Abstract
Candida parapsilosis is an important opportunistic pathogen with increasing prevalence. Extracellular lipases have been shown to play an important role in the virulence of pathogenic Candida species. However, studying the role of secreted lipase in C. albicans is challenging due to the lack of a mutant strain deficient in all 10 lipase genes. In contrast, we have previously constructed a lipase mutant C. parapsilosis strain lacking both CpLIP1 and CpLIP2, and shown that it has significantly decreased virulence in various infection models, and is killed more efficiently by mouse macrophages. In the present study, we compared the response of human peripheral blood monocyte-derived macrophages to a wild type (wt) as well as a lipase-deficient (lip−/−) C. parapsilosis strain that has been previously established in our lab. Although macrophages phagocytosed both strains with similar efficiency, lipase mutants were killed more efficiently according to fluorescent microscopic analysis. The more efficient killing of lip−/− cells was confirmed by CFU-determinations. Phagocytosis of wt and lip−/−C. parapsilosis was also examined by flow cytometry, revealing that both strains were internelized to the similar extent by macrophages. Additionally, quantitative imaging analysis revealed that the rate of phagolysosome fusion was higher in case of lip−/−C. parapsilosis. Interestingly, macrophages stimulated with lip−/−C. parapsilosis showed at least 1.5-fold higher expression of TNFα, IL-1β, IL-6, IL-8, and PTGS-2 after 12 h compared with those infected with wt C. parapsilosis, as determined by qRT-PCR. Furthermore, the lip−/−C. parapsilosis strain induced significantly higher TNFα, IL-1β, IL-6, and IL-10 protein production in macrophages after 24 h compared with the wt strain. These findings confirm the role of fungal lipases as important virulence factors during C. parapsilosis infection.
Introduction
Candida parapsilosis is an opportunistic fungal pathogen that causes disseminated infection in immunocompromised patients.Citation1 Although C. albicans remains the leading cause of invasive candidiasis, the incidence of infections due to C. parapsilosis has significantly increased over the past two decades.Citation2 Unlike C. albicans, C. parapsilosis typically causes invasive disease without colonizing the host before dissemination, and is frequently nosocomially transmitted horizontally by hospital personnel or via medical equipment.Citation2 Additionally, C. parapsilosis is the predominant species responsible for invasive candidiasis in premature infants, and it is often associated with neonatal mortality.Citation2 Although its clinical importance is increasing, the pathogenesis of C. parapsilosis infection is poorly understood.
Secretion of hydrolytic enzymes has been associated with virulence in pathogenic bacteria, parasites,Citation3 and fungi.Citation4 Secreted aspartyl proteinases have been extensively studied and investigations have demonstrated that they play a role in the virulence of both C. albicansCitation5 and C. parapsilosis.Citation6 In contrast, much less is known about the role of extracellular lipases during infection, although there is a growing body of evidence showing that they are important virulence factors. Lipases belong to the family of carboxylic ester hydrolases and catalyze the hydrolysis and synthesis of triacylglycerols.Citation7 Secreted microbial lipases are thought to have a role in many processes, including nutrient acquisition by liberation of free fatty acids from lipid molecules, adhesion to host cells and generation of inflammatory mediators.Citation8 We recently demonstrated that C. parapsilosis isolates lacking extracellular lipase activity are killed more efficiently by primary human macrophages compared with lipase producing strains.Citation9 Furthermore, genetically engineered lipase mutants of C. parapsilosis and C. albicans have significantly decreased virulence in various infection models.Citation8,Citation10,Citation11 Another paper supporting the role of lipases as virulence factors details that inhibition of fungal lipases during infection of reconstituted human tissue models with C. albicans and C. parapsilosis results in significantly decreased tissue damage.Citation12
While 10 lipase genes have been identified in C. albicans (LIP1–10),Citation13 C. parapsilosis possesses only two LIP genes, CpLIP1 and CpLIP2.Citation14 In 2007, a CpLIP1–CpLIP2 knockout mutant (lip−/−) strain of C. parapsilosis was established by Gácser et al., providing a useful tool for studying the role of extracellular lipases in virulence.Citation8 This well characterized lipase-deficient strain has limited growth capacity in lipid-rich media, and decreased virulence in in vitro and in vivo infection models.Citation8 We recently found that lip−/− C. parapsilosis strains were significantly more efficiently phagocytosed and killed by human monocyte-derived dendritic cells and that the expression of dendritic cell effector molecules differed in co-cultures with either the mutant or wild type (wt) cells.Citation15
The aim of the current study was to examine the response of primary human monocyte-derived macrophages to infection with a wt C. parapsilosis strain in comparison with the lip−/− mutant. Macrophages are central players of both innate and adaptive immunity. They are able to sense and ingest a wide range of pathogens, contributing to the first line of host defense and are known to have a crucial role in fungal infections.Citation16 Additionally, they can mediate the adaptive immune response by producing specific cytokines. Here we show how the lack of C. parapsilosis lipase influences the host responses during infection, further supporting the role of lipases as important virulence factors.
Results
C. parapsilosis lip−/− cells are phagocytosed to the same extent as wt yeasts, but the lip−/− cells are killed more efficiently by primary human monocyte-derived macrophages
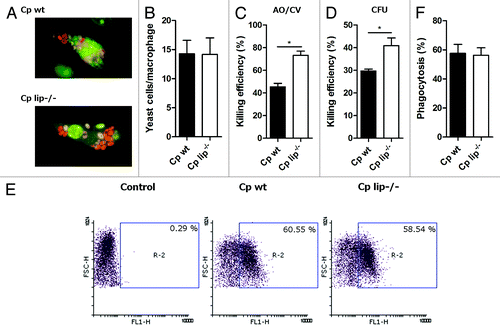
First, we examined the phagocytosis and killing of C. parapsilosis cells by fluorescence microscopy using acridin orange dye to differentiate between dead and live yeast cells. Human macrophages efficiently phagocytosed and killed the wt and lip−/− cells of C. parapsilosis (). The total numbers of yeast cells engulfed by macrophages were similar between the two C. parapsilosis strains (), but the lip−/− cells were more efficiently killed (mean ± SEM, wt vs. lip−/−, 45.40 ± 3.04 vs. 73.18 ± 3.72% killing efficiency, P < 0.05, ). The killing efficacy was confirmed by CFU (colony forming unit) determinations, which showed that macrophages were more efficient in eliminating the lip−/− strain (mean ± SEM, 40.87 ± 3.40% killing efficiency) compared with wt C. parapsilosis (29.70 ± 0.81%, P < 0.05, ). We also determined the phagocytosis of wt and lip−/− C. parapsilosis using flow cytometry following fluorescent labeling of yeast cells, and found that the phagocytosis of lipase mutant C. parapsilosis cells (mean ± SEM, 57.57 ± 6.12%) was comparable to that of wt cells (56.16 ± 5.23%, ).
Figure 1. Phagocytosis and killing of wt and lip−/−C. parapsilosis cells by human monocyte-derived macrophages. (A) Representative fluorescent microscopic images of human macrophages co-cultured for 3 h wt or lip−/−C. parapsilosis. Green fluorescence of acridin orange dye indicates live cells with intact DNA, while dead cells with degraded DNA show red fluorescence. (B) Phagocytosis of wt and lip−/−C. parapsilosis as determined by fluorescent microscopic analysis following acridin orange staining. Data are from three independent experiments and represent the average numbers of yeast cells engulfed by one macrophage (only phagocytosing macrophages were included in the analysis). (C) Killing of wt and lip−/−C. parapsilosis as determined by fluorescent microscopic analysis. Data represent the percent of dead yeast cells ± SEM. AO/CV, acridin orange/crystal violet. (D) Killing of wt and lip−/−C. parapsilosis cells by human macrophages as measured by CFU-determinations. Experiments were performed in triplicate. Data represent killing efficiency ± SEM for three donors. (E and F) Phagocytosis of wt and lip−/−C. parapsilosis cells by human macrophages analyzed by flow cytometry. Yeast cells were labeled with FITC or AlexaFluor647 and co-cultured with macrophages for 2 h. Data are from three independent experiments which were performed in duplicate. Representative dot plots (E) and summarized data (F) of the flow cytometric analysis are shown. Cp, C. parapsilosis; wt, wild type; lip−/−, lipase mutant; *P < 0.05, **P < 0.01.
Phagosome–lysosome fusion in human macrophages is more intense with lip−/− C. parapsilosis than with wt yeasts
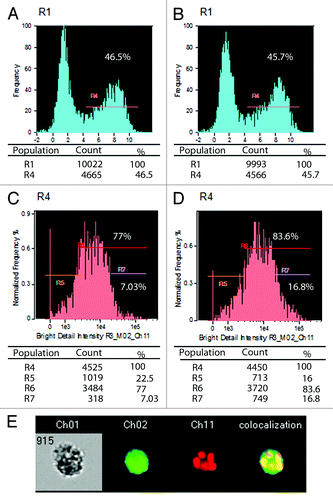
Next we addressed the question whether the lack of lipase influences phagosome-lysosome fusion following the phagocytosis of C. parapsilosis cells. Therefore, we assessed the phagosome-lysosome co-localization by quantitative imaging following AlexaFluor647-labeling of C. parapsilosis cells, and LysoTracker Green staining of macrophages. Again, we found that there was no difference in the phagocytosis of the two C. parapsilosis strains after two hours (). However, we found an increase in phagosome-lysosome co-localization in human macrophages infected with lip−/− C. parapsilosis in contrast to wt yeasts (macrophages with intense co-localization: 16.8 vs 7.03% for lip−/− and wt, respectively ). These results provide mechanistic insights into the more efficient elimination of the lip−/− strain by macrophages.
Figure 2. Phagocytosis of wt and lip−/−C. parapsilosis by human primary macrophages analyzed by quantitative imaging. (A and B) Phagocytosis of wt (A) and lip−/− (B) C. parapsilosis by human macrophages. R1: Single cell events, R4: macrophage population containing yeast cells discriminated by the presence of red fluorescence due to ingestion of AlexaFluor647-labeled Candida cells. (C and D) Phagosome-lysosome colocalization in human macrophages following the phagocytosis of AlexaFluor647 labeled wt (C) or lip−/− (D) C. parapsilosis; macrophages were labeled with LysoTracker Green following phagocytosis. Histograms show the intensity of phagosome-lysosome colocalization. R5: low co-localization, R6: moderate and high co-localization, R7: high co-localization. (E) Representative picture of a phagocytosing macrophage during quantitative imaging analysis. Ch1: brightfield image, Ch2: green fluorescence channel (LysoTracker Green), Ch11: red fluorescence channel (AlexaFluor647).
C. parapsilosis lip−/− cells induce higher gene expression of inflammatory mediators in human macrophages than wt yeasts
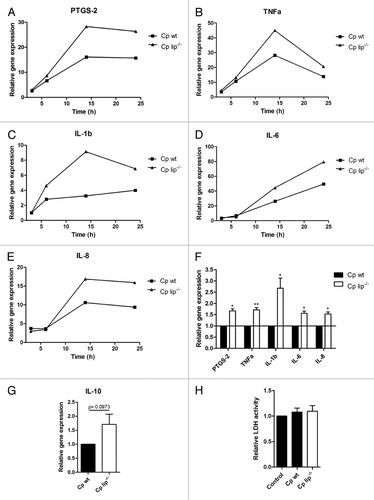
To examine the effect of C. parapsilosis lipase on the induction of inflammatory mediators, the expression of several host genes was determined following the stimulation of macrophages with wt and lip−/− C. parapsilosis. We found that the genes for prostaglandin-endoperoxide synthase 2 (PTGS-2, cyclooxygenase-2), tumor necrosis factor α (TNFα), interleukin 1 β (IL-1β), interleukin 6 (IL-6) and interleukin 8 (IL-8) were highly induced in macrophages following stimulation with either strain of C. parapsilosis (), although the gene expression varied greatly between donors. Interestingly, after 14 h of incubation, the expression of PTGS-2 was 1.67 higher in macrophages stimulated with lip−/− C. parapsilosis compared with macrophages stimulated with the wt strain (fold change in lip−/−-stimulated macrophages relative to that in wt-stimulated cells ± SEM, 1.67 ± 0.10, n = 3, P < 0.05, ). Similarly, the lip−/− C. parapsilosis cells induced at least 1.5-fold higher expression of the genes for inflammatory cytokines compared with wt yeasts: TNFα (1.72 ± 0.10, P < 0.01), IL-1β (2.68 ± 0.45, P < 0.05), IL-6 (1.57 ± 0.09, P < 0.05) and IL-8 (1.53 ± 0.09, P < 0.05, ). Furthermore, we found that the expression of the anti-inflammatory cytokine interleukin-10 (IL-10) was not induced at 14 h post-infection, but after 24 h it was higher in macrophages stimulated with lip−/− C. parapsilosis compared with wt-stimulated cells (fold change compared with wt stimulation ± SEM, 1.71 ± 0.37, n = 3, P = 0.097 [NS], ). Importantly, there was no difference in the host-cell damaging capacity of wt and lip−/− C. parapsilosis strains in our experimental setting, as determined 24 h post-infection by LDH (lactate dehydrogenase) measurements ().
Figure 3. Expression of inflammatory mediators in human macrophages induced by wt and lip−/−C. parapsilosis. (A–E) Expression kinetics of PTGS-2 (A), TNFα (B), IL-1β (C), IL-6 (D), and IL-8 (E) in human macrophages induced by wt and lip−/−C. parapsilosis. Macrophages were co-incubated with wt or lip−/−C. parapsilosis for 3, 6, 14, or 24 h and the gene expression was determined by qRT-PCR by the ΔΔCT method using B2MG as an endogenous control gene. Data indicate fold changes in mRNA expression compared with unstimulated control samples. Graphs are representatives of three independent experiments. (F and G) Expression of PTGS-2, TNFα, IL-1β, IL-6, and IL-8 after 14 h (F) and IL-10 after 24 h (G) in human macrophages following stimulation with wt or lip−/−C. parapsilosis. Data indicate fold changes in mRNA expression ± SEM for 3 donors. Data were normalized for each donor to mRNA levels induced by wt C. parapsilosis to minimize donor-to-donor variability. (H) Host cell-damaging capacity of wt and lip−/−C. parapsilosis determined by LDH assay. Macrophages were co-incubated with the different C. parapsilosis strains for 24 h, and the LDH activity was measured in cell culture supernatants. Data represent relative LDH activity ± SEM for 6 donors. Cp, C. parapsilosis; wt, wild type; lip−/−, lipase mutant; *P < 0.05, **P < 0.01.
Macrophages produce more inflammatory cytokines in response to lipase-deficient C. parapsilosis
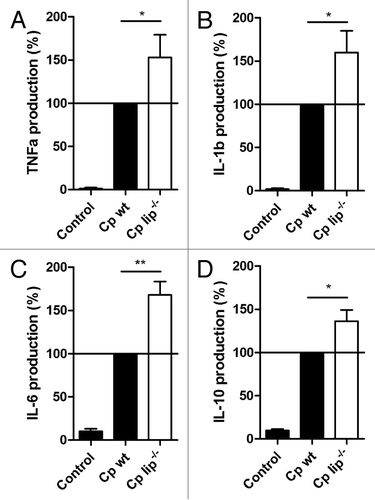
To correlate the expression results with the presence of cytokines, we measured the cytokine production of human macrophages following stimulation with wt and lip−/− C. parapsilosis. In accordance with the gene expression data, macrophages stimulated with the lip−/− C. parapsilosis strain produced more of the inflammatory cytokines TNFα, IL-1β and IL-6 compared with wt-stimulated cells (TNFα, 15.98 ± 3.72 vs. 12.22 ± 3.13 ng/mL, P < 0.05, n = 8; IL-1β, 178.27 ± 38.96 vs. 138.41 ± 42.12 pg/mL, P = 0.055, n = 8; IL-6, 6162.96 ± 1826.46 vs. 4098.24 ± 1276.82 pg/mL, P < 0.01, n = 8). The level of secreted IL-10 was also slightly higher following stimulation with lip−/− C. parapsilosis (517.95 ± 171.54 pg/mL, vs. 408.29 ± 154.99 pg/mL [wt stimulation], P < 0.01, n = 8). shows the relative cytokine production of macrophages stimulated with wt or lip−/− C. parapsilosis (cytokine levels were normalized for each donor to cytokine levels induced by wt C. parapsilosis [100%] to minimize donor-to-donor variability).
Figure 4. Cytokine secretion of human macrophages in response to wt and lip−/−C. parapsilosis. Secreted TNFα (A), IL-1β (B), IL-6 (C), and IL-10 (D) levels were measured by ELISA after stimulation of macrophages with wt or lip−/−C. parapsilosis for 24 h. Data were normalized for each donor to cytokine levels induced by wt C. parapsilosis (100%) to minimize donor-to-donor variability. Data represent % cytokine production ± SEM for 8 donors. Actual cytokine levels (in pg/mL or ng/mL) are described in the text (see Results). Cp, C. parapsilosis; wt, wild type; lip−/−, lipase mutant; *P < 0.05, **P < 0.01.
Discussion
Secretion of lipolytic enzymes is associated with the virulence of Candida species.Citation4,Citation8-Citation10 Lipase mutants of C. parapsilosis and C. albicans have decreased virulence in murine models of disseminated candidiasis, indicated by significantly lower mortality rates and decreased tissue fungal burdens.Citation8,Citation10 Extracellular lipase secretion by C. parapsilosis affects the adhesive properties and biofilm-forming ability of the microorganisms, as well as its phagocytosis and survival in macrophages.Citation8 Although the role of secreted Candida lipases as virulence factors has been recognized, little is known about the influence of these enzymes on the host’s immune system.
One approach to study the role of lipases duing infection is the use of gene knockout mutants. However, while a C. albicans lipase knockout mutant lacking all the LIP genes has not been constructed yet (most likely due to the high number of LIP genes), a lipase knockout mutant strain of C. parapsilosis (lacking both LIP1 and LIP2) has been established by Gácser et al.Citation8 These lipase mutants have no extracellular lipase activityCitation8; therefore, they provide a useful tool for studying the role of lipase in C. parapsilosis virulence. Recently, we investigated the interactions of wt and lip−/− C. parapsilosis strains with human monocyte-derived dendritic cells,Citation15 and found that dendritic cells displayed a stronger inflammatory response against lip−/− C. parapsilosis in comparison to wt cells.
In this study, we investigated the interactions of wt and lip−/− C. parapsilosis with primary human monocyte-derived macrophages. Macrophages are central players of the immune system, connecting the innate and adaptive arms of the host’s immune responses. They bear a variety of pattern recognition receptors, which are able to recognize invading pathogens and initiate potent inflammatory responses.Citation17 Additionally, macrophages efficiently phagocytose and kill pathogens, and further augment adaptive immune responses by antigen presentation.
Microscopic analysis following acridin orange staining of co-cultures revealed that pimary human monocyte-derived macrophages efficiently ingest and kill both the wt and lip−/− strain of C. parapsilosis in vitro. When comparing the killing of the wt and the lip−/− strain of C. parapsilosis, we found that lipase mutants were killed more efficiently by macrophages, which is in line with earlier studies using mouse cells linesCitation8 or human monocyte-derived dendritic cells.Citation15 These results confirm that secreted lipases promote the survival of Candida cells in macrophages. However, we found no differences in the phagocytosis of wt and lip−/− C. parapsilosis cells by macrophages after 2 h of co-incubation.
Following phagocytosis, phagosome-lysosome fusion is a key event that facilitates the killing of the ingested pathogen by lysosomal enzymes and other antimicrobial components.Citation18 There are several bacterial and fungal species, such as Mycobacterium tuberculosis and Histoplasma capsulatum, which are able to inhibit phagolysosome fusion, thereby avoiding killing by macrophages.Citation19,Citation20 Among Candida species, C. albicans and C. glabrata can inhibit phagosome maturation.Citation18 Ingestion of lip−/− C. parapsilosis results in stronger phagosome-lysosome colocalization in human monocyte-derived dendritic cells.Citation15 Here we show that in the absence of secreted fungal lipase, the phagosome-lysosome colocalization following ingestion of C. parapsilosis occurs with greater frequency in human macrophages as well. These results are in line with the enhanced killing of lip−/− C. parapsilosis cells and suggest that secreted fungal lipases may promote the survival of microbes in macrophages by interfering with phagolysosome fusion.
In addition to the intracellular killing of microbes, the secretion of inflammatory mediators by macrophages is essential for the efficient eradication of pathogens. TNFα, IL-1β, and IL-6 are potent inflammatory cytokines with pleiotropic effects, including the enhancement of antimicrobial capacity of neutrophils and macrophages, modification of vascular permeability, and recruitment of leukocytes to the site of inflammation.Citation21,Citation22 The chemokine IL-8 is the most potent activator of neutrophil recruitment.Citation23 PTGS-2 (cyclooxigenase 2, COX-2) is responsible for the production of prostaglandins, which are important mediators of inflammation.Citation24 When we investigated the systhesis of several inflammatory mediators in macrophages in response to wt and lip−/− C. parapsilosis infection, we found that both strains induced a high expression of PTGS-2, TNFα, IL-1β, IL-6, and IL-8 genes with a peak at 14 h post-infection. Interestingly, at this time point, macrophages stimulated with the lipase mutant C. parapsilosis showed at least 1.5 times higher expression of these molecules compared with those infected with wt cells. Differences could also be seen at the protein level, as macrophages stimulated with lip−/− C. parapsilosis produced more TNFα, IL-1β, and IL-6 after 24 h compared with wt-stimulated human cells. These results are in line with the findings of our prior studies with dentritic cells, which showed that both immature and mature human dendritic cells have elevated cytokine secretion when infected with lip−/− C. parapsilosis compared with wt cells.Citation15 In the current work, we additionally found the level of the anti-inflammatory cytokine IL-10 produced by macrophages was higher with lip−/− C. parapsilosis stimulation relative to wt yeast cells. The most plausible explanation for this finding is that IL-10 is known to be induced by TNF, in order to prevent the host from the effects of excessive inflammation.Citation25 Although the underlying molecular mechanisms are yet to be clarified, our results suggest that C. parapsilosis lipase may have anti-inflammatory potential. As there are diverse bioactive lipid mediators—such as prostaglandins, leukotrienes, and cannabinoids—released by macrophages during infection,Citation26 it is conceivable that fungal lypolytic enzymes are able to modify the immune response by affecting the synthesis and/or degradation of these molecules.
In conclusion, our data show that secreted lipase of C. parapsilosis promotes the survival of fungal cells in macrophages and mitigate the inflammatory response of the host, thereby interfering with the efficient clearing of the pathogen. Further experiments are needed to determine the mechanisms of action of C. parapsilosis lipase on the induction of inflammatory mediators.
Materials and Methods
Candida strains and growth conditions
Candida parapsilosis sensu stricto strains (GA1 wild type [wt],Citation8 lipase mutant Δcplip1–2/Δcplip1–2 [lip−/−]Citation8) were grown overnight in liquid YPD medium (1% yeast extract, 2% bactopepton, and 2% glucose) at 30 °C. Cells were harvested by centrifugation, washed twice with PBS (phosphate-buffered saline; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4; pH 7.4) and counted in a Bürker-chamber prior to experiments.
Isolation of human peripheral blood mononuclear cells (PBMCs) and differentiation of monocyte-derived macrophages
Isolation of human PBMCs from buffy coats of healthy donors, under approval of the institutional review board of Szeged University, by Ficoll Paque PLUS (GE Healthcare) density gradient centrifugation was performed as previously described.Citation9 PBMCs were washed with PBS and treated with ACK lysis buffer (150 mM NH4Cl, 10 mM KHCO3, and 0.1 mM Na2EDTA) in order to eliminate erythrocytes. Isolated PBMCs were suspended in RPMI 1640 medium (Lonza) supplemented with 1% 100× penicillin–streptomycin solution (Sigma-Aldrich), and plated on 12-well cell culture plates (107 PBMCs/well) to isolate monocytes by plastic adherence. Plates were incubated for 2 h (37 °C, 5% CO2, and 100% relative humidity), and the attached monocytes were gently washed with PBS. To differentiate macrophages, isolated monocytes were cultured for 7 d in RPMI 1640 medium supplemented with 10% human serum (Lonza) and 1% 100× penicillin–streptomycin solution or in X-VIVO 15 medium (Lonza) supplemented with 10 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF, Sigma-Aldrich) and 1% 100× penicillin–streptomycin solution.
In vitro stimulation of monocyte-derived macrophages
Differentiated human macrophages were stimulated with wt and lip−/− C. parapsilosis strains in 12-well cell culture plates at an effector/target ratio of 1:5. Total RNA was isolated from macrophages at 3, 6, 14, and 24 h post-infection. Cell culture supernatants were collected after 24 h and stored at −20 °C until assayed for cytokine production.
Fluorescent microscopy
Human macrophages were cultured on round plastic slides in 24-well cell culture plates. Yeast cells were added to macrophages at an effector/target ratio of 1:5, and the co-cultures were incubated at 37 °C for 3 h. Subsequently, the samples were washed with PBS, and the slides were stained with 0.01% acridine orange dye (Sigma) for 45 s as described by Pruzanski and Saito.Citation27 This method allows live/dead cell differentiation, as acridin orange binding to intact (ds) DNA shows green fluorescence, while it emits red fluorescence when binding to degraded (ss) DNA in dead cells. Afterwards, the slides were gently washed with PBS and stained for 45 s with 0.05% crystal violet dye (Sigma) to quench the fluorescence of acridin orange originating from non-phagocytosed yeast cells. The slides were then rinsed 3 times with PBS and then coverslips were placed and sealed at the edge with nail polish. Samples were analyzed with a fluorescence microscope with appropriate filters in place (Olympus DP-72). The killing efficiency was calculated for each counted macrophage as follows: (number of dead yeast cells / number of engulfed yeast cells) × 100. Only macrophages that phagocytosed at least one yeast cell were included in the analysis.
Killing assay
Human macrophages (5 × 104/well) were co-incubated with wt or lip−/− C. parapsilosis strains in 96-well plastic cell culture plates at an effector/target ratio of 1:5. As a control, the same number of yeast cells were incubated in the appropriate cell culture medium without macrophages. After 3 h of incubation, macrophages were treated with distilled water and lysed using a 26-gauge needle. The lysates were serially diluted, plated on YPD agar plates and incubated for 2 d at 30 °C. After 2 d, the number of CFUs was determined and multiplied by the dilution factor to calculate the original cell number. The killing efficiency was calculated as follows: (number of live Candida cells in control wells − number of live Candida cells in co-cultures) / number of live Candida cells in control wells × 100.
Phagocytosis assay (flow cytometry)
C. parapsilosis strains (wt and lip−/−) were labeled with fluorescein-isothiocyanate (FITC, 1 mg/mL) in hydrogen-carbonate buffer (100 mM NaCl, 50 mM NaHCO3). After overnight incubation, cell suspensions were washed four times with PBS and adjusted to the proper concentration. Macrophages were co-cultured in 12-well plates with the FITC-labeled C. parapsilosis cells at a ratio of 1:5 for 2 h to allow for phagocytosis. After the incubation period, the cell culture medium was removed, and macrophages were washed twice with PBS in order to eliminate non-phagocytosed Candida cells. Subsequently, 500 µL TrypLE™ Express solution (Gibco) was added to the cultures and incubated for 45 min at 37 °C in order to detach cells from the bottom of cell culture plates. Macrophages were then gently removed by pipetting, and the collected cells were washed with FACS buffer (0.5% FBS in PBS), suspended in 400 µL FACS buffer and measured on a FACSCalibur instrument. Data were analyzed using the Flowing Software 2.5 software.
Quantitative imaging
Yeast cells were labeled with the fluorescent dye Alexa Fluor 647 carboxylic acid, succinimidyl ester (Invitrogen), as described,Citation9 and then washed four times and adjusted to the appropriate cell concentration. The labeled cells were co-cultivated with primary human macrophages and the co-cultures prepared for FACS analysis according to the methods described above, except measurements were performed on a FlowSight instrument (Amnis). Data were analyzed using the IDEAS Software (Amnis). For the analysis of phagosome-lysosome colocalization, macrophages were stained with 25 nM Lysotracker Green dye (Life Technologies) following phagocytosis.
RNA isolation, cDNA synthesis, and qRT-PCR
Total RNA from human macrophages was isolated using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. The concentration and integrity of isolated RNA was confirmed by Bioanalyzer (Agilent Technologies). The integrity index of RNA samples was ≥9. DNA was eliminated from RNA samples using the RNase-Free DNase Set (Qiagen) as instructed by the manufacturer. cDNA was synthesized from 500 ng total RNA using the RevertAidTM First Strand cDNA Synthesis Kit (Thermo Scientific) according to the manufacturer’s protocol. qRT-PCR was performed using Maxima SYBR Green qPCR Master Mix (Thermo Scientific), in a C1000TM Thermal Cycler (BIO-RAD) equipped with a CFX96™ Real-Time Detector System (BIO-RAD). β2-microglobulin (B2MG) was used as an endogenous control gene and fold changes were calculated by the ΔΔCt method.Citation28 PCR product specificity was confirmed by melting analysis. Primer sequences were as follows: B2MG, forward 5′-CCGTGTGAAC CATGTGACTT TGTC-3′, reverse 5′-GCTGCTTACA TGTCTCGATC CC-3′; PTGS-2, forward 5′-CACAAGATGG CAAAATGCTG-3′, reverse 5′-GCATCATGGA AGATGCATTG-3′; TNFA forward 5′-CACAGTGAAG TGCTGGCAAC-3′, reverse 5′-GTAGGCCCCA GTGAGTTCTG-3′; IL1B, forward 5′-TTCCAGGAGA ATGACCTGAG-3′, reverse 5′-TAAGCCTCGT TATCCCATGT-3′; IL6, forward 5′-TAAGGAGTTC CTGCAGTCCA-3′, reverse 5′-AGGAATGCCC ATTAACAACA-3′; IL10, forward, 5′-TTAAGGGTTA CCTGGGTTGC-3′, reverse 5′-GGTCTTGGTT CTCAGCTTGG-3′; IL8, forward 5′-GCTCTGTGTG AAGGTGCAGT-3′, reverse 5′-GGGTGGAAAG GTTTGGAGT-3′.
Cytokine measurements
The concentration of TNFα, IL-1β, IL-6, and IL-10 in cell culture supernatants was determined by DuoSet ELISA Kits (R&D Systems) according to the manufacturer’s instructions.
LDH assay
The concentration of lactate dehydrogenase in cell culture supernatants was measured using the Cytotoxicity Detection Kit (LDH; Roche) according to the manufacturer’s instructions.
Statistical analysis
Differences between groups were analyzed by paired t test using GraphPad Prism 5 software. Differences were considered statistically significant at P ≤ 0.05. All experiments were performed in duplicate or triplicate, with the different iterations using macrophages from at least three independent donors (see Results and figure legends for details).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP 4.2.4. A/2-11-1-2012-0001 “National Excellence Program”. A.G. is supported by OTKA NN100374, NF84006, and by EMBO Installation Grant 1813. A.G. supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. C.V. is partially supported by GOP-1.1.1-11-2011-0003.
References
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20:133 - 63; http://dx.doi.org/10.1128/CMR.00029-06; PMID: 17223626
- Trofa D, Gácser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev 2008; 21:606 - 25; http://dx.doi.org/10.1128/CMR.00013-08; PMID: 18854483
- Corvo I, O’Donoghue AJ, Pastro L, Pi-Denis N, Eroy-Reveles A, Roche L, McKerrow JH, Dalton JP, Craik CS, Caffrey CR, et al. Dissecting the active site of the collagenolytic cathepsin L3 protease of the invasive stage of Fasciola hepatica. PLoS Negl Trop Dis 2013; 7:e2269; http://dx.doi.org/10.1371/journal.pntd.0002269; PMID: 23875031
- Park M, Do E, Jung WH. Lipolytic enzymes involved in the virulence of human pathogenic fungi. Mycobiology 2013; 41:67 - 72; http://dx.doi.org/10.5941/MYCO.2013.41.2.67; PMID: 23874127
- Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev 2003; 67:400 - 28; http://dx.doi.org/10.1128/MMBR.67.3.400-428.2003; PMID: 12966142
- Horváth P, Nosanchuk JD, Hamari Z, Vágvölgyi C, Gácser A. The identification of gene duplication and the role of secreted aspartyl proteinase 1 in Candida parapsilosis virulence. J Infect Dis 2012; 205:923 - 33; http://dx.doi.org/10.1093/infdis/jir873; PMID: 22301631
- Brockerhoff H. Model of interaction of polar lipids, cholesterol, and proteins in biological membranes. Lipids 1974; 9:645 - 50; http://dx.doi.org/10.1007/BF02532169; PMID: 4414692
- Gácser A, Trofa D, Schäfer W, Nosanchuk JD. Targeted gene deletion in Candida parapsilosis demonstrates the role of secreted lipase in virulence. J Clin Invest 2007; 117:3049 - 58; http://dx.doi.org/10.1172/JCI32294; PMID: 17853941
- Németh T, Tóth A, Szenzenstein J, Horváth P, Nosanchuk JD, Grózer Z, Tóth R, Papp C, Hamari Z, Vágvölgyi C, et al. Characterization of virulence properties in the C. parapsilosis sensu lato species. PLoS One 2013; 8:e68704; http://dx.doi.org/10.1371/journal.pone.0068704; PMID: 23874732
- Gácser A, Stehr F, Kröger C, Kredics L, Schäfer W, Nosanchuk JD. Lipase 8 affects the pathogenesis of Candida albicans.. Infect Immun 2007; 75:4710 - 8; http://dx.doi.org/10.1128/IAI.00372-07; PMID: 17646357
- Trofa D, Soghier L, Long C, Nosanchuk JD, Gacser A, Goldman DL. A rat model of neonatal candidiasis demonstrates the importance of lipases as virulence factors for Candida albicans and Candida parapsilosis.. Mycopathologia 2011; 172:169 - 78; http://dx.doi.org/10.1007/s11046-011-9429-3; PMID: 21667319
- Trofa D, Agovino M, Stehr F, Schäfer W, Rykunov D, Fiser A, Hamari Z, Nosanchuk JD, Gácser A. Acetylsalicylic acid (aspirin) reduces damage to reconstituted human tissues infected with Candida species by inhibiting extracellular fungal lipases. Microbes Infect 2009; 11:1131 - 9; http://dx.doi.org/10.1016/j.micinf.2009.08.007; PMID: 19703582
- Hube B, Stehr F, Bossenz M, Mazur A, Kretschmar M, Schäfer W. Secreted lipases of Candida albicans: cloning, characterisation and expression analysis of a new gene family with at least ten members. Arch Microbiol 2000; 174:362 - 74; http://dx.doi.org/10.1007/s002030000218; PMID: 11131027
- Neugnot V, Moulin G, Dubreucq E, Bigey F. The lipase/acyltransferase from Candida parapsilosis: molecular cloning and characterization of purified recombinant enzymes. Eur J Biochem 2002; 269:1734 - 45; http://dx.doi.org/10.1046/j.1432-1327.2002.02828.x; PMID: 11895444
- Nagy I, Filkor K, Németh T, Hamari Z, Vágvölgyi C, Gácser A. In vitro interactions of Candida parapsilosis wild type and lipase deficient mutants with human monocyte derived dendritic cells. BMC Microbiol 2011; 11:122; http://dx.doi.org/10.1186/1471-2180-11-122; PMID: 21619700
- Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol 2011; 29:1 - 21; http://dx.doi.org/10.1146/annurev-immunol-030409-101229; PMID: 20936972
- Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 2008; 6:67 - 78; http://dx.doi.org/10.1038/nrmicro1815; PMID: 18079743
- Seider K, Brunke S, Schild L, Jablonowski N, Wilson D, Majer O, Barz D, Haas A, Kuchler K, Schaller M, et al. The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J Immunol 2011; 187:3072 - 86; http://dx.doi.org/10.4049/jimmunol.1003730; PMID: 21849684
- Vergne I, Fratti RA, Hill PJ, Chua J, Belisle J, Deretic V. Mycobacterium tuberculosis phagosome maturation arrest: mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol Biol Cell 2004; 15:751 - 60; http://dx.doi.org/10.1091/mbc.E03-05-0307; PMID: 14617817
- Strasser JE, Newman SL, Ciraolo GM, Morris RE, Howell ML, Dean GE. Regulation of the macrophage vacuolar ATPase and phagosome-lysosome fusion by Histoplasma capsulatum.. J Immunol 1999; 162:6148 - 54; PMID: 10229858
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140:805 - 20; http://dx.doi.org/10.1016/j.cell.2010.01.022; PMID: 20303872
- Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest 2006; 116:1642 - 50; http://dx.doi.org/10.1172/JCI27114; PMID: 16710478
- Hammond ME, Lapointe GR, Feucht PH, Hilt S, Gallegos CA, Gordon CA, Giedlin MA, Mullenbach G, Tekamp-Olson P. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J Immunol 1995; 155:1428 - 33; PMID: 7636208
- Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol 2006; 38:1654 - 61; http://dx.doi.org/10.1016/j.biocel.2006.03.021; PMID: 16713323
- Volk HD. Immunodepression in the surgical patient and increased susceptibility to infection. Crit Care 2002; 6:279 - 81; http://dx.doi.org/10.1186/cc1507; PMID: 12225595
- Suram S, Silveira LJ, Mahaffey S, Brown GD, Bonventre JV, Williams DL, Gow NA, Bratton DL, Murphy RC, Leslie CC. Cytosolic phospholipase A(2)α and eicosanoids regulate expression of genes in macrophages involved in host defense and inflammation. PLoS One 2013; 8:e69002; http://dx.doi.org/10.1371/journal.pone.0069002; PMID: 23950842
- Pruzanski W, Saito S. Comparative study of phagocytosis and intracellular bactericidal activity of human monocytes and polymorphonuclear cells. Application of fluorochrome and extracellular quenching technique. Inflammation 1988; 12:87 - 97; http://dx.doi.org/10.1007/BF00915894; PMID: 3366485
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402 - 8; http://dx.doi.org/10.1006/meth.2001.1262; PMID: 11846609
