Figures & data
Figure 1 Crystal structure (PDB ID: 1IE4) of TTR tetramer with T4 interacting with the two T4-binding sits is shown. (A) The two T4-binding sites located at the dimer–dimer interfaces are framed by the white boxes. (B) The specific interaction between T4 and amino acids at the binding pockets is shown. The yellow rod structure is indicated as T4, and the green solid lines are hydrogen bonds. These pictures are prepared using the program UCSF Chimera developed by the University of California.
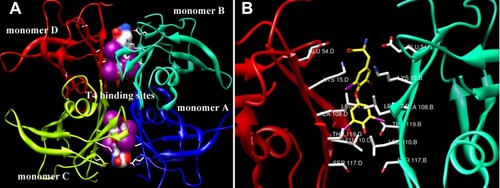
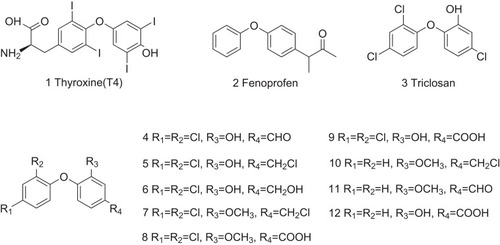
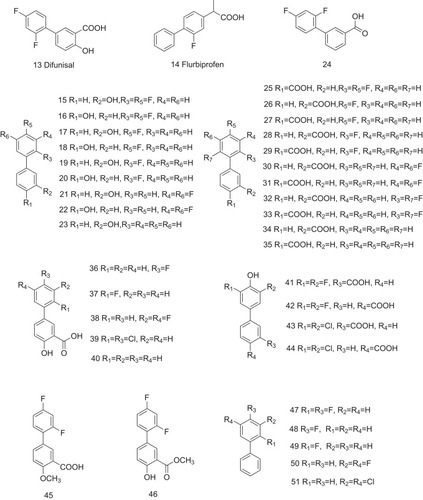
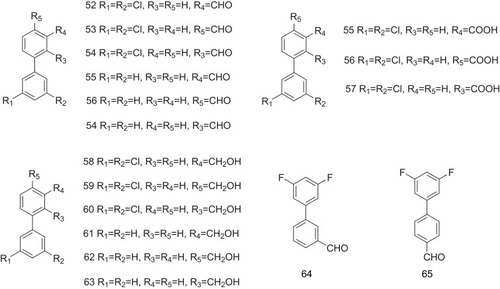
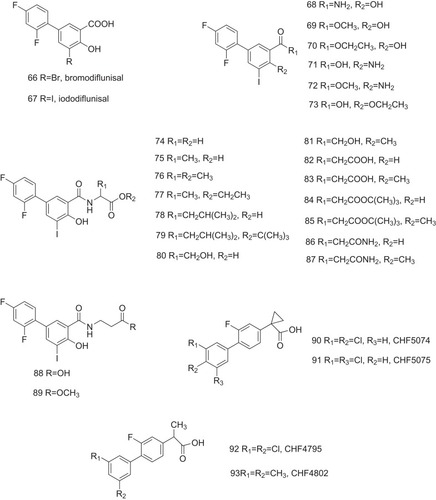
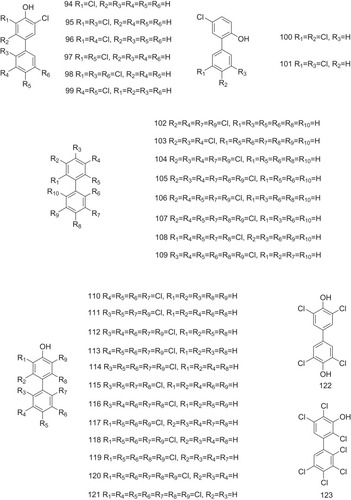
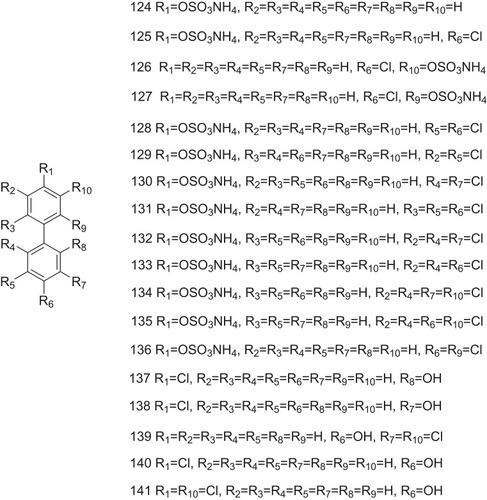
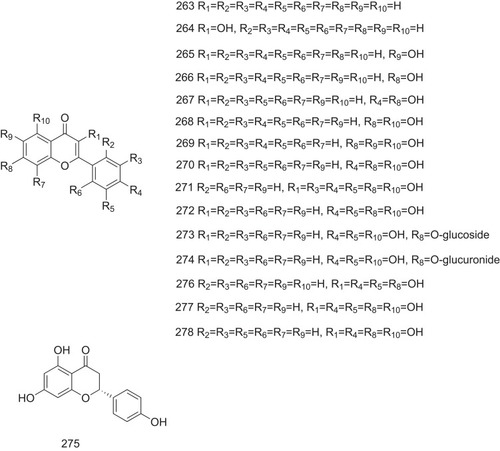
Table 1 The Structure–Activity Relationship of TTR Amyloidogenesis Inhibitors
Figure 2 The dissociation of TTR tetramer. TTR tetramer dissociates into monomers, which can be dimerized and further tetramerized by interacting with diflunisal. The unfolded/misfolded monomers of TTR aggregate to form amyloid fibrils, which may be inhibited by inhibitors, such as diflunisal.
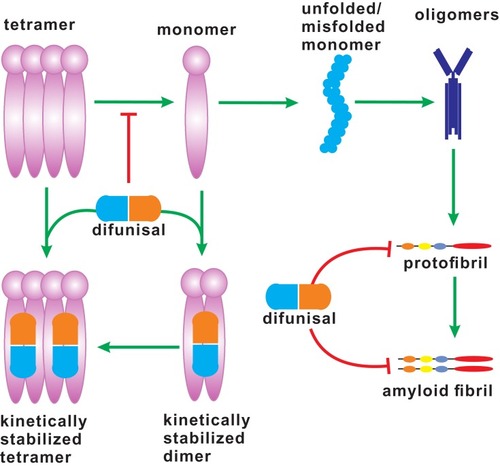
Figure 3 The substructure-combinational strategy is used for producing potent and selective ATTR inhibitors (A). The binding model is indicated within the T4-binding pockets (B). The indicated structure may be considered as the possible pharmacophoric elements (C), and the alternative substitutions may be showed as Z, Y, and X.
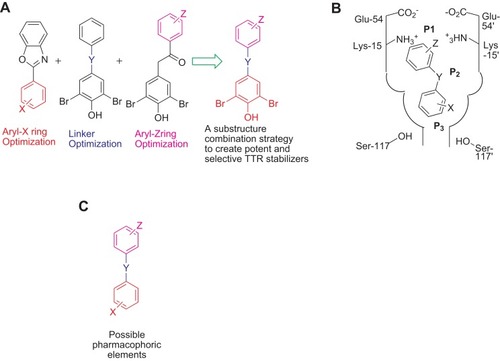
Table 2 The Efficacy Scores of Compounds 142–181
Figure 9 Compounds 142–181 with the different linkers as the potent inhibitors against ATTR are indicated.
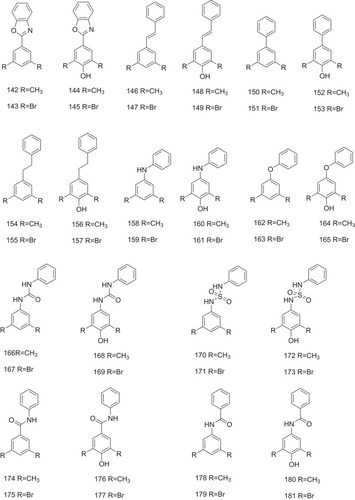
Figure 10 Compounds 182–262 with different substitution positions of benzamides as the potent inhibitors against ATTR are indicated.
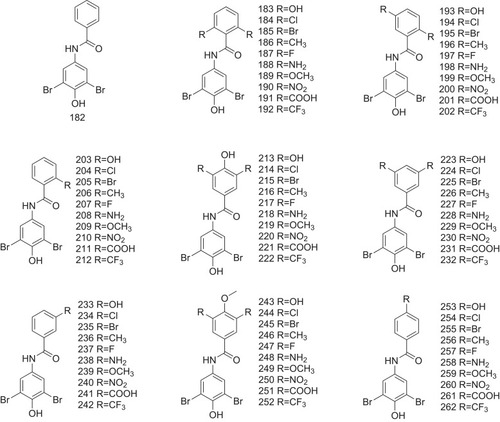
Table 3 The Efficacy Scores of Compounds 178–258
Figure 11 Summary of the structure–activity relationships of small molecule ATTR inhibitors composed of two aryl rings and variable linkers.
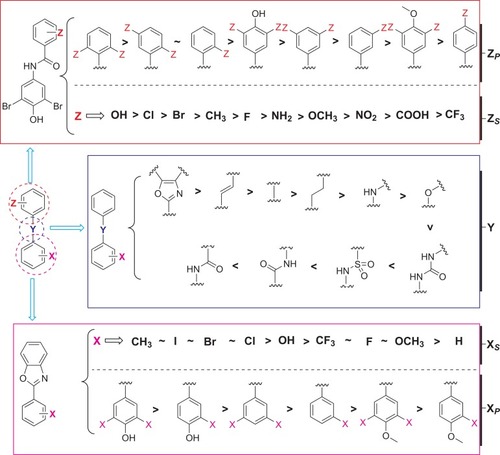
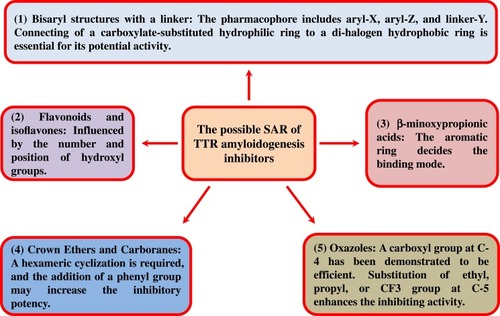
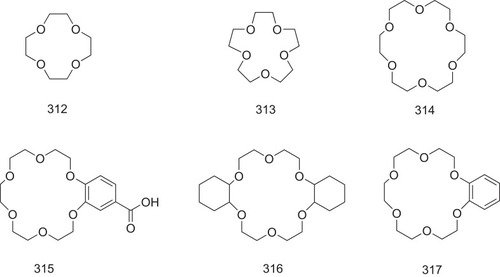
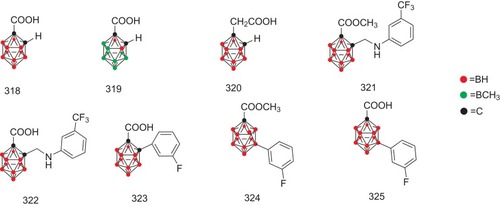
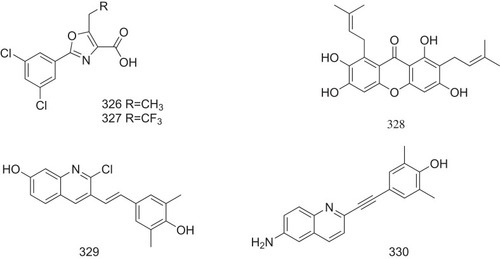
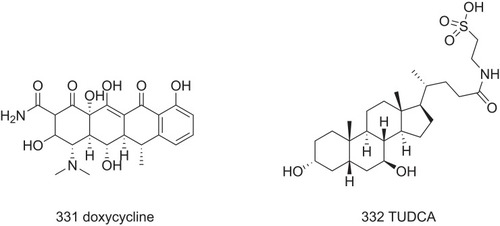
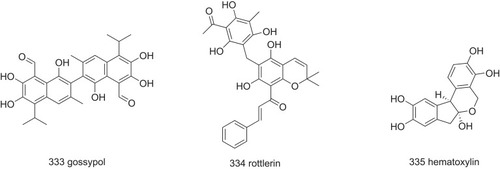
Figure 22 The possible structure–activity relationship of TTR amyloidogenesis inhibitors, including bisayl structures with a linker, flavonoids and isoflavones, β-minoxypropionic acids, crown ethers and carboranes, and oxazoles.