Abstract
Fungal melanins are important in the virulence of many pathogenic fungi. In this study, we examined the role of melanin in the interaction between Cryptococcus neoformans and the invertebrate host, Galleria mellonella. C. neoformans was able to melanize in the presence of G. mellonella homogenate, indicating the presence of melanin substrates. Melanization was confirmed by the recovery of acid-resistant particles that were recognized by anti-melanin antibodies. In addition, we tested the effect of fungal melanization on virulence. Surprisingly, G. mellonella larvae infected with melanized fungal cells lived longer than those infected with non-melanized fungi. When the cellular immune response of G. mellonella to melanized and non-melanized cells was compared, inflammatory nodules were observed in both groups. However the response was stronger in larvae infected with melanized cells. These results suggest that fungal melanin activates the immune response of G. mellonella, thereby resulting in the decreased virulence observed with melanized cells.
Introduction
Infectious disease research relies on experimental animal models for investigation of virulence, treatment, and immunity. Vertebrates, such as mice, are traditional models of choice for these studies. However, concerns about animal welfare and costs have prompted researchers to seek out alternatives. Larvae of the waxmoth, Galleria mellonella, are an increasingly popular alternative host for the study of numerous fungal and bacterial pathogens.Citation1,Citation2 Some of the advantages to using the larvae are their low cost, commercial availability, and ease of use.Citation3
Cryptococcus neoformans is a human fungal pathogen that causes one million cases of cryptococcosis annually worldwide.Citation4 Disease is prevalent among populations that are immune compromised, such as AIDS patients. C. neoformans infections result from the inhalation of spores or yeast into the lungs. In the absence of an effective immune response, infection of the brain and central nervous system can lead to life-threatening illness.Citation5 In the laboratory, C. neoformans can infect a variety of organisms, including mice, insects, amoeba and plants.Citation6-Citation8G. mellonella larvae are susceptible to systemic infection with C. neoformans. Furthermore, several C. neoformans virulence factors that are important in mammals are also important in infection of G. mellonella, including laccase, an enzyme that is required for the production of melanin.Citation9
Melanins are pigments that are found in the cell walls of many fungal pathogens and have roles in fungal virulence as well as survival in the environment. Melanins are polymers of phenolic or indolic subunits. They share certain physical and chemical characteristics, such as dark color, low solubility and resistance to acid hydrolysis. Research suggests that fungal melanin is composed of aggregates of small particles or granules. Exogenous substrates, such as the catecholamine l-3,4-dihydroxyphenylalanine (l-DOPA) are required for melanin production by C. neoformans. Synthesis of melanin occurs when laccase catalyzes the initial oxidation of l-DOPA to form dopaquinone, a highly reactive molecule. Further spontaneous steps produce melanin, an amorphous polymer.Citation10-Citation12
Melanin also plays a central role in the host defense of Galleria mellonella and other invertebrates. These organisms have an innate immune system that consists of both humoral and cellular defenses. The humoral responses include a variety of secreted molecules with antimicrobial properties, including phenoloxidase, which defends against pathogens by catalyzing the formation of melanin and toxic melanin intermediates. The cellular immune responses include phagocytosis and nodulation. Nodulation occurs when microbes are recognized by the immune system and surrounded by layers of hemocytes, where they may be trapped and killed. The humoral and cellular components can also work together as melanization occurs inside of nodules.Citation13,Citation14
The goal of this study was to analyze the role of melanin in the interaction of C. neoformans and G. mellonella. Specifically, we tested for the presence of melanin substrates in G. mellonella larvae. In addition, we examined the effect of fungal melanization on virulence and host defense.
Results
Detection of melanin substrates in G. mellonella larvae
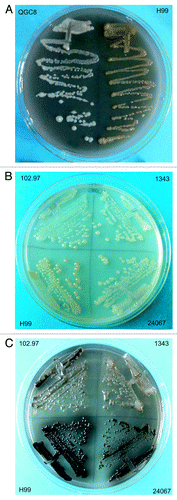
The ability of C. neoformans to produce melanin depends on the availability of substrates. Therefore, we tested for the presence of melanin substrates in Galleria mellonella larvae. This information is key to understanding the role of laccase and melanization in the virulence of C. neoformans in G. mellonella. Medium was made with homogenized larvae and inoculated with C. neoformans. After five days of incubation, growth of the wild-type strain appeared light tan. The color intensity increased over time until a brown color was achieved at two weeks of incubation (). Longer incubation times did not produce darker pigmentation, nor did doubling the concentration of larvae homogenate in the medium (data not shown). Furthermore, pigmentation was dependent on the laccase activity of C. neoformans, because a laccase deficient mutant did not become pigmented on this medium (). In general, the pigmentation observed with G. mellonella homogenate was not as dark as with l-DOPA. Additional wild-type cryptococcal strains of other serotypes were tested for melanization in the presence of G. mellonella homogenate. Of the strains tested, H99 (serotype A) appeared the most pigmented in the presence of l-DOPA or Galleria homogenate ().
Figure 1. Melanization of C. neoformans in the presence of G. mellonella homogenate. (A) Wild type (right) or laccase deletion (left) strains of C. neoformans were inoculated onto G. mellonella medium. Plates were incubated for 2 wk at 30 °C. (B and C) Indicated cryptococcal isolates were incubated in medium with G. mellonella homogenate (B) or 1 mM l-DOPA (C), including H99 (serotype A), 24067 (serotype D), 102.97 (serotype B), and 1343 (serotype D). Plates were incubated for 2 wk at 32 °C.
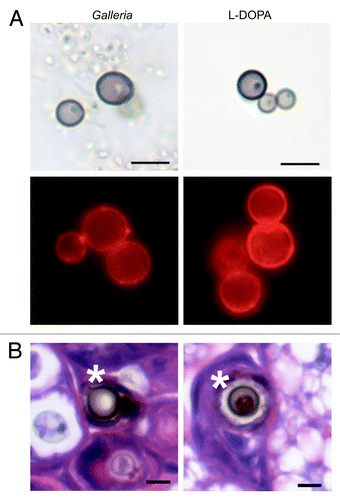
The pigment produced in the presence of G. mellonella homogenate was analyzed to determine whether it was melanin. Acid-resistant particles resembling cryptococcal cells (melanin “ghosts”) were recovered from cultures grown in the presence of homogenate (). Furthermore, the particles were detected by immunofluorescence with an anti-melanin antibody (). No fluorescence was observed when the particles were incubated with the secondary antibody alone (data not shown). On the basis of dark color, acid insolubility, and reactivity with a melanin-binding mAb we tentatively concluded that the pigment was a melanin. Next, histology of larvae infected with C. neoformans was performed to determine whether melanized cells could be detected in G. mellonella. C. neoformans cells surrounded by a brown pigment were observed in the histological sections (). Together, these data suggested that melanization of C. neoformans in G. mellonella larvae is possible.
Figure 2. Analysis of melanin produced by C. neoformans. (A) Melanin “ghosts” were isolated from fungal cells grown in the presence of G. mellonella homogenate or l-DOPA. The melanin ghosts were visualized by light microscopy (top row) and immunofluorescence with a melanin-binding antibody (bottom row). (B) G. mellonella was infected with C. neoformans strain H99. After 5 d, larvae were sacrificed and fixed. Sections were stained with hematoxylin and eosin. Asterisks indicate pigmented C. neoformans cells. Scale bars, 5 μm.
Effect of pre-melanization of C. neoformans on virulence
To determine the optimal dosage of fungi, larvae were inoculated with 105, 106, or 107 CFUs. Larvae infected with 105 CFUs survived over one week, while inoculums containing more than 105 CFUs killed the caterpillars in less than one week (data not shown). Therefore, inoculums of 105 CFU were used in subsequent trials. Next, the effect of melanization on virulence was tested by infecting larvae with wild type C. neoformans cells that had been cultured with or without l-DOPA. At 32 °C pre-melanization of C. neoformans decreased virulence in G. mellonella or had no effect (; ). In three out of four separate trials the mean survival times for larvae infected with melanized cells was longer than for those infected with non-melanized cells. The experiment was then repeated at human physiological temperature. A slight decrease in virulence of melanized cells was observed at 37 °C (). The effect of laccase on virulence was evaluated under similar conditions. There was no difference in the survival of G. mellonella infected with either a wild-type strain (H99) or a double laccase mutant (QGC8) at both 32 °C and 37 °C (P > 0.05).
Figure 3. Kaplan–Meier survival curves for larvae infected with melanized and non-melanized C. neoformans cells. Larvae infected with melanized C. neoformans survived longer than those infected with non-melanized cells. The results of one representative infection are shown (n = 10).
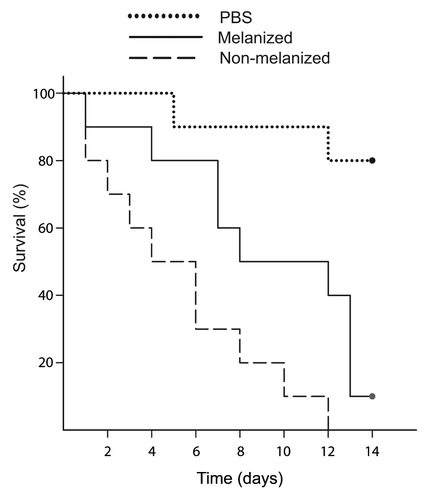
Table 1. Summary of survival experiments
Since there was some variability in the survival results, the experiment was repeated with larvae from another supplier (Vanderhorst). Although there was a trend toward longer survival of larvae infected with melanized cells, the results were not significant (). Thus, we were more likely to observe a significant difference between melanized and non-melanized cells when using larvae supplied from New York Worms rather than Vanderhorst.
Fungal burden was assessed in addition to virulence. For both melanized and non-melanized cells, there was little change in the amount of CFUs present in the larvae at day two. By day five, both melanized and non-melanized cells had increased CFUs by approximately 100-fold. To determine how well the yeast reproduced in the G. mellonella larvae, a growth curve was generated by plotting the starting inoculum and day five fungal burden. The slope of the resulting line was used as a measure of growth. The growth of the non-melanized C. neoformans was higher than the melanized (6.6 × 106 and 1.29 × 106 CFU/day, respectively). However, this trend was not significant (P > 0.05).
Nodule formation in response to melanized fungi
To determine whether there were differences in the fates of melanized and non-melanized fungal cells, infected larvae were analyzed by histology. Larvae were fixed at 2, 5, and 8 d post infection, corresponding to early, middle, and late time points, respectively. Fixed larvae were embedded in paraffin and stained with hematoxylin and eosin. The size and number of nodules in each larva was measured (). At day 2 post-infection, a robust cellular immune response was observed, with fungal cells visible inside inflammatory nodules (). This response was stronger in larvae infected with melanized cells. In these larvae the nodules were significantly larger. The average nodule size was 4298 μm2 for the melanized group and 1490 μm2 for the non-melanized group (P < 0.02). At day 2, there was a trend toward more nodules in the melanized group.
Table 2. Nodule formation in G. mellonella larvae
Figure 4. Histology of infected larvae. (A) Two or five days after infection with C. neoformans, larvae were sacrificed and fixed. Sections were stained with hematoxylin and eosin. C. neoformans cells were observed surrounded by hemocytes in inflammatory nodules. Arrows point to nodules Scale bar, 10 μm. (B) Left: five-day larvae with some tissue damage. Right: seven-day larvae with extensive tissue damage. Scale bars, 100 μm.
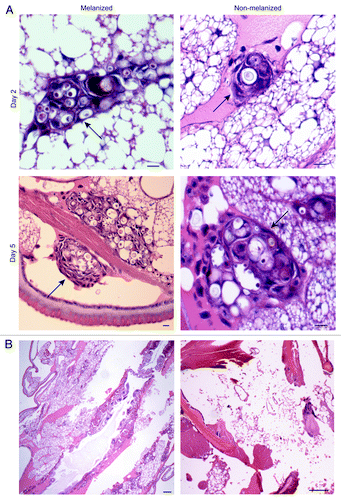
By day 5, very large nodules were observed with numerous yeast cells inside (). Similar to day 2, the melanized group had significantly larger nodules (11 417 μm2 for melanized and 5096 μm2 for nonmelanized (P < 0.02). There was no consistent trend in the number of nodules at day 5 for the melanized vs. non-melanized groups. Some tissue damage was apparent, with empty spaces seen in the larvae sections for both the melanized and non-melanized groups, which possibly confounded the nodule counts (). By 7 d post-infection, the interiors of the larvae showed extensive tissue damage and large areas were filled with fungal cells for both groups ().
Since the histology results suggested an enhanced immune response in the larvae infected with melanized cells, a qualitative evaluation of phenoloxidase activation was made by observing the color of the larvae. No obvious differences in melanization were observed between the melanized and non-melanized groups. However, the larvae from New York worms were more heavily melanized than the Vanderhorst larvae ().
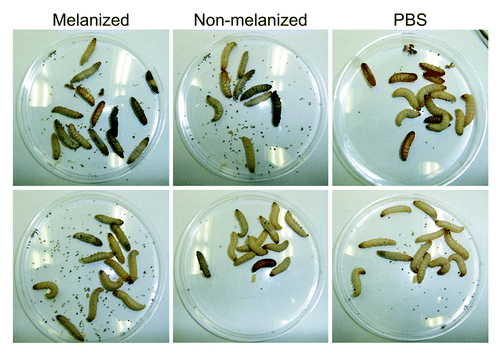
Figure 5. Melanization of infected larvae. Larvae from each group at 32 °C were photographed at day 5 post-infection. Top row: larvae from New York Worms. Bottom row: larvae from Vanderhorst. Each group contained both live and dead larvae. The number of dead larvae in each group was 6, 5, and 1 (top row, left to right) and 3, 3, and 0 (bottom row, left to right).
Discussion
Melanin is produced by many fungal pathogens and has an important role in the ability of fungi to infect hosts. G. mellonella are a useful tool in infectious disease research. Studies can be done easily and with low cost. In this study, the role of melanization in C. neoformans infection of G. mellonella larvae was analyzed. Specifically, our goal was to address the potential use of the G. mellonella model for investigating melanin as a virulence factor.
C. neoformans cannot produce melanin without being supplied a substrate. This trait has allowed researchers to easily control melanin production and determine that it is important for C. neoformans to survive UV irradiation, predation by amoeba, and killing by host phagocytes.Citation8,Citation15,Citation16 A fundamental question in understanding the role of melanin in the interaction of C. neoformans and G. mellonella is whether melanin substrates are present in the larvae. We observed laccase-dependent pigment production when C. neoformans was cultured on medium containing G. mellonella homogenate. Acid-resistant particles that were recognized by an anti-melanin antibody were recovered from the homogenate cultures. Together, these results suggest that melanin substrates are present in the larvae and that C. neoformans may be able to produce melanin inside of the larvae. Pigmented cells were visible in histology sections of infected larvae. However the possibility that this was melanin produced by G. mellonella phenoloxidase cannot be ruled out.
Melanin is an established virulence factor in numerous fungal species. To further explore the question of melanin and virulence, we assessed melanized and non-melanized cells in G. mellonella infection by growing wild-type cells with l-DOPA. In a mouse model, pre-melanization of C. neoformans resulted in higher fungal burdens in the lungs and brain at early times post-infection.Citation17 This suggested that melanin is important for fungal virulence in mice. In contrast, our results suggest that melanin is not a critical virulence factor in G. mellonella. Pre-melanization of C. neoformans with l-DOPA resulted in decreased virulence in G. mellonella. In addition, the presence of the genes for laccase (LAC1 and LAC2) had no effect on virulence under the experimental conditions of this study. This was somewhat surprising since previous research showed that a LAC1 deletion strain had reduced virulence in G. mellonella.Citation9 However, this may be explained by the fact that the studies were done using C. neoformans strains with different genetic backgrounds (serotype A and serotype D). The two serotypes diverged over 18 million years ago in evolutionCitation18 and differences in their overall virulence have been reported in miceCitation19 as well as in G. mellonella.Citation9 Furthermore, the effects of certain virulence factors varied between serotypes A and D, including protein kinase A,Citation20 mating type,Citation21 and macrophage killing.Citation22
We found variability in the survival of larvae in our experiments, including larvae from different suppliers as well as different batches of larvae from the same supplier. The variability was not surprising since G. mellonella larvae are not maintained for scientific research purposes and there may be differences in overall health, genetics, and rearing conditions that affect the outcome of survival experiments. Previous research showed that cold or hot thermal shock of the larvae resulted in differences in survival outcomes and activation of the immune response.Citation23 This implies that results may be affected by temperatures experienced by the larvae during storage and transport. Larvae in our experiments were stored at cold temperature prior to infection, which may have enhanced the immune effects and differences in survival.
Although the virulence results were surprising in terms of what is known about melanin as a virulence factor, they agree with other studies on fungal virulence in G. mellonella. The decreased virulence of the melanized C. neoformans cells in G. mellonella is consistent with what has been observed with Aspergillus fumigatus. This fungus produces melanin via a multi-enzyme polyketide synthase pathway. When color mutants in this pathway were used to infect G. mellonella, many of them were more virulent than the melanin-producing parent strain.Citation24 Similarly, when the virulence of multiple pathogenic and non-pathogenic Cryptococcus species was tested in G. mellonella the ability of the fungi to melanize (among other known virulence factors) did not predict virulence.Citation25
A potential explanation for the decreased virulence of melanized cells lies in the immune response of the larvae. Like other invertebrates, G. mellonella has a phenoloxidase system that defends against microbes using melanin and melanin-intermediates. Phenoloxidase is found in a proenzyme form in insects. It gets activated when microbial components (such as β-1,3-glucan or peptidoglycan) are recognized and bound by proteins that initiate a serine protease cascade that results in cleavage of the prophenoloxidase.Citation26 Phenoloxidase then catalyzes the formation of melanin from l-DOPA or similar precursors.Citation27 It is possible that the decreased virulence was due to an interaction of fungal melanin with the phenoloxidase pathway. The increased nodule formation by the host in the presence of melanized C. neoformans supports the hypothesis that the G. mellonella immune system responds to fungal melanin. Thus, the decreased virulence observed may be due to an increased immune response in G. mellonella.
Materials and Methods
Strains and larvae
C. neoformans strains H99, QGC8, and 24067 were used in this study. H99 (serotype A) and 24067 (serotype D) are clinical isolates. QGC8 is a laccase mutant in which the LAC1 and LAC2 genes are deleted in the H99 background.Citation28 The C. neoformans var gattii isolates 102.97 (serotypes B) and 1343 (serotype C) were obtained from Stuart Chaskes. G. mellonella larvae were obtained from New York Worms and Vanderhorst Wholesale, Inc. Larvae were stored at 4 °C prior to experiments.
Medium and culture conditions
Fungal cells were prepared for infection by culturing the C. neoformans H99 strain in chemically defined minimal medium (15 mM dextrose, 10 mM magnesium sulfate, 29.4 mM potassium phosphate, 13 mM glycine, 3 μM thiamine, pH 5.5) at 30 °C and 150 RPM for one week. To obtain melanized cells, l-DOPA (1 mM) was added to the cultures.
G. mellonella larvae medium
G. mellonella larvae were homogenized in 2× chemically defined minimal medium. Homogenization was performed on ice. The homogenate was centrifuged to pellet debris (5 min at 2500 rpm, 4 °C) and filter sterilized. The resulting solution was mixed with molten agar (3%) and immediately poured into petri dishes. The medium was inoculated the following day. Twenty larvae (approximately 5 g) were homogenized to make 200 mL of medium. The liquid medium was made in a similar manner, but without the addition of agar. The medium was briefly heated to inactivate G. mellonella phenoloxidase.
Infection of G. mellonella
Washed fungal cells were suspended in PBS at a concentration of 5 × 106 CFU/mL. G. mellonella larvae weighing approximately 250 mg (± 25) were selected and randomly sorted into equal sized groups per treatment. G. mellonella larvae were infected by injection into the last proleg. A repeating dispenser fitted with a disposable syringe delivered precisely 20 μL of inoculum to each larva. Larvae were infected with 1 × 105 CFU. To prevent contamination with bacteria, streptomycin (20 mg/kg bodyweight) was administered. After infection, larvae were placed in a sterile petri dish and maintained in a 32 or 37 ± 2 °C incubator. G. mellonella survival was monitored daily. Between 10 and 15 larvae were infected per treatment group. This sample size represents a power of 0.53–0.69 to correctly reject the null hypothesis that melanized and non-melanized C. neoformans have equal virulence in G. mellonella (with an α of 0.05 and an effect size of 0.8). Fungal burden was determined by sacrificing 4–5 larvae on days 2 and 5 post-infection and homogenizing the larvae in PBS with a hand-held homogenizer equipped with a 7 mm probe (Omni International). Separate infections were performed for the survival, histology, and fungal burden analyses.
Immunofluorescence of melanin ghosts
C. neoformans strain H99 was cultured in medium with G. mellonella larvae homogenate for approximately 1 mo. Melanin was then isolated from the cultures in a multi-step procedure that included enzymatic digestion, chemical denaturation, chloroform extraction, and boiling in concentrated acid, as described previously.Citation29 The resulting particles were analyzed by immunofluorescence with a melanin-binding monoclonal antibody (6D2).Citation30 mAb 6D2 was purified from cell culture supernatants. The secondary antibody was a goat anti-mouse IgM conjugated to TRITC. Each antibody was diluted to a concentration of 10 μg/mL in 1% BSA. Antibody incubations were done for 1 h at 37 °C. As a control, the particles were incubated with secondary antibody alone, showing that 6D2 binding to the melanin ghosts was required to observe fluorescence.
Analysis of virulence
Three groups of larvae were infected for each experiment: (1) melanized C. neoformans (2) non-melanized C. neoformans, and (3) PBS control. The number of surviving larvae was recorded daily. Larvae were considered dead if they did not respond to sustained touch stimulus. Kaplan–Meier survival curves were created with the statistics program SPSS 18 (SPSS, Inc.). Differences between treatment groups were compared by the Log-rank method.
Fixation and histology
At indicated time points larvae were collected and incubated on ice for 15 min. Larvae were then fixed in 4% paraformaldehyde in PBS for 10 d. A needle was used to poke holes in the larvae to facilitate entry of fixative. After 10 d, the larvae were transferred to 30% sucrose overnight, and then transferred to 70% ethanol for storage. Fixation was performed at 4 °C. Larvae were embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Analysis of slides
Slides were scanned visually with a microscope to identify nodules. Digital microscope images of the nodules were analyzed with ImageJ software (NIH) to determine their size by the following method. First, a scale of pixels to microns was established with a stage micrometer. The size was then determined by drawing an outline around each nodule and measuring its area with the software. Nodule area was analyzed using the non-parametric Wilcoxon Rank Sums test. A P value < 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We wish to thank Arturo Casadevall and Monica Garcia-Solache for helpful discussions and suggestions. We thank Antonio Nakouzi and Johanna Rivera for assistance with the immunofluorescence analysis. In addition, we thank the Histotechnology and Comparative Pathology Facility at Albert Einstein College of Medicine. Support for this project was provided by a PSC-CUNY Award, jointly funded by The Professional Staff Congress and The City University of New York, and the Eugene M. Lang Junior Faculty Research Fellowship of Baruch College. Some of the experiments were supported by NIH grant 5R01AI052733-10.
References
- Ramarao N, Nielsen-Leroux C, Lereclus D. The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. J Vis Exp 2012; e4392; PMID: 23271509
- Lionakis MS. Drosophila and Galleria insect model hosts: new tools for the study of fungal virulence, pharmacology and immunology. Virulence 2011; 2:521 - 7; http://dx.doi.org/10.4161/viru.2.6.18520; PMID: 22186764
- Mylonakis E. Galleria mellonella and the study of fungal pathogenesis: making the case for another genetically tractable model host. Mycopathologia 2008; 165:1 - 3; http://dx.doi.org/10.1007/s11046-007-9082-z; PMID: 18060516
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009; 23:525 - 30; http://dx.doi.org/10.1097/QAD.0b013e328322ffac; PMID: 19182676
- Sabiiti W, May RC. Mechanisms of infection by the human fungal pathogen Cryptococcus neoformans.. Future Microbiol 2012; 7:1297 - 313; http://dx.doi.org/10.2217/fmb.12.102; PMID: 23075448
- Xue C, Tada Y, Dong X, Heitman J. The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell Host Microbe 2007; 1:263 - 73; http://dx.doi.org/10.1016/j.chom.2007.05.005; PMID: 18005707
- Apidianakis Y, Rahme LG, Heitman J, Ausubel FM, Calderwood SB, Mylonakis E. Challenge of Drosophila melanogaster with Cryptococcus neoformans and role of the innate immune response. Eukaryot Cell 2004; 3:413 - 9; http://dx.doi.org/10.1128/EC.3.2.413-419.2004; PMID: 15075271
- Steenbergen JN, Shuman HA, Casadevall A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci U S A 2001; 98:15245 - 50; http://dx.doi.org/10.1073/pnas.261418798; PMID: 11742090
- Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, Ausubel FM, Diener A. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun 2005; 73:3842 - 50; http://dx.doi.org/10.1128/IAI.73.7.3842-3850.2005; PMID: 15972469
- Eisenman HC, Casadevall A. Synthesis and assembly of fungal melanin. Appl Microbiol Biotechnol 2012; 93:931 - 40; http://dx.doi.org/10.1007/s00253-011-3777-2; PMID: 22173481
- Nosanchuk JD, Casadevall A. The contribution of melanin to microbial pathogenesis. Cell Microbiol 2003; 5:203 - 23; http://dx.doi.org/10.1046/j.1462-5814.2003.00268.x; PMID: 12675679
- Williamson PR. Laccase and melanin in the pathogenesis of Cryptococcus neoformans.. Front Biosci 1997; 2:e99 - 107; PMID: 9342305
- Gillespie JP, Kanost MR, Trenczek T. Biological mediators of insect immunity. Annu Rev Entomol 1997; 42:611 - 43; http://dx.doi.org/10.1146/annurev.ento.42.1.611; PMID: 9017902
- Kavanagh K, Reeves EP. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev 2004; 28:101 - 12; http://dx.doi.org/10.1016/j.femsre.2003.09.002; PMID: 14975532
- Wang Y, Aisen P, Casadevall A. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect Immun 1995; 63:3131 - 6; PMID: 7622240
- Wang Y, Casadevall A. Decreased susceptibility of melanized Cryptococcus neoformans to UV light. Appl Environ Microbiol 1994; 60:3864 - 6; PMID: 7986054
- Bryan RA, Jiang Z, Howell RC, Morgenstern A, Bruchertseifer F, Casadevall A, Dadachova E. Radioimmunotherapy is more effective than antifungal treatment in experimental cryptococcal infection. J Infect Dis 2010; 202:633 - 7; http://dx.doi.org/10.1086/654813; PMID: 20594103
- Xu J, Vilgalys R, Mitchell TG. Multiple gene genealogies reveal recent dispersion and hybridization in the human pathogenic fungus Cryptococcus neoformans.. Mol Ecol 2000; 9:1471 - 81; http://dx.doi.org/10.1046/j.1365-294x.2000.01021.x; PMID: 11050543
- Barchiesi F, Cogliati M, Esposto MC, Spreghini E, Schimizzi AM, Wickes BL, Scalise G, Viviani MA. Comparative analysis of pathogenicity of Cryptococcus neoformans serotypes A, D and AD in murine cryptococcosis. J Infect 2005; 51:10 - 6; http://dx.doi.org/10.1016/j.jinf.2004.07.013; PMID: 15979484
- Hicks JK, D’Souza CA, Cox GM, Heitman J. Cyclic AMP-dependent protein kinase catalytic subunits have divergent roles in virulence factor production in two varieties of the fungal pathogen Cryptococcus neoformans.. Eukaryot Cell 2004; 3:14 - 26; http://dx.doi.org/10.1128/EC.3.1.14-26.2004; PMID: 14871933
- Nielsen K, Cox GM, Wang P, Toffaletti DL, Perfect JR, Heitman J. Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and alpha isolates. Infect Immun 2003; 71:4831 - 41; http://dx.doi.org/10.1128/IAI.71.9.4831-4841.2003; PMID: 12933823
- Ben-Abdallah M, Sturny-Leclère A, Avé P, Louise A, Moyrand F, Weih F, Janbon G, Mémet S. Fungal-induced cell cycle impairment, chromosome instability and apoptosis via differential activation of NF-κB. PLoS Pathog 2012; 8:e1002555; http://dx.doi.org/10.1371/journal.ppat.1002555; PMID: 22396644
- Mowlds P, Kavanagh K. Effect of pre-incubation temperature on susceptibility of Galleria mellonella larvae to infection by Candida albicans.. Mycopathologia 2008; 165:5 - 12; http://dx.doi.org/10.1007/s11046-007-9069-9; PMID: 17922218
- Jackson JC, Higgins LA, Lin X. Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella.. PLoS One 2009; 4:e4224; http://dx.doi.org/10.1371/journal.pone.0004224; PMID: 19156203
- Garcia-Solache MA, Izquierdo-Garcia D, Smith C, Bergman A, Casadevall A. Fungal virulence in a lepidopteran model is an emergent property with deterministic features. MBio 2013; 4:e00100 - 13; http://dx.doi.org/10.1128/mBio.00100-13; PMID: 23631914
- Cerenius L, Lee BL, Söderhäll K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol 2008; 29:263 - 71; http://dx.doi.org/10.1016/j.it.2008.02.009; PMID: 18457993
- Gonzalez-Santoyo I, Cordoba-Aguilar A. Phenoloxidase: A key component of the insect immune system. Entomol Exp Appl 2012; 142:1 - 16; http://dx.doi.org/10.1111/j.1570-7458.2011.01187.x
- Pukkila-Worley R, Gerrald QD, Kraus PR, Boily MJ, Davis MJ, Giles SS, Cox GM, Heitman J, Alspaugh JA. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot Cell 2005; 4:190 - 201; http://dx.doi.org/10.1128/EC.4.1.190-201.2005; PMID: 15643074
- Eisenman HC, Mues M, Weber SE, Frases S, Chaskes S, Gerfen G, Casadevall A. Cryptococcus neoformans laccase catalyses melanin synthesis from both D- and L-DOPA. Microbiology 2007; 153:3954 - 62; http://dx.doi.org/10.1099/mic.0.2007/011049-0; PMID: 18048910
- Rosas AL, Nosanchuk JD, Feldmesser M, Cox GM, McDade HC, Casadevall A. Synthesis of polymerized melanin by Cryptococcus neoformans in infected rodents. Infect Immun 2000; 68:2845 - 53; http://dx.doi.org/10.1128/IAI.68.5.2845-2853.2000; PMID: 10768981
