Abstract
In humans, exposure to early life adversity has profound implications for susceptibility to developing neuropsychiatric disorders later in life. Studies in rodents have shown that stress experienced during early postnatal life can have lasting effects on brain development. Glucocorticoids and sex steroids are produced in endocrine glands and the brain from cholesterol; these molecules bind to nuclear and membrane-associated steroid receptors. Unlike other steroids that can also be made in the brain, neurosteroids bind specifically to neurotransmitter receptors, not steroid receptors. The relationships among steroids, neurosteroids, and stress are multifaceted and not yet fully understood. However, studies demonstrating altered levels of progestogens, androgens, estrogens, glucocorticoids, and their neuroactive metabolites in both developmental and adult stress paradigms strongly suggest that these molecules may be important players in stress effects on brain circuits and behavior. In this review, we discuss the influence of developmental and adult stress on various components of the brain, including neurons, glia, and perineuronal nets, with a focus on sex steroids and neurosteroids. Gaining an enhanced understanding of how early adversity impacts the intricate systems of brain steroid and neurosteroid regulation could prove instrumental in identifying novel therapeutic targets for stress-related conditions.
Introduction
The word “hormone” was first used over 100 years ago to describe “the chemical messengers which, speeding from cell to cell along the bloodstream, may coordinate the activities and growth of different parts of the body” (Starling, Citation1905). Originating from the Greek root hormon, which means “to arouse or excite,” the term was coined to describe secretin, a substance derived from the digestive system. As more molecules that are produced in the periphery, released into the bloodstream, and act on distant targets were discovered and characterized, the term hormone became generally accepted by the scientific community. Hormones can be roughly divided into three major biochemical classes: (1) steroids; (2) peptides; and (3) amino acid derivatives (Tata, Citation2005). This review will focus only on the first in this list – steroids.
Many of the molecules originally designated as hormones can be produced in non-endocrine tissue where they exert local action. This is common in the brain where molecules originally designated as hormones are produced by neurons and glia, and have paracrine, autocrine, and intracrine functions. This means they can act on cells nearby the synthesizing cell, act on the synthesizing cell itself, or act within the synthesizing cell, respectively (Rubinow, Citation2018; Saldanha et al., Citation2011). Despite compelling evidence for brain synthesis and non-endocrine action of these molecules, the widespread adoption of the term hormone to describe molecules that have broader mechanisms of action has led to common misunderstandings and obstacles to progress in the field. This is particularly true for some steroid molecules that were originally discovered as originating from the gonads (progestogens, estrogens, androgens) and adrenals (corticosteroids). These molecules were first characterized for their roles in reproductive function and stress responses respectively. It is now abundantly clear that progestogens, estrogens, androgens, and glucocorticoids can be made in the brain where they act on steroid receptors locally (Lloyd-Evans & Waller-Evans, Citation2020), or in some cases are further metabolized into neurosteroids that can serve as allosteric modulators of neurotransmitter receptors (Wang, Citation2011). Nonetheless, common misperceptions exist due to the original discovery and characterization of these molecules as hormones. For example, estradiol is widely considered to be a “female sex hormone” due to its peripheral actions, but it is present in much higher concentrations in the brains of adult males than females (Hojo & Kawato, Citation2018). Furthermore, molecules originally associated with female reproduction, such as progesterone, are increased in the periphery and brains of both males and females in response to stress (Shors et al., Citation2001; Romeo et al., Citation2007; Laham et al., Citation2022).
The broad regional synthesis of steroids and their multiple mechanisms of action have complicated attempts to understand their roles in stress effects, particularly in the context of sex differences. This review attempts to integrate information about peripheral and brain concentrations and actions of progestogens, androgens, estrogens, and glucocorticoids, and their metabolites (), in the context of early life stress effects on neurons, glia, and the extracellular matrix in the brain. The review focuses primarily on brain regions that have been implicated in stress effects and cognitive/emotional processing, including the hippocampus (HIP), basolateral amygdala (BLA), and prefrontal cortex (PFC). To set the stage for this endeavor, we start with a brief description of steroid synthesis in the periphery and brain.
Table 1. Chemical Names, Common Names, and Abbreviations for Neuroactive Derivatives of Sex Steroids and Glucocorticoids.
Sex steroid and glucocorticoid synthesis begins with cholesterol
Progestogens, estrogens, androgens, and corticosteroids are made from cholesterol. Cholesterol is produced primarily in the body with a lower percentage coming from the diet (Miller & Bose, Citation2011; Rone et al., Citation2009). Synthesis of these steroids begins in the mitochondria where cholesterol is transported from the outer mitochondrial membrane (OMM) to the inner mitochondrial membrane (IMM) by a lipid binding protein called steroidogenic acute regulatory protein (StAR) (Miller & Bose, Citation2011) (). Another downstream protein to StAR, mitochondrial translocator protein (TSPO), is involved in transporting cholesterol from OMM to IMM (Papadopoulos et al., Citation1997). The mitochondria contain important enzymes for steroid synthesis, including P450 side-chain cleavage (P450 scc) enzymes (Omura, Citation2006). The first enzymatic step occurs in the IMM where cholesterol is converted to pregnenolone by the catalyzing action of a P450 scc enzyme (Koritz & Kumar, Citation1970; Manna et al., Citation2013; Stocco et al., Citation2005; Stone & Hechter, Citation1955).
Figure 1. Subcellular Compartments for Steroid/Neurosteroid Synthesis. Diagram showing that steroid/neurosteroid synthesis occurs in the mitochondria and endoplasmic reticulum.
Cholesterol enters the mitochondria facilitated by StAR and TSPO protein where it is cleaved into pregnenolone by enzyme P450scc. Pregnenolone is converted to progesterone (P4) by 3β- HSD. In the endoplasmic reticulum, progesterone is converted to testosterone by a two-step pathway. 17-hydroxy progesterone and androstenedione catalyzed by P450c17 and 17β-HSD respectively. T is reduced to 5α-DHT and then 3α-diol. P450 aromatase enzyme (P450 aro) reduces androstenedione and T into estrone and estradiol respectively. In the cytoplasm, progesterone is also catalyzed into 5α-DHP and then into allopregnanolone by 5α-reductase and 3α-HSD). Estrone is reversibly converted to estradiol by 17β- HSD. Progesterone is converted into DOC by 21-hydroxylase which undergoes two subsequent reduction steps by 5α-reductase and 3α-HSD to form THDOC. DOC can also be converted into corticosterone by 11β-hydroxylase. Enzymes are denoted in red, steroids and neurosteroids in blue boxes. The arrows and directional arrows indicate irreversible and reversible reactions respectively. Abbreviations; StAR; steroidogenic acute regulatory protein; TSPO, translocator protein; p450scc, P450 side chain cleavage; P4, progesterone; HSD, hydroxysteroid dehydrogenase; 17-OH progesterone, 17-hydroxyprogesterone; 5α- DHT, 5α- dihydrotestosterone; 5α- DHP, 5α- dihydroprogestrone; DOC, deoxycorticosterone; THDOC, tetrahydrodeoxycorticosterone
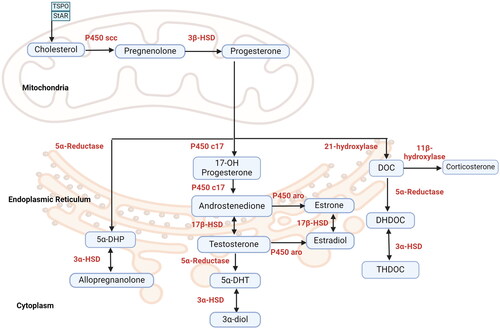
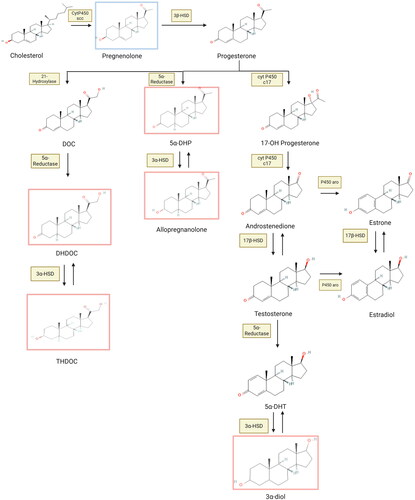
Pregnenolone can serve as the precursor to many different steroids that have hormonal action, including progestogens, estrogens, androgens, and glucocorticoids (Baulieu et al., Citation2001) (). The presence of relevant enzymes is a main determining factor of whether tissue that generates pregnenolone synthesizes bioactive amounts of these other substances. On its own, pregnenolone does not exert influence over sex steroid receptors, but it does have neurosteroid properties (Vallée et al., Citation2014) ().
Figure 2. Chemical Structures of Neurosteroid Synthesis. Neurosteroids are closely related structurally, which allows many to serve as ligands for the same receptors. Neurosteroids binding to GABAa receptors and endocannabinoid receptors are surrounded by red and blue boxes respectively. Abbreviations: HSD, hydroxysteroid dehydrogenase; P450 scc, P450 side chain cleavage enzyme; P450 aro, P450 aromatase; DOC, deoxycorticosterone; DHDOC, dihydrodeoxycorticosterone; THDOC, tetrahydrodeoxycorticosterone; 5α-DHP, 5α- dihydroprogesterone; 17-OH progesterone, 17-hydroxyprogesterone; 5α-DHT, 5α- dihydrotestosterone.
Progestogens
In the mitochondria, pregnenolone can be oxidized into progesterone (P4), the main progestogen, by the enzyme 3β-hydroxysteroid dehydrogenase (3β-HSD) (Simard et al., Citation2005) (). Once synthesized, P4 diffuses across the OMM to the cytosol where it can bind to intracellular nuclear progesterone receptors (PRs), diffuse across the cell membrane and bind with membrane-associated PRs on other cells, or enter nearby cells to exert its action on nuclear PRs. P4 can also enter the endoplasmic reticulum where, depending on the presence of specific enzymes, it can be converted into other metabolites, some of which also bind to PRs, such as 5α-dihydroprogesterone (DHP) (Eicheler et al.,Citation1994; Guennoun, Citation2020). It should be noted that DHP also binds to GABAa receptors so it works as a steroid and neurosteroid (Guennoun, Citation2020).
Androgens
An alternative biochemical path for progesterone is oxidation to 17-OH progesterone and then to the androgen androstenedione by other cytochrome P450 enzymes in the endoplasmic reticulum (Fevold et al., Citation1989; Giatti et al., Citation2020). Androstenedione can be converted to testosterone by the enzyme 17β-hydroxysteroid dehydrogenase (17β-HSD).
Testosterone is reduced to 5α-dihydrotestosterone (5α-DHT) by the action of 5α-reductase (Jin & Penning, Citation2001). Androstenedione, testosterone and DHT can bind to either nuclear androgen receptors (ARs), which serve as transcription factors and alter gene expression, or membrane-associated androgen receptors, which activate G proteins and alter second messenger cascades (Hiipakka & Liao, Citation1998; Zucker et al.,Citation1996).
Estrogens
Progesterone-derived androstenedione can be converted to the estrogen estrone by the enzyme P450 aro and then to estradiol, the most prevalent estrogen in mammals, by the enzyme 17β-HSD (Osawa et al., Citation1993). Estradiol can also be synthesized by the aromatization of testosterone also through P450 aro (MacLusky et al., Citation1994). Both of these biochemical reactions occur in the endoplasmic reticulum. Estrogen receptors (ERs) include those that are nuclear, whose activation alters gene expression, or membrane-associated, whose activation alters G protein signaling cascades (McEwen, Citation2002).
Glucocorticoids
An additional biochemical pathway for progesterone is its conversion to deoxycorticosterone (DOC) by the enzyme 21-hydroxylase. Next, DOC can be converted to corticosterone, the main rodent glucocorticoid, by the action of the enzyme 11β-hydroxylase. In primates and many other nonrodent mammals, the enzyme 17-α hydroxylase, which rodents lack (Raff, Citation2016), enables the production of 17α- hydroxyprogesterone, a substrate that is converted to cortisol by 21-hydroxylase and 11β-hydroxylase (Porcu et al., Citation2016; Van Belle, Citation2017). The steroids involved in both of these pathways have been shown to bind to both nuclear and membrane-associated glucocorticoid receptors (GRs) with the former altering gene expression and the latter activating G-protein coupled signaling cascades (Heitzer et al., Citation2007).
Determining factors of sex steroid and glucocorticoid synthesis
There are many similarities among the biochemical pathways involved in the synthesis of progestogens, androgens, estrogens, and glucocorticoids. First, all of the relevant steroids derive from the same precursor molecule, pregnenolone (). Second, androgens, estrogens, and glucocorticoids all derive from progesterone. Third, many of the biochemical pathways use the same enzymes, with different products arising from the action on different substrates. For example, 17β-HSD can convert estrone to estradiol as well as androstenedione to testosterone (Fevold et al., Citation1989).
Given the shared biochemical pathways, it is not surprising that most tissues that synthesize one type of these steroids also synthesize the others, albeit at different concentrations. For example, the adrenal glands, the testes, the ovaries, and the brain all produce progestogens, androgens, estrogens, and glucocorticoids (Dufau et al., Citation1971; Holst et al.,Citation2004; Nelson & Bulun, Citation2001; Rosol et al.,Citation2001). Differential concentrations of these steroids in specific tissues depend on both the expression of specific enzymes as well as on the concentration of specific substrates.
Not surprisingly given their shared biochemical pathways, these molecules also have similar molecular structures (). The molecular similarity of many of these steroids confers cross-steroid receptor ligand status to several of them. For example, progesterone binds to PRs with highest affinity, but it also binds to GRs (Beato et al., Citation1995; Kontula et al., Citation1983), and testosterone binds with highest affinity to ARs, but it also binds to PRs and ERs (Chang et al., Citation1995; Rochefort & Garcia, Citation1976). These features make it difficult to find agonists and antagonists of these receptors that are specific. For example, mifepristone, a well-known PR antagonist, is also a potent GR antagonist (Im & Appleman, Citation2010). In addition to the complex interactions of sex steroids and glucocorticoids with their receptors, these molecules exert action in the brain through their conversion to neurosteroids.
Sex steroids and glucocorticoids can be converted to neurosteroids
Neurosteroids are steroids synthesized in the central nervous system by both neurons and glial cells (Baulieu, et al., Citation2001). Neurosteroids are different from sex steroids and glucocorticoids in that they exert their effects by binding to neurotransmitter receptors as opposed to nuclear or membrane bound-steroid receptors (Mellon & Griffin, Citation2002; Penning et al., Citation2004; Schverer et al., Citation2018). In addition to serving as a precursor to progesterone, androgens, estrogens, and glucocorticoids, pregnenolone itself can act as a neurosteroid by binding to endocannabinoid receptors where it acts as an allosteric inhibitor (Vallée et al., Citation2014). Progesterone, androgens, and glucocorticoids can also all be converted to neurosteroids that bind to GABA receptors (Reddy & Jian, Citation2010).
As mentioned above, progesterone can be metabolized to DHP by the action of 5α-reductase. DHP is then converted to 3α, 5α tetrahydroprogesterone (3α, 5α-THP), or allopregnanolone, in the presence of the enzyme 3α-HSD (Penning et al., Citation2004). When acting on testosterone, 5α-reductase produces DHT, which is further converted to the neurosteroid 3α-androstanediol (3α-diol) in the presence of 3α-HSD (Jin & Penning, Citation2001). The glucocorticoid deoxycorticosterone is reduced to dihydrodeoxycorticosterone (DHDOC) by the action of 5α-reductase and then the enzyme 3α-HSD converts it to the neurosteroid tetrahydrodeoxycorticosterone (THDOC) (Purdy et al., Citation1991). Thus, allopregnanolone, 3α-diol, and THDOC are all produced by the action of the same enzymes (5α-reductase and 3α-HSD) with the specific end products determined by whether the substrates are progestogens, androgens, or glucocorticoids. DHP, allopregnanolone, 3α-diol, and THDOC have very similar biological actions as positive allosteric modulators of GABAa receptors (Crawley et al., Citation1986; Guennoun, Citation2020; Puia et al., Citation2003; Reddy & Jian, Citation2010). GABAa receptors are ligand-gated chloride channels whose activation inhibits cells. Accordingly, these neurosteroids have been shown to inhibit cells with GABAa receptors (Wang, Citation2011). Allopregnanolone has been most extensively studied and shown to bind to GABAa receptors with delta (δ) subunits, which are the most common extrasynaptic receptors, whose activation increases tonic inhibition (Belelli et al., Citation2002; Stell et al., Citation2003).
Like steroid synthesis within neurons and glia, the synthesis of neurosteroids occurs in the mitochondria (Miller & Bose, Citation2011). Studies have shown that many cells in the brain are equipped with the enzymes necessary to convert cholesterol or peripheral steroids to neurosteroids (Kimoto et al., Citation2001), including neurons and glia of the neocortex, hippocampus, amygdala, thalamus and olfactory regions (Agís-Balboa et al., Citation2006).
Sex differences in brain synthesis and levels of steroids and neurosteroids
There are obvious sex differences in the peripheral levels of sex steroids and glucocorticoids in both rodents and primates. Female rodents have higher peripheral levels of progestogens, estrogens, and glucocorticoids than males while males have higher peripheral levels of androgens (Nelson & Bulun, Citation2001; Tyagi et al., Citation2017). Despite these clear differences and the ease with which these molecules can cross the blood brain barrier, brain concentrations often do not reflect patterns in the periphery in part because of the presence of binding globulins in the blood. A good example of this is the female > male sex difference in peripheral glucocorticoids in rodents, which does not appear to exist in humans (Bangasser & Wicks, Citation2017; Moisan, Citation2021; Reschke-Hernández et al., Citation2017). The exact reason for the species difference in whether males and females exhibit different levels of peripheral glucocorticoids remains unclear, although it is possible that there are sex differences in the regulation of adrenal steroids in rodents that do not exist in humans. Nonetheless, the peripheral sex difference in rodents does not correspond to a similar sex difference in brain glucocorticoid concentrations because females have more corticosteroid binding globulin than males, which limits glucocorticoid access to the brain (Mataradze et al., Citation1992). Thus, the functional consequences of this rodent sex difference remain unclear.
Another reason that brain concentrations do not reflect peripheral sex differences is brain region-specific expression of enzymes that convert peripherally synthesized steroids into other molecules. Perhaps the best example of this is the sex difference in peripheral estradiol levels in rodents, which is reversed in the brain. Adult female rats and mice have higher levels of circulating estradiol than males, but because of the presence of aromatase in the brain as well as high concentrations of the main substrate of aromatase, testosterone, males have higher concentrations of estradiol in some brain regions (Amateau et al., Citation2004; Gillies & McArthur, Citation2010; Hokenson et al., Citation2022). Taken together, these findings emphasize the need to examine brain concentrations of bioactive molecules directly, rather than inferring their relative concentrations by extrapolating from peripheral levels.
With regard to brain concentrations of sex steroids and glucocorticoids, studies have shown that baseline progestogen levels are higher in female than male rodent brains (Meffre et al., Citation2007; Pesaresi et al., Citation2010; Sze et al., Citation2018). Conversely, baseline androgen concentrations, like estradiol levels, are higher in male than female rodent brains (Hojo & Kawato, Citation2018; Pesaresi et al., Citation2010; Sze et al., Citation2018). By contrast, no baseline differences in brain glucocorticoid concentrations exist between the sexes. The relative concentrations of neurosteroids more closely reflect the concentrations of their precursors, in that female > male sex differences in progesterone parallel those of allopregnanolone (Meffre et al., Citation2007), while male > female sex differences in testosterone parallel those in 3α-diol. In female rodents, the estrous cycle produces fluctuating changes in peripheral progesterone. These estrous cycle differences in progesterone parallel differences in allopregnanolone concentrations with higher levels of during diestrus (Caruso et al., Citation2010; Laham et al., Citation2022). Collectively, these findings suggest that brain concentrations of neurosteroids are dependent on both steroid concentrations in the periphery and the presence of enzymes necessary for the production of neurosteroid precursors or neurosteroids themselves.
Impact of stress on sex steroid, glucocorticoid, and neurosteroid levels
Steroid and neurosteroid levels across brain regions fluctuate dynamically in response to different conditions such as stress (Purdy et al., Citation1991; Barbaccia, Citation2004). In addition to the baseline differences in neuroactive steroids as described above, sex-specific differences in steroid and neurosteroid levels have been observed in response to stress.
Exposure to adverse conditions during early life alters levels of sex steroids, glucocorticoids, and neurosteroids in rodents and humans (). For example, male rats reared under limited bedding and nesting conditions show increased serum estradiol levels (Eck et al., Citation2022; 2019). Maternal separation leads to decreased testosterone levels in serum of male mice (Miyaso et al., Citation2021; Tsuda et al., Citation2011).
Figure 3. Diagram Illustrating Some of the Effects of Developmental Stress on Steroid and Neurosteroid Levels, Neuronal Morphology, Glial Expression, and PNN Integrity. Note: Stress influences various aspects of brain function that extend beyond the factors mentioned in the diagram. P4, Progesterone; E2, Estradiol; T, Testosterone; ALLO, Allopregnanolone; CORT, Corticosterone; DHP, Dihydroprogesterone; DHT, Dihydrotestosterone; DOC, Deoxycorticosterone; THDOC, Tetrahydrodeoxycorticosterone; BLA, Basolateral amygdala; PFC, prefrontal cortex; HIP, hippocampus; dHIP, dorsal hippocampus; vHIP, ventral hippocampus; Iba1, ionized calcium binding adaptor molecule 1; GFAP, Glial fibrillary acidic protein; PNN, Perineuronal nets; PV, Parvalbumin; CSPG, Chondroitin sulfate proteoglycan. ♂: male ♀: female.
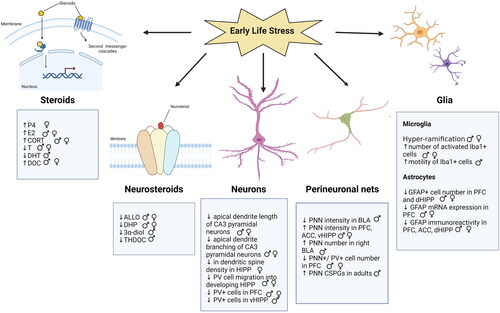
Maternal separation with early weaning has been shown to increase serum and brain concentrations of progesterone in diestrus female mice (Laham et al., Citation2022). Along with these increases in progesterone levels, allopregnanolone levels are lower (Laham et al., Citation2022). Changes in the expression of enzymes associated with steroid and neurosteroid synthesis have been reported in response to both prenatal and early postnatal stress in rodents. Juvenile offspring of rat dams exposed to restraint during late gestation showed decreased conversion of progesterone to 5α-reduced metabolites such as DHP and allopregnanolone in the medial prefrontal cortex (mPFC) (Paris & Frye, Citation2011b). A reduction in allopregnanolone was noted in the hippocampus of female offspring of rat dams exposed to immune challenge during the late gestation period (Paris et al., Citation2011). Frye et al. (Citation2010) reported that male rat offspring of dams exposed to prenatal stress show increased corticosterone levels, decreased 3α-diol concentration, and decreased hippocampal DHT levels accompanied by increased avoidance behavior. Similarly, gestational and acute restraint stress decreased hippocampal 3α-diol levels and increased avoidance behavior (Walf & Frye, Citation2012). Our laboratory has found that female diestrus mice subjected to maternal separation and early weaning have reduced levels of 5α-reductase in the ventral hippocampus, which likely contributes to the elevated ratio of progesterone to allopregnanolone (Laham et al., Citation2022). Similarly, male juvenile mice reared in isolation have lower expression of 5α-reductase in the mPFC and nucleus accumbens and reduced allopregnanolone and THDOC in the frontal cortex (Bortolato et al., Citation2011). Postweaning social isolation has also been shown to result in a downregulation of cortical and plasma concentrations of allopregnanolone, This was also associated with increased avoidance behavior (Serra et al., Citation2005; Serra et at., 2000). These studies indicate a dysregulation in neurosteroid synthesis in rodent models of developmental stress (). Although it is difficult to draw connections between studies done in rodents and the human condition, it seems relevant to note that childhood Holocaust survivors have diminished serum levels of 5α- reductase (Yehuda et al., Citation2009).
Table 2. Impact of Stress on Sex Steroid, Glucocorticoid, and Neurosteroid Levels.
In adulthood, acute stress increases the levels of certain steroids in rodents, including progesterone, testosterone, estrogen, and corticosterone/cortisol, in both the periphery and brain (López-Calderón et al., Citation1990; Romeo et al., Citation2007; Shors et al., Citation1999). Stress increases levels of these steroids, as well as their neurosteroid derivatives, in males and females, with increases in allopregnanolone being greater in the frontal cortex, amygdala and brainstem in females. A significant increase in allopregnanolone was only noted in the frontal cortex in stress-induced male rats (Sze et al., Citation2018). Studies indicate that plasma and brain levels of THDOC and allopregnanolone rapidly increase in rats exposed to acute stress (Reddy & Rogawski, Citation2002). Vallée et al. (Citation2000) quantified the neurosteroid concentration in rat plasma and brain following forced swim stress and reported a significant elevation in allopregnanolone and pregnenolone levels. Peak brain and plasma levels of allopregnanolone and THDOC are noted in rats exposed to acute stressors such as cold swim stress, foot shock, or carbon dioxide exposure (Barbaccia et al., Citation1996, Citation1997). Similarly, other studies have shown a 4-20 fold rapid elevation in THDOC and allopregnanolone in the rodent cortex and hypothalamus following exposure to acute swim test (Purdy et al., Citation1991). Investigators have reported a reduction in avoidance behavior in response to the administration of allopregnanolone and THDOC in rodents (Bitran et al., Citation1991; Wieland et al., Citation1991). Studies suggest that this increase in the levels of allopregnanolone is a potential homeostatic mechanism, to increase the GABAa receptor threshold and promote stress resistance in response to acute challenges (Shirayama et al., Citation2011; Yoshizawa et al., Citation2017). Blockade of allopregnanolone production in response to acute stress using finasteride, a 5α-reductase inhibitor, decreases sensorimotor gating index in response to acute stress, an effect observed in several neuropsychiatric disorders (Pallarès et al., Citation2015).
Also in adulthood, chronic stress alters levels of peripheral sex steroids and glucocorticoids. Studies have shown that chronic stress elevates serum levels of progesterone and glucocorticoids in male and female rodents, while decreasing levels of testosterone and estradiol in rats and mice (Gao et al., Citation2020; Retana-Márquez et al., Citation2003). Studies have also shown that chronic stress-induced changes in peripheral steroid levels do not always parallel changes in neurosteroid concentrations. Indeed, adult male mice exposed to a social isolation paradigm, show a reduction in 5α-reductase activity, which produces decreased allopregnanolone levels in the frontal cortex and olfactory bulb. These findings were not observed in female mice (Dong et al., Citation2001; Pinna et al., Citation2004). The available evidence suggests that compensatory mechanisms exist in the brain to mitigate peripheral changes in steroid levels after acute stress. These mechanisms appear to be diminished after chronic stress, possibly through a downregulation of enzymes that convert steroids to neurosteroids with buffering action on avoidance behavior (). In humans, individuals with neuropsychiatric disease associated with stress, including posttraumatic stress disorder and major depressive disorder, have lower levels of serum 5α-reductase (Agis-Balboa et al., Citation2014), which likely influences levels of neurosteroids, as some studies have suggested (Römer et al., Citation2010; Römer & Gass, Citation2010).
Impact of stress on neurons: involvement of steroids and neurosteroids
Stress is known to have profound effects on neuronal excitability, biochemistry, and structure, some of which are mediated by stress-induced changes in steroids and neurosteroids. The most obvious steroids to be implicated in stress effects on neurons are glucocorticoids, also known as stress hormones. A large literature has shown that developmental stress influences neurotransmission, gene expression, dendritic architecture, and postnatal neurogenesis (De Kloet et al., Citation1996; McEwen et al., Citation2016; Mirescu et al., Citation2004; Monroy et al., Citation2010). For example, prenatal and postnatal stress, as well as chronic stress in adulthood, result in atrophied apical dendrites of CA3 pyramidal cells and diminished hippocampal, but not prefrontal or hypothalamic, synaptic markers in rodents (Liu et al., Citation2016; Paris & Frye, Citation2011a; Watanabe et al., Citation1992). Some of these effects have been attributed to changes in glucocorticoids (De Kloet et al., Citation1996; McEwen et al., Citation2016; Mirescu et al., Citation2004) and others are very similar to what has been observed with chronic stress in adulthood, where a causal link with glucocorticoids has been established (Horchar & Wohleb, Citation2019; Kvarta et al., Citation2015) (). However, it should be emphasized that elevated glucocorticoid levels do not explain many of the persistent effects of early life adversity as well as those of stress in adulthood (Faturi et al., Citation2010; Kim et al., Citation2015).
Table 3. Impact of Stress on Neurons: Involvement of Steroids and Neurosteroids.
In addition to glucocorticoids, other steroids known to be altered by developmental stress, as well as acute and chronic adult stress, influence neurons and as such, may contribute to stress responses. For example, estrogen and progesterone, which are both elevated in response to postnatal and acute adult stress in rodents (Paris & Frye, Citation2011a, Citation2011b; Shors et al., Citation1999), are known to increase dendritic spine density in the hippocampus (Gould et al., Citation1990; Woolley et al., Citation1990). Allopregnanolone has a similar effect to progesterone of increasing dendritic spine density (Barreto-Cordero et al., Citation2020; Shimizu et al., Citation2015) raising the possibility that progesterone acts through its derivative allopregnanolone to modulate dendritic spines. Studies have shown that ovariectomized female rats treated with estrogen replacement and subjected to acute stress showed an increase in spine density in basolateral amygdala (BLA) (Shansky et al., Citation2010). A similar increase in BLA spine density was noted in male rats exposed to chronic stress (Mitra et al., Citation2005). In adulthood, acute stress exerts differential effects on CA1 pyramidal cell dendritic spine density in male and female rats (Shors et al., Citation1999). Stress reduces dendritic spine density in female rats when they are in proestrus, while it has the opposite effect in male rats (Shors et al., Citation2001). Similar results were observed in female mice where acute stress in the context of higher estrogen leads to reduced dendritic spine density and impaired hippocampus-dependent memory, while similar stress exposure during a low estrogen state has no effects (Hokenson et al., Citation2021). Furthermore, while acute stress in adulthood increases dendritic spines in males, developmental stress has the opposite effect in male mice and rats (Monroy et al., Citation2010; Xu et al., Citation2022) (). These findings suggest a complex interplay between stress effects and sex that may be not only dependent on baseline levels of steroids and neurosteroids but also on the age at which the stress occurs.
Testosterone also influences dendritic spine density in the hippocampus with parallel results in performance on hippocampus-dependent learning tasks in rats (Muthu et al., Citation2022). Studies have also shown that both testosterone and its derivative DHT can stimulate hippocampal spine density in rats (Hatanaka et al., Citation2015). Taken together, these findings raise the possibility that stress-induced changes in progestogens, androgens, estrogens, and glucocorticoids, as well as their neurosteroid derivatives may be responsible for some stress-induced effects on neurons, including on dendritic structure.
In addition to changes in excitatory neurons, developmental and adult stress have been shown to influence inhibitory interneurons in both the hippocampus and prefrontal cortex. Maternal separation diminishes the migration of parvalbumin positive (PV+) interneurons into the developing hippocampus (Katahira et al., Citation2018). Similarly, exposure to early life adversity has been shown to reduce the number of PV + interneurons in the prefrontal cortex of female rats (Gildawie et al., Citation2021). We have found a reduction in the number of PV + interneurons, and somatostatin + interneurons, in the ventral hippocampus of adult male mice previously subjected to maternal separation with early weaning (Murthy et al., Citation2019) although in the case of PV + cells, this reduction likely reflects diminished production of parvalbumin rather than an actual decrease in the number of these cells. Studies in adult rats have shown that chronic, but not acute, stress also reduces parvalbumin expression in hippocampus interneurons (Filipović et al., Citation2013) (). Although inhibitory interneurons, including PV + cells, are known to express GRs and ERs (Hernández-Vivanco et al., Citation2022; Kraus et al., Citation2022), it remains unexplored whether the effects of stress on this population are mediated through these signaling mechanisms.
Likewise, although both principal neurons and inhibitory interneurons express GABAa receptors, which are known targets of neurosteroid derivatives of sex steroids and glucocorticoids, the connection between stress effects and neurosteroid action remains unexplored.
Impact of stress on perineuronal nets: involvement of steroids and neurosteroids?
Many neurons shown to be affected by early life stress are enwrapped in perineuronal nets (PNNs), extracellular matrix structures known to regulate neural underpinnings of cognition and behavior, including but not limited to, synaptic plasticity, neuronal oscillations, and synchrony across brain regions (Sorg et al., Citation2016; Wingert & Sorg, Citation2021). Numerous studies have investigated the impact of postnatal and adult stress on PNNs and shown complex effects that seem to depend on multiple factors, including brain region, sex, and timing/type of stressor (Laham & Gould, Citation2021).
Postnatal stress using the maternal separation and early weaning stress paradigm has been shown to increase PNN intensity in the ventral hippocampus in adulthood along with increases in avoidance behavior (Catale et al., Citation2022; Dimatelis et al., Citation2013; Murthy et al., Citation2019). An upregulation of PNN CSPGs such as tenascin, brevican, neurocan was also noted in animals subjected to maternal separation (Dimatelis et al., Citation2013). Other results suggest an increase in PNN number in the BLA and HIP animals exposed to stress during the postnatal period or in adulthood (Guadagno et al., Citation2020; Riga et al., Citation2017), including an adult paradigm of enrichment loss (Smail et al., Citation2023). However, additional studies have shown a downregulation in PNN intensity in PFC, BLA (Gildawie et al., Citation2021; Santiago et al., Citation2018) and PNN number (Gildawie et al., Citation2020; Klimczak et al., Citation2021; Ueno et al., Citation2018; Yu et al., Citation2020) in PFC, HIP, prelimbic cortex, and BLA in animals exposed to postnatal and adult stress paradigms such as maternal separation, social isolation, social defeat, and chronic mild stress. Sex- dependent effects of postnatal stress have also been observed in the development of PNNs around PV + cells in the PFC and BLA of male and female rats, with a higher PNN intensity noted in the PFC of male rats exposed to maternal separation and juvenile social isolation, while a higher PNN intensity reported in BLA of female rats (Gildawie et al., Citation2021). Similarly, increase in PNN intensity was reported in the right amygdala of male juvenile rats but not females after exposure to early life stress (Guadagno et al., Citation2020). Studies have shown that changes in PNN intensity/number are associated with increased in avoidance behavior and impairments in stress regulation (Laham et al., Citation2022; Lee & Lee, Citation2021; Murthy et al., Citation2019; Santiago et al., Citation2018; Yu et al., Citation2020). Collectively, these studies suggest that a dysregulation in PNN intensity and number occurs after exposure to several stress paradigms. It is important to consider factors such as type of stressor, duration of time after stress exposure (Gildawie et al., Citation2020, Citation2021; Laham & Gould, Citation2021; Ueno et al., Citation2018), sex, and stage of development (Drzewiecki et al., Citation2020) when addressing the impact of stress on PNNs ().
Table 4. Impact of Stress on Perineuronal Nets: Involvement of Steroids and Neurosteroids?.
The potential involvement of steroids and neurosteroids in stress-induced PNN changes remains relatively unexplored. Studies have shown that chronic corticosterone treatment in adult mice leads to an increase in PNN intensity in the hippocampus (Alaiyed et al., Citation2020), which is consistent with the possibility that stress-induced increases in glucocorticoids, or their derivatives, influence PNNs. Studies have shown that inhibition of estradiol synthesis increases PNN intensity around PV+ interneurons in the dorsal CA1 region of the hippocampus in female mice, a finding that has been causally linked to diminished hippocampus-dependent learning and memory and hippocampal neuronal oscillations (Hernández-Vivanco et al., Citation2022). Given that studies have shown early life and adult stress affect estradiol levels, it is possible that some stress effects may exert their action through modulation of circulating or brain-derived estradiol although the possibility remains to be investigated. Allopregnanolone inhibition in adult female mice has been shown to alter PNN composition in the ventral CA1, increasing the numbers of PNNs that contain the C4S sulfation pattern, a PNN characteristic known to inhibit plasticity (Laham et al., Citation2022). Similar changes were observed in ventral CA1 PNNs in adult female mice after early life stress (maternal separation with early weaning), an experience that diminishes ventral hippocampal allopregnanolone concentrations (Laham et al., Citation2022). Taken together, these findings suggest that early life stress may alter PNN composition through actions on allopregnanolone. This is an area that is clearly in need of further investigation. It is also worth noting that neuronal activity has been shown to modulate PNN intensity (Evans et al., Citation2022), thus, the influence of these molecules on PNNs may occur indirectly, through effects on neuronal function.
Impact of stress on glia: involvement of steroids and neurosteroids?
Microglia and astrocytes are highly sensitive to environmental changes and involved in inflammatory processes. Several studies indicate that stress may be associated with the dysregulation of immune system response (Black, Citation2003; Kiecolt-Glaser et al., Citation1996). Microglia respond to stress in a variety of ways, including increasing in number, producing pro-inflammatory cytokines, and expressing cell surface antigens (Calcia et al., Citation2016; Kettenmann et al., Citation2011). One of the key cell surface antigens is the intracellular ionized calcium-binding adaptor protein, Iba1, which is found in microglia and is upregulated during inflammation. Upregulation in Iba1 expression in the hippocampus has been reported in rodents exposed to different stress paradigms when compared to unstressed animals (Bian et al., Citation2012; Kojo et al., Citation2010; Park et al., Citation2011; Wohleb et al., Citation2011). A similar increase in Iba1 expression is observed in other regions of the brain such as the amygdala, nucleus accumbens, and PFC in animals subjected to stress (Bian et al., Citation2012; Schiavone et al., Citation2009; Tynan et al., Citation2010; Wohleb et al., Citation2011) ().
Table 5. Impact of Stress on Microglia and Astrocytes: Involvement of Steroids and Neurosteroids?
Maternally separated male and female rats exposed to a “second hit” of stress during adulthood, show an upregulation of Iba1 activity in the PFC, along with hyper-ramified microglial morphology, indicating microglial activation or a priming process increasing the susceptibility of a heightened response during subsequent stressors (Ganguly et al., Citation2018; Hoeijmakers et al., Citation2017; Roque et al., Citation2016; Saavedra et al., Citation2017; Tay et al., Citation2018). Takatsuru and colleagues report that ELA increases the number and motility of microglial processes (Takatsuru et al., Citation2015). Additionally, exposure to early life adversity results in a decrease in the arborization area of Iba1+ cells (Baldy et al., Citation2018). During normal hippocampus growth in mice, there is a decrease in microglial density and phagocytic activity. However, in mice exposed to ELA, there is an increase in phagocytic activity during hippocampal development as well as an increase in microglial density and surface area in the hippocampus at PND 14 (Delpech et al., Citation2016) ().
In adulthood, acute stress increases microglial number (Frank et al., Citation2007), and induces de-ramification (Sugama et al., Citation2007) and according to more recent studies, adult exposure to acute stress reduces microglial phagocytic activity. (Fonken et al., Citation2018; Frank et al., Citation2019). Chronic stress in adulthood causes inflammation, which is characterized by an increase in brain cytokine levels, and this elevation is associated with changes in microglia morphology (Calcia et al., Citation2016; Yirmiya et al., Citation2015).
Exposure to chronic stress is associated with an increase in Iba1+ cell density, Iba1 area and microglial phagocytic function (Frank et al., Citation2019; Hinwood et al., Citation2012; Nair & Bonneau, Citation2006; Tynan et al., Citation2010). Microglia hyper-ramification has also been observed in the PFC and hippocampus of animals subjected to chronic restraint stress (Hinwood et al., Citation2013) or repeated swimming tests (Hellwig et al., Citation2016), while an increase in de-ramified microglia were noted in the amygdala, PFC and hippocampus of mice subjected to repeated social defeat paradigm (Wohleb et al., Citation2011) ().
Stress hormones and cytokines influence glial fibrillary acidic protein (GFAP), a cytoskeletal astroglia protein, and an increase in these levels indicates astrocyte activity. An upregulation in astrocytic activity and neuroinflammatory cytokines was reported in animals subjected to early life stress paradigm such as maternal deprivation (Giridharan et al., Citation2019; Réus et al., 2017, 2019). Conversely, a decrease in GFAP mRNA expression and GFAP + cell number was noted in the PFC of mice exposed to maternal separation (Musholt et al., Citation2009; Yamawaki et al., Citation2018).
Additionally, a decrease in GFAP immunoreactivity was reported in the PFC, ACC, dorsal hippocampus of animals exposed to ELA (Abbink et al., Citation2020; Banqueri et al., Citation2019). In adulthood, exposure to acute stressors resulted in a decrease in astrocyte density (Saur et al., Citation2016), GFAP expression in the hippocampus (Xia et al., Citation2013) and induces astrocytic hypertrophy (Murphy-Royal et al., Citation2019). Chronic stress in adulthood is associated with a reduction in astrocyte number and somatic volume in the hippocampus (Altshuler et al.,Citation2010; Banasr et al., Citation2010; Czéh et al., Citation2006; Li et al., Citation2013, Liu et al., Citation2009, Liu et al., Citation2011, Tynan et al., Citation2013). Exposure to chronic mild unpredictable stress during adulthood is also associated with a decrease in structural complexity of astrocytes (Yamawaki et al., Citation2018) ().
The involvement of steroids and neurosteroids in stress-induced changes in microglia and astrocytes has been incompletely explored, but chronic stress-induced changes in microglial activation and astrocyte number can be blocked by GR antagonism (Horchar & Wohleb, Citation2019; Lou et al., Citation2018) and stress-related memory formation can be blocked by deleting GRs from astrocytes (Tertil et al., Citation2018; Wiktorowska et al., Citation2021). Evidence showing that allopregnanolone increases microglial cell processes and decreases microglia migration in mice, suggests a role for allopregnanolone in modulating microglial morphology and inhibiting phagocytic function. (Jolivel et al., Citation2021). Given these insights into the interplay between glial cells and neurosteroids, and considering the known effects of stress on these entities individually, it seems increasingly important to explore the impact of stress on glial functionality through the modulation of steroids and neurosteroids.
Conclusions
Early adverse experiences are known to alter neurons, glia, and the extracellular matrix and many of these effects have been causally linked to behavioral change that lasts into adulthood. Understanding the connection between aversive experience and brain changes will require considering all these brain components, as well as the many signaling molecules that can be altered by early life stress. Early adversity is known to alter the levels of glucocorticoids and sex steroids in both the periphery and brain, and these molecules can be synthesized in both compartments. While studies have investigated the causal role of glucocorticoids in stress effects, less attention has been paid to the potential involvement of glucocorticoid derivatives, as well as to sex steroids produced in the periphery and brain, and their neurosteroid end products. Additional studies directly investigating the potential involvement of these signaling molecules in stress influences on neurons, glia, and the extracellular matrix will help to shed light on the complex cascade of events that produce lasting changes after early life stress.
Acknowledgements
The authors thank Casey Brown for helpful comments on the manuscript and Biorender for assistance with the figure schematics.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
Notes on contributors
Isha R. Gore
Isha R. Gore is a PhD student at the Princeton Neuroscience Institute (PNI) studying the effects of stress on neural mechanisms underlying avoidance behavior in mice. Prior to joining PNI, Gore received a Master’s degree from Columbia University.
Elizabeth Gould
Elizabeth Gould, PhD is a Professor of Neuroscience and head of laboratory at the Princeton Neuroscience Institute. Her lab focuses on plasticity mechanisms affected by genes, experience, and steroids in developing and adult mice. Prior to joining the Princeton faculty, Gould received her PhD from UCLA and was a postdoctoral fellow and assistant professor at The Rockefeller University.
References
- Abbink, M. R., Kotah, J. M., Hoeijmakers, L., Mak, A., Yvon-Durocher, G., van der Gaag, B., Lucassen, P. J., & Korosi, A. (2020). Characterization of astrocytes throughout life in wildtype and APP/PS1 mice after early-life stress exposure. Journal of Neuroinflammation, 17(1), 1. https://doi.org/10.1186/s12974-020-01762-z
- Agis-Balboa, R. C., Guidotti, A., & Pinna, G. (2014). 5α-reductase type I expression is downregulated in the prefrontal cortex/Brodmann’s area 9 (BA9) of depressed patients. Psychopharmacology, 231(17), 3569–18. https://doi.org/10.1007/s00213-014-3567-5
- Agís-Balboa, R. C., Pinna, G., Zhubi, A., Maloku, E., Veldic, M., Costa, E., & Guidotti, A. (2006). Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proceedings of the National Academy of Sciences, 103(39), 14602–14607. https://doi.org/10.1073/pnas.0606544103
- Alaiyed, S., McCann, M., Mahajan, G., Rajkowska, G., Stockmeier, C. A., Kellar, K. J., Wu, J. Y., & Conant, K. (2020). Venlafaxine stimulates an MMP-9-dependent increase in excitatory/inhibitory balance in a stress model of depression. The Journal of Neuroscience., 40(22), 4418–4431. https://doi.org/10.1523/JNEUROSCI.2387-19.2020
- Altshuler, L. L., Abulseoud, O. A., Foland-Ross, L., Bartzokis, G., Chang, S., Mintz, J., Hellemann, G., & Vinters, H. V. (2010). Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disorders, Banqueri, M., Méndez, M2(5), 541–549. https://doi.org/10.1111/j.1399-5618.2010.00838.x
- Amateau, S. K., Alt, J. J., Stamps, C. L., & McCarthy, M. M. (2004). Brain estradiol content in newborn rats: Sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology, 145(6), 2906–2917. https://doi.org/10.1210/en.2003-1363
- Baldy, C., Fournier, S., Boisjoly-Villeneuve, S., Tremblay, M.-È., & Kinkead, R. (2018). The influence of sex and neonatal stress on medullary microglia in rat pups. Experimental Physiology, 103(9), 1192–1199. https://doi.org/10.1113/EP087088
- Banasr, M., Chowdhury, G. M. I., Terwilliger, R., Newton, S. S., Duman, R. S., Behar, K. L., & Sanacora, G. (2010). Glial pathology in an animal model of depression: Reversal of stress- induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Molecular Psychiatry, 15(5), 501–511. https://doi.org/10.1038/mp.2008.106
- Bangasser, D. A., & Wicks, B. (2017). Sex-specific mechanisms for responding to stress. Journal of Neuroscience Research, 95(1-2), 75–82. https://doi.org/10.1002/jnr.23812
- Banqueri, M., Méndez, M., Gómez-Lázaro, E., & Arias, J. L. (2019). Early life stress by repeated maternal separation induces long-term neuroinflammatory response in glial cells of male rats. Stress, 22(5), 563–570. https://doi.org/10.1080/10253890.2019.1604666
- Barbaccia, M. L. (2004). Neurosteroidogenesis: Relevance to neurosteroid actions in brain and modulation by psychotropic drugs. Critical Reviews in Neurobiology, 16(1–2), 67–74. https://doi.org/10.1615/critrevneurobiol.v16.i12.70
- Barbaccia, M. L., Roscetti, G., Trabucchi, M., Mostallino, M. C., Concas, A., Purdy, R. H., & Biggio, G. (1996). Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology, 63(2), 166–172. https://doi.org/10.1159/000126953
- Barbaccia, M. L., Roscetti, G., Trabucchi, M., Purdy, R. H., Mostallino, M. C., Concas, A., & Biggio, G. (1997). The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. British Journal of Pharmacology, 120(8), 1582–1588. https://doi.org/10.1038/sj.bjp.0701046
- Barreto-Cordero, L. M., Ríos-Carrillo, J., Roldán-Roldán, G., Rasia-Filho, A. A., Flores, G., Bringas, M. E., Briones-Aranda, A., & Picazo, O. (2020). Cyclic changes and actions of progesterone and allopregnanolone on cognition and hippocampal basal (stratum oriens) dendritic spines of female rats. Behavioural Brain Research, 379, 112355. https://doi.org/10.1016/j.bbr.2019.112355
- Baulieu, E. E., Robel, P., & Schumacher, M. (2001). Neurosteroids: Beginning of the story. International Review of Neurobiology, 46, 1–32. https://doi.org/10.1016/s0074-7742(01)46057-0
- Beato, M., Herrlich, P., & Schütz, G. (1995). Steroid hormone receptors: Many actors in search of a plot. Cell, 83(6), 851–857. https://doi.org/10.1016/0092-8674(95)90201-5
- Belelli, D., Casula, A., Ling, A., & Lambert, J. J. (2002). The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology, 43(4), 651–661. https://doi.org/10.1016/s0028-3908(02)00172-7
- Bian, Y., Pan, Z., Hou, Z., Huang, C., Li, W., & Zhao, B. (2012). Learning, memory, and glial cell changes following recovery from chronic unpredictable stress. Brain Research Bulletin, 88(5), 471–476. https://doi.org/10.1016/j.brainresbull.2012.04.008
- Bitran, D., Hilvers, R. J., & Kellogg, C. K. (1991). Anxiolytic effects of 3 alpha-hydroxy-5 alpha[beta]-pregnan-20-one: Endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Research, 561(1), 157–161. https://doi.org/10.1016/0006-8993(91)90761-j
- Black, P. H. (2003). The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain, Behavior, and Immunity, 17(5), 350–364. https://doi.org/10.1016/s0889-1591(03)00048-5
- Bortolato, M., Devoto, P., Roncada, P., Frau, R., Flore, G., Saba, P., Pistritto, G., Soggiu, A., Pisanu, S., Zappala, A., Ristaldi, M. S., Tattoli, M., Cuomo, V., Marrosu, F., & Barbaccia, M. L. (2011). Isolation rearing-induced reduction of brain 5α-reductase expression: Relevance to dopaminergic impairments. Neuropharmacology, 60(7-8), 1301–1308. https://doi.org/10.1016/j.neuropharm.2011.01.013
- Calcia, M. A., Bonsall, D. R., Bloomfield, P. S., Selvaraj, S., Barichello, T., & Howes, O. D. (2016). Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology, 233(9), 1637–1650. https://doi.org/10.1007/s00213-016-4218-9
- Caruso, D., Pesaresi, M., Maschi, O., Giatti, S., Garcia-Segura, L. M., & Melcangi, R. C. (2010). Effect of short-and long-term gonadectomy on neuroactive steroid levels in the central and peripheral nervous system of male and female rats. Journal of Neuroendocrinology, 22(11), 1137–1147. https://doi.org/10.1111/j.1365-2826.2010.02064.x
- Catale, C., Martini, A., Piscitelli, R. M., Senzasono, B., Iacono, L. L., Mercuri, N. B., Guatteo, E., & Carola, V. (2022). Early-life social stress induces permanent alterations in plasticity and perineuronal nets in the mouse anterior cingulate cortex. The European Journal of Neuroscience, 56(10), 5763–5783. https://doi.org/10.1111/ejn.15825
- Chang, C., Saltzman, A., Yeh, S., Young, W., Keller, E., Lee, H. J., Wang, C., & Mizokami, A. (1995). Androgen receptor: An overview. Critical Reviews in Eukaryotic Gene Expression, 5(2), 97–125. https://doi.org/10.1615/critreveukargeneexpr.v5.i2.10
- Compagnone, N. A., & Mellon, S. H. (2000). Neurosteroids: Biosynthesis and function of these novel neuromodulators. Frontiers in Neuroendocrinology, 21(1), 1–56. https://doi.org/10.1006/frne.1999.0188
- Crawley, J. N., Glowa, J. R., Majewska, M. D., & Paul, S. M. (1986). Anxiolytic activity of an endogenous adrenal steroid. Brain Research, 398(2), 382–385. https://doi.org/10.1016/0006-8993(86)91500-3
- Czéh, B., Simon, M., Schmelting, B., Hiemke, C., & Fuchs, E. (2006). Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology, 31(8), 1616–1626. https://doi.org/10.1038/sj.npp.1300982
- De Kloet, E. R., Rots, N. Y., & Cools, A. R. (1996). Jun Brain-corticosteroid hormone dialogue: Slow and persistent. Cellular and Molecular Neurobiology, 16(3), 345–356. https://doi.org/10.1007/BF02088100
- Delpech, J. C., Wei, L., Hao, J., Yu, X., Madore, C., Butovsky, O., & Kaffman, A. (2016). Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain, Behavior, and Immunity, 57, 79–93. https://doi.org/10.1016/j.bbi.2016.06.006
- Dimatelis, J. J., Hendricks, S., Hsieh, J., Vlok, N. M., Bugarith, K., Daniels, W. M. U., & Russell, V. A. (2013). Exercise partly reverses the effect of maternal separation on hippocampal proteins in 6-hydroxydopamine-lesioned rat brain. Experimental Physiology, 98(1), 233–244. https://doi.org/10.1113/expphysiol.2012.066720
- Dong, E., Matsumoto, K., Uzunova, V., Sugaya, I., Takahata, H., Nomura, H., Watanabe, H., Costa, E., & Guidotti, A. (2001). Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proceedings of the National Academy of Sciences, 98(5), 2849–2854. https://doi.org/10.1073/pnas.051628598
- Drzewiecki, C. M., Willing, J., & Juraska, J. M. (2020). Influences of age and pubertal status on number and intensity of perineuronal nets in the rat medial prefrontal cortex. Brain Structure & Function, 225(8), 2495–2507. https://doi.org/10.1007/s00429-020-02137-z
- Dufau, M. L., Catt, K. J., & Tsuruhara, T. (1971). Gonadotrophin stimulation of testosterone production by the rat testis in vitro. Biochimica et Biophysica Acta, 252(3), 574–579. https://doi.org/10.1016/0304-4165(71)90161-9
- Eck, S. R., Ardekani, C. S., Salvatore, M., Luz, S., Kim, E. D., Rogers, C. M., Hall, A., Lee, D. E., Famularo, S. T., Bhatnagar, S., & Bangasser, D. A. (2020). The effects of early life adversity on growth, maturation, and steroid hormones in male and female rats. The European Journal of Neuroscience, 52(1), 2664–2680. https://doi.org/10.1111/ejn.14609
- Eck, S. R., Palmer, J. L., Bavley, C. C., Karbalaei, R., Ordoñes Sanchez, E., Flowers, J., Holley, A., Wimmer, M. E., & Bangasser, D. A. (2022). Effects of early life adversity on male reproductive behavior and the medial preoptic area transcriptome. Neuropsychopharmacology, 47(6), 1231–1239. https://doi.org/10.1038/s41386-022-01282-9
- Eicheler, W., Tuohimaa, P., Vilja, P., Adermann, K., Forssmann, W. G., & Aumüller, G. (1994). Immunocytochemical localization of human 5 alpha-reductase 2 with polyclonal antibodies in androgen target and non-target human tissues. The Journal of Histochemistry and Cytochemistry, 42(5), 667–675. https://doi.org/10.1177/42.5.8157936
- Evans, A., Terstege, D. J., Scott, G. A., Tsutsui, M., & Epp, J. R. (2022). Neurogenesis mediated plasticity is associated with reduced neuronal activity in CA1 during context fear memory retrieval. Scientific Reports, 12(1), 7016. https://doi.org/10.1038/s41598-022-10947-w
- Faturi, C. B., Tiba, P. A., Kawakami, S. E., Catallani, B., Kerstens, M., & Suchecki, D. (2010). Disruptions of the mother-infant relationship and stress-related behaviours: Altered corticosterone secretion does not explain everything. Neuroscience and Biobehavioral Reviews, 34(6), 821–834. https://doi.org/10.1016/j.neubiorev.2009.09.002
- Fevold, H. R., Lorence, M. C., McCarthy, J. L., Trant, J. M., Kagimoto, M., Waterman, M. R., & Mason, J. I. (1989). Rat P450(17 alpha) from testis: Characterization of a full-length cDNA encoding a unique steroid hydroxylase capable of catalyzing both delta 4- and delta 5-steroid-17,20-lyase reactions. Molecular Endocrinology, 3(6), 968–975. https://doi.org/10.1210/mend-3-6-968
- Filipović, D., Zlatković, J., Gass, P., & Inta, D. (2013). The differential effects of acute vs. chronic stress and their combination on hippocampal parvalbumin and inducible heat shock protein 70 expression. Neuroscience, 236, 47–54. https://doi.org/10.1016/j.neuroscience.2013.01.033
- Fonken, L. K., Frank, M. G., Gaudet, A. D., D’Angelo, H. M., Daut, R. A., Hampson, E. C., Ayala, M. T., Watkins, L. R., & Maier, S. F. (2018). Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain, Behavior, and Immunity, 70, 257–267. https://doi.org/10.1016/j.bbi.2018.03.005
- Frank, M. G., Baratta, M. V., Sprunger, D. B., Watkins, L. R., & Maier, S. F. (2007). Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain, Behavior, and Immunity, 21(1), 47–59. https://doi.org/10.1016/j.bbi.2006.03.005
- Frank, M. G., Fonken, L. K., Watkins, L. R., & Maier, S. F. (2019). Microglia: Neuroimmune-sensors of stress. Seminars in Cell & Developmental Biology, 94, 176–185. https://doi.org/10.1016/j.semcdb.2019.01.001
- Frye, C., Edinger, K., Lephart, E., & Walf, A. (2010). 3α-androstanediol, but not testosterone, attenuates age-related decrements in cognitive, anxiety, and depressive behavior of male rats. Frontiers in Aging Neuroscience, 2, 15. https://doi.org/10.3389/fnagi.2010.00015
- Ganguly, P., Thompson, V., Gildawie, K., & Brenhouse, H. C. (2018). Adolescent food restriction in rats alters prefrontal cortex microglia in an experience-dependent manner. Stress (Amsterdam, Netherlands), 21(2), 162–168. https://doi.org/10.1080/10253890.2017.1423054
- Gao, L., Zhao, F., Zhang, Y., Wang, W., & Cao, Q. (2020). Diminished ovarian reserve induced by chronic unpredictable stress in C57BL/6 mice. Gynecological Endocrinology, 36(1), 49–54. https://doi.org/10.1080/09513590.2019.1631274
- Giatti, S., Diviccaro, S., Serafini, M. M., Caruso, D., Garcia-Segura, L. M., Viviani, B., & Melcangi, R. C. (2020). Sex differences in steroid levels and steroidogenesis in the nervous system: Physiopathological role. Frontiers in Neuroendocrinology, 56, 100804. https://doi.org/10.1016/j.yfrne.2019.100804
- Gildawie KR, Ryll LM, Hexter JC, Peterzell S, Valentine AA, Brenhouse HC. (2021). A two-hit adversity model in developing rats reveals sex-specific impacts on prefrontal cortex structure and behavior. Dev Cogn Neurosci. 48:100924. https://doi.org/10.1016/j.dcn.2021.100924.
- Gildawie, K. R., Ryll, L. M., Hexter, J. C., Peterzell, S., Valentine, A. A., & Brenhouse, H. C. (2021). A two- hit adversity model in developing rats reveals sex-specific impacts on PFC structure and behavior. Developmental Cognitive Neuroscience, 48, 100924. https://doi.org/10.1016/j.dcn.2021.100924
- Gildawie, K. R., Honeycutt, J. A., & Brenhouse, H. C. (2020). Region-specific effects of maternal separation on perineuronal net and parvalbumin-expressing interneuron formation in male and female rats. Neuroscience, 428, 23–37. https://doi.org/10.1016/j.neuroscience.2019.12.010
- Gillies, G. E., & McArthur, S. (2010). Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacological Reviews, 62(2), 155–198. https://doi.org/10.1124/pr.109.002071
- Giridharan, V. V., Réus, G. Z., Selvaraj, S., Scaini, G., Barichello, T., & Quevedo, J. (2019). Maternal deprivation increases microglial activation and neuroinflammatory markers in the PFC and hippocampus of infant rats. Journal of Psychiatric Research, 115, 13–20. https://doi.org/10.1016/j.jpsychires.2019.05.001
- Gould, E., Woolley, C. S., Frankfurt, M., & McEwen, B. S. (1990). Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. The Journal of Neuroscience, 10(4), 1286–1291. https://doi.org/10.1523/JNEUROSCI.10-04-01286.1990
- Guadagno, A., Verlezza, S., Long, H., Wong, T. P., & Walker, C.-D. (2020). It is all in the right amygdala: Increased synaptic plasticity and perineuronal nets in male, but not female, juvenile rat pups after exposure to early-life stress. The Journal of Neuroscience, 40(43), 8276–8291. https://doi.org/10.1523/JNEUROSCI.1029-20.2020
- Guennoun, R. (2020). Progesterone in the brain: Hormone, neurosteroid and neuroprotectant. International Journal of Molecular Sciences, 21(15), 5271. https://doi.org/10.3390/ijms21155271
- Hatanaka, Y., Hojo, Y., Mukai, H., Murakami, G., Komatsuzaki, Y., Kim, J., Ikeda, M., Hiragushi, A., Kimoto, T., & Kawato, S. (2015). Rapid increase of spines by dihydrotestosterone and testosterone in hippocampal neurons: Dependence on synaptic androgen receptor and kinase networks. Brain Research, 1621, 121–132. https://doi.org/10.1016/j.brainres.2014.12.011
- Heitzer, M. D., Wolf, I. M., Sanchez, E. R., Witchel, S. F., & DeFranco, D. B. (2007). Glucocorticoid receptor physiology. Reviews in Endocrine & Metabolic Disorders, 8(4), 321–330. https://doi.org/10.1007/s11154-007-9059-8
- Hellwig, S., Brioschi, S., Dieni, S., Frings, L., Masuch, A., Blank, T., & Biber, K. (2016). Altered microglia morphology and higher resilience to stress-induced depression-like behavior in CX3CR1-deficient mice. Brain, behavior, and immunity, 55, 126–137. https://doi.org/10.1016/j.bbi.2015.11.008
- Hernández-Vivanco, A., Cano-Adamuz, N., Sánchez-Aguilera, A., González-Alonso, A., Rodríguez-Fernández, A., Azcoitia, Í., de la Prida, L. M., & Méndez, P. (2022). Sex-specific regulation of inhibition and network activity by local aromatase in the mouse hippocampus. Nature Communications, 13(1), 3913. https://doi.org/10.1038/s41467-022-31635-3
- Hiipakka, R. A., & Liao, S. (1998). Molecular mechanism of androgen action. Trends in Endocrinology and Metabolism, 9(8), 317–324. https://doi.org/10.1016/s1043-2760(98)00081-2
- Hinwood, M., Morandini, J., Day, T. A., & Walker, F. R. (2012). Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial PFC. Cerebral Cortex, 22(6), 1442–1454. https://doi.org/10.1093/cercor/bhr229
- Hinwood, M., Tynan, R. J., Charnley, J. L., Beynon, S. B., Day, T. A., & Walker, F. R. (2013). Chronic stress induced remodeling of the prefrontal: Structural re-organization of microglia and the inhibitory effect of minocycline. Cerebral Cortex, 23(8), 1784–1797. https://doi.org/10.1093/cercor/bhs151
- Hoeijmakers, L., Ruigrok, S. R., Amelianchik, A., Ivan, D., van Dam, A.-M., Lucassen, P. J., & Korosi, A. (2017). Early-life stress lastingly alters the neuroinflammatory response to amyloid pathology in an Alzheimer’s disease mouse model. Brain, Behavior, and Immunity, 63, 160–175. https://doi.org/10.1016/j.bbi.2016.12.023
- Hojo, Y., & Kawato, S. (2018). Neurosteroids in adult hippocampus of male and female rodents: Biosynthesis and actions of sex steroids. Frontiers in Endocrinology, 9, 183. https://doi.org/10.3389/fendo.2018.00183
- Hokenson, R. E., Alam, Y. H., Short, A. K., Jung, S., Jang, C., & Baram, T. Z. (2022). Sex-dependent effects of multiple acute concurrent stresses on memory: A role for hippocampal estrogens. Frontiers in Behavioral Neuroscience, 16, 984494. https://doi.org/10.3389/fnbeh.2022.984494
- Hokenson, R. E., Short, A. K., Chen, Y., Pham, A. L., Adams, E. T., Bolton, J. L., Swarup, V., Gall, C. M., & Baram, T. Z. (2021). Unexpected role of physiological estrogen in acute stress-induced memory deficits. The Journal of Neuroscience. 41(4), 648–662. https://doi.org/10.1523/JNEUROSCI.2146-20.2020
- Holst, J. P., Soldin, O. P., Guo, T., & Soldin, S. J. (2004). Steroid hormones: Relevance and measurement in the clinical laboratory. Clinics in Laboratory Medicine, 24(1), 105–118. https://doi.org/10.1016/j.cll.2004.01.004
- Horchar, M. J., & Wohleb, E. S. (2019). Glucocorticoid receptor antagonism prevents microglia-mediated neuronal remodeling and behavioral despair following chronic unpredictable stress. Brain, Behavior, and Immunity, 81, 329–340. https://doi.org/10.1016/j.bbi.2019.06.030
- Im, A., & Appleman, L. J. (2010). Mifepristone: Pharmacology and clinical impact in reproductive medicine, endocrinology and oncology. Expert Opinion on Pharmacotherapy, 11(3), 481–488. https://doi.org/10.1517/14656560903535880
- Jin, Y., & Penning, T. M. (2001). Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: Key enzymes in androgen metabolism. Best Practice & Research. Clinical Endocrinology & Metabolism, 15(1), 79–94. https://doi.org/10.1053/beem.2001.0120
- Jolivel, V., Brun, S., Binamé, F., Benyounes, J., Taleb, O., Bagnard, D., De Sèze, J., Patte-Mensah, C., & Mensah-Nyagan, A.-G. (2021). Microglial cell morphology and phagocytic activity are critically regulated by the neurosteroid allopregnanolone: A possible role in neuroprotection. Cells, 10(3), 698. https://doi.org/10.3390/cells10030698
- Katahira, T., Miyazaki, N., & Motoyama, J. (2018). Immediate effects of maternal separation on the development of interneurons derived from medial ganglionic eminence in the neonatal mouse hippocampus. Development, Growth & Differentiation, 60(5), 278–290. https://doi.org/10.1111/dgd.12540
- Kehoe, P., Mallinson, K., McCormick, C. M., & Frye, C. A. (2000). Central allopregnanolone is increased in rat pups in response to repeated, short episodes of neonatal isolation. Developmental Brain Research, 124(1-2), 133–136. https://doi.org/10.1016/S0165-3806(00)00106-1
- Kettenmann, H., Hanisch, U.-K., Noda, M., & Verkhratsky, A. (2011). Physiology of microglia. Physiological Reviews, 91(2), 461–553. https://doi.org/10.1152/physrev.00011.2010
- Kiecolt-Glaser, J. K., Glaser, R., Gravenstein, S., Malarkey, W. B., & Sheridan, J. (1996). Chronic stress alters the immune response to influenza virus vaccine in older adults. Proceedings of the National Academy of Sciences, 93(7), 3043–3047. https://doi.org/10.1073/pnas.93.7.3043
- Kim, E. J., Pellman, B., & Kim, J. J. (2015). Stress effects on the hippocampus: A critical review. Learning & Memory, 22(9), 411–416. https://doi.org/10.1101/lm.037291.114
- Kimoto, T, Tsurugizawa, T, Ohta, Y, Makino, J, Hojo, Y, Takata, N, Kawato, S., & Ho, S. (2001). Neurosteroid synthesis by cytochrome p450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium- dependent synthesis. Endocrinology, 142(8), 3578–3589. https://doi.org/10.1210/endo.142.8.8327
- Klimczak, P., Rizzo, A., Castillo-Gómez, E., Perez-Rando, M., Gramuntell, Y., Beltran, M., & Nacher, J. (2021). Parvalbumin interneurons and perineuronal nets in the hippocampus and retrosplenial cortex of adult male mice after early social isolation stress and perinatal NMDA receptor antagonist treatment. Frontiers in Synaptic Neuroscience, 13, 733989. https://doi.org/10.3389/fnsyn.2021.733989
- Kojo, A., Yamada, K., Kubo, K. Y., Yamashita, A., & Yamamoto, T. (2010). Occlusal disharmony in mice transiently activates microglia in hippocampal CA1 region but not in dentate gyrus. The Tohoku Journal of Experimental Medicine, 221(3), 237–243. https://doi.org/10.1620/tjem.221.237
- Kontula, K., Paavonen, T., Luukkainen, T., & Andersson, L. C. (1983). Binding of progestins to the glucocorticoid receptor. Correlation to their glucocorticoid-like effects on in vitro functions of human mononuclear leukocytes. Biochemical Pharmacology, 32(9), 1511–1518. https://doi.org/10.1016/0006-2952(83)90474-4
- Koritz, S. B., & Kumar, A. M. (1970). On the mechanism of action of the adrenocorticotrophic hormone. The stimulation of the activity of enzymes involved in pregnenolone synthesis. The Journal of Biological Chemistry, 245(1), 152–159. https://doi.org/10.1016/S0021-9258(18)63433-7
- Kraus, K. L., Chordia, A. P., Drake, A. W., Herman, J. P., & Danzer, S. C. (2022). Hippocampal interneurons are direct targets for circulating glucocorticoids. The Journal of Comparative Neurology, 530(12), 2100–2112. https://doi.org/10.1002/cne.25322
- Kvarta, M. D., Bradbrook, K. E., Dantrassy, H. M., Bailey, A. M., & Thompson, S. M. (2015). Corticosterone mediates the synaptic and behavioral effects of chronic stress at rat hippocampal temporoammonic synapses. Journal of Neurophysiology, 114(3), 1713–1724. https://doi.org/10.1152/jn.00359.2015
- Laham, B. J., & Gould, E. (2022). How stress influences the dynamic plasticity of the brain’s extracellular matrix. Frontiers in Cellular Neuroscience, 15, 814287. https://doi.org/10.3389/fncel.2021.814287
- Laham, B. J., Murthy, S. S., Hanani, M., Clappier, M., Boyer, S., Vasquez, B., & Gould, E. (2022). The estrous cycle modulates early-life adversity effects on mouse avoidance behavior through progesterone signaling. Nature Communications, 13(1), 7537. https://doi.org/10.1038/s41467-022-35068-w
- Lee, J., & Lee, K. (2021). Parvalbumin-expressing GABAergic interneurons and perineuronal nets in the prelimbic and orbitofrontal cortices in association with basal anxiety-like behaviors in adult mice. Behavioural Brain Research, 398, 112915. https://doi.org/10.1016/j.bbr.2020.112915
- Li, L. F., Yang, J., Ma, S. P., & Qu, R. (2013). Magnolol treatment reversed the glial pathology in an unpredictable chronic mild stress-induced rat model of depression. European Journal of Pharmacology, 711(1-3), 42–49. https://doi.org/10.1016/j.ejphar.2013.04.008
- Liu, Q., Li, B., Zhu, H.-Y., Wang, Y.-Q., Yu, J., & Wu, G.-C. (2009). Clomipramine treatment reversed the glial pathology in a chronic unpredictable stress-induced rat model of depression. European Neuropsychopharmacology, 19(11), 796–805. https://doi.org/10.1016/j.euroneuro.2009.06.010
- Liu, Q., Li, B., Zhu, H.-Y., Wang, Y.-Q., Yu, J., & Wu, G.-C. (2011). Glia atrophy in the hippocampus of chronic unpredictable stress-induced depression model rats is reversed by electroacupuncture treatment. Journal of Affective Disorders, 128(3), 309–313. https://doi.org/10.1016/j.jad.2010.07.007
- Liu, R., Yang, X. D., Liao, X. M., Xie, X. M., Su, Y. A., Li, J. T., Wang, X. D., & Si, T. M. (2016). Early postnatal stress suppresses the developmental trajectory of hippocampal pyramidal neurons: The role of CRHR1. Brain Structure & Function, 221(9), 4525–4536. https://doi.org/10.1007/s00429-016-1182-4
- Lloyd-Evans, E., & Waller-Evans, H. (2020). Biosynthesis and signalling functions of central and peripheral nervous system neurosteroids in health and disease. Essays in Biochemistry, 64(3), 591–606. https://doi.org/10.1042/EBC20200043
- López-Calderón, A., Gonzaléz-Quijano, M. I., Tresguerres, J. A., & Ariznavarreta, C. (1990). Role of LHRH in the gonadotrophin response to restraint stress in intact male rats. The Journal of Endocrinology, 124(2), 241–246. https://doi.org/10.1677/joe.0.1240241
- Lou, Y. X., Li, J., Wang, Z. Z., Xia, C. Y., & Chen, N. H. (2018). Glucocorticoid receptor activation induces decrease of hippocampal astrocyte number in rats. Psychopharmacology, 235(9), 2529–2540. https://doi.org/10.1007/s00213-018-4936-2
- Luine, V., Villegas, M., Martinez, C., & McEwen, B. S. (1994). Repeated stress causes reversible impairments of spatial memory performance. Brain Research, 639(1), 167–170. https://doi.org/10.1016/0006-8993(94)91778-7
- MacLusky, N. J., Walters, M. J., Clark, A. S., & Toran-Allerand, C. D. (1994). Aromatase in the cerebral cortex, hippocampus, and mid-brain: Ontogeny and developmental implications. Molecular and Cellular Neurosciences, 5(6), 691–698. https://doi.org/10.1006/mcne.1994.1083
- Manna, P. R., Cohen-Tannoudji, J., Counis, R., Garner, C. W., Huhtaniemi, I., Kraemer, F. B., & Stocco, D. M. (2013). Mechanisms of action of hormone-sensitive lipase in mouse Leydig cells: Its role in the regulation of the steroidogenic acute regulatory protein. The Journal of Biological Chemistry, 288(12), 8505–8518. https://doi.org/10.1074/jbc.M112.417873
- Mataradze, G. D., Kurabekova, R. M., & Rozen, V. B. (1992). The role of sex steroids in the formation of sex-differentiated concentrations of corticosteroid-binding globulin in rats. The Journal of Endocrinology, 132(2), 235–240. https://doi.org/10.1677/joe.0.1320235
- McEwen, B. (2002). Estrogen actions throughout the brain. Recent Progress in Hormone Research, 57(1), 357–384. https://doi.org/10.1210/rp.57.1.357
- McEwen, B. S., Nasca, C., & Gray, J. D. (2016). Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41(1), 3–23. https://doi.org/10.1038/npp.2015.171
- Meffre, D., Pianos, A., Liere, P., Eychenne, B., Cambourg, A., Schumacher, M., Stein, D. G., & Guennoun, R. (2007). Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: Analysis by gas chromatography/mass spectrometry. Endocrinology, 148(5), 2505–2517. https://doi.org/10.1210/en.2006-1678
- Mellon, S. H., & Griffin, L. D. (2002). Neurosteroids: Biochemistry and clinical significance. Trends in Endocrinology and Metabolism, 13(1), 35–43. https://doi.org/10.1016/s1043-2760(01)00503-3
- Miller, W. L., & Bose, H. S. (2011). Early steps in steroidogenesis: Intracellular cholesterol trafficking. Journal of Lipid Research, 52(12), 2111–2135. https://doi.org/10.1194/jlr.R016675
- Mirescu, C., Peters, J. D., & Gould, E. (2004). Early life experience alters response of adult neurogenesis to stress. Nature Neuroscience, 7(8), 841–846. https://doi.org/10.1038/nn1290
- Mitra, R., Jadhav, S., McEwen, B. S., Vyas, A., & Chattarji, S. (2005). Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences, 102(26), 9371–9376. https://doi.org/10.1073/pnas.0504011102
- Miyaso, H., Nagahori, K., Takano, K., Omotehara, T., Kawata, S., Li, Z. L., Kuramasu, M., Wu, X., Ogawa, Y., & Itoh, M. (2021). Neonatal maternal separation causes decreased numbers of sertoli cell, spermatogenic cells, and sperm in mice. Toxicology Mechanisms and Methods, 31(2), 116–125. https://doi.org/10.1080/15376516.2020.1841865
- Moisan, M.-P. (2021). Sexual dimorphism in glucocorticoid stress response. International Journal of Molecular Sciences, 22(6), 3139. https://doi.org/10.3390/ijms22063139
- Monroy, E., Hernández-Torres, E., & Flores, G. (2010). Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. Journal of Chemical Neuroanatomy, 40(2), 93–101. https://doi.org/10.1016/j.jchemneu.2010.05.005
- Murphy-Royal, C., Gordon, G. R., & Bains, J. S. (2019). Stress-induced structural and functional modifications of astrocytes—Further implicating glia in the central response to stress. Glia, 67(10), 1806–1820. https://doi.org/10.1002/glia.23610
- Murthy, S., Kane, G. A., Katchur, N. J., Lara Mejia, P. S., Obiofuma, G., Buschman, T. J., McEwen, B. S., & Gould, E. (2019). Perineuronal nets, inhibitory interneurons, and anxiety-related ventral hippocampal neuronal oscillations are altered by early life adversity. Biological Psychiatry, 85(12), 1011–1020. https://doi.org/10.1016/j.biopsych.2019.02.021
- Musholt, K., Cirillo, G., Cavaliere, C., Rosaria Bianco, M., Bock, J., Helmeke, C., Braun, K., & Papa, M. (2009). Neonatal separation stress reduces glial fibrillary acidic protein- and S100beta-immunoreactive astrocytes in the rat medial precentral cortex. Developmental Neurobiology, 69(4), 203–211. https://doi.org/10.1002/dneu.20694
- Muthu, S. J., Lakshmanan, G., Shimray, K. W., Kaliyappan, K., Sathyanathan, S. B., & Seppan, P. (2022). Testosterone influence on microtubule-associated proteins and spine density in hippocampus: Implications on learning and memory. Developmental Neuroscience, 44(6), 498–507. https://doi.org/10.1159/000525038
- Nair, A., & Bonneau, R. H. (2006). Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. Journal of Neuroimmunology, 171(1-2), 72–85. https://doi.org/10.1016/j.jneuroim.2005.09.012
- Nelson, L. R., & Bulun, S. E. (2001). Estrogen production and action. Journal of the American Academy of Dermatology, 45(3 Suppl), S116–S124. https://doi.org/10.1067/mjd.2001.117432
- Omura, T. (2006). Mitochondrial P450s. Chemico-Biological Interactions, 163(1-2), 86–93. https://doi.org/10.1016/j.cbi.2006.06.008
- Osawa, Y., Higashiyama, T., Shimizu, Y., & Yarborough, C. (1993). Multiple functions of aromatase and the active site structure; aromatase is the placental estrogen 2- hydroxylase. The Journal of Steroid Biochemistry and Molecular Biology, 44(4-6), 469–480. https://doi.org/10.1016/0960-0760(93)90252-r
- Pallarès, M., Llidó, A., Mòdol, L., Vallée, M., & Darbra, S. (2015). Finasteride administration potentiates the disruption of prepulse inhibition induced by forced swim stress. Behavioural Brain Research, 289, 55–60. https://doi.org/10.1016/j.bbr.2015.04.023
- Papadopoulos, V., Amri, H., Boujrad, N., Cascio, C., Culty, M., Garnier, M., Hardwick, M., Li, H., Vidic, B., Brown, A. S., Reversa, J. L., Bernassau, J. M., & Drieu, K. (1997). Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids, 62(1), 21–28. https://doi.org/10.1016/s0039-128x(96)00154-7
- Paris, J. J., & Frye, C. A. (2011a). Gestational exposure to variable stressors produces decrements in cognitive and neural development of juvenile male and female rats. Current Topics in Medicinal Chemistry, 11(13), 1706–1713.
- Paris, J. J., & Frye, C. A. (2011b). Juvenile offspring of rats exposed to restraint stress in late gestation have impaired cognitive performance and dysregulated progestogen formation. Stress (Amsterdam, Netherlands), 14(1), 23–32. https://doi.org/10.3109/10253890.2010.512375
- Paris, J. J., Brunton, P. J., Russell, J. A., & Frye, C. A. (2011). Immune stress in late pregnant rats decreases length of gestation and fecundity, and alters later cognitive and affective behaviour of surviving pre-adolescent offspring. Stress, 14(6), 652–664. https://doi.org/10.3109/10253890.2011.628719
- Park, J. H., Yoo, K.-Y., Lee, C. H., Kim, I. H., Shin, B. N., Choi, J. H., Park, J. H., Hwang, I. K., & Won, M.-H. (2011). Comparison of glucocorticoid receptor and ionized calcium-binding adapter molecule 1 immunoreactivity in the adult and aged gerbil hippocampus following repeated restraint stress. Neurochemical Research, 36(6), 1037–1045. https://doi.org/10.1007/s11064-011-0444-z
- Penning, T. M., Jin, Y., Steckelbroeck, S., Lanisnik Rizner, T., & Lewis, M. (2004). Structure-function of human 3 alpha-hydroxysteroid dehydrogenases: genes and proteins. Molecular and cellular endocrinology, 215(1-2), 63–72. https://doi.org/10.1016/j.mce.2003.11.006
- Pesaresi, M., Maschi, O., Giatti, S., Garcia-Segura, L. M., Caruso, D., & Melcangi, R. C. (2010). Sex differences in neuroactive steroid levels in the nervous system of diabetic and non-diabetic rats. Hormones and Behavior, 57(1), 46–55. https://doi.org/10.1016/j.yhbeh.2009.04.008
- Pinna, G., Agis-Balboa, R. C., Doueiri, M.-S., Guidotti, A., & Costa, E. (2004). Brain neurosteroids in gender-related aggression induced by social isolation. Critical Reviews in Neurobiology, 16(1-2), 75–82.
- Porcu, P., Barron, A. M., Frye, C. A., Walf, A. A., Yang, S.-Y., He, X.-Y., Morrow, A. L., Panzica, G. C., & Melcangi, R. C. (2016). Neurosteroidogenesis today: Novel targets for neuroactive steroid synthesis and action and their relevance for translational research. Journal of Neuroendocrinology, 28(2), 12351. https://doi.org/10.1111/jne.12351
- Puia, G., Mienville, J. M., Matsumoto, K., Takahata, H., Watanabe, H., Costa, E., & Guidotti, A. (2003). On the putative physiological role of allopregnanolone on GABAA receptor function. Neuropharmacology, 44(1), 49–55. https://doi.org/10.1016/S0028-3908(02)00341-6
- Purdy, R. H., Morrow, A. L., Moore, P. H., & Paul, S. M. (1991). Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proceedings of the National Academy of Sciences, 88(10), 4553–4557. https://doi.org/10.1073/pnas.88.10.4553
- Raff, H. (2016). CORT, Cort, B, Corticosterone, and now cortistatin: Enough already!. Endocrinology, 157(9), 3307–3308. https://doi.org/10.1210/en.2016-1500
- Reddy, D. S., & Jian, K. (2010). The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. The Journal of Pharmacology and Experimental Therapeutics, 334(3), 1031–1041. https://doi.org/10.1124/jpet.110.169854
- Reddy, D. S., & Rogawski, M. A. (2002). Stress-induced deoxycorticosterone-derived neurosteroids modulate GABAA receptor function and seizure susceptibility. The Journal of Neuroscience, 22(9), 3795–3805. https://doi.org/10.1523/JNEUROSCI.22-09-03795.2002
- Reschke-Hernández, A. E., Okerstrom, K. L., Bowles Edwards, A., & Tranel, D. (2017). Sex and stress: Men and women show different cortisol responses to psychological stress induced by the Trier social stress test and the Iowa singing social stress test. Journal of Neuroscience Research, 95(1-2), 106–114. https://doi.org/10.1002/jnr.23851
- Retana-Márquez, S., Bonilla-Jaime, H., Vázquez-Palacios, G., Martínez-García, R., & Velázquez-Moctezuma, J. (2003). Changes in masculine sexual behavior, corticosterone and testosterone in response to acute and chronic stress in male rats. Hormones and Behavior, 44(4), 327–337. https://doi.org/10.1016/j.yhbeh.2003.04.001
- Réus, G. Z., Fernandes, G. C., de Moura, A. B., Silva, R. H., Darabas, A. C., de Souza, T. G., Abelaira, H. M., Carneiro, C., Wendhausen, D., Michels, M., Pescador, B., Dal-Pizzol, F., Macêdo, D. S., & Quevedo, J. (2017). Early life experience contributes to the developmental programming of depressive-like behaviour, neuroinflammation and oxidative stress. Journal of Psychiatric Research, 95, 196–207. https://doi.org/10.1016/j.jpsychires.2017.08.020
- Réus, G. Z., Giridharan, V. V., de Moura, A. B., Borba, L. A., Botelho, M. E. M., Behenck, J. P., Generoso, J. S., Selvaraj, S., Bhatti, G., Barichello, T., & Quevedo, J. (2021). The impact of early life stress and immune challenge on behavior and glia cells alteration in late adolescent rats. International Journal of Developmental Neuroscience, 81(5), 407–415. https://doi.org/10.1002/jdn.10108
- Réus, G. Z., Silva, R. H., de Moura, A. B., Presa, J. F., Abelaira, H. M., Abatti, M., Vieira, A., Pescador, B., Michels, M., Ignácio, Z. M., Dal-Pizzol, F., & Quevedo, J. (2019). Early maternal deprivation induces microglial activation, alters glial fibrillary acidic protein immunoreactivity and indoleamine 2,3-dioxygenase during the development of offspring rats. Molecular Neurobiology, 56(2), 1096–1108. https://doi.org/10.1007/s12035-018-1161-2
- Riga, D., Kramvis, I., Koskinen, M. K., van Bokhoven, P., van der Harst, J. E., Heistek, T. S., Jaap Timmerman, A., van Nierop, P., van der Schors, R. C., Pieneman, A. W., de Weger, A., van Mourik, Y., Schoffelmeer, A. N. M., Mansvelder, H. D., Meredith, R. M., Hoogendijk, W. J. G., Smit, A. B., & Spijker, S. (2017). Hippocampal extracellular matrix alterations contribute to cognitive impairment associated with a chronic depressive-like state in rats. Science Translational Medicine, 9(421), eaai8753. https://doi.org/10.1126/scitranslmed.aai8753
- Rochefort, H., & Garcia, M. (1976). Androgen on the estrogen receptor. I—binding and in vivo nuclear translocation. Steroids, 28(4), 549–560. https://doi.org/10.1016/0039-128x(76)90023-4
- Romeo, R. D., Karatsoreos, I. N., Ali, F. S., & McEwen, B. S. (2007). The effects of acute stress and pubertal development on metabolic hormones in the rat. Stress (Amsterdam, Netherlands), 10(1), 101–106. https://doi.org/10.1080/10253890701204270
- Römer, B., & Gass, P. (2010). Finasteride‐induced depression: New insights into possible pathomechanisms. Journal of Cosmetic Dermatology, 9(4), 331–332. https://doi.org/10.1111/j.1473-2165.2010.00533.x
- Römer, B., Pfeiffer, N., Lewicka, S., Ben-Abdallah, N., Vogt, M. A., Deuschle, M., Vollmayr, B., & Gass, P. (2010). Finasteride treatment inhibits adult hippocampal neurogenesis in male mice. Pharmacopsychiatry, 43(5), 174–178. https://doi.org/10.1055/s-0030-1249095
- Rone, M. B., Fan, J., & Papadopoulos, V. (2009). Cholesterol transport in steroid biosynthesis: Role of protein-protein interactions and implications in disease states. Biochimica et Biophysica Acta, 1791(7), 646–658. https://doi.org/10.1016/j.bbalip.2009.03.001
- Roque, A., Ochoa-Zarzosa, A., & Torner, L. (2016). Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain, Behavior, and Immunity, 55, 39–48. https://doi.org/10.1016/j.bbi.2015.09.017
- Rosol, T. J., Yarrington, J. T., Latendresse, J., & Capen, C. C. (2001). Adrenal gland: Structure, function, and mechanisms of toxicity. Toxicologic Pathology, 29(1), 41–48. https://doi.org/10.1080/019262301301418847
- Rubinow, K. B. (2018). An intracrine view of sex steroids, immunity, and metabolic regulation. Molecular Metabolism, 15, 92–103. https://doi.org/10.1016/j.molmet.2018.03.001
- Saavedra, L. M., Fenton Navarro, B., & Torner, L. (2017). Early life stress activates glial cells in the hippocampus but attenuates cytokine secretion in response to an immune challenge in rat pups. Neuroimmunomodulation, 24(4-5), 242–255. https://doi.org/10.1159/000485383
- Saldanha, C. J., Remage-Healey, L., & Schlinger, B. A. (2011). Synaptocrine signaling: Steroid synthesis and action at the synapse. Endocrine Reviews, 32(4), 532–549. https://doi.org/10.1210/er.2011-0004
- Santiago, A. N., Lim, K. Y., Opendak, M., Sullivan, R. M., & Aoki, C. (2018). Early life trauma increases threat response of peri‐weaning rats, reduction of axo‐somatic synapses formed by parvalbumin cells and perineuronal net in the basolateral nucleus of amygdala. The Journal of Comparative Neurology, 526(16), 2647–2664. https://doi.org/10.1002/cne.24522
- Saur, L., Baptista, P. P. A., Bagatini, P. B., Neves, L. T., de Oliveira, R. M., Vaz, S. P., Ferreira, K., Machado, S. A., Mestriner, R. G., & Xavier, L. L. (2016). Experimental post-traumatic stress disorder decreases astrocyte density and changes astrocytic polarity in the CA1 hippocampus of male rats. Neurochemical Research, 41(4), 892–904. https://doi.org/10.1007/s11064-015-1770-3
- Schiavone, S., Sorce, S., Dubois-Dauphin, M., Jaquet, V., Colaianna, M., Zotti, M., Cuomo, V., Trabace, L., & Krause, K.-H. (2009). Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biological Psychiatry, 66(4), 384–392. https://doi.org/10.1016/j.biopsych.2009.04.033
- Schverer, M., Lanfumey, L., Baulieu, E.-E., Froger, N., & Villey, I. (2018). Neurosteroids: Non-genomic pathways in neuroplasticity and involvement in neurological diseases. Pharmacology & Therapeutics, 191, 190–206. https://doi.org/10.1016/j.pharmthera.2018.06.011
- Serra, M., Pisu, M. G., Floris, I., & Biggio, G. (2005). Social isolation-induced changes in the hypothalamic–pituitary–adrenal axis in the rat. Stress, 8(4), 259–264. https://doi.org/10.1080/10253890500495244
- Serra, M., Pisu, M. G., Littera, M., Papi, G., Sanna, E., Tuveri, F., Usala, L., Purdy, R. H., & Biggio, G. (2000). Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. Journal of Neurochemistry, 75(2), 732–740. https://doi.org/10.1046/j.1471-4159.2000.0750732.x
- Shansky, R. M., Hamo, C., Hof, P. R., Lou, W., McEwen, B. S., & Morrison, J. H. (2010). Estrogen promotes stress sensitivity in a PFC–amygdala pathway. Cerebral Cortex, 20(11), 2560–2567. https://doi.org/10.1093/cercor/bhq003
- Shimizu, H., Ishizuka, Y., Yamazaki, H., & Shirao, T. (2015). Allopregnanolone increases mature excitatory synapses along dendrites via protein kinase A signaling. Neuroscience, 305, 139–145. https://doi.org/10.1016/j.neuroscience.2015.07.079
- Shirayama, Y., Muneoka, K., Fukumoto, M., Tadokoro, S., Fukami, G., Hashimoto, K., & Iyo, M. (2011). Infusions of allopregnanolone into the hippocampus and amygdala, but not into the nucleus accumbens and medial PFC, produce antidepressant effects on the learned helplessness rats. Hippocampus, 21(10), 1105–1113. https://doi.org/10.1002/hipo.20824
- Shors, T. J., Chua, C., & Falduto, J. (2001). Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience, 21(16), 6292–6297. https://doi.org/10.1523/JNEUROSCI.21-16-06292.2001
- Shors, T. J., Chua, C., & Falduto, J. (2001). Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. The Journal of Neuroscience, 21(16), 6292–6297. https://doi.org/10.1523/JNEUROSCI.21-16-06292.2001
- Shors, T. J., Pickett, J., Wood, G., & Paczynski, M. (1999). Acute stress persistently enhances estrogen levels in the female rat. Stress, 3(2), 163–171. https://doi.org/10.3109/10253899909001120
- Simard, J., Ricketts, M.-L., Gingras, S., Soucy, P., Feltus, F. A., & Melner, M. H. (2005). Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocrine Reviews, 26(4), 525–582. https://doi.org/10.1210/er.2002-0050
- Smail, M. A., Smith, B. L., Shukla, R., Alganem, K., Eby, H. M., Bollinger, J. L., Parikh, R. K., Chambers, J. B., Reigle, J. K., Moloney, R. D., Nawreen, N., Wohleb, E. S., Pantazopoulos, H., McCullumsmith, R. E., & Herman, J. P. (2023). Molecular neurobiology of loss: A role for basolateral amygdala extracellular matrix. Molecular Psychiatry. https://doi.org/10.1038/s41380-023-02231-8
- Sorg, B. A., Berretta, S., Blacktop, J. M., Fawcett, J. W., Kitagawa, H., Kwok, J. C., & Miquel, M. (2016). Casting a wide net: Role of perineuronal nets in neural plasticity. The Journal of Neuroscience, 36(45), 11459–11468. https://doi.org/10.1523/JNEUROSCI.2351-16.2016
- Starling, E. H. (1905). The Croonian lectures on the chemical correlation of the functions of the body. Lancet, 166(4275), 339–341.
- Stell, B. M., Brickley, S. G., Tang, C. Y., Farrant, M., & Mody, I. (2003). Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proceedings of the National Academy of Sciences, 100(24), 14439–14444. https://doi.org/10.1073/pnas.2435457100
- Stocco, D. M., Wang, X., Jo, Y., & Manna, P. R. (2005). Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: More complicated than we thought. Molecular Endocrinology, 19(11), 2647–2659. https://doi.org/10.1210/me.2004-0532
- Stone, D., & Hechter, O. (1955). Studies on ACTH action in perfused bovine adrenals: Aspects of progesterone as an intermediary in corticosteroidogenesis. Archives of Biochemistry and Biophysics, 54(1), 121–128. https://doi.org/10.1016/0003-9861(55)90014-x
- Sugama, S., Fujita, M., Hashimoto, M., & Conti, B. (2007). Stress induced morphological microglial activation in the rodent brain: Involvement of interleukin-18. Neuroscience, 146(3), 1388–1399. https://doi.org/10.1016/j.neuroscience.2007.02.043
- Sze, Y., Gill, A. C., & Brunton, P. J. (2018). Sex-dependent changes in neuroactive steroid concentrations in the rat brain following acute swim stress. Journal of Neuroendocrinology, 30(11), e12644. https://doi.org/10.1111/jne.12644
- Takatsuru, Y., Nabekura, J., Ishikawa, T., Kohsaka, S., & Koibuchi, N. (2015). Early-life stress increases the motility of microglia in adulthood. The Journal of Physiological Sciences: JPS, 65(2), 187–194. https://doi.org/10.1007/s12576-015-0361-z
- Tata, J. R. (2005). One hundred years of hormones: A new name sparked multidisciplinary research in endocrinology, which shed light on chemical communication in multicellular organisms. EMBO Reports, 6(6), 490–496. https://doi.org/10.1038/sj.embor.7400444
- Tay, T. L., Béchade, C., D’Andrea, I., St-Pierre, M.-K., Henry, M. S., Roumier, A., & Tremblay, M.-E. (2018). Microglia gone rogue: Impacts on psychiatric disorders across the lifespan. Frontiers in Molecular Neuroscience, 10, 421. https://doi.org/10.3389/fnmol.2017.00421
- Tertil, M., Skupio, U., Barut, J., Dubovyk, V., Wawrzczak-Bargiela, A., Soltys, Z., Golda, S., Kudla, L., Wiktorowska, L., Szklarczyk, K., Korostynski, M., Przewlocki, R., & Slezak, M. (2018). Glucocorticoid receptor signaling in astrocytes is required for aversive memory formation. Translational Psychiatry, 8(1), 255. https://doi.org/10.1038/s41398-018-0300-x
- Tsuda, M. C., Yamaguchi, N., & Ogawa, S. (2011). Early life stress disrupts peripubertal development of aggression in male mice. Neuroreport, 22(6):, 259–263. https://doi.org/10.1097/WNR.0b013e328344495a
- Tyagi, V., Scordo, M., Yoon, R. S., Liporace, F. A., & Greene, L. W. (2017). Revisiting the role of testosterone: Are we missing something? Reviews in Urology, 19(1), 16–24.
- Tynan, R. J., Beynon, S. B., Hinwood, M., Johnson, S. J., Nilsson, M., Woods, J. J., & Walker, F. R. (2013). Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathologica, 126(1), 75–91. https://doi.org/10.1007/s00401-013-1102-0
- Tynan, R. J., Naicker, S., Hinwood, M., Nalivaiko, E., Buller, K. M., Pow, D. V., Day, T. A., & Walker, F. R. (2010). Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain, Behavior, and Immunity, 24(7), 1058–1068. https://doi.org/10.1016/j.bbi.2010.02.001
- Ueno, H., Suemitsu, S., Murakami, S., Kitamura, N., Wani, K., Matsumoto, Y., Okamoto, M., Aoki, S., & Ishihara, T. (2018). Juvenile stress induces behavioral change and affects perineuronal net formation in juvenile mice. BMC Neuroscience, 19(1), 41. https://doi.org/10.1186/s12868-018-0442-z
- Vallée, M., Rivera, J. D., Koob, G. F., Purdy, R. H., & Fitzgerald, R. L. (2000). Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Analytical Biochemistry, 287(1), 153–166. https://doi.org/10.1006/abio.2000.4841
- Vallée, M., Vitiello, S., Bellocchio, L., Hébert-Chatelain, E., Monlezun, S., Martin-Garcia, E., Kasanetz, F., Baillie, G. L., Panin, F., Cathala, A., Roullot-Lacarrière, V., Fabre, S., Hurst, D. P., Lynch, D. L., Shore, D. M., Deroche-Gamonet, V., Spampinato, U., Revest, J. M., Maldonado, R., … Piazza, P. V. (2014). Pregnenolone can protect the brain from cannabis intoxication. Science, 343(6166), 94–98. https://doi.org/10.1126/science.1243985
- Van Belle, S. (2017). Cortisol and other glucocorticoids. In Agustín Fuentes (Ed.), The international encyclopedia of primatology (pp. 1-5). Hoboken, NJ: Wiley.
- Walf, A. A., & Frye, C. A. (2012). Gestational or acute restraint in adulthood reduces levels of 5α-reduced testosterone metabolites in the hippocampus and produces behavioral inhibition of adult male rats. Frontiers in Cellular Neuroscience, 6, 40. https://doi.org/10.3389/fncel.2012.00040
- Wang, M. (2011). Neurosteroids and GABA-A receptor function. Frontiers in Endocrinology, 2, 44. https://doi.org/10.3389/fendo.2011.00044
- Watanabe, Y., Gould, E., & McEwen, B. S. (1992). Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research, 588(2), 341–345. https://doi.org/10.1016/0006-8993(92)91597-8
- Wieland, S., Lan, N. C., Mirasedeghi, S., & Gee, K. W. (1991). Anxiolytic activity of the progesterone metabolite 5 alpha-pregnan-3 alpha-o1-20-one. Brain Research, 565(2), 263–268. https://doi.org/10.1016/0006-8993(91)91658-n
- Wiktorowska, L., Bilecki, W., Tertil, M., Kudla, L., Szumiec, L., Mackowiak, M., & Przewlocki, R. (2021). Knockdown of the astrocytic glucocorticoid receptor in the central nucleus of the amygdala diminishes conditioned fear expression and anxiety. Behavioural Brain Research, 402, 113095. https://doi.org/10.1016/j.bbr.2020.113095
- Wingert, J. C., & Sorg, B. A. (2021). Impact of perineuronal nets on electrophysiology of parvalbumin interneurons, principal neurons, and brain oscillations: A review. Frontiers in Synaptic Neuroscience, 13, 673210. https://doi.org/10.3389/fnsyn.2021.673210
- Wohleb, E. S., Hanke, M. L., Corona, A. W., Powell, N. D., Stiner, L. M., Bailey, M. T., Nelson, R. J., Godbout, J. P., & Sheridan, J. F. (2011). β-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. The Journal of Neuroscience, 31(17), 6277–6288. https://doi.org/10.1523/JNEUROSCI.0450-11.2011
- Woolley, C. S., Gould, E., Frankfurt, M., & McEwen, B. S. (1990). Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. The Journal of Neuroscience, 10(12), 4035–4039. https://doi.org/10.1523/JNEUROSCI.10-12-04035.1990
- Xia et al., 2013: Xia, L., Zhai, M., Wang, L., Miao, D., Zhu, X., & Wang, W. (2013). FGF2 blocks PTSD symptoms via an astrocyte-based mechanism. Behavioural brain research, 256, 472–480.
- Xu, B., Zhang, X., He, Y., Liu, C., Li, L., Liu, Q., Huang, Y., Chen, M., Ren, B., Guo, Y., & Chen, Y. (2022). The impacts of early-life adversity on striatal and hippocampal memory functions. Neuroscience, 490, 11–24. https://doi.org/10.1016/j.neuroscience.2022.02.029
- Yamawaki, Y., Nishida, M., Harada, K., & Akagi, H. (2018). Data on the effect of maternal separation coupled with social isolation in a forced swim test and gene expression of glial fibrillary acid protein in the PFC of rats. Data in Brief, 18, 496–500. https://doi.org/10.1016/j.dib.2018.03.055
- Yehuda, R., Bierer, L. M., Andrew, R., Schmeidler, J., & Seckl, J. R. (2009). Enduring effects of severe developmental adversity, including nutritional deprivation, on cortisol metabolism in aging Holocaust survivors. Journal of Psychiatric Research, 43(9), 877–883. https://doi.org/10.1016/j.jpsychires.2008.12.003
- Yirmiya, R., Rimmerman, N., & Reshef, R. (2015). Depression as a microglial disease. Trends in Neurosciences, 38(10), 637–658. https://doi.org/10.1016/j.tins.2015.08.001
- Yoshizawa, K., Okumura, A., Nakashima, K., Sato, T., & Higashi, T. (2017). Role of allopregnanolone biosynthesis in acute stress-induced anxiety-like behaviors in mice. Synapse, 71(8), e21978. https://doi.org/10.1002/syn.21978
- Yu, Z., Chen, N., Hu, D., Chen, W., Yuan, Y., Meng, S., Zhang, W., Lu, L., Han, Y., & Shi, J. (2020). Decreased density of perineuronal net in prelimbic cortex is linked to depressive-like behavior in young-aged rats. Frontiers in Molecular Neuroscience, 13, 4. https://doi.org/10.3389/fnmol.2020.00004
- Zucker, T. P., Higashiura, K., Mathur, R. S., & Halushka, P. V. (1996). Androstenedione increases thromboxane A2 receptors in human erythroleukemia cells. Life Sciences, 58(8), 683–690. https://doi.org/10.1016/s0024-3205(96)80007-5

