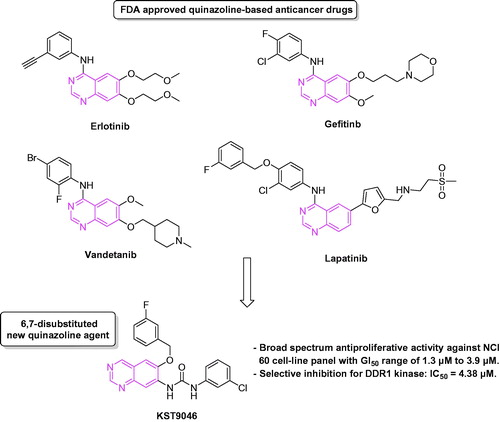
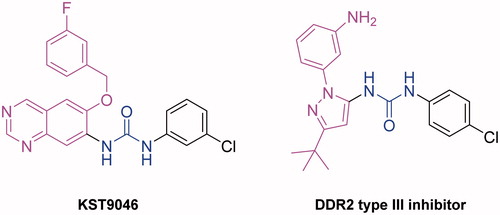
Figures & data
Figure 2. Dose-response curves (% growth versus sample concentration at NCI fixed protocol, µM) obtained from the NCI’s in vitro disease-oriented human tumor cells line of compound KST9046 on nine cancer disease. The different color and shape of NCI subpanel cell lines indicative of growth percentage inhibition with concentration of sample.
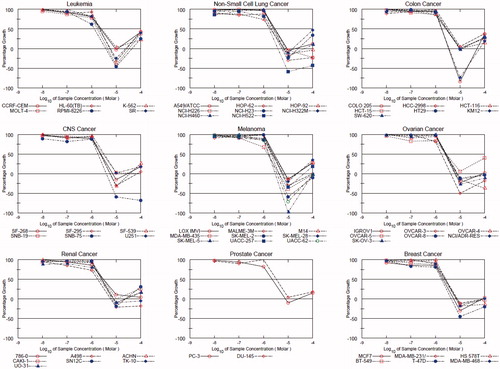
Table 1. % Growth inhibition at 10 µM, GI50, TGI and LC50 of compound KST9046 over the NCI cell-line panel.
Table 2. Summary of kinase inhibitory profile of the new agent KST9046 at 10 µM over a panel of 347 kinases.
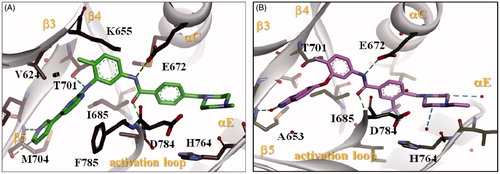
Figure 4. Docking of compound KST9046 in the binding site of DDR1 kinase catalytic domain (PDB ID: 4BKJ) in 3D style.
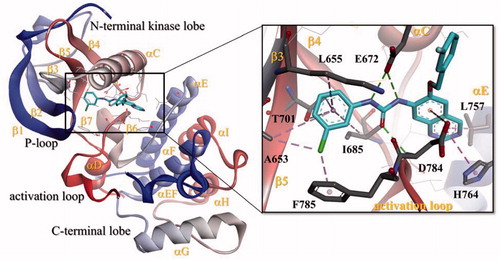
Figure 5. Docking of Imatinib (A) and DDR1-1N-1 (B) (type II DDR1 inhibitors) in the binding site of DDR1 kinase catalytic domain in 3D style.
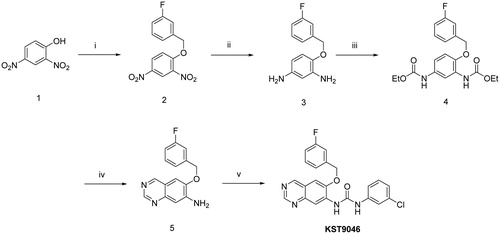
Scheme 1. Reagents and conditions: (i) 3-fluorobenzyl bromide, K2CO3, KI, CH3CN, 75 °C, 8 h; (ii) H2, 10% Pt/C, CH3OH, rt, 6 h; (iii) Ethyl chloroformate, TEA, THF, rt, 2 h; (iv) (a) HMTA, TFA, rt, 1 h, (b) 10% KOH aqueous ethanolic (1:1), K3Fe(CN)6, 100 °C, 4 h; (v) 3-chlorophenyl isocyanate, THF, 85 °C, overnight.