Abstract
Invasive aspergillosis is a life-threatening disease mainly caused by Aspergillus fumigatus. Patients at risk are generally immunocompromised and lungs are assumed to provide the primary site for infection and invasive disease manifestation. Contrarily, visceral organ involvement appears to result from a subsequent hematogenous spread. To compare the kinetics of dissemination within deep organs in immunosuppressed vs. immunocompetent mice, we used a bioluminescent A. fumigatus strain in an intravenous infection model. By applying an immunosuppressive regimen with corticosteroids, dissemination to the liver and kidneys was observed already 24 h after inoculation accompanied by a marked inflammatory response within the liver. In contrast, in the immunocompetent condition, fungal growth and inflammation were mainly restricted to the kidneys and only small amounts of fungal biomass and a weak inflammatory response were detected in the liver. Additionally, disease progressed much slower compared with the immunosuppressed condition. This is the first study underlying the duality between liver and renal tropism of A. fumigatus in relation to the immune status of the host.
Introduction
The rising incidence of pulmonary aspergillosis is unlikely to diminish because of the constant increase of patients with prolonged neutropenia. A. fumigatus causes a myriad of diseases ranging from non-invasive disease with superficial colonization to disseminated invasive disease with an associated high mortality rate. Immunosuppression markedly increases the risk for invasive disease characterized by tissue invasion and secondary bloodstream dissemination to multiple organs.Citation1 Extra-pulmonary aspergillosis may be present in 25–60% of cases and is almost universally described in the context of disseminated diseases.Citation2 Isolated extra-pulmonary aspergillosis located in the central nervous system, the skin, the liver, the urinary tract and the digestive tract has been mentioned in case reports, but potential portals of entry other than the respiratory tract are speculative.Citation3
Murine disseminated aspergillosis has rarely been documented. Indeed, mice with pulmonary infection either overcome the infection by residual immune responses in case of low dose infections or succumb from inflammatory responses or tissue infarction.Citation4 Thus, systemic infections using intravenous inoculation of A. fumigatus conidia have been used in immunocompetent and immunosuppressed animals to mimic the dissemination observed in humans regardless of the natural entry site of A. fumigatus.Citation5-Citation7 These investigations revealed that intravenous infection results in reproducible organ infection and eventual healing in immunocompetent animals.Citation8
To monitor the disease progression, two primary parameters of infection are generally followed: survival rate and fungal burden in infected tissues. In the case of invasive aspergillosis, the best suited method for determination of the infectious burden is controversial. Besides quantification of fungal genomic DNA by qPCR, determination of cell wall compounds by chitin assays, CFU determinations, or evaluation of the tissue burden from histopathologic analyses are common.Citation9 All assays have in common that fungal burden can only be determined from sacrificed animals.
In this study, we addressed the following questions: (1) How does the host respond to A. fumigatus after intravenous inoculation, under immunosuppression by corticosteroids and in the immunocompetent condition, (2) what is the kinetics of disease progression, (3) does the amount of the fungal burden differ between organs and immune status and (4) what is the impact of the immune status on the inflammatory response within liver and kidneys? To answer these questions we used the bioluminescent A. fumigatus strain C3, which constitutively expresses the firefly luciferase under the control of the gpdA promoter.Citation10
In vivo bioluminescence imaging allowed studying the progression of infection in individual animals. Significant differences in the temporal and spatial manifestation of organ infections were observed in dependence of the immune status of infected animals. Results from in vivo imaging were confirmed by histopathologic analyses, showing severe inflammatory lesions only observed in the liver of immunosuppressed mice while fungal growth and inflammation were mainly restricted to the kidneys of immunocompetent infected mice.
Materials and Methods
Strain culturing and mouse infection
A. fumigatus strain C3
The bioluminescent A. fumigatus strain C3 was cultured on malt extract agar slants as described earlierCitation10 and bioluminescence of the strain was confirmed in vitro by determination of light emission from cultures grown for 8 h in RPMI medium by using an IVIS 100 system (Xenogen Corporation).
Mice and immunosuppressive treatment
Male Balb/C (25 g, 6-week-old) were cared for in accordance with Institut Pasteur guidelines in compliance with the European animal welfare regulation. Immunosuppression with cortisone acetate was performed as previously described.Citation11
Infection
For immunocompetent and immunosuppressed mice, two groups of five animals were infected via the lateral tail vein with 2 × 106 conidia in a total volume of 100 μl.
In all experiments mice were kept for a maximum of 15 d post-inoculation. A weight curve for individual mice was recorded in 24 h intervals starting three days prior to infection.
Bioluminescence imaging and histopathology
All bioluminescence images were acquired using an IVIS 100 system as previously described.Citation10 For the histopathological analyses, organs were collected between 45 h and 69 h for immunosuppressed mice and between day 8 and 11 for immunocompetent mice. Organs of interest were immediately fixed in 4% neutral-buffered formalin and embedded in paraffin. Five micrometer sections were cut and stained with hematoxylin and eosin (HE) and Grocott’s methenamine silver, for detection of fungi as already described.Citation12
Statistical analysis
Comparisons between groups were performed using t tests. Significance was determined with the Wilcoxon rank test. A p value of < 0.05 was considered statistically significant. Data are reported in the figures as means ± standard deviation.
Results
Effect of the immune status on disease progression and survival
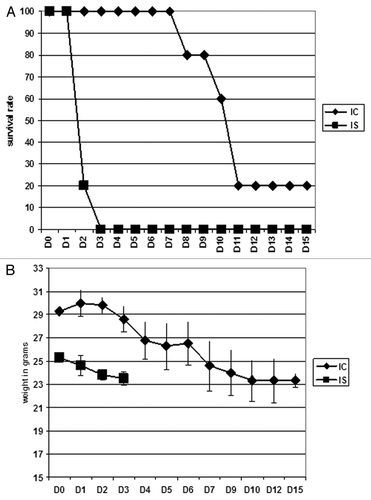
Investigating the survival of cortisone acetate treated vs. immunocompetent mice after intravenous infection with 2 × 106 conidia showed significant differences in the disease patterns. All immunosuppressed animals died within three days after inoculation and lost an average of 13% of their initial body weight. In contrast, all immunocompetent animals survived the first eight days after inoculation, but started to succumb to infection the following days. Only 20% survived the observation period of 15 d accompanied by a weight loss of between 3 to 20% starting at day three after infection ().
Figure 1. Mice survival following intravenous infection with 2 × 106 conidia of A. fumigatus. In each experiment, groups of 5 mice were immunosuppressed (IS) with cortisone acetate at day −3 and day 0 prior to intravenous infection with 2 × 106 conidia of the bioluminescent A. fumigatus strain C3. Untreated infected mice were designated as immunocompetent (IC). Weight loss and survival were monitored for 15 d (A) and (B). The experiment was repeated once and combined data are presented.
In vivo bioluminescence imaging
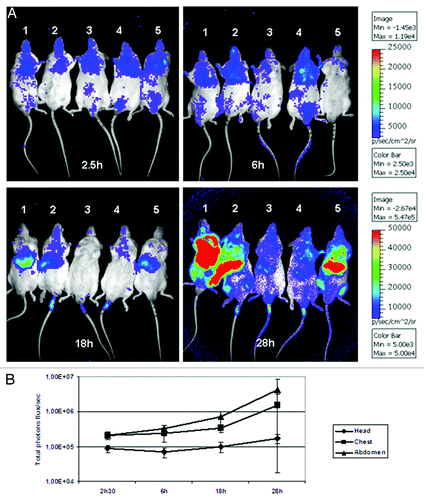
Bioluminescence imaging on the cortisone acetate treated animals revealed that luminescence signals were already detected from the chest and brain between 2.5 to 6 h after inoculation (). After 18 h, the luminescence increased within the abdominal region suggesting that the liver became severely infected. After 28 h, the luminescence signal dramatically increased with an average of total photon fluxes of 4.25 × 106 from the abdominal, 1.50 × 106 from the chest and 1.72 × 105 from the head region (). These results clearly indicate that conidia were trapped in specific organs and possessed a rapid germination rate within these tissues, which resulted in the death of all mice within 3 d.
Figure 2. Time dependent monitoring of light emission from immunosuppressed mice. (A) Luminescence was acquired with an IVIS 100 system, 10 min after intraperitoneal injection of D-luciferin. Light emission from live animals was recorded for 5 min. Three independent regions of interest (ROI) from the head, chest and abdomen for each individual animal were analyzed and the total luminescence (total flux/second) from each ROI was quantified using the living image 3.1 software. In (B) is shown the average of luminescence from head, chest and abdomen of all mice.
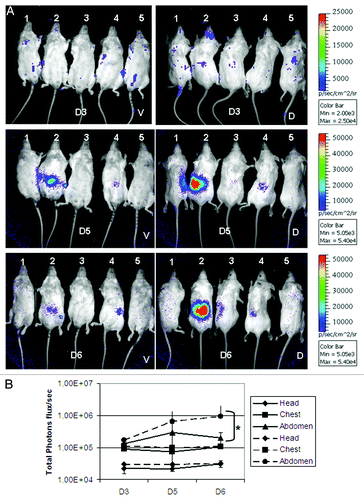
In contrast to the immunosuppressed animals, emergence of luminescence signals was delayed in immunocompetent animals. As seen in , unlike the low constant signal measured from head and chest areas, significant increasing signals appeared 5 d after inoculation from the abdominal region reaching peak values of 2.1 × 105 and 9.5 × 105 average total photon fluxes from the ventral and dorsal sides of the abdominal area at day 6 post infection (p < 0.05). In addition, as shown in , immunocompetent mice succumbed to infection much later than cortisone acetate treated mice (days 8–11 vs. days 2–3). Collectively, these results confirmed a higher degree of fungal growth restriction under the immunocompetent condition.
Figure 3. Time dependent monitoring of light emission from immunocompetent mice. Luminescence was acquired from mice as indicated in . (A) The luminescence was measured from ROIs from both ventral (V) and dorsal (D) sides. In (B) is shown the average of luminescence from head, chest and abdomen of all mice from ventral (full line) and dorsal (dashed line) sides. *p < 0.05.
Histopathological analysis
Histopathological analyses revealed a good correlation between the luminescence signal and the presence of fungi within these tissues (). In the immunosuppressed condition, intravenously injected conidia massively disseminated to the liver and kidneys. In the immunocompetent condition fungal growth was mainly restricted to the kidneys and only to a very minor extent observed in the liver.
Figure 4. Comparison of histopathologic lesion profiles between immunocompetent and immunosuppressed mice. Organs were collected between 45 and 69 h after infection for immunosuppressed mice and between day 8 and day 11 for immunocompetent mice. Liver: In the immunosuppressed model, marked zonal hepatitis characterized by (A) centrilobular necrosis and neutrophil infiltration, centered on a centrilobular vein and second extending to blood vessel wall (inset, arrowhead) and parenchyma. (B) Numerous intralesional hyphae were observed. In the immunocompetent model (C), no inflammation is detected and the lesions were only characterized by centrilobular necrosis, with hepatocytes displaying hypercondensed (pycnosis) or fragmented (karyorrhexis) nuclei (inset, arrowheads). (D) Rarely conidia were observed in the cytoplasm of Kupffer cells (inset). Kidney: in the immunosuppressed model, (E) lesions centered on cortical glomeruli and were characterized by neutrophil infiltration (inset) associated with (F) A. fumigatus hyphae. In the immunocompetent model, (G) severe lesions centered on the pelvis and the papilla, characterized by a massive neutrophil and macrophage infiltration (inset), associated with (H) a very high density of fungal hyphae. (A, C, E, G) HE staining. (B, D, F, H) Grocott staining.
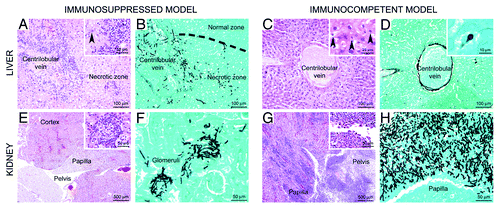
A more detailed analysis of fungal growth and histological lesions of the liver of corticosteroid treated mice revealed a marked zonal lesion centered on portal tracts and centrilobular veins, but secondly extending to sinusoids. It was characterized by large zones of hepatocyte necrosis associated with infiltration of neutrophils that were often fragmented (suppuration), lymphocytes, plasma cells, and macrophages. The cellular infiltrates often involved blood vessel walls (veins and arteries) and were associated with fibrinous thrombi (). Intralesional Aspergillus hyphae were detected ().
Lesions were markedly different in the immunocompetent model. In the liver of two out of five mice investigated, a minimal zonal multifocal lesion, centered on centrilobular veins, was observed (). It was characterized by necrosis of hepatocytes of the centrilobular zone (, inset), associated with the presence of conidia in the cytoplasm of Kupffer cells (). As these conidia remained in a resting state even at late stages of infection (day 6 post-infection and beyond), it can be assumed, that Kupffer cells were able to phagocytose conidia and prevent the rapid germination within this organ.
In the kidneys of corticosteroid treated mice (), a mild inflammatory lesion usually centered on cortical glomeruli was observed. It was characterized by necrosis of mesangial cells sometimes associated with mesangial infiltrates of lymphocytes, plasma cells and neutrophils (, inset). Intralesional fungal hyphae were numerous and extended across the Bowman’s capsule ().
Concerning the kidneys of immunocompetent mice, significant differences could be noted between mice that survived or not. Surviving mice indeed displayed no significant histological lesion in the kidney except one mouse with only very minimal inflammatory infiltrates in the papilla (data not shown). However, mice that died of infection rather displayed severe lesions (), centered on the pelvis and secondarily extending to renal tubes of the medulla and cortex (ascending infection: pyelonephritis), in contrast to corticosteroid treated mice, in which the cortical glomeruli were targeted (descending infection: glomerulonephritis). These lesions were characterized by a severe infiltration of neutrophils, very often fragmented (suppuration) (, inset). Additionally, a high density of A. fumigatus hyphae was detected in the lesions of the renal pelvis ().
Discussion
Since aspergillosis is an airborne infection, lungs are the primary organ for disease manifestation. However, other organs such as the brain and the liver can become infected by dissemination through the blood stream.Citation13 Although intracerebral or intravenous infections are not assumed to play an important natural route for the acquisition of invasive aspergillosis, such infections are frequently used to test the efficiency of antifungal drugs and to study the dissemination and disease progression in brain and kidneys.Citation5-Citation7,Citation14 The ability to detect luminescence from all deep tissues including liver and kidneys is an essential prerequisite for a potential use of luminescence based systems for monitoring drug efficiency from in vivo conditions. Our previous studies using an intranasal infection model revealed a localization of fungi that was mainly restricted to the lung and, in some cases, the nasal sinus.Citation4 Thus, to confirm that bioluminescence can be tracked from all body sites, we took advantage of intravenous conidia injections to confirm the general suitability of the system. Indeed, luminescence signals were detected from liver and kidneys and correlated with histologic analyses. Despite previous assumptions that distribution of firefly luciferin may provide a limiting factor for imaging the dissemination of infections, tracking of A. fumigatus infections from diverse body sites indicates that substrate availability is not a limiting factor of this technique.Citation15,Citation16
In this study, we were additionally interested in the kinetics of fungal distribution, the velocity of the manifestation and progression of infection, the main organs targeted and the fungal load in these organs after intravenous inoculation.
An important observation from our study was the effect of corticosteroid treatment on the rapid manifestation of infection in the liver. This infection was accompanied by a moderate to marked inflammatory reaction characterized by multifocal infiltrates of neutrophils, lymphocytes and macrophages. The nature of this inflammatory infiltrate appears similar to that observed in the lungs of corticosteroid immunosuppressed mice following intranasal inoculation. In the lung of immunocompetent mice alveolar macrophages phagocytose inhaled conidia and restrict their rapid germination. This function is hampered under corticosteroid treatment resulting in a rapid escape of A. fumigatus conidia from alveolar macrophages accompanied by massive neutrophil influx with severe tissue destruction.Citation4
In the liver, nonspecific phagocytosis is mediated primarily by Kupffer cells.Citation17 In agreement, in the immunocompetent condition conidia were detected in the cytoplasm of some Kupffer cells. As these conidia remained in a resting state even at late stages of infection (day 6 post-infection and beyond), it can be assumed that Kupffer cells efficiently phagocytose conidia and inhibit their germination. This is in agreement with a previous observation on A. fumigatus and Candida albicans infections in which a TLR4 independent release of proinflammatory cytokines has been observed pointing to a vital immune response of Kupffer cells toward fungal infections.Citation18
We assume that treatment with cortisone acetate might alter the efficiency of Kupffer cells to restrict fungal germination. In this respect it has been shown that high glucocorticoid concentrations reduce the production of nitric oxide, oxygen radicals and prostaglandins and impair phagocytosis.Citation19 Thus, such an impairment of function may allow the rapid germination of conidia. However, corticosteroids may also be responsible for the inflammation observed in the liver of treated animals. Systemic administration of corticosteroids increases the number of circulating neutrophils by 3- to 5-fold,Citation20 leading to a significant increase in neutrophil recruitment to the site of infection. Due to the germination of conidia, the neutrophil recruitment could be further stimulated by inflammatory cytokine secretion regulated by NK and DC cells in response to fungal antigens presented on the surface of hyphae.Citation21
The second major observation concerned the kidney lesions, and more specifically the significant differences in severity and pathogenesis of these lesions between immunocompetent and immunosuppressed mice. In the immunosuppressed condition kidney lesions were characteristic for a descending infection with a glomerular capillary starting point (glomerulonephritis). These lesions were minimal and infected mice would probably have died from their liver destruction before kidney lesions could become more severe. In contrast, in the immunocompetent condition, in which the liver displayed no obvious severe pathology, kidney lesions were severe and characteristic for an ascending infection, with a pelvis starting point (pyelonephritis). These inflammatory lesions (massive neutrophil infiltration and renal parenchyma destruction) were severe enough to cause the death of infected animals. A pelvis and medulla targeting after intravenous inoculation of A. fumigatus was already described in the literatureCitation8 occurring 9 d after infection, but the exact mechanism of this targeting and differences concerning the immune status were not described. Our results suggest that the prolonged time course of infection in immunocompetent mice allowed the development of pelvis lesions. In contrast, the immunosuppressed mice died before any lesion could be detected in the medulla of kidneys.
Further studies are required to elucidate the exact mechanisms of kidney infections and could include other imaging techniques such as two-photon imaging. At current, such differences in kidney infections by A. fumigatus have not been described, but addressing these questions in future studies might enhance the knowledge on fungal pathogenicity mechanisms.
In conclusion, this is, to our knowledge, the first report describing the suitability of bioluminescence imaging to detect disseminated fungal infections with a qualitative assessment of the fungal burden from deep tissues such as the liver or kidneys. Although depth of infection, light absorption by hemoglobin and light scattering from bones make a transfer of light intensity signals into colony forming units difficult,Citation22 this technique reflects the increase of fungal burdens in various organs from individual animals. Previous studies on disseminated candidiasis using C. albicans strains that produced either an intracellular firefly luciferase or a cell wall-bound Gaussia luciferase failed.Citation23,Citation24 These negative results for monitoring systemic disease were mainly attributed to limited substrate distribution of coelenterazine in case of Gaussia luciferase or a limited uptake of D-luciferin by Candida hyphae in the case of the firefly luciferase. However, our studies on A. fumigatus show that bioluminescence imaging is, in general, suitable to study disease progression of fungal pathogens and substrate availability does not appear as a major limiting factor. Thus, this technique provides a perfect tool to identify the main target organs during an infection process and allows monitoring differences in disease progression depending on the immune status of the host. In addition, this technique provides a potential tool in prospective studies to monitor the efficacy of antifungal drugs in animal models and may be combined with additional monitoring techniques using labeled antimicrobial peptides.Citation25
Acknowledgments
We express our gratitude to T. Angelique and K. Sebastien for their consistent aid in the animal facilities. This work was supported by grants of the Hans Knoell-Institute (MB) and funding from the Institut Pasteur through a Programme Tansversal de Recherche (OI-G).
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
References
- Alderson JW, Van Dinter TG Jr., Opatowsky MJ, Burton EC. Disseminated aspergillosis following infliximab therapy in an immunosuppressed patient with Crohn's disease and chronic hepatitis C: a case study and review of the literature. MedGenMed 2005; 7:7; PMID: 16369233
- Bartlett JG. Aspergillosis update. Medicine (Baltimore) 2000; 79:281 - 2; http://dx.doi.org/10.1097/00005792-200007000-00009; PMID: 10941357
- Eggimann P, Chevrolet JC, Starobinski M, Majno P, Totsch M, Chapuis B, et al. Primary invasive aspergillosis of the digestive tract: report of two cases and review of the literature. Infection 2006; 34:333 - 8; http://dx.doi.org/10.1007/s15010-006-5660-0; PMID: 17180588
- Ibrahim-Granet O, Jouvion G, Hohl TM, Droin-Bergere S, Philippart F, Kim OY, et al. In vivo bioluminescence imaging and histopathopathologic analysis reveal distinct roles for resident and recruited immune effector cells in defense against invasive aspergillosis. BMC Microbiol 2010; 10:105; http://dx.doi.org/10.1186/1471-2180-10-105; PMID: 20377900
- Mavridou E, Bruggemann RJ, Melchers WJ, Verweij PE, Mouton JW. Impact of cyp51A mutations on the pharmacokinetic and pharmacodynamic properties of voriconazole in a murine model of disseminated aspergillosis. Antimicrob Agents Chemother 2010; 54:4758 - 64; http://dx.doi.org/10.1128/AAC.00606-10; PMID: 20733046
- Olson JA, George A, Constable D, Smith P, Proffitt RT, Adler-Moore JP. Liposomal amphotericin B and echinocandins as monotherapy or sequential or concomitant therapy in murine disseminated and pulmonary Aspergillus fumigatus infections. Antimicrob Agents Chemother 2010; 54:3884 - 94; http://dx.doi.org/10.1128/AAC.01554-09; PMID: 20606065
- Clemons KV, Stevens DA. The contribution of animal models of aspergillosis to understanding pathogenesis, therapy and virulence. Med Mycol 2005; 43:Suppl 1 S101 - 10; http://dx.doi.org/10.1080/13693780500051919; PMID: 16110800
- Kretschmar M, Buchheidt D, Hof H, Nichterlein T. Galactomannan enzyme immunoassay for monitoring systemic infection with Aspergillus fumigatus in mice. Diagn Microbiol Infect Dis 2001; 41:107 - 12; http://dx.doi.org/10.1016/S0732-8893(01)00282-6; PMID: 11750162
- Vallor AC, Kirkpatrick WR, Najvar LK, Bocanegra R, Kinney MC, Fothergill AW, et al. Assessment of Aspergillus fumigatus burden in pulmonary tissue of guinea pigs by quantitative PCR, galactomannan enzyme immunoassay, and quantitative culture. Antimicrob Agents Chemother 2008; 52:2593 - 8; http://dx.doi.org/10.1128/AAC.00276-08; PMID: 18474582
- Brock M, Jouvion G, Droin-Bergere S, Dussurget O, Nicola MA, Ibrahim-Granet O. Bioluminescent Aspergillus fumigatus, a new tool for drug efficiency testing and in vivo monitoring of invasive aspergillosis. Appl Environ Microbiol 2008; 74:7023 - 35; http://dx.doi.org/10.1128/AEM.01288-08; PMID: 18820063
- Schöbel F, Ibrahim-Granet O, Ave P, Latge JP, Brakhage AA, Brock M. Aspergillus fumigatus does not require fatty acid metabolism via isocitrate lyase for development of invasive aspergillosis. Infect Immun 2007; 75:1237 - 44; http://dx.doi.org/10.1128/IAI.01416-06; PMID: 17178786
- Sinha BK, Monga DP, Prasad S. A combination of Gomori-Grocott methenamine silver nitrate and hematoxylene and eosin staining technique for the demonstration of Candida albicans in tissue. Quad Sclavo Diagn 1988; 24:129 - 32; PMID: 2479048
- Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008; 46:327 - 60; http://dx.doi.org/10.1086/525258; PMID: 18177225
- Clemons KV, Parmar R, Martinez M, Stevens DA. Efficacy of Abelcet alone, or in combination therapy, against experimental central nervous system aspergillosis. J Antimicrob Chemother 2006; 58:466 - 9; http://dx.doi.org/10.1093/jac/dkl236; PMID: 16760192
- Berger F, Paulmurugan R, Bhaumik S, Gambhir SS. Uptake kinetics and biodistribution of 14C-D-luciferin–a radiolabeled substrate for the firefly luciferase catalyzed bioluminescence reaction: impact on bioluminescence based reporter gene imaging. Eur J Nucl Med Mol Imaging 2008; 35:2275 - 85; http://dx.doi.org/10.1007/s00259-008-0870-6; PMID: 18661130
- Lee KH, Byun SS, Paik JY, Lee SY, Song SH, Choe YS, et al. Cell uptake and tissue distribution of radioiodine labelled D-luciferin: implications for luciferase based gene imaging. Nucl Med Commun 2003; 24:1003 - 9; http://dx.doi.org/10.1097/00006231-200309000-00009; PMID: 12960600
- Parker GA, Picut CA. Liver immunobiology. Toxicol Pathol 2005; 33:52 - 62; http://dx.doi.org/10.1080/01926230590522365; PMID: 15805056
- Overland G, Stuestol JF, Dahle MK, Myhre AE, Netea MG, Verweij P, et al. Cytokine responses to fungal pathogens in Kupffer Cells are Toll-like receptor 4 independent and mediated by tyrosine kinases. Scand J Immunol 2005; 62:148 - 54; http://dx.doi.org/10.1111/j.1365-3083.2005.01653.x; PMID: 16101821
- Lim HY, Muller N, Herold MJ, van den Brandt J, Reichardt HM. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology 2007; 122:47 - 53; http://dx.doi.org/10.1111/j.1365-2567.2007.02611.x; PMID: 17451463
- Schleimer RP. Glucocorticoids suppress inflammation but spare innate immune responses in airway epithelium. Proc Am Thorac Soc 2004; 1:222 - 30; http://dx.doi.org/10.1513/pats.200402-018MS; PMID: 16113438
- Krueger PD, Lassen MG, Qiao H, Hahn YS. Regulation of NK cell repertoire and function in the liver. Crit Rev Immunol 2011; 31:43 - 52; PMID: 21395510
- Andreu N, Zelmer A, Wiles S. Noninvasive biophotonic imaging for studies of infectious disease. FEMS Microbiol Rev 2011; 35:360 - 94; http://dx.doi.org/10.1111/j.1574-6976.2010.00252.x; PMID: 20955395
- Doyle TC, Nawotka KA, Kawahara CB, Francis KP, Contag PR. Visualizing fungal infections in living mice using bioluminescent pathogenic Candida albicans strains transformed with the firefly luciferase gene. Microb Pathog 2006; 40:82 - 90; http://dx.doi.org/10.1016/j.micpath.2005.11.003; PMID: 16426810
- Enjalbert B, Rachini A, Vediyappan G, Pietrella D, Spaccapelo R, Vecchiarelli A, et al. A multifunctional, synthetic Gaussia princeps luciferase reporter for live imaging of Candida albicans infections. Infect Immun 2009; 77:4847 - 58; http://dx.doi.org/10.1128/IAI.00223-09; PMID: 19687206
- Lupetti A, de Boer MG, Erba P, Campa M, Nibbering PH. Radiotracers for fungal infection imaging. Med Mycol 2011; 49:Suppl 1 S62 - 9; http://dx.doi.org/10.3109/13693786.2010.508188; PMID: 20795767