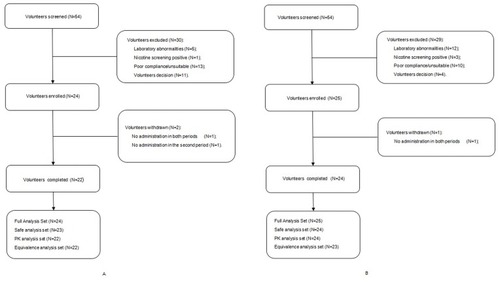
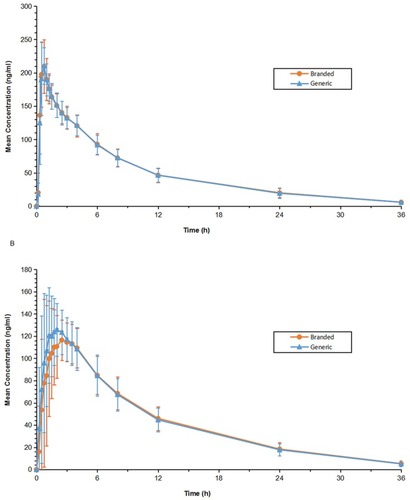
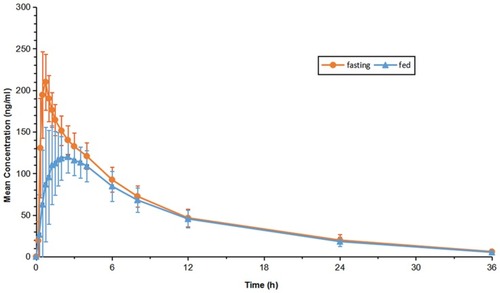
Figures & data
Table 1 Demographic Characteristics Of Volunteers In The Fasting And Fed Studies (Mean ± SD [Range])
Table 2 Main Pharmacokinetic Parameters For The Generic And Branded Formulations In Fasting And Fed Volunteers
Table 3 Geometric Mean, Ratio, Intra-Individual Variability, And 90% Confidence Intervals For Levocetirizine In Fasting And Fed Conditions
Table 4 P Values Of Multivariate Analysis Of The Generic And The Branded Formulations In Fasting And Fed Conditions