ABSTRACT
A diet rich in dietary fiber (DF) is considered healthy and recommended dietary intake of DF is established all over the world. The physiological effect of DF is mostly related to its behavior during digestion. In this review, the behavior of DF in the human digestive tract is discussed and linked to its physiological effect with special attention to four aspects of such behavior: (i) the modulation of bioavailability by the plant cell walls, (ii) the effect of DF on the rheological and colloidal state of digesta, (iii) the binding of DF with phenolic compounds, bile salts, mineral ions, and digestive enzymes, and (iv) DF fermentation in the large intestine and the corresponding effect on microbiota composition. It is stressed that the detailed chemical characterization of DF is crucial to explain its effect on health and that DF behavior in the digestive tract can be modulated by interactions with other food and meal components so that information of the bare content in DF of food is not sufficient to predict its physiological effect.
1. Introduction
It is widely recognized that populations consuming diets high in dietary fiber (DF) have a lower incidence of chronic diseases compared to populations that consume diets lower in DF (Institute of Medicine, Citation2001; Carrera-Bastos et al., Citation2011). From the early 70s, when the “dietary fiber hypothesis” relating the lack of DF to some diseases typical of western populations has been put forward (Burkitt et al., Citation1972; Trowell, Citation1972), epidemiological evidence has accumulated for the protecting effect of DF on obesity (Liu et al., Citation2003; Du et al., Citation2010; Maki et al., Citation2010), insulin sensitivity and diabetes (Meyer et al., Citation2000; Liese et al., Citation2003; Montonen et al., Citation2003; Lau et al., Citation2005; de Munter et al., Citation2007; Rave et al., Citation2007; Weickert and Pfeiffer, Citation2008), cardiovascular and coronary hearth diseases (Brown et al., Citation1999; Bazzano et al., Citation2003; Ajani et al., Citation2004; Jensen et al., Citation2004; Pereira et al., Citation2004), and cancer (Nomura et al., Citation2007; Schatzkin et al., Citation2007, Citation2008; Dahm et al., Citation2010; Murphy et al., Citation2012). A negative association between DF intake and mortality, particularly from circulatory, digestive, and non-CVD and non-cancer inflammatory diseases was also observed in the European Prospective Investigation into Cancer and Nutrition (EPIC; Chuang et al., Citation2012). DF is generally considered as an important element of a healthy diet and this has led to dietary recommendations for an optimal consumption level of DF. Compared to other dietary components, which beneficial effect on health is either related to their intrinsic nutritional value or driven by direct elicitation of specific biological responses, DF beneficial effect on health is explained by its behavior in the gastrointestinal tract (GIT). Direct effects of DF on human health have been demonstrated, e.g., bacterial and fungal β-glucan can interact with specific receptors of epithelial immune cells and boost immunological responses (Mikkelsen et al., Citation2014a). However, this is the exception rather than the rule. Reviews where the physiological effect of DF is described in relation to behavior during digestion can be found in the scientific literature but they mainly address one or few aspects of this behavior (Gidley, Citation2013; Baye et al., Citation2015; Dhital et al., Citation2015b; Lovegrove et al., Citation2015; Padayachee et al., Citation2015) and a comprehensive and detailed account is needed, especially in the light of the extensive work carried out in this field in the last 2 years. In this review, the behavior of DF during digestion is discussed and the physiological implications of such behavior are highlighted. In particular, four aspects of DF behavior in the GIT are described that contributes to its health effect: (i) the effect of plant cell walls on bioavailability, (ii) the effect of DF on the rheological and colloidal state of digesta, (iii) the binding of DF with phenolic compounds, bile salts, mineral ions, and digestive enzymes, and (iv) DF fermentation in the large intestine and the effect on gut microbiota composition.
2. What is DF?
Since its introduction in 1953 (Hipsley, Citation1953), the definition of DF has been much debated and to date multiple definitions are available from several official national or international organizations (see in Jones, Citation2014). In all those definitions DF means carbohydrate polymers that escape digestion and absorption in the upper human intestine. However, they differ in the inclusion or not of synthetic or isolated compounds (i.e., not naturally part of food) and in the requirement of documented physiological health effects (and the type of physiological effect). In addition, several analytical procedures are available that differ in the types of carbohydrate that can be measured (Zielinski et al., Citation2013). CODEX alimentarius published its definition of DF in 2009 (Codex alimentarius, Citation2010). It states that “Dietary fiber means carbohydrate polymers with 10 or more monomeric units, which are not hydrolyzed by the endogenous enzymes in the small intestine of humans and belong to the following categories: (1) Edible carbohydrate polymers naturally occurring in the food as consumed, (2) Carbohydrate polymers, which have been obtained from food raw material by physical, enzymatic or chemical means and which have been shown to have a physiological effect of benefit to health as demonstrated by generally accepted scientific evidence to competent authorities, (3) Synthetic carbohydrate polymers, which have been shown to have a physiological effect of benefit to health as demonstrated by generally accepted scientific evidence to competent authorities.” The CODEX definition left to each national authority to decide whether to include oligosaccharides with a degree of polymerization (DP) between 3 and 9 (Jones, Citation2014). However, none of the analytical methods available so far is able to discriminate between DF with a DP < or > than 10 (Zielinski et al., Citation2013) and a cut-off at a chain length of 9 can be considered arbitrary since no abrupt change in physiological effect can be proved between DP 9 and 10 (Lupton et al., Citation2009). By 2014, most countries (including EU) have included oligosaccharides with DP 3-9 into their definition of DF whereas US Food and Drug Administration is still awaiting a decision. CODEX also proposed a standardized method for the analysis of DF, i.e., AOAC method 2009.01|AACCI approved method 32-45.01 that is a gravimetric method coupled with HPLC analysis (AOAC, Citation2012). A new method, AOAC method 2011.25|AACCI approved method 32-50.01 has the same performance of AOAC method 2009.01 but can also discriminate between soluble and insoluble DF. To date, these methods are the most inclusive methods of analysis for DF even though not absolutely comprehensive (Zielinski et al., Citation2013).
Table 1. Chemical composition and source of the most common classes of dietary fiber.
Whatever the definition used, DF refers to a chemically very heterogenous group of substances. DF may differ in the type(s) and relative content in monosaccharides constituents, the anomeric form of the monosaccharides (α or β), the type of chemical linkage between monosaccharides, the length of the linear polymer chain, the presence, distribution, and composition of branches attached to the linear chain. Based on the length of the linear chain, DF oligosaccharides with DP 3-9 and DF polysaccharides with DP ≥10 can be distinguished (Berlitz et al., 2009). DF polysaccharides include resistant starch (RS) and non-starch polysaccharides (NSP). RS is defined as the fraction of starch that escapes digestion and enters the colon. Resilience to digestion in the upper intestine is not an intrinsic property for starch and for that reason RS that is measured analytically in a food may not coincide with the amount of indigested starch that enters the large intestine after a meal because the latter may depend on the meal composition, storage, and cooking conditions and physiological factors (Dhital et al., Citation2015b). Lignin is not a carbohydrate but a heterogenous group of phenol polymers, which is included in all the DF definitions. In , the chemical composition and the sources of the most common classes of DF is provided. Whereas oligosaccharides and starch have a primary role as energy reservoir in plants, NSP are almost always constituents of the plant (or fungal) cell walls. In addition to DF naturally occurring in food, isolated DF molecules or DF-rich ingredients can be added to food products for their technological properties, e.g., water-holding, texturizing, emulsifying, and stabilizing properties or to provide extra health benefits.
3. Physicochemical properties of DF
Certain physicochemical properties of DF are key to understand their behavior during digestion: particle size, solubility, hydration properties, and viscosity (Guillon and Champ, Citation2000). DF solubility depends on its chemical structure and the interactions with water molecules. Linear DF that adopts ordered (semi)crystalline conformations in solution have limited solubility, e.g., cellulose. Branched DFs are less likely to form ordered structures in solutions and are therefore water-soluble. DF with charged and/or strongly polarizable groups (e.g., pectins) is generally water-soluble but solubility depends on pH and ionic strength of the medium. Based on their solubility in water DF is frequently classified in soluble (pectins, gums, oligosaccharides, etc.) and insoluble (cellulose, lignin, etc.) DF. Needless to say, this dichotomous distinction is arbitrary and does not reflect the vast range of solubility that can be found within DF also from the same class. Hydration properties refer to thermodynamics and kinetics of water absorption/desorption. It includes the swelling capacity, i.e., the volume occupied, after hydration under specified conditions, by a unit mass of DF, and water retention capacity (also expressed as water holding or water binding capacity depending on the way it is measured), i.e., the amount of water retained under specified conditions by a unit mass of DF. This water includes extra-particulate water, trapped between the DF particles and intra-particulate water, trapped within pores or surface of DF particles. The former depends on particle degree of compaction, i.e., on their size, shape, and elasticity. The latter depends on the total surface area of DF particles and that of intra-particle voids. Typically soluble DF has a much higher water holding capacity than insoluble DF. Viscosity is defined as the resistance to flow and DF has a varying capacity of producing viscous solution upon dissolution in water. This capacity strongly depends on DF molecular weight and concentration and is positively correlated to its solubility.
4. What food scientists should know about digestion physiology
Digestion is a complex physiological event that has evolved to ensure the most efficient absorption of nutrients from food. Digestion takes place in the GIT that can be thought of as an open-end canal that comprises oral cavity, esophagus, stomach, intestine (composed of duodenum, jejunum, and ileum), and large intestine (ascending, transverse, descending colon, rectum, and anus). A transversal section of the GIT is composed by the following tissues: mucosa, submucosa, muscularis mucosa, and serosa. The epithelium is the innermost layer of the mucosa, where most of the secretive, digestive, and absorptive processes take place. The epithelium is always covered in a thin layer of mucus, which has protective and mechanical functions. Secretive glands are associated with the GIT and supply water, digestive enzymes, hormones, and other components necessary for the digestive processes (protons, bile salts, etc.). The ab-oral progression of the food bolus through the GIT is provided by a highly coordinated wave-like movement known as peristalsis, which comprises contraction of the muscular mucosae behind the bolus and its relaxation in the section immediately after the bolus. Rhythmical contraction of small segments of the GIT provides mixing of the digesta. Oral processing is the first step in food digestion. During oral processing solid foods are mechanically disrupted to smaller particle, by means of chewing and biting. Food trituration allows the maximum extraction of nutrients from the ensuing digestion steps. Mixing with saliva contributes to turn non-flowing solid materials to a food bolus that can be swallowed and driven to the stomach by peristaltic movements of the esophageal muscles. Human saliva contains salivary amylase and lingual lipase that start the digestion of starch and lipid in the mouth as well as lysozyme, lactoferrin, and mucosal glycoproteins (mucins) (Humphrey and Williamson, Citation2001). The amount of starch hydrolyzed in the mouth is significant (Hoebler et al., Citation1998), but the extent of lipid hydrolysis in the mouth is negligible and its physiological significance is not clear (Kulkarni and Mattes, Citation2014). In the stomach, food disintegration continues even further for the simultaneous action of acidic gastric juice, mechanical mixing, and the enzymatic activity of pepsin (a proteolytic enzyme) and gastric lipase. Stomach pH ranges from 1 to 3 in the fasted state but after food ingestion it can increase to 5–7 depending on the buffering capacity of the meal (Carriere et al., Citation1991). Because of the low pH salivary amylase is denatured once entering the gastric environment whereas lipid hydrolysis can continue by the acid-resistant lingual lipase and gastric lipase. After gastric processing, food particles' sizes are in the order of magnitude of mm and the bolus is now referred to as chyme. The chyme is emptied from the stomach at a variable rate depending on the food ingested but it is usually complete after 150–180 minutes (N'Goma et al., Citation2012). Liquid or poorly viscous fluids are emptied faster than solid foods. The small intestine is the main site for food digestion and nutrients absorption. Thanks to pancreatic juice containing bicarbonate salts, intestine pH increases to 5–7. Pancreatic juice contains a variety of enzymes for the digestion of starch, proteins, and fat. Macromolecules are broken down to component monomers, i.e., sugars, amino acids and peptides and fatty acids that are efficiently absorbed by a variety of membrane-bound brush-border transporters. Proteins and peptides are hydrolyzed by trypsin, chymotrypsin, elastase, carboxypeptidase. Starch is hydrolyzed by pancreatic α-amylase and the resulting oligosaccharides are further converted by membrane-bound α-glucosidase into glucose. Membrane-bound sucrase and lactase phlorizin hydrolase complete the hydrolysis of oligo- and disaccharides into monosaccharides. Lipids are hydrolyzed by pancreatic lipase (together with co-lipase in a complex), cholesterol esterase, and phospholipase A2. The resulting free fatty acids and lipophilic compounds (e.g., vitamin A, D, carotenoids, etc.) are incorporated in mixed micelles stabilized by bile salts (acting as surfactants) and unilamellar vesicles and absorbed through pinocytosis by the epithelial cells. Other nutrients and molecules are taken up by specific transporters or pass through the intestinal epithelium by passive diffusion. Human small intestine provides an absorptive surface of approximately 200 m2 for the presence of the plicae circularis and villi as well as of the brush border on epithelial cells (Moffet et al., Citation1993). Digestion of food is completed in the large intestine where what escapes absorption in the upper intestine (mainly DF) is fermented by the local microbiota. Short chain fatty acids are typical products of bacterial fermentation of DF in the colon. In the large intestine, some nutrient absorption can occur along with the re-absorption of a substantial amount of water from the chyme before faeces are expelled. Digestive processes (secretion of enzymes and other factors, gut motility) is governed by a series of complex neuro-hormonal pathways (summarized in from Brownlee, Citation2014) that are controlled by luminal chemical profile and bulk. The former is sensed by specialized epithelial chemosensor entero-endocrine cells (Sternini et al., Citation2008), and the latter by mechanoreceptors (stretch activated neural cells) occurring within the muscularis mucosa (Furness, Citation2000).
5. Structural and chemical changes of DF during digestion
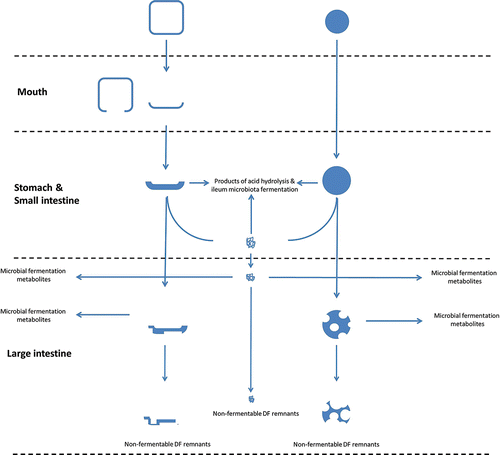
Despite the view of DF as an inert material in the GIT, it indeed undergoes structural and chemical changes that impact its behavior during digestion. A schematic picture of such changes can be found in . In vegetal tissues, DF is part of intact cell walls whereas DF-rich commercial preparation or ingredients occurs as particles of varying size, purity, and composition where DF can be still part of cell walls or isolated from it. During oral processing, the mechanical stress causes the cell walls to break open and to be reduced to smaller fragments. The level of fragmentation has a major impact on nutrients bioavailability as discussed in the next section. During passage through the stomach and the intestine, DF will absorb water and swell to a varying extent depending on its specific hydration property. Swelling is followed by partial solubilization of soluble DF from cell walls (fragments) and particulate material into the digestive fluids. Despite certain DFs are readily soluble when in isolated form, solubilization is limited when DF is still part of a cell wall structure because of strong chemical interactions with other cell wall components. Those interactions can become less strong as digestion proceeds. For instance, digestion of protein components of the cell wall, which are cross-linked to DF increases DF solubility (Robertson et al., Citation1997). Thus, even if thermodynamically favored, the solubilization process may not be complete within the time scale of meal digestion. As effect of solubilization the amount of soluble DF in the liquid fraction of the GIT increases. Once in solution, DF molecules can remain disperse or aggregate to a varying extent producing colloidal particles (Ulmius et al., Citation2012). Simultaneously, solubilization of DF from cell wall (fragments) alters the permeability of the cell wall to digestive fluids (see Section 6.1) whereas solubilization of DF material from particles may reduce their size (Hardacre et al., Citation2014) and change the particle surface and structure, e.g., increasing the inter-particles' voids and the intra-particles' pores and thus the hydration and binding properties of the remaining material. Beside structural changes, DF undergoes chemical changes in the upper intestine. By definition DF is resistant to hydrolysis by human digestive enzymes yet pancreatic lipase and other esterases might exert some hydrolytic activity toward methyl esters, O-acetyl esters, and N-acetylamide groups in DF molecules (Miller et al., Citation1995). At the very low pH of the stomach, the glycosidic linkages between component monosaccharides and ester linkages can suffer acid hydrolysis. The stability of the glycosidic bond to acid hydrolysis depends on the sugars involved, the anomeric form of the sugar, the position within the molecule, and the presence of substituents on the sugar molecule (Berlitz et al., 2009). In general, neutral polysaccharides are more stable at low pH compared to polyanionic polysaccharides (Smidsrod et al., Citation1966). Based on a limited number of studies, it can be concluded that DF largely retains its chemical structure during gastric digestion but some hydrolysis may occur, especially if gastric pH remains low. For instance, arabinose moieties may be limitedly released from arabinoxylans side chains but the xylan linear chain remains intact (Zhang et al., Citation2003). In contrast, Mikhaleva et al. (Citation2011) reported a 7–18% of pectin hydrolysis after digestion at pH 1.9 for four hours. The hydrolysis resulted in release of reducing sugars and fragments of the pectin linear chain. Miller et al. (Citation1995) also reported limited hydrolysis of ester bonds in spinach cell wall pectins, being the methyl ester groups more resistant of the O-acetyl groups at pH 1.8 whereas the opposite was observed at pH 8.8. In comparison to pectins and arabinoxylans, β-glucan is resistant to very low pH for long times (Johansson et al., Citation2006). Moreover, a microbial community is harbored in the ileum, which also relies on carbohydrate fermentation as energy source. The fermentative activity of the ileum microflora is very intense in certain animals, e.g., pigs (Millar and Chesson, Citation1984), but very little is known in humans. Studies on ileostomy patients suggest that a minor but significant fraction of DF can be degraded by the ileum microflora (Tornquist et al., Citation1986; Livesey et al., Citation1995; Sundberg et al., Citation1996). Once in the colon DF undergoes a very intense fermentation by the local microbiota as described in the next sections. As an effect all the fermentable DF (both solubilized and incorporated in particles of colloidal size) is degraded leaving remnants and soluble non-fermentable DF molecules that are then expelled as waste.
Figure 1. Changes of structural properties of DF during digestion. DF occurs in food in cell walls (a) or particles of varying size (b). Grinding and chewing open up cells and produced fragments of cell wall material. In the stomach and small intestine, DF will absorb water and swell to a degree that depends on its chemical and structural properties which is followed by the solubilization of the soluble DF fraction. Acid hydrolysis in the stomach and ileum microbiota fermentation can occur in the upper intestine. Fermentable DF (both soluble and incorporated in cell walls and particles) is fermented in the large intestine leaving remnants made up of non-fermentable DF.
6. The behavior of DF during digestion explains its healthy effect
The behavior of DF during digestion is key to understand its physiological effect. In the following sections, four aspects of such behavior are described. Clearly, the systemic health effect exerted by DF stems from a complex interplay between different mechanisms operating simultaneously. In , the behavior of DF during each step of digestion and its physiological implications for human health is schematically described. The readers can use the table as a guidance through the following sections.
Table 2. Overview of the effects of DF in each digestion step and their physiological implications.
6.1. The cell wall DF and nutrient bio-accessibility
Dietary DF is mostly part of plant (and fungal) cell wall. The plant cell wall is a supramolecular structure that confers protection and rigidity to the cell and modulates its communication with the surrounding environment. The plant cell wall is made up of two distinct layers: a primary cell wall that forms during plant growth and a secondary cell wall that forms after plants have completed their growth. The most common structural model for plant primary cell walls described a network of cellulose fibrils-hemicellulose (xyloglucan, arabinoxylans, β-glucan, etc.) embedded in a network of pectins (Carpita and Gibeaut, Citation1993; Cosgrove, Citation2001). Within this structure, cellulose and hemicelluloses function as a load-bearing structure, while pectin confers plasticity and controls the porosity of the cell wall. However, recent studies proposed a new paradigm for the cell wall structure, with a single network of pectin, cellulose, and hemicellulose (Dick-Perez et al., Citation2011). In grains and seeds (but not in rice), cell walls are comparatively poorer in pectins and cellulose whereas arabinoxylans and β-glucans dominate (Burton and Fincher, Citation2014). Secondary cell walls are made up of a network of cellulose and lignin whereas pectins are low or absent (Pettolino et al., Citation2012). Compared to primary cell walls, secondary cell walls are minor components of human diet. Pectins are also the main component of the middle lamella, a thin layer that is responsible for the adhesion of adjacent cells in plant tissues. The chemical structure of the cell wall and its organization varies across plant species and across tissues within the same plant and is influenced by environmental and developmental factors (Boerjan, Citation2003; Ralph, Citation2010; Vanholme et al., Citation2010). Cell wall structure and composition plays a pivotal role in the bioavailability of micro- and macronutrients from plant-based foods. In order to be available for absorption, intracellular compounds must pass through the physical barrier represented by the cell wall. If cells are still intact when ingested, they can efficiently encapsulate intracellular nutrients. Intracellular nutrients can still pass through pores in the cell wall but this may occur slowly and may be restricted by the pore size. Digestion of macromolecules can also occur inside the cells provided that digestive fluids can enter the cell.
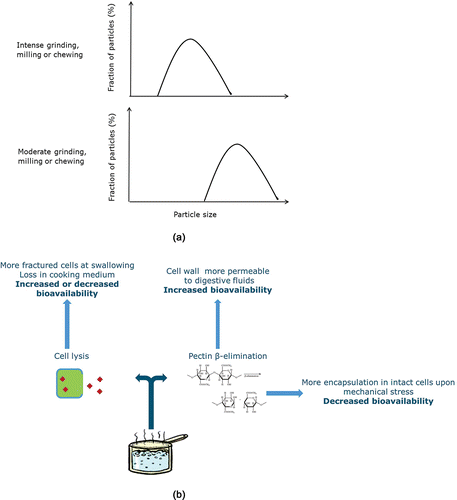
The barrier effect exerted by the plant cell wall is the result of a very complex interplay between cell wall chemical composition and structure and modifications suffered upon digestion, as well as industrial, domestic, and oral processing (). Structural integrity of plant cell walls is largely retained during gastrointestinal digestion (Berg et al., Citation2012; Brummer et al., Citation2014; Edwards et al., Citation2015a). However, minor chemical modifications to cell wall may occur when exposed to harsh gastric conditions (Miller et al., Citation1995). Noah et al., (Citation1998) reported distinctive changes in the cellular structure of white beans (Phaseolus vulgaris L.) containing digesta collected from ileostomy subjects after ≈12 hours of digestion. Hydration of the cell wall during digestion and solubilization of soluble components may change its structural organization. Mandalari et al. (Citation2008) observed a two-fold increase in almond cell wall thickness upon 3 hours digestion and six-fold increase after 12 hours and suggested a possible facilitation of diffusion through the swollen cell walls. Tydemans et al. also reported swelling of cell wall in raw carrots tissues and solubilization of pectins. In raw fruits and vegetables, reduction of particle size by intense mechanical grinding, milling, or chewing increases the fraction of fractured cells that are exposed on the particles' surface (). This explains the inverse relationship between food particle size and bioavailability that is frequently reported. Upon thermal treatments changes in the chemical structure of the cell wall material occur (). At higher temperature, the β-eliminative hydrolysis of pectins is accelerated and a significant amount of pectins is solubilized from the cell wall and middle lamella. The solubilization of pectins from middle lamella would change the cell wall strength in relation to the strength of cell–cell adhesion and thus the fracture behavior of plant tissue upon sub-sequent mechanical and oral processing (Waldron et al., Citation2003; Tydemans et al., 2010). In raw crunchy fruits and vegetables where cell adhesion is quite strong, tissue fracture occurs mainly through cell walls. In this case, under mechanical stress or oral processing the number of fractured cells is relatively high. In mealy fruits or in fruits and vegetables after thermal treatment, cell adhesion is weakened and tissue fraction would occur mainly along cell walls, producing, upon mechanical stress, clusters of intact cells. On the other hand, the solubilization of pectins (and possibly of other constituents of the cell wall) may increase the permeability of the cell wall by increasing the cell wall pore size. Aribas-Agusti et al. (Citation2015) proved that permeability of carrot cell wall toward molecules of limited size is enhanced after heat treatment because of the solubilization of native pectin. Lemmens et al. (Citation2010) clearly demonstrated that whereas particle size is a limiting factor for β-carotene bio-accessibility in raw carrots, the effect is partially or completely lost upon gentle (three minutes) and intense (25 minutes) boiling, respectively, i.e., β-carotene was efficiently released also from large particle size because of the solubilization of cell wall pectin. In addition, thermal treatments are also accompanied by cell lysis as a consequence of the pressure generated within the cell by steam. In the light of this complex interplay between integrity and permeability, it is difficult to predict the effect of thermal treatments on nutrient bio-accessibility in plant-based foods. Incidentally, during maturation/ripening, solubilization of pectins by enzymes pectin methyl esterase (PME) and polygalacturonase (PG) can also change fracture behavior of the plant tissue and cell wall permeability. Despite an effect of the fruit/vegetable maturity/ripeness stage on bio-accessibility can be easily envisaged, this issue has not been addressed in the scientific literature so far.
Figure 2. Effect of industrial, domestic, or oral processing and bio-availability of a generic intracellular nutrient/phytochemical. (a) In raw fruits and vegetables, a more intense mechanical grinding, milling, or chewing increases the fraction of fractured cells that are exposed on the particle surface and thus the nutrient bioavailability. (b) Upon thermal treatment (e.g., domestic cooking), cell wall structure and permeability changes and thus its barrier effect. A fraction of plant cells may lyse releasing their content in the digestive fluids or in the cooking medium, if any. Simultaneously, hydrolysis of pectins in the primary cell wall increases permeability to intracellular compounds whereas hydrolysis of pectin in middle lamella changes the fracture behavior of vegetal tissue, which may result in more cells intact upon chewing or grinding.
The barrier effect of cell walls on bioavailability can be either beneficial or detrimental depending on the nutrient. In raw almond, when constrained in intact cells lipids body cannot be efficiently emulsified and digested compared to extracellular lipids, which greatly reduced almond caloric content (Ellis et al., Citation2004; Mandalari et al., Citation2014; Grundy et al., Citation2015a, Citation2015b). Transmission electron microscope (TEM) micrographs of almond pieces collected from ileostomy patients showed loss of intracellular fluids in the five most adjacent cell layers to the fractured surface of the almond particles after ≈12 hours of small intestinal digestion (Mandalari et al., Citation2008). Conversely, after three hours of vitro digestion of almond particles, only lipid digestion from the layer of fractured cells on the particles surface was observed. Based on this observation, Grassby et al. (Citation2014) have developed a theoretical model to predict the bio-accessibility of lipids from almonds from particle size distribution of almond after chewing/grinding. The theoretical model was developed from simple geometrical principles and correlates the amount of nutrient released from the almond matrix to the total area of ruptured cells exposed on the surface of almond particles. Studies on ileostomy patients have also shown that starch locked up into intact cells is digested more slowly compared to free starch in wheat endosperm (Edwards et al., Citation2015a) and barley meals (Livesey et al., Citation1995), which is beneficial for the management and prevention of diabetes. However, the amount of resistant starch was unaffected in wheat endosperm (Edwards et al., Citation2015a). The microscopic analysis of 10 hours digesta of wheat endosperm meal showed that starch is digested even in apparently intact cells, which demonstrates the permeability of the cell walls to pancreatic α-amylase. Intact cells also hinder starch hydrolysis in legume/pulses (Berg et al., Citation2012; Brummer et al., Citation2014), which explains their relatively low glycemic index. The barrier effect to mass transfer may not be the sole cause for the partial and slowed starch digestion. When constrained within intact cells, starch granules are tightly packed compared to gelatinized starch leached out from broken cells. This reduces the effective surface for hydrolysis (Berg et al., Citation2012). Furthermore, a rigid cell wall may limit the degree of gelatinization of starch upon cooking, which makes it more resistant to digestion. Edwards et al. (Citation2015b) have demonstrated that chickpea starch is only partially gelatinized during hydrothermal processes and the degree of gelatinization highly correlate with the degree of starch hydrolysis. This effect was not seen in wheat particles, likely because wheat cell wall imposes less restriction to water and heat transfer and thus to starch gelatinization. Cell wall integrity has also been demonstrated to limit bio-accessibility of nutrients/phytochemicals, which has a detrimental effect on health. This has been demonstrated for instance for β-carotene in carrots (Bengtsson et al., Citation2010; Lemmens et al., Citation2010; Tydemans et al., 2010; Netzel et al., Citation2011; Moelants et al., Citation2012) and mango (Low et al., Citation2015) and lycopene in red carrots (Palmero et al., Citation2013) as well as for proteins (Melito and Tovar, Citation1995).
Finally, it must be remembered that nutrients that have escaped absorption in the small intestine because encapsulated in intact cells can be released in the large intestine upon fermentation of cell wall material by the local microbiota. Some (but not all) of these compounds, e.g., polyphenols, may be absorbed by the colon epithelium as such or after conversion by microbiota. The interrelations between plant cell wall composition, effect of processing, microbial fermentation, and bioavailability in the large intestine is an issue that warrants further investigation in the future.
6.2. DF and the physical state of digesta
Interaction of DF with digestive fluids and other dietary components contributes to shape the colloidal state and the rheological properties of the digesta. Among these properties viscosity has been the most thoroughly investigated. The capacity of certain DF to produce viscous solutions in aqueous media is considered the major mechanism behind the capacity of soluble DF to lowering post-prandial blood glucose (Jenkins et al., Citation1987; Dikeman and Fahey, Citation2006; Scazzina et al., Citation2013) and serum cholesterol (Jenkins et al., Citation1975; Brown et al., Citation1999; Vuksan et al., Citation2011). Increasing digesta viscosity would change the digesta flow regime within the GIT from turbulent to laminar impairing an efficient mechanical mixing of digesta with digestive fluids and nutrient transport from the GIT lumen to the mucosa. Furthermore, an increase in the viscosity of the unstirred water layer at the surface of the mucosa would slow down the diffusion of nutrients (Eastwood and Morris, Citation1992; Wursch and Pi-Sunyer, Citation1997; Malkki, 2001). Increased viscosity in the stomach would slow down gastric emptying and thus the rate of nutrients' appearance in the small intestine. Which mechanism is actually responsible for the observed effect on glycaemia and blood cholesterol is still controversial (Jarijs et al., 1984; Edwards et al., Citation1987; Leclere et al., Citation1994). Recent data from in vitro models of digestion point at a modest decrease in starch hydrolysis rate and diffusion coefficients for glucose (1.5–2.5 times) against a 100-fold increase of digesta viscosity and suggest the delay of gastric emptying as the major mechanism contributing to the lowering of post-prandial glycaemia (Dhital et al., Citation2014). It is also apparent that the relationship between viscosity and enzymatic/absorptive processes is not always linear. Hardacre et al., (Citation2015) have measured the evolution of apparent viscosity of digesta upon in vitro small intestinal digestion of gelatinized starch/guar gums mixtures and reported that the rate of starch hydrolysis did not slowdown in parallel to a decrease of digesta viscosity (caused by progressive digestion of starch). An increased digesta viscosity may also impair the efficiency of emulsification in the small intestine, which produces less and smaller lipid droplets upon mechanical mixing (Pasquier et al., Citation1996), which reduces bioavailability of lipophilic compounds. It has been also suggested that the delayed and reduced absorption of nutrients in the duodenum and jejune may contribute triggering the ileal break in the distal ileum, i.e., a feedback mechanism that results in inhibition of proximal gastrointestinal motility and secretion, which induces feelings of satiety and reduces ad libitum food intake (Maljaars et al., Citation2009).
Upon consumption of a standard meal, digesta consists of a suspension of particulate matter in a fluid phase that becomes increasingly less fluid as the chyme proceeds toward distal intestinal segments as a result of the progressive absorption of water. Hence, the behavior of digesta changes progressively from that of a pseudoplastic fluid, which viscosity depends on the liquid fraction and the concentration, shape, size, and buoyancy of the particulate matter, to that of a particulate aggregate, which properties depend mostly on the mechanical properties of the solid elements (Lentle and Janssen, Citation2008). Since whole digesta viscosity is higher than viscosity of digesta liquid fraction, viscosity of digesta increases along the GIT. DF can increase the viscosity of digesta by both increasing the viscosity of its liquid fraction and by affecting the physical properties of the digesta particulate material. The capacity to increase viscosity of digesta liquid fraction is typical of soluble DF such as pectins, gums, arabinoxylans, and β-glucans and depends on several factors. The first is DF chemical structure. In general, rigid or rod-like polymers that occur in solution as extended molecules have higher viscosity than polymers of equivalent molecular mass, which exhibit a more compact conformation. This is because viscosity reflects the effective volume of the polymer in solution, i.e., the volume of a sphere which diameter is equal to the longest dimension of the macromolecule. In this respect, branched polymers tend to have a smaller effective volume than linear polymers of equivalent mass (Berlitz et al., 2009). For the same reason, high molecular weight (HMW) polysaccharides produce more viscous solutions than their corresponding lower MW depolymerization products. Depolymerization of DF can occur during food processing. Pectins are hydrolyzed upon thermal treatments. β-glucan can be also partially hydrolyzed during baking, partially losing its viscosity upon bread baking (Jaskari et al., Citation1995). Cereals β-glucanase can hydrolyze β-glucan during dough mixing and proofing thus reducing viscosity of final bakery products (Izydorczyk et al., Citation2000). When extracted from different sources and with different extraction procedures, DF molecules of the same class may largely vary in MW. Differences in the chemical structure for DF from the same class may also generate differences in viscosity. For instance, viscosity of pectin solutions decreases with the DM (Yoo et al., Citation2006). Arabinoxylans extracted from different lines of rye may produce solutions with substantial different viscosity, which is related to their MW and to the degree of branching (Ragaee et al., Citation2001).
The viscosity of a polysaccharide solution is also related to its concentration and often increases with its concentration with a power-law relationship (Berlitz et al., 2009). When the concentration is lower than the overlap concentration, c* (concentration at which the polysaccharides chains begin to overlap), viscosity increases little with c whereas at c > c* viscosity steeply increases with c. Dilution of DF with digestive fluids will therefore reduce (often dramatically) viscosity of a DF solution but this effect varies with the DF type (Edwards et al., Citation1987). Fabek et al., (Citation2014) reported that only xanthan gum was able to significantly retain its viscosity after mixing with digestive fluids compared to other gums (locust bean, guar, fenugreek, and flaxseed). The level of dilution may vary within certain range depending, e.g., on the level of secreted digestive fluids (which depends on meal characteristics) and amount of water consumed at meal. In addition, DF concentration in the liquid fraction of digesta depends on the amount that solubilizes from particulate material and solubilization can be only partial (see ). For instance, solubility in the intestinal fluids of β-glucan from fine barley flour is higher than in coarse barley flour (Lazaridou et al., Citation2014). The viscosity of charged polysaccharide solutions also depends on ionic strength and pH of the solution. At higher ionic strength, the electrical charges are more shielded and a charged polymer may adopt a more compact or globular conformation in solution. At lower ionic strength, the negative charges tend to increase their relative distance and the polymer takes on a more linear conformation, which increases its effective volume and thus the viscosity of the solution. Ionic strength of digestive fluids is known to vary among individuals and also among days within the same individual (Lindahl et al., Citation1997) and an additional source of variation is expected from the diet. Similarly, pH changes the ionization of charged groups, which impacts on the polymer conformation, e.g., polyanions are expected to produce more viscous solutions at neutral than acidic pH. Interactions with other dietary components are also crucial. After a typical meal, several different DF polysaccharides may occur in the GIT and this may have synergic or antinergic effect (Edwards et al., Citation1987; Pal, Citation1996) on viscosity. The same holds true for interactions with other dietary biopolymers such as proteins (Schmidt and Smith, Citation1992; Berlitz et al., 2009). At relatively high concentrations, a mixed DF/proteins solution may phase separate because of thermodynamic incompatibility among biopolymers and the rheological behavior of the resulting system may differ significantly from that of individual phases (Bourriot et al., Citation1999). It has been hypothesized that chyme may exist as a phase separated system of protein dispersed particles in a continuous gel-like phase containing the polysaccharides (Tolstoguzov, Citation2000). This phase separation might have an effect on enzymatic digestive processes also because of the potential distribution of reactants and enzymes between phases (Li et al., Citation2002).
The capacity of DF to change the physical properties of the digesta particulate material depends on its ability to change the disintegration kinetics of foods or the size distribution of digesta particles, e.g., by gluing together food particles or by contributing itself to the insoluble particulate material. In this respect also insoluble DF may increase digesta viscosity and slow down nutrients absorption, as reported, e.g., for crystalline cellulose (Takahashi et al., Citation2005), wheat bran particles (Sakata et al., Citation2007; Hardacre et al., Citation2015), or wood particles (Hardacre et al., Citation2015). The way of administration of DF, whether in its native cell wall form, as purified compound incorporated in food or co-digested with other food in a complete meal will therefore differently affect the viscosity of digesta but this has been poorly investigated. Viscosity of in vitro digesta of alginate is two-fold higher than when an equivalent amount of alginate is either incorporated or co-digested with bread (Houghton et al., Citation2015). When isolated DF is formulated into food, additional mechanisms can contribute to explain the observed effects on nutrients absorption beside viscosity. In bread and pasta, DF can form a physical ‘‘barrier” around the starch granules that impairs access to α-amylase (Brennan et al., Citation1996) but may also alter the gluten network in such a way to protect or expose (depending on formulation) the starch granules to α-amylase (Foschia et al., Citation2015). Finally, viscosity also depends on the shear rate applied, being lower at higher shear rates and dependence from shear rate depends on the polysaccharide concentration. The solution behaves as a Newtonian fluids at c < c*, i.e.. the deformation is proportional to the applied shear rate at each shear rate and as a shear-tinning fluids at c > c*, i.e., the deformation decreases as the shear rate increases. Since digesta are likely exposed to variable shear rates during transit along the GIT, viscosity is expected to vary locally.
Some DFs are also able to form gels under the physiological conditions of the GIT. Gels are solids, which consist of polymers cross-linked to form an interconnected network immersed in a liquid medium, typically water (Oakenfull, Citation1987). From a rheological standpoint, gel is a viscoelastic system with a storage modulus (G') larger than the loss modulus (G”) (de Vries, Citation2004). The gelation properties of DF hydrocolloids has been studied mostly in the gastric environment and poorly investigated under small intestinal conditions, even though there are evidences that β-glucan and chitosan may form gels there (Rodriguez and Albertengo, Citation2005; Ulmius et al., Citation2012). Gelation of the gastric content has been reported to enhance satiety and reduce the rate of gastric emptying (Hoad et al., Citation2004). The gelation mechanism that is of interest here is the ionotropic gelation of anionic polysaccharides, i.e., gelation triggered by cross-linking of anionic polysaccharides by cations, mainly calcium ions. The gel properties may depend on the polymer concentration, its molecular structure (e.g., DM of pectin), pH and time of exposure, and the concentration in cations, mainly calcium ions (Norton et al., Citation2007). Gelation kinetics and gel mechanical properties are equally important for the physiological effect. Gel has to resist the mechanical stress exerted by the stomach. Moreover, gel formation should be fast enough to allow the gel structuring in the stomach but slow enough to allow most of the gastric content to be incorporated in the gel. Once formed, the gels should also retain their physical properties upon variable exposure to gastric acidic fluids. A typical example of DF that forms gels in the stomach is alginate. However, its gelation in the stomach is too fast and the gel properties are too sensitive to gastric conditions (Hoad et al., Citation2004; Norton et al., Citation2011). An alternative to alginate is represented by gellan gum, a complex bacterial polysaccharide, and pectins (Norton et al., Citation2007; Spyropoulos et al., Citation2011). Gastric gelation of DF hydrocolloids can also be modulated by other dietary components. It has been showed that a mixture of whey protein and pectin at pH<PI of the protein is able to form an intragastric gel at much lower polysaccharide concentrations because of the electrostatic interactions between anionic polysaccharides and cationic proteins, although no gelation was observed in a single biopolymer system (Zhang et al., Citation2014a). Also xanthan gum and carragenans are able to form gels upon mixing with gastric juices and whey protein isolates (Zhang et al., 2104b) and gel strength was stronger for carragenans/whey protein mixtures likely because of the higher negative charge of the carragenans. Blending of different polysaccharides may also offer an alternative option for the modulation of intragastric gelation. Gelation induced by some DF hydrocolloids may be a suitable strategy to control satiety and nutrient intake for simple liquid or soft food but its efficacy on complex food or complete meals should be proved.
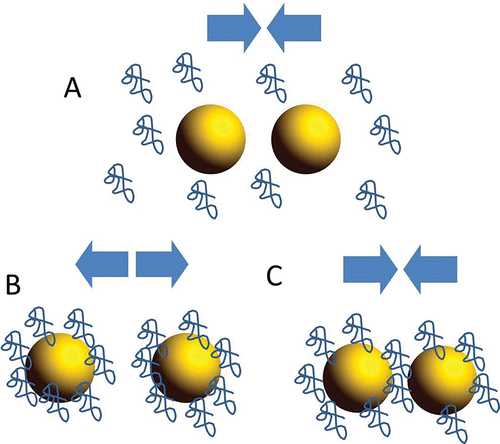
Another effect of DF concerns the effect on lipid emulsion stability. Due to their water insoluble nature, dietary lipids have to be emulsified to be digested and this occurs in the stomach and in the duodenum by means of mechanical mixing combined with the emulsifying activity of bile salts and other endogenous or dietary surfactants (e.g., phospholipids). This emulsification results in lipid droplets which size ranges between 10 and 50 μm (Pasquier et al., Citation1996). Since gastric and pancreatic lipases act at the interface between the oil droplets and water, the higher the amount of emulsified lipids and the smaller the droplet size the higher is the water/lipid interface and the higher the lipolysis rate. Controlling the rate of lipid digestion can be used as a strategy to reduce obesity and the incidence of other fat-rich diets related diseases. DF polymers can either stabilize or destabilize emulsions in the gastric and duodenal compartments depending on DF type, concentration, presence of other biopolymers in solution, pH, and ionic strength, and the nature of the emulsion-stabilizing surfactant, in a very complex interplay that has not been completely elucidated (). For instance, DF can destabilize emulsions by promoting extensive depletion or bridging flocculation of the fat globules. Depletion flocculation is a phenomenon first described by Asakura and Oosawa (Citation1954). In the presence of polymers that do not interact nor absorb on droplet surface, a depletion zone is formed around the lipid droplets that is sterically inaccessible to the polymers. In such a zone, the concentration of the polymers is lower than in the bulk of the dispersed phase. In such conditions, it is thermodynamically favorable for the droplets to coalesce so as to reduce the depletion zone and “dilute” the polymer in the bulk. A depletion flocculation mechanism to explain the effect of DF on lipid digestion was first proposed by Minekus et al., (Citation2004) who observed that partially hydrolyzed guar gum reduced lipolysis rate and absorption of cholesterol from full-fat yoghurt. When DF irreversibly absorbs on the droplet surface forming a stable thick layer, approaching droplets will repel each other (steric repulsion), which results in stabilization of the emulsion. This may be counteracted by the fact that absorption of DF to the droplet interface may hinder the access of lipase to the fat globules. Bridging flocculation requires instead that the polymer reversibly absorbs to droplets surface forming a dilute or incomplete absorbed layer. When this happens multiple droplet can bridge together, which promotes their flocculation. Beysseriat et al. (Citation2006) showed that, at neutral pH of the small intestine, positively charged chitosan can destabilize corn oil in water emulsion stabilized by tween by adsorbing on the surface of negatively charged droplets and inducing their coalescence through a bridging flocculation mechanism. Instead, negatively charged pectin cannot adsorb onto lipid droplet but is able to destabilize the emulsion by inducing depletion flocculation. Espinoza-Ruiz et al. (2016) also reported increased coalescence of corn oil in water emulsions stabilized with tween 80 in the presence of pectins extracted from banana passion fruit and corresponding decrease in the rate of lipid digestion. The depletion attraction induced by pectin molecules increased as the MW and the total electric charge (which depends on pectin DM as well as ionic strength and pH of the medium) of the pectin molecules increases with the molecular weight being a much more important factor than DM. Beside pectin, also methyl cellulose is able to induce substantial depletion flocculation of tween stabilized corn oil/water emulsions at high concentrations (Espinal-Ruiz et al., Citation2014a).
Figure 3. Schematic representation of mode of stabilization and destabilization of oil droplets (yellow spheres) in an oil-water emulsion by dietary fiber (blue random coils). (A) When DF does not absorb to the droplet surface, flocculation of lipid droplets may result from the steric effect due to the presence of polymers in the continuous phase (depletion flocculation). (B) when DF stably absorbs on droplet surface forming thick layers, steric repulsion ensues that stabilizes the emulsion. (C) When DF forms incomplete layers on droplet surface, bridging of droplets may cause their flocculation (bridging flocculation).
6.3. Binding properties of DF
DF polysaccharides may non-covalently bind, adsorb, or entrap other dietary components during transit along the GIT and this capacity can also explain its physiological effect. The first relevant case is the binding with phenolic compounds. Phenolic compounds (henceforth PC) are plant secondary metabolites constituted by one (phenolic acids) or multiple (polyphenols) phenol rings. The number of rings, their linkage, and the number and type of functional groups attached to them define different classes of PC. PC, especially polyphenols, have raised interest because of their potential antioxidant (Hertog et al., Citation1993), anti-estrogenic (Yuan et al., Citation2007), anti-inflammatory, immunomodulatory (Ruiz and Haller, Citation2006; Park et al., Citation2007; Ruiz et al., Citation2007), cardioprotective, and anti-carcinogenic (Ganry, Citation2002; Liu, Citation2004) activities. PCs have mainly intracellular localization in intact plants whereas some phenolic acids are covalently bound to arabinoxylans, lignin, and pectins of the cell wall. These latter must be considered as structural part of DF (Vitaglione et al., Citation2008) and will not be considered here. Non-covalent complexes between DF and PC may already form in the food product during processes that break down cells and release the intracellular content, e.g., after blending or pureeing. The amount of PC bound to DF varies according to DF content and composition, processing, PC content of food, and storage time. Complexes may also form in the mouth as soon as PCs are released from broken cells upon mastication or even in the GIT.
The nature of the non-covalent interactions between DF and PC has been thoroughly reviewed (Bordenave et al., Citation2014; Jacobek, Citation2015). The interactions between DF and PC may be hydrogen bonding and electrostatic interactions but also hydrophobic interactions. Under specified conditions, the amount of PC that bounds to DF depends on DF and PC type. Moreover, since absorption is a surface phenomenon, the amount of PC bound would also depend on the total surface of DF, i.e., it would be inversely proportional to DF particle size (le Bourvellec and Renard, Citation2005a). Since most of DF is ingested as cell walls, the interaction between PC and plant cell walls is of particular importance. Studies on apple polyphenols have found that the interactions mainly involve pectins through ionic interactions and increase with the PC MW (Le Burvellec et al., Citation2005a, Citation2005b). Padayachee et al. (Citation2012a) have investigated the interactions of cell wall analogs (in the form of bacterial pectin-cellulose composites) with anthocyanins (a class of polyphenols) and phenolic acids. The authors reported a two-steps mechanism of binding. The first step is very quick (few seconds) and resulted in up to 18% of anthocyanins bound at neutral pH. Binding ability was slightly higher for non-acetylated compared to acetylated anthocyanins. The second step occurs slowly through unspecific stacking of anthocyanins on pectin-cellulose composites. Pectins seem to bind relatively higher amount of anthocyanins through hydrogen bonding or electrostatic interactions, compared to cellulose that binds PC through hydrophobic interactions. The same two-step mechanism was described by the same authors in the case of cell wall/phenolic acid interactions (Padayachee et al., Citation2012b). Cellulose seems more important than pectin in binding phenolic acid probably due to the electrostatic repulsion between negatively charged pectin and phenolic acids and no differences were found among phenolic acids. The interactions between isolated DF molecules and PC have been also investigated. Phan et al. (Citation2015) reported that the binding capacity of cellulose for PC can be as high as 0.6 w/w depending on pH and PC concentration and is relatively fast. β-glucans has also been studied in much detail. The interactions between tea PC (catechin, epicatechin, epigallocatechin, and epigallocatechin gallate) and β-glucan mainly involve hydrogen bonding (Wu et al., Citation2011a; Gao et al., Citation2012) and oat β-glucan is able to bind not < than 40 mg/g at body temperature (Wu et al., Citation2011b). These interactions do not depend on β-glucan structure but rather on the level of hydroxylation, glycosylation, and galloylation of PC. From a physiological perspective the stability of pre-formed complexes to digestion is as important as the kinetics and thermodynamics of complexes formation in food but this has been poorly investigated so far. Since the dissociation of DF-PC complexes is an endothermic process, complexes pre-formed in food will partially dissociate when exposed to body temperature. Analogously, when the interactions between DF and PC are electrostatic, i.e., through charged or ionizable DF and PC functional groups, during residence in the stomach the relatively low pH is expected to favor dissociation because of reduced charge on DF and PC molecules. Padayachee et al. (Citation2013) showed that the amount of PC released from cell wall analogs-polyphenols/phenolic acid complexes after gastric and small intestinal in vitro digestion is very limited (<2%).
The physiological implications of DF-PC interactions are multiple. First, they modulate PC bioavailability. When absorbed to DF, PP will escape absorption in the small intestine but can be released upon fermentation of the carrier DF in the colon and absorbed therein as such or prior modification. It has been hypothesized that such transportation of PC to the lower gut is a major physiological function of DF (Saura-Callixto, Citation2011) and has been estimated that 50% of dietary PC are transported to the lower gut by DF (Saura-Callixto and Goni, Citation2006). Furthermore, when bound to DF, labile PC can be protected along passage through the GIT. For instance, anthocyanins are unstable at neutral pH of the small intestine, which contributes to their very low bioavailability in vivo. When absorbed on soybean flour or soybean proteins, anthocyanins are more stable at neutral pH and body temperature compared to free anthocyanins (Roopchand et al., Citation2012; Ribnicky et al., Citation2014). It is plausible to assume that interactions with DF may be as effective in protecting PC as protein–PC interactions. The second physiological consequence of DF-PC binding is that they may modulate PC biological activity. Since PC remains in the large intestine for relatively long time, they are able to quench the reactive radicals contributing to keep a reducing environment therein. In addition, several PC possesses inhibitory activity toward pancreatic enzymes (He et al., Citation2007; McDougall et al., Citation2008; Williamson, Citation2013). The interactions between DF and PC would reduce the amount of PC that can interact with digestive enzymes thus reducing their efficacy. This has been actually demonstrated in ternary systems procyanidins/trypsin/DF (Gonçalves et al., Citation2011) and in ternary systems procyanidins/α-amylase/DF (Soares et al., Citation2012) where the presence of DF increased the amount of free enzyme because of competition of procyanidins with DF.
The second relevant binding phenomenon involving DF is with bile acids. Bile acids are steroic acids synthetized in the liver and secreted with the bile. Taurocholic acid, glycocholic acid, taurochenodeoxycholic acid, and glycochenodeoxycholic acid are the most abundant bile acids in the human bile and are known as primary bile acids. Deoxycholic acid and litocholic acid are products of bacterial fermentation of bile acids in the large intestine and are known as secondary bile acids. Bile acids are essential for the emulsification of dietary lipids, formation of mixed micelles, and proper digestion and absorption of lipophilic compounds. The binding between DF and bile acids has important implications. First, it will facilitate the elimination of bile salts in the faeces, which is thought to be one of the major mechanisms for the cholesterol lowering effect of DF. Approximately 95% of parent bile acids and their bacterial metabolites are efficiently re-absorbed in the distal part of the small intestine in a process known as enterohepatic circulation, transported to the liver and used therein for the synthesis of new bile acids. When bound to DF bile acids escape re-absorption in the small intestine and the liver must use endogenous cholesterol to replenish the bile acids pool thus lowering the level of circulating cholesterol. In vivo studies have repeatedly demonstrated an increased excretion of bile salts in the faeces after administration of DF (Arjmandi and Reevesm, Citation1992; Lia et al., Citation1995; Madar and Stark, Citation1995). However, the anticholesterolemic effect of certain DF can be also ascribed to other mechanisms as reviewed by Gunness et al., (Citation2010a). Second, the binding to DF would make bile acids unavailable as surfactants in the small intestine thus disturbing lipid emulsification, formation of mixed micelles, and the complete digestion of lipids and their absorption. This may result in lower levels of circulating triglycerides but also to a reduced bioavailability of lipophilic nutrients.
The exact nature of the interactions between DF and bile acids depends on the DF and bile acid chemical structure. Lignin is known to be an excellent absorbent of bile acids (Kosikova et al., Citation2002). The binding capacity has been ascribed to hydrophobic interactions since it increases with acidity, degree of methylation of lignin, and hydrophobicity of the bile acid (Eastwood and Hamilton, Citation1968). Cellulose is reported to have a lower binding capacity compared to other DF classes (Story and Kritchevsky, Citation1976; Vahouny et al., Citation1980) but cellulose ethers are more effective than cellulose. Cellulose ethers bind via hydrophobic interactions with bile acids and the affinity is higher for more hydrophobic bile acids and for hydroxypropyl and hydroxypropylmethyl cellulose (Torcello-Gomez et al., Citation2015). Among soluble DF, β-glucan supplementation is known to increase the faecal excretion of bile acids (Lia et al., Citation1995; Ellegard and Andersson, Citation2007) but the actual mechanism behind the effect may be not (only) related to its bile acids binding capacity. Removal of β-glucan from oat flour is known to reduce the amount of bile acids bound in vitro (Sayar et al., Citation1996) but a study by Bowles et al. (Citation1996) using solid state 13C NMR could not find evidences of molecular interactions between glycocholic acid and barley β-glucan. Moreover, HMW β-glucan is able to lower cholesterol more than an equivalent amount of low molecular weight (LMW) β-glucan (Malkki et al., Citation1992; Wolever et al., Citation2010), which suggests a substantial contribution from β-glucan ability to form viscous solutions in the GIT (see previous section). However, recent evidence has been provided of dynamic and transient interactions between β-glucan and taurodesoxychenocolate by using 13C NMR and 1H−13C NMR (Gunness and Gidley, Citation2010b; Mikkelsen et al., Citation2014). Guar gum also increases the faecal excretion of bile acids and repeatedly showed hypocholesterolemic effect in vivo as reviewed by Butt et al., (Citation2007). Vahouny et al. (Citation1980) have reported binding capacity for guar gum equal or even higher then lignin depending on the bile acid. Similarly to β-glucan, the hypocholesterolemic effect of guar gum may be partially ascribed to its viscosity. Cholesterol lowering effect of pectin is also long known and has been reviewed previously (Reiser Citation1987; Brown et al., Citation1999; Theuwissen and Mensink, Citation2008) but this capacity greatly varies depending on pectin structure, e.g., substantial differences between pectins extracted from different sources or different fractions from the same source have been reported (Rubio-Senent et al., Citation2015). Chitosan, a cationic polysaccharide is also able to bind to negatively charged (at neutral pH) bile acids (Thongngam and McClements, Citation2005). The interaction between chitosan (or chitin) and bile acids may also occur at the water/oil interface, i.e., chitosan can adsorb on bile acids-stabilized lipid droplets, effectively inhibiting the access of lipase to its substrate (Han et al., Citation1999; Tsuijta et al., 2007). Apart from DF and bile acids molecular structure, the surface area of DF is another key factor in determining the extent of binding, i.e., the higher the surface area, the higher the amount of bile salts bound on DF at equilibrium (Huang and Dural, Citation1995). Moreover, DF incorporated in cell wall may bind bile acids less efficiently than purified DF in solution. In vitro binding capacity of lignin fractions extracted from olives stones decreases when lignin is intertwined with cellulose in the “native” cell wall setting (Rodriguez-Gutierrez et al., Citation2014). Incorporation of DF in food and the presence of additional dietary components in a complete meal may also alter the binding capacity determined under relatively “clean” conditions. For instance, incorporation of DF did not increase the binding capacity of control cookies and muffins (Dziedzic et al., Citation2015).
Another important property of DF is the capacity to bind mineral ions. The issue has been recently reviewed (Baye et al., Citation2015). This capacity has been showed in several in vitro studies (Ismail-Beigi et al., Citation1977; Fernandez and Phillips, Citation1982; Debon and Tester, Citation2001; Bosscher et al., Citation2003; Miyada et al., Citation2011). The interactions are essentially electrostatic and involve primarily divalent cations rather than monovalent cations (Espinal-Ruiz et al., Citation2014b). Carboxylic groups of, e.g., pectins, alginate, gums, and carboxymethyl cellulose are the main functional groups involved but the sulfate groups of carragenans may also be involved. Debon and Tester (Citation2001) reported that pectins, xanthan gum, and carragenans have higher affinity toward divalent cations than agar and guar gum. Furthermore, low methoxylated pectins bind more mineral ions than high methoxylated pectins because of the higher number of carboxylic acid moieties (Nair et al., Citation1987). However, cellulose and lignin also are reported to bind a certain amount of cations even though they do not possess acidic moieties but only ionizable hydroxyl groups. pH of the medium obviously affects the binding properties of DF. The ion exchange capacity is obviously higher at neutral pH of the small intestine compared to the lower pH of the stomach because of the higher proportion of charged acidic groups. Ionic strength of the digestive fluids has also to be taken into account. Schlemmer et al. (Citation1989) reported that at ionic strength ≈ 0.1 pectins and gums do not bind divalent cations. It must be underlined that, when non-purified DF is considered, the binding capacity may relate to the variable amount of co-passenger compounds, such as phytic acid, organic acids, and polyphenols, which have known mineral binding capacity. The consequences of the interaction of DF with minerals are multiple. First, it may impair their bioavailability. However, in vivo studies have reported contradictory results (Baye et al., Citation2015). This may have several explanations: (1) DF fermentation in the colon may release bound minerals making them available in the large intestine. In this respect, it is interesting to note that acetate and propionate (typical fermentation products of DF in the colon) may enhance the absorption of calcium in the colon (Scholz-Arendt et al., Citation2007), (2) as cation exchanger, dietary anionic DF is provided in the diet as salt which counterions have to be considered in the mineral balance (Debon and Tester, Citation2001). Sequestration of calcium ions by DF may also impair lipid digestion and absorption. Calcium ions are important cofactors of pancreatic lipase activity (Whayne and Felts, Citation1971). Formation of calcium salts of free fatty acids is essential for fatty acids removal from the lipid droplet surface, which otherwise would hinder access to lipase to the droplet (Zangenberg et al., Citation2001).
Several DF can inhibit the catalytic activity of digestive enzymes by interacting directly with the enzyme at molecular level. Those interactions (either electrostatic, hydrogen bonding, or hydrophobic) can alter enzyme conformation and therefore its catalytic activity. Alternatively, DF can compete for the active site of the enzyme with the enzyme natural substrate. Alginate and pectins are known to inhibit pancreatic lipase. The mechanism of pectin inhibition has not been elucidated completely. It has been suggested that pectin can form a complex with the enzyme that inhibits the binding with lipid droplets but also protonation of lipase active site by carboxylic acid residues of pectin, which would also explain the higher inhibition observed for low methoxylated pectins (Isaksson et al., Citation1982; Kumar and Chauhan, Citation2010). A similar mechanism has been hypothesized for alginate that also possesses carboxylic groups. For alginate, the inhibitory activity increases with the ratio between guluronic acid blocks and mannuronic acid blocks (Wilcox et al., Citation2014). Interestingly, alginate is able to inhibit pepsin but not trypsin (Chater et al., Citation2015) and the inhibitory activity against pepsin is inversely correlated with the ratio guluronic acid blocks and mannuronic acid blocks (Strugala et al., Citation2005). This selective inhibition may be explained by the formation of electrostatic complexes between positively charged pepsin and (partially) negative charged alginate in the stomach, which would not form in the small intestine. Inhibitory activity of DF has been described also toward α-amylase. Guar gum is able to form a complex with α-amylase that renders the enzyme inactive (Slaughter et al., Citation2002; Hardacre et al., Citation2015). A specific inhibitory effect has been also recently reported for cellulose on α-amylase (Dhital et al., Citation2015a) and for fucoidan (a bioactive sulfonated polysaccharide found in brown algae) on α-amylase and β-glucosidase (Kim et al., Citation2014). In the former case, the authors hypothesized an unspecific interaction between enzyme and cellulose, which is not mediated by the active site of the enzyme and a linear mixed inhibition of amylase activity by cellulose in the presence of maltose. The direct inhibition of digestive enzymes activity by DF is a topic of relatively recent inquiry that deserves further investigations in the future.
6.4. DF and gut microbiota fermentation
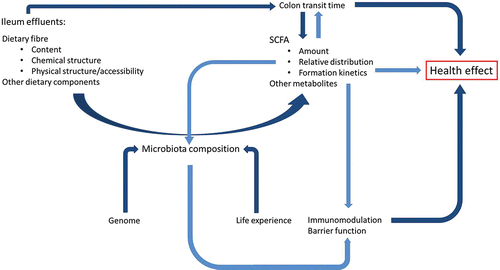
Humans host a very complex bacterial community in the large intestine that is composed of as much as 1000 different species with large inter-individual variability in its composition. DF that reaches the colon is extensively fermented by the local microbiota and represents the most important fuel for human microbiota (Flint et al., Citation2012). Gut microbiota possesses a range of polysaccharides degrading enzymes (CAZymes), which largely complement the few expressed by the human host (Kurokawa et al., Citation2007). Microbiota can be seen thus as a “digestive partner” or an “additional digestive organ” that has co-evolved with the human host and that allows the host to benefit of food components that would otherwise be lost as waste (Tuhoy et al., Citation2012). The complex interplay between diet (and especially DF), microbiota, and health is schematically depicted in . Microbial community inhabiting the human large intestine is essential to maintain immune homeostasis and intestinal barrier function. This may occur directly by ecological pressure of commensal microbiota against pathogenic bacteria or via bacterial products (components of bacterial cell wall, SCFA, etc.), which stimulate innate immune receptors expressed on epithelial cells to restore immune homeostasis in the intestine (Jarchum and Pamer, Citation2011; Salonen and De Vos, Citation2014).
Figure 4. The complex interplay between diet and microbiota determines the final health effect on the host. Microbiota composition is affected by genetic and individual factors (life experience) and determines how a certain ileum effluent (what enters the large intestine) is converted in SCFA (from DF) and other metabolites (from proteins, lipids, melanoidins, etc.). In turn, bacterial cell wall constituents and products of microbial fermentation contribute to shift the microbiota composition and to modulate the immunological response of the host. Gut microbiota and its metabolic products ultimately contribute to the host health.
On one hand, the structure of microbiota population determines which metabolites are produced from ileum effluents. Large inter-individual variability in microbiota composition is well known. This variability arises partly from genotype and life history and mainly from long-term dietary habits. Since different bacterial population will express different pools of CAZymes, the same DF will be differently fermented by different individuals/groups. The concept of Microbiota-Accessible Carbohydrates (MAC) has been introduced to refer to the carbohydrates that are metabolically available to gut microbes and thus differ among individuals or dietary groups of individuals (Sonnenburg and Sonnenburg, Citation2013). DF fermentation by the gut microbiota results in the production of short chain fatty acids (SCFA), mainly acetate, propionate, and butyrate which concentration is proportional to the amount of DF provided. SCFA have several effects on human host. Most of the SCFA is absorbed by the colon providing supplemental energy to the host (estimated to be around 10% of the energy from the diet, McNeil, Citation1984). Production of SCFA also results in the decrease of luminal pH. This contributes to the modification of the microbial population (bacteria from phylum firmicutes are favored over bacteroides, Walker et al., Citation2005; Duncan et al., Citation2009), increase of the solubility of certain minerals like calcium (Scholz-Ahrens et al., Citation2007), inhibition of the growth of Gram-negative Enterobacteriaceae including familiar pathogens Salmonella spp. and Escherichia coli (Walker et al., Citation2005; Duncan et al., Citation2009), decrease of protein fermentation and production of associated peptide-derived toxic metabolites (Russell et al., Citation2011). In addition, propionate has been showed to increase satiety and glucose tolerance (Arora et al., Citation2011) and butyrate is known for its anti-inflammatory and anti-carcinogenic effects (Canani et al., Citation2011). In particular, butyrate increases cell proliferation in colonocytes and induces apoptosis of colon cancer cells (Lupton et al., Citation1993; Hague et al., Citation1995). The beneficial effect of DF does not depend only on the amount of SCFA but also on the relative concentration of acetate, propionate, and butyrate as well as on the location where DF are fermented and SCFA produced, i.e., being the production of SCFA throughout the whole colon preferable over just in the proximal segment. On the other hand, the DF composition of diet will change the bacterial population in the colon. In general, a DF-rich diet will shift the bacterial population toward a more beneficial composition (De Filippo et al., Citation2010). Certain DFs have recognized prebiotic effect, i.e., the capacity of inducing specific changes in gut microbiota activity or composition, which results in beneficial effects for the host. Recently, Hamaker and Tuncil (Citation2014) introduced the concept of “discrete structure,” i.e., a unique chemical structure of a DF molecule that would align encoded genes for CAZymes in bacterial DNA. The multitude of discrete structures in DF molecules would explain the differences in the prebiotic effect associated with even chemically very similar DF and may be perhaps exploited for the design of food ingredients that can shape microbiota in the desired way.
Fermentation dynamics of DF and its prebiotic effect depends on several factors. First, DF chemical structure has a large effect on its fermentability. In general, soluble polysaccharides are fermented faster and to a higher extent than insoluble polysaccharides (Hamaker and Tuncil, Citation2014). For instance, cellulose and lignin, typical insoluble DFs are poorly fermented by the gut microbiota (Slavin et al., Citation1981; Monro and Mishra, Citation2010). Oligosaccharides are fermented more rapidly than polysaccharides but the fermentation rate depends on the chain length. Inulin with DP < 10 is fermented faster than inulin with DP > 10 (Roberfroid et al., Citation1998). While arabinoxylans oligosaccharides (AOX) with DP < 15 were completely fermented in the ascending and transverse colon, 30% of AOX with DP > 15 was fermented in the descending colon with distinctive prebiotic effects in the two segments (Sanchez et al., Citation2009). Also minor chemical differences within DF of the same class can change their fermentability. For instance, the degree of methylation of pectins influences its utilization in vitro and in animal models (Dongowski et al., Citation2002). Recently, structural features of the arabinoxylan structure that may impact their fermentation rate have been identified (Runpagaporn et al., Citation2015). Second, the composition of the ileal effluents may affect the order of utilization of available nutrients by generating a temporal hierarchy based on which more accessible nutrients are metabolized first. Typically several different types of DF are present in the colon affluent. Fermentation is a non-additive process and therefore the outcomes of fermentation of a mixture of two DF structure is expected to be not equal to the sum outcome of the fermentation of each DF individually. Rendering starch physically inaccessible to bacteria by encapsulation in porous alginate-based gelled matrix produced a shift in the fermentation pattern toward a higher level of butyrate, which suggests also a favoring of butyrogenic bacteria (Rose et al., Citation2009, Citation2010). It may be hypothesized that fermentation of starch encapsulated within intact cell walls may be delayed compared to free starch (having the same gelatinization level) because of the (partial) inaccessibility of starch granules to bacteria. Indeed bacteria will have first to consume the plant cell wall before the starch can be fermented. The same may hold true for starch embedded in a strong protein network resistant to digestion in the upper intestine such as in pasta (Petitot et al., Citation2009; Stuknyte' et al., Citation2014). Even if some bacteria can secrete extracellular enzymes, most of them must access to, and colonize, the DF to start ferment it (van Wey et al., Citation2011). Particle size, porosity, and total surface area of DF particles may therefore affect the fermentation rate but their role has not been completely elucidated. It has been reported that finer particles of wheat bran are fermented faster than coarser particles (Stewart and Slavin, Citation2009). An interesting study from Day et al., (Citation2012), however, shows that this correlation is not trivial as it seems. The authors showed a faster fermentation in bigger carrot cell clusters compared to small cell cluster, individual cells, and cell wall fragments. The authors explained their counterintuitive finding by arguing that cell wall junction (pectin-rich middle lamella) may provide an environment that fosters bacterial growth thus counteracting the effect of a smaller total surface area exposed by bigger cell clusters to bacterial enzymatic activity. Furthermore, isolated soluble polysaccharides are more rapidly fermented compared to those organized in complex supramolecular structures such as intact cell walls but the opposite is observed for insoluble DF such as cellulose (Mikkelsen et al., Citation2011). The structure/organization of the cell wall is also important. Ensilation of chicory root pulp increases the fermentability of pectins because of the more loosen structure induced by the fermentation process that makes pectins more accessible to bacterial enzymes (Ramasamy et al., Citation2014). Third, a significant fraction of the ileum effluent is composed of dietary compounds other than DF, e.g., melanoidins, i.e., nitrogen-containing sugar-based polymers produced during Millard reaction in cooked plant-based foods, but also proteins, lipids, and phenolic compounds. These dietary components may impact DF fermentability and prebiotic effect. Aprikian et al. (Citation2003) and Bazzocco et al. (Citation2008) showed that when the apple cell wall polysaccharides and apple polyphenols are fermented together in an in vitro digestion model, the production of SCFA was different compared to apple DF fermented alone. This may depend on the inhibition of extracellular microbial enzyme exerted by polyphenols or by the partial occupancy of fermentation sites by the polyphenols absorbed on the DF molecule.
On top of that, the speed at which digesta progresses along the colon affects the contact time with the microbiota and thus the dynamics of SCFA production but also the contact time of potentially toxic compounds with colon mucosa, which is thought to have a protective effect. Stool transit time in the colon is inversely correlated with its consistency (Heaton and O'Donnell, Citation1994; Russo et al., Citation2013) and the latter is very sensitive to its water content (McRorie et al., Citation1998). In the distal segment of the colon, DF is rapidly fermented with production of gases and increase of the microbial biomass, which keeps high the water-holding capacity of stools. As moving toward the distal segments of the colon, all the fermentable DF is consumed and the water-holding capacity of the feces is determined by the microbial biomass rather than by remaining DF (Gidley, Citation2013). Stools slow down because of the slower biomass growth and the re-absorption of water. However, it is mostly the insoluble, poorly fermented DF that has the major effect on stool mass and water content and in reducing colon transit time. This effect is not related to DF water holding capacity, which is very low for insoluble DF, but rather on the mechanical stimulation of mucus secretion and intestinal peristalsis triggered by DF particles of proper size and shape. Bigger and coarser particles are indeed more effective than small and smooth particles (Tomlin and Read, Citation1988; Lewis and Heaton, Citation1999). Recent investigations suggest that soluble DF can also increase colon transit time but only if the DF is slowly and partially fermentable (Ruiz et al., Citation2015). In this case, the fermentation in the distal part of the colon would produce SCFA, which reduces the colon transit time by stimulating local intestinal peristalsis and by maintaining the local osmolarity. In addition, the presence of indigested material with substantial water holding capacity would contribute to stool softening and intestinal regularity.
7. Conclusions and future perspectives
There is general consensus that a diet rich in DF helps protecting against a vast array of diseases. Compared to other dietary components, DF's beneficial effect on health almost uniquely depends on the modulation of digestive processes rather than on intrinsic nutritional properties or the elicitation/triggering of specific biological activities. In this review, the behavior of DF during digestion of food has been discussed and related to the physiological effects on health. Despite our knowledge of the behavior of DF in the GIT has rapidly increased over the last years, also thanks to the development and improvement of reliable and realistic in vitro models of digestion, there are still several points of attention for future research.
The first is that a strong structure–function relationship exists that explains the physiological effect of DF. DF behavior during digestion depends on its chemical structure but the term DF is a black box including a wide range of different chemical structures even within the same “class.” A detailed characterization of the DF molecular structure is essential to predict its physiological behavior, something that has not always been considered in a number of intervention and epidemiological studies and that may, at least partially, explain their contradictory outcomes (Gemen et al., Citation2011). Considering the effect that food processing may have on DF, that characterization should be carried out on food products at the moment of consumption rather than on raw ingredients or intermediates. The second consideration is that interactions of DF with other meal components are essential and must be incorporated when the physiological effect is to be predicted for a certain DF. The same DF structure may produce different effect depending on the micro- and macronutrients it will interact with, i.e., on food/meal composition. Third, the physiological effect also depends upon the modality of DF administration. The effect is predictably different whether a certain DF is administrated free in solution (e.g., in a beverage), it is part of naturally occurring cell wall material or is incorporated as ingredient/additive into a solid food matrix.
All this means that the bare chemical determination of the total DF content of a food does not allow the prediction of its biological activity. This has implications for dietary recommendation for optimal DF consumption and in intervention and epidemiological studies where the target physiological effect should not be just related to the intake of DF but also to DF chemical structure, modality of administration, and food/meal composition. Finally, in designing in vitro experiments it is time to move toward more realistic experimental settings where the effect of DF is investigated in relation to the food matrix/meal with which it is provided. This will represent a contribution to the shifting from the reductionist approach to nutrition where a food is merely regarded as the sum of its nutritive components to a more integrated food-based approach where the effect of the interactions between individual components and thus of the foods as a whole is considered.
Acknowledgments
The author gratefully acknowledges Prof. Vincenzo Fogliano for the revision of the manuscript. The author declares no conflict of interest.
References
- Ajani, U. A., Ford, E. S. and Mokdad, A. H. (2004). Dietary fiber and C reactive protein: Findings from national health and nutrition examination survey data. J. Nutr. 134:1181–1185.
- AOAC. (2012). AOAC Official Methods of Analysis, 18th ed. AOAC International, Gaithersburg, USA.
- Aprikian, O., Duclos, V., Guyot, S. and Besson, C. (2003). Apple pectin and a polyphenol- rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. J. Nutr. 133:1860–1865.
- Aribas-Agusti, A., Van Buggenhout, S., Palmero, P., Hendrickx, M. and Van Loey, A. (2015). Investigating the role of pectin in carrot cell wall changes during thermal processing: A microscopic approach. Innov. Food Sci. Emerg. 24:113–120.
- Arjmandi, B. H. and Reevesm, R. D. (1992). Dietary soluble fiber and cholesterol affect serum cholesterol concentration, hepatic portal venous short-chain fatty acid concentrations and fecal sterol excretion in rats. J. Nutr. 122:246–253.
- Arora, T., Sharma, R. and Frost, G. (2011). Anti-obesity and satiety enhancing factor? Appetite 56:511–515.
- Asakura, S. and Oosawa, F. (1954). On the interactions between two bodies immersed in a solution of macromolecules. J. Chem. Phys. 22:1255–1256.
- Baye, K., Guyot, J.-P. and Mouquet-Rivier, C. (2017). The unresolved role of dietary fibers on mineral absorption. Crit. Rev. Food Sci. Nutr. 57:949–957.
- Bazzano, L. A., He, J., Ogden, L. G., Loria, C. M. and Whelton, P. K. (2003). Dietary fiber intake and reduced risk of coronary heart disease in US men and women: The national health and nutrition examination survey|epidemiologic follow-up study. Arch. Intern. Med. 163:1897–1904.
- Bazzocco, S., Mattila, I., Guyot, S., Renard, C. M. G. C. and Aura, A.-M. (2008). Factors affecting the conversion of apple polyphenols to phenolic acids and fruit matrix to short-chain fatty acids by human faecal microbiota in vitro. Eur. J. Nutr. 47:442–452.
- Belitz, H. D., Grosch, W. and Schieberle, P. (2009). Food Chemistry, 4th ed. Springer, Berlin, Germany.
- Bengtsson, A., Brackmann, C., Enejder, A., Alminger, M. L. and Svanberg, U. (2010). Effects of thermal processing on the in vitro bioaccessibility and microstructure of b-carotene in orange-fleshed sweet potato. J. Agric. Food Chem. 58(20):11090–11096.
- Berg, T., Singh, J., Hardacre, A. and Boland, M. J. (2012). The role of cotyledon cell structure during in vitro digestion of starch in navy beans. Carbohyd. Polym. 87:1678–1688.
- Beysseriat, M., Decker, E. A. and McClements, D. J. (2006). Preliminary study of the influence of dietary fiber on the properties of oil-in-water emulsions passing through an in vitro human digestion model. Food Hydrocoll. 20:800–809.
- Boerjan, W., Ralph, J. and Baucher, M. (2003). Lignin biosythesis. Annu. Rev. Plant Biol. 54:519–546.
- Bordenave, N., Hamaker, B. R. and Ferruzzi, M. G. (2014). Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 5:18–34.
- Bosscher, D., Van Caillie-Bertrand, M., Van Cauwenbergh, R. and Deelstra, H. (2003). Availabilities of calcium, iron, and zinc from dairy infant formulas is affected by soluble dietary fibers and modified starch fractions. Nutrition 19(7):641–645.
- Bourriot, S., Garnier, C. and Doublier, J.-L. (1999). Phase separation, rheology and microstructure of micellar casein-guar gum mixtures. Food Hydrocoll. 13:43–49.
- Bowles, R. K., Morgan, K. R., Furneaux, R. H. and Coles, G. D. (1996). 13C CP/MAS NMR study of the interaction of bile acids with barley β-d-glucan. Carbohydr. Polym. 29:7–10.
- Brennan, C. S., Blake, D. E., Ellis, P. R. and Schofield, J. D. (1996). Effects of guar galactomannan on wheat bread microstructure and on the in vitro and in vivo digestibility of starch in bread. J. Cereal Sci. 24:151–160.
- Brown, L., Rosner, B., Willett, W. W. and Sacks, F. M. (1999). Cholesterol lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 69:30–42.
- Brownlee, I. (2014). The impact of dietary fibre intake on the physiology and health of the stomach and upper gastrointestinal tract. Bioact. Carbohydr. Diet. Fibre 4:155–169.
- Brummer, Y., Kaviani, M. and Tosh, S. M. (2014). Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Res. Int. 67:117–125.
- Burkitt, D. P., Walker, A. R. and Painter, N. S. (1972). Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet 2:1408–1412.
- Burton, R. A. and Fincher, G. B. (2014). Evolution and development of cell walls in cereal grains. Front. Plant Sci. 5:1–15.
- Butt, M. S., Shahazadi, N., Sharif, M. K. and Nasir, M. (2007). Guar gum: A miracle therapy for hypercholesterolemia, hyperglycemia and obesity. Crit. Rev. Food Sci. Nutr. 47(4):389–396.
- Canani, R. B., Costanzo, M. D., Leone, L., Pedata, M., Meli, R. and Calignano, A. (2011). Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 17:1519–1528.
- Carpita, N. C. and Gibeaut, D. M. (1993). Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3:1—30.
- Carrera-Bastos, P., Fontes-Villalba, M., O'Keefe, J., Lindeberg, S. and Cordain, L. (2011). The western diet and lifestyle and diseases of civilization. Res. Reports Clin. Cardiol. 2:15–35.
- Carriere, F., Moreau, H., Raphel, V., Laugier, R., Benicourt, C., Junien, J. L. and Verger, R. (1991). Purification and biochemical characterization of dog gastric lipase. Eur. J. Biochem. 202:75–83.
- Chater, P. I., Wilcox, M. D., Brownlee, I. A. and Pearson, J. P. (2015). Alginate as a protease inhibitor in vitro and in a model gut system; selective inhibition of pepsin but not trypsin. Carbohydr. Polym. 131:142–151.
- Chuang, S.-C., Norat, T., Murphy, N., Olsen, A., Tjønneland, A., Overvad, K., Boutron-Ruault, M. C., Perquier, F., Dartois, L., Kaaks, R., Teucher, B., Bergmann, M. M., Boeing, H., Trichopoulou, A., Lagiou, P., Trichopoulos, D., Grioni, S., Sacerdote, C., Panico, S., Palli, D., Tumino, R., Peeters, P. H., Bueno-de-Mesquita, B., Ros, M. M., Brustad, M., Åsli, L. A., Skeie, G., Quirós, J. R., González, C. A., Sánchez, M. J., Navarro, C., Ardanaz Aicua, E., Dorronsoro, M., Drake, I., Sonestedt, E., Johansson, I., Hallmansm, G., Key, T., Crowe, F., Khaw, K. T., Wareham, N., Ferrari, P., Slimani, N., Romieu, I., Gallo, V., Riboli, E. and Vineis, P. (2012). Fiber intake and total and cause-specific mortality in the European prospective investigation into cancer and nutrition cohort. Am. J. Clin. Nutr. 96:164–174.
- CODEX Alimentarius (CODEX). (2010). Guidelines on Nutrition Labeling CAC/GL 2–1985 as Last Amended 2010. FAO, Rome.
- Cosgrove, D. J. (2001). Wall structure and wall loosening. A look backwards and forwards. Plant Physiol. 125:131–134.
- Dahm, C. C., Keogh, R. H., Spencer, E. A., Greenwood, D. C., Key, T. J., Fentiman, I. S., Shipley, M. J., Brunner, E. J., Cade, J. E., Burley, V. J., Mishra, G., Stephen, A. M., Kuh, D., White, I. R., Luben, R., Lentjes, M. A., Khaw, K. T. and Rodwell Bingham, S. A. (2010). Dietary fiber and colorectal cancer risk: A nested case–control study using food diaries. J. Natl. Cancer Inst. 102:614–626.
- Day, L., Gomez, J., Oiseth, S. K., Gidley, M. J. and Williams, B. A. (2012). Faster fermentation of cooked carrot cell clusters compared to cell wall fragments in vitro by porcine feces. J. Agric. Food Chem. 60(12):3282–3290.
- Debon, S. J. J. and Tester, R. F. (2001). In vitro binding of calcium, iron and zinc by non-starch polysaccharides. Food Chem. 73(4):401–410.
- De Filippo, C., Cavalieri, D., Di Paola, M., Ramazzotti, M., Poullet, J. B., Massart, S., Collini, S., Pieraccini, G. and Lionetti, P. (2010). Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 107:14691–14696.
- de Munter, J. S., Hu, F. B., Spiegelman, D., Franz, M. and Van Dam, R. M. (2007). Whole grain, bran, and germ intake and risk of type 2 diabetes: A prospective cohort study and systematic review. PLoS Med. 4:1385–1395.
- de Vries, J. (2004). Hydrocolloid gelling agents and their applications. In: Gums and Stabilizers for the Food Industry, Vol. 12, Philips, G. O. and Williams, P. A., Eds., The Royal Society of Chemistry, UK. pp. 22–30.
- Dhital, S., Dolan, G., Stokes, J. R. and Gidley, M. J. (2014). Enzymatic hydrolysis of starch in the presence of cereal soluble fibre polysaccharides. Food Funct. 5:579–586.
- Dhital, S., Gidley, M. J. and Warren, F. J. (2015a). Inhibition of α-amylase activity by cellulose: Kinetic analysis and nutritional implications. Carbohydr. Polym. 123:305–312.
- Dhital, S., Warren, F. J., Butterworth, P. J., Ellis, P. R. and Gidley, M. J. (2017). Mechanisms of starch digestion by α-amylase–structural basis for kinetic properties. Crit. Rev. Food Sci. Nutr. 57:875–892.
- Dick-Perez, M., Zhang, Y., Hayes, J., Salazar, A., Zabotina, O. A. and Hong, M. (2011). Structure and interactions of plant cell-wall polysaccharides by two- and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry 50:989–1000.
- Dikeman, C. L. and Fahey, G. C. Jr. (2006). Viscosity as related to dietary fiber: A review. Crit. Rev. Food Sci. Nutr. 46(8):649–663.
- Dongowski, G., Lorenz, A. and Proll, A. (2002). The degree of methylation influences the degradation of pectin in the intestinal tract of rats and in vitro. J. Nutr. 132:1935–1944.
- Du, H., van der A, D. L., Boshuizen, H. C., Forouhi, N. G., Wareham, N. J., Halkjaer, J., Tjonneland, A., Overvad, K., Jakobsen, M. U., Boeing, H., Buijsse, B., Masala, G., Palli, D., Sorensen, T. J., Saris, W. H. and Feskens, E. J. (2010). Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am. J. Clin. Nutr. 91:329–336.
- Duncan, S. H., Louis, P., Thomson, J. M. and Flint, H. J. (2009). The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 11:2112–2122.
- Dziedzic, K., Gorecka, D., Szwengiel, A., Smoczyńska, P., Czaczyk, K. and Komolka, P. (2015). Binding of bile acids by pastry products containing bioactive substances during in vitro digestion. Food Funct. 6:1011–1020.
- Eastwood, M. A. and Hamilton, D. (1968). Studies on the adsorption of bile salts to non-absorbed components of diet. Biochim. Biophys. Acta 152:165–173.
- Eastwood, M. A. and Morris, E. R. (1992). Physical properties of dietary fiber that influence physiological function: A model for polymers along the gastrointestinal tract. Am. J. Clin. Nutr. 55:436–442.
- Edwards, C. A., Blackburn, N. A., Craigen, L., Davison, P., Tomlin, J., Sugden, K., Johnson, I. T. and Read, N. W. (1987). Viscosity of food gums determined in vitro related to their hypoglycemic actions. Am. J. Clin. Nutr. 46:72–77.
- Edwards, C. H., Grundy, M. M. L., Grassby, T., Vasilopoulou, D., Frost, G. S., Butterworth, P. J., Berry, S. E. E., Sanderson, J. and Ellis, P. R. (2015a). Manipulation of starch bioaccessibility in wheat endosperm to regulate starch digestion, postprandial glycemia, insulinemia, and gut hormone responses: A randomized controlled trial in healthy ileostomy participants. Am. J. Clin. Nutr. 102:791–800.
- Edwards, C. H., Warren, F. J., Campbell, G. M., Gaisford, S., Royall, P. G., Butterworth, P. J. and Ellis, P. R. (2015b). A study of starch gelatinisation behaviour in hydrothermally-processed plant food tissues and implications for in vitro digestibility. Food Funct. 6:3634–3641.
- Ellegard, L. and Andersson, H. (2007). Oat bran rapidly increases bile acid excretion and bile acid synthesis: an ileostomy study. Eur. J. Clin. Nutr. 61:938–945.
- Ellis, P. R., Kendall, C. W. C., Ren, Y., Parker, C., Pacy, J. F., Waldron, K. W. and Jenkins, D. J. A. (2004). Role of cell walls in the bioaccessibility of lipids in almond seeds. Am. J. Clin. Nutr. 80:604–613.
- Espinal-Ruiz, M., Parada-Alfonso, F., Restrepo-Sanchez, L.-P., Narvaez-Cuenca, C.-E. and McClements, D. J. (2014a). Impact of dietary fibers methyl cellulose, chitosan, and pectin on digestion of lipids under simulated gastrointestinal conditions. Food Funct. 5:3083–3095.
- Espinal-Ruiz, M., Parada-Alfonso, F., Restrepo-Sanchez, L.-P., Narvaez-Cuenca, C.-E. and McClements, D. J. (2014b). Interaction of a dietary fiber (pectin) with gastrointestinal components (bile salts, calcium, and lipase): A calorimetry, electrophoresis, and turbidity study. J. Agric. Food Chem. 62:12620–12630.
- Espinal-Ruiz, M., Restrepo-Sanchez, L.-P., Narvaez-Cuenca, C. E. and McClements, D. J. (2016). Impact of pectin properties on lipid digestion under simulated gastrointestinal conditions: Comparison of citrus and banana passion fruit (Passiflora tripartita var. mollissima) pectins. Food Hydrocoll. 52:329–342.
- Fabek, H., Messerschmidt, S., Brulport, V. and Goff, H. D. (2014). The effect of in vitro digestive processes on the viscosity of dietary fibres and their influence on glucose diffusion. Food Hydrocoll. 35:718–726.
- Fernandez, R. and Phillips, S. (1982). Components of fiber bind iron in vitro. Am. J. Clin. Nutr. 35(1):100–106.
- Flint, H. J., Scott, K. P., Duncan, S. H., Louis, P. and Forano, E. (2012). Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 3(4):289–306.
- Foschia, M., Peressini, D., Sensidoni, A., Brennan, M. A. and Brennan, C. S. (2015). Synergistic effect of different dietary fibres in pasta on in vitro starch digestion?. Food Chem. 172:245–250.
- Furness, J. B. (2000). Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 81(1–3):87–96.
- Ganry, O. (2002). Phytoestrogen and breast cancer prevention. Eur. J. Cancer Prev. 11:519–522.
- Gao, R., Liu, H., Peng, Z., Wu, Z., Wang, Y. and Zhao, W. G. (2012). Adsorption of (−)-epigallocatechin-3-gallate (EGCG) onto oat b-glucan. Food Chem. 132:1936–1943.
- Gemen, R., de Vries, J. F. and Slavin, J. L. (2011). Relationship between molecular structure of cereal dietary fiber and health effects: Focus on glucose/insulin response and gut health. Nutr. Rev. 69(1):22–33.
- Gidley, M. J. (2013). Hydrocolloids in the digestive tract and related health implications. Curr. Opin. Coll. Interf. Sci. 18:371–378.
- Golay, A., Coulston, A. M., Hollenbeck, C. B., Kaiser, L. L., Würsch, P. and Reaven, G. M. (1986). Comparison of metabolic effects of white beans processed into two different physical forms. Diabetes Care 9(3):260–266.
- Gonçalves, R., Mateus, N. and de Freitas, V. (2011). Influence of carbohydrates on the interaction of procyanidin B3 with trypsin. J. Agric. Food Chem. 59:11794–11802.
- Grassby, T., Picout, D. V., Mandalari, G., Faulks, R. M., Kendall, C. W. C., Rich, G. T., Wickham, M. S. J., Lapsley, K. and Ellis, P. R. (2014). Modelling of nutrient bioaccessibility in almond seeds based on the fracture properties of their cell walls. Food Funct. 5:3096–3106.
- Grundy, M. M.-L., Grassby, T., Mandalari, G., Waldron, K. W., Butterworth, P. J., Berry, S. E. and Ellis, P. R. (2015a). Effect of mastication on lipid bioaccessibility of almonds in a randomized human study and its implications for digestion kinetics, metabolizable energy, and postprandial lipemia. Am. J. Clin. Nutr. 101(1):25–33.
- Grundy, M. M.-L., Wilde, P. J., Butterworth, P. J., Gray, R. and Ellis, P. R. (2015b). Impact of cell wall encapsulation of almonds on in vitro duodenal lipolysis. Food Chem. 185:405–412.
- Guillon, F. and Champ, M. (2000). Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res. Int. 33:233–245.
- Gunness, P., Flanagan, B. M. and Gidley, M. (2010a). Molecular interactions between cereal soluble dietary fibre polymers and a model bile salt deduced from 13C NMR titration. J. Cereal Sci. 52:444–449.
- Gunness, P. and Gidley, M. (2010b). Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 1:149–155.
- Hague, A., Elder, D. J., Hicks, D. J. and Paraskeva, C. (1995). Apoptosis in colorectal tumour cells: Induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int. J. Cancer. 60:400–406.
- Hamaker, B. R. and Tuncil, Y. (2014). A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 426(23):3838–3850.
- Han, L. K., Kimura, Y. and Okuda, H. (1999). Reduction in fat storage during chitin–chitosan treatment in mice fed a high-fat diet. Int. J. Obesity. 23(2):174–179.
- Hardacre, A. K., Yap, S.-Y., Lentle, R. G., Janssen, P. W. M. and Monro, J. A. (2014). The partitioning of water in aggregates of undigested and digested dietary particles. Food Chem. 142:446–454.
- Hardacre, A. K., Yap, S.-Y., Lentle, R. G. and Monro, J. A. (2015). The effect of fibre and gelatinised starch type on amylolysis and apparent viscosity during in vitro digestion at a physiological shear rate. Carbohydr. Polym. 123:80–88.
- He, Q., Lv, Y. and Yao, K. (2007). Effects of tea polyphenols on the activities of α-amylase, pepsin, trypsin and lipase. Food Chem. 101:1178–1182.
- Heaton, K. W. and O'Donnell, L. J. (1994). An office guide to whole-gut transit time. Patients' recollection of their stool form. J. Clin. Gastroenterol. 19:28–30.
- Hertog, M. G., Feskens, E. J., Hollman, P. C., Katan, M. B. and Kromhout, D. (1993). Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen elderly study. Lancet 342:1007–1011.
- Hipsley, E. H. (1953). Dietary “fibre” and pregnancy toxaemia. Brit. Med. J. 2:420–422.
- Hoad, C. L., Rayment, P., Spiller, R. C., Marciani, L., Alonso, B. D., Traynor, C., Mela, D. J., Peters, H. P. F. and Gowland, P. A. (2004). In vivo imaging of intragastric gelation and its effect on satiety in humans. J. Nutr. 134(9):2293–2300.
- Hoebler, C., Karinthi, A., Devaux, M. F., Guillon, F., Gallant, D. J., Bouchet, B., Melegari, C. and Barry, J. L. (1998). Physical and chemical transformations of cereal food during oral digestion in human subjects. Br. J. Nutr. 80:429–436.
- Houghton, D., Wilcox, M. D., Chater, P. I., Brownlee, I. A., Seal, C. J. and Pearson, J. P. (2015). Biological activity of alginate and its effect on pancreatic lipase inhibition as a potential treatment for obesity. Food Hydrocoll. 49:18–24.
- Huang, C.-M. and Dural, N. (1995). Adsorption of bile acids on cereal type food fibers. J. Food Process. Eng. 18:243–266.
- Humphrey, S. P. and Williamson, R. T. (2001). A review of saliva: Normal composition, flow, and function. Prosthet. Dent. 85:162–169.
- Institute of Medicine. (2001). Dietary Reference Intakes: Proposed Definition of Dietary Fiber. National Academies Press, Washington, DC.
- Isaksson, G., Lundquist, I. and Ihse, I. (1982). In vitro inhibition of pancreatic enzyme activities by dietary fiber. Digestion. 24(1):54–59.
- Ismail-Beigi, F., Faraji, B. and Reinhold, J. (1977). Binding of zinc and iron to wheat bread, wheat bran, and their components. Am. J. Clin. Nutr. 30(10):1721–1725.
- Izydorczyk, M. S., Storsley, J., Labossiere, D., MacGregor, A. W. and Rossnagel, B. G. (2000). Variation in total and soluble β-glucan content in hulless barley: effects of thermal, physical, and enzymic treatments. J. Agric. Food Chem. 48:982–989.
- Jacobek, L. (2015). Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 175:556–567.
- Jarchum, I. and Pamer, E. G. (2011). Regulation of innate and adaptive immunity by the commensal microbiota. Curr. Opin. Immunol. 23:353–360.
- Jarjis, H. A., Blackburn, N. A., Redfern, J. S. and Read, N. W. (1984). The effect of ispaghula husk (Fybogel and Metamucil) and guar gum on glucose tolerance in man. Br. J. Nutr. 51:371–378.
- Jaskari, J., Henriksson, K., Nieminen, A., Suortti, T., Salovaara, H. and Poutanen, K. (1995). Effect of hydrothermal and enzymic treatments on the viscous behavior of dry- and wet-milled oat brans. Cereal Chem. 72:625–631.
- Jenkins, D. J. A., Jenkins, A. L., Wolever, T. M. S., Collier, G. R., Rao, A. V. and Thompson, L. U. (1987). Starchy foods and fibre—reduced rate of digestion and improved carbohydrate metabolism. Scand. J. Gastroenterol. 22:132–141.
- Jenkins, D. J., Newton, C., Leeds, A. R. and Cummings, J. H. (1975). Effect of pectin, guar gum, and wheat fibre on serum-cholesterol. Lancet 1:1116–1117.
- Jensen, M. K., Koh-Banerjee, P., Hu, F. B., Franz, M., Sampson, L., Gronbaek, M. and Rimm, E. B. (2004). Intakes of whole grains, bran, and germ and the risk of coronary heart disease in men. Am. J. Clin. Nutr. 80:1492–1499.
- Johansson, L., Virkki, L., Anttila, H., Esselstrom, H., Tuomainen, P. and Sontag-Strohm, T. (2006). Hydrolysis of β-glucan. Food Chem. 97:71–79.
- Jones, J. M. (2014). CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap'. Nutr. J. 13:34–43.
- Kim, K.-T., Rioux, L.-E. and Turgeon, S. (2014). Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 98:27–33.
- Kosikova, B., Slamenova, D., Mikulasova, M., Horvathova, E. and Labaj, J. (2002). Reduction of carcinogenesis by bio-based lignin derivatives. Biomass Bioenerg. 23:153–159.
- Kulkarni, B. V. and Mattes, R. D. (2014). Lingual lipase activity in the orosensal detection of fat in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306:R879–R885.
- Kumar, A. and Chauhan, G.S. (2010). Extraction and characterization of pectin from apple pomace and its evaluation as lipase (steapsin) inhibitor. Carbohydr. Polym. 82(2):454–459.
- Kurokawa, K., Itoh, T., Kuwahara, T., Oshima, K., Toh, H., Toyoda, A., Takami, H., Morita, H., Sharma, V. K., Srivastava, T. P., Taylor, T. D., Noguchi, H., Mori, H., Ogura, Y., Ehrlich, D. S., Itoh, K., Takagi, T., Sakaki, Y., Hayashi, T. and Hattori, M. (2007). Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 14:169–181.
- Lau, C., Faerch, K., Glumer, C., Tetens, I., Pedersen, O., Carstensen, B., Jorgensen, T. and Borch-Jonsen, K. (2005). Dietary glycemic index, glycemic load, fiber, simple sugars, and insulin resistance. Diabetes Care 28:1397–1403.
- Lazaridou, A., Marinopoulou, A., Matsoukas, N. P. and Biliaderis, C. G. (2014). Impact of flour particle size and autoclaving on β-glucan physicochemical properties and starch digestibility of barley rusks as assessed by in vitro assays. Bioact. Carbohydr. Diet Fibre 1:58–73.
- Le Bourvellec, C., Bouchet, B. and Renard, C. M. G. C. (2005b). Non-covalent interaction between procyanidins and apple cell wall material. Part III; study on model polysaccharides. Biochim. Biophys. Acta Gen. Subj. 1725:10–18.
- Le Bourvellec, C. and Renard, C. M. G. C. (2005a). Non-covalent interaction between procyanidins and cell wall material. Part II: Quantification and impact of the cell wall drying. Biochim. Biophys. Acta Gen. Subj. 1725:1–9.
- Leclere, C. J., Champ, M., Boillot, J., Guille, G., Lecannu, G., Molis, C., Bornet, F., Krempf, M., Delort-Laval, J. and Galmich, J.-P. (1994). Role of viscous guar gums in lowering the glycemic response after a solid meal. Am. J. Clin. Nutr. 59:914–921.
- Lemmens, L., Van Buggenhout, S., Van Loey, A. and Hendrickx, M. (2010). Particle size reduction leading to cell wall rupture is more important for b-carotene bio-accessibility of raw compared to thermally processed carrots. J. Agric. Food Chem. 58:12769–12776.
- Lentle, R. G. and Janssen, P. W. M. (2008). Physical characteristics of digesta and their influence on flow and mixing in the mammalian intestine: A review. J. Comp. Physiol. B 178:673–690.
- Lewis, S. J. and Heaton, K. W. (1999). Roughage revisited: the effect on intestinal function of inert plastic particles of different sizes and shape. Dig. Dis. Sci. 44:744–758.
- Li, M., Kim, J.-W. and Peeples, T. L. (2002). Amylase partitioning and extractive bioconversion of starch using thermoseparating aqueous two-phase systems. J. Biotechnol. 93:15–26.
- Lia, A., Hallmans, G., Sandberg, A. S., Sundberg, B., Aman, P. and Andersson, H. (1995). Oat beta-glucan increases bile acid excretion and a fiberrich barley fraction increases cholesterol excretion in ileostomy subjects. Am. J. Clin. Nutr. 62:1245–1251.
- Liese, A. D., Roach, A. K., Sparks, K. C., Marquart, L., D'Agostino, R. B. Jr, and Mayer-Davis, E. J. (2003). Whole-grain intake and insulin sensitivity: the insulin resistance atherosclerosis study. Am. J. Clin. Nutr. 78:965–971.
- Lindahl, A., Ungell, A. L., Knutson, L. and Lennernas, H. (1997). Characterization of fluids from the stomach and proximal jejunum in men and women. Pharmaceut. Res. 14(4):497–502.
- Liu, R. H. (2004). Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 134:3479S–3485S.
- Liu, S., Willett, W. C., Manson, J. E., Hu, F. B., Rosner, B. and Colditz, G. (2003). Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am. J. Clin. Nutr. 78:920–927.
- Livesey, G., Wilkinson, J. A., Roe, M., Faulks, R., Clark, S., Brown, J. C., Kennedy, H. and Elia, M. (1995). Influence of the physical form of barley grain on the digestion of its starch in the human small intestine and implications for health. Am. J. Clin. Nutr. 61:75–81.
- Lovegrove, A., Edwards, C. H., De Noni, I., Patel, H., El, S. N., Grassby, T., Zielke, C., Ulmius, M., Nilsson, L., Butterworth, P. J., Ellis, P. R. and Shewry, P. R. (2017). Role of polysaccharides in food, digestion and health. Crit. Rev. Food Sci. Nutr. 57:237–253.
- Low, D. Y., D'Arcy, B. and Gidley, M. (2015). Mastication effects on carotenoid bioaccessibility from mango fruit tissue. Food Res. Int. 67:238–246.
- Lupton, J. R., Betteridge, V. A. and Pijls, L. T. J. (2009). Codex final definition of dietary fibre: Issues of implementation. Qual. Assur. Saf. Crops. Food. 1:206–212.
- Lupton, J. R. and Kurtz, P. P. (1993). Relationship of colonic luminal short-chain fatty acids and pH to in vivo cell proliferation in rats. J. Nutr. 123:1522–1530.
- Madar, Z. and Stark, A. (1995). Possible mechanism by which dietary fibres affect lipid metabolism. Agro Food Ind. Hi-Tech. 6:40–42.
- Maki, K. C., Beiseigel, J. M., Jonnalagadda, S. S., Gugger, C. K., Reeves, M. S., Farmer, M. V., Kaden, V. N. and Rains, T. N. (2010). Whole-grain ready-to-eat oat cereal, as part of a dietary program for weight loss, reduces low-density lipoprotein cholesterol in adults with overweight and obesity more than a dietary program including low-fiber control foods. J. Am. Diet Assoc. 110:205–214.
- Maljaars, P. W. J., Peters, H. P. F., Mela, D. J. and Masclee, A. A. M. (2009). Ileal brake: A sensible food target for appetite control. A review. Physiol. Behav. 95:271–281.
- Malkki, Y., Autio, K., Hanninen, O., Myllymaki, O., Pelkonen, K., Suortti, T. and Torronen, R. (1992). Oat bran concentrates: Physical properties of β;-glucan and hypercholesterolemic effects in rats. Cereal Chem. 69:647–653.
- Mandalari, G., Grundy, M. M.-L., Grassby, T., Parker, M. L., Cross, K. L., Chessa, S., Bisignano, C., Barreca, D., Bellocco, E., Lagana', G., Butterworth, P. J., Faulks, R. M., Wilde, P. J., Ellis, P. R. and Waldron, K. W. (2014). The effects of processing and mastication on almond lipid bioaccessibility using novel methods of in vitro digestion modelling and micro-structural analysis. Br. J. Nutr. 112(9):1521–1529.
- Mandalari, R., Faulks, G. T., Rich, V., Lo Turco, D., Picout, R. B., Lo Curto, C., Bisignano, G., Dugo, K. W., Waldron, P., Ellis, P. S. and Wickham, M. S. (2008). Release of protein, lipid, and vitamin E from almond seeds during digestion J. Agric. Food Chem. 56:3409–3416.
- McDougall, G. J., Kulkarni, N. N. and Stewart, D. (2008). Current developments on the inhibitory effects of berry polyphenols on digestive enzymes. BioFactors 34:73–80.
- McNeil, N. I. (1984). The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 39:338–342.
- McRorie, J., Pepple, S. and Rudolph, C. (1998). Effects of fiber laxatives and calcium docusate on regional water content and viscosity of digesta in the large intestine of the pig. Dig. Dis. Sci. 43:738–745.
- Melito, C. and Tovar, J. (1995). Cell walls limit in vitro protein digestibility in processed legume seeds. Food Chem. 53:305–307.
- Meyer, K. A., Kushi, L. H., Jacobs, D. R. Jr, Slavin, J., Sellers, T. A. and Folsom, A. R. (2000). Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 71:921–930.
- Mikhaleva, N. Y., Borisenkov, M. F., Gyunter, E. A., Popeiko, O. V. and Ovodov, Y. S. (2011). Effect of successive acid and enzymatic hydrolysis on the structure and antioxidant activity of pectins. Russ. J. Bioorg. Chem. 37:822–828.
- Mikkelsen, D., Gidley, M. J. and Williams, B. A. (2011). In vitro fermentation of bacterial cellulose composites as model dietary fibers. J. Agric. Food Chem. 59:4025–4032.
- Mikkelsen, M. S., Cornali, S. B., Jensen, M. G., Nilsson, M., Beeren, S. R. and Meier, S. (2014). Probing Interactions between β-Glucan and bile salts at atomic detail by 1H−13C NMR assays. J. Agric. Food Chem. 62:11472–11478.
- Mikkelsen, M. S., Jespersen, B. M., Mehlsen, A., Engelsen, S. B. and Frokiaer, A. (2014a). Cereal β-glucan immune modulating activity depends on the polymer fine structure. Food Res. Int. 6:829–836.
- Millar, P. and Chesson, A. (1984). Modifications to swede (Brassica napus L.) anterior to the terminal ileum of pigs: some implications for the analysis of dietary fibre. Br. J. Nutr. 52:583–594.
- Miller, J. C., Buchanan, C. J., Eastwood, E. A. and Fry, S. C. (1995). The solubilisation and hydrolysis of spinach cell wall polysaccharides in gastric and pancreatic fluids. J. Sci. Food Agric. 68:389–394.
- Minekus, M., Jelier, M., Xiao, J.-Z., Kondo, S., Iwatsuki, K., Kokubo, S., Bos, M., Dunnewind, B. and Havenaar, R. (2004). Effect of partially hydrolyzed guar gum (PHGG) on the bioaccessibility of fat and cholesterol. Biosci. Biotechnol. Biochem. 69:932–938.
- Miyada, T., Nakajima, A. and Ebihara, K. (2011). Iron bound to pectin is utilised by rats. Br. J. Nutr. 106(1):73–78.
- Moelants, K. R. N., Lemmens, L., Vandebroeck, M., Van Buggenhout, S., Van Loey, A. M. and Hendrickx, M. E. (2012). Relation between particle size and carotenoid bioaccessibility in carrot- and tomato-derived suspensions. J. Agric. Food Chem. 60:11995–12003.
- Moffett, D. F., Moffett, S. B. and Schauf, C. L. (1993). Human Physiology–Foundations & Frontiers, 2nd ed. Mosby Elsevier, US.
- Monro, J. A. and Mishra, S. (2010). Digestion-resistant remnants of vegetable vascular and parenchyma tissues differ in their effects in the large bowel of rats. Food Dig. 1:47–56.
- Montonen, J., Knekt, P., Jarvinen, R., Aromaa, A. and Reunanen, A. (2003). Whole-grain and fiber intake and the incidence of type 2 diabetes. Am. J. Clin. Nutr. 77:622–629.
- Murphy, N., Norat, T., Ferrari, P., Jenab, M., Bueno-de-Mesquita, B., Skeie, G., Dahm, C. C., Overvad, K., Olsen, A., Tjønneland, A., Clavel-Chapelon, F., Boutron-Ruault, M. C., Racine, A., Kaaks, R., Teucher, B., Boeing, H., Bergmann, M. M., Trichopoulou, A., Trichopoulos, D., Lagiou, P., Palli, D., Pala, V., Panico, S., Tumino, R., Vineis, P., Siersema, P., van Duijnhoven, F., Peeters, P. H., Hjartaker, A., Engeset, D., González, C. A., Sánchez, M. J., Dorronsoro, M., Navarro, C., Ardanaz, E., Quirós, J. R., Sonestedt, E., Ericson, U., Nilsson, L., Palmqvist, R., Khaw, K. T., Wareham, N., Key, T. J., Crowe, F. L., Fedirko, V., Wark, P. A., Chuang, S. C. and Riboli, E. (2012). Dietary fibre intake and risks of cancers of the colon and rectum in the European prospective investigation into cancer and nutrition (EPIC). Plos One 7:1–10.
- Nair, B. M., Asp, N.-G., Nyman, M. and Persson, H. (1987). Binding of mineral elements by some dietary fibre components-in vitro (I). Food Chem. 23:295–303.
- Netzel, M., Netzel, G., Zabaras, D., Lundin, L., Day, L., Addepalli, R., Osborne, S. and Seymour, R. (2011). Release and absorption of carotenes from processed carrots (Daucus carota) using in vitro digestion coupled with a Caco-2 cell trans-well culture model. Food Res. Int. 44:868–874.
- N'Goma, J.-C. B., Amara, S., Dridi, K., Jannin, V. and Carriere, F. (2012). Understanding the lipid digestion processes in the GI tract before designing lipid-based drug-delivery systems. Ther. Deliv. 3:105–124.
- Noah, L., Guillon, F., Bouchet, B., Buleon, A., Molis, C., Gratas, M. and Champ, M. (1998). Digestion of carbohydrate from white beans (Phaseolus vulgaris L.) in healthy humans. J. Nutr. 128:977–985.
- Nomura, A. M., Hankin, J. H., Henderson, B. E., Wilkens, L. R., Murphy, S. P., Pike, M. C., LeMarchand, L., Stram, D. O., Monroe, K. R. and Kolonel, L. N. (2007). Dietary fiber and colorectal cancer risk: The multiethnic cohort study. Cancer Causes Control 18:753–764.
- Norton, A. B., Cox, P. W. and Spyropoulos, F. (2011). Acid gelation of low acyl gellan gum relevant to self-structuring in the human stomach. Food Hydrocoll. 25:1105–1111.
- Norton, I., Moore, S. and Fryer, P. (2007). Understanding food structuring and breakdown: Engineering approaches to obesity. Obes. Rev. 8:83–88.
- Oakenfull, D. (1987). Gelling agents. Crit. Rev. Food Sci. Nutr. 26:1–31.
- Padayachee, A., Day, L., Howell, K. and Gidley, M. J. (2017). Complexity and health functionality of plant cell wall fibres from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 57:59–81.
- Padayachee, A., Netzel, G., Netzel, M., Day, L., Zabaras, D., Mikkelsen, D. and Gidley, M. J. (2012a). Binding of polyphenols to plant cell wall analogues – part 1: Anthocyanins. Food Chem. 134:155–161.
- Padayachee, A., Netzel, G., Netzel, M., Day, L., Zabaras, D., Mikkelsen, D. and Gidley, M. J. (2012b). Binding of polyphenols to plant cell wall analogues – Part 2: Phenolic acids. Food Chem. 135:2287–2292.
- Padayachee, G., Netzel, M., Netzel, L., Day, D., Zabaras, D., Mikkelsen, D. and Gidley, M. J. (2013). Lack of release of bound anthocyanins and phenolic acids from carrot plant cell walls and model composites during simulated gastric and small intestinal digestion. Food Funct. 4:906–916.
- Pal, R. (1996). Rheological properties of mixed polysaccharides and polysaccharide-thickened emulsions. AIChE J. 42(7):1824–1832.
- Palmero, P., Lemmens, L., Ribas-Agusti', A., Sosa, C., Met, K., Umutoni, J. d. D., Hendrickx, M. and Van Loey, A. (2013). Novel targeted approach to better understand how natural structural barriers govern carotenoid in vitro bioaccessibility in vegetable based systems. Food Chem. 141:2036–2043.
- Park, J. S., Woo, M. S., Kim, D. H., Hyun, J. W., Kim, W. K., Lee, J. C. and Kim, H. S. (2007). Anti-inflammatory mechanisms of isoflavone metabolites in lipopolysaccharide-stimulated microglial cells. J. Pharmacol. Exp. Ther. 320:1237–1245.
- Pasquier, B., Armand, M., Guillon, F., Castelain, C., Borel, P., Barry, J. L., Pieroni, G. and Lairon, D. (1996). Viscous soluble dietary fibers alter emulsification and lipolysis of triacylglycerols in duodenal medium in vitro. J. Nutr. Biochem. 7:293–302.
- Pereira, M. A., O'Reilly, E., Augustsson, K., Fraser, G. E., Goldbourt, U., Hitmann, B. L., Hallmans, G., Knekt, P., Liu, S., Pietinen, P., Spiegelman, D., Stevens, J., Virtamo, J., Willet, W. C. and Ascherio, A. (2004). Dietary fiber and risk of coronary heart disease: A pooled analysis of cohort studies. Arch. Intern. Med. 164:370–376.
- Petitot, M., Abecassis, J. and Micard, V. (2009). Structuring of pasta components during processing: Impact on starch and protein digestibility and allergenicity. Trends Food Sci. Tech. 20:521–532.
- Pettolino, F. A., Walsh, C., Fincher, G. B. and Bacic, A. (2012). Determining the polysaccharide composition of plant cell walls. Nat. Protoc. 7:1590–1607.
- Phan, A. D. T., Netzel, G., Wang, D., Flanagan, B. M., D'Arcy, B. and Gidley, M. J. (2015). Binding of dietary polyphenols to cellulose: Structural and nutritional aspects. Food Chem. 171:388–396.
- Ragaee, S. M., Campbell, G. L., Scoles, G. J., McLeod, J. G. and Tyler, R. T. (2001). Studies on rye (Secale cereale L.) lines exhibiting a range of extract viscosities. 1. Composition, molecular weight distribution of water extracts, and biochemical characteristics of purified water-extractable arabinoxylan. J. Agric. Food Chem. 49:2437–2445.
- Ralph, J. (2010). Hydroxycinnamates in lignification. Phytochem. Rev. 9(1):65–83.
- Ramasamy, U. S., Venema, K., Schols, H. and Gruppen, H. (2014). Effect of soluble and insoluble fibers within the in vitro fermentation of chicory root pulp by human gut bacteria. J. Agric. Food Chem. 62:6794–6802.
- Rave, K., Roggen, K., Dellweg, S., Heise, T. and tom Dick, H. (2007). Improvement of insulin resistance after diet with a whole-grain based dietary product: Results of a randomized, controlled crossover study in obese subjects with elevated fasting blood glucose. Br. J. Nutr. 98:929–936.
- Reiser, S. (1987). Metabolics effects of dietary pectins related to human health. Food Technol. 41:91–99.
- Ribnicky, D. M., Roopchand, D. E., Poulev, A., Kuhn, P., Oren, A., Cefalu, W. T. and Raskin, I. (2014). Artemisia dracunculus L. polyphenols complexed to soy protein show enhanced bioavailability and hypoglycemic activity in C57BL/6 mice. Nutrition. 30:S4–S10.
- Roberfroid, M. B., Van Loo, J. A. E. and Gibson, G. R. (1998). The bifidogenic nature of chicory inulin and its hydrolysis products. J. Nutr. 128:11–19.
- Robertson, J. A., Majsak-Newman, G., Ring, S. G. and Selvendran, R. R. (1997). Solubilization of mixed linkage (1-3),(1-4)-b-D-glucans from barley: Effects of cooking and digestion. J. Cereal Sci. 25:275–283.
- Rodriguez, M. S. and Albertengo, L. E. (2005). Interaction between chitosan and oil under stomach and duodenal digestive chemical conditions. Biosci. Biotechnol. Biochem. 69:2057–2062.
- Rodriguez-Gutierrez, G., Rubio-Senent, F., Lama-Munoz, A., Garcia, A. and Fernandez-Bolanos, J. (2014). Properties of lignin, cellulose, and hemicelluloses isolated from olive cake and olive stones: binding of water, oil, bile acids, and glucose. J. Agric. Food Chem. 62:8973–8981.
- Roopchand, D. E., Grace, M. H., Kuhn, P., Cheng, D. M., Plundrich, N., Poulev, A., Howell, A., Friedlender, B., Lila, M. A. and Raskin, I. (2012). Efficient sorption of polyphenols to soybean flour enables natural fortification of foods. Food Chem. 131:1193–1200.
- Rose, D. J., Keshavarzian, A., Patterson, J. A., Venkatachalam, M., Gillevet, P. and Hamaker, B. R. (2009). Starch-entrapped microspheres extend in vitro fecal fermentation, increase butyrate production, and influence microbiota pattern. Mol. Nutr. Food Res. 53:S121–S130.
- Rose, D. J., Venema, K., Keshavarzian, A. and Hamaker, B. R. (2010). Starch entrapped microspheres show a beneficial fermentation profile and decrease in potentially harmful bacteria during in vitro fermentation in faecal microbiota obtained from patients with inflammatory bowel disease. Br. J. Nutr. 103:1514–1524.
- Rubio-Senent, F., Rodriguez-Gutierrez, G., Lama-Munoz, A. and Fernandez-Bolanos, J. (2015). Pectin extracted from thermally treated olive oil by-products: Characterization, physico-chemical properties, in vitro bile acid and glucose binding. Food Hydrocoll. 43:311–321.
- Ruiz, M. S. A., Espinosa, M. D. B., Contreras Fernandez, C. J., Luque Rubia, A. J., Sanchez Ayllon, F., Aldeguer Garcia, M., Santamaria, C. G. and Lopez Roman, F. J. (2016). Digestion-resistant maltodextrin effects on colonic transit time and stool weight: A randomized controlled clinical study. Eur. J. Nutr. 55:2389–2397.
- Ruiz, P. A., Braune, A., Hölzwimmer, G., Quintanilla-Fend, L. and Haller, D. (2007). Quercetin inhibits TNF-induced NF-kappaB transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells. J. Nutr. 137:1208–1215.
- Ruiz, P. A. and Haller, D. (2006). Functional diversity of flavonoids in the inhibition of the proinflammatory NF-kappaB, IRF, and Akt signaling pathways in murine intestinal epithelial cells. J. Nutr. 136:664–671.
- Runpagaporn, P., Reuhs, B. L., Kaur, A., Patterson, J. A., Keshavarzian, A. and Hamaker, B. R. (2015). Structural features of soluble cereal arabinoxylan fibers associatedwith a slow rate of in vitro fermentation by human fecal microbiota. Carbohydr. Polym. 130:191–197.
- Russell, W. R., Gratz, S. W., Duncan, S. H., Holtrop, G., Ince, J., Scobbie, L., Duncan, G., Johnstone, A. M., Lobley, G. E., Wallace, R. J., Duthie, G. G. and Flint, H. J. (2011). High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 93:1062–1072.
- Russo, M., Martinelli, M., Sciorio, E., Botta, C., Miele, E., Vallone, G. and Staiano, A. (2013). Stool consistency, but not frequency, correlates with total gastrointestinal transit time in children. J. Pediatr. 162:1188–1192.
- Sakata, T. and Saito, M. (2007). Insoluble Dietary Fiber of Wheat Bran Increased Viscosity of Pig Whole Cecal Contents in Vitro. J. Nutr. Sci Vitaminol. 53, 380–381.
- Salonen, A. and De Vos, W. (2014). Impact of diet on human intestinal microbiota and health. Annu. Rev. Food Sci. Technol. 5:239–262.
- Sanchez, J. I., Marzorati, M., Grootaert, C., Baran, M., Van Craeyveld, V., Courtin, C. M., Broekaert, W. E., Delcour, J. A., Verstaete, W. and Van de Wiele, T. (2009). Arabinoxylan-oligosaccharides (AXOS) affect the protein/carbohydrate fermentation balance and microbial population dynamics of the Simulator of Human Intestinal Microbial Ecosystem. J. Microb. Biotechnol. 2:101–113.
- Saura-Callixto, F. (2011). Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 59:43–49.
- Saura-Callixto, F. and Goni, I. (2006). Antioxidant capacity of the Spanish mediterranean diet. Food Chem. 94:442–447.
- Saura-Callixto, F., Jimenez, J. P., Tourino, S., Serrano, J., Fuguet, E., Torres, J. L. and Goni, I. (2010). Proanthocyanidin metabolites associated with dietary fibre from in vitro colonic fermentation and proanthocyanidin metabolites in human plasma. Mol. Nutr. Food Res. 54:939–946.
- Sayar, S., Jannink, J. L. and White, P. J. (1996). In vitro bile acid binding activity within flour fractions from oat lines with typical and high α-Glucan amounts. J. Agric. Food Chem. 54:5142–5148.
- Scazzina, F., Siebenhandl-Ehn, S. and Pellegrini, N. (2013). The effect of dietary fibre on reducing the glycaemic index of bread. Br. J. Nutr. 109:1163–1174.
- Schatzkin, A., Mouw, T., Park, Y., Subar, A. F., Kipnis, V., Hollenbeck, A., Leitzmann, M. F. and Thompson, F. E. (2007). Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and health study. Am. J. Clin. Nutr. 85:1353–1360.
- Schatzkin, A., Park, Y., Leitzmann, M. F., Hollenbeck, A. R. and Cross, A. J. (2008). Prospective study of dietary fiber, whole grain foods, and small intestinal cancer. Gastroenterology. 135:1163–1167.
- Schlemmer, U. (1989). Studies of the binding of copper, zinc and calcium to pectin, alginate, carrageenan and gum guar in HCO3−-CO2 buffer. Food Chem. 32:223–234.
- Schmidt, K. A. and Smith, D. E. (1992). Milk reactivity of gums and milk proteins solutions. J. Dairy Sci. 75:3290–3295.
- Scholz-Ahrens, K. E., Ade, P., Marten, B., Weber, P., Timm, W., Aςil, Y., Glüer, C.-C. and Schrezenmeir, J. (2007). Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J. Nutr. 137(3):838S–846S.
- Slaughter, S. L., Ellis, P. R., Jackson, E. C. and Butterworth, P. J. (2002). The effect of guar galactomannan and water availability during hydrothermal processing on the hydrolysis of starch catalysed by pancreatic α-amylase. Biochim. Biophys. Acta 1571:55–63.
- Slavin, J. L., Brauer, P. M. and Marlett, J. A. (1981). Neutral detergent fiber, hemicellulose and cellulose digestibility in human subjects. J. Nutr. 111:287–297.
- Smidsrod, O., Haug, A. and Larsen, B. (1966). The influence of pH on the rate of hydrolysis of acidic polysaccharides. Acta Chem. Scand. 20:1026–1034.
- Soares, S., Mateus, N. and de Freitas, V. (2012). Carbohydrates inhibit salivary proteins precipitation by condensed tannins. J. Agric. Food Chem. 60:3966–3972.
- Sonnenburg, E. D. and Sonnenburg, J. L. (2013). Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 20:779–786.
- Spyropoulos, F., Norton, A. B. and Norton, I. T. (2011). Self-structuring foods based on acid-sensitive mixed biopolymer to impact on satiety. Procedia Food Sci. 1:1487–1493.
- Sternini, C., Anselmi, L. and Rozengurt, E. (2008). Enteroendocrine cells: A site of ‘taste’ in gastrointestinal chemosensing. Curr. Opin. Endocrinol. Diabetes Obes. 15(1):73–78.
- Stewart, M. L. and Slavin, J. L. (2009). Particle size and fraction of wheat bran influence short-chain fatty acid production in vitro. Br. J. Nutr. 102:1404–1407.
- Story, J. A. and Kritchevsky, D. (1976). Comparison of the binding of various bile acids and bile salts in vitro by several types of fiber. J. Nutr. 106:1292–1294.
- Strugala, V., Kennington, E. J., Campbell, R. J., Skjak-Braek, G. and Dettmar, P. W. (2005). Inhibition of pepsin activity by alginates in vitro and the effect of epimerization. Int. J. Pharm. 304:40–50.
- Stuknyte', M., Cattaneo, S., Pagani, M. A., Marti, A., Micard, V., Hogenboom, J. and De Noni, I. (2014). Spaghetti from durum wheat: Effect of drying conditions on heat damage, ultrastructure and in vitro digestibility. Food Chem. 149:40–46.
- Sundberg, B., Wood, P. J., Lia, A., Andersson, H., Sandberg, A.-S., Hallmans, G. and Aman, P. (1996). Mixed-linked b-glucan from breads of different cereals is partly degraded in the human ileostomy model. Am. J. Clin. Nutr. 64:878–885.
- Takahashi, T., Karita, S., Ogawa, N. and Goto, M. (2005). Crystalline cellulose reduces plasma glucose concentrations and stimulates water absorption by increasing the digesta viscosity in rats. J. Nutr. 135:2405–2410.
- Theuwissen, E. and Mensink, R. P. (2008). Water-soluble dietary fibers and cardiovascular disease. Physiol. Behav. 94:285–292.
- Thongngam, M. and McClements, D. J. (2005). Isothermal titration calorimetry study of the interactions between chitosan and a bile salt (sodium taurocholate). Food Hydrocoll. 19:813–819.
- Tolstoguzov, V. B. (2000). Phase behaviour of macromolecular components in biological and food systems. Rev. Nahrung 44:299–308.
- Tomlin, J. and Read, N. W. (1988). Laxative properties of indigestible plastic particles. Br. Med. J. 297:1175–1176.
- Torcello-Gomez, A., Fernandez Fraguas, C., Ridout, M. J., Woodward, N. C., Wilde, P. J. and Foster, T. F. (2015). Effect of substituent pattern and molecular weight of cellulose ethers on interactions with different bile salts. Food Funct. 6:730–739.
- Tornquist, H., Rissanen, A. and Andersson, H. (1986). Balance studies in patients with intestinal resection, how long is enough?. Br. J. Nutr. 56:11–16.
- Trowell, H. (1972). Ischemic heart disease and dietary fiber. Am. J. Clin. Nutr. 25:926–932.
- Tsujita, T., Takaichi, H., Takaku, T., Sawai, T., Yoshida, N. and Hiraki, J. (2007). Inhibition of lipase activities by basic polysaccharide. J. Lipid Res. 48(2):358–365.
- Tuhoy, K. M., Conterno, L., Gasperotti, M. and Viola, R. (2012). Up-regulating the human intestinal microbiome using whole plant foods, polyphenols, and/or fiber. J. Agric. Food Chem. 60:8776–8782.
- Tydeman, E. A., Parker, M. L., Wickham, M., Rich, G. T., Faulks, R., Gidley, M. J., Fillery-Travis, A. and Waldron, K. (2010). Effect of carrot (Daucus carota) microstructure on carotene bioaccessibility in the upper gastrointestinal tract. 1. In vitro simulations of carrot digestion. J. Agric. Food Chem. 58:9847–9854.
- Ulmius, M., Adapa, S., Onning, G. and Nilsson, L. (2012). Gastrointestinal conditions influence the solution behaviour of cereal β-glucans in vitro. Food Chem. 1:536–540.
- Vahouny, G. V., Tombes, R., Cassidy, M. M., Kritchevsky, D. and Gallo, D. D. (1980). Dietary fibers: V. Binding of bile salts, phospholipids and cholesterol from mixed micelles by bile acid sequestrants and dietary fibers. Lipids 15:1012–1018.
- Vanholme, R., Demedts, B., Morreel, K., Ralph, J. and Boerjan, W. (2010). Lignin biosynthesis and structure. Plant Physiol. 153:895–905.
- Van Wey, A. S., Cookson, A. L., Roy, N. C., McNabb, W. C., Soboleva, T. K. and Shorten, P. R. (2011). Bacterial biofilms associated with food particles in the human large bowel. Mol. Nutr. Food Res. 55:969–978.
- Vitaglione, P., Napolitano, A. and Fogliano, V. (2008). Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 19:451–463.
- Vuksan, V., Jenkins, A. L., Rogovik, A. L., Fairgrieve, C. D., Jovanovski, E. and Leiter, L. A. (2011). Viscosity rather than quantity of dietary fibre predicts cholesterol-lowering effect in healthy individuals. Br. J. Nutr. 106:1349–1352.
- Waldron, K. W., Parker, M. L. and Smith, A. C. (2003). Plant cell walls and food quality. Compr. Rev. Food Sci. F. 2:101–119.
- Walker, A. W., Duncan, S. H., McWilliam Leitch, E. C., Child, M. W. and Flint, H. J. (2005). pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 71:3692–3700.
- Weickert, M. O. and Pfeiffer, A. F. (2008). Metabolic effects of dietary fiber consumption and prevention of diabetes. J. Nutr. 138:439–442.
- Whayne, T. F. and Felts, J. M. (1971). Activation of lipoprotein lipase. Evaluation of calcium, magnesium, and ammonium as cofactors. Circ. Res. 28:649–654.
- Wilcox, M. D., Brownlee, I. A., Richardson, J. C., Dettmar, P. W. and Pearson, J. P. (2014). The modulation of pancreatic lipase activity by alginates. Food Chem. 146:479–484.
- Williamson, G. (2013). Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 57:48–57.
- Wolever, T. M., Tosh, S. M., Gibbs, A. L., Brand-Miller, J., Duncan, A. M., Hart, V., Lamarche, B., Thomson, B. A., Duss, R. and Wood, P. J. (2010). Physicochemical properties of oat b-glucan influence its ability to reduce serum LDL cholesterol in humans: A randomized clinical trial. Am. J. Clin. Nutr. 92:723–732.
- World Health Organization. (2013). Diet, Nutrition and the Prevention of Chronic Disease. Report of a Joint WHO/FAO Expert Consultation. World Health Organization, Geneva, Switzerland. WHO Technical Report Series 916.
- Wu, Z., Li, H., Ming, J. and Zhao, G. (2011b). Optimization of adsorption of tea polyphenols into oat β-Glucan using response surface methodology. J. Agric. Food Chem. 59:378–385.
- Wu, Z., Ming, J., Gao, R., Wang, Y., Liang, Q., Yu, H. and Zhao, G. (2011a). Characterization and antioxidant activity of the complex of tea polyphenols and oat β-Glucan. J. Agric. Food Chem. 59:10737–10746.
- Wursch, P. and Pi-Sunyer, X. (1997). The role of viscous soluble fiber in the metabolic control of diabetes. Diabet. Care 20:1774–1780.
- Yoo, S.-H., Fishman, M. L., Hotchkiss, Jr, A. T. and Lee, H. G. (2006). Viscometric behavior of high-methoxy and low-methoxy pectin solutions. Food Hydrocoll. 20:62–67.
- Yuan, J., Wang, J. and Liu, X. (2007). Metabolism of dietary soy isoflavones to equol by human intestinal microflora; implications for health. Mol. Nutr. Food Res. 51:765–781.
- Zangenberg, N. H., Mullertz, A., Kristensen, H. G. and Hovgaard, L. (2001). A dynamic in vitro lipolysis model I. Controlling the rate of lipolysis by continuous addition of calcium. Eur. J. Pharm. Sci. 14(2):115–122.
- Zhang, P., Zhang, Q. and Whistler, R. L. (2003). L-Arabinose release from arabinoxylan and arabinogalactan under potential gastric acidities. Cereal Chem. 80(3):252–254.
- Zhang, R., Zhang, Z., Zhang, H., Decker, E. A. and McClements, D. J. (2015). Influence of emulsifier type on gastrointestinal fate of oil-in-water emulsions containing anionic dietary fiber (pectin). Food Hydrocoll. 45:175–185.
- Zhang, S. and Vardhanabhuti, B. (2014a). Intragastric gelation of whey protein–pectin alters the digestibility of whey protein during in vitro pepsin digestion. Food Funct. 5:102–110.
- Zhang, S., Zhang, Z. and Vardhanabhuti, B. (2014b). Effect of charge density of polysaccharides on selfassembled intragastric gelation of whey protein/ polysaccharide under simulated gastric conditions. Food Funct. 5:1829–1838.
- Zielinski, G., DeVries, J. W., Craig, S. A. and Bridges, A. R. (2013). Dietary fiber in codex alimentarius: Current status and ongoing discussion. Cereal Food Worlds 58(3):148–152.

