Abstract
Nitric oxide (NO) is a critical component of host defense against invading pathogens; however, its therapeutic utility is limited due to a lack of practical delivery systems. Recently, a NO-releasing nanoparticulate platform (NO-np) was shown to have in vitro broad-spectrum antimicrobial activity and in vivo pre-clinical efficacy in a dermal abscess model. To extend these findings, both topical (TP) and intralesional (IL) NO-np administration was evaluated in a MRSA intramuscular murine abscess model and compared with vancomycin. All treatment arms accelerated abscess clearance clinically, histologically, and by microbiological assays on both days 4 and 7 following infection. However, abscesses treated with NO-np via either route demonstrated a more substantial, statistically significant decrease in bacterial survival based on colony forming unit assays and histologically revealed less inflammatory cell infiltration and preserved muscular architecture. These data suggest that the NO-np may be an effective addition to our armament for deep soft tissue infections.
Keywords: :
Introduction
Pyomyositis is a suppurative infection of the large skeletal muscles without an apparent origin from contiguous structures. The most commonly involved muscles are those in the thigh and gluteal region, though infection of multiple other muscles have been reported.Citation1 In recent years, the incidence of muscle abscesses has increased.Citation2 Staphylococcus aureus is responsible for greater than 70% of these infections.Citation1 Cases of methicillin-resistant S. aureus (MRSA) pyomyositis have been increasingly reported to date, and inappropriate use of broad-spectrum antibiotics has further spurred the emergence of many antibiotic-resistant strains.Citation3,Citation4 Patients with MRSA intramuscular abscesses are often hospitalized for intravenous antibiotics and may require interventional radiological or surgical intervention to drain abscesses and fluid collections, adding to the overall cost of care and ultimately associated suffering with this condition. With both the rising incidence of resistant isolates and cost of care, new therapies are needed in order to unburden the strain and difficulty of preventing the progression of and treating these deep tissue infections.
Nanoparticles can be used to transport biologically active agents that in most clinical settings are quite difficult or not feasible to deliver, such as nitric oxide (NO). NO is known to possess impressively broad antimicrobial activity due to both its inherent ability to inhibit growth and kill pathogens as well as its function as a potent immunostimulatory signaling molecule.Citation5,Citation6 Our data and work by other investigators show that NO is a potentially effective therapeutic for severe skin and soft-tissue infections.Citation7-Citation13 However, as a highly reactive gas, NO has proven difficult to deliver in a convenient format and this has largely precluded its use, even in hospital settings.Citation14 We have recently described a new nanoplatform comprised of nitric oxide releasing nanoparticles (NO-np).Citation15 NO is generated within the particles through the thermally induced reduction of nitrite to NO. The unique make-up of the nanoparticles allows for long range electron and proton transfer, facilitating the redox chemistry. This technology is a novel NO generator without many of the shortcomings of organic nitrates such as nitroglycerin, the most commonly used NO-donor in clinical practice. The main limitation of organic nitrates is decreased efficacy with prolonged continuous use; a so-called “nitrate tolerance,” resulting from depletion of tissue thiols, an acquired desensitization of soluble guanylyl cycylase to NO, or increase in breakdown of cyclic guanosine monophosphate (GMP) by phosphodiesterases.Citation16 As NO-np do not require an external reducing agent, it bypasses many of these limitations while retaining and perhaps improving efficacy.
The antimicrobial utility of the NO-np has been previously demonstrated in vitroCitation10,Citation12,Citation17 and has also been evaluated in several infection pre-clinical models including MRSACitation12 and A. baumanniiCitation10 infected wounds. Furthermore, as a means of capitalizing on and demonstrating the unique properties of nano-sized materials, the use of the NO-np in treating MRSA subcutaneous abscess was also evaluated and demonstrated.Citation18 To extend these results further and investigate the reach with which the NO-np can exert their antimicrobial impact, we investigated the comparative efficacy of both topically (TP) applied and intralesional (IL) NO-np in a murine MRSA intramuscular abscess model to the current “gold standard” for invasive MRSA disease, systemic vancomycin.
Methods and Materials
Ethics statement
All animal studies were conducted according to the experimental practices and standards approved by the Animal Welfare and Research Ethics Committee at the Albert Einstein College of Medicine.
NO nanoparticle (NO-np) synthesis
The generation of NO-np has been previously reported.Citation15,Citation19 Briefly a hydrogel/glass composite was synthesized using a mixture of tetramethylorthosilicate (TMOS), polyethylene glycol (PEG), glucose, chitosan, and sodium nitrite in a 0.5 M sodium phosphate buffer (pH 7). The nitrite was reduced to NO within the matrix because of the glass properties of the composite effecting redox reactions initiated with thermally generated electrons from glucose. Subsequently, the ingredients were combined and dried using a lyophilizer, resulting in a fine powder comprising nanoparticles containing NO. Once exposed to an aqueous environment, the hydrogel properties of the composite allow for an opening of the water channels inside the particles, facilitating the release of the trapped NO over extended time periods. Control nanoparticles (np) were generated in a similar manner without the inclusion of nitrite.
In vivo abscess model and NO-np treatment
To investigate the antimicrobial efficacy of NO-np for intramuscular abscesses formed by MRSA, female Balb/c mice (6 to 8 weeks old; National Cancer Institute, MD) were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine, the hair on their flanks removed, and the skin disinfected with iodine. Then, a suspension of 100 μL with 107 colony forming units (CFU) MRSA 6524 in PBS was inoculated intramuscularly in each flank of the animals (two abscesses per mouse). At times points 2, 24, 48 and 72 h following infection, 5 mg np or NO-np were applied (TP) to the skin overlying the abscess or a suspension of 100 μL with 5 mg/mL of NO-np or np dissolved in PBS was injected into (IL) the abscesses. The topical application was aided by the use of a cone with a 5 mm opening applied over the targeted tissue. The nanoparticles were applied into the distal cone followed by 50 μL PBS, which resulted in the formation of a gel that was absorbed within 30 min. For the vancomycin group, mice received 5 mg/kg vancomycin subcutaneously (mid back) at 2 h post-infection and then subsequently at 24, 48 and 72 h. Vancomycin dosing was based on previous studies utilizing animal models of soft tissue MRSA infections.Citation20,Citation21 The np (without NO) were used as a control at the same TP and IL doses according to the NO-np schedule. Untreated, infected mice were used as an additional control. On days 4 and 7, mice were euthanized and the abscesses were excised, homogenized and cultured quantitatively by plating on tryptic soy agar. The experiment was performed twice, each experiment consisting of six animals per experimental arm. The percentage of CFU survival was determined by comparing the survival of the treatment arms relative to the survival of untreated bacteria.
Histological processing
At day 4 and 7 following MRSA intramuscular abscess formation after infection and treatment as above, abscess tissues from one hind limb were excised from euthanized mice, six per treatment group, fixed in 10% formalin for 24 h, processed, and embedded in paraffin. Four micron vertical sections were fixed to glass slides and subjected to H&E staining to observe the muscular morphology and pathology. Slides were examined by light microscopy with an Olympus AX70 microscope, and images were obtained (QImaging Retiga 1300 digital camera) with QCapture Suite V2.46 software (QImaging).
Statistical analysis
All data were subjected to statistical analysis using GraphPad Prism 5.0 (GraphPad Software). P values were calculated by analysis of variance and were adjusted by use of the Bonferroni correction. P values of 0.05 were considered significant.
Results
Intramuscular and topical administrations of NO-np decreased MRSA burden in intramuscular abscesses
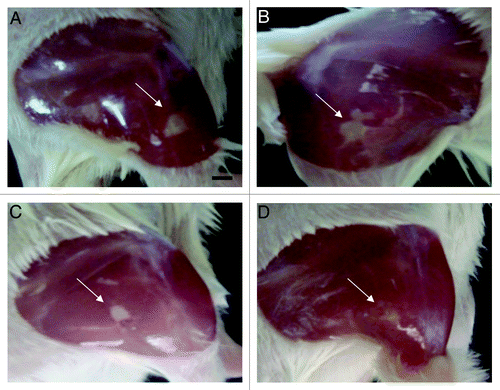
The effect of NO-np on intramuscular MRSA abscesses in Balb/c mice was investigated. TP and direct intramuscular injections into abscesses of NO-np decreased the size and purulence of abscesses clinically on day 4 following initial infection. Animals treated with subcutaneous vancomycin also demonstrated some clinical improvement as compared with the control group (), whereas lesions in mice treated with either IL or TP np (without NO) were similar to controls (data not shown). Intramuscular abscesses on day 7 in all groups were not clinically or grossly visually evident.
Figure 1. NO-nps clinically accelerate the clearance of intramuscular abscesses. Induced MRSA abscesses in Balb/c mice were evaluated clinically on day 4 following infection. The four images, (A) untreated, (B) vancomycin treated, (C) NO-np TP treated and (D) NO-np IL, are representative of the clinical appearance of these lesions. Arrows indicate abscesses.
Histological examination of excised tissue from the various groups of mice differed significantly. In the control animals, which included untreated mice and topically np or intralesional np treated mice, lesions at day 4 were large and well circumscribed with intense, neutrophil rich inflammatory infiltrates and scattered macrophages resulting in central caseous necrosis destroying the normal architecture (; np controls not shown). Sections from vancomycin treated animals revealed a similar pattern of inflammation and tissue destruction, though not as intense as the control groups. In contrast, abscesses from mice topically treated with NO-np demonstrated an interstitial inflammatory cell infiltrate in proximity to a poorly defined and comparatively smaller neutrophilic collection which did not resemble the well circumscribed inflammation appreciated in the control groups as well as vancomycin treated groups. Even less inflammation was appreciated in the mice that received NO-np intralesionally in which neutrophils were splayed between muscle fiber bundles and there was no evidence of a defined abscess. Day 7 histologic examination demonstrated reduced inflammation in all groups, with near complete restoration of the tissue architecture at the sites of NO-np treated lesions (data not shown).
Figure 2. NO-nps effectively kill MRSA and limit muscular infection damage. (A) Histological analysis of Balb/c mice untreated, Vancomycin treated, NO-np TP treated, and NO-np IL treated MRSA-infected intramuscular abscesses on day 4. Mice were intramuscularly infected with 107 MRSA. Representative H&E-stained sections of the skin lesions are shown with the Scale bars: 4X: 25 mm; 10X: 10 mm. (B) Abscess bacterial burden (CFU; colony forming units) in mice infected subcutaneously with 107 MRSA and treated with NO-np is significantly lower than untreated or np-treated mice (n = 12 abscesses per group) as well as vancomycin treated. The percent survival at days 4 (a) and 7 (b) was tabulated based on CFU/gm abscess and compared with control. Error bars denote standard deviations. Symbols denote P value significance calculated by analysis of variance and adjusted by use of the Bonferroni correction. *p = 0.0001, for comparisons of NO-np IL or TP compared with vancomycin. ϕp = 0.001, for comparison of NO-np IL to NO-np TP at day 4. #p = 0.045, for comparison of NO-np IL to NO-np TP at day 7.
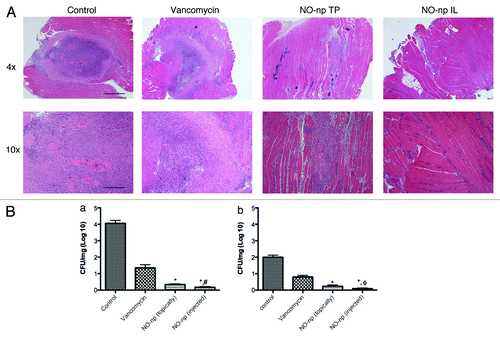
Tissue cultures from abscesses at both days 4 and 7 following infection demonstrated that vancomycin, TP NO-np and intramuscular NO-np significantly decreased bacterial burden as compared with controls (). At days 4 and 7 following infection, control intramuscular abscesses contained 4.13 ± 0.53 log10 CFU/mg and 2.03 ± 0.43 log10 CFU/mg, respectively. There were no significant differences between untreated controls and the TP and IL np (without NO) groups (data not shown). On day 4 (), the CFUs in the vancomycin treated group decreased to 1.36 ± 0.62 log10 CFU/mg and 0.80 ± 0.32 log10 CFU/mg at day 4 and 7, respectively (, p < 0.001).The CFUs in the abscesses from mice treated topically with NO-np decreased to 0.34 ± 0.13 log10 CFU/mg and 0.23 ± 0.08 log10 CFU/mg at day 4 and 7, respectively. The topically delivered NO-np was significantly more effective at both intervals compared with vancomycin (p = 0.0001). The IL NO-np treatment was the most effective treatment approach against MRSA abscesses resulting in CFUs of 0.17 ± 0.0.03 log10 CFU/mg and 0.09 ± 0.02 at day 4 and 7, respectively. Compared with vancomycin and TP NO-np, IL NO-np administration was significantly more effective at both time points (day 4: p = 0.0001 and p = 0.001, respectively; day 7: p = 0.001 and p = 0.045, respectively).
Discussion
The present study investigated the ability of NO-np to effectively clear intramuscular abscesses as compared with vancomycin. Based on previous findings from our in vivo studies, moistened 5 mg of NO-np was applied topically or 5 mg/ml of NO-np was delivered intramuscularly. The results demonstrate a significant decrease in CFU/mg of infected tissue in the NO-np treated animals as compared with control and vancomycin treated, which is consistent with past investigations using this NO-releasing technology. Similarly, the histology of involved muscle from the NO-np treated arms demonstrated limited inflammation and damage to muscle fibers.
Although a seemingly simple diatomic molecule, NO exhibits a wide array of functional activities. NO can interact directly with pathogenic microbes due to the ease with which it can transverse the lipid bilayer; reaching important metabolic enzymes and DNA to cripple essential biological processes. NO can be oxidized to reactive nitrogen species (RNS) such as peroxynitrite. RNS themselves exert antimicrobial effects via a variety of reactions, including the nitrosation of protein thiols and the nitrosylation of metal centers (Fe-S), ultimately modifying the functions of proteins that are essential to cellular processes.Citation22 Peroxynitrite specifically can disrupt the microbial membrane through lipid peroxidation, accelerating degradation of cellular integrity. Given these antimicrobial properties, in concert with the ability of NO to accelerate wound healing, there has been tremendous interest in the development of NO donors and delivery systems.
The use of nanoparticles as delivery vehicles for bactericidal agents represents a new paradigm in the design of both topical and systemic agents. Over the last few decades, the applications of nanotechnology in medicine have been extensively explored in many medical arenas. Nanotechnology concerns the understanding and control of matters in the 1–100 nm range, at which scale materials have unique physicochemical properties including ultra-small size, large surface to volume ratio, high reactivity and unique interactions with biological systems.Citation23,Citation24 By loading bioactive agents through physical encapsulation, adsorption, or chemical conjugation, the pharmacokinetics and therapeutic index of the drugs can be significantly improved in contrast to the free drug counterparts. Many advantages of nanoparticle-based drug delivery have been recognized, including releasing drugs at a sustained and controlled manner. The use of nano-vehicles also allows for the delivery of highly reactive and short-lived biomolecules such as nitric oxide.Citation25
In the present study, NO-np was found to be effective at accelerating the resolution of MRSA intramuscular abscesses when applied topically or intralesionally, and both approaches were more effective then systemic treatment with vancomycin. Both the biofilm-like nature of and poor perfusion associated with bacterial abscesses often undermine the efficacy of conventional antibiotic therapy such as vancomycin. However, as a small, lipophilic gas, NO can transverse physical barriers such as biofilms, as well as even prevent their formation.Citation11
Vancomycin is a branched tricyclic glycosylated nonribosomal peptide that inhibits proper cell wall synthesis in Gram-positive bacteria such as S. aureus by two mechanism—it prevents the synthesis of the log polymers of N-acetylmuramic acid (NAM) and N-acetylglucosamine (NAG) that form the backbone strands of the bacterial cell wall, and it prevents the backbone polymers that do manage to form from cross-linking with each other.Citation26 Unlike most antibiotics, NO exerts its antimicrobial effects through several mechanisms. By targeting protein thiols and metal centers, NO can block essential microbial physiologic processes including respiration and DNA replication. NO can also directly damage microbial DNA through the generation of peroxynitrite. In addition, NO is an immunomodulatory signaling molecule, enhancing and accelerating the host’s own immune response by recruiting macrophages and T lymphocytes.Citation27 Interestingly, the histopathology of tissue from the NO treated arms demonstrated decreased inflammatory infiltrate as compared with controls and vancomycin groups. The decrease in neutrophilic infiltrate is likely a result of the rapid clearance of bacterial burden from tissue due to the sustained release of NO. However, it is known that NO can limit neutrophil migration by downregulating the expression of ICAM-1 which is required for neutrophil diapedesis from the vasculature.Citation28 This feature is important as although neutrophils can aid in clearing invading pathogens, their robust generation of oxidative stress can result in extensive tissue damage. By both directly killing MRSA and limiting neutrophil infiltration, the NO-np accelerated resolution of the intramuscular abscesses.
The NO-np platform evaluated in this study has previously undergone in vitro broad spectrum antimicrobial testing against multiple clinical isolates of both Gram-positive and -negative bacteria.Citation12,Citation17 The rate and amount of NO released from our nanoparticles allows the molecule to alter peripheral and integral structures on the bacterial plasma membrane, particularly membrane-bound proteins and lipids.Citation15,Citation19 The potent efficacy of the NO-nps both for the topical treatment of wound infectionsCitation10,Citation12 and subcutaneous MRSA abscessesCitation18 has been previously reported. In the current study, we extrapolated upon these past investigations to evaluate the potential utility of the NO-np in the setting of pyomyositis, and compared the NO-np to a first line antibiotic for this complicated soft tissue infection, vancomycin. All three treatment modalities—vancomycin, TP NO-np and IL NO-np—were effective in accelerating abscess clearance as compared with controls based on tissue cultures and histology; however the TP and IL NO-np treated lesions demonstrated statistically significant improvement as compared with the vancomycin.
The growing danger of life-threatening infections and the rising economic burden of resistant bacteria have underscored the demand for new antibacterial therapeutics.Citation29,Citation30 There is an urgent need to expand treatment options for infections caused by resistant pathogens such as MRSA. This situation is further aggravated by a decline in the development of new antibiotics.Citation31
In summary, this study extends the potential application of NO as an antimicrobial agent in the setting of skin and soft tissue infections by demonstrating the efficacy of the TP or IL application of NO-np in the setting of intramuscular abscesses, and showing superiority to vancomycin. The data presented suggests that this technology could be used as an adjunctive therapy prior to or in addition to surgical drainage of bacterial abscesses, which is the current standard approach to treatment of an abscess. The antibacterial effects witnessed utilizing the limited dosing schedule in this study suggests that clinically relevant and realistic dosing would also yield a similar outcome. Both NO’s direct bactericidal and immunomodulatory properties provide several advantages over antibiotics that frequently have one mechanism of action, and ultimately limit the risk of resistance developing against this agent. It is further possible that IL NO-np could be effective in the setting of other deep abscesses, such as lesions in the lung or liver. Together, these data suggest that the NO-np platform has the potential to serve as a novel, easily administered class of topical or injectable antimicrobials for the treatment of deep tissue infections and abscesses.
Acknowledgments
A.J.F gratefully acknowledges support by the Dermatology Foundation and Centocor Ortho Biotech. L.R.M. gratefully acknowledges support from Long Island University.
Declaration of Potential Conflicts of Interest
A.J.F, J.M.F, and J.D.N. report serving on the scientific advisory board of Makefield Therapeutics, Inc.
References
- Olson DP, Soares S, Kanade SV. Community-acquired MRSA pyomyositis: case report and review of the literature. J Trop Med 2011; 2011:970848; PMID: 21461362
- Pannaraj PS, Hulten KG, Gonzalez BE, Mason EO Jr., Kaplan SL. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2006; 43:953 - 60; http://dx.doi.org/10.1086/507637; PMID: 16983604
- Boucher HW. Challenges in anti-infective development in the era of bad bugs, no drugs: a regulatory perspective using the example of bloodstream infection as an indication. Clin Infect Dis 2010; 50:Suppl 1 S4 - 9; http://dx.doi.org/10.1086/647937; PMID: 20067391
- Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis 2008; 46:155 - 64; http://dx.doi.org/10.1086/524891; PMID: 18171244
- Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest 1997; 99:2818 - 25; http://dx.doi.org/10.1172/JCI119473; PMID: 9185502
- Han G, Zippin JH, Friedman A. From Bench to Bedside: The Therapeutic Potential of Nitric Oxide in Dermatology. J Drugs Dermatol 2009; 8:586 - 94; PMID: 19537386
- Jones ML, Ganopolsky JG, Labbe A, Prakash S. A novel nitric oxide producing probiotic patch and its antimicrobial efficacy: preparation and in vitro analysis. Appl Microbiol Biotechnol 2010; 87:509 - 16; http://dx.doi.org/10.1007/s00253-010-2490-x; PMID: 20300748
- Seabra AB, Martins D, Simoes M, da Silva R, Brocchi M, de Oliveira MG. Antibacterial nitric oxide-releasing polyester for the coating of blood-contacting artificial materials. Artif Organs 2010; 34:E204 - 14; http://dx.doi.org/10.1111/j.1525-1594.2010.00998.x; PMID: 20497163
- Major TA, Panmanee W, Mortensen JE, Gray LD, Hoglen N, Hassett DJ. Sodium nitrite-mediated killing of the major cystic fibrosis pathogens Pseudomonas aeruginosa, Staphylococcus aureus, and Burkholderia cepacia under anaerobic planktonic and biofilm conditions. Antimicrob Agents Chemother 2010; 54:4671 - 7; http://dx.doi.org/10.1128/AAC.00379-10; PMID: 20696868
- Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, Martinez LR. The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence 2010; 1:62 - 7; http://dx.doi.org/10.4161/viru.1.2.10038; PMID: 21178416
- Hetrick EM, Shin JH, Paul HS, Schoenfisch MH. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009; 30:2782 - 9; http://dx.doi.org/10.1016/j.biomaterials.2009.01.052; PMID: 19233464
- Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, et al. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against staphylococcus aureus skin infection. J Invest Dermatol 2009; 129:2463 - 9; http://dx.doi.org/10.1038/jid.2009.95; PMID: 19387479
- Hetrick EM, Shin JH, Stasko NA, Johnson CB, Wespe DA, Holmuhamedov E, et al. Bactericidal efficacy of nitric oxide-releasing silica nanoparticles. ACS Nano 2008; 2:235 - 46; http://dx.doi.org/10.1021/nn700191f; PMID: 19206623
- Friedman A, Friedman J. New biomaterials for the sustained release of nitric oxide: past, present and future. Expert Opin Drug Deliv 2009; 6:1113 - 22; http://dx.doi.org/10.1517/17425240903196743; PMID: 19663720
- Friedman AJ, Han G, Navati MS, Chacko M, Gunther L, Alfieri A, et al. Sustained release nitric oxide releasing nanoparticles: Characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide 2008; 19:12 - 20; http://dx.doi.org/10.1016/j.niox.2008.04.003; PMID: 18457680
- Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, et al. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation 2001; 104:2338 - 43; http://dx.doi.org/10.1161/hc4401.098432; PMID: 11696475
- Friedman A, Blecher K, Sanchez D, Tuckman-Vernon C, Gialanella P, Friedman JM, et al. Susceptibility of Gram-positive and -negative bacteria to novel nitric oxide-releasing nanoparticle technology. Virulence 2011; 2:217 - 21; http://dx.doi.org/10.4161/viru.2.3.16161; PMID: 21577055
- Han G, Martinez LR, Mihu MR, Friedman AJ, Friedman JM, Nosanchuk JD. Nitric oxide releasing nanoparticles are therapeutic for Staphylococcus aureus abscesses in a murine model of infection. PLoS ONE 2009; 4:e7804; http://dx.doi.org/10.1371/journal.pone.0007804; PMID: 19915659
- Han G, Friedman AJ, Friedman JM. Nitric oxide releasing nanoparticle synthesis and characterization. Methods Mol Biol 2011; 704:187 - 95; http://dx.doi.org/10.1007/978-1-61737-964-2_14; PMID: 21161638
- Fernandez J, Hilliard JJ, Abbanat D, Zhang W, Melton JL, Santoro CM, et al. In vivo activity of ceftobiprole in murine skin infections due to Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2010; 54:116 - 25; http://dx.doi.org/10.1128/AAC.00642-09; PMID: 19884364
- Patel MV, De Souza NJ, Gupte SV, Jafri MA, Bhagwat SS, Chugh Y, et al. Antistaphylococcal activity of WCK 771, a tricyclic fluoroquinolone, in animal infection models. Antimicrob Agents Chemother 2004; 48:4754 - 61; http://dx.doi.org/10.1128/AAC.48.12.4754-4761.2004; PMID: 15561853
- Tavares AF, Nobre LS, Melo AM, Saraiva LM. A novel nitroreductase of Staphylococcus aureus with S-nitrosoglutathione reductase activity. J Bacteriol 2009; 191:3403 - 6; http://dx.doi.org/10.1128/JB.00022-09; PMID: 19286809
- Farokhzad OC. Nanotechnology for drug delivery: the perfect partnership. Expert Opin Drug Deliv 2008; 5:927 - 9; http://dx.doi.org/10.1517/17425247.5.9.927; PMID: 18754745
- Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano 2009; 3:16 - 20; http://dx.doi.org/10.1021/nn900002m; PMID: 19206243
- Friedman A, Friedman J. New biomaterials for the sustained release of nitric oxide: past, present and future. Expert Opin Drug Deliv 2009; 6:1113 - 22; http://dx.doi.org/10.1517/17425240903196743; PMID: 19663720
- Griffith RS. Introduction to vancomycin. Rev Infect Dis 1981; 3:Suppl S200 - 4; PMID: 7043707
- Mowbray M, Tan X, Wheatley PS, Rossi AG, Morris RE, Weller RB. Topically applied nitric oxide induces T-lymphocyte infiltration in human skin, but minimal inflammation. J Invest Dermatol 2008; 128:352 - 60; http://dx.doi.org/10.1038/sj.jid.5701096; PMID: 17914444
- Dal Secco D, Paron JA, de Oliveira SH, Ferreira SH, Silva JS, Cunha Fde Q. Neutrophil migration in inflammation: nitric oxide inhibits rolling, adhesion and induces apoptosis. Nitric Oxide 2003; 9:153 - 64; http://dx.doi.org/10.1016/j.niox.2003.11.001; PMID: 14732339
- Breen JO. Skin and soft tissue infections in immunocompetent patients. Am Fam Physician 2010; 81:893 - 9; PMID: 20353147
- Giamarellou H, Poulakou G. Multidrug-resistant Gram-negative infections: what are the treatment options?. Drugs 2009; 69:1879 - 901; http://dx.doi.org/10.2165/11315690-000000000-00000; PMID: 19747006
- Spellberg B. The antibiotic crisis: can we reverse 65 years of failed stewardship?. Arch Intern Med 2011; 171:1080 - 1; http://dx.doi.org/10.1001/archinternmed.2011.26; PMID: 21357798