Figures & data
Table 1. The list of critical keywords and setting selected for document searching in Scopus database (2000–2016)
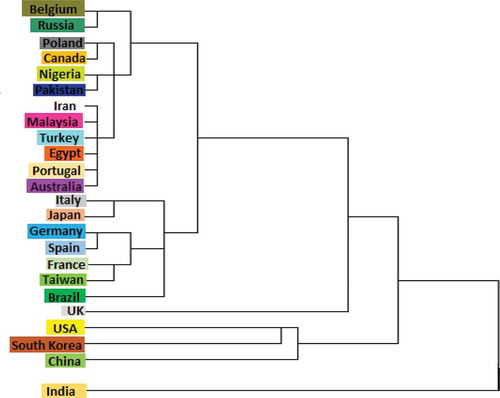
Table 2. Frequency of the most studied PCs per country (2000–2016)
Table 3. The role of dietary catechins in epigenetics events
Table 4. The total number of documents published regarding research on PCs for HD per country (2000–2016)
Figure 4. Number of published papers per country for (A) polyphenols; (B) flavonoids; (C) lignans; and (D) stilbenes.
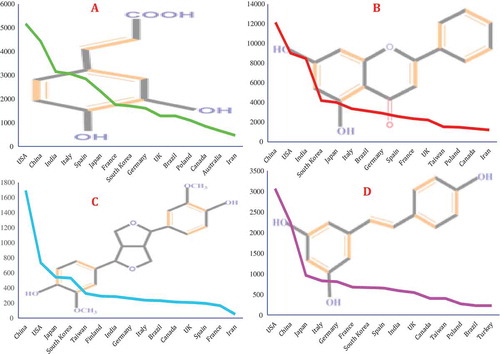
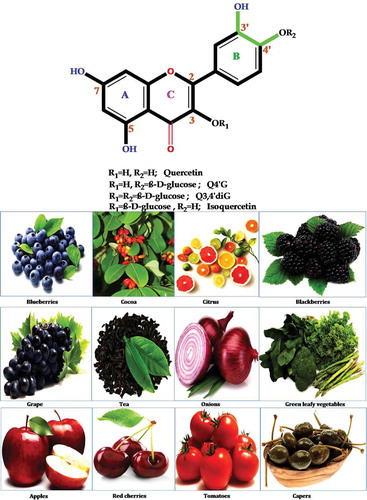
Figure 7. Possible pathway of metabolic conversion of quercetin, an onion flavonoid, during intestinal absorption and inflammation. First, Q4ʹG converts to quercetin by mediating the β-glucosidase enzyme. Second, UGT changes quercetin to Q3GA. In the next step, during inflammation by the mediating of β-glucuronidase and COMT enzymes, Q3GA converts to quercetin and isorahamentin.
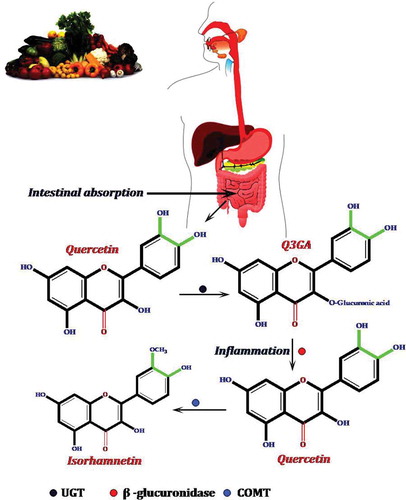
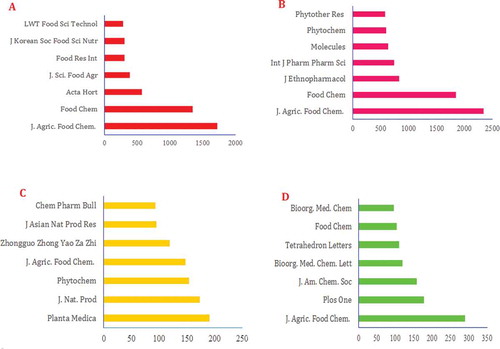
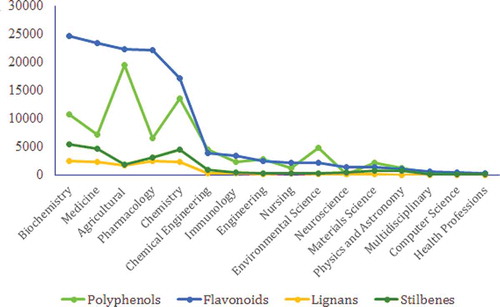
Figure 18. The most popular journals covering research on (A) polyphenols, (B) flavonoids, (C) lignans, and (D) stilbenes (2000–2016).