gene expression in the midgut tissue of larvae fed on bacterial diets compared with larvae grown on naïve (bacteria-free) diets. (B) Gene expression in the eggs laid by parents fed on bacterial diets compared with naïve (bacteria-free) diets. Relative fold changes for each gene were set to 1 for the control treatment. Results represent the mean values of 3 independent biological replicates ± SE.
Specific trans-generational immune responses
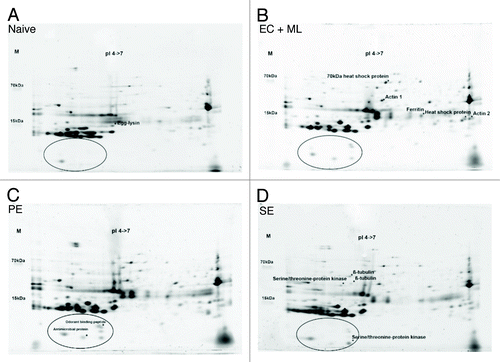
Lepidopteran eggs are known to respond to bacterial infection by producing antimicrobial proteins in non-embryonic tissues.Citation20 We therefore used two-dimensional gelelectrophoresis (2DGE) to look for differences between the eggs laid by females fed on the normal and contaminated diets. We found several protein spots that were unique to the eggs laid by females that were fed on bacteria, as well as other proteins that were present in both samples but more abundant in the eggs laid by females that were fed on bacteria. Proteins with a molecular mass less than 10 kDa featured prominently among this group (). These spots were picked and analyzed by mass spectrometry, leading to the identification of at least one antimicrobial peptide (anionic antimicrobial peptide 2) that was either unique to (or more abundant in) the eggs that were laid after the consumption of bacteria. The latter is known as anionic antimicrobial peptide 2.Citation21 Our data therefore suggest that species-dependent trans-generational immune priming in G. mellonella involves the differential expression of particular immunity-related proteins in the eggs ().
Figure 3. Proteomic analysis of G. mellonella eggs by 2DGE. (A) Eggs laid by naïve parents fed on an uncontaminated diet as larvae. (B) Eggs laid by parents fed on a diet supplemented with a mixture of E. coli and M. luteus. (C) Eggs laid by parents fed on a diet supplemented with P. entomophila. (D) Eggs laid by parents fed on a diet supplemented with S. entomophila. Circles highlight the differences in protein expression in the lower acidic part of the gel. Protein spots identified by peptide mass fingerprinting are marked with an asterisk and labeled accordingly.
Maternal transfer of bacteria
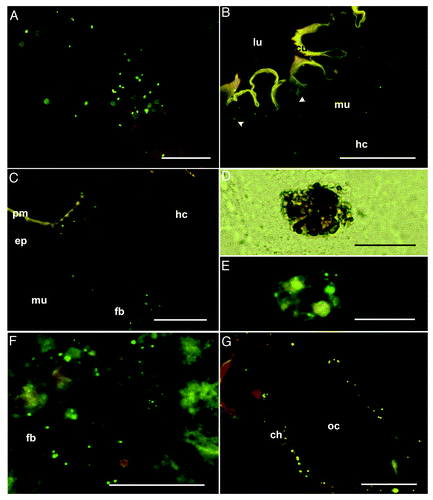
We established a hypothesis to explain how information concerning the presence of bacteria in the early maternal diet could be translated into differences in immunity-related gene expression in the eggs laid by the same female. This hypothesis proposes that trans-generational immune priming could be realized simply by the transfer of ingested bacteria or bacterial fragments from the larval gut into the eggs. We tested the hypothesis by feeding G. mellonella larvae with non-viable E. coli labeled with the fluorescent dye Texas Red (; Fig. S1). We added the bacteria to the larval diet and monitored their fate by fluorescence microscopy. We discovered that the fluorescent bacteria could penetrate the gut and enter the hemocoel (; Fig. S1A–D). In the pupae developing from larvae that were fed on these bacteria, we observed bacteria that were either free or attached to the fat body, and also nodules comprising aggregated hemocytes with entrapped bacteria (; Fig. S1E–F). G. mellonella hemocytes share structural and functional similarities with mammalian phagocytes.Citation22 Remarkably, we discovered that some of the labeled bacteria were ultimately deposited in the ovary and ovipositioned eggs. Observation by microscopy revealed the deposition of bacteria in the chorion () and to a lesser extent the yolk of the developing eggs (Fig. S1K), allowing them to elicit the expression of immunity-related genes ().
Figure 4. Analysis of the maternal transfer of bacteria in G. mellonella by fluorescence microscopy (A) Artificial diet mixed with fluorescently-labeled E. coli (green spots), cryo-section, scale bar = 50 μm. (B–C) Final-larval instar (stage V) fed with bacteria-contaminated diet. (B) Higher magnification of the foregut epithelium to show fluorescent E. coli beneath the cuticle. Ingested bacteria (green spots) are scattered in the epithelium (arrowhead), in the intercellular space between the epithelium and muscular tissue, and in the musculature and hemocoel, cryo-section, scale bar = 100 μm. (C) Fluorescence images of the midgut region showing translocated labeled bacterial probes (green spots) in association with the midgut epithelium as well as the fat body cells in the hemocoel, cryo-section, scale bar = 100 μm. (D–F) Pupal stage injected with labeled bacterial probes. (D) Higher magnification shows additional distributed nodule formations of circulating hemocytes in the pupal hemocoel, cryo-section, scale bar = 50 μm. (E) Nodules include bacterial probes (green spots), cryo-section, scale bar = 50 μm. (F) Bacteria attached to the fat body of pupae, cryo-section, scale bar = 100 μm. (G) Oviposited eggs of females injected with fluorescent bacteria during final-instar stage. The oocyte is lined with a chorion containing fluorescent E. coli (green spots), cryo-section, scale bar = 100 μm. Fluorescent photomicrographs were acquired with overlays of DSR and GFP2 fluorescence filters to optimize fluorescence visualization. Abbreviations: ch, chorion; cu, cuticle; ep, epithelium; fb, fat body; hc, hemocoel; lu, gut lumen; mu, musculature; oc, oocyte; pm, peritrophic membrane.
Discussion
Feeding experiments involving the lepidopteran model host G. mellonella showed that contamination of the larval diet with bacteria is sufficient to elicit immune responses in the larval gut including the induced expression of immunity-related genes. In contrast, the addition of a mixture of antibiotics to the G. mellonella larval diet has recently been shown to suppress immunity-related genes in the gut, suggesting there is homeostasis between e.g., the expression of antimicrobial peptides secreted into the gut lumen and the microbiota therein.Citation15 Our findings are in agreement with a previous study in which bacteria were added to the larval diet of another lepidopteran species, the cabbage looper T. ni.Citation9,Citation10 However, we found that pathogenic bacteria such as P. entomophila and S. entomophila activated stronger immune responses than non-pathogenic ones such as E. coli and M. luteus.
Strikingly, we found that immunity-related gene expression was stronger in eggs laid by females that consumed bacteria when they were larvae, compared with those laid by females that were never exposed to the bacteria. Similar observations have been reported for other insects. For example, MoretCitation23 reported that mealworm beetle larvae (T. molitor) challenged with bacterial lipopolysaccharide (LPS) produced offspring with enhanced phenoloxidase and antibacterial activities in the hemolymph, providing evidence for facultative trans-generational immune priming. Furthermore, T. ni larvae fed on a bacteria-rich diet undergo substantial changes in immunity-related gene expression that persists in larvae of the next generation.Citation9 Our data confirm the occurrence of such trans-generational immune priming in G. mellonella, which was detectable when E. coli and M. luteus were added to the diet but was more pronounced when the larvae were fed with S. entomophila. In agreement with MoretCitation23 and Freitak et al.Citation9,Citation10 we observed the strong induction of prophenoloxidases and the antimicrobial protein gloverin.
Our results indicate the presence of a bacterial species-dependent specific trans-generational immune response in G. mellonella. To investigate the specificity of this response in more detail we analyzed the egg proteome by 2DGE followed by mass spectrometry for protein identification. By taking advantage of our previously established comprehensive G. mellonella transcriptomic database,Citation18 we were able to identify several differentially-expressed proteins including serine/threonine protein kinases and an antimicrobial peptide. Comparative proteomic analysis of the eggs laid by females fed as larvae on diets with and without specific bacteria elucidated specific changes in the egg protein pattern and provided, therefore, more evidence of bacterial species-dependent trans-generational immune priming. However, our results did not explain how the information concerning the presence of particular bacteria in the larval gut was transmitted to the offspring.
The second part of our investigation was inspired by the hypothesis that bacteria in the larval gut could be translocated (complete or as fragments) into the eggs. We therefore added E. coli cells carrying a fluorescent label to the G. mellonella larval diet and monitored their fate by fluorescence microscopy, revealing that the bacteria can pass across the gut epithelium into the hemocoel. The ability of bacteria or their products to cross the intestinal barrier is known in mammals, including humans,Citation24 and has also been demonstrated in the powerful insect model Drosophila melanogaster fed on diets containing the pathogen S. entomophila.Citation25 The assumption that S. entomophila can also cross the intestinal barrier in G. mellonella would explain why the ingestion of this species elicited the particularly strong induction of defense genes such as gloverin in the larvae.
In the pupae of larvae fed on labeled bacteria, the fluorescent cells were attached to the fat body or entrapped within nodules suggesting they can also activate cellular immune responses. Most strikingly, we demonstrated that the labeled bacteria accumulate in the ovaries and ultimately in the chorion (and to a lesser extent the yolk) of the developing eggs. The translocation of bacteria through the gut epithelium and their deposition in the eggs suggests that the maternal transfer of bacteria or bacterial fragments can plausibly mediate trans-generational immune priming in Lepidoptera.
The physiological mechanism underlying the translocation of bacteria from the gut via the hemocoel into the eggs is not yet understood. We postulate that the bacteria are translocated from the hemocoel into the developing eggs passively, concomitant with the uptake of lipophorins and vitellogenins during oogenesis.Citation26 Vitellogenin is a yolk precursor which acts as a ligand to promote the massive uptake of lipophorins by endocytosis. Apolipoproteins such as apolipophorin III in the G. mellonella hemolymph are known to bind bacteria and bacterial cell wall compounds such as lipopolysaccharides and β-1,3-glucans and to contribute to the extracellular encapsulation of entrapped bacteria within nodules.Citation27 Developing lepidopteran eggs take up larger amounts of hemolymph protein by endocytosis.Citation26 Therefore, we speculate that endocytosis could also mediate the passive translocation of bacteria (or bacterial fragments) which are bound to or covered by hemolymph proteins. Even the deposition of ingested bacteria in the developing egg shells could contribute to trans-generational immune priming in the Lepidoptera because the egg shell is the primary food source of the newly-hatched larvae.
The observed maternal transfer of food-derived or injected bacteria into the developing eggs would explain the reported strain-specific nature of trans-generational immune priming.Citation4,Citation8 However, the maternal transfer of bacteria or bacterial fragments does not explain the paternal immune priming of offspring in the red flour beetle T. castaneum, suggesting other mechanisms can be involved in this phenomenon.Citation11,Citation28 In contrast, we observed the highest levels of immunity-related gene expression in G. mellonella eggs from individuals that had fed as larvae on pathogenic S. entomophila. This discrepancy can be explained by the different effects of injected soluble microbial elicitors and orally-administered bacterial pathogens on immunity-related transcriptional reprogramming, as shown in T. castaneum.Citation29 Whereas soluble microbial elicitors such as LPS and peptidoglycans interact passively with the immune system, living entomopathogens can actively interfere with it, e.g., by suppressing the expression of immunity-related genes. When either heat-inactivated or living pathogens were injected into G. mellonella larvae, there was direct evidence for the active role of pathogens in manipulating both immunity and development during infection.Citation14,Citation30 The coincidence between immune suppression and delayed development during infections has recently been attributed to the ability of bacterial and fungal pathogens to interfere with host epigenetic mechanisms including histone acetylation, which plays an essential role in the regulation of transcriptional reprogramming.Citation31
To summarize, we have discovered an unexpectedly simple mechanism by which trans-generational immune priming can be achieved in insects. The translocation of ingested bacteria from the gut lumen into the hemocoel, and their subsequent deposition in the eggs, can plausibly explain the observed bacterial species-dependent effects on maternal fecundity and offspring immunity. We are currently investigating whether epigenetic mechanisms such as DNA methylation and histone acetylation also play a role in trans-generational immune priming.
Materials and Methods
Insect rearing and treatment
G. mellonella third-instar larvae were purchased from Fauna Topics GmbH (Marbach), fed on a standard wax moth diet acquired from the same company and kept in the dark at 32 °C. The larvae were allowed to pupate and the resulting adults were mated. Eggs were counted and removed daily for three consecutive days, based on our observation that all eggs are laid within 3 d after the onset of the mating. Ten crosses were performed per treatment and we analyzed the eggs from all 10 females.
The larvae were then fed on a standard wax moth diet drenched with the following overnight cultures: (1) control group, sterile LB broth, (2) mixture of Escherichia coli and Micrococcus luteus, (3) Serratia entomophila, and (4) Pseudomonas entomophila. We used 30 mL overnight bacterial culture per 125 g of diet (15 mL each of the E. coli and M. luteus cultures) which was well mixed with the substrate. The food was replaced twice weekly to maintain a constant concentration of microbes throughout the experiment.
Analysis of immune responses
Lysozyme activity in the hemolymph was estimated using a lytic zone assay, in which petri dishes (8 cm diameter) were filled with 10 mL of autoclaved Sörensen buffer containing 17 mg lyophilized M. luteus cells (Sigma), 1.6 mg streptomycin sulfate (Calbiochem) and a final concentration of 1.5% agar. Wells 2 mm in diameter were punched in the agar, filled with 4 µL fresh hemolymph and incubated at 37 °C for 24 h. A dilution series of chicken egg white lysozyme (Sigma) (2 mg/mL, 1 mg/mL, 0.750 mg/mL, 0.500 mg/mL, 0.250 mg/mL, 0.125 mg/mL, 0.62 mg/mL, and 0.31 mg/mL) was added to each plate allowing a calibration curve to be produced based on these standards. Lytic activity was determined by measuring the diameter of the clear zone around each well.
Hemolymph phenoloxidase activity was measured by diluting 10 µL of hemolymph in 500 µL ice-cold PBS followed by flash freezing. The samples were then thawed on ice, centrifuged (10 min, 4 °C, and 9000 × g) and 100 µL of the supernatant was mixed with 200 µL 3 mM L-DOPA (Sigma). The kinetic properties of the enzyme were analyzed by measuring the absorbance at 490 nm and 30 °C in a BIO-TEK EL 808 spectrophotometer, taking samples at 1 min intervals for 45 min. The slope of the curve during the linear reaction phase (15–45 min after adding the substrate) was used for analysis.
Analysis of fluorescent bioparticles
G. mellonella larvae were fed on a standard wax moth diet mixed with E. coli K-12 cells conjugated with Texas Red (BioParticles, Sigma), using 100 µL of a 1 mg/mL bacterial cell suspension in PBS per 1 g of diet. Because the labeled particles were diluted when they crossed the midgut–hemocoel barrier, we included an additional treatment in which the larvae were injected with the particles, making it easier to monitor their fate in the hemocoel and increasing the chances of detecting fluorescent particles in the eggs.
RNA isolation and quantitative real-time PCR
One-day-old eggs, and midguts dissected from early-stage last-instar larvae, were homogenized in TRIzol Reagent (Invitrogen) and total RNA was isolated according to the manufacturer’s protocol. The isolated RNA was treated with Turbo DNase (Ambion) to eliminate any contaminating genomic DNA and then purified using RNeasy MinElute columns (Qiagen). The integrity of the RNA was verified by agarose gel electrophoresis and the quantity was determined using a NanoDrop spectrophotometer (PeqLab).
First-strand cDNA was synthesized using the First Strand cDNA Synthesis Kit (Fermentas, Thermo Scientfic) with 500 ng of DNA-free total RNA as the template and a mixture of random and dT20 primers. Real-time PCR oligonucleotide primers were designed using Primer3 (http://frodo.wi.mit.edu) by applying the rules of highest maximum efficiency and sensitivity to avoid the formation of dimers, hairpins and other artifacts (Table S1). Gene-specific primers were designed against selected G. mellonella genes, with 18S RNA and ubiquitin genes used for normalization. Quantitative real-time PCR was performed in optical 96-well plates on a CFX96 Real-Time PCR Detection System (Biorad) using the SSoFast Eva Green Supermix (Biorad). Each assay comprised three biological replicates (each representing pooled mRNA from 15 individuals or a pool of ~500 eggs) and two technical replicates.
Two-dimensional gel electrophoresis and protein identification
We homogenized ~500 1-d-old eggs per cross in 300 µL ice-cold lysis buffer (7 M urea, 2 M thiourea, 2% CHAPS, 60 mM DTT, and 2% carrier ampholytes) and stored the samples at −20 °C. After thawing on ice and centrifugation (10 min, 4 °C, and 9000 × g), the protein content of the supernatant was determined using the 2D Quant Kit (GE Healthcare) and then separated by 2DGE using an XT-MES buffer system. We loaded 50 µg of total protein on 11-cm 4–7NL IPG strips (Biorad), rehydrated the strips overnight in sample buffer containing 0.02% bromophenol blue and performed first-dimension isoelectric focusing using an IPG manifold (GE Healthcare). Orthogonal separation was performed using 4–12% Bis-Tris Criterion XT Precast gels (Bio-Rad) running at 70 V for ~4 h until the dye front reached the end of the gel. The gels were then washed three times in double-distilled water and stained for 2–3 h with Flamingo™ Fluorescent Gel Stain (Biorad). Spots of interest were excised with the ExQuest spot cutter and proteins were digested with trypsin using a robotic liquid-handling system (MicroStarlet, HamiltonRobotics).
The digested proteins were analyzed by MALDI-TOF mass spectrometry on an Ultraflex TOF/TOF mass spectrometer equipped with a nitrogen laser and a LIFT-MS/MS facility. The instrument was operated in positive-ion reflectron mode using 2,5-dihydroxybenzoic acid and methylenediphosphonic acid as the matrix. We acquired readings comprising 200–400 individual spectra. We used the Compass 1.1 software package, comprising FlexControl 2.4, FlexAnalysis 3.0, and BioTools 3.0, for data processing and instrument control. A peptide standard (Bruker Daltonics) was used for external calibration.
Proteins were identified by carrying out a MASCOT peptide mass fingerprint search (http://www.matrixscience.com) using an in-house G. mellonella databaseCitation18 and SwissProt (Table S2), applying a mass tolerance of 75 ppm and allowing cysteine carbamidomethylation as a global modification and the oxidation of methionine as a variable modification.
Statistical analysis
Statistical analysis was performed using the software package Statistica 9 (StatSoft), the distribution of the data was tested using the Shapiro–Wilkoxon and Levene tests. Where assumptions for normality and homogeneity were not violated, our hypotheses were tested using an ANOVA model. Otherwise a nonparametric Kruskall–Wallis ANOVA test was used. The 2−ΔΔCt method was used to calculate the relative fold differences in gene expression.Citation32
Additional material
Download Zip (401.2 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Hessian Ministry of Science, Higher Education and Art (HMWK) via the funded LOEWE center for Insect Biotechnology. A.V. thanks the German Research Foundation (Deutsche Forschungsgemeinschaft) for funding the projects embedded within the DFG Priority Programme 1399 “Host-Parasite Coevolution – rapid reciprocal adaptations and its genetic basis (VI 219/3-2).” The authors are indebted to Dr Richard M Twyman for careful editing of the manuscript.
References
- Schmid-Hempel P. Natural insect host-parasite systems show immune priming and specificity: puzzles to be solved. Bioessays 2005; 27:1026 - 34; http://dx.doi.org/10.1002/bies.20282; PMID: 16163710
- Sadd BM, Schmid-Hempel P. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr Biol 2006; 16:1206 - 10; http://dx.doi.org/10.1016/j.cub.2006.04.047; PMID: 16782011
- Zanchi C, Troussard JP, Moreau J, Moret Y. Relationship between maternal transfer of immunity and mother fecundity in an insect. Proc Biol Sci 2012; 279:3223 - 30; http://dx.doi.org/10.1098/rspb.2012.0493; PMID: 22535782
- Little TJ, O’Connor B, Colegrave N, Watt K, Read AF. Maternal transfer of strain-specific immunity in an invertebrate. Curr Biol 2003; 13:489 - 92; http://dx.doi.org/10.1016/S0960-9822(03)00163-5; PMID: 12646131
- Sadd BM, Kleinlogel Y, Schmid-Hempel R, Schmid-Hempel P. Trans-generational immune priming in a social insect. Biol Lett 2005; 1:386 - 8; http://dx.doi.org/10.1098/rsbl.2005.0369; PMID: 17148213
- Sadd BM, Schmid-Hempel P. Facultative but persistent trans-generational immunity via the mother’s eggs in bumblebees. Curr Biol 2007; 17:R1046 - 7; http://dx.doi.org/10.1016/j.cub.2007.11.007; PMID: 18088585
- Moret Y. “Trans-generational immune priming”: specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor.. Proc Biol Sci 2006; 273:1399 - 405; http://dx.doi.org/10.1098/rspb.2006.3465; PMID: 16777729
- Roth O, Sadd BM, Schmid-Hempel P, Kurtz J. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum.. Proc Biol Sci 2009; 276:145 - 51; http://dx.doi.org/10.1098/rspb.2008.1157; PMID: 18796392
- Freitak D, Heckel DG, Vogel H. Dietary-dependent trans-generational immune priming in an insect herbivore. Proc Biol Sci 2009; 276:2617 - 24; http://dx.doi.org/10.1098/rspb.2009.0323; PMID: 19369263
- Freitak D, Heckel DG, Vogel H. Bacterial feeding induces changes in immune-related gene expression and has trans-generational impacts in the cabbage looper (Trichoplusia ni).. Front Zool 2009; 6:7; http://dx.doi.org/10.1186/1742-9994-6-7; PMID: 19422678
- Roth O, Joop G, Eggert H, Hilbert J, Daniel J, Schmid-Hempel P, Kurtz J. Paternally derived immune priming for offspring in the red flour beetle, Tribolium castaneum.. J Anim Ecol 2010; 79:403 - 13; http://dx.doi.org/10.1111/j.1365-2656.2009.01617.x; PMID: 19840170
- Seppola M, Johnsen H, Mennen S, Myrnes B, Tveiten H. Maternal transfer and transcriptional onset of immune genes during ontogenesis in Atlantic cod. Dev Comp Immunol 2009; 33:1205 - 11; http://dx.doi.org/10.1016/j.dci.2009.06.013; PMID: 19577592
- Richards EJ. Inherited epigenetic variation--revisiting soft inheritance. Nat Rev Genet 2006; 7:395 - 401; http://dx.doi.org/10.1038/nrg1834; PMID: 16534512
- Mukherjee K, Hain T, Fischer R, Chakraborty T, Vilcinskas A. Brain infection and activation of neuronal repair mechanisms by the human pathogen Listeria monocytogenes in the lepidopteran model host Galleria mellonella. Virulence 2013; 4:324 - 32; http://dx.doi.org/10.4161/viru.23629; PMID: 23348912
- Mukherjee K, Raju R, Fischer R, Vilcinskas A. Galleria mellonella as a model host to study gut microbe homeostasis and brain infection by the human pathogen listeria monocytogenes.. Adv Biochem Eng Biotechnol 2013; 135:27 - 39; http://dx.doi.org/10.1007/10_2013_203; PMID: 23708825
- Banville N, Browne N, Kavanagh K. Effect of nutrient deprivation on the susceptibility of Galleria mellonella larvae to infection. Virulence 2012; 3:497 - 503; http://dx.doi.org/10.4161/viru.21972; PMID: 23076277
- Vilcinskas A. Anti-infective therapeutics from the Lepidopteran model host Galleria mellonella.. Curr Pharm Des 2011; 17:1240 - 5; http://dx.doi.org/10.2174/138161211795703799; PMID: 21470117
- Vogel H, Altincicek B, Glöckner G, Vilcinskas A. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella.. BMC Genomics 2011; 12:308; http://dx.doi.org/10.1186/1471-2164-12-308; PMID: 21663692
- Jiang H, Vilcinskas A, Kanost MR. Immunity in lepidopteran insects. Adv Exp Med Biol 2010; 708:181 - 204; http://dx.doi.org/10.1007/978-1-4419-8059-5_10; PMID: 21528699
- Gorman MJ, Kankanala P, Kanost MR. Bacterial challenge stimulates innate immune responses in extra-embryonic tissues of tobacco hornworm eggs. Insect Mol Biol 2004; 13:19 - 24; http://dx.doi.org/10.1111/j.1365-2583.2004.00454.x; PMID: 14728663
- Sowa-Jasiłek A, Zdybicka-Barabas A, Stączek S, Wydrych J, Mak P, Jakubowicz T, Cytryńska M. Studies on the role of insect hemolymph polypeptides: Galleria mellonella anionic peptide 2 and lysozyme. Peptides 2014; forthcoming http://dx.doi.org/10.1016/j.peptides.2014.01.012; PMID: 24472857
- Browne N, Heelan M, Kavanagh K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 2013; 4:597 - 603; http://dx.doi.org/10.4161/viru.25906; PMID: 23921374
- Moret Y. “Trans-generational immune priming”: specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor.. Proc Biol Sci 2006; 273:1399 - 405; http://dx.doi.org/10.1098/rspb.2006.3465; PMID: 16777729
- Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol 2007; 22:464 - 71; http://dx.doi.org/10.1111/j.1440-1746.2007.04933.x; PMID: 17376034
- Nehme NT, Liégeois S, Kele B, Giammarinaro P, Pradel E, Hoffmann JA, Ewbank JJ, Ferrandon D. A model of bacterial intestinal infections in Drosophila melanogaster.. PLoS Pathog 2007; 3:e173; http://dx.doi.org/10.1371/journal.ppat.0030173; PMID: 18039029
- Telfer WH. Egg formation in lepidoptera. J Insect Sci 2009; 9:1 - 21; http://dx.doi.org/10.1673/031.009.5001; PMID: 20050770
- Altincicek B, Stötzel S, Wygrecka M, Preissner KT, Vilcinskas A. Host-derived extracellular nucleic acids enhance innate immune responses, induce coagulation, and prolong survival upon infection in insects. J Immunol 2008; 181:2705 - 12; PMID: 18684961
- Zanchi C, Troussard JP, Martinaud G, Moreau J, Moret Y. Differential expression and costs between maternally and paternally derived immune priming for offspring in an insect. J Anim Ecol 2011; 80:1174 - 83; http://dx.doi.org/10.1111/j.1365-2656.2011.01872.x; PMID: 21644979
- Freitak D, Knorr E, Vogel H, Vilcinskas A. Gender- and stressor-specific microRNA expression in Tribolium castaneum.. Biol Lett 2012; 8:860 - 3; http://dx.doi.org/10.1098/rsbl.2012.0273; PMID: 22628099
- Vilcinskas A, Matha V. Effect of the entomopathogenic fungus Beauveria bassiana on humoral immune response of Galleria mellonella larvae (Lepidoptera: Pyralidae). Eur J Entomol 1997; 94:461 - 72
- Mukherjee K, Fischer R, Vilcinskas A. Histone acetylation mediates epigenetic regulation of transcriptional reprogramming in insects during metamorphosis, wounding and infection. Front Zool 2012; 9:25; http://dx.doi.org/10.1186/1742-9994-9-25; PMID: 23035888
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402 - 8; http://dx.doi.org/10.1006/meth.2001.1262; PMID: 11846609
