Abstract
Bacterial pathogens, including those of humans, animals, and plants, encounter phosphate (Pi)-limiting or Pi-rich environments in the host, depending on the site of infection. The environmental Pi-concentration results in modulation of expression of the Pho regulon that allows bacteria to regulate phosphate assimilation pathways accordingly. In many cases, modulation of Pho regulon expression also results in concomitant changes in virulence phenotypes. Under Pi-limiting conditions, bacteria use the transcriptional-response regulator PhoB to translate the Pi starvation signal sensed by the bacterium into gene activation or repression. This regulator is employed not only for the maintenance of bacterial Pi homeostasis but also to differentially regulate virulence. The Pho regulon is therefore not only a regulatory circuit of phosphate homeostasis but also plays an important adaptive role in stress response and bacterial virulence. Here we focus on recent findings regarding the mechanisms of gene regulation that underlie the virulence responses to Pi stress in Vibrio cholerae, Pseudomonas spp., and pathogenic E. coli.
Introduction
Phosphorous (P) is the sixth most abundant element in living organisms. Its primary biological importance in bacteria, in the form of phosphate (Pi), is as a component of nucleotides, which serve as energy storage within cells (ATP) or when linked together to form the nucleic acids DNA and RNA. The Pi group is also an important component of membrane phospholipids and it is incorporated into proteins during post-translational modifications, particularly as a regulatory device such as employed during signal transduction of bacterial two-component systems (TCS). For bacteria, the primary sources of P are inorganic phosphate, pyrophosphate and metaphosphate. They are acquired by the Pi-specific transport system (Pst) and low Pi-affinity transporter (Pit). Secondary P sources used by bacteria are phosphonates and organophosphates (i.e., Glycerol-3-P) that require separate transporters (Ugp and Phn systems) and enzymatic hydrolysis by alkaline phosphatase (PhoA).
In this review, we focus on recent findings in the mechanisms of gene regulation that underlie the virulence responses to Pi stress in Vibrio cholerae, Pseudomonas spp., and pathogenic E. coli. We also briefly discuss the relationship between Pi regulation and bacterial virulence in additional non-enteric pathogens.
The Pho Regulon and Pi Availability
TCSs are signal transduction pathways commonly used by prokaryotes to sense and adapt to stimuli in the environment. As many as 50 different TCSs may exist in a single bacterium such as E. coli which has at least 30 TCSs.Citation1-3 These systems are characterized by a sensor histidine-kinase (HK) that undergoes auto phosphorylation on a histidine residue. This phosphoryl group is then transferred to an aspartate residue of a response regulator protein that usually acts as a transcriptional activator (). In E. coli, there are at least 40 gene members of the Pho regulon that are primarily involved in Pi assimilation and metabolism.Citation4-6 The core members of the Pho regulon comprise 9 transcriptional units: eda, phnCDEFGHIJKLMNOP, phoA, phoBR, phoE, phoH, psiE, pstSCAB-phoU, and ugpBAECQ. These units encode proteins which ensure Pi homeostasis including the PhoB-R TCS, alkaline phosphatase and transporters of Pi, carbon-P, glycerol-3-P, and phosphonates. Transcription of genes within the Pho regulon is regulated in response to extracellular Pi concentrations via the TCS PhoB-R. The Pst system also plays an important role in Pi homeostasis. It has two related but distinct functions: high-affinity uptake of Pi (in low Pi conditions = Pho ON) and the maintainance of Pho regulon expression at basal level (in high Pi conditions = Pho OFF).Citation7 The pst operon (pstSCAB–phoU) encodes a transport complex belonging to the ABC transporter superfamily. The periplasmic protein PstS binds Pi that is able to freely diffuse across the Gram-negative outer membrane, whereas PstA and PstC are membrane channel-forming proteins. The ATPase PstB provides the energy for translocation and interacts with PstA and PstC.Citation8 PhoU has no evident role in Pi transport, but is required for Pho regulon expression.
Figure 1. Model for transcription regulation by PhoB and transmembrane signal transduction by environmental Pi and the Pst system in E. coli (adapted from refs. 5 and 56). Two processes are involved in Pho regulon control: inhibition when Pi is in excess and activation under Pi limitation. The Pst transport system is associated with the PhoR histidine kinase/phosphatase, which controls the phosphorylation state of the response regulator PhoB. At high Pi concentrations (>4 μM), PhoR might interact with the PstSCAB-PhoU complex, which represses its autophosphorylation and acts as a PhoB phosphatase. In contrast, low extracellular Pi concentrations sensed by Pst system, which increases the kinase activity of PhoR, resulting in the accumulation of phosphorylated PhoB∼P. Likewise, loss-of-function mutations in the pst genes lead to the constitutive phosphorylation of PhoB, regardless of the external Pi concentration. The Pi binding protein PstS acts as the primary sensor of external Pi concentration. When the Pi concentration is low (<4 μM), PhoR undergoes conformational change, resulting in its release from the repressor complex and autophosphorylation. PhoR is the histidine kinase/phosphatase that donates a phosphoryl group to PhoB when environmental Pi is limiting and removes the phosphoryl group from PhoB∼P when environmental Pi is abundant. PhoB is a typical winged-helix response regulator that upon aspartyl phosphorylation forms a dimer, which binds to DNA sequences upstream of Pho regulon genes to recruit RNA polymerase (RNAP) and initiate transcription. In some cases the transcription is repressed possibly by disruption of RNAP interaction with other activators.
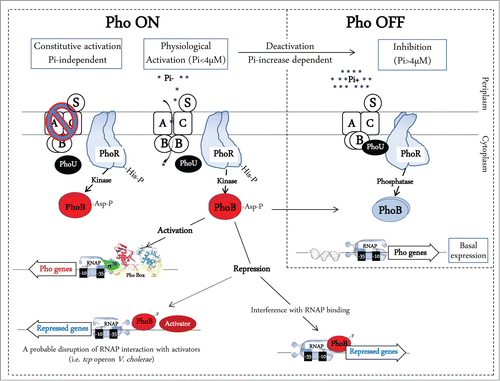
During Pi limitation, the HK PhoR phosphorylates PhoB (PhoB∼P). PhoB∼P is then able to activate the Pho regulon by binding to a consensus Pho box sequence within the promoters of Pho regulon genes.Citation5 The Pho boxes are formed by direct repeat units consisting of 11 nucleotides, where the first 7 nucleotides are well conserved (consensus sequence GTTCACC). Conversely, when Pi is abundant, the Pho regulon is not induced (OFF state = basal expression), which is mediated by an interaction between the Pst-PhoU complex and PhoR, that prevents PhoR-mediated phosphorylation of PhoB.Citation9 In these high Pi conditions, PhoB is maintained in the non-phosphorylated form by PhoR phosphatase activity, and expression of the Pst system becomes deactivated while the expression of the Pit system becomes de-repressed. Pit does not require chemical energy to drive Pi transport when it is abundant, but when conditions are limiting, the cell uses ATP to scavenge Pi and similar P-containing compounds from the environment via the Pst system.Citation10
PhoB is a typical winged-helix response regulator that upon aspartyl phosphorylation forms a dimer, which binds in head-to-tail arrangement to DNA sequences upstream of Pho regulon genes to recruit RNA polymerase (RNAP)Citation11-14 (). PhoB∼P interacts specifically with the σCitation70 subunit of RNAP, and this interaction facilitates the entry of the RNA polymerase into the pho promoters for transcription initiation.Citation15 In most cases PhoB∼P acts as a positive transcriptional factor, but there are a few promoters in which PhoB works as a repressor. This is the case for hrdA and rpoZ encoding respectively the σ-factor and ω-factor of RNAP in Streptomyces species.Citation16,17 This repression is due to promoter occlusion when PhoB∼P binds to the −10 to +1 region of the promoter thus interfering with RNAP binding. Another example is PhoB∼P repressing tcpPH by binding to a Pho-box located between the promoter and the binding sites for the AphA and AphB positive regulators (). In this case, the repression may be due to disruption of RNAP interaction with other transcriptional activators.Citation18
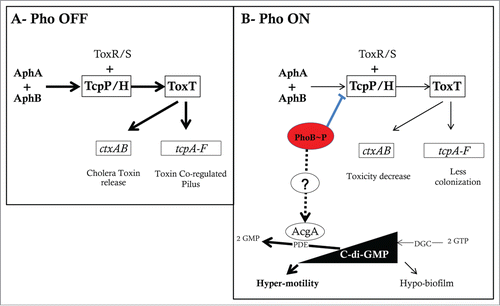
Figure 2. The V. cholerae virulence cascade and the mechanisms of Pi/Pho regulation of virulence, motility, and biofilm formation. (A) V. cholerae virulence cascade. In high Pi conditions (Pho OFF) and in conditions favoring gene expression of AphA and AphB regulators, AphA cooperates with AphB to activate the tcpPH promoter. TcpPH cooperatively with ToxR/S activates the toxT promoter. ToxT then activates the tcpA-F and ctxAB promoters. This increases colonization through formation of the toxin co-regulated pilus (TCP) and cytotoxicity through Cholera toxin (CT) release. (B) In low Pi conditions (or Δpst mutation), PhoB∼P binds to the Pho-box located between the tcpPH promoter and the binding sites for AphA and AphB. This results in tcpPH repression which reduces the downstream ToxT-dependent virulence cascade. C-di-GMP is synthesized from 2 GTP molecules by diguanylate cyclases (DGCs) and degraded to 2 GMP molecules by phosphodiesterases (PDEs). Studies in V. cholerae have shown that c-di-GMP increases biofilm formation and reduces the motility and toxT expression. In low Pi (or Δpst mutation) PhoB induces indirectly the expression of acgAB which results in degradation of c-di-GMP leading to an increase in motility and a decrease of biofilm formation.
In certain circumstances, PhoB is activated in response to other environmental signals through cross-activation by non-partner HK proteins. Indeed, in addition to the well- known CreC, it has been shown that 5 other non-partner HK (ArcB, KdpD, QseC, BaeS, and VanS) could also activate PhoB.Citation19,20 Within a genetically homogeneous cell population, bacteria evolved strategies that display a stochastic character of gene expression in a cross-regulation network of TCSs. This would allow for non-genetic diversity, and thereby a rapid adaption to different environments.Citation21
Mechanisms by Which PhoB Controls the Virulence of Vibrio cholerae
PhoB-mediated regulation of V. cholerae toxicity and colonization
In order to cause the cholera diarrheal disease, V. cholerae induces expression of genes encoding the colonization factor toxin-co-regulated pilus (TCP) and the ADP ribosylating cholera toxin (CT).Citation22-24 Dependent on a transcriptional cascade, this is triggered by a pair of independent activators, AphA and AphB (A). These 2 activators cooperatively induce the tcpPH operon encoding TcpP/H, a transcriptional activator of toxT expression. ToxT in turn increases gene transcription of the ctx (CT) and tcp (TCP) genes. The low Pi response regulator PhoB was capable of shorting this regulation cascade by binding and repressing the tcpPH promoter (B). This resulted in impaired colonization by a V. cholerae Δpst strain in the infant mouse and decreased expression of CT and TCP.Citation18 However, under the normally high Pi conditions of the gut, the AphA/AphB regulatory cascade would serve to promote TCP and CT production.
Cyclic-di-GMP: A Pi/PhoB messenger of motility and biofilm formation
During the last decade, several investigations point-out the implication of c-di-GMP in various bacterial phenotypes and fundamental processes including Pi homeostasis and bacterial virulence. c-di-GMP [bis-(3′-5′)-cyclic dimeric guanosine monophosphate] is a ubiquitous soluble molecule and a bacterial second messenger. c-di-GMP induces biofilm formation by stimulating the biosynthesis of various adhesins and exopolysaccharides and inhibits bacterial motility by controlling the switch between planktonic and sedentary lifestyles (for a review, see refs. 25 and 26). The level of c-di-GMP is enzymatically and spatio-temporally controlled in bacterial cells by the GGDEF and the EAL domains of many diguanylate cyclases (DGC) and phosphodiesterases (PDE) (for a review, see ref. 27).
The link between Pi/Pho and bacterial virulence via c-di-GMP signaling has been investigated in V. cholerae. Twenty-two V. cholerae genes have been predicted to encode an EAL domain protein including VieA.Citation28 It has been shown that expression of the PDE VieA positively regulates virulence gene expression (ctx and toxT),Citation29 while ectopic expression of a DGC reduced the induction of virulence genes during infection using the infant mouse model of cholera.Citation30 VieA also positively regulates motility and flagellar synthesis genes,Citation31 and negatively regulates the expression of Vibrio exopolysaccharide synthesis (vps) genes.Citation32 These findings suggest that c-di-GMP assists in the transition from environment to host via regulation of V. cholerae behavior. Indeed, Tischler et al.Citation32 and Pratt et al.Citation33 have shown that the vieA(E170A) mutation abrogates PDE activity, and increases intracellular c-di-GMP levels leading to hypo-motility and hyper-biofilm phenotypes. Sometime after, the same team demonstrated that a Δpst mutation could restore motility and decrease biofilm formation of the vieA(E170A) strain.Citation34 In the study, the authors also demonstrated that the V. cholerae WT strain grown in low Pi increased its motility and decreased biofilm formation (B). This was due to degradation of [c-di-GMP] by AcgA, another EAL domain containing PDE. In this condition, PhoB indirectly activated the transcription of the acgAB operon that encodes two enzymes with opposing activities on the intracellular c-di-GMP. It seems that in low Pi, the PDE activity (AcgA) overrides the DGC activity (AcgB).Citation34
The link between Pi homeostasis and c-di-GMP signaling, suggests a regulatory model in which biofilm formation would be promoted during the course of infection. However, in late infection stages, PhoB activation shuts down virulence gene expression. This also occurs when extracellular Pi becomes limiting and results in a concomitant induction of gene expression that promotes bacterial survival and dissemination in low Pi aquatic environments.Citation34
Roles of PhoB and Pi Response in Various Virulence-Related Phenotypes of Pseudomonas spp.
Many bacterial species belonging to the genus Pseudomonas have been characterized, demonstrate metabolic variability, and are able to colonize a wide range of niches such as soil, water, and plant or animal tissue. The most-studied Pseudomonas species include the opportunistic pathogen P. aeruginosa and the plant growth-promoting Pseudomonas fluorescens.
Pi limitation switches on harmful and lethal phenotypes in P. aeruginosa
P. aeruginosa is responsible for severe nosocomial infections and chronically colonizes lungs of cystic fibrosis (CF) patients leading to morbidity and mortality.Citation35,36 Infection with Gram-negative pathogens, leads to Pi reduction in the plasma to a suboptimal level for bacterial growth,Citation37 and the Pi concentration measured from sputum of CF patients ranged from 12 to 14 mM.Citation38 In these conditions, P. aeruginosa synthesizes several exo-products, including the hemolytic and non-hemolytic C phospholipases, PLC-H and PLC-N.Citation39 These PLCs are also induced by osmoprotectant compounds from eukaryotic and prokaryotic cells.Citation40 It was demonstrated that induction of PLC-H by the osmoprotective compounds is independent of Pi concentration or PhoB, while induction of PLC-N by these compounds requires Pi-deficient conditions and PhoB.Citation41 This indicates a relevant pathogenic potential of PLC in the hyperosmotic conditions of the lungs of cystic fibrosis patients. Furthermore, PLC is also known to degrade phosphatidylcholine, a component of lung surfactant that may provide essential Pi nutrients with the potential to enhance lung colonization and cause atelectasis. In addition, mucoidy has been shown to be induced in P. aeruginosa by growth on minimal medium with phosphorylcholine as the sole carbon and Pi source.Citation42
It was reported that Pi-starvation of P. aeruginosa in the distal gut coupled with the administration of analgesic opioids led to PhoB induction of the expression of phzA1/2 (phenazine biosynthesis), and rhlR, and lasR (which encode response regulators of the quorum sensing systems Rhl and Las). This was directly linked to production of the toxic phenazine, pyocyanin and drug resistance during development of human sepsis.Citation43-45 Increased pyocyanin toxin production was PhoB-dependent via RhlR and PhzA1/2 when Pi was limited. However, in Pi-sufficient conditions, RhlR stimulated pyocyanin production in a PhoB-independent way through PQS (Pseudomonas quinolone signal) and/or low iron availability.Citation43 By contrast, the host supplemented with excess Pi was less susceptible to P. aeruginosa infectionCitation46. The excess of Pi attenuated bacterial virulence by decreasing opioid-induced pyocyanin production.
Highly virulent multidrug-resistant clinical strains of P. aeruginosa showed an over-production of PstS and the ability to kill 60% of surgically injured mice.Citation47 This virulence phenotype was associated with formation of PstS-rich surface structures induced by PhoB in low Pi that allows scavenging of Pi within the host while remaining less accessible to the immune system. The mechanism by which these PstS-containing structures are formed remains to be determined.Citation48 In contrast, the Δpst strain was harmless and did not produce PstS.Citation47,49 Furthermore, the rough cells (producing PstS-rich appendages at cell surfaces) formed less biofilm than smooth cells.Citation49 P. aeruginosa could also cause red death in 60% of Caenorhabditis elegans nematodes.Citation50 This lethal phenotype appears to be activated by a triangulated response to low Pi between PhoB, MvfR-PQS quorum sensing, and the pyoverdine iron acquisition system.
All these reports emphasize the central role of Pi in switching on the virulence of P. aeruginosa. They also suggest that this opportunistic pathogen can use Pi deficiency as an environmental signal of host injury and then shift to virulent and lethal phenotypes.
Pi stress changes the motility and biofilm formation of Pseudomonas spp.
The studies on motility and biofilm formation phenotypes of Pseudomonas spp. due to Pi stress reported changes similar to those occurring in V. cholerae. In P. aeruginosa, Pi limitation promoted bacterial hyper-swarming through PhoB upregulation of rhlR expression resulting in increased rhamnolipid production.Citation51 The rhamnolipid biosurfactants facilitate swarming motility. This may explain the hyper-motile phenotype exhibited by the Δpst mutant and the motility defect observed in the ΔphoB mutant.Citation52
Moreover, Monds et al.Citation53 showed that P. fluorescens lost its ability to form biofilm in response to Pi limitation through Pho regulon activation. For biofilm formation, P. fluorescens requires production of the LapA adhesin that is responsible for the transition from reversible to irreversible attachment, and that is stimulated by c-di-GMP. In low Pi conditions, the level of c-di-GMP was decreased through PhoB inducing the PDE activity of RapA. This inhibition of P. fluorescens biofilm formation in low Pi also requires the Pho-dependent activation of ApaH, an enzyme that degrades Ap4A, the dinucleoside polyphosphate suggested to be an “alarmone” that signals the onset of oxidative stress.Citation54
Effect of Phosphate Availability on Virulence Gene Expression in Pathogenic E. coli
We previously reviewed the importance of the Pho regulon for Escherichia coli virulence and the relationship between the Pi metabolism and pathogenicity.Citation55,56 More recently, other studies have reported virulence mechanisms depending on the environmental Pi or the Pho/Pst systems.
Extra-intestinal pathogenic E. coli (ExPEC)
ExPEC are an important group of pathogenic E. coli that cause a wide range of human and animal diseases. The constitutive expression of the Pho regulon through inactivation of the Pst system was demonstrated in ExPEC strain 5131 causing porcine septicemia,Citation57,58 the avian pathogenic E. coli (APEC) serogroup O78 strain χ7122,Citation45 and uropathogenic E. coli (UPEC) strain CFT073.Citation59 In all these strains, the Pho constitutive expression resulting from deletion of the Pst system attenuated virulence and virulence attributes, including sensitivity to hydrogen peroxide and serum, decreased production of type 1 fimbriae and cell surface modification. In the case of UPEC strain CFT073, the constitutive activation of the Pho regulon altered expression of regulators fimB, ipuA, and ipbA (encoding recombinases, mediating inversion of the fim promoter), and decreased the level of the alarmone ppGpp that led to reduced type 1 fimbriae production and mouse bladder colonization.Citation59 However, in APEC, the PhoR-mediated constitutive activation of the Pho regulon was specifically shown to be critical for virulence, rather than inactivation of the Pst system alone. Indeed, a point mutation in phoR, which resulted in constitutive activation of the Pho regulon, independent of Pi transport and or inactivation of the Pst system, also resulted in attenuation of APEC virulence.Citation60
Diarrheagenic E. coli and Citrobacter rodentium
For intestinal pathogenic E. coli, the implication of the Pho regulon in virulence modulation was demonstrated for enteropathogenic E. coli (EPEC) and enterohemorrhagic E. coli (EHEC). These 2 diarrheagenic E. coli groups, together with C. rodentium, belong to a class labeled as attaching-effacing E. coli (AEEC). They share the locus of enterocyte effacement (LEE) encoding the type III-secretion system (T3SS) and its effectors and the ability to cause the A/E phenotype. A deletion of the Pst system in different strains among the EPEC showed either an in vitro or ex vivo defect of the attachment and adhesion to epithelial cells. Moreover, it has been shown that a pst mutant of C. rodentium is less virulent for mice, its natural host,Citation61 and recent studies reported that the Pho regulon regulates the virulence of C. rodentium through PhoB control of DegP (serine protease) and NleG8 (a T3SS protein).Citation62 In EHEC, PhoB directly modulated LEE gene expression as well as proteins secreted by T3SS.Citation63 Moreover, EHEC can release Shiga toxins (Stx) that cause bloody diarrhea and hemolytic uremic syndrome (HUS). In vitro, growth in Pi-limiting media seems to increase production and secretion of Stx2 through increased stx2 transcription mediated by PhoB.Citation63 Also, we previously showed that the PhoB protein is critical for EHEC persistence in co-culture with amoebae.Citation64 This could be linked to an adaptive response of EHEC to low Pi after exit from the mammalian host, as EHEC has been found in aquatic poor nutrient environments,Citation65 where it might co-exist with protozoan predators that would promote its survival outside of the mammalian gut.
Other Phenotypes and Bacterial Virulence Tied to Phosphate and the Pho Regulon
In recent years, additional reports have linked extracellular Pi availability, the PhoB regulator and/or the Pst system to different bacterial phenotypes and virulence-related determinants. Here we describe some virulence phenotypes where the genetic mechanisms are not yet fully elucidated.
Biofilm formation and antibiotic resistance
Biofilm formation by the plant pathogen Agrobacterium tumefaciens is enhanced in low Pi conditions through increased attachment by unipolar polysaccharide (UPP), and is controlled by the PhoB-PhoR regulatory system that is also essential for viability of A. tumefaciens.Citation66,67 Proteus mirabilis is the most common etiological agent responsible for complicated urinary tract infections (UTIs). Its survival in the urinary tract depends to its ability to produce a battery of virulence factors, including urease, flagella, fimbriae, hemolysin and formation of crystalline biofilm on indwelling catheters.Citation68-70 The abolition of the Pst system in P. mirabilis caused a defect in biofilm formation when grown in human urine.Citation71 As long as bacteria use the Pho regulon to regulate attachment to surfaces and aggregate to form biofilms, formation of these sessile communities and their inherent resistance to antimicrobial agents are at the root of many persistent and chronic bacterial infections.Citation66
The lower susceptibility of biofilm-grown bacteria to antimicrobial agents (antibiotics, biocides) has been extensively investigated.Citation72-74 Vilain et al.Citation75 showed that Pi deprivation of E. coli in gel-entrapped conditions (like bacteria in natural biofilms), is associated with a high β-lactam antibiotic resistance (latamoxef). Furthermore, it is known that Pi excess in the medium suppresses the biosynthesis of many secondary metabolites such as antibiotics.Citation76,77 As mentioned above, cross-talk activation of PhoB comes from VanS, the HK partner of the VanR–VanS TCS that orchestrates vancomycin resistance in Enterococcus faecium.Citation20 These findings imply a role for Pi homeostasis and PhoB in a complex regulation of antibiotic stress resistance.
Oxidative stress resistance
Several studies reported the activation of oxidative stress resistance in conditions of Pi starvation or when Pst is abolished, and that both situations activate the Pho regulon. These reports concerned different bacterial species: Sinorhizobium meliloti, P. aeruginosa and A. tumefaciens,Citation78 V. cholerae biotype El Tor,Citation79 avian pathogenic E. coli,Citation80 and C. rodentium.Citation61 Taken together, these studies indicate that Pho regulon activation leads to increased gene expression of catalases that protect bacteria by degrading hydrogen peroxide. There was also greater gene expression of genes encoding DNA protection proteins and superoxide dismutase. However, since the major oxidative stress response regulators, OxyR and SoxRS seem not to be under control of the Pho regulon induction, the pathway linking the reduction of oxidative stress functions to Pi management remains unknown.
Bacterial secretion systems
Gram-negative bacteria use a variety of secretion systems to target proteins to both prokaryotic and eukaryotic cells. Secretion systems such as the type III, IV, and VI secretion (T3SS, T4SS, and T6SS) pathways, translocate substrates directly into recipient cells in a contact-dependent manner, and constitute important virulence factors. Recent observations link the regulation of Pi homeostasis with Sec-independent secretion systems in some bacterial species. A well-studied example occurs in the pathogen Edwardsiella tarda.
E. tarda colonizes a broad range of aquatic species in addition to fish.Citation81 It can also be found in the intestinal tract of birds, reptiles, and mammals including humans.Citation82 The T3SS and T6SS were determined as the two most important virulence mechanisms in E. tarda.Citation83,84 In response to both, Pi and iron availabilities, Chakraborty et al.Citation85 investigated the effects of PhoB and Fur regulators on T3SS and T6SS in this organism. The authors demonstrated that EsrC (a positive regulator of both T3SS and T6SS), and PhoB, both bind to the evpA promoter (E. tarda virulence protein) to regulate directly and positively gene transcription of T6SS. In addition, PhoB interacts with PhoU to activate esrC. Fur however, senses high iron and inhibits EsrC-binding to evpA. It was then concluded that PhoB and Fur negatively and indirectly cross-talk via unidentified factors to regulate the T3SS and T6SS in E. tarda. As such, the regulation of virulence genes through the PhoB/Pi pathway becomes more complex through interactions with other master regulators.
Pho regulon expression during infection and immune system hijack
Depletion of extracellular Pi after a surgical injury via phosphatonin induction of phosphaturia resulted in a reduction of Pi in serum.Citation86 Under these Pi-limiting conditions, it has been shown that in Bacteroides fragilis PhoB was necessary for successful infection and survival in peritoneal abscesses.Citation87 In this study, activation of the Pho regulon induced a shift of B. fragilis from gut symbiosis to pathogenicity. Recently, it has been shown that Mycobacterium tuberculosis requires the Pst system to negatively regulate activity of RegX3 (a PhoB analog) in response to in vivo available Pi.Citation88 The authors showed that the M. tuberculosis Pst system was essential for virulence in mice and to counteract IFN-γ-dependent host immunity. Using selective capture of transcribed sequences (SCOTS), we previously showed that APEC O78 strain χ7122 can express PhoB in vivo in an infectious context, in infected chickens.Citation89 However, the pst deletion increases susceptibility of APEC O78 to rabbit serum, while this strain was not killed by chicken serum.Citation90 This suggests the presence of species specific differences in host innate immune defenses and complement-mediated killing.
Summary
All bacterial species including pathogens and commensals are equipped to cope and adapt to nutritional limitation. In most cases, the same input signal such as Pi availability is directly controlled by Pst and the PhoB/R TCS, allowing a Pi homeostasis response. Depending on the level of Pi signal, pathogenic bacteria regulate different downstream systems such as quorum sensing regulatory networks and production of c-di-GMP, adhesins, or cytotoxins. This results in virulence variation and different adaptive changes including biofilm formation, motility, and antimicrobial and oxidative stress resistance.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
Acknowledgments
We thank Judith Kashul for editing services.
Funding
We thank the Natural Sciences and Engineering Research Council of Canada (RGPIN 250129-07) for financial support to C.M.D. and (RGPIN SD-25120-09) to J.H. and Fonds de la recherche du Québec en nature et technologies to J.H. (FRQNT PT165375) and a studentship to S.M.C. from Fonds CRIPA (FRQNT Regroupements stratégiques 111946).
References
- Parkinson JS. Signal transduction schemes of bacteria. Cell 1993; 73:857-71; PMID:8098993; http://dx.doi.org/10.1016/0092-8674(93)90267-T
- Oshima T, Aiba H, Masuda Y, Kanaya S, Sugiura M, Wanner BL, Mori H, Mizuno T. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol Microbiol 2002; 46:281-91; PMID:12366850; http://dx.doi.org/10.1046/j.1365-2958.2002.03170.x
- Mizuno T. Compilation of all genes encoding two-component phosphotransfer signal transducers in the genome of Escherichia coli. DNA Res 1997; 4:161-8; PMID:9205844; http://dx.doi.org/10.1093/dnares/4.2.161
- Yang C, Huang TW, Wen SY, Chang CY, Tsai SF, Wu WF, Chang CH. Genome-wide PhoB binding and gene expression profiles reveal the hierarchical gene regulatory network of phosphate starvation in Escherichia coli. PLoS One 2012; 7:e47314; PMID:23071782; http://dx.doi.org/10.1371/journal.pone.0047314
- Hsieh YJ, Wanner BL. Global regulation by the seven-component Pi signaling system. Curr Opin Microbiol 2010; 13:198-203; PMID:20171928; http://dx.doi.org/10.1016/j.mib.2010.01.014
- Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 2009; 7:263-73; PMID:19287449; http://dx.doi.org/10.1038/nrmicro2109
- Rao NN, Torriani A. Molecular aspects of phosphate transport in Escherichia coli. Mol Microbiol 1990; 4:1083-90; PMID:1700257; http://dx.doi.org/10.1111/j.1365-2958.1990.tb00682.x
- Chan FY, Torriani A. PstB protein of the phosphate-specific transport system of Escherichia coli is an ATPase. J Bacteriol 1996; 178:3974-7; PMID:8682808
- Wanner BL. Signal transduction in the control of phosphate-regulated genes of Escherichia coli. Kidney Int 1996; 49:964-7; PMID:8691745; http://dx.doi.org/10.1038/ki.1996.136
- Van Dien SJ, Keasling JD. A dynamic model of the Escherichia coli phosphate-starvation response. J Theor Biol 1998; 190:37-49; PMID:9473389; http://dx.doi.org/10.1006/jtbi.1997.0524
- Makino K, Amemura M, Kim SK, Nakata A, Shinagawa H. Role of the sigma 70 subunit of RNA polymerase in transcriptional activation by activator protein PhoB in Escherichia coli. Genes Dev 1993; 7:149-60; PMID:8422984; http://dx.doi.org/10.1101/gad.7.1.149
- Bachhawat P, Swapna GV, Montelione GT, Stock AM. Mechanism of activation for transcription factor PhoB suggested by different modes of dimerization in the inactive and active states. Structure 2005; 13:1353-63; PMID:16154092; http://dx.doi.org/10.1016/j.str.2005.06.006
- McCleary WR. The activation of PhoB by acetylphosphate. Mol Microbiol 1996; 20:1155-63; PMID:8809768; http://dx.doi.org/10.1111/j.1365-2958.1996.tb02636.x
- Ritzefeld M, Walhorn V, Kleineberg C, Bieker A, Kock K, Herrmann C, Anselmetti D, Sewald N. Cooperative binding of PhoB(DBD) to its cognate DNA sequence-a combined application of single-molecule and ensemble methods. Biochemistry 2013; 52:8177-86; PMID:24199636; http://dx.doi.org/10.1021/bi400718r
- Kim SK, Makino K, Amemura M, Nakata A, Shinagawa H. Mutational analysis of the role of the first helix of region 4.2 of the sigma 70 subunit of Escherichia coli RNA polymerase in transcriptional activation by activator protein PhoB. Mol Gen Genet 1995; 248:1-8; PMID:7651320; http://dx.doi.org/10.1007/BF02456607
- Sola-Landa A, Rodríguez-García A, Apel AK, Martín JF. Target genes and structure of the direct repeats in the DNA-binding sequences of the response regulator PhoP in Streptomyces coelicolor. Nucleic Acids Res 2008; 36:1358-68; PMID:18187507; http://dx.doi.org/10.1093/nar/gkm1150
- Santos-Beneit F, Barriuso-Iglesias M, Fernández-Martínez LT, Martínez-Castro M, Sola-Landa A, Rodríguez-García A, Martín JF. The RNA polymerase omega factor RpoZ is regulated by PhoP and has an important role in antibiotic biosynthesis and morphological differentiation in Streptomyces coelicolor. Appl Environ Microbiol 2011; 77:7586-94; PMID:21908625; http://dx.doi.org/10.1128/AEM.00465-11
- Pratt JT, Ismail AM, Camilli A. PhoB regulates both environmental and virulence gene expression in Vibrio cholerae. Mol Microbiol 2010; 77:1595-605; PMID:20659293; http://dx.doi.org/10.1111/j.1365-2958.2010.07310.x
- Haldimann A, Daniels LL, Wanner BL. Use of new methods for construction of tightly regulated arabinose and rhamnose promoter fusions in studies of the Escherichia coli phosphate regulon. J Bacteriol 1998; 180:1277-86; PMID:9495769
- Fisher SL, Jiang W, Wanner BL, Walsh CT. Cross-talk between the histidine protein kinase VanS and the response regulator PhoB. Characterization and identification of a VanS domain that inhibits activation of PhoB. J Biol Chem 1995; 270:23143-9; PMID:7559459; http://dx.doi.org/10.1074/jbc.270.39.23143
- Zhou L, Grégori G, Blackman J, Robinson JP, Wanner BL. Stochastic activation of the response regulator PhoB by noncognate histidine kinases. J Integr Bioinform 2005; 2:11-24
- Karaolis DK, Johnson JA, Bailey CC, Boedeker EC, Kaper JB, Reeves PR. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci U S A 1998; 95:3134-9; PMID:9501228; http://dx.doi.org/10.1073/pnas.95.6.3134
- Waldor MK, Mekalanos JJ. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 1996; 272:1910-4; PMID:8658163; http://dx.doi.org/10.1126/science.272.5270.1910
- Kaper JB, Morris JG Jr., Levine MM. Cholera. Clin Microbiol Rev 1995; 8:48-86; PMID:7704895
- Ryan RP, Fouhy Y, Lucey JF, Dow JM. Cyclic di-GMP signaling in bacteria: recent advances and new puzzles. J Bacteriol 2006; 188:8327-34; PMID:17028282; http://dx.doi.org/10.1128/JB.01079-06
- Jenal U, Malone J. Mechanisms of cyclic-di-GMP signaling in bacteria. Annu Rev Genet 2006; 40:385-407; PMID:16895465; http://dx.doi.org/10.1146/annurev.genet.40.110405.090423
- Römling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 2013; 77:1-52; PMID:23471616; http://dx.doi.org/10.1128/MMBR.00043-12
- Tamayo R, Tischler AD, Camilli A. The EAL domain protein VieA is a cyclic diguanylate phosphodiesterase. J Biol Chem 2005; 280:33324-30; PMID:16081414; http://dx.doi.org/10.1074/jbc.M506500200
- Tischler AD, Camilli A. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect Immun 2005; 73:5873-82; PMID:16113306; http://dx.doi.org/10.1128/IAI.73.9.5873-5882.2005
- Tamayo R, Schild S, Pratt JT, Camilli A. Role of cyclic Di-GMP during el tor biotype Vibrio cholerae infection: characterization of the in vivo-induced cyclic Di-GMP phosphodiesterase CdpA. Infect Immun 2008; 76:1617-27; PMID:18227161; http://dx.doi.org/10.1128/IAI.01337-07
- Beyhan S, Tischler AD, Camilli A, Yildiz FH. Transcriptome and phenotypic responses of Vibrio cholerae to increased cyclic di-GMP level. J Bacteriol 2006; 188:3600-13; PMID:16672614; http://dx.doi.org/10.1128/JB.188.10.3600-3613.2006
- Tischler AD, Camilli A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol Microbiol 2004; 53:857-69; PMID:15255898; http://dx.doi.org/10.1111/j.1365-2958.2004.04155.x
- Pratt JT, Tamayo R, Tischler AD, Camilli A. PilZ domain proteins bind cyclic diguanylate and regulate diverse processes in Vibrio cholerae. J Biol Chem 2007; 282:12860-70; PMID:17307739; http://dx.doi.org/10.1074/jbc.M611593200
- Pratt JT, McDonough E, Camilli A. PhoB regulates motility, biofilms, and cyclic di-GMP in Vibrio cholerae. J Bacteriol 2009; 191:6632-42; PMID:19734314; http://dx.doi.org/10.1128/JB.00708-09
- Gaspar MC, Couet W, Olivier JC, Pais AA, Sousa JJ. Pseudomonas aeruginosa infection in cystic fibrosis lung disease and new perspectives of treatment: a review. Eur J Clin Microbiol Infect Dis 2013; 32:1231-52; PMID:23619573; http://dx.doi.org/10.1007/s10096-013-1876-y
- Tümmler B, Koopmann U, Grothues D, Weissbrodt H, Steinkamp G, von der Hardt H. Nosocomial acquisition of Pseudomonas aeruginosa by cystic fibrosis patients. J Clin Microbiol 1991; 29:1265-7; PMID:1907611
- Weinberg ED. Iron and susceptibility to infectious disease. Science 1974; 184:952-6; PMID:4596821; http://dx.doi.org/10.1126/science.184.4140.952
- Sanders NN, Franckx H, De Boeck K, Haustraete J, De Smedt SC, Demeester J. Role of magnesium in the failure of rhDNase therapy in patients with cystic fibrosis. Thorax 2006; 61:962-8; PMID:17071834; http://dx.doi.org/10.1136/thx.2006.060814
- Ostroff RM, Vasil ML. Identification of a new phospholipase C activity by analysis of an insertional mutation in the hemolytic phospholipase C structural gene of Pseudomonas aeruginosa. J Bacteriol 1987; 169:4597-601; PMID:2820937
- Lucchesi GI, Lisa TA, Domenech CE. Choline and betaine as inducer agents of Pseudomonas aeruginosa phospholipase C activity in high phosphate medium. FEMS Microbiol Lett 1989; 48:335-8; PMID:2498157; http://dx.doi.org/10.1111/j.1574-6968.1989.tb03359.x
- Shortridge VD, Lazdunski A, Vasil ML. Osmoprotectants and phosphate regulate expression of phospholipase C in Pseudomonas aeruginosa. Mol Microbiol 1992; 6:863-71; PMID:1602966; http://dx.doi.org/10.1111/j.1365-2958.1992.tb01537.x
- Terry JM, Piña SE, Mattingly SJ. Environmental conditions which influence mucoid conversion Pseudomonas aeruginosa PAO1. Infect Immun 1991; 59:471-7; PMID:1898904
- Jensen V, Löns D, Zaoui C, Bredenbruch F, Meissner A, Dieterich G, Münch R, Häussler S. RhlR expression in Pseudomonas aeruginosa is modulated by the Pseudomonas quinolone signal via PhoB-dependent and -independent pathways. J Bacteriol 2006; 188:8601-6; PMID:17028277; http://dx.doi.org/10.1128/JB.01378-06
- Shor R, Halabe A, Rishver S, Tilis Y, Matas Z, Fux A, Boaz M, Weinstein J. Severe hypophosphatemia in sepsis as a mortality predictor. Ann Clin Lab Sci 2006; 36:67-72; PMID:16501239
- Glattard E, Welters ID, Lavaux T, Muller AH, Laux A, Zhang D, Schmidt AR, Delalande F, Laventie BJ, Dirrig-Grosch S, et al. Endogenous morphine levels are increased in sepsis: a partial implication of neutrophils. PLoS One 2010; 5:e8791; PMID:20098709; http://dx.doi.org/10.1371/journal.pone.0008791
- Zaborin A, Gerdes S, Holbrook C, Liu DC, Zaborina OY, Alverdy JC. Pseudomonas aeruginosa overrides the virulence inducing effect of opioids when it senses an abundance of phosphate. PLoS One 2012; 7:e34883; PMID:22514685; http://dx.doi.org/10.1371/journal.pone.0034883
- Long J, Zaborina O, Holbrook C, Zaborin A, Alverdy J. Depletion of intestinal phosphate after operative injury activates the virulence of P aeruginosa causing lethal gut-derived sepsis. Surgery 2008; 144:189-97; PMID:18656625; http://dx.doi.org/10.1016/j.surg.2008.03.045
- Shah M, Zaborin A, Alverdy JC, Scott K, Zaborina O. Localization of DING proteins on PstS-containing outer-surface appendages of Pseudomonas aeruginosa. FEMS Microbiol Lett 2014; 352:54-61; PMID:24372739; http://dx.doi.org/10.1111/1574-6968.12368
- Zaborina O, Holbrook C, Chen Y, Long J, Zaborin A, Morozova I, Fernandez H, Wang Y, Turner JR, Alverdy JC. Structure-function aspects of PstS in multi-drug-resistant Pseudomonas aeruginosa. PLoS Pathog 2008; 4:e43; PMID:18282104; http://dx.doi.org/10.1371/journal.ppat.0040043
- Zaborin A, Romanowski K, Gerdes S, Holbrook C, Lepine F, Long J, Poroyko V, Diggle SP, Wilke A, Righetti K, et al. Red death in Caenorhabditis elegans caused by Pseudomonas aeruginosa PAO1. Proc Natl Acad Sci U S A 2009; 106:6327-32; PMID:19369215; http://dx.doi.org/10.1073/pnas.0813199106
- Blus-Kadosh I, Zilka A, Yerushalmi G, Banin E. The effect of pstS and phoB on quorum sensing and swarming motility in Pseudomonas aeruginosa. PLoS One 2013; 8:e74444; PMID:24023943; http://dx.doi.org/10.1371/journal.pone.0074444
- Bains M, Fernández L, Hancock RE. Phosphate starvation promotes swarming motility and cytotoxicity of Pseudomonas aeruginosa. Appl Environ Microbiol 2012; 78:6762-8; PMID:22773629; http://dx.doi.org/10.1128/AEM.01015-12
- Monds RD, Newell PD, Gross RH, O’Toole GA. Phosphate-dependent modulation of c-di-GMP levels regulates Pseudomonas fluorescens Pf0-1 biofilm formation by controlling secretion of the adhesin LapA. Mol Microbiol 2007; 63:656-79; PMID:17302799; http://dx.doi.org/10.1111/j.1365-2958.2006.05539.x
- Monds RD, Newell PD, Wagner JC, Schwartzman JA, Lu W, Rabinowitz JD, O’Toole GA. Di-adenosine tetraphosphate (Ap4A) metabolism impacts biofilm formation by Pseudomonas fluorescens via modulation of c-di-GMP-dependent pathways. J Bacteriol 2010; 192:3011-23; PMID:20154123; http://dx.doi.org/10.1128/JB.01571-09
- Lamarche MG, Wanner BL, Crépin S, Harel J. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev 2008; 32:461-73; PMID:18248418; http://dx.doi.org/10.1111/j.1574-6976.2008.00101.x
- Crépin S, Chekabab SM, Le Bihan G, Bertrand N, Dozois CM, Harel J. The Pho regulon and the pathogenesis of Escherichia coli. Vet Microbiol 2011; 153:82-8; PMID:21700403; http://dx.doi.org/10.1016/j.vetmic.2011.05.043
- Lamarche MG, Harel J. Membrane homeostasis requires intact pst in extraintestinal pathogenic Escherichia coli. Curr Microbiol 2010; 60:356-9; PMID:19937031; http://dx.doi.org/10.1007/s00284-009-9549-x
- Daigle F, Fairbrother JM, Harel J. Identification of a mutation in the pst-phoU operon that reduces pathogenicity of an Escherichia coli strain causing septicemia in pigs. Infect Immun 1995; 63:4924-7; PMID:7591158
- Crépin S, Houle S, Charbonneau ME, Mourez M, Harel J, Dozois CM. Decreased expression of type 1 fimbriae by a pst mutant of uropathogenic Escherichia coli reduces urinary tract infection. Infect Immun 2012; 80:2802-15; PMID:22665376; http://dx.doi.org/10.1128/IAI.00162-12
- Bertrand N, Houle S, LeBihan G, Poirier É, Dozois CM, Harel J. Increased Pho regulon activation correlates with decreased virulence of an avian pathogenic Escherichia coli O78 strain. Infect Immun 2010; 78:5324-31; PMID:20921144; http://dx.doi.org/10.1128/IAI.00452-10
- Cheng C, Tennant SM, Azzopardi KI, Bennett-Wood V, Hartland EL, Robins-Browne RM, Tauschek M. Contribution of the pst-phoU operon to cell adherence by atypical enteropathogenic Escherichia coli and virulence of Citrobacter rodentium. Infect Immun 2009; 77:1936-44; PMID:19255191; http://dx.doi.org/10.1128/IAI.01246-08
- Cheng C, Wakefield MJ, Yang J, Tauschek M, Robins-Browne RM. Genome-wide analysis of the Pho regulon in a pstCA mutant of Citrobacter rodentium. PLoS One 2012; 7:e50682; PMID:23226353; http://dx.doi.org/10.1371/journal.pone.0050682
- Chekabab SM, Jubelin G, Dozois CM, Harel J. PhoB activates Escherichia coli O157:H7 virulence factors in response to inorganic phosphate limitation. PLoS One 2014; 9:e94285; PMID:24710330; http://dx.doi.org/10.1371/journal.pone.0094285
- Chekabab SM, Daigle F, Charette SJ, Dozois CM, Harel J. Survival of enterohemorrhagic Escherichia coli in the presence of Acanthamoeba castellanii and its dependence on Pho regulon. Microbiologyopen 2012; 1:427-37; PMID:23233434; http://dx.doi.org/10.1002/mbo3.40
- Vital M, Hammes F, Egli T. Competition of Escherichia coli O157 with a drinking water bacterial community at low nutrient concentrations. Water Res 2012; 46:6279-90; PMID:23062788; http://dx.doi.org/10.1016/j.watres.2012.08.043
- Danhorn T, Hentzer M, Givskov M, Parsek MR, Fuqua C. Phosphorus limitation enhances biofilm formation of the plant pathogen Agrobacterium tumefaciens through the PhoR-PhoB regulatory system. J Bacteriol 2004; 186:4492-501; PMID:15231781; http://dx.doi.org/10.1128/JB.186.14.4492-4501.2004
- Xu J, Kim J, Danhorn T, Merritt PM, Fuqua C. Phosphorus limitation increases attachment in Agrobacterium tumefaciens and reveals a conditional functional redundancy in adhesin biosynthesis. Res Microbiol 2012; 163:674-84; PMID:23103488; http://dx.doi.org/10.1016/j.resmic.2012.10.013
- Stickler DJ, King JB, Winters C, Morris SL. Blockage of urethral catheters by bacterial biofilms. J Infect 1993; 27:133-5; PMID:8228293; http://dx.doi.org/10.1016/0163-4453(93)94620-Q
- Mobley HL, Island MD, Massad G. Virulence determinants of uropathogenic Escherichia coli and Proteus mirabilis. Kidney Int Suppl 1994; 47:S129-36; PMID:7869662
- Peerbooms PG, Verweij AM, MacLaren DM. Vero cell invasiveness of Proteus mirabilis. Infect Immun 1984; 43:1068-71; PMID:6365782
- O’May GA, Jacobsen SM, Longwell M, Stoodley P, Mobley HL, Shirtliff ME. The high-affinity phosphate transporter Pst in Proteus mirabilis HI4320 and its importance in biofilm formation. Microbiology 2009; 155:1523-35; PMID:19372157; http://dx.doi.org/10.1099/mic.0.026500-0
- Mah TF. Biofilm-specific antibiotic resistance. Future Microbiol 2012; 7:1061-72; PMID:22953707; http://dx.doi.org/10.2217/fmb.12.76
- Donlan RM. Role of biofilms in antimicrobial resistance. ASAIO J 2000; 46:S47-52; PMID:11110294; http://dx.doi.org/10.1097/00002480-200011000-00037
- Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 2001; 9:34-9; PMID:11166241; http://dx.doi.org/10.1016/S0966-842X(00)01913-2
- Vilain S, Cosette P, Junter GA, Jouenne T. Phosphate deprivation is associated with high resistance to latamoxef of gel-entrapped, sessile-like Escherichia coli cells. J Antimicrob Chemother 2002; 49:315-20; PMID:11815573; http://dx.doi.org/10.1093/jac/49.2.315
- Martin JF, Demain AL. Control of antibiotic biosynthesis. Microbiol Rev 1980; 44:230-51; PMID:6991900
- Martin JF. Control of antibiotic synthesis by phosphate. In: Heidelberg SB, ed. Advances in Biochemical Engineering, 1977:105-27.
- Yuan ZC, Zaheer R, Finan TM. Phosphate limitation induces catalase expression in Sinorhizobium meliloti, Pseudomonas aeruginosa and Agrobacterium tumefaciens. Mol Microbiol 2005; 58:877-94; PMID:16238634; http://dx.doi.org/10.1111/j.1365-2958.2005.04874.x
- Sultan SZ, Silva AJ, Benitez JA. The PhoB regulatory system modulates biofilm formation and stress response in El Tor biotype Vibrio cholerae. FEMS Microbiol Lett 2010; 302:22-31; PMID:19909344; http://dx.doi.org/10.1111/j.1574-6968.2009.01837.x
- Crépin S, Lamarche MG, Garneau P, Séguin J, Proulx J, Dozois CM, Harel J. Genome-wide transcriptional response of an avian pathogenic Escherichia coli (APEC) pst mutant. BMC Genomics 2008; 9:568; PMID:19038054; http://dx.doi.org/10.1186/1471-2164-9-568
- Schlenker C, Surawicz CM. Emerging infections of the gastrointestinal tract. Best Pract Res Clin Gastroenterol 2009; 23:89-99; PMID:19258189; http://dx.doi.org/10.1016/j.bpg.2008.11.014
- Janda JM, Abbott SL, Kroske-Bystrom S, Cheung WK, Powers C, Kokka RP, Tamura K. Pathogenic properties of Edwardsiella species. J Clin Microbiol 1991; 29:1997-2001; PMID:1774326
- Zheng J, Leung KY. Dissection of a type VI secretion system in Edwardsiella tarda. Mol Microbiol 2007; 66:1192-206; PMID:17986187; http://dx.doi.org/10.1111/j.1365-2958.2007.05993.x
- Tan YP, Zheng J, Tung SL, Rosenshine I, Leung KY. Role of type III secretion in Edwardsiella tarda virulence. Microbiology 2005; 151:2301-13; PMID:16000720; http://dx.doi.org/10.1099/mic.0.28005-0
- Chakraborty S, Sivaraman J, Leung KY, Mok YK. Two-component PhoB-PhoR regulatory system and ferric uptake regulator sense phosphate and iron to control virulence genes in type III and VI secretion systems of Edwardsiella tarda. J Biol Chem 2011; 286:39417-30; PMID:21953460; http://dx.doi.org/10.1074/jbc.M111.295188
- Salem RR, Tray K. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg 2005; 241:343-8; PMID:15650646; http://dx.doi.org/10.1097/01.sla.0000152093.43468.c0
- Wakimoto S, Nakayama-Imaohji H, Ichimura M, Morita H, Hirakawa H, Hayashi T, Yasutomo K, Kuwahara T. PhoB regulates the survival of Bacteroides fragilis in peritoneal abscesses. PLoS One 2013; 8:e53829; PMID:23342014; http://dx.doi.org/10.1371/journal.pone.0053829
- Tischler AD, Leistikow RL, Kirksey MA, Voskuil MI, McKinney JD. Mycobacterium tuberculosis requires phosphate-responsive gene regulation to resist host immunity. Infect Immun 2013; 81:317-28; PMID:23132496; http://dx.doi.org/10.1128/IAI.01136-12
- Dozois CM, Daigle F, Curtiss R 3rd. Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc Natl Acad Sci U S A 2003; 100:247-52; PMID:12506201; http://dx.doi.org/10.1073/pnas.232686799
- Lamarche MG, Dozois CM, Daigle F, Caza M, Curtiss R 3rd, Dubreuil JD, Harel J. Inactivation of the pst system reduces the virulence of an avian pathogenic Escherichia coli O78 strain. Infect Immun 2005; 73:4138-45; PMID:15972503; http://dx.doi.org/10.1128/IAI.73.7.4138-4145.2005
