Figures & data
Figure 1. Schematic overview of the production (e.g., fed-batch production) of a genetically modified microorganism (GMM) to deliver a therapeutic molecule. Example of the in situ production and of a protein with anti-inflammatory properties by a GM L. lactis strain in the context of intestinal inflammation.
Figure 2. Family of vectors that allow controlled expression and export of proteins in L. lactis. (A) Schematic structures of different expression cassettes (left) under the control of the lactococcal PnisA promoter for the indicated specific bacterial cellular localization and carried by the specified plasmids. For details of the plasmid constructions see the text. Stems topped with circles indicate the tryptophan transcriptional terminator (trpA). Not to scale. (B) Graphical representation on the production of the desired protein by using the plasmid indicated for the different bacterial localization of interest in L. lactis. pCYT: to obtain the expression of a protein in the cytoplasm, the gene of interest is fused only to the PnisA promoter. pSEC: in which the secretion pathway used is the Sec-dependent pathway. It recognizes proteins synthesized with an N-terminal signal peptide (SP) and ensures their export and translocation. It is worth highlighting that the nature of the SP used to secrete a protein can greatly influence the secretory efficiency of the protein. Thus, one of the most efficient SP for secreting heterologous proteins in L. lactis is that of the Usp45 protein (i.e. SPUsp45), which is the majority protein secreted by L. lactis 26. Indeed, this SPUsp45 has been used to export many heterologous proteins in L. lactis 27. pCWA: to obtain a protein anchored to the bacterial wall, the gene of interest is fused to SPUsp45 and the anchoring domain of the S. pyogenes M6 protein (CWAM6). This domain contains the necessary signals for wall anchoring 28. This figure was created with Biorender.com (accessed date: 9th June 2022).
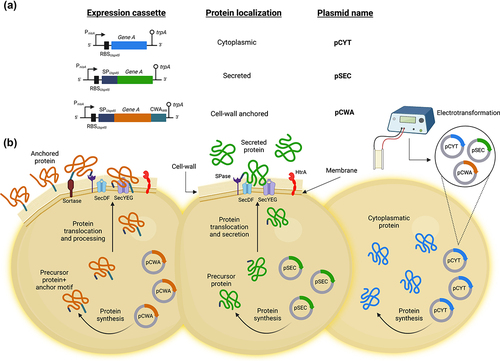
Table 1. Some examples of heterologous protein production in L. lactis for vaccination purposes.
Table 2. Some examples of heterologous protein production in lactobacilli for vaccination purposes.
