ABSTRACT
Nut consumption is clearly related to human health outcomes. Its beneficial effects have been mainly attributed to nut fatty acid profiles and content of vegetable protein, fiber, vitamins, minerals, phytosterols and phenolics. However, in this review we focus on the prebiotics properties in humans of the non-bioaccessible material of nuts (polymerized polyphenols and polysaccharides), which provides substrates for the human gut microbiota and on the formation of new bioactive metabolites and the absorption of that may partly explain the health benefits of nut consumption.
Introduction
Nut intake is inversely associated with all-cause and cancer mortality, type 2 diabetes in women, and coronary heart disease and its intermediate biomarkers (Bao et al., Citation2013; Estruch et al., Citation2013; Berryman et al., Citation2015; Grosso et al., Citation2015; van den Brandt and Schouten, Citation2015). In the PREDIMED (PREvención con DIeta MEDiterránea) trial, a Mediterranean diet supplemented with olive oil or nuts was associated with a significantly greater reduction in cardiovascular events (an aggregate of fatal and nonfatal cardiovascular disease events, namely CHD and stroke), after a median of 4.8 years of follow-up, in comparison with a control diet consisting of advice to reduce all types of dietary fat (Estruch et al., Citation2013). Moreover, nut supplementation seems to exert an additional beneficial effect on stroke occurrence (Estruch et al., Citation2013; Salas-Salvadó et al., Citation2014), formation of a less atherogenic LDL subfraction (Damasceno et al., Citation2013) and incidence of metabolic syndrome than supplementation with virgin olive oil (Salas-Salvadó et al., Citation2008; Babio et al., Citation2014).
In the recent Dietary Guidelines for Americans (USDA, n.d.), the Committee concluded that a high consumption of nuts, among other healthy foods, is clearly associated with beneficial health outcomes (Dietary Guidelines for Americans, 2015). Although there is a concern about consuming nuts due to their high energy density, current data indicate that nut consumption is not related to body weight increase (Rajaram and Sabaté, Citation2006; Flores-Mateo et al., Citation2013; Tan et al., Citation2014) in certain situations. In the US, although 14.4% of men and 11.8% of women consume more than the FDA recommended daily amount of 43 g (1.5 oz) of nuts, around 60% of the population does not consume any nuts on a given day, and this proportion increases to up to 70% among Blacks and Hispanics (Nielsen et al., Citation2014).
The health benefits of nuts have been mainly attributed to their fatty acid profile, and content of vegetable protein, fiber, vitamin phytosterols and phenolics. However, the aim of the present review is to examine the current evidence supporting the notion that the beneficial dietary role of nuts is also based on their prebiotic properties. These are mainly due to high levels of dietary fiber and polymerized polyphenols, which are metabolized by gut microbiota to form bioactive molecules that can benefit the health of the human host.
Prebiotic effect
Microorganisms within the human gastrointestinal (GI) tract (approximately 100 trillion) are estimated to outnumber somatic cells within the body by tenfold. The collective genomes of these microbiome are a source of functional attributes that humans have not had to evolve themselves (Gill et al., Citation2006; Turnbaugh et al., Citation2007). As well as bacteria, gut microbial communities include yeasts, single-cell eukaryotes, viruses and small parasitic worms. The number and diversity of microbes fluctuates along the GI tract, but the majority is found within the large intestine. Human gut bacteria can be beneficial (Bifidobacterium and Lactobacillus) or harmful (Clostridia and Bacteroides) to the host. Overall, the intestinal microbiota has a great impact on human health (Slavin, Citation2013; Zhang et al., Citation2015).
The prebiotic effect is defined as “the selective stimulation of growth and/or activity(ies) of one or a limited number of microbial genus(era)/species in the gut microbiota that confer(s) health benefits to the host” (Roberfroid et al., Citation2010). There are very few studies describing the prebiotic effect of nuts and their impact on gut microflora and the metabolome. Early in vitro studies showed the prebiotic effect of chestnut extract (Blaiotta et al., Citation2013), and almonds and their skins (Mandalari et al., Citation2008). More recently, two papers (Liu et al., Citation2014; Ukhanova et al., Citation2014) have described these prebiotic properties in different intervention human clinical trials. Ukhanova et al (Ukhanova et al., Citation2014) performed two separate randomized clinical feeding studies, administering 0, 1.5 (43 g) and 3 (86 g) servings per day of almonds or pistachios to 18 and 16 healthy volunteers, respectively, for 18 d. Although the effective dose is not defined, an increase in the number of presumed butyrate producers was observed in both groups, and it was concluded that almonds and especially pistachios can affect the composition of the fecal bacterial and fungal microbiota. However, neither almond nor pistachio intake increased the numbers of Lactobacillus or Bifidobacteria. In the same year, Liu et al (Citation2014) reported a study with 48 volunteeers that were randomly assigned to three diferent intervention groups: a control group supplied with 8 g/d of fructooligosaccharides (4 g at lunch and 4 g at dinner), a group that had 10 g/d of almond skins (5 g at lunch and 5 g at dinner), and finally, a third group that had 56 g/d of roasted, unsalted, whole almonds (28 g at lunch and 28 g at dinner). After 6 weeks, significant increases in Bifidobacterium spp. and Lactobacillus spp. were observed in the almond and almond skin groups, although the populations of Escherichia coli showed little change, and the growth of Clostridium perfringens was significantly repressed. In addition, significant changes were observed in some bacterial enzyme activities, such as increased β-galactosidase activity and reduced fecal β-glucuronidase, nitroreductase and azoreductase activities. The differing results of these two studies could be due to their duration, since Ukhanova et al. (Citation2014) administered nuts only for 18 days in contrast with 6 weeks in the case Liu et al. (Citation2014).
Therefore, almonds or almond skins, if supplied for almost 6 weeks, seem to fulfill the criteria for a prebiotic, as defined by Roberfroid (Citation2007), by promoting (1) resistance to gastric acidity, hydrolysis by mammalian enzymes, and GI absorption; (2) fermentation by intestinal microflora; and (3) selective stimulation of the growth and/or activity of those intestinal bacteria that contribute to health and well-being. Nevertheless, more clinical intervention studies with nuts are required.
The skin covering nut kernels is particularly rich in fiber and polyphenols (see ), including both flavonoids and nonflavonoids that may help to explain this prebiotic effect.
Table 1. Fiber and polyphenols content in raw nuts, with kernel.
Substrates of gut microbiota
Polymerized polyphenols
Nuts are rich in polymerized polyphenols, representing one of the richest food sources of polymerized polyphenols per serving (Abe et al., Citation2010; Pérez-Jiménez et al., Citation2010), they are metabolized by gut microbiota, altering the ecosystem and changing the microbiota profile (see ). Polymerized polyphenols that are present in nuts are mainly ellagitannins and proanthocyanidins. Chestnuts, pecans, and walnuts are highly rich in ellagitannins (Abe et al., Citation2010; Regueiro et al., Citation2014), while hazelnuts, pecans, pistachios and almonds are rich in proanthocyanidins (see ).
Polymerized polyphenols may provide a substrate for gut microbiota and the compounds arising from this metabolism may modulate and cause fluctuations in the composition of the microflora population through selective prebiotic effects and antimicrobial activities against gut pathogenic bacteria. The formation of new bioactive metabolites due to breakdown by microbiota and the modulation of the colonic microbiota may both contribute to health benefits for the host, although the mechanisms have not been clearly resolved (Selma et al., Citation2009; Cardona et al., Citation2013).
Ellagitannins
Ellagitannins (ETs) are polyphenols included within the so-called “hydrolyzable tannins.” ETs can occur in chestnuts, pecans, and walnuts, as complex polymers reaching molecular weights of up to 4000 units? (Cerdá et al., Citation2005) in which hexahydroxydiphenic acid forms diesters with sugars (most often d-glucose). The basic structure is that of ellagic acid, which in the colon is metabolized by microbiota to urolithins (Tulipani et al., Citation2011; Tulipani et al., Citation2012; Espín et al., Citation2013; Puupponen-Pimiä et al., Citation2013; Nuñez-Sánchez et al., Citation2014; Garcia-Aloy et al., Citation2014; Pfundstein et al., Citation2014; Tomás-Barberán et al., Citation2014; Sánchez-González et al., Citation2015).
In vitro assays have shown that the prebiotic properties of ETs increase the growth of Lactobacillus and Bifidobacterium (Kamijo et al., Citation2008; Li et al., Citation2015). However, in an 8-week randomized controlled intervention study, no effect of ET was observed in the diversity of predominant bacterial populations in 32 individuals showing symptoms of metabolic syndrome. Participants were randomized to receive ET-rich fruits providing a daily intake of 789 ± 27 mg of ETs (n = 20) or control (n = 12). The estimated ET intake of the control group was close to zero. Despite the lack of effect on bacterial diversity, ET bioavailability and urolithin formation were found to be conditioned by the composition of gut microbiota (Puupponen-Pimiä et al., Citation2013). To our knowledge, no study has been published to evaluate the prebiotic effect of the administration of ET-rich food or nuts in healthy individuals. Therefore, to evaluate the possible role of these polyphenols as prebiotic molecules, there is a clear need for more human intervention clinical trials in different population groups, both healthy and at risk of inflammatory or metabolic diseases.
Although the bioavailability of ETs and ellagic acid is very low, these molecules undergo extensive metabolism by gut microbiota to produce urolithins, which are much easier to absorb and become highly bioavailable as phase II conjugates in plasma, mainly in the glucuronide conjugate form, at concentrations in the range of 0.2–20 μM (Espín et al., Citation2013; Pfundstein et al., Citation2014). Tomás-Barberan et al. (Citation2014) described three distinct urolithin phenotypes according to their excretion after ET and ellagic acid intake. These structural differences can modulate the physiological activity of urolithins, and therefore their potential health effects. Urolithin A is the most active in inhibiting TNF-α production, while urolithin C is the only compound that inhibits IL-6 synthesis (Piwowarski, Granica, Zwierzyńska et al., Citation2014). It is therefore conceivable that the health effects of ET-containing products are associated with these gut-produced urolithins, synthesized in different forms and proportions depending on the microflora (Puupponen-Pimiä et al., Citation2013; Pfundstein et al., Citation2014; Tomás-Barberán et al., Citation2014).
Proanthocyanidins (PAC)
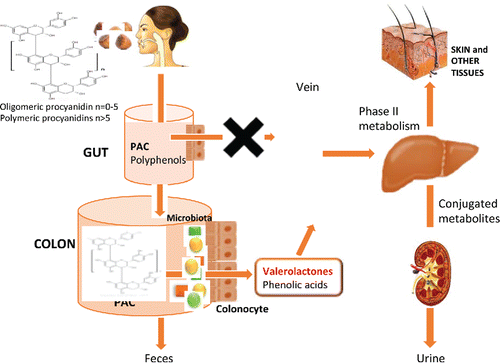
PAC are among the most abundant polyphenols in the human diet and one of the least absorbed polyphenols, mostly due to their size and structural complexity, since PAC consist of oligomers and polymers of monomeric unit flavan-3-ols (−)-epicatechin or (+)-catechins, (including the gallated forms)(Bittner et al., Citation2013). As shown in , they occur mainly in hazelnuts (622 mg/cup), pecans (615 mg/cup), pistachios (291 mg/cup), and almonds (225 mg/cup). Epidemiological data show that PAC intake was inversely associated with the risk of developing type 2 diabetes (Zamora-Ros et al., Citation2014) and certain cancers: endometrial (Rossi et al., Citation2013), colorectal, particularly cancer of the rectum (Rossi et al., Citation2010), pancreas (Rossi et al., Citation2012), and non-Hodgkin's lymphoma (Frankenfeld et al., Citation2008; Thompson et al., Citation2010). However, in humans, PAC are poorly absorbed so these effects can only be explained by their action in the colon, due to a direct effect on intestinal cell and/or for their prebiotic properties and by the active metabolites originated by this microbiota metabolism.
Dietary intervention clinical studies with PAC-rich foods have shown the prebiotic properties of these compounds. One study was performed in two populations, a group of 9 healthy adults (age 37–42 years) and a group of eight elderly patients (age 67–98 years) to assess the prebiotic effects of grape seeds, which are rich in PAC. The younger group received a dose of 190 mg/day of PAC whereas the elderly patients received 380 mg/day, for 2 weeks. Despite the different dose administered, both healthy adults and elderly patients showed a similar response with a significant increase in the fecal number of Bifidobacterium, after the 2 weeks of treatment, whereas the number of putrefactive bacteria such as enterobacteria tended to decrease. The level of putrefactive substances, including ammonia, phenol, p-cresol, 4-ethylphenol, indole, and skatols tended to decrease after the PAC-rich extract intake, and fecal pH also tended to decrease (Yamakoshi et al., Citation2001).The interaction between PAC and intestinal bacteria was also confirmed in a randomized, double blind, crossover, and controlled intervention study with twenty volunteers. A drink with a low PAC content (23 mg of cocoa flavanols/d) and another containing a high PAC dose (494 mg of cocoa flavanols/d), were administered for 4 weeks. Daily consumption of the high PAC cocoa drink significantly increased bifidobacteria and lactobacilli populations but significantly reduced clostridia counts (Tzounis et al., Citation2011). Studies performed in pigs treated with grape seeds showed a similar trend, with PAC intake increasing Lachnospiraceae, Clostridales, Lactobacillus, and Ruminococcus. Lachnospiraceae and Ruminococcus represent the major butyrate and propionate producers in human fecal samples (Choy et al., Citation2014).
Most PAC remain in the digestive tract until reaching the colon and can be quantified in feces. Still mainly intact, they exert their biological action on the colon cells, where they can regulate cell signaling pathways by interacting with cell membrane proteins (Choy et al., Citation2013), and protect intestinal barrier integrity through different mechanisms, including their antioxidant capacity and anti-inflammation activity (Choy and Waterhouse, Citation2014). However, the unabsorbed PAC are also extensively metabolized by gut microbiota to produce smaller phenolic acids, including valerolactones, hydroxybenzoic acid, hydroxyphenylacetic acid, hydroxyphenylpropinoic acid, hyrdroxyphenylvaleric acid or hydroxycinnamic acids.
Fiber or non-bioaccessible cell walls
After cereals, nuts are the foods richer in fiber (Salas-Salvadó et al., Citation2006), the nuts with the highest content are almonds (12%), which contain mainly insoluble fiber (14.30 g/cup) and a small amount of soluble fiber (1.60 g/cup). The dietary fiber or the plant cell walls consist of mainly non-starch polysaccharides, a non-extractable material that is neither digested nor absorbed in the small intestine (Phillips and Cui, Citation2011; Saura-Calixto, Citation2012; Grassby et al., Citation2014). These plant cell walls are formed by supramolecular assemblies of cellulose, hemicelluloses, pectic substances, non-carbohydrate components and water (Grassby et al., Citation2014). These cell walls retain a high amount of lipids, and are composed of protein and polyphenols that remain in the colon after duodenal digestion, being therefore available for fermentation in the colon by gut microbiota. The lipid encapsulation mechanism provides an explanation for why almonds have a low metabolizable energy content (Novotny et al., Citation2012), an attenuated impact on postprandial lipemia (Grundy et al., Citation2015) and this lipid composition may influence on gut microbiota and subsequent fermentation products (Shen et al., Citation2014). The cell walls act as barriers to prevent the physical release of lipids from cells that arrive intact in the colon (Mandalari et al., Citation2014), where they are metabolized by microbiota, releasing lipids, proteins and polyphenols of smaller molecular size.
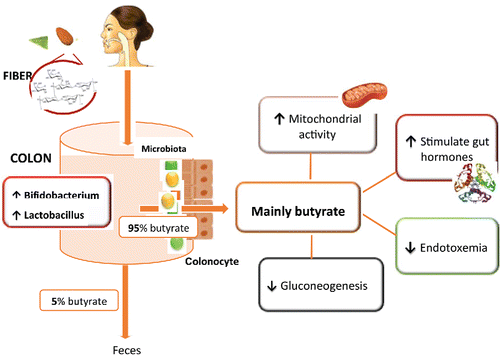
Studies on nut fiber bioaccessibility have been largely restricted to almonds (Ellis et al., Citation2004; Cassady et al., Citation2009; G. Mandalari et al., Citation2010; Novotny et al., Citation2012; Grassby et al., Citation2014; Grundy et al., Citation2015), and should be extended to other nuts, since part of the health outcomes attributed to nuts may be partly due to this fibrous material being unavailable for digestion until metabolized in the colon. Evidence of microbial degradation of almond polysaccharides (non-starch polysaccharides) was observed by Ellis et al., when analyzing the human stool after almond consumption (Ellis et al., Citation2004). Indigestible nut polysaccharides are fermented by the intestinal bacteria to short-chain fatty acids (SCFA), apparently mainly butyrate (Mandalari et al., Citation2008; Ukhanova et al., Citation2014) (see ).
Biological effects of the main metabolites formed by host microbiota from nut polyphenols or fiber
Human microbiota metabolize non bioavailable polyphenols facilitating the bioactivation of polyphenols that may exert their effect on the colonocytes; however they are also absorbed, arriving to different tissues where they can exert their protective activity. Nut fiber that arrives to the colon is metabolized by microbiota to form SCFA that are good energy substrate for colonocytes and they are signaling molecules that participate in various cellular processes.
From ellagitannins
Ellagic acid and urolithin, are the main ET metabolites formed by the intestinal microbiota (Espín et al., Citation2013; Puupponen-Pimiä et al., Citation2013; Nuñez-Sánchez et al., Citation2014; Pfundstein et al., Citation2014; Tomás-Barberán et al., Citation2014; Sánchez-González et al., Citation2015). These compounds have shown high activity in vitro as anti-inflammatory (Giménez-Bastida et al., Citation2012; Piwowarski, Granica and Kiss, Citation2014; Piwowarski, Granica, Zwierzyńska, et al., Citation2014) and as anticarcinogenic agents (Sánchez-González et al., Citation2014; Cho et al., Citation2015; González-Sarrías et al., Citation2015), increasing the effectiveness of chemotherapeutic drug treatment (Giménez-Bastida et al., Citation2012). Although ellagic acid is poorly absorbed, it may exert by itself a protective effect on colon cells (Ramírez de Molina et al., Citation2015). However, as has been pointed out previously, the main ET metabolites circulating in human plasma (Espín et al., Citation2013) and prostate tissue (González-Sarrías et al., Citation2010; Freedland et al., Citation2013) are the glucuronidated and methylated urolithins A and B. These compounds may exert their effect in the colon and have a protective effective on the systemic system and also on prostate cells; however, when they are glucuronidated they have lower antiproliferative activity (González-Sarrías et al., Citation2014). Due to the affinity of urolithin for prostatic cells and their possible role on prostate cancer prevention, research on these compounds has mainly focused on their role on prostatic cells.
Animal models clearly show that walnut intake or ETs decrease the incidence of prostate cancer (Reiter et al., Citation2013; Hardman, Citation2014; Kim et al., Citation2014) but results in human intervention trials after intake of ET-rich foods are still inconclusive (see ). In a preliminary study done by González-Sarrías et al. (González-Sarrías et al., Citation2010) 63 men with prostate cancer or benign prostatic hyperplasia were randomized: 14 received 35 g/day of walnuts (288 mg of ET [24]), 19 consumed 200 mL/day of pomegranate juice (874 mg of ET [73]) and 30 consumed no ET-containing foods for 3 days before surgery [68] (see ). Metabolites of urolithin A and traces of B were detected in the prostate gland in both groups receiving the ET-rich food, and more metabolites were detected in the prostate samples from patients who consumed walnuts than those from the pomegranate juice group. While both foods are rich in ETs, the major ET in walnuts is perduncagalin (Cerdá et al., Citation2006) and in pomegranate punicalagin (Seeram et al., Citation2006). Nevertheless, the expression levels of several cancer-related markers, with a key role in cell cycle and cell proliferation regulation in prostate tissues, could not be correlated with these metabolites. Simon et al. (Simon et al., Citation2007) reported a lack of effect on serum prostate-specific antigen (PSA) levels among 40 middle-aged normal weight? men when they increased walnut consumption (daily mean of 35 g, 12% total energy equivalent to 288 mg of ETs (Abe et al., Citation2010)) for 6 months relative to their habitual intake (Simon et al., Citation2007). PSA levels are considered a biomarker of prostate enlargement, inflammation, and cancer. Spaccarotella et al. (Spaccarotella et al., Citation2008) did not observe changes in PSA levels after consumption of walnuts for 8 weeks. However, they observed a trend towards an increase in the ratio of free PSA:total PSA at the end of the walnut phase in a randomized, cross-over intervention study with 21 men between 45 and 74 years of age. A higher ratio of free:total PSA may be associated with less aggressive cancer. In that study, participants consumed 75 g of walnuts per day (approximately 24% of energy intake from walnuts, based on a diet with 2000 kcal/day), equivalent to 617 mg of ETs for 8 weeks or their usual “Average American Diet”, and then, following a 2-week break, switched to the other diet for 8 weeks (see ).
Table 2. Intervention studies with walnut interventions performed on men to evaluate prostatic biomarkers.
Walnuts are not only rich in ETs they also contain other nutrients (alpha-linolenic, phyosterols) that may help to explain this protective effect on prostate hyperplasia. However unless animal studies show that walnuts or ETs reduce prostate tumor growth (Reiter et al., Citation2013; Kim et al., Citation2014). In humans, the results are still inconclusive. According to the American Institute for Cancer Research (American Institute for Cancer Research, Citation2015) “the evidence is currently too limited to draw any conclusions about walnuts – or any nuts – and cancer risk.”
From proanthocyanidins (PAC)
PAC are oligomers and polymers of monomeric units of flavan-3-ols (−)-epicatechin or (+)-catechins (including the gallated forms), that are present in the nut kernel of many nuts, representing one of the richest food sources per serving. Unless epidemiological data clearly show the benefits of PAC consumption however PAC are not bioavailable for human gut (see ). So they arrive intact to the colon however there they are metabolized by gut microbiota forming the bioactive compounds: gamma-valerolactones and phenolic acids so the benefits of PAC consumption has to be explained by a direct effect of these compounds in the intestine or by the colon bioactive metabolites originated. Hydroxyphenylvalerolactones are generated from the C-ring opening of flavanols (procyanidins B and (epi)catechin) by the action of colonic microflora, followed by further lactonization. They are described as the main microbial metabolites derived from the biotransformation of these PAC or flavan-3-ols (Appeldoorn et al., Citation2009; Ou et al., Citation2014) and they may be considered a biomarker of epicatechin and procyanidin consumption, since they increase significantly after the consumption of these specific compounds (Ottaviani et al., Citation2012; Rodriguez-Mateos et al., Citation2015). After almond skins consumption, since PAC are the major polymerized polyphenols in almonds, the main metabolite produced quantified in plasma and urine were hydroxyphenylvalerolactones (Urpi-Sarda et al., Citation2009; Garrido et al., Citation2010; Llorach et al., Citation2010). However, the hydrolysis of the C-rings depends on the microbiota present, since only some specific microbial organisms seem to have the capacity to open this ring (Kutschera et al., Citation2011; Takagaki and Nanjo, Citation2015). The physiological activity of gamma-valerolactone metabolites has not been extensively studied, but preliminary data are promising. In a study with two volunteers, after consumption of pine bark extract rich in flavan-3-ol, valerolactone metabolites accumulated in human blood cells (Mülek et al., Citation2015; Mülek and Högger, Citation2015). In an open oral intervention study, with sixteen healthy human subjects that received given an oral administration of 540 mg of flavanols (tea extract) per day with vitamin C (50 mg), for 12 weeks, valerolactones were detected in skin tissue and fluid, inhibiting skin inflammation induced by 12-hydroxyeicosatetraenoic acid after UV exposure (Rhodes et al., Citation2013). More studies are necessary to explore the possible mechanism of action of gamma-valerolactones and other microbiota metabolites, since these results may help to explain the beneficial health outcomes attributed to procyanidin or flavanol intake (Holt et al., Citation2012; McCullough et al., Citation2012; Wang et al., Citation2014), which are otherwise difficult to explain given their low bioavailability (Ottaviani et al., Citation2012).
Short-chain fatty acids (SCFAs) from nut polysaccharides
Polysaccharide fermentation in the colon releases SCFAs: acetate, propionate and butyrate, and gases such as hydrogen and carbon dioxide. Long-term exposure to certain foods seems to modulate SCFA production. Butyrate production has been correlated with fecal donor intake of grain-, nut-, and vegetable-based foods (Yang and Rose, Citation2014).
However, not many studies have focused on the changes in SCFA profile after nut consumption. In vitro studies with almonds have shown an increase in the levels of lactic, acetic, propionic and butyric acids (Mandalari et al., Citation2008).In vivo, nut polysaccharides are fermented by human intestinal bacteria to SCFA, apparently mainly butyrate (Mandalari et al., Citation2008; Ukhanova et al., Citation2014). The relative proportions of the SCFA produced will likely alter cholesterol synthesis (St-Onge et al., Citation2000). 95% of the produced SCFAs are rapidly absorbed by the colonocytes while the remaining 5% are secreted in the feces. SCFAs are metabolized by colon epithelial cells to which they constitute approximately 60–70% of the energy requirement. Moreover SCFA act as signaling molecules in various cellular processes. Recent in vivo and in vitro studies suggest that SCFAs stimulate gut hormone secretion. Therefore, the SCFA signal is likely to be important for gut physiological functions (Kaji et al., Citation2014). The beneficial effect of butyrate on human host health has been extensively described in several review papers (Leonel and Alvarez-Leite, Citation2012; Brahe et al., Citation2013; Hartstra et al., Citation2014). Butyrate (see ) may induce beneficial metabolic effects through enhancement of mitochondrial activity, prevention of metabolic endotoxemia, and activation of intestinal gluconeogenesis via different routes of gene expression and hormone regulation. Recent studies indicate a role for SCFAs, in particular propionate and butyrate, in metabolic and inflammatory disorders such as obesity, diabetes and inflammatory bowel diseases (Brahe et al., Citation2013; Puertollano et al., Citation2014). A butyrate-increasing diet may be applied in prevention and treatment of obesity related metabolic diseases
Future research should focus on how nut consumption may affect butyrate production and health outcomes.
Conclusion
Non-bioaccessible material from nuts, constituting mainly of polymerized polyphenols and polysaccharides, arriving in the colon intact seems to have a prebiotic effect. When a sufficient amount (56 g) is administered for at least 8 weeks, an increase in Bifidobacterium and Lactobacillus is observed. However, more human intervention studies, administering different doses, over a sufficiently long period of time, should be performed to evaluate these prebiotic properties further. Polymerized polyphenols from nuts are metabolized by colon microbiota to produce smaller molecular size polyphenols that are bioavailable in the colon. Urolithins are the main metabolites after the intake of ellagitannins (the main polyphenols in walnuts). While these compounds have demonstrated in vitro, in animal models their protective effect on prostate cells, more intervention clinical trials are necessary to support any recommendation. When low bioavailable proanthocyanidins are administered, the main biomarker is gamma-valerolactones, which may be, with other phenolic metabolites, responsible for the significant beneficial effect on health outcomes attributed to proanthocynidins intake. Butyrate is the main product of nut polysaccharide fermentation by microbiota and its benefits for the human host via changes in gene expression and hormone regulation have been extensively described.
Acknowledgments
RLR would like to thank to the University of Barcelona for allowing her sabbatical stage at the Obesity Center in Columbia University.
Funding
RLR would like to express thanks for the financial support from CICYT (AGL2013-49083-C3-1-R) and the Instituto de Salud Carlos III, ISCIII (CIBEROBN) from the Ministerio de Economía y Competitividad (MEC) and Generalitat de Catalunya (GC) 2014 SGR 773. MPSO is funded from the Almond Board of California.
References
- Abe, L. T., Lajolo, F. M. and Genovese, M. I. (2010). Comparison of phenol content and antioxidant capacity of nuts. Ciência e Tecnol. Aliment. 30:254–259.
- American Institute for Cancer Research (2015). No Title [Internet]. Available from: <fight-cancer/tab-content/walnuts-research.html>.
- Appeldoorn, M. M., Vincken, J.-P., Aura, A.-M., Hollman, P. C. H. and Gruppen, H. (2009). Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-gamma-valerolactone as the major metabolites. J. Agric. Food Chem. 57:1084–1092.
- Babio, N., Toledo, E., Estruch, R., Ros, E., Martínez-González, M. A., Castañer, O., Bulló, M., Corella, D., Arós, F., Gómez-Gracia, E., Ruiz-Gutiérrez, V., Fiol, M., Lapetra, J., Lamuela-Raventos, R. M., Serra-Majem, L., Pintó, X., Basora, J., Sorlí, J. V. and Salas-Salvadó, J. (2014). Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ. 186:E649–E657.
- Bao, Y., Han, J., Hu, F. B., Giovannucci, E. L., Stampfer, M. J., Willett, W. C. and Fuchs, C. S. (2013). Association of nut consumption with total and cause-specific mortality. N. Engl. J. Med. 369:2001–2011.
- Berryman, C. E., West, S. G., Fleming, J. A., Bordi, P. L. and Kris-Etherton, P. M. (2015). Effects of daily almond consumption on cardiometabolic risk and abdominal adiposity in healthy adults with elevated LDL-cholesterol: A randomized controlled trial. J. Am. Heart Assoc. 4:e000993–e000993.
- Bittner, K., Rzeppa, S. and Humpf, H. U. (2013). Distribution and quantification of flavan-3-ols and procyanidins with low degree of polymerization in nuts, cereals, and legumes. J. Agric. Food Chem. 61:9148–9154.
- Blaiotta, G., La Gatta, B., Di Capua, M., Di Luccia, A., Coppola, R. and Aponte, M. (2013). Effect of chestnut extract and chestnut fiber on viability of potential probiotic Lactobacillus strains under gastrointestinal tract conditions. Food Microbiol. 36:161–169.
- Brahe, L. K., Astrup, A. and Larsen, L. H. (2013) Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes. Rev. 14:950–959.
- Cardona, F., Andrés-Lacueva, C., Tulipani, S., Tinahones, F. J. and Queipo-Ortuño, M. I. (2013). Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 24:1415–1422.
- Cassady, B. A., Hollis, J. H., Fulford, A. D., Considine, R. V. and Mattes, R. D. (2009). Mastication of almonds: Effects of lipid bioaccessibility, appetite, and hormone response. Am. J. Clin. Nutr. 89:794–800.
- Cerdá, B., Soto, C., Albaladejo, M. D., Martínez, P., Sánchez-Gascón, F., Tomás-Barberán, F. and Espín, J. C. (2006). Pomegranate juice supplementation in chronic obstructive pulmonary disease: A 5-week randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 60:245–253.
- Cerdá, B., Tomás-Barberán, F. A. and Espín, J. C. (2005). Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 53:227–235.
- Cho, H., Jung, H., Lee, H., Yi, H. C., Kwak, H.-K. and Hwang, K. T. (2015). Chemopreventive activity of ellagitannins and their derivatives from black raspberry seeds on HT-29 colon cancer cells. Food Funct. 6:1675–1683.
- Choy, Y. Y., Jaggers, G. K., Oteiza, P. I. and Waterhouse, A. L. (2013). Bioavailability of intact proanthocyanidins in the rat colon after ingestion of grape seed extract. J. Agric. Food Chem. 61:121–127.
- Choy, Y. Y., Quifer-Rada, P., Holstege, D. M., Frese, S. A., Calvert, C. C., Mills, D. A., Lamuela-Raventos, R. M. and Waterhouse, A. L. (2014). Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct. 5(9):2298–2308.
- Choy, Y. Y. and Waterhouse, A. L. (2014). Proanthocyanidin Metabolism, a mini review. Nutr. Aging. 2:111–116.
- Damasceno, N. R. T., Sala-Vila, A., Cofán, M., Pérez-Heras, A. M., Fitó, M., Ruiz-Gutiérrez, V., Martínez-González, M.-Á. Á., Corella, D., Arós, F., Estruch, R. and Ros, E. (2013). Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis. 230:347–353.
- Ellis, P. R., Kendall, C. W. C., Ren, Y., Parker, C., Pacy, J. F., Waldron, K. W. and Jenkins, D. J. A. (2004). Role of cell walls in the bioaccessibility of lipids in almond seeds. Am. J. Clin. Nutr. 80:604–613.
- Espín, J. C., Larrosa, M., García-Conesa, M. T. and Tomás-Barberán, F. (2013). Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: The evidence so far. Evid. Based. Complement. Alternat. Med. 2013:270418.
- Estruch, R., Ros, E., Salas-Salvadó, J., Covas, M.-I., Corella, D., Arós, F., Gómez-Gracia, E., Ruiz-Gutiérrez, V., Fiol, M., Lapetra, J., Lamuela-Raventos, R. M., Serra-Majem, L., Pintó, X., Basora, J., Muñoz, M. A., Sorlí, J. V, Martínez, J. A. and Martínez-González, M. A. (2013). Primary prevention of cardiovascular disease with a mediterranean diet. New Engl. J. Med. 368:1279–1290.
- Flores-Mateo, G., Rojas-Rueda, D., Basora, J., Ros, E. and Salas-Salvadó, J. (2013). Nut intake and adiposity: Meta-analysis of clinical trials. Am. J. Clin. Nutr. 97:1346–1355.
- Frankenfeld, C. L., Cerhan, J. R., Cozen, W., Davis, S., Schenk, M., Morton, L. M., Hartge, P. and Ward, M. H. (2008). Dietary flavonoid intake and non-Hodgkin lymphoma risk. Am. J. Clin. Nutr. 87:1439–1445.
- Freedland, S. J., Carducci, M., Kroeger, N., Partin, A., Rao, J.-Y., Jin, Y., Kerkoutian, S., Wu, H., Li, Y., Creel, P., Mundy, K., Gurganus, R., Fedor, H., King, S. A., Zhang, Y., Heber, D. and Pantuck, A. J. (2013). A double-blind, randomized, neoadjuvant study of the tissue effects of POMx pills in men with prostate cancer before radical prostatectomy. Cancer Prev. Res. (Phila). 6:1120–1127.
- Garcia-Aloy, M., Llorach, R., Urpi-Sarda, M., Tulipani, S., Estruch, R., Martínez-González, M. A., Corella, D., Fitó, M., Ros, E., Salas-Salvadó, J. and Andres-Lacueva, C. (2014). Novel multimetabolite prediction of walnut consumption by a urinary biomarker model in a free-living population: The PREDIMED Study. J. Proteome Res. 13:3476–3483.
- Garrido, I., Urpi-Sarda, M., Monagas, M., Gómez-Cordovés, C., Martín-Alvarez, P. J., Llorach, R., Bartolomé, B. and Andrés-Lacueva, C. (2010). Targeted analysis of conjugated and microbial-derived phenolic metabolites in human urine after consumption of an almond skin phenolic extract. J. Nutr. 140:1799–1807.
- Gill, S. R., Pop, M., Deboy, R. T., Eckburg, P. B., Turnbaugh, P. J., Samuel, B. S., Gordon, J. I., Relman, D. A., Fraser-Liggett, C. M. and Nelson, K. E. (2006). Metagenomic analysis of the human distal gut microbiome. Science. 312:1355–1359.
- Giménez-Bastida, J. A., González-Sarrías, A., Larrosa, M., Tomás-Barberán, F., Espín, J. C. and García-Conesa, M.-T. (2012). Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-α-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol. Nutr. Food Res. 56:784–796.
- González-Sarrías, A., Giménez-Bastida, J. A., García-Conesa, M. T., Gómez-Sánchez, M. B., García-Talavera, N. V, Gil-Izquierdo, A., Sánchez-Alvarez, C., Fontana-Compiano, L. O., Morga-Egea, J. P., Pastor-Quirante, F. A., Martínez-Díaz, F., Tomás-Barberán, F. A. and Espín, J. C. (2010). Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol. Nutr. Food Res. 54:311–322.
- González-Sarrías, A., Giménez-Bastida, J. A., Núñez-Sánchez, M. Á., Larrosa, M., García-Conesa, M. T., Tomás-Barberán, F. A. and Espín, J. C. (2014). Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur. J. Nutr. 53:853–864.
- González-Sarrías, A., Tomé-Carneiro, J., Bellesia, A., Tomás-Barberán, F. A. and Espín, J. C. (2015). The ellagic acid-derived gut microbiota metabolite, urolithin A, potentiates the anticancer effects of 5-fluorouracil chemotherapy on human colon cancer cells. Food Funct. 6:1460–1469.
- Grassby, T., Picout, D. R., Mandalari, G., Faulks, R. M., Kendall, C. W. C., Rich, G. T., Wickham, M. S. J., Lapsley, K. and Ellis, P. R. (2014). Modelling of nutrient bioaccessibility in almond seeds based on the fracture properties of their cell walls. Food Funct. 5:3096–3106.
- Grosso, G., Yang, J., Marventano, S., Micek, A., Galvano, F. and Kales, S. N. (2015). Nut consumption on all-cause, cardiovascular, and cancer mortality risk: A systematic review and meta-analysis of epidemiologic studies. Am. J. Clin. Nutr. 101:783–793.
- Grundy, M. M. L., Grassby, T., Mandalari, G., Waldron, K. W., Butterworth, P. J., Berry, S. E. E. and Ellis, P. R. (2015). Effect of mastication on lipid bioaccessibility of almonds in a randomized human study and its implications for digestion kinetics, metabolizable energy, and postprandial lipemia. Am. J. Clin. Nutr. 101:25–33.
- Hardman, W. E. (2014). Walnuts have potential for cancer prevention and treatment in mice. J. Nutr. 144:p.555S–560S.
- Hartstra, A. V., Bouter, K. E. C., Backhed, F. and Nieuwdorp, M. (2014). Insights into the role of the microbiome in obesity and Type 2 diabetes. Diabetes Care. 38:159–165.
- Holt, R. R., Heiss, C., Kelm, M. and Keen, C. L. (2012). The potential of flavanol and procyanidin intake to influence age-related vascular disease. J. Nutr. Gerontol. Geriatr. 31:290–323.
- Kaji, I., Karaki, S. and Kuwahara, A. (2014). Short-chain fatty acid receptor and its contribution to glucagon-like peptide-1 release. Digestion. 89:31–36.
- Kamijo, M., Kanazawa, T., Funaki, M., Nishizawa, M. and Yamagishi, T. (2008). Effects of Rosa rugosa petals on intestinal bacteria. Biosci. Biotechnol. Biochem. 72:773–777.
- Kim, H., Yokoyama, W. and Davis, P. A. (2014). TRAMP prostate tumor growth is slowed by walnut diets through altered IGF-1 levels, energy pathways, and cholesterol metabolism. J. Med. Food. 17:1281–1286.
- Kutschera, M., Engst, W., Blaut, M. and Braune, A. (2011). Isolation of catechin-converting human intestinal bacteria. J. Appl. Microbiol. 111: 165–175.
- Leonel, A. J. and Alvarez-Leite, J. I. (2012). Butyrate: Implications for intestinal function. Curr. Opin. Clin. Nutr. Metab. Care. 15:474–479.
- Li, Z., Summanen, P. H., Komoriya, T., Henning, S. M., Lee, R.-P., Carlson, E., Heber, D. and Finegold, S. M. (2015). Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe. 34:164–168.
- Liu, Z., Lin, X., Huang, G., Zhang, W., Rao, P. and Ni, L. (2014). Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe. 26:1–6.
- Llorach, R., Garrido, I., Monagas, M. M., Urpi-Sarda, M., Tulipani, S., Bartolome, B. B. and Andres-Lacueva, C. (2010). Metabolomics study of human urinary metabolome modifications after intake of almond (Prunus dulcis (Mill.) D.A. Webb) skin polyphenols. J. Proteome Res. 9:5859–5867.
- Mandalari, G., Faulks, R. M., Bisignano, C., Waldron, K. W., Narbad, A. and Wickham, M. S. J. (2010). In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiol. Lett. 304:116–122.
- Mandalari, G., Grundy, M. M.-L., Grassby, T., Parker, M. L., Cross, K. L., Chessa, S., Bisignano, C., Barreca, D., Bellocco, E., Laganà, G., Butterworth, P. J., Faulks, R. M., Wilde, P. J., Ellis, P. R. and Waldron, K. W. (2014). The effects of processing and mastication on almond lipid bioaccessibility using novel methods of in vitro digestion modelling and micro-structural analysis. Br. J. Nutr. 112:1521–1529.
- Mandalari, G., Nueno-Palop, C., Bisignano, G., Wickham, M. S. J. and Narbad, A. (2008). Potential prebiotic properties of almond (Amygdalus communis L.) seeds. Appl. Environ. Microbiol. 74:4264–4270.
- Mandalari, G., Tomaino, A., Rich, G.T., Lo Curto, R., Arcoraci, T., Martorana, M., Bisignano, C., Saija, A., Parker, M. L., Waldron, K. W. and Wickham, M. S. J. (2010). Polyphenol and nutrient release from skin of almonds during simulated human digestion. Food Chem. 122:1083–1088.
- McCullough, M. L., Peterson, J. J., Patel, R., Jacques, P. F., Shah, R. and Dwyer, J. T. (2012). Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 95:454–464.
- Mülek, M., Fekete, A., Wiest, J., Holzgrabe, U., Mueller, M. J. and Högger, P. (2015). Profiling a gut microbiota-generated catechin metabolite's fate in human blood cells using a metabolomic approach. J. Pharm. Biomed. Anal. 114:71–81.
- Mülek, M. and Högger, P. (2015). Highly sensitive analysis of polyphenols and their metabolites in human blood cells using dispersive SPE extraction and LC-MS/MS. Anal. Bioanal. Chem. 407:1885–1899.
- Nielsen, S. J., Ph, D., Div, M., Kit, B. K., Ogden, C. L. and Ph, D. (2014). Nut Consumption Among U.S. Adults. 2009–2010.
- Novotny, J. A., Gebauer, S. K. and Baer, D. J. (2012). Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets. Am. J. Clin. Nutr. 96:296–301.
- Nuñez-Sánchez, M. A., García-Villalba, R., Monedero-Saiz, T., García-Talavera, N. V, Gómez-Sánchez, M. B., Sánchez-Álvarez, C., García-Albert, A. M., Rodríguez-Gil, F. J., Ruiz-Marín, M., Pastor-Quirante, F. A., Martínez-Díaz, F., Yáñez-Gascón, M. J., González-Sarrías, A., Tomás-Barberán, F. A. and Espín, J. C. (2014). Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol. Nutr. Food Res. 58:1199–1211.
- Ottaviani, J. I., Kwik-Uribe, C., Keen, C. L. and Schroeter, H. (2012). Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans. Am. J. Clin. Nutr. 95:851–858.
- Ou, K., Sarnoski, P., Schneider, K. R., Song, K., Khoo, C. and Gu, L. (2014). Microbial catabolism of procyanidins by human gut microbiota. Mol. Nutr. Food Res. 58:2196–2205.
- Pérez-Jiménez, J., Neveu, V., Vos, F. and Scalbert, A. (2010). Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 64(Suppl 3):S112–S120.
- Pfundstein, B., Haubner, R., Würtele, G., Gehres, N., Ulrich, C. M. and Owen, R. W. (2014). Pilot walnut intervention study of urolithin bioavailability in human volunteers. J. Agric. Food Chem. 62:10264–10273.
- Phillips, G. O. and Cui, S. W. (2011). An introduction: Evolution and finalisation of the regulatory definition of dietary fibre. Food Hydrocoll. 25:139–143.
- Piwowarski, J. P., Granica, S. and Kiss, A. K. (2014). Influence of gut microbiota-derived ellagitannins’ metabolites urolithins on pro-inflammatory activities of human neutrophils. Planta Med. 80:887–895.
- Piwowarski, J.P., Granica, S., Zwierzyńska, M., Stefańska, J., Schopohl, P., Melzig, M. F. and Kiss, A. K. (2014). Role of human gut microbiota metabolism in the anti-inflammatory effect of traditionally used ellagitannin-rich plant materials. J. Ethnopharmacol. 155:801–809.
- Puertollano, E., Kolida, S. and Yaqoob, P. (2014). Biological significance of short-chain fatty acid metabolism by the intestinal microbiome. Curr. Opin. Clin. Nutr. Metab. Care. 17:139–144.
- Puupponen-Pimiä, R., Seppänen-Laakso, T., Kankainen, M., Maukonen, J., Törrönen, R., Kolehmainen, M., Leppänen, T., Moilanen, E., Nohynek, L., Aura, A.-M., Poutanen, K., Tómas-Barberán, F. A., Espín, J. C. and Oksman-Caldentey, K.-M. (2013). Effects of ellagitannin-rich berries on blood lipids, gut microbiota, and urolithin production in human subjects with symptoms of metabolic syndrome. Mol. Nutr. Food Res. 57:2258–2263.
- Rajaram, S. and Sabaté, J. (2006). Nuts, body weight and insulin resistance. Br. J. Nutr. 96(Suppl 2):S79–S86.
- Ramírez de Molina, A., Vargas, T., Molina, S., Sánchez, J., Martínez-Romero, J., González-Vallinas, M., Martín-Hernández, R., Sánchez-Martínez, R., Gómez de Cedrón, M., Dávalos, A., Calani, L., Del Rio, D., González-Sarrías, A., Espín, J. C., Tomás-Barberán, F. A. and Reglero, G. (2015). The ellagic acid derivative 4,4’-di-O-methylellagic acid efficiently inhibits colon cancer cell growth through a mechanism involving WNT16. J. Pharmacol. Exp. Ther. 353:433–444.
- Regueiro, J., Sánchez-González, C., Vallverdú-Queralt, A., Simal-Gándara, J., Lamuela-Raventós, R. and Izquierdo-Pulido, M. (2014). Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap-Orbitrap mass spectrometry. Food Chem. 152:340–348.
- Reiter, R. J., Tan, D.-X., Manchester, L. C., Korkmaz, A., Fuentes-Broto, L., Hardman, W. E., Rosales-Corral, S. A. and Qi, W. (2013). A walnut-enriched diet reduces the growth of LNCaP human prostate cancer xenografts in nude mice. Cancer Invest. 31:365–373.
- Rhodes, L. E., Darby, G., Massey, K. A., Clarke, K. A., Dew, T. P., Farrar, M. D., Bennett, S., Watson, R. E. B., Williamson, G. and Nicolaou, A. (2013). Oral green tea catechin metabolites are incorporated into human skin and protect against UV radiation-induced cutaneous inflammation in association with reduced production of pro-inflammatory eicosanoid 12-hydroxyeicosatetraenoic acid. Br. J. Nutr. 110: 891–900.
- Roberfroid, M. (2007). Prebiotics: The Concept Revisited. J. Nutr. 137:830S–837.
- Roberfroid, M., Gibson, G.R., Hoyles, L., McCartney, A.L., Rastall, R., Rowland, I., Wolvers, D., Watzl, B., Szajewska, H., Stahl, B., Guarner, F., Respondek, F., Whelan, K., Coxam, V., Davicco, M.-J., Léotoing, L., Wittrant, Y., Delzenne, N.M., Cani, P.D., Neyrinck, A.M. and Meheust, A. (2010). Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 104(Suppl):S1–S63.
- Rodriguez-Mateos, A., Cifuentes-Gomez, T., Gonzalez-Salvador, I., Ottaviani, J. I., Schroeter, H., Kelm, M., Heiss, C. and Spencer, J. P. E. (2015). Influence of age on the absorption, metabolism, and excretion of cocoa flavanols in healthy subjects. Mol. Nutr. Food Res.
- Rossi, M., Edefonti, V., Parpinel, M., Lagiou, P., Franchi, M., Ferraroni, M., Decarli, A., Zucchetto, A., Serraino, D., Dal Maso, L., Negri, E. and La Vecchia, C. (2013). Proanthocyanidins and other flavonoids in relation to endometrial cancer risk: A case-control study in Italy. Br. J. Cancer 109: 1914–1920.
- Rossi, M., Lugo, A., Lagiou, P., Zucchetto, A., Polesel, J., Serraino, D., Negri, E., Trichopoulos, D. and La Vecchia, C. (2012) Proanthocyanidins and other flavonoids in relation to pancreatic cancer: A case-control study in Italy. Ann. Oncol. 23:1488–1493.
- Rossi, M., Negri, E., Parpinel, M., Lagiou, P., Bosetti, C., Talamini, R., Montella, M., Giacosa, A., Franceschi, S. and La Vecchia, C. (2010) Proanthocyanidins and the risk of colorectal cancer in Italy. Cancer Causes Control. 21:243–250.
- Salas-Salvadó, J., Bulló, M., Estruch, R., Ros, E., Covas, M.-I., Ibarrola-Jurado, N., Corella, D., Arós, F., Gómez-Gracia, E., Ruiz-Gutiérrez, V., Romaguera, D., Lapetra, J., Lamuela-Raventós, R. M., Serra-Majem, L., Pintó, X., Basora, J., Muñoz, M. A., Sorlí, J. V and Martínez-González, M. A. (2014). Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 160:1–10.
- Salas-Salvadó, J., Bulló, M., Pérez-Heras, A. and Ros, E. (2006). Dietary fibre, nuts and cardiovascular diseases. Br. J. Nutr. 96(Suppl 2):S46–S51.
- Salas-Salvadó, J., Fernández-Ballart, J., Ros, E., Martínez-González, M.-A., Fitó, M., Estruch, R., Corella, D., Fiol, M., Gómez-Gracia, E., Arós, F., Flores, G., Lapetra, J., Lamuela-Raventós, R., Ruiz-Gutiérrez, V., Bulló, M., Basora, J. and Covas, M.-I. (2008). Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: One-year results of the PREDIMED randomized trial. Arch. Intern. Med. 168:2449–2458.
- Sánchez-González, C., Ciudad, C. J., Izquierdo-Pulido, M. and Noé, V. (2016). Urolithin A causes p21 up-regulation in prostate cancer cells. Eur. J. Nutr. 55:1099–1112.
- Sánchez-González, C., Ciudad, C. J., Noé, V. and Izquierdo-Pulido, M. (2014). Walnut polyphenol metabolites, urolithins A and B, inhibit the expression of the prostate-specific antigen and the androgen receptor in prostate cancer cells. Food Funct. 5:2922–2930.
- Saura-Calixto, F. (2012). Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. J. Agric. Food Chem. 60:11195–11200.
- Seeram, N. P., Henning, S. M., Zhang, Y., Suchard, M., Li, Z. and Heber, D. (2006). Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J. Nutr. 136:2481–2485.
- Selma, M. V, Espín, J. C. and Tomás-Barberán, F. A. (2009). Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 57:6485–6501.
- Shen, W., Gaskins, H. R. and McIntosh, M. K. (2014). Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. J. Nutr. Biochem. 25:270–80. Available from: <http://www.ncbi.nlm.nih.gov/pubmed/24355793>[ Accessed 18 August 2015].
- Simon, J. A., Tanzman, J. S. and Sabaté, J. (2007). Lack of effect of walnuts on serum levels of prostate specific antigen: A brief report. J. Am. Coll. Nutr. 26:317–20.
- Slavin, J. (2013). Fiber and prebiotics: Mechanisms and health benefits. Nutrients 5:1417–1435.
- Spaccarotella, K. J., Kris-Etherton, P. M., Stone, W. L., Bagshaw, D. M., Fishell, V. K., West, S. G., Lawrence, F. R. and Hartman, T. J. (2008). The effect of walnut intake on factors related to prostate and vascular health in older men. Nutr. J. 7:13.
- St-Onge, M. P., Farnworth, E. R. and Jones, P. J. H. (2000). Consumption of fermented and nonfermented dairy products: Effects on cholesterol concentrations and metabolism. Am. J. Clin. Nutr. 71:674–681.
- Takagaki, A. and Nanjo, F. (2015). Bioconversion of (−)-epicatechin, (+)-epicatechin, (−)-catechin, and (+)-catechin by (−)-epigallocatechin-metabolizing bacteria. Biol. Pharm. Bull. 38:789–794.
- Tan, S. Y., Dhillon, J. and Mattes, R. D. (2014). A review of the effects of nuts on appetite, food intake, metabolism, and body weight. Am. J. Clin. Nutr. 100(Suppl 1):412S–422S.
- Thompson, C. A., Habermann, T. M., Wang, A. H., Vierkant, R. A., Folsom, A. R., Ross, J. A. and Cerhan, J. R. (2010). Antioxidant intake from fruits, vegetables and other sources and risk of non-Hodgkin's lymphoma: The Iowa Women's Health Study. Int. J. Cancer. 126:992–1003.
- Tomás-Barberán, F. A., García-Villalba, R., González-Sarrías, A., Selma, M. V and Espín, J.C. (2014). Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 62:6535–6538.
- Tulipani, S., Llorach, R., Jáuregui, O., López-Uriarte, P., Garcia-Aloy, M., Bullo, M., Salas-Salvadó, J. and Andrés-Lacueva, C. (2011). Metabolomics unveils urinary changes in subjects with metabolic syndrome following 12-week nut consumption. J. Proteome Res. 10:5047–5058.
- Tulipani, S., Urpi-Sarda, M., García-Villalba, R., Rabassa, M., López-Uriarte, P., Bulló, M., Jáuregui, O., Tomás-Barberán, F., Salas-Salvadó, J., Espín, J. C. and Andrés-Lacueva, C. (2012). Urolithins are the main urinary microbial-derived phenolic metabolites discriminating a moderate consumption of nuts in free-living subjects with diagnosed metabolic syndrome. J. Agric. Food Chem. 60:8930–8940.
- Turnbaugh, P. J., Ley, R. E., Hamady, M., Fraser-Liggett, C. M., Knight, R. and Gordon, J. I. (2007). The human microbiome project. Nature. 449:804–810.
- Tzounis, X., Rodriguez-Mateos, A., Vulevic, J., Gibson, G. R., Kwik-Uribe, C. and Spencer, J. P. E. (2011). Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 93:62–72.
- Ukhanova, M., Wang, X., Baer, D. J., Novotny, J. A., Fredborg, M. and Mai, V. (2014). Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. Br. J. Nutr. 111:2146–2152.
- Urpi-Sarda, M., Garrido, I., Monagas, M., Gómez-Cordovés, C., Medina-Remón, A., Andres-Lacueva, C. and Bartolomé, B. (2009). Profile of plasma and urine metabolites after the intake of almond [Prunus dulcis (Mill.) D.A. Webb] polyphenols in humans. J. Agric. Food Chem. 57:10134–10142.
- USDA Scientific Report of the 2015 Dietary Guidelines Advisory Committee.
- Van den Brandt, P.A. and Schouten, L. J. (2015). Relationship of tree nut, peanut and peanut butter intake with total and cause-specific mortality: A cohort study and meta-analysis. Int. J. Epidemiol. 44:1038–1049.
- Wang, X., Ouyang, Y. Y., Liu, J. and Zhao, G. (2014). Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 111:1–11.
- Yamakoshi, J., Tokutake, S., Kikuchi, M., Kubota, Y., Konishi, H. and Mitsuoka, T. (2001). Effect of Proanthocyanidin-Rich Extract from Grape Seeds on Human Fecal Flora and Fecal Odor. Microb. Ecol. Health Dis. 13:25–31. Available from: <http://www.scopus.com/inward/record.url?eid=2-s2.0-0035081663&partnerID=tZOtx3y1>[ Accessed 18 June 2015].
- Yang, J. and Rose, D. J. (2014). Long-term dietary pattern of fecal donor correlates with butyrate production and markers of protein fermentation during in vitro fecal fermentation. Nutr. Res. 34:749–759.
- Zamora-Ros, R., Forouhi, N. G., Sharp, S. J., González, C. A., Buijsse, B., Guevara, M., van der Schouw, Y. T., Amiano, P., Boeing, H., Bredsdorff, L., Fagherazzi, G., Feskens, E. J., Franks, P. W., Grioni, S., Katzke, V., Key, T. J., Khaw, K.-T., Kühn, T., Masala, G., Mattiello, A., Molina-Montes, E., Nilsson, P. M., Overvad, K., Perquier, F., Redondo, M. L., Ricceri, F., Rolandsson, O., Romieu, I., Roswall, N., Scalbert, A., Schulze, M., Slimani, N., Spijkerman, A. M. W., Tjonneland, A., Tormo, M. J., Touillaud, M., Tumino, R., van der A, D. L., van Woudenbergh, G. J., Langenberg, C., Riboli, E. and Wareham, N. J. (2014). Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. J. Nutr. 144:335–343.
- Zhang, Y.-J., Li, S., Gan, R.-Y., Zhou, T., Xu, D.-P. and Li, H.-B. (2015). Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 16:7493–7519.