 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
There is increasing evidence that nutrient differences observed among crop varieties or animal breeds belonging to the same species are sometimes greater than differences between species. Parkia biglobosa is an important tree species that provides edible products and income to rural households in West Africa. To better understand intra-species nutrient variability of P. biglobosa edible products, a review on the nutrient content of its pulp and seeds (raw and fermented) was conducted. Google scholar and the keywords “P. biglobosa” AND “nutrition” were used to screen the available literature from 1980 onwards, and the Zotero software was used to manage references. A step-wise assessment of titles, abstracts and full papers, led to a selection of 69 papers from which data were retrieved following FAO INFOODS guidelines. After data harmonization and quality checks, 42 papers were retained and used to extract data to populate a nutrient database. Despite an apparent abundance of nutrient analyses focused on P. biglobosa’s edible products, the quality of data available was poor and very few authors presented additional information, such as soil characteristics, climate, maturity at harvest, etc. that could influence the nutritional content of the products. Many data gaps remain. The present study will stimulate further investigations into nutrient composition of P. biglobosa products and ultimately will contribute to selecting nutritionally “+” trees for multiplication and/or domestication of the species.
Introduction
Within-species differences in nutrient content between crop varieties and animal breeds can sometimes be greater than the differences between species (CBD/WHO, 2015; Lutaladio, Burlingame, and Crews Citation2010). For example, nutrient differences from 5.6 up to 14.6 g protein/100 g edible portion in rice have been documented (Burlingame, Charrondiere, and Mouille Citation2009; Kennedy et al. Citation2017). Consequently, the consumption of 200 g of rice per day can represent from less than 25% to more than 65% of the recommended daily intake of proteins, depending on the variety consumed (Kennedy and Burlingame Citation2003). For bananas, nutrient differences across varieties from less than 1 up to 8500 μg β-carotene/100 g of edible portion have been documented (Burlingame, Charrondiere, and Mouille Citation2009). In other words, consuming one dessert banana a day or consuming one orange-fleshed high β-carotene variety could make the difference between being Vitamin-A deficient or not (Ekesa et al. Citation2015; Englberger et al. Citation2006). These findings have led to an increased awareness of the importance of documenting and understanding within-species nutrient differences in food composition (Burlingame Citation2004) and to the development of the FAO nutrition indicators for biodiversity: food composition (1) and food consumption (2) (FAO Citation2008; FAO Citation2010).
In Burkina Faso, Parkia biglobosa (Jacq.) R.Br. ex G. Don f. (Fabaceae; Mimosoideae) is a tree of utmost importance as a source of edible products and income for the vast majority of rural households (Thiombiano et al. Citation2013; Vinceti et al. Citation2018). The species, known as néré in Francophone Africa, is indigenous to sub-Saharan Africa (Hopkins Citation1983) and has a very wide distribution range (). It has been ranked by local people among the top priority tree species in Burkina Faso (ICRAF Citation2006; Kristensen and Lykke 2013), and its importance is documented all across West African countries (Hall et al. Citation1997; Teklehaimanot Citation2004). Parkia biglobosa pulp is a good source of energy and vitamin C, while the fermented grains contribute calcium, lipids and proteins to the diets of vulnerable populations in West-Africa (Orwa et al. Citation2009) (). Although the quantity of “soumbala” (fermented seeds) consumed at each meal is rather low, the fact that soumbala is consumed regularly makes it an important source of nutrients (Boedecker et al. Citation2014).
Figure 1. Geographic distribution of Parkia biglobosa in relation to annual rainfall (adapted from Hall et al. Citation1997).
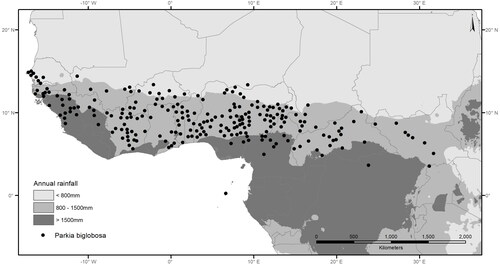
Figure 2. An individual of Parkia biglobosa from a Parkland in Burkina Faso (credit: Barbara Vinceti, Bioversity International).

Figure 3. Fruits (pods) of Parkia biglobosa from a Parkland in Burkina Faso. Pulp and seeds are consumed as edible products (credit: Barbara Vinceti, Bioversity International).

Figure 4. Balls of soumbala derived from the fermented seeds of Parkia biglobosa, sold as a condiment at the market (credit: Barbara Vinceti, Bioversity International).

Several studies on tree population structure of P. biglobosa have shown poor regeneration and ageing of the stands, which may result in complete disappearance over time (Bouda and Nikiema Citation1996; Ouedraogo Citation1995; Raebild, Hansen, and Kambou Citation2012). Factors such as overexploitation, shortening of the fallow period and a drier climate have been suggested as responsible for this decline (Boffa Citation1999; Bouda and Nikiema Citation1996; Gijsbers, Kessler, and Knevel Citation1994; Nikiema Citation1993; Teklehaimanot Citation2004). In some areas, due to extreme competition in accessing the resource, people harvest P. biglobosa pods before their complete maturity, and this is likely to affect the regeneration of the species and the quality (taste and nutritional properties) of the edible products derived from the pods (Pehou et al. Citation2020).
Differences in growth, drought-resistance and morphology between P. biglobosa individuals from different populations across its distribution range have been described (Bouda et al. Citation2013; Ouedraogo et al. Citation2012). Similarly, published values derived from different studies about the nutritional composition of edible products from P. biglobosa present huge variability. This seems to be common for other tree species. Stadlmayer et al. (2013) reported large variability in values after compiling data from published food composition studies for 10 different indigenous fruit species from Sub-Saharan Africa. Some studies mention factors such as climate, soils or provenances as potential explanatory variables for these differences, but their specific influence has not been studied so far.
The objective of this literature review is to compile a nutrient database with values extracted from publicly available data sources for P. biglobosa pulp and seeds (raw and fermented) to better understand the within-species variability in the nutritional properties of this tree. This will underpin a new research agenda that combines results from the genetic characterization of different populations of P. biglobosa, with an understanding of the variation in nutrients in the resource base. The ultimate objective is to inform the selection of optimal sources of planting material for forest restoration projects at different sites.
A systematic literature review was conducted to address the following questions: what is the level of within-species variation in proximate, mineral and vitamin content in P. biglobosa pulp, raw and fermented seeds? Are there any patterns in nutritional values identifiable across studies and different sites located in different regions of the species distribution range? Are there studies conducted in multiple locations combining research on nutritional content with environmental and/or genetic aspects? Are there studies assessing the relative weight of different factors determining the observed within-species differences in nutritional composition (e.g. soil, rainfall regime, level of maturity at the time of harvesting)? Are there research gaps?
Methodology
Literature search
An initial scoping search of scientific literature was performed screening multiple search engines and bibliographic databases (Google Scholar, Microsoft Academic, CiteSeer, Bioline International, Science Direct, Plos One, African Journals online, Directory of Open Access Journals, Web of Science, Medline, Pubmed, Agris, Agricola, CAB Abstracts, Food Science and Technical abstracts – FSTA). Searches included both open access and standard subscription-based journals, both peer-reviewed and gray literature.
After the initial search iterations, Google Scholar appeared to be the broadest source of information, including the same hits generated by other search engines or databases. A broad range of search terms was tested including the scientific name of the species (Parkia biglobosa), the English (African locust bean) and French (Nére) common names, and local names of the edible raw products or products processed from fermentation of P. biglobosa seeds (dawadawa). These were combined with the following additional search terms: nutrition, nutritional composition, nutritional analysis, vitamin or mineral content. The combination of keywords Parkia biglobosa AND nutrition turned out to be the most comprehensive and included all results derived from other combinations of terms. After this initial testing of search terms, in June 2018 a literature search was performed in Google Scholar using the search terms Parkia biglobosa AND nutrition, to gather information on the nutrient composition of the different edible products derived from P. biglobosa. The review focused on scientific publications released from January 1980 onwards.
The reference list obtained from the search in Google Scholar was imported in Zotero, an open-source reference management software (www.zotero.org) and duplicates were removed. Subsequently, titles and abstracts were screened, and full papers of relevant documents were collated for further reading. Based on a screening of the full-text, the following articles were further discarded: papers presenting secondary data (reported from previous papers), papers with results shown only in graphs or in the form of value ranges without precise figures, papers dealing with processed edible tree products mixed with additional ingredients, papers focused on other tree species of the same genus or papers only presenting amino acid or fatty acid profiles, which were outside the scope of this review.
Database compilation
After the selection, the remaining papers were used to extract nutrient data and to compile a first draft nutrient database in excel, using the INFOODS food component identifiers (tagnames) and the FAO/INFOODS compilation tool as a guide (FAO/INFOODS 2009; INFOODS Citation2012). Nutrients were entered in excel exactly in the way they were presented in the original paper, accompanied with units and methods used for the nutritional analysis or reference thereto. Huge variability was observed in the expression of units and denominators. Data were harmonized and recalculated into g (proximate), mg (minerals and antinutrients), or μg (vitamins) per 100 g fresh weight of edible portion (EP) to enable comparisons across studies. Some data were expressed on a dry matter (DM) basis, without providing moisture or DM percentages; these were recalculated on the basis of a 100 g fresh weight of edible portion (EP), using the average moisture content calculated from our database. The results of these recalculations were presented in a second draft nutrient database with standardized data. A data quality check was performed, further excluding any papers with implausible data. The sum of proximates (water, fat, protein, carbohydrate, fiber and ash) was checked and papers with values outside of the 95–105 g range were excluded. Some papers did not mention whether the data were expressed on a dry or a fresh weight basis. Therefore, assumptions had to be made following the example found in Stadlmayer et al. (2013). If the sum of proximates, excluding moisture content, totaled 100 or more, the assumption was that the values were reported on a dry weight basis and were therefore converted to a fresh weight basis. When only a few proximate values were reported, they were compared with values in the West African Food Composition Tables (WAFCT) for plausibility and papers with divergent values were excluded. Some papers were inconsistent/incoherent: e.g. the proximate composition was within the 95–105 range for one product and outside the range for another product. These papers were also completely excluded from the study. Mineral values were added up and those papers in which the sum of minerals was more than double the ash value were discarded (Stadlmayer et al. 2013). For vitamins or anti-nutrients, thresholds for quantitative checks were not available. After excluding all the cases above, a final database with harmonized values from selected papers was compiled and used as the basis for the review of nutrient values presented in this paper. Micro-nutrient values for Parkia biglobosa products available in the WAFCT were extracted and presented in our result tables (in the first line) in order to enable comparisons.
Data presentation and analysis
An overview table was constructed presenting the main characteristics of the studies selected for this review: author and year of publication, P. biglobosa product(s) analyzed, nutrients analyzed, number of lab replications carried out, origin of the samples (market or collected directly from the trees), geographical region and any further information regarding environmental or seasonal factors that might have influenced the nutrient composition presented in the paper. Some papers contained an imprecise description of sampling methods, sample preparation procedures, and/or about sampling sites. Furthermore, some papers did not specify the exact edible product analyzed and/or whether the lab analyses were conducted in replicates or not. Simple descriptive statistics were used to summarize results.
A geographic map was developed showing all the sampling sites reported in the final selection of articles retained for this review. This enabled to visualize the geographic distribution of the research conducted across the main ecoregions in West Africa, and to compare the spatial distribution of nutritional studies with the range of P. biglobosa. Those studies missing precise information regarding the sampling sites (beyond the country level) were positioned on the map using the geographic centroid value of the country were the research had been conducted, reported in the article.
Nutrient data from the final excel database were extracted and presented in separate tables for each of the nutrient categories considered (proximate, minerals, vitamins and antinutrients) and grouped based on the different P. biglobosa edible product examined (raw product, fermented product and pulp) for ease of comparison. Values presented in this review might slightly differ from the values found in the original papers, due to the conversions applied to harmonize units and denominators for all values.
A preliminary attempt to identify patterns in nutrient composition was made. However, the objective was not to directly compare values found in the various papers, given that the studies presented in the literature differed to a large extent in sampling strategy, product preparation and/or methods for laboratory analyses. Methodological procedures were compared with FAO/INFOODS recommended methods. Nutrient values derived from unknown or non-recommended methods were flagged in our tables; exceptions were made for vitamins (non-recommended methods were mostly used) and for anti-nutrients (no recommendations on methods were available). Papers referring to AOAC methods, without presenting further details or explanations, were assumed to have used the recommended methods for proximates and minerals. This assumption was not extended to vitamins and minerals.
Results
Metadata
shows an overview of the literature search process and data flow. The main search was performed in June 2018 in Google Scholar, using the keywords “Parkia biglobosa” AND nutrition; it generated 3500 hits which were all scanned for relevance against title and abstract. By repeating a thorough search in Food Science and Technology Abstracts (FSTA, the second most relevant database, as revealed by the first pilot screening of the literature), a total of five additional relevant references, not found in the previous search in Google Scholar, were included in the list of articles to be considered. This resulted in 108 papers for which full texts were collected in Zotero. Just one document, a dissertation, had to be excluded because it was inaccessible.
Figure 5. A flow diagram describing the different phases of the literature review, the number of records discarded and retained at each step, and the reasons for exclusion.
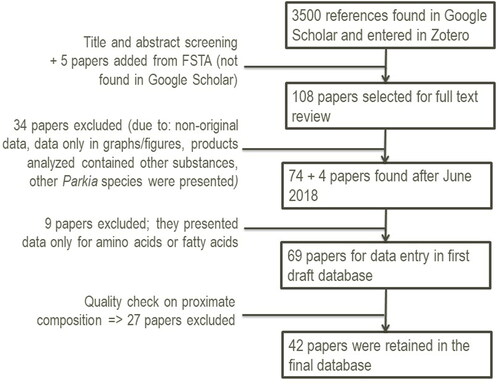
After reading the full content, 34 papers were excluded from the database based on the following reasons: they were review papers presenting non-original data, did not present exact values (data were only presented in graphs or ranges), only processed products had been analyzed mixing other ingredients with P. biglobosa seeds or pulp. Some papers reported the same data found in others. In cases of dataset “duplicates” between two different papers, only the first one published was retained. Finally, some papers did not match our expectations regarding content, e.g. the full text focused on a different Parkia species.
Subsequently, another four relevant papers published in the two months following the formal ending of the literature search were added. Nine papers were further excluded because they were only dealing with amino acids or fatty acid profiles. This brought down the number to 69 papers that were used to extract nutrient values for the different P. biglobosa edible products. On this basis, a first draft nutrient database was developed. Data were harmonized into the same units and denominators and this resulted in a second draft database. Further data quality checks on proximate values in the second draft database, led to the exclusion of another 27 papers. Finally, 42 papers provided the data for the final nutrient database. Only seven out of these 42 papers were consistent in mentioning the denominator (fresh weight EP or dry weight) for all nutrient categories presented. Eight papers mentioned a denominator for one nutrient category, but not for another. For 22 papers, we made assumptions regarding the denominator, based on the sum of the proximate values (see methods 2.2). Five papers did not present full proximate range data and nutrient values had to be compared with the WAFCT. Assumptions made on how the proximate values were expressed were extended to the other nutrients.
provides an overview of key content from the 42 papers that made it into the final nutrient database: author and journal names, specific products analyzed, nutrient categories analyzed, number of replicate analyses in the laboratory, sample origins, geographical area and other environmental characteristics. The 42 papers selected were published in a wide range of journals in different science categories, from food and nutrition sciences to biology, chemistry, ethnobotany, agriculture, livestock, technology and applied biosciences. Papers from food and nutrition journals were not necessarily better in presenting correct units, denominators, sampling and analysis methods than papers from other journal categories. The analyzed products were treated and described in many different ways in the papers. Four papers did not clearly describe the edible products analyzed and referred to seed or pulp in general terms. In one case, it was necessary to make a “reasonable” guess about whether the product actually analyzed was pulp or seed. Given these heterogeneous elements across the selected papers, comparisons between papers to identify potential patterns in the nutrient composition according to geographical location, climate, soil or genetic parameters were not carried out. Other parameters that differed across papers were sampling strategies and laboratory methods used. Seven papers did not indicate whether laboratory analyses were carried out in duplicates or not, and four papers presented values of standard deviations in their results but did not mention the number of replicate analyses.
Table 1. Summary characteristics of papers used for the review.
summarizes the food product categories and nutrients analyzed in the different papers. Most studies analyzed fermented seed and presented proximate data. shows a map with the bioclimatic regions of West Africa and the location of the sampling sites of all 42 articles retained in this review, based on indication provided in the articles themselves. When only the region of sampling was indicated in the paper, we tagged the capital of that region on the map. Often, papers dealing with fermented products only mentioned the market where the product was bought (and not the origin of the product). Two papers (9 and 30) did not include further information on the sample location beyond the country; these studies were positioned in the centroid of the country and were marked with a different symbol (triangle). Four papers covered multiple sampling sites. The research presented in the 42 selected articles was mainly conducted in Nigeria (28), followed by Ghana (4), Benin, Burkina Faso, Chad and Ivory Coast (2 studies in each country) and Cameroon and Mali (1 study in each). Most study sites were located in the Guinean ecozone (23), followed by the Sudanian ecozone (17) and only six study sites fell in the Guineo-Congolian ecozone.
Figure 6. Map with location of the sampling sites reported in the 42 articles retained for this literature review. The main bioclimatic regions of West Africa are delineated based on annual rainfall (adapted from CILSS Citation2016). The positioning of the sampling sites is based on indications provided in each individual paper. The numbering of the sites in the map corresponds with the numbering of the articles in this review (see list of the 42 references in ). If the original article mentioned only the region of sampling and not the precise site, the point on the map was located in correspondence of the capital of that region. When only the country was indicated in the article (n. 9 and 30), the point on the map was marked with a different symbol (triangle), positioned in correspondence with the geographic centroid of the country. Multiple letters associated with the same number indicate that multiple sites were investigated in the specific paper.
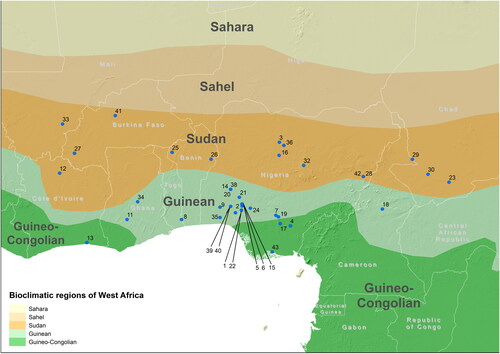
Table 2. Summary table with number of papers presenting data for the different Parkia biglobosa’s food products and the different nutrient categories.
Nutrient database observations
present the proximate, mineral, vitamin and antinutrient content for raw seeds, fermented seeds and pulp products of P. biglobosa.
Table 3a. Proximate composition for raw Parkia biglobosa seed products in g/100g fwb.
Table 3b. Proximate composition for fermented Parkia biglobosa seed products in g/100g fwb.
Table 3c. Proximate composition for Parkia biglobosa pulp products in g/100g fwb.
Table 4a. Mineral composition of raw Parkia biglobosa seed products in mg/100g fwb.
Table 4b. Mineral composition of fermented Parkia biglobosa seed products in mg/100g fwb. Values are mean of independent replications as per the author ± standard deviation. Blank spaces indicate the author did not determine the value. No author analyzed cobalt. Only one author analyzed selenium (0.48 ± 0.01).
Table 4c. Mineral composition of Parkia biglobosa pulp products in mg/100g fwb.
Table 5a. Vitamin composition for raw Parkia biglobosa seed products in µg/100g (except Vit C, expressed in mg/100g).
Proximate composition (, b and c)
The default methods used in the selected literature were mostly the FAO/INFOODS recommended methods for proximate composition: oven drying for moisture content, Kjeldahl method with 6.25 conversion factor for crude protein, Soxhlet solvent extraction for crude fat, dry ashing for ash and (total or available) carbohydrates by difference. Exceptions to these methods were indicated in . Most fiber values were expressed as the least recommended crude fiber component, unless otherwise indicated (see details in ).
Moisture content for the unfermented seeds ranged from 1.2 to 13.06 g/100 g EP, protein content from 20.93 to 36.46 g/100 g EP, fat content from 8.06 to 32.02 g/100 g EP; ash content from 1.2 to 5.9 g/100 EP, fibers from 0.39 to 17.37 g/100 g EP and available carbohydrates from 13.2 to 54.21 g/100 g EP. These values were within the range of the values presented in the WAFCT (Stadlmayer et al. 2012).
Moisture content for the fermented seeds ranged from 4.92 to 63.5 g/100 g EP, protein content from 3.22 to 49.69 g/100 g EP, fat content from 7.44 to 37.13 g/100 g EP; ash content from 0.56 to 8.26 g/100 g EP, fibers from 1.49 to 14.3 g/100 g EP and available carbohydrates from 0.09 to 53.5 g/100 g EP. No values for fermented seeds were available in the WAFCT. Moisture content for pulp ranged from 4.5 to 13.2 g/100 g EP, protein content from 0.62 to 6.89 g/100 g EP, fat content from 0.8 to 25.62 g/100 g EP; ash content from 2.47 to 8.36 g/100 g EP, fibers from 2.55 to 14.23 g/100 g EP and available carbohydrates from 32.2 to 80.8 g/100 g EP. All values, except for paper 4 (lowest range for protein and fiber; low in carbohydrates and very high in fats) and 30 (highest ranges for protein and fat; very low in carbohydrates) were within the range of the WAFCT values. Some papers presented total carbohydrates, other papers available carbohydrates. Carbohydrates were mostly calculated by difference (100 minus the other proximate values, including or not fiber). The comparison of nutritional values for unfermented and fermented products within the same paper, when both products were analyzed, showed that moisture and fat content mostly increased with fermentation, while ash, fiber and carbohydrate content decreased. For protein, the pattern was unclear (sometimes it increased, sometimes it decreased).
Four papers (13, 26, 29, 42) compared nutritional data of samples collected at different locations. The first paper (13) compared fermented products bought from three different markets in Ivory Coast and indicated that the products were processed differently according to the customs of the different ethnic groups. Products bought on the Korhogo market were processed locally, products on the Abidjan market originated from Korhogo (Ivory Coast), Guinea or Burkina Faso, and products on the Bouake market from Bouake (Ivory Coast), Guinea or Burkina Faso. The second paper (29) presented data for P. biglobosa pulp bought in three markets from different regions in Chad (all within the Sudanian agro-ecological zone, see ). Unfortunately, the exact origin of the pulp bought on the market could not be determined, so this study did not enable an assessment of nutrient differences among provenances. At the same time, the differences in proximate composition of different samples within this paper were quite small compared to the differences with values reported in other papers. The third paper presented data for whole seeds, dehulled seeds and fermented seeds from the Guineo-Congolian zone (42a and 42c on the map in ) and a location at the border of the Sudanian and Guinean agro-ecological zone (42b). No pattern could be identified for any nutrient, looking for consistently highest or lowest values within a certain origin for all three products. The fourth paper (26) compared data for dehulled seeds from three villages in Atacora Department (Sudanian agro-ecological zone) and three villages in Borgou Department (Guinean agro-ecological zone) in Benin. The moisture content of dehulled P. biglobosa seeds was higher for all three villages in Borgou compared to all three villages in Atacora, while the protein content was systematically higher in Atacora than in Borgou. Fat content (higher in Atacora) and ash content (higher in Borgou) followed a similar pattern, but with very small differences. We observed that values extracted from within a paper (comparing different locations or product treatments, etc.) presented less variation than the values for the same product treatment presented by different authors.
Minerals (, b and c)
Less data were available for mineral composition of edible P. biglobosa products. Calcium, magnesium, iron and zinc were the most analyzed minerals, with fewer papers dealing with manganese, sodium, potassium, copper or phosphorus. Most papers followed the FAO/INFOODS recommended methods: Atomic Absorption Spectroscopy for iron, zinc, calcium, magnesium, manganese and cupper; and flame photometry for the analysis of sodium and potassium. In , values obtained from unknown or non-recommended methods were indicated with superscripts. For phosphorus, FAO/INFOODS recommendations suggest either colorimetry or ICP-MS (Inductively Coupled plasma Mass Spectrometry). In four papers the phosphovanado molybdate (yellow) colorimetric method was used; in all other papers, methods that were not in the FAO/INFOODS lists were used, therefore they were indicated as unknown or non-recommended (“other methods used”). All values were recalculated as mg/100 g EP, however, in many cases, assumptions had to be made regarding denominators. Ranges of mineral content varied widely among authors, up to five orders of magnitude, from as low as 0.21 mg/100 g EP up to 49780 mg/100 g EP for sodium content in fermented seeds. Paper 35 reported the highest values for magnesium, potassium and phosphorus, the second highest level of calcium in unfermented seeds, the highest values of calcium and phosphorus in the pulp. Paper 34 seemed to present systematically high values for several minerals (iron, zinc, calcium, magnesium, manganese and sodium) in the fermented product analyzed, while paper 22 reported the highest calcium values in unfermented and fermented seeds. Paper 11 presented systematically low values for minerals in both unfermented and fermented products. Papers 5 and 23 also showed low mineral values (except phosphorus) for the fermented product, while paper 40 systematically reported the lowest mineral values for pulp (except for iron). Like for the values of proximate composition, variation was much greater among papers than within the same paper. Six papers presented mineral data on fermented and unfermented seeds; however, no clear pattern relating a possible increase or decrease of mineral content due to fermentation could be found. One paper presenting mineral data was a multilocation study, analyzing nutrient values for P. biglobosa pulp bought in three different markets in Chad.
Vitamins (, b and c)
Very little data was available regarding vitamin content of edible P. biglobosa products. The most analyzed were beta-carotene and other pro-vitamin A components, followed by Vitamin C and Vitamin E. Few papers presented Vitamin B or D data. The methods used for vitamin analyses were in most cases unknown or not found among the FAO/INFOODS recommended methods. Only 3 papers used an FAO/INFOODS recommended method for beta-carotene and/or other pro-vitamin A carotenes (papers 14, 19 and 32). None of the other analysis were carried out using FAO/INFOODS recommended methods. Furthermore, most papers did not present (correct) equivalents and/or units. Four papers mentioned that ascorbic acid was analyzed (5, 10, 12 and 14); the others reported they conducted Vitamin C analyses. Regarding Vitamin E, paper 5 mentioned tocopherol as equivalent, paper 10 alpha-tocopherol and paper 38 total tocopherol; the other papers mentioned Vitamin E. To the extent possible, all values were recalculated on a μg/100 g EP basis (except for Vitamin C on a mg/100g EP basis). Similarly to the case of minerals, a lot of assumptions had to be made regarding denominators. Ranges of vitamin content varied widely among authors, up to five orders of magnitude, from 0.76 ug/100 g EP to 23480 ug/100 g EP for Vitamin E. Similarly to what was found for proximate and mineral composition, variation among authors was much larger than variation in values from comparisons within the same paper. Vitamin E content in paper 38 seemed to have gone down substantially after fermentation. The study comparing pulp from three markets in Chad was the only multilocation study reporting vitamin data (Vitamin C).
Anti-nutrients and other nutrients (annex 1)
The most analyzed anti-nutrients and other compounds were oxalates, tannins and phytates; few papers presented values for trypsin inhibitor, saponins or cyanides. There are no FAO/INFOODS recommended methods for anti-nutrients and large heterogeneity was observed in the way antinutrients were analyzed and expressed, with differences in methods, active components, units, denominators, often without properly describing methods and/or the actual components analyzed, making conversions into mg/100 g EP extremely prone to errors. The antinutrient values should be interpreted with care and are presented in this paper only to illustrate how difficult it is to compare data if authors do not describe adequately what sampling approach, sample preparation, analytical methods and/or units, denominators and equivalents were used. Different papers most likely measured different equivalents. In our database (), we reported data as found in the original articles, harmonized them to the extent possible, using the same unit and denominator (mg/100 g EP). Anti-nutrient values derived from the same analytical method were marked with the same superscript in . We noted that paper 42 systematically recorded high oxalate values across the different locations sampled, for whole and dehulled unfermented seeds as well as fermented seeds. Paper 17 presented high values for tannins in the fermented seeds, while paper 3 presented systematically low values for all anti-nutrients in the pulp.
Table 6a. Anti-nutrient and other nutrient composition for raw Parkia biglobosa seed products in mg/100g fwb.
Table 6b. Anti-nutrient and other nutrient composition for fermented Parkia biglobosa seed products in mg/100g fwb.
Table 6c. Anti-nutrient and other nutrient composition for Parkia biglobosa pulp products in mg/100g fwb.
Discussion
To better understand within-species variability in nutritional properties of P. biglobosa’s pulp and seeds, a nutrient database was developed with values extracted from available data for P. biglobosa pulp, raw and fermented seeds. Out of a total of 69 relevant papers, 27 had to be excluded after a quality check on the proximate composition. Like in Stadlmayer et al. (2013) and McBurney et al. (Citation2004), we had to collate data highly inconsistent in quality, accompanied by unclear descriptions of sampling methods, of the specific products analyzed, of laboratory protocols used, and presented without sufficient level of detail in the papers (e.g. lack of denominators). Thus, it can be concluded that, despite an apparent wealth of information on P. biglobosa pulp and seed nutrient composition, the quality of this information is low and presents several gaps.
Given the many different products analyzed and the different and/or unclear methods used, it was not possible to provide a precise answer to our first research question, about the level of within-species variation in proximate, mineral and vitamin content in P. biglobosa pulp, raw and fermented seeds. While the proximate composition was more or less in line with the data in the WAFCT, mineral and vitamin values showed huge variance. The selected papers that presented systematically high(er) or low(er) nutrient values than in other studies did not necessarily use different analytical methods. The variance found could be associated to a multitude of factors, including differences in sample preparation: for example, the extreme high value for sodium in paper 34 might be simply due to a contamination of the sample during the fermentation process. While we discarded those studies that explicitly added particular substances to the fermentation process, we did not filter out articles where a questionable descriptions of sample preparation was presented, or where the samples tested came from the market and could have contained additives. Paper 13 mentioned that differences in seed processing existed between different cultural groups and indeed the largest differences were found among fermented products derived from P. biglobosa’ seeds. Significant differences were recorded also for vitamin values in the pulp (beta-carotene and vitamin C); this pattern was confirmed by data from the WACFT, that presented very different values of Vitamin C in flour versus sun dried mature pulp. It has to be noted that many vitamins are heat sensitive and that vitamin content is influenced by the time interval between harvest and lab analyses. Finally, slight differences in fruit maturation can be associated with large differences in vitamin content.
We noted that, in general, values extracted from the same papers (comparing different locations or product treatments, etc.) showed much less variation than the values reported by different authors for the same product treatment or the same location. Only four papers (13, 26, 29, 42) compared nutrient composition of P. biglobosa products across different locations. Two papers compared fermented seeds from different markets, so the differences found could have been due to different processing methods. Another paper presented only one data point per each location, per type of product, so the sampling was too small to draw any conclusions. Only a paper (26) presented data points for three villages in the Atacora Department in Benin (Sudanian agro-ecological zone) and for three villages in the Bourgou Department (Guinean agro-ecological zones) with some clear patterns in the differences detected for proximate content between the Atacora and Bourgou samples. Whether these differences are due to soil characteristics, climate (rainfall patterns and/or temperature) or different P. biglobosa provenances remains to be studied. No studies assessed the relative weight of different factors determining the observed within-species differences in nutritional composition (e.g. soil, rainfall regime, level of maturity at the time of harvesting). Some papers (17 out of the 42 selected) compared their findings with other studies and attempted to provide tentative explanations for the differences observed. These related to different stages of maturity/ripeness, differences in sample preparation and handling, storage conditions, laboratory analysis methods, soil, climate and seasonality. Eleven of these 17 papers also attributed the observed variation in nutritional data to potential differences among varieties/provenances/genotypes.
Recommendations
Many gaps in P. biglobosa nutrient composition data remain despite the wealth of studies on this species. Data quality is generally low and comparability of data among authors is very problematic. Besides pointing out the inadequate expression of nutrients, McBurney et al. (Citation2004) also refer to issues with (incorrect) recycling of data from previous papers, leading to a 1000-fold increase in nutrient contents in some cases. Non-original data were excluded from this review, but we noticed in analogy with McBurney an issue with recycling vague methods and/or descriptions as well as lacking or incorrect nutrient expressions.
We recommend that authors provide clear descriptions of (a) the specific products analyzed (seeds with hull, without hull, mechanically dehulled or dehulled after boiling, dried or not, and the processing methods used for fermented seeds); (b) sampling methods, indicating how many samples were collected, whether they were analyzed as composite sample or not, how the samples were processed and how many replicate analyses were carried out in the laboratory; (c) analytical methods with proper references; and (d) nutrient expressions (correct equivalents, units plus mention of the denominator).
Given the growing interest in intra-species variability of nutrient composition, we furthermore strongly recommend presenting complementary data in addition to nutrient composition data, to make nutrient data location/ecoregion-specific. Soil characteristics, rainfall patterns, temperature, agro-ecological zone, harvesting time, degree of product maturity, tree management practices and genetic provenance, all influence the nutrient composition of our foods. We are only at the beginning of understanding the influence of these variables on some crops. Despite the great focus of nutritional studies on P. biglobosa, not much research on these aspects has been carried out for P. biglobosa edible products. Disentangling the complexity in the relationship between environmental and genetic factors and the nutrient composition of edible products from P. biglobosa will help to identify nutritionally plus trees for multiplication and/or breeding purposes, and to ultimately contribute to better nutrition for populations living in resource poor settings such as rural West Africa.
Conclusion
A nutrient composition database was collated with data about P. biglobosa pulp and both raw and fermented seeds, through a systematic online search of relevant scientific literature and a careful compilation of nutrient data into an excel file. A total of 42 papers were retained for the construction of the final nutrient database.
Several gaps in nutrient data were identified. Particularly for vitamins, data were very scarcely reported. It was not possible to detect any patterns in nutrient composition based on environmental characteristics or genetic provenance due to a lack of (quality) data and very deficient documentation of the environmental context where sampling has been carried out. The paper by Koura et al. (Citation2014) however seemed to indicate that such differences exist for P. biglobosa edible products. Some recommendations were formulated regarding minimum standards for reporting nutrient data as well as for presenting complementary information that would make nutritional data better interpretable and usable.
The huge differences in nutrient content found could not be clearly attributed to specific factors, such as, for example, differences in analytical methods used, sample processing, maturity of the samples, environmental characteristics (e.g., climate, soil) of the collection site, provenance of the samples, etc., This means that it would be difficult, or impossible, with the data available, to formulate any recommendation on intakes of P. biglobosa edible products that would meet daily needs for specific nutrients (especially for minerals and vitamins). The quantities of fermented products derived from P. biglobosa consumed per meal are small, but intakes are regular and complement otherwise monotonous staple-based diets, so P. biglobosa edible products constitute an important source of nutrients. A better understanding of the main factors determining variation in their nutrient content would indicate whether it is possible to identify “+” trees, and/or management practices for improved nutrient content of P. biglobosa edible products.
Acknowledgements
This work was coordinated by Bioversity International with financial support of the Austrian Development Agency and of the CGIAR Research Programs on Forests, Trees and Agroforestry (FTA) and on Agriculture for Nutrition and Health (A4NH). Special thanks go to Francesca Giampieri, librarian at Bioversity International, who assisted in accessing the scientific literature on which the tables presented in this review are based.
Disclosure statement
No financial interest or benefit has arisen from the direct applications of this research.
Table 5b. Vitamin composition for fermented Parkia biglobosa seed products in µg/100g (except Vit C, expressed in mg/100g).
Table 5c. Vitamin composition for Parkia biglobosa pulp products in µg/100g (except Vit C, expressed in mg/100g).
Additional information
Funding
References
- Abey, S., and N. O. Abey. 2016. Effects of gamma irradiation and cooking on the physico-chemical properties of African locust bean (Parkia biglobosa) seeds. Food and Public Health 6 (1):8–14.
- Adamu, A. S., and J. G. Oyetunde. 2013. Comparison of dietary proximate and mineral values of two varieties of bean. Asian Journal of Natural and Applied Sciences 2 (2):103–6.
- Afolayan, M., G. S. Bawa, A. A. Sekoni, F. O. Abeke, V. O. Inekwe, and E. O. Odegbile. 2014. Phytochemical and nutritional evaluation of locust bean fruit pulp. Journal of Emerging Trends in Engineering and Applied Sciences 5 (7):44–7.
- Aja, P. M., C. E. Offor, and O. U. Orji. 2015. Proximate and antinutrient compositions of Parkia biglobosa fruits in Abakaliki, Ebonyi State, Nigeria. International Journal of Current Microbiology and Applied Sciences 4 (2):394–8.
- Ajayi, K., O. T. Adepoju, O. M. Taiwo, S. T. Omojola, and M. E. Aladetuyi. 2018. Nutritional potential of underutilized gum arabic tree seeds (Acacia nilotica) and locust bean seeds (Parkia biglobosa). African Journal of Food Science 12 (8):196–203. doi: https://doi.org/10.5897/AJFS2017.1650.
- Akintayo, E. T. 2004. Characteristics and composition of Parkia biglobosa. Bioresource Technology 92 (3):307–10. doi: https://doi.org/10.1016/S0960-8524(03)00197-4.
- Akubor, P., J. Onuh, and C. Orishagbemi. 2017. Chemical composition and functional properties of African locust bean pulp flour and wheat flour. Asian Journal of Biotechnology and Bioresource Technology 1 (4):1–53. doi: https://doi.org/10.9734/AJB2T/2017/36478.
- Appiah, F., I. Oduro, W. O. Ellis, and G. Adu. 2012. Comparative assessment of the performance of Parkia biglobosa, Glycine max and Treculia africana in the production of a local condiment (dawadawa) in Ghana. African Journal of Food Science 6 (5):111–6.
- Azokpota, P., D. J. Hounhouigan, and M. C. Nago. 2006. Microbiological and chemical changes during the fermentation of African locust bean (Parkia biglobosa) to produce afitin, iru and sonru, three traditional condiments produced in Benin. International Journal of Food Microbiology 107 (3):304–9. doi: https://doi.org/10.1016/j.ijfoodmicro.2005.10.026.
- B. A. O., O. S. F. O. U. B. O.O. A. O., and S. R. A. A. 2004. The effect of seasoning salts and local condiments on mineral availability from two nigerian vegetables. Pakistan Journal of Nutrition 3 (3):146–53. doi: https://doi.org/10.3923/pjn.2004.146.153.
- Bamigboye, A. Y., O. T. Adepoju, O. Oyinsan, and H. Kabir. 2012. Micronutrient potentials and contribution to nutrient intake of four commonly consumed local condiments and spices in South-Western Nigeria. Nigerian Journal of Nutritional Sciences 33 (1):57–61.
- Boateng, M., D. B. Okai, Y. O. Frimpong, and C. O. Asabere. 2014. A comparative study of the nutritional and microbial profiles of the raw and processed seeds of the African locust bean (Parkia biglobosa). Livestock Research for Rural Development 26 (178). Accessed September 2, 2020. http://www.lrrd.org/lrrd26/10/boat26178.htm
- Boedecker, J., C. Termote, A. E. Assogbadjo, P. Van Damme, and C. Lachat. 2014. Dietary contribution of wild edible Plants to women’s diets in Benin – an underutilized potential. Food Security 6 (6):833–49. doi: https://doi.org/10.1007/s12571-014-0396-7.
- Boffa, J.-M. 1999. Agroforestry parklands in sub-Saharan Africa. FAO Conservation guide No 34:1–230. Rome: FAO.
- Borquaye, L. S., G. Darko, M. K. Laryea, E. N. Gasu, N. A. A. Amponsah, and E. N. Appiah. 2017. Nutritional and anti-nutrient profiles of some Ghanaian spices. Cogent Food and Agriculture 3:1–12.
- Bouda, Z. H., J. Bayala, B. Markussen, J. S. Jensen, and A. Raebild. 2013. Provenance variation in survival, growth and dry matter partitioning of Parkia biglobosa (Jacq.) R.Br. ex G.Don seedlings in response to water stress. Agroforestry Systems 87 (1):59–71. doi: https://doi.org/10.1007/s10457-012-9521-9.
- Bouda, Z. H. N., and A. Nikiema. 1996. Etude de la Dynamique des peuplements de Parkia biglobosa Jacq. (Benth). Rapport technique. ISSN 1018–7065. CNSF, Ougadougou, Burkina Faso.
- Burlingame, B. 2004. Fostering quality data in food composition databases: Visions for the future. Journal of Food Composition and Analysis 17 (3-4):251–8. doi: https://doi.org/10.1016/j.jfca.2004.03.026.
- Burlingame, B., R. Charrondiere, and B. Mouille. 2009. Food composition is fundamental to the cross-cutting initiative on biodiversity for food and nutrition. Journal of Food Composition and Analysis 22 (5):361–5. doi: https://doi.org/10.1016/j.jfca.2009.05.003.
- Camara,F., S. Soronikpoho, T. Souleymane, B. Kouakou, and D. K. Marcellin. 2016a. Centesimal composition and bioactive compounds in African mustards used as condiments in Ivory Coast. African Journal of Food Science 10 (7):87–93. doi: https://doi.org/10.5897/AJFS2015.1377.
- Camara, F., S. Soronikpoho, T. Souleymane, B. Kouakou, and K. Marcellin. 2016b. Caractéristiques biochimiques et microbiologiques de moutardes Africaines produites à base de graines fermentées de Parkia biglobosa et de Glycine max, Vendues En Côte d’Ivoire. International Journal of Biological and Chemical Sciences 10 (2):506–18. doi: https://doi.org/10.4314/ijbcs.v10i2.5.
- CBD (Convention on Biological Diversity) and WHO (World Health Organization) (CBD/WHO). 2015. Connecting Global Priorities: Biodiversity and Human Health. A State of Knowledge Review. Geneva: World Health Organization and Secretariat for the Convention on Biological Diversity.
- CILSS. 2016. Landscapes of West Africa – A Window on a Changing World. Garreston, United States: U.S. Geological Survey.
- Dosumu, O. O., O. O. Oluwaniyi, G. V. Awolola, and O. O. Oyedeji. 2012. Nutritional composition and antimicrobial properties of three Nigerian condiments. Nigerian Food Journal 30 (1):43–52. doi: https://doi.org/10.1016/S0189-7241(15)30012-6.
- Egbebi, A. O., K. T. Seidu, and A. A. Muhammad. 2016. Nutritional and microbiological analyses of fermented locust beans (Parkia Biglobosa) and fermented melon (Citrullus vulgaris). Savant Journal of Agricultural Research 2 (1):001–6.
- Ekesa, B., D. Nabuuma, G. Blomme, and I. Van den Bergh. 2015. Provitamin A carotenoid content of unripe and ripe banana cultivars for potential adoption in eastern Africa. Journal of Food Composition and Analysis 43:1–6. doi: https://doi.org/10.1016/j.jfca.2015.04.003.
- Englberger, L., R. B. H. Wills, B. Blades, L. Dufficy, J. W. Daniells, and T. Coyne. 2006. Carotenoid content and flesh color of selected banana cultivars growing in Australia. Food and Nutrition Bulletin 27 (4):281–91. doi: https://doi.org/10.1177/156482650602700401.
- Esenwah, C. N., and M. J. Ikenebomeh. 2008. Processing effects on the nutritional and anti-nutritional contents of African locust bean (Parkia biglobosa Benth.) seed. Pakistan Journal of Nutrition 7 (2):214–7. doi: https://doi.org/10.3923/pjn.2008.214.217.
- Ezugwu, E. N., J. I. Okoye, and G. I. Ene. 2018. Effects of processing on the nutrient and anti- nutrient contents of African locust bean (Parkia biglobosa) flour. International Journal of Food Science and Nutrition 3 (4):107–11.
- FAO. 2008. Expert consultation on nutrition indicators for biodiversity 1. Food composition. Rome, Italy: FAO.
- FAO. 2010. Expert consultation on nutrition indicators for biodiversity 2. Food consumption. Rome, Italy: FAO.
- FAO/INFOODS. 2009. FAO/INFOODS compilation tools version 1.2.1. http://fao.org/infoods/infoods/software-tools/en/
- Gijsbers, H. J. M., J. J. Kessler, and M. K. Knevel. 1994. Dynamics and natural regeneration of woody species in farmed parklands in the Sahel region (Province of Passore. Forest Ecology and Management 64 (1):1–12. doi: https://doi.org/10.1016/0378-1127(94)90122-8.
- Gouado, I., R. Aba Ejoh, T. Some Issa, F. J. Schweigert, and M. F. Tchouanguep. 2007. Carotenoids content of some locally consumed fruits and yams in Cameroon. Pakistan Journal of Nutrition 6 (5):497–501. doi: https://doi.org/10.3923/pjn.2007.497.501.
- Hall, J. B., H. F. Thomlinson, P. I. Oni, M. Buchy, and D. P. Aebischer. 1997. A monograph of Parkia biglobosa. School of Agricultural and Forest Sciences Publication N.9. Bangor: University of Wales.
- Hopkins, H. C. 1983. The taxonomy, reproductive biology and economic potential of Parkia (Leguminosae: Mimosoideae) in Africa and Madagascar. Botanical Journal of the Linnean Society 87 (2):135–67. doi: https://doi.org/10.1111/j.1095-8339.1983.tb00987.x.
- Ibeabuchi, J. C., M. Ojukwu, and O. Ijeoma. 2013. Comparative study of proximate and sensory qualities of iru powder, ogiri powder and iru-ogiri blend. Natural Products 9 (7):288–93.
- ICRAF. 2006. Renforcement des stratégies de subsistance à travers une utilisation et une gestion améliorées des parcs agroforestiers au Sahel. Rapport d’étape, Projet IFAD 799. Bamako, Mali: World Agroforestry Centre.
- Ifesan, B. O. T., A. O. Akintade, and R. A. O. Gabriel-Ajobiewe. 2017. Physicochemical and nutritional properties of Mucuna pruriens and Parkia biglobosa subjected to controlled fermentation. International Food Research Journal 24 (5):2177–84.
- Iheke, E., A. Oshodi, A. Omoboye, and O. Ogunlalu. 2017. Effect of fermentation on the physicochemical properties and nutritionally valuable minerals of locust bean (Parkia biglobosa). American Journal of Food Technology 12 (6):379–84. doi: https://doi.org/10.3923/ajft.2017.379.384.
- Ijarotimi, O. S., and O. O. Keshinro. 2012. Comparison between the amino acid, fatty acid, mineral and nutritional quality of raw, germinated and fermented African locust bean (Parkia biglobosa) flour. Acta Scientarium Polonorum 11 (2):151–65.
- INFOODS. 2012. Tagnames for food components.http://www.fao.org/infoods/infoods/standards-guidelines/en/
- Jide, A. O., A. Gbenga, O. Karimu, and J. O. Alademeyin. 2018. Proximate and physicochemical properties of flour and oil extracted from raw and boil locust bean (Parkia biglobosa). The Pharmaceutical and Chemical Journal 5 (4):1–5.
- Kayalto, B., C. Zongo, R. W. Compaore, A. Savadogo, B. B. Otchom, and A. S. Traore. 2013. Study of the nutritional value and hygienic quality of local infant flours from Chad, with the aim of their use for improved infant flours preparation. Food and Nutrition Sciences 4 (9):59–68. doi: https://doi.org/10.4236/fns.2013.49A2009.
- Kennedy, G., and B. Burlingame. 2003. Analysis of food composition data on rice from a plant genetic resources perspective. Food Chemistry 80 (4):589–96. doi: https://doi.org/10.1016/S0308-8146(02)00507-1.
- Kennedy, G., D.Stoian, D.Hunter, E.Kikulwe, C.Termote, R.Alders, B.Burlingame, R.Jamnadass, S. McMullin, and S. Thilsted. 2017. Food Biodiversity for Healthy, Diverse Diets. In Bioversity international. Mainstreaming Agrobiodiversity in sustainable food systems: Scientific foundations for an Agrobiodiversity index, 23. Italy.
- Koura, K., P. I. G. Ouidoh, P. Azokpota, J. C. Ganglo, and D. J. Hounhouigan. 2014. Caractérisation physique et composition chimique des graines de Parkia biglobosa (Jacq.) R. Br. en usage au Nord-Bénin. Journal of Applied Biosciences 75 (1):6239–49. doi: https://doi.org/10.4314/jab.v75i1.4.
- Kristensen, M., and A. M. Lykke. 2003. Informant-based valuation of use and conservation preferences of savanna trees in Burkina Faso. Economic Botany 57 (2):203–17. doi: https://doi.org/10.1663/0013-0001(2003)057[0203:IVOUAC]2.0.CO;2.
- Kronborg, M., J. B. Iboudo, A. S. Bassole, H. W. Banford, H. W. Ravn, and A. M. Lykke. 2014. Correlates of product quality of soumbala, a West African non-timber forest product. Ethnobotany Research and Applications 12:25–37.
- Lockett, C. T., C. C. Calvert, and L. E. Grivetti. 2000. Energy and micronutrient composition of dietary and medicinal wild plants consumed during drought. Study of rural Fulani, Northeastern Nigeria. International Journal of Food Science and Nutrition 51:195–208.
- Lutaladio, N., B. Burlingame, and J. Crews. 2010. Horticulture, biodiversity and nutrition. Journal of Food Composition and Analysis 23 (6):481–5. doi: https://doi.org/10.1016/j.jfca.2010.08.001.
- Makalao, M. M., A. Savadogo, C. Zongo, and A. S. Traore. 2016. Composition nutritionnelle de 10 fruits sauvages consommés dans trois départements du Tchad. Nutritional composition of 10 wild fruits consumed in three divisions in Chad. International Journal of Biological and Chemical Sciences 9 (5):2385–400. doi: https://doi.org/10.4314/ijbcs.v9i5.11.
- McBurney, R. P. H., C. Griffin, A. A. Paul, and D. C. Greenberg. 2004. The nutritional composition of African wild food plants: From compilation to utilization. Journal of Food Composition and Analysis 17 (3-4):277–89. doi: https://doi.org/10.1016/j.jfca.2004.03.008.
- Nadro, M. S., and H. A. Umaru. 2004. Comparative chemical evaluation of locust bean (Parkia biglobosa) fruit pulp harvested during the dry and wet season. Nigerian Journal of Biotechnology 15 (1):42–7.
- Ndukwe, M. N., and M. D. Solomon. 2017. Proximate and antinutrient composition of some local food condiments in their raw and fermented forms. International Journal of Biochemistry Research & Review 20 (1):1–8. doi: https://doi.org/10.9734/IJBCRR/2017/37727.
- Nikiema, A. 1993. Regeneration of Parkia biglobosa (Jacq.) R. Br. ex G. Don in an agroforestry system. A pilot study in Burkina Faso. MSc Thesis., Wageningen Agricultural University, The Netherlands.
- Nordeide, M. B., A. Hatloy, M. Folling, E. Lied, and A. Oshaug. 1996. Nutrient composition and nutritional importance of green leaves and wild food resources in an agricultural district, Koutiala, in Southern Mali. International Journal of Food Sciences and Nutrition 47 (6):455–68. doi: https://doi.org/10.3109/09637489609031874.
- Nyadanu, D., R. Adu Amoah, B. Obeng, A. O. Kwarteng, R. Akromah, L. M. Aboagye, and H. Adu-Dapaah. 2017. Ethnobotany and analysis of food components of African locust bean (Parkia biglobosa (Jacq.) Benth.) in the transitional zone of Ghana: Implications for domestication, conservation and breeding of improved varieties. Genetic Resources and Crop Evolution 64 (6):1231–40. doi: https://doi.org/10.1007/s10722-016-0432-x.
- Ogunyinka, B. I., B. E. Oyinloye, F. O. Osunsanmi, A. P. Kappo, and A. R. Opoku. 2017. Comparative study on proximate, functional, mineral, and antinutrient composition of fermented, defatted, and protein isolate of Parkia biglobosa seed. Food Science & Nutrition 5 (1):139–47. doi: https://doi.org/10.1002/fsn3.373.
- Okpala, J. O. 1990. Analyses of some macronutrients and food substances in the mature fruit parts of Parkia biglobosa. Nigerian Journal of Botany 3:209–14.
- Oladele, O. O., and M. A. Agunbiade. 2018. Effect of salt treatment on fungi spoilage, proximate and mineral compositions of fermented African locust bean (Parkia biglobosa) seeds during ambient storage. Octa Journal of Biosciences 6 (1):19–22.
- Oluwaniyi, O. O., and I. O. Bazambo. 2014. Antinutritional and phytochemical evaluation of raw and fermented African locust bean (Parkia biglobosa) seeds. Global Journal of Pure and Applied Sciences 20 (2):105–9. doi: https://doi.org/10.4314/gjpas.v20i2.4.
- Omafuvbe, B. O., S. O. Falade, B. A. Osuntogun, and S. R. A. Adewusi. 2004. Chemical and biochemical changes in African locust bean (Parkia biglobosa) and melon (Citrullus vulgaris) seeds during fermentation to condiments. Pakistan Journal of Nutrition 3 (3):140–5. doi: https://doi.org/10.3923/pjn.2004.140.145.
- Orwa, C., A. Mutua, R. Kindt, R. Jamnadass, and S. Anthony. 2009. Agroforestree Database: A tree reference and selection guide. Version 4.0 http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp.
- Osuntogun, B. A., F. S. Olumuyiwa, U. Onome, B. Omafuvbe, A. Oladipo, and S. Adewusi. 2004. The effect of seasoning salts and local condiments on mineral availability from two nigerian vegetables. Pakistan Journal of Nutrition 3 (3):146–153.
- Ouedraogo, M., A. Raebild, A. Nikiema, and E. D. Kjaer. 2012. Evidence for important genetic differentiation between provenances of Parkia biglobosa from the Sudano-Sahelian zone of West Africa. Agroforestry Systems 85 (3):489–503. doi: https://doi.org/10.1007/s10457-011-9463-7.
- Ouedraogo, A. S. 1995. Parkia biglobosa (Leguminosae) en Afrique de l’Ouest: biosystematique et amelioration. PhD diss., Wageningen University, Institute for Forestry and Nature Research, The Netherlands.
- Pehou, C.,H. Djoudi,B. Vinceti, andM. Elias. 2020. Intersecting and dynamic gender rights to néré, a food tree species in Burkina Faso. Journal of Rural Studies 76:230–9. doi: https://doi.org/10.1016/j.jrurstud.2020.02.011.
- Raebild, A., U. B. Hansen, and S. Kambou. 2012. Regeneration of Vitellaria paradoxa and Parkia biglobosa in a Parkland in Southern Burkina Faso. Agroforestry Systems 85 (3):443–53. doi: https://doi.org/10.1007/s10457-011-9397-0.
- Stadlmayr, B., U. R. Charrondière, S. Eisenwagen, R. Jamnadass, and K. Kehlenbeck. 2013. Nutrient composition of selected indigenous fruits from sub-Saharan Africa. Journal of the Science of Food and Agriculture 93 (11):2627–36. doi: https://doi.org/10.1002/jsfa.6196.
- Stadlmayr, B., N. V. Enujiugha, G. R. Bayili, G. E. Fagbohoun, B. Samb, P. Addy, I. Barikmo, F. Ouattara, A. Oshaug, I. Akinyele, and U. R. Charrondiere. 2012. West African food composition table. Rome: Food and Agriculture Organization of the United Nations.
- Teklehaimanot, Z. 2004. Exploiting the potential of indigenous agroforestry trees: Parkia biglobosa and Vitellaria paradoxa in sub-Saharan Africa. Agroforestry Systems 61-62 (1-3):207–20. doi: https://doi.org/10.1023/B:AGFO.0000029000.22293.d1.
- Thiombiano, D. N. E., N. Lamien, A. M. Castro-Euler, B. Vinceti, D. Agundez, and I. J. Boussim. 2013. Local communities demand for food tree species and the potentialities of their landscapes in two ecological zones of Burkina Faso. Open Journal of Forestry 3 (3):79–87. doi: https://doi.org/10.4236/ojf.2013.33014.
- Thiombiano, D. N. E., C. Parkouda, N. Lamien, A. Sr, A. M. Castro-Euler, and I. J. Boussim. 2014. Nutritional composition of five food tree species products used in human diet during food shortage period in Burkina Faso. African Journal of Biotechnology 13 (17):1807–12. doi: https://doi.org/10.5897/AJB2013.13462.
- Umaru, H. A., R. Adamu, D. Dahiru, and M. S. Nadro. 2007. Levels of antinutritional factors in some wild edible fruits of northern Nigeria. African Journal of Biotechnology 6 (16):1935–8. doi: https://doi.org/10.5897/AJB2007.000-2294.
- Urua, I. S., E. A. Uyoh, V. O. Ntui, and E. C. Okpako. 2013. Effect of processing on proximate composition, anti-nutrient status and amino acid content in three accessions of African locust bean (Parkia biglobosa (jacq.) benth . International Journal of Food Sciences and Nutrition 64 (1):94–102. doi: https://doi.org/10.3109/09637486.2012.704903.
- Vinceti, B., C. Termote, N. Thiombiano, D. Agundez, and N. Lamien. 2018. Food tree species consumed during periods of food shortage in Burkina Faso and their threats. Forest Systems 27 (2):e006. doi: https://doi.org/10.5424/fs/2018272-12157.
- Zotero. Version 5.0. Accessed May 2018. http://www.zotero.org/download

