Abstract
The haptoglobin-hemoglobin receptor (HpHbR) of African trypanosomes plays a critical role in human innate immunity against these parasites. Localized to the flagellar pocket of the veterinary pathogen Trypanosoma brucei brucei this receptor binds Trypanosome Lytic Factor-1 (TLF-1), a subclass of human high-density lipoprotein (HDL) facilitating endocytosis, lysosomal trafficking and subsequent killing. Recently, we found that group 1 Trypanosoma brucei gambiense does not express a functional HpHbR. We now show that loss of the TbbHpHbR reduces the susceptibility of T. b. brucei to human serum and TLF-1 by 100- and 10,000-fold, respectively. The relatively high concentrations of human serum and TLF-1 needed to kill trypanosomes lacking the HpHbR indicates that high affinity TbbHpHbR binding enhances the cytotoxicity; however, in the absence of TbbHpHbR, other receptors or fluid phase endocytosis are sufficient to provide some level of susceptibility. Human serum contains a second innate immune factor, TLF-2, that has been suggested to kill trypanosomes independently of the TbbHpHbR. We found that T. b. brucei killing by TLF-2 was reduced in TbbHpHbR-deficient cells but to a lesser extent than TLF-1. This suggests that both TLF-1 and TLF-2 can be taken up via the TbbHpHbR but that alternative pathways exist for the uptake of these toxins. Together the findings reported here extend our previously published studies and suggest that group 1 T. b. gambiense has evolved multiple mechanisms to avoid killing by trypanolytic human serum factors.
Introduction
African trypanosomes are eukaryotic pathogens that cause important human and animal diseases. These parasites have evolved a variety of mechanisms to escape innate and acquired immunity, including the use of the variant surface glycoprotein (VSG) coat to cover the plasma membrane of the parasite providing a barrier against attack by complement.Citation1 The VSG coat also serves as the molecular decoy during antigenic variation, presenting an ever-changing target to the adaptive immune system of the mammal, thus allowing the parasites to evade antibody-mediated killing.Citation2 The subspecies of trypanosomes that infect humans face the additional challenge of encountering a unique innate defense mechanism mediated by two related serum proteins complexes. In the circulation of humans, TLF-1 is a minor subclass of HDL containing apolipoprotein A-1 (apoA-1), the defining protein of all HDLs, and two primate specific proteins, apolipoprotein L-1 (apoL-1) and haptoglobin-related protein (Hpr).Citation3-Citation7 In addition to these apolipoproteins, Hpr binds free hemoglobin (Hb) in the circulation, which is likely released from erythrocytes during early infection.Citation8 The heterodimeric Hpr/Hb complex is proposed to be bifunctional, serving both as the ligand for the T. b. brucei HpHbRCitation9,Citation10 and directly contributing to high specific activity killing by catalyzing the peroxidation of lysosomal membrane lipids.Citation6,Citation7,Citation11 The other primate specific apolipoprotein in TLF-1, apoL-1, is also directly involved in T. b. brucei killing.Citation5,Citation12,Citation13 An ion channel forming protein, apoL-1 undergoes conformation changes at lysosomal pH and can integrate into membranes.Citation5,Citation12,Citation14 The combined action of Hpr/Hb and apoL-1 results in the osmotic lysis of the parasite.Citation15,Citation16 The other trypanolytic serum complex is called TLF-2 and, while largely devoid of lipids it contains Hpr and apoA-1Citation17 and apoL-1 (this paper) suggesting that these complexes share a common origin and perhaps have a similar mechanism of trypanosome killing.Citation18
The two subspecies of human sleeping sickness trypanosomes have evolved distinct mechanisms to survive in the human host. Trypanosoma brucei rhodesiense produces the serum resistance associated (SRA) protein that binds and inhibits TLF-1.Citation19-Citation21 SRA, an intracellular protein largely found in endosomes, co-localizes with TLF-1 in early endosomes and traffics to the lysosome.Citation22 Thus, T. b. rhodesiense survives in humans largely because it is able to produce an antidote to TLF-1. While untested, it is likely that TLF-2 is inhibited by SRA by the same mechanism since both serum complexes contain apoL-1. In contrast, we recently showed that group 1 T. b. gambiense does not bind or take up TLF-1, suggesting that these cells have evolved a different mechanism to avoid the cytotoxicity of TLF-1.Citation23 The underlying basis for reduced TLF-1 uptake is 2-fold. First, TbgHpHpR is expressed at very low levels by group 1 T. b. gambiense and second, TbgHpHbR contains a number of point mutations within the coding sequence that render the receptor non-functional.Citation23 The combination of mutations to the TbgHpHbR and reduced expression abolished TLF-1 binding and uptake resulting in resistance to TLF-1. Thus, in contrast to T. b. rhodesiense, it appears the mechanism of group 1 T. b. gambiense resistance to TLF-1 involves reduced uptake and avoidance of the toxin. To date no evidence for an inhibitory protein with SRA-like characteristics has been described in T. b. gambiense. In this short addendum to the Kieft et al. paperCitation23 we now show that while the TbbHpHbR enhances susceptibility to human serum, TLF-2 and TLF-1 other receptors or fluid phase endocytosis also contribute to trypanosome killing. Further, our results suggest that the resistance of group 1 T. b. gambiense to human serum and TLF involves other mechanisms beyond the simple loss of a single receptor.
Materials and Methods
TLF-1 and TLF-2 purification
Total serum was obtained from a healthy human donor. As previously described, two sequential flotations on sodium bromide gradients (ρ = 1.063 and 1.26 g/ml) resulted in an HDL-rich fraction (TLF-1; top third of the gradient) and a lipoprotein-deficient fraction (TLF-2; bottom third of the gradient).Citation4 The TLF-1 fraction was passed over an anti-IgM column (Sigma, A9935). The unbound material was then passed over an anti-Hpr column, washed with PBSE (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 10 mM KH2PO4, 3 mM EDTA) and bound protein was eluted in 100 mM glycine (pH 2.5) and neutralized with 1 M Tris (pH 7.5). The TLF-2 fraction was passed over an anti-Hpr column and washed with PBSE. Bound protein was eluted in 100 mM glycine (pH 2.5), neutralized with 1 M Tris (pH 7.5) and immediately added to an anti-IgM column and washed with PBSE. Bound protein was eluted in 100 mM glycine (pH 2.5), neutralized with 1 M Tris (pH 7.5). All protein samples were aliquoted and stored at −80°C.
Size exclusion chromatography and protein gel blot analysis
Size exclusion chromatography was performed on a 1 X PBSE equilibrated Superose 6 10/300 GL column (GE Healthcare). Individual protein standards were used to estimate the molecular weights of TLF-1 and TLF-2. Samples of TLF-1 and TLF-2 from immuno-affinity purification (70 μg) were run on the Superose 6 column at a flow rate of 0.5 ml/min. Fractions were collected (0.5 ml), proteins concentrated 6-fold with microspin S-300HR columns (GE, 27513001) and the distribution of Hpr, apoL-1 and IgM determined by SDS-PAGE, silver staining and protein gel blot analysis. Characterization of antibodies against Hpr and apoL-1 has previously been described.Citation7 Anti-IgM was purchased from Sigma and used according to the manufacturer’s recommendation (Sigma, I0759).
Results
TLF-1 resistant T. b. brucei
During the course of our studies on the mechanism of TLF-1 resistance in group 1 T. b. gambiense we developed a laboratory model for TLF-1 resistance using well-characterized clonal cell lines of T. b. brucei that had been selected for resistance to human HDLs.Citation23,Citation24 We isolated TLF-1 resistant (R) or susceptible (S) T. b. brucei lines expressing either the VSG800 or VSG060.Citation23 The T. b. brucei 427-800R and T. b. brucei 427-060R lines showed reduced uptake of TLF-1 relative to the TLF-1 susceptible parental T. b. brucei 427-221S cells and TLF-1 susceptible cells expressing either VSG800 or VSG060. In addition, we showed that the expression of TbbHpHbR mRNA was reduced approximately 20-fold in resistant cells.Citation23 These findings led us to examine group 1 T. b. gambiense where we found that not only was expression of the TbgHpHbR mRNA reduced but that mutations to the gene abolished function.Citation23
Purification and characterization of TLF-1 and TLF-2
In order to determine whether loss of TbbHpHbR was sufficient to provide complete protection from human serum, TLF-1 and TLF-2 activity we developed a purification protocol exploiting physical and compositional differences in these human serum innate immune complexes (). Freshly collected human plasma was initially separated by density gradient ultracentrifugation to produce HDL-enriched (ρ 1.063–1.26 g/ml) and lipoprotein-deficient fractions (ρ < 1.063 g/ml) that were used as the starting materials for TLF-1 and TLF-2 purification respectively. During the purification of TLF-1 small amounts of contaminating TLF-2 were removed from the HDL-containing fraction by absorption with anti-IgM. TLF-1 was subsequently bound to anti-Hpr resin, washed extensively at neutral pH to remove human HDLs lacking Hpr and eluted at low pH. TLF-2 was purified from the lipoprotein-deficient serum by sequential affinity chromatography with anti-Hpr followed by binding and elution from an anti-IgM column. The purity of TLF-1 and TLF-2 was evaluated by size exclusion chromatography on Superose 6 and protein gel blot analysis with anti-Hpr, apoL-1 and IgM (). Based on size exclusion chromatography, TLF-1 and TLF-2 have estimated relative sizes of 576 kDa and 1.6 MDa respectively.Citation17 Superose 6 chromatography of purified TLF-1 and TLF-2 revealed somewhat dispersed distributions consistent with particle heterogeneity but there was minimal overlap of the TLF-1 and TLF-2 absorbance peaks at 280 nM (). Protein gel blot analysis revealed no contaminating TLF-2 in our purified TLF-1 preparations based on the lack of anti-IgM reactive material on protein gel blots (; data not shown). TLF-2 preparations were highly enriched in particles containing Hpr, apoL-1 and IgM; however, these preparations also contained small amounts of IgM deficient complexes with an elution time (~28 min) from the Superose 6 column consistent with TLF-1. Based on the distribution of the Hpr dimer and IgM across the size exclusion fractions we estimate the amount of contaminating TLF-1 in these preparations to be ~10%.
Figure 1. Characterization of purified TLF-1 and TLF-2. (A) Superose 6 size exclusion chromatography of TLF-1 and TLF-2. Absorbance profiles (280 nM) of TLF-1 and TLF-2, superimposed on individually ran marker proteins [1, thyroglobulin (660 kDa); 2, apoferritin (480 kDa); 3, conalbumin (67 kDa); 4, ovalbumin (45 kDa)]. (B) Analysis of individual Superose 6 column fractions of TLF-1 and TLF-2 separated on non-denaturing 12% SDS-PAGE and silver stained (top panel). Hpr, apoL1 and IgM were detected by protein gel blot. NA, not analyzed.
![Figure 1. Characterization of purified TLF-1 and TLF-2. (A) Superose 6 size exclusion chromatography of TLF-1 and TLF-2. Absorbance profiles (280 nM) of TLF-1 and TLF-2, superimposed on individually ran marker proteins [1, thyroglobulin (660 kDa); 2, apoferritin (480 kDa); 3, conalbumin (67 kDa); 4, ovalbumin (45 kDa)]. (B) Analysis of individual Superose 6 column fractions of TLF-1 and TLF-2 separated on non-denaturing 12% SDS-PAGE and silver stained (top panel). Hpr, apoL1 and IgM were detected by protein gel blot. NA, not analyzed.](/cms/asset/17ab197f-2f65-45a6-aad2-227782a58cd0/kvir_a_10918295_f0001.gif)
Susceptibility of T. b. brucei to human serum, TLF-1 and TLF-2
Our previous studies compared the short-term killing of trypanosomes to TLF-1.Citation23 Here we have re-examined the susceptibility of these T. b. brucei lines using a long-term growth assay (). Consistent with previous studies, the parental T. b. brucei 427-221S, T. b. brucei 427-800S, and T. b. brucei 427-060S were highly susceptible to TLF-1 with a calculated LG50 of 0.8–6 ng/ml. T. b. brucei 427-800R and T. b. brucei 427-060R were > 10,000-fold more resistant to TLF-1 than wild-type T. b. brucei, suggesting the TbbHbHbR is important in TLF-1 susceptibility. However, concentrations of > 10 μg/ml overcame the TbbHpHbR deficiency leading to reduced survival (). Since the concentration of TLF-1 needed to kill T. b. brucei 427-800R and T. b. brucei 427-060R is similar to that found in human serum it is likely that TbbHpHbR-independent mechanisms of TLF-1 uptake play a significant role in trypanosome killing.
Figure 2. In vitro activity of human serum, TLF-1 and TLF-2. TLF-1 resistant (R) and susceptible (S) clonal cell lines of bloodstream form T. b. brucei Lister 427 expressing VSG221, 800 and 060 were prepared as previously described.Citation24,Citation25 The percentage surviving cells was determined, using phase contrast microscopy, 14 h following the addition of TLF-1, TLF-2 or complete human serum to exponentially growing cultures at 37°C. (A) TLF-1 susceptibility of T. b. brucei 427-221S (black), T. b. brucei 427-800S (blue), T. b. brucei 427-800R (red), T. b. brucei 427-060S (yellow) and 427-060R (green). (B) Normal human serum (NHS) susceptibility of T. b. brucei 427-221S (black), T. b. brucei 427-800S (blue), T. b. brucei 427-800R (red), T. b. brucei 427-060S (yellow) and 427-060R (green). (C) TLF-2 susceptibility of T. b. brucei 427-221S (black), T. b. brucei 427-800S (blue), T. b. brucei 427-800R (red), T. b. brucei 427-060S (yellow) and 427-060R (green).
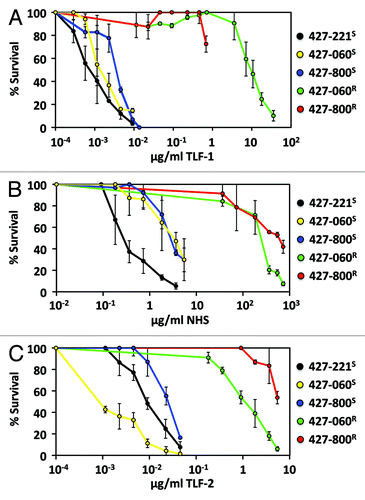
Based on our studies with both the group 1 T. b. gambiense and the TLF-1 resistant T. b. brucei lines we predicted that loss of a functional TbgHpHbR played a critical role in human infection by African trypanosomes.Citation23 The dramatic reduction in susceptibility to TLF-1 in the TbbHpHbR-deficient cell lines supports this prediction (). However, the possibility remained that human serum contained additional innate immune factors, such as TLF-2, that might not require the TbbHpHbR. To test this possibility, we treated TLF-1 resistant and susceptible T. b. brucei lines with human serum (). We found that T. b. brucei 427-800R and T. b. brucei 427-060R were approximately 100-fold more resistant to human serum killing than either T. b. brucei 427-800S or T. b. brucei 427-060S (). Based on these results, we conclude that loss of TbbHpHbR expression contributes to the overall resistance of these cells to human serum; however the level of resistance is much less than the high level of resistance seen for TLF-1 (10,000-fold). A possible interpretation of these findings is that other human serum components, such as TLF-2, are less dependent on TbbHpHbR binding than TLF-1.
It has been proposed that TLF-2 can bind to T. b. brucei in the absence of the TbbHpHbR.Citation18 We tested whether highly purified TLF-2 was able to kill T. b. brucei 427-800R and T. b. brucei 427-060R (). Similar to our findings with complete human serum, these TbbHpHbR-deficient cells were more resistant to TLF-2 relative to the wild-type TbbHpHbR-expressing cell lines. Thus, reduced expression of TbbHpHbR expression caused a reduced susceptibility of TLF-2 killing suggesting that TLF-2 can bind to the TbbHpHbR. However, the toxic concentration of TLF-2 is >10-fold less than TLF-1, indicating that TbbHpHbR-independent mechanisms may play a greater role in TLF-2 binding, uptake and killing.
Discussion
In the studies presented here human serum, TLF-1 and TLF-2 susceptibility was examined in isogenic lines of T. b. brucei differing in TbbHpHbR expression. Cells deficient in TbbHpHbR expression were 10,000-fold more resistant to TLF-1 relative to wild-type susceptible cells. However, at concentrations of TLF-1 typically found in serum (>10 μg/ml), both resistant and susceptible cell lines were killed. Human serum killing was also reduced approximately 100-fold in cell lines expressing reduced levels of TbbHpHbR. However, significant killing was still observed at human serum concentrations above 100 μg/ml. Since the only difference in the susceptible and resistance cell lines is the levels of expression of TbbHpHbR, it seems likely that human serum contains a second trypanolytic activity that interacts with T. b. brucei independently of the TbbHpHbR. Based on this interpretation of the human serum killing results we decided to investigate whether TLF-2 killing of T. b. brucei was independent on the level of expression of TbbHpHbR. We found that TLF-2 killing was reduced 500–1000-fold in cell lines with reduced levels of TbbHpHbR, suggesting that TLF-2 also binds TbbHpHbR. These results are in apparent contrast to previous studies on TLF-2 showing that TLF-2 killing was not inhibited by the addition of haptoglobin, an inhibitor of HpHb binding to the TbbHpHbR.Citation17 These results have been used subsequently to argue that TLF-2 does not bind the TbbHpHbR.Citation10 It is possible that our results are influenced by the small amount of contaminating TLF-1 in our TLF-2 preparations. Clearly, a detailed characterization of the TLF-2 binding, uptake and cellular location is needed.
Our results are consistent with previous findings indicating the importance of the TbbHpHbR in TLF-1 killing but also suggest that other mechanisms of TLF-1 binding and uptake may contribute to trypanosome killing. The most likely pathways for TbbHpHbR-independent uptake of TLF-1 is either by fluid phase endocytosis or the trypanosome lipoprotein scavenger receptorCitation18,Citation25. The findings presented here further support the findings of others that TLF-2 killing is less dependent on the TbbHpHbR than is TLF-1Citation10,Citation17. Finally, we propose that group 1 T. b. gambiense have evolved multiple mechanisms, including but not limited to the loss of a functional HpHbR, to avoid the cytotoxicity of the trypanosome lytic factors. We are currently exploring these mechanisms.
Acknowledgments
We thank current members of the Hajduk Laboratory for comments and suggestions on TLF and its mechanism of killing trypanosomes. The work on TLF in the Hajduk lab is supported by NIH (AI039033). C.M.R.T. and A.M.L. thank the Wellcome Trust for financial support. A.M.L. is a Wellcome Trust Research Career Development Fellow. P.C. is a UK Biotechnology and Biological Sciences Research Council research student.
References
- Pays E. The variant surface glycoprotein as a tool for adaptation in African trypanosomes. Microbes Infect 2006; 8:930 - 7; http://dx.doi.org/10.1016/j.micinf.2005.10.002; PMID: 16480910
- Taylor JE, Rudenko G. Switching trypanosome coats: what’s in the wardrobe. Trends Genet 2006; 22:614 - 20; http://dx.doi.org/10.1016/j.tig.2006.08.003; PMID: 16908087
- Rifkin MR. Identification of the trypanocidal factor in normal human serum: high-density lipoprotein. Proc Natl Acad Sci USA 1978; 75:3450 - 4; http://dx.doi.org/10.1073/pnas.75.7.3450; PMID: 210461
- Hajduk SL, Moore DR, Vasudevacharya J, Siqueira H, Torri AF, Tytler EM, et al. Lysis of Trypanosoma brucei by a toxic subspecies of human high density lipoprotein. J Biol Chem 1989; 264:5210 - 7; PMID: 2494183
- Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P, Nolan DP, Lins L, Van Den Abbeele J, et al. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 2003; 422:83 - 7; http://dx.doi.org/10.1038/nature01461; PMID: 12621437
- Smith AB, Esko JD, Hajduk SL. Killing of trypanosomes by human haptoglobin related protein. Science 1995; 268:284 - 6; http://dx.doi.org/10.1126/science.7716520; PMID: 7716520
- Shiflett AM, Bishop JR, Pahwa A, Hajduk SL. Human high density lipoproteins are platforms for the assembly of multi-component innate immune complexes. J Biol Chem 2005; 280:32578 - 85; http://dx.doi.org/10.1074/jbc.M503510200; PMID: 16046400
- Widener J, Nielsen MJ, Shiflett A, Moestrup S, Hajduk S. Hemoglobin is a co-factor of human trypanosome lytic factor. PLoS Pathog 2007; 3:1250 - 61; http://dx.doi.org/10.1371/journal.ppat.0030129; PMID: 17845074
- Drain J, Bishop R, Hajduk SL. Haptoglobin-related protein mediates trypanosome lytic factor binding to trypanosomes. J Biol Chem 2001; 276:30254 - 60; http://dx.doi.org/10.1074/jbc.M010198200; PMID: 11352898
- Vanhollebeke B, De Muylder G, Nielsen MJ, Pays A, Tebabi P, Dieu M, et al. A haptoglobin-hemoglobin receptor conveys innate immunity to Trypanosoma brucei in humans. Science 2008; 320:677 - 81; http://dx.doi.org/10.1126/science.1156296; PMID: 18451305
- Bishop JR, Shimamura M, Hajduk SL. Insight into the mechanism of trypanosome lytic factor-1 killing of Trypanosoma brucei brucei.. Mol Biochem Parasitol 2001; 118:33 - 40; http://dx.doi.org/10.1016/S0166-6851(01)00361-9; PMID: 11704271
- Pérez-Morga D, Vanhollebeke B, Paturiaux-Hanocq F, Nolan DP, Lins L, Homble F, et al. Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science 2005; 309:469 - 72; http://dx.doi.org/10.1126/science.1114566; PMID: 16020735
- Molina-Portela MP, Samanovic M, Raper J. Distinct roles of apolipoprotein components within the trypanosome lytic factor complex revealed in a novel transgenic mouse model. J Exp Med 2008; 205:1721 - 8; http://dx.doi.org/10.1084/jem.20071463; PMID: 18606856
- Molina-Portela MP, Lugli EB, Recio-Pinto E, Raper J. Trypanosome lytic factor, a subclass of high-density lipoprotein, forms cation-selective pores in membranes. Mol Biochem Parasitol 2005; 144:218 - 26; http://dx.doi.org/10.1016/j.molbiopara.2005.08.018; PMID: 16202458
- Rifkin MR. Trypanosoma brucei: Biochemical and morphological studies of cytotoxicity caused by normal human serum. Exp Parasitol 1984; 58:81 - 93; http://dx.doi.org/10.1016/0014-4894(84)90023-7; PMID: 6745390
- Vanhollebeke B, Lecordier L, Perez-Morga D, Amiguet-Vercher A, Pays E. Human serum lyses Trypanosoma brucei by triggering uncontrolled swelling of the parasite lysosome. J Eukaryot Microbiol 2007; 54:448 - 51; http://dx.doi.org/10.1111/j.1550-7408.2007.00285.x; PMID: 17910690
- Raper J, Fung R, Ghiso J, Nussenzweig V, Tomlinson S. Characterization of a novel trypanosome lytic factor in human serum. Infect Immun 1999; 67:1910 - 6; PMID: 10085035
- Vanhollebeke B, Pays E. The trypanolytic factor of human serum; many ways to enter the parasite, a single way to kill. Mol Microbiol 2010; 76:806 - 14; http://dx.doi.org/10.1111/j.1365-2958.2010.07156.x; PMID: 20398209
- De Greef C, Hamers R. The serum resistance-associated (SRA) gene of Trypanosoma brucei rhodesiense encodes a variant surface glycoprotein-like protein. Mol Biochem Parasitol 1994; 68:277 - 84; http://dx.doi.org/10.1016/0166-6851(94)90172-4; PMID: 7739673
- Turner CMR, McLellan S, Lindergard LAG, Bisoni L, Tait A, MacLeod A. Human infectivity trait in Trypanosoma brucei: stability, heritability and relation to sra expression. Parasitology 2004; 129:445 - 54; http://dx.doi.org/10.1017/S0031182004005906; PMID: 15521633
- Xong HV, Vanhamme L, Chamekh M, Chimfwembe CE, Van Den Abbeele J, Pays A, et al. A VSG expression site-associated gene confers resistance to human serum in Trypanosoma rhodesiense. Cell 1998; 95:839 - 46; http://dx.doi.org/10.1016/S0092-8674(00)81706-7; PMID: 9865701
- Stephens NA, Hajduk SL. Endosomal Localization of the serum resistance-associated protein in African trypanosomes confers human infectivity. Eukaryot Cell 2011; 10:1023 - 33; http://dx.doi.org/10.1128/EC.05112-11; PMID: 21705681
- Kieft R, Capewell P, Turner CM, Veitch NJ, MacLeod A, Hajduk S. Mechanism of Trypanosoma brucei gambiense (group 1) resistance to human trypanosome lytic factor. Proc Natl Acad Sci USA 2010; 107:16137 - 41; http://dx.doi.org/10.1073/pnas.1007074107; PMID: 20805508
- Faulkner SD, Oli MW, Kieft R, Cotlin L, Widener J, Shiflett A, et al. In vitro generation of human high density lipoprotein resistant Trypanosoma brucei brucei. Eukaryot Cell 2006; 5:1276 - 86; http://dx.doi.org/10.1128/EC.00116-06; PMID: 16896212
- Green HP, Pilar Molina Portela M, St. Jean EM, Lugli EB, Raper J. Evidence for a Trypanosoma brucei lipoprotein scavenger receptor. J Biol Chem 2003; 278:422 - 7; http://dx.doi.org/10.1074/jbc.M207215200; PMID: 12401813