Abstract
Soybeans are a rich source of isoflavones, which are classified as phytoestrogens. Despite numerous proposed benefits, isoflavones are often classified as endocrine disruptors, based primarily on animal studies. However, there are ample human data regarding the health effects of isoflavones. We conducted a technical review, systematically searching Medline, EMBASE, and the Cochrane Library (from inception through January 2021). We included clinical studies, observational studies, and systematic reviews and meta-analyses (SRMA) that examined the relationship between soy and/or isoflavone intake and endocrine-related endpoints. 417 reports (229 observational studies, 157 clinical studies and 32 SRMAs) met our eligibility criteria. The available evidence indicates that isoflavone intake does not adversely affect thyroid function. Adverse effects are also not seen on breast or endometrial tissue or estrogen levels in women, or testosterone or estrogen levels, or sperm or semen parameters in men. Although menstrual cycle length may be slightly increased, ovulation is not prevented. Limited insight could be gained about possible impacts of in utero isoflavone exposure, but the existing data are reassuring. Adverse effects of isoflavone intake were not identified in children, but limited research has been conducted. After extensive review, the evidence does not support classifying isoflavones as endocrine disruptors.
Introduction
Over the past 30 years, the health effects of soyfoods and soybean isoflavones have been rigorously investigated. Modern interest in isoflavones began with the US National Cancer Institute funding research aimed at understanding the role of these soybean constituents in cancer prevention and treatment (Messina and Barnes Citation1991) Within a decade the number of purported health benefits of isoflavones being investigated greatly expanded to include areas such as bone health (Blair et al. Citation1996; Potter et al. Citation1998), hot flash alleviation (Murkies et al. Citation1995) and cognitive function (Pan, Anthony, and Clarkson Citation1999a, Citation1999b).
Research interest in soyfoods coincided with the interest in isoflavones because, although they are only one of >100 potentially biologically active components in soybeans and soyfoods (Kang et al. Citation2010; Fang, Yu, and Badger Citation2004), among commonly consumed foods the soybean is a uniquely rich source of these diphenolic molecules (Thompson et al. Citation2006; Franke et al. Citation1998). To this point, mean isoflavone intake in Japan among older adults ranges from approximately 30 to 50 mg/d (Messina, Nagata, and Wu Citation2006; Konishi et al. Citation2019) whereas per capita intake in the United States (Bai, Wang, and Ren Citation2014; Sebastian et al. Citation2015; Chun, Chung, and Song Citation2007; Chun et al. Citation2007) and Europe (Zamora-Ros et al. Citation2013; Ziauddeen et al. Citation2019) is only a few mg. Thus, soyfoods are often equated with isoflavones.
Genistein (molecular weight, 270 g/mol), daidzein (molecular weight, 254.2 g/mol) and glycitein (molecular weight, 284.3 g/mol), and their respective glycosides (the predominate form in soybeans and unfermented soyfoods) account for approximately 50, 40 and 10%, respectively, of the total isoflavone content of soybeans (Murphy, Barua, and Hauck Citation2002). In fermented soyfoods, the percentage of isoflavones in aglycone form increases but is quite variable depending upon the food and duration of fermentation (Murphy, Barua, and Hauck Citation2002; Jang et al. Citation2008; Chan et al. Citation2009; Fukutake et al. Citation1996; Wei, Chen, and Chen Citation2008). Commonly consumed fermented foods include tempeh, miso and natto whereas unfermented soyfoods include tofu, soymilk and edamame. In this manuscript, isoflavone amounts refer to the aglycone equivalent weight. When converting the glycoside to the aglycone amount, a conversion factor of 0.6 is used since the sugar component of the glycoside accounts for approximately 40% of the total mass.
Of the three soybean isoflavones, genistein is generally considered the most potent based on in vitro assays measuring estrogenic potential (Gramec Skledar et al. Citation2020; Matsumura et al. Citation2005). In addition to the isoflavones in soybeans, upon ingestion, endogenous metabolites with varying biological activity are produced. Especially notable in this regard is that approximately 25% of Westerners and 50% of Asians host the gut microbiome composition that convert daidzein into equol, a conversion that some speculate will benefit the health of those consuming isoflavones (Setchell, Brown, and Lydeking-Olsen Citation2002).
Although in observational studies involving Asian cohorts isoflavone exposure occurs via traditional soyfoods, which are made from whole soybeans, clinical studies typically utilize isoflavone supplements or soy ingredients, commonly referred to as soy protein products, because they are easily standardized and incorporated into the diet. These products include soy flour, soy protein concentrate (SPC) and soy protein isolate (SPI), which range in protein content from 50% (flour) to 90% (SPI). Soy protein products are widely used by the food industry for their functional properties. For example, they are added to foods to increase shelf life and moisture retention and to modify texture (Thrane et al. Citation2017). Because only small amounts of soy protein products are added to foods for functional purposes, when used in this way (as ingredients) they make a negligible contribution to nutrient intake. However, soy protein products are also used as a base for making meat alternatives and nondairy beverages and are often added to foods in larger amounts, such as breakfast cereals and energy bars, to boost protein content. When used in this way, they can greatly contribute to protein intake.
Despite interest in the proposed benefits of isoflavones, for almost the entire time their benefits have been investigated, they have been embroiled in controversy. In some sense this controversy is not surprising when recognizing that isoflavones first came to the attention of the scientific community in the 1940s as a result of breeding problems experienced by female sheep in Western Australia (Bennetts, Underwood, and Shier Citation1946; Adams Citation1995) grazing on a type of clover rich in isoflavones (Bradbury and White Citation1954; Lundh, Pettersson, and Martinsson Citation1990). Even those researchers who recognized the potential health benefits of isoflavones acknowledged the possibility that they could impair female fertility (Setchell et al. Citation1984). This particular concern gained support when in 1987, it was determined that the addition of soy meal to the diet of the captive cheetah contributed to its inability to reproduce (Setchell et al. Citation1987). Despite the large populations of soyfood-consuming countries, concerns that isoflavones impair fertility continue to be discussed and debated (West et al. Citation2005; Cooper Citation2019; Cederroth, Zimmermann, and Nef Citation2012). Patisaul and Jefferson (Citation2010) reviewed the animal data related to this topic.
The estrogen-like effects of isoflavones underlie both the proposed benefits as well as concerns about adverse effects. By the 1950s, investigators had already demonstrated the estrogen-like properties of isoflavones in experimental animals, which led them to be viewed as possible growth promoters for use by the animal feed industry (Carter, Matrone, and Smart Citation1955; Carter, Smart, and Matrone Citation1953; Cheng et al. Citation1953). In the 1960s, Folman and Pope (Cederroth, Zimmermann, and Nef Citation2012; Patisaul and Jefferson Citation2010) established the relative binding affinities of soybean isoflavones for the only estrogen receptor (estrogen receptor alpha, ERα) known at that time (Folman and Pope Citation1966; Folman and Pope Citation1969). Somewhat prophetically, their work led them to conclude that the importance of genistein “… might lie as much in its ability to antagonize the natural steroid estrogens as in its own estrogenic activity” (Folman and Pope Citation1966).
Although an antiestrogenic effect of isoflavones has only infrequently been observed in clinical studies, early on it served as a partial theoretical basis for enthusiasm about a protective effect of isoflavones against hormone-dependent cancers, especially breast cancer (Messina and Barnes Citation1991; Stewart, Westley, and May Citation1992). It also formed a theoretical basis for classifying isoflavones as selective estrogen receptor modulators (SERMs) (Brzezinski and Debi Citation1999). This classification gained support with the discovery in 1996 of a second estrogen receptor – ERβ (Kuiper et al. Citation1996) – and the recognition that in contrast to estrogen, which binds with equal affinity to ERα and ERβ, isoflavones preferentially bind to the latter (Kuiper et al. Citation1998; Jiang et al. Citation2013). In general, activation of ERα and ERβ are seen as exerting proliferative and anti-proliferative effects, respectively (Paruthiyil et al. Citation2004).
The isoflavone controversy began in earnest in the late 1990s as a result of research showing that in ovariectomized athymic mice implanted with MCF-7 cells (an estrogen-dependent human breast cancer cell line), genistein stimulated the growth of existing mammary tumors (Hsieh et al. Citation1998). Published at nearly the same time were the results of two human studies that intervened with isoflavone-rich soy protein that appeared to support these findings, one, a pilot study examining nipple aspirate fluid (Petrakis et al. Citation1996) and the other, a preliminary analysis of research examining in vivo breast cell proliferation (McMichael-Phillips et al. Citation1998).
However, prior to the breast cancer controversy, questions about the safety of soy infant formula (SIF) had already been raised (Irvine et al. Citation1995; Irvine, Fitzpatrick, and Alexander Citation1998; Setchell et al. Citation1997), despite it having been widely used for many decades (Merritt and Jenks Citation2004) and the conclusion by the Committee on Nutrition of the American Academy of Pediatrics in 1983, that SIF produces normal growth and development in full-term infants (Committee on Nutrition Citation1983). In subsequent years, much in the same way interest in the benefits of isoflavones expanded, concern about isoflavones expanded to include areas such as thyroid function (Divi, Chang, and Doerge Citation1997; Divi and Doerge Citation1996) and cognitive function (White et al. Citation2000). In fact, both isoflavones (and other phytoestrogens) and soy are routinely referred to as endocrine disruptors (Lee et al. Citation2019; Bar-El and Reifen Citation2010; Chung et al. Citation2019; Fernandez-Lopez et al. Citation2016; Beszterda and Frański Citation2018; Patisaul Citation2017; Salsano et al. Citation2019; Kwack et al. Citation2009; Xiao et al. Citation2018; Rietjens, Louisse, and Beekmann Citation2017; Min, Wang, and Liang Citation2020), a designation first made >20 years ago (Ginsburg Citation1996), although there has also been pushback against this classification, especially in the case of soyfoods (Messina Citation2011).
Endocrine disruptors were defined at a US Environmental Protection Agency workshop in 1996 as “exogenous agents that interfere with the production, release, transport, metabolism, binding action or elimination of natural hormones in the body responsible for the maintenance of homoeostasis and the regulation of developmental processes” (Kavlock et al. Citation1996). The World Health Organization defines an endocrine disruptor as “an exogenous substance or mixture that alters the function(s) of the endocrine system and consequently causes adverse effects in an intact organism, or its progeny, or (sub) populations” (Solecki et al. Citation2017). An adverse effect is defined as a “… change in morphology, physiology, growth, reproduction, development or lifespan of an organism which results in impairment of functional capacity or impairment of capacity to compensate for additional stress or increased susceptibility to the harmful effects of other environmental influences.” Endocrine disruptors have been linked with increased risks for obesity, diabetes mellitus and cardiovascular diseases, impaired male and female reproduction, hormone sensitive cancers, thyroid disruption and neurodevelopmental and neuroendocrine abnormalities (Gore et al. Citation2015).
While there is general recognition that isoflavones are endocrine-active substances, as discussed by the European Food Safety Authority (EFSA), endocrine active substances are not necessarily endocrine disrupting chemicals (European Food Safety Authority Citation2010). According to EFSA, an endocrine active substance is any chemical that can interact directly or indirectly with the endocrine system, and subsequently result in an effect on the endocrine system, target organs and tissues. Whether the effect is adverse (“disruptive”) or not will depend on the type of effect, the dose and the background physiological situation (European Food Safety Authority Citation2010).
Over the past 20 years, several scientific and regulatory bodies and organizations have evaluated the safety of isoflavones and soyfoods. For example, in 1999, in the process of reviewing evidence in support of a proposed health claim for soyfoods and coronary heart diseases based on the hypocholesterolemic effect of soy protein, the US Food and Drug Administration (FDA) concluded that when consumed at a level of 25 g/d, soy protein was safe and the claim was lawful (Food Labeling: Health Claims; Soy Protein and Coronary Heart Disease Citation1999). Although the claim was based on soy protein and not isoflavones, health concerns examined by the FDA were those primarily related to the latter. Nearly 20 years later, in the process of reevaluating evidence in support of the existing health claim, the FDA reached the same conclusion (Department of Health and Human Services Citation2017). Other organizations that evaluated isoflavones (as supplements and/or in foods) include the UK Committee on Toxicity of Chemicals in Foods, Consumer Products and the Environment (COT) (2003, 2013), the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) (2005), the Japanese Food Safety Commission, Novel Foods Expert Committee (2006), the US National Toxicology Program (2006, 2009), the EFSA (2015) and the Permanent Senate Commission on Food Safety of the German Research Foundation (SKLM) (2018). Recently, both the ANSES and the COT have announced their intention to consider reevaluating isoflavone safety in young children. From a public health perspective, as interest in plant-based diets increases, it is reasonable to expect that the consumption of soyfoods will also, so it is critical to have a clear understanding of the health effects of isoflavones.
Therefore, this technical review will examine claims that isoflavones are endocrine disruptors as well as concerns raised about soy/isoflavones that may not be limited to those typically associated with endocrine disruption. This examination will focus primarily on the clinical data; and secondarily, on observational data. With respect to the latter, because with rare exceptions (e.g., Adventist Health Studies 1 (Beeson et al. Citation1989) and 2 (Butler et al. Citation2008) and the Oxford Arm of the European Prospective Investigation into Cancer (Davey et al. Citation2003)), soy consumption is extremely low among Western cohorts, the focus will be on studies involving Asian cohorts. This perspective on the epidemiological data was articulated nearly two decades ago (Messina Citation2004). Cohorts involving low-intake populations will be cited, but with the appropriate caveat. For the most part, in vitro and animal studies will be highlighted only as part of the background information needed to understand the origins of the claims of harmful effects.
This approach to the literature has been adopted not because of the failure of supporters of using animals to study endocrine disruptors (Gore et al. Citation2015; Patisaul, Fenton, and Aylor Citation2018) to recognize limitations of such models (Jocsak et al. Citation2019), in particular the differences in isoflavone metabolism between rodents and humans that make extrapolating from the latter to the former especially problematic (Gu et al. Citation2006; Setchell et al. Citation2011). Rather, it is because there are ample human data upon which to reach conclusions about the possible endocrine-disrupting effects of soy and isoflavones in nearly all areas for which concerns have been raised. Unlike other compounds classified as endocrine disruptors, human intervention studies have routinely examined the health impact, and in many cases, the long-term health impact, of isoflavone exposure via supplements and foods. Areas for which there may be some question about whether enough data exist to reach conclusions will be highlighted
Finally, soy infant formula (SIF) will be occasionally cited but for the following reasons it will not be a focus of this review:
SIF has been extensively reviewed by previous authors (Vandenplas et al. Citation2014; Testa et al. Citation2018; Jefferson, Patisaul, and Williams Citation2012; Badger et al. Citation2009; Stevens Citation2017) and committees (McCarver et al. Citation2011; Bhatia and Greer Citation2008).
On a body weight (bw) basis, isoflavone exposure is much higher in infants fed SIF than in children or adults consuming soyfoods in amounts compatible with Asian consumption, as are blood isoflavone levels () (Setchell et al. Citation1997; Badger et al. Citation2002). Isoflavone intake of older Asian children is approximately 1 mg/kg bw whereas in infants it is 1.8 to 9.5- fold higher. For comparison, the UK COT estimated that mean isoflavone intake among British infants aged 6 to 18 months and children aged 18 months to 5 years would be 2.88 and 2.29 mg/kg bw respectively, if current dairy product intake was replaced by soy-based dairy alternatives. Intake was primarily due to the replacement of cow’s milk with soymilk; the isoflavone content of the latter was estimated to contain 100 mg/l. Isoflavone intakes at the 97.5 percentile for the younger and older age groups were 8.97 and 7.21 mg/kg body weight, respectively.
During the first few weeks and months of life (prior to the introduction of foods or non-formula beverages), infants are likely more sensitive to hormonal influences than are children or adults (Patisaul Citation2017; Jefferson, Patisaul, and Williams Citation2012; Yilmaz et al. Citation2020; WHO & UNEP Citation2013). This point has been highlighted by the US National Institute of Environmental Health Sciences (Soy Infant Formula 2019) and the Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals (Gore et al. Citation2015).
Recommendations to increase soy consumption based on nutritional and health attributes, especially related to the prevention of chronic disease, do not apply to SIF, although one very preliminary report found that SIF use was associated with a reduced breast cancer risk (Boucher et al. Citation2008).
Table 1. Isoflavone intake in infants using soy infant formula and free-living Asian children and adolescents.
Background
Isoflavone intake
The isoflavone content (aglycone equivalent weight) of soyfoods derived from whole soybeans varies but expressed on a per gram protein basis, is approximately 3.5 mg/g (Messina, Nagata, and Wu Citation2006). For such soyfoods, one serving (e.g. 200 or 250 ml soymilk, 100 g tofu, 1 ounce [28 g] soynuts) provides approximately 25 mg isoflavones. In contrast, the isoflavone content of SPI and SPC processed in the usual manner which involves the use of alcohol, is generally only ∼1 mg/g protein, because during processing as much as 90% of the isoflavone content is eliminated (Murphy, Barua, and Hauck Citation2002; Murphy et al. 1998).
Extensive isoflavone intake data are available for adults because of the many large cohort and case-control studies that have been published from several high-soy-consuming Asian countries. As noted previously, mean isoflavone intake in Japan among older adults ranges from approximately 30 to 50 mg/d (Messina, Nagata, and Wu Citation2006; Konishi et al. Citation2019) whereas per capita intake in the United States is <3 mg/d (Bai, Wang, and Ren Citation2014; Sebastian et al. Citation2015; Chun, Chung, and Song Citation2007; Chun et al. Citation2007; Vieux, Maillot, and Rehm Citation2020). Isoflavone intake in Shanghai, China (Yang et al. Citation2005; Lee et al. Citation2007; Shu et al. Citation2015), is comparable to Japan, but is much higher than other regions within China (Liu et al. Citation2004). Examples of the range of isoflavone intake in China and Japan are shown in . Isoflavone intake in Korea (Lee and Kim Citation2007; Kim et al. Citation2015) is lower than in Japan but higher than in Singapore (Talaei et al. Citation2014; Mueller et al. Citation2012) and Hong Kong (Ho et al. Citation2003; Koh et al. Citation2005). Most soy consumed throughout the world is in unfermented form, as ethnic Chinese consume relatively little fermented soy whereas in Japan, about half of all soy consumed is fermented (Messina, Nagata, and Wu Citation2006). shows the isoflavone intake among participants in several long-term intervention trials. As can be seen, intake was quite a bit higher than typical Asian isoflavone intake. The trial identified with the highest exposure was a Taiwanese study involving postmenopausal women that intervened with 300 mg/d isoflavones (Tai et al. Citation2012).
Table 2. Isoflavone intake reported in large prospective epidemiologic studies from China and Japan.
Table 3. Selected examples of isoflavone exposure in intervention trials of at least approximately 2 years duration.
Isoflavone occurrence and function in plants
Isoflavones are distinct from the much more common flavonoids in the position of the phenyl B ring – in isoflavonoids, it is a substituent at the 3-position of the heterocyclic ring (flavonoids have the B-ring at the 2-position). Isoflavonoids are formed from flavonoids by the enzyme isoflavone isomerase. The main source of isoflavones are legumes from the family Fabaceae (Dixon and Sumner Citation2003), namely soybeans (Glycine max) which contain daidzein (7,4′-dihydroxyisoflavone), genistein (5,7,4′-trihydroxyisoflavone) and glycitein (7,4′-dihydroxy-6-methoxyisoflavone), and red clover (Trifolium pratense), which contains the methylated isoflavones formononetin (7-hydroxy-4′-methoxyisoflavone) and biochanin A (5,7-dihydroxy-4′-methoxyisoflavone). Less common sources of isoflavones in plants used as foods are the tubers of the American groundnut, Apios americana, where they are as glycosyl-glycosides and in the root of Pueraria lobata (Kudzu) as C-glucosides (Barnes et al. Citation2002; Ichige et al. Citation2013).
Gut bacterial metabolism of isoflavones in mammals results in reduction of the heterocyclic ring to form the isoflavanones, dihydrodaidzein and dihydroxygenistein, and the ring cleaved O-desmethylangolensin. Further bacterial reduction of isoflavonones leads to the isoflavan equol (4′,7-dihydroxyisoflavan) (Setchell, Brown, and Lydeking-Olsen Citation2002). None of these metabolites are normally found in foods although they are a consequence of consuming isoflavone-containing foods. Small amounts, however, are present in dairy products from cows, goats and sheep consuming soybeans or isoflavone-containing clover.
In plants, isoflavones function as phytoalexins, low-molecular compounds synthesized and accumulated in plants during stress and microbe attacks. These active defense compounds have fungistatic, antibacterial, antiviral, and antioxidant properties (Dakora and Phillips Citation1996). Isoflavone concentration rises during stress (e.g., lowered humidity, pathogen attack, or plant diseases) and is, to a large extent, influenced by environmental and climatic conditions such as temperature, precipitation, harvest period and soil fertility (Scilewski da Costa Zanatta et al. 2017; Bobby et al. Citation2014; Hasanah et al. Citation2015). However, an even greater determinant of isoflavone concentration is soybean variety as isoflavone content has been shown to vary as much as 10-fold among varieties (Eldridge and Kwolek Citation1983; Kim et al. Citation2014; ILSI range is from ILSI Crop Composition Database Citation2019), although the genetic modification of soybeans does not appear to appreciably alter isoflavone concentration (Padgette et al. Citation1996; Taylor et al. Citation2017; Lepping, Herman, and Potts Citation2013; Harrigan et al. Citation2007; McCann et al. Citation2005; Novak and Haslberger Citation2000; Lappé et al. Citation1998).
In addition to functioning as phytoalexins, isoflavones play many roles in plant-microbe interactions, including rhizobia-legume symbiosis (Rípodas et al. Citation2013). Isoflavones are essential for nodulation because of their ability to induce the nodulation genes (Subramanian, Stacey, and Yu Citation2006). They are therefore responsible for natural enrichment of fixed nitrogen in soils, a role well known to farmers who unknowingly used plants producing isoflavones in a crop rotation strategy.
As noted previously, genistein, daidzein and glycitein account for approximately 50, 40 and 10%, respectively, of the total isoflavone content of soybeans, although this can vary from strain to strain (Murphy, Barua, and Hauck Citation2002). The hypocotyls of soybeans contain much higher isoflavone concentrations than cotyledons with a much higher proportion of glycitein (up to 45%). Isoflavones in soybeans prior to food processing are β-glycosides esterified with malonic acid (Kudou et al. Citation1991). In fermented soyfoods, much of the isoflavones present are in aglycone form but the percentage varies markedly (Murphy, Barua, and Hauck Citation2002; Jang et al. Citation2008; Chan et al. Citation2009; Fukutake et al. Citation1996; Wei, Chen, and Chen Citation2008; Kuo et al. Citation2006; Chun, Chung, and Song Citation2007; Chun et al. Citation2007). If the fermentation is a lengthy process (for miso or some forms of soy sauce this can be up to nine months), additional oxidative metabolism can occur introducing hydroxyl groups into the 6- and 8-positions on the A-ring (Esaki et al. Citation1999).
Hexane extraction to recover the oil fraction of soybeans does not alter the isoflavone content of soybean protein. However, the boiling water extraction of soybeans to make soymilk (and then tofu) causes the hydrolysis of the malonyl group, yielding simple β-glucosides (Barnes, Kirk, and Coward Citation1994). This also occurs during the hot aqueous alcohol extraction of soy flour and accordingly, the remaining protein fraction is largely depleted of isoflavones. When the alcohol is evaporated from the extract, the residue is soy molasses, a rich source of isoflavone β-glucosides. When heating soy in a dry format (extrusion of soy protein concentrate or toasting of soybeans, soy flour or the hypocotyls), the malonyl group is decarboxylated to form the 6′’-O-acetyl-7-O-β-glucoside (Barnes, Kirk, and Coward Citation1994).
Isoflavone absorption and metabolism
According to EFSA, no estimate of bioavailability of isoflavones in humans can be given although it concluded bioavailability was low (EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) 2015). In rats, absolute bioavailability of genistein was 7% and 15% in males and females, respectively. Setchell et al. (Citation2001, Citation2003) showed that the bioavailability of genistein is greater than that of daidzein as determined by the area under the curve for the serum isoflavones. In cheetahs and domestic cats, the UDP-glucuronyltransferase gene specific for β-glucuronidation of genistein (UGT1A6) contains a stop codon (Court and Greenblatt Citation2000). In these animals, the predominant form of isoflavones in their blood is their sulfonates (Whitehouse-Tedd et al. Citation2011).
Unlike the aglycones, β-glycosides cannot be absorbed due to their higher hydrophilicity and higher molecular mass (Hur et al. Citation2000). They become bioavailable and can be absorbed only when hydrolyzed (Setchell, Brown, and Lydeking-Olsen Citation2002), which can occur along the entire length of the gastrointestinal tract, including colonic bacteria (Hur et al. Citation2000), but mostly, they are hydrolyzed in the jejunum (Zubik and Meydani Citation2003) by brush border membrane and β-glucosidases (Németh et al. Citation2003), such as lactase-phlorizin hydrolase, which are active from relatively early life (Day et al. Citation2000). Lactase-phlorizin hydrolase is also responsible for lactose hydrolysis (Rivera-Sagredo et al. Citation1992). Once the glycosides are hydrolyzed, the resulting aglycone can be absorbed via passive diffusion (Decroos et al. Citation2005); typically this occurs within 1–2 h (King, Broadbent, and Head Citation1996; Sfakianos et al. Citation1997). The isoflavones in the root of kudzu vine (Pueraria lobata) are C-glycosides. Because of this, puerarin, the C-glycoside of daidzein, is absorbed without deconjugation with a peak concentration in the blood within the hour (Prasain et al. Citation2007). It is inferred that intestinal absorption occurs via the Na+-dependent glucose transporter since puerarin depresses glucose absorption whereas daidzin does not (Meezan et al. Citation2005).
In rats, intraduodenally administered genistein is almost completely absorbed; however, it is converted in enterocytes to its 7-O-β-glucuronide, the principal form of genistein and other isoflavones found systemically (Cheng et al. Citation1953). Genistein-7-O-β-glucuronide is excreted into bile. Only about 20% of intraduodenally administered genistein-7-O-β-glucuronide is recovered in bile over a 4-h period; however, when it is distally administered, biliary recovery is 60–70% (Cheng et al. Citation1953). This suggests that genistein-7-O-β-glucuronide undergoes bacterially-induced hydrolysis and hence undergoes enterohepatic circulation. In addition, genistein is converted to p-ethylphenol and its sulfonate and β-glucuronide metabolites. Genistein can also be converted to double conjugates (di-β-glucuronide and β-glucuronide/sulfonates) (Soukup et al. Citation2016; Paul et al. Citation2017). In mice, absolute bioavailability amounted to 9–14% for genistein and 29–34% for daidzein (Andrade et al. Citation2010). Urinary or plasma isoflavones have been shown to be reliable biomarkers of soy consumption (Atkinson et al. Citation2002; Grace et al. Citation2004; Wu et al. Citation2003) and, when the timing of specimen collection is considered, the urinary appearance of isoflavones has been shown to accurately reflect circulating levels (Franke, Custer, and Hundahl Citation2004; Franke et al. Citation2006).
There is a biphasic isoflavone appearance pattern in plasma and urine of humans after the consumption of soy or purified isoflavone preparations with peak isoflavone levels occurring 1–2 h and again 4–8 h after intake (Setchell et al. Citation2003; Zubik and Meydani Citation2003; Fanti et al. Citation1999; Franke et al. Citation1999; King and Bursill Citation1998). The time of the first peak represents small intestinal absorption via passive absorption (Setchell et al. Citation2003) of aglycones and glycosides that were hydrolyzed (Day et al. Citation2000), whereas the second peak represents absorption of isoflavone glycosides by the large intestine after hydrolysis by gut bacteria (Franke, Custer, and Hundahl Citation2004; Franke, Lai, and Halm Citation2014).
After absorption, genistein and daidzein are metabolized by UDP-glucuronyl transferase to glucuronides, and to a lesser extent by sulfotransferases to sulfate esters in the intestinal mucosa cells and liver (Ronis et al. Citation2006). Conjugation can occur in one or two (4′ or 7′) locations of the isoflavone ring. These metabolites (mono- and diglucuronides, mono- and disulfates, and sulfoglucuronides of daidzein and genistein), which can be found in the plasma (Hosoda et al. Citation2010), are excreted in the bile and deconjugated in the distal part of the intestine. Deconjugation allows them to be absorbed again and be part of the enterohepatic circulation (Barnes Citation2010).
The isoflavone daidzein is metabolized to S-equol and O-desmethylangolensin (ODMA) by gut bacteria and excreted predominantly through the urine (Axelson et al. Citation1984). Most people are able to make ODMA, but the ability to produce S-equol is limited to 25–50% of the population; whether this metabolic feature results in more beneficial health effects from soy consumption remains uncertain (Setchell, Brown, and Lydeking-Olsen Citation2002; Atkinson, Frankenfeld, and Lampe Citation2005).
The extent of isoflavone metabolism varies among individuals and may be influenced by dietary factors (Lampe et al. 1999; Rowland et al. Citation2000). There is a wide inter-individual variability in serum levels among individuals in response to isoflavone ingestion (Wiseman et al. Citation2004; Mathey et al. Citation2006). For example, in two studies involving postmenopausal women who consumed 100 mg/d isoflavones, in one the mean (SD) genistein level after 10 weeks (N = 25) was 806 nmol/l (1238) (Wiseman et al. Citation2004), whereas in the other (N = 12), after 60 days it was 2.42 µmol/l (0.84–4.15) (Mathey et al. Citation2006).
Setchell et al. (Citation2011) extensively examined phase II isoflavone conjugation and found major differences between humans and rodents. Namely, the proportion of unconjugated genistein in plasma from adults and infants who consumed different soyfoods, pure genistein, or an isoflavone supplement was <1% in steady state and <2% at peak concentrations. By contrast, the plasma percentages of unconjugated genistein concentrations in Sprague-Dawley rats and C57BL/6, nude, and transgenic AngptL4B6 mice were 4.0 ± 0.6%, 4.6 ± 0.6%, 11.6 ± 0%, and 30.1 ± 4.3%, respectively, which represent 20, 23, 58, and 150 times that in humans. Similar results were reported by Gu et al. (Citation2006); for example, they found <1% of the isoflavones in human plasma were present as aglycones whereas in monkey serum, 6% was. Soukup et al. (Citation2016) concluded that “… there are marked differences between humans, rats and mice in the profile of major metabolites following IF [isoflavone] phase II metabolism.”
That much less unconjugated isoflavone is present in human compared to rodent serum is an important observation because the conjugate has relatively little biological activity (Yuan et al. Citation2012; Islam et al. Citation2015). It is the isoflavone aglycones that show an affinity for ERs and have other non-hormonal effects (Setchell Citation2000).
Pharmacokinetic studies show that peak plasma level (Cmax) is achieved faster when aglycones as opposed to glucosides are consumed (Setchell et al. Citation2001; Izumi et al. Citation2000; Kano et al. Citation2006; Cassidy et al. Citation2006). Cmax may also be higher, but whether total isoflavone absorption is greater in response to the ingestion of aglycones vs glycosides is unclear as the data are quite conflicting with some studies showing no difference in urinary isoflavone excretion between fermented and unfermented foods or glycosides and aglycones (Zubik and Meydani Citation2003; Beeson et al. Citation1989; Butler et al. Citation2008; Davey et al. Citation2003; Messina Citation2004; Patisaul, Fenton, and Aylor Citation2018; Jocsak et al. Citation2019; Gu et al. Citation2006; Setchell et al. Citation2011; Vandenplas et al. Citation2014; Testa et al. Citation2018; Jefferson, Patisaul, and Williams 2012; Badger et al. 2009; Stevens Citation2017; McCarver et al. Citation2011; Bhatia and Greer Citation2008; Badger et al. 2002; Yilmaz et al. Citation2020; WHO & UNEP Citation2013; Soy Infant Formula 2019; Boucher et al. 2008; Murphy et al. Citation1999; Vieux, Maillot, and Rehm Citation2020; Yang et al. Citation2005; Lee et al. Citation2007; Shu et al. Citation2015; Liu et al. Citation2004; Lee and Kim Citation2007; Kim et al. Citation2015; Talaei et al. Citation2014; Mueller et al. Citation2012; Ho et al. Citation2003; Koh et al. Citation2005; Tai et al. Citation2012; Dixon and Sumner Citation2003; Barnes et al. Citation2002; Ichige et al. Citation2013; Dakora and Phillips Citation1996; Scilewski da Costa Zanatta et al. 2017; Bobby et al. Citation2014; Hasanah et al. Citation2015; Eldridge and Kwolek Citation1983; Kim et al. Citation2014; ILSI range is from ILSI Crop Composition Database Citation2019; Padgette et al. Citation1996; Taylor et al. Citation2017; Lepping, Herman, and Potts Citation2013; Harrigan et al. Citation2007; McCann et al. Citation2005; Novak and Haslberger Citation2000; Lappé et al. Citation1998; Rípodas et al. Citation2013; Subramanian, Stacey, and Yu Citation2006; Kudou et al. Citation1991; Kuo et al. Citation2006; Chun, Chung, and Song Citation2007; Chun et al. Citation2007; Esaki et al. Citation1999; Barnes, Kirk, and Coward Citation1994; EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) 2015; Setchell et al. Citation2003; Setchell et al. Citation2001; Court and Greenblatt Citation2000; Whitehouse-Tedd et al. Citation2011; Hur et al. Citation2000; Setchell, Brown, and Lydeking-Olsen Citation2002; Zubik and Meydani Citation2003; Németh et al. Citation2003; Day et al. Citation2000; Rivera-Sagredo et al. Citation1992; Decroos et al. Citation2005; King, Broadbent, and Head Citation1996; Sfakianos et al. Citation1997; Prasain et al. Citation2007; Meezan et al. Citation2005; Soukup et al. Citation2016; Paul et al. Citation2017; Andrade et al. Citation2010; Atkinson et al. Citation2002; Grace et al. Citation2004; Wu et al. Citation2003; Franke, Custer, and Hundahl Citation2004; Franke et al. Citation2006; Fanti et al. Citation1999; Franke et al. Citation1999; King and Bursill Citation1998; Franke, Lai, and Halm Citation2014; Ronis et al. Citation2006; Hosoda et al. Citation2010; Barnes Citation2010; Axelson et al. Citation1984; Atkinson, Frankenfeld, and Lampe Citation2005; Lampe et al. 1999; Rowland et al. Citation2000; Wiseman et al. Citation2004; Mathey et al. Citation2006; Yuan et al. Citation2012; Islam et al. Citation2015; Setchell Citation2000; Izumi et al. Citation2000; Kano et al. Citation2006; Cassidy et al. Citation2006; Tsangalis et al. Citation2005; Richelle et al. Citation2002; Xu et al. Citation2000; Tsunoda, Pomeroy, and Nestel Citation2002; Maskarinec et al. Citation2008) whereas other studies show greater absorption with fermented foods/aglycone isoflavones (Setchell et al. Citation2001; Izumi et al. Citation2000; Kano et al. Citation2006; Cassidy et al. Citation2006; Hutchins et al. Citation1995; Silva et al. Citation2020; Jang et al. Citation2020).
Finally, although estimates vary somewhat, the elimination half-life for isoflavones is approximately 8 h (Setchell et al. Citation2003; Setchell et al. Citation2001; King and Bursill Citation1998; Watanabe et al. Citation1998; Shelnutt et al. Citation2002; Busby et al. Citation2002; Burnett et al. Citation2011; Takimoto et al. 2003). Therefore, as concluded by Setchell et al. (Citation2003), steady state plasma concentrations would be more readily maintained by repeated ingestion of isoflavones throughout the day than by ingestion just once a day. Also, the bioavailability of isoflavones is nonlinear at higher intakes, suggesting that uptake is rate-limiting and saturable (Setchell et al. Citation2003). Work in postmenopausal women showed that doubling isoflavone intake increased plasma concentrations by 55–62% for daidzein, genistein and equol (only for producers) (van der Velpen et al. Citation2014).
Serum and tissue isoflavone levels
Adlercreutz, Markkanen, and Watanabe (Citation1993) were the first to report serum isoflavone levels in free living native Japanese (). They found that among 14 middle-aged men the geometric means (95% CI (Confidence Interval)) for serum total genistein and daidzein were 276 (116, 652) and 107 (47, 237) nmol/l, respectively. Samples were collected in the morning but no information about the dietary intake of the study participants was provided. These levels dwarfed the mean genistein and daidzein levels of 6.3 and 6.2 nmol/l, respectively, in Finnish men (Adlercreutz, Markkanen, and Watanabe Citation1993). For comparison, plasma genistein and daidzein levels in 7 infants fed SIF were 2.5 and 1.2 µmol/l, respectively (Setchell et al. Citation1997). Also for comparison, serum genistein and daidzein concentrations among vegetarian and vegan participants of the Oxford center (UK) of the European Prospective Investigation into Cancer and Nutrition were 148 and 79 nmol/l, respectively, which were 5–50 times higher than those in the other study center regions which involved almost exclusively non-vegetarians (Peeters et al. Citation2007). The values reported by Adlercreutz, Markkanen, and Watanabe (Citation1993) are generally in line with those of several other investigators as shown in .
Table 4. Serum isoflavone levels in native Japanese adults.
Two studies nicely illustrate the circulating levels of isoflavones that can be reached in response to an isoflavone intake that greatly exceeds typical Asian intake. In a study by Busby et al. (Busby et al. Citation2002), 30 healthy men ingested a single dose of one of two soy-derived isoflavone preparations. The delivered doses of genistein were 1, 2, 4, 8, or 16 mg/kg bw. Formulation A was composed of approximately 90% genistein, 10% daidzein, and 1% glycitein whereas formulation B was composed of 43% genistein, 21% daidzein, and 2% glycitein. As shown in , in response to a single dose (formulation B) that provided 4, 8 or 16 mg genistein per kg bw, serum total genistein concentrations were approximately 9, 18 and 27 µmol/l, respectively. The data included in also show the extremely low percentage of isoflavones in serum in the unconjugated form.
Table 5. Serum isoflavone levels in men in response to varying isoflavone doses (Busby et al. Citation2002).
In the other study, Tai et al. (Citation2012) found that among postmenopausal women, genistein levels reached approximately 7 µmol/l in response to the consumption of 300 mg/d isoflavones (172.5 mg genistein + 127.5 mg daidzein) at 4 weeks, although levels decreased as the 2-year study progressed, which may reflect a decrease in compliance ().
Table 6. Mean (SD) of serum genistein and daidzein concentrations at each visit among postmenopausal women from three medical centers in Taiwan participating in a 2-year clinical trial in which participants received either 300 mg/d isoflavones or a placebo (Tai et al. Citation2012).
Finally, research in humans confirms that isoflavones end up in tissues (Pumford et al. Citation2002; Hong et al. Citation2002; Brössner et al. Citation2006). For example, in women who consumed approximately 60 mg/d isoflavones for five days in the form of either soymilk or as a supplement, total genistein and daidzein concentrations (aglycone equivalents) ranged from 135.1 to 2831 nmol/L and 105.1 to 1397 nmol/L, respectively, in hydrolyzed serum and from 92.33 to 493.8 pmol/g and 22.15 to 770.8 pmol/g, respectively, in hydrolyzed breast tissue (Bolca et al. Citation2010). Pumford et al. (Citation2002) reported that in four women who consumed 45 mg/d isoflavones for two weeks prior to surgery, mean tissue genistein and daidzein concentration was 0.665 nmol/g and 0.145 nmol/g wet breast tissue, respectively.
Interestingly, Gardner et al. (Citation2009) concluded that prostate tissue may have the ability to concentrate dietary soy isoflavones. In their study, men received 82 mg/d isoflavones for two weeks prior to radical prostatectomy. The median total isoflavone concentration in the isoflavone supplemented group was 2.3 mmol/L in the prostate tissue and 0.7 mmol/L in the serum. Total isoflavone concentrations in this group were an average of 6-fold greater in prostate tissue compared to serum; the tissue versus serum ratio was significantly lower for genistein than daidzein, 4-fold versus 10-fold. The conclusion that the prostate concentrates isoflavones relative to the serum was also reached by Rannikko et al. (Citation2006) and Hedlund et al. (Citation2005) (daidzein only).
Receptor binding
In general, phytoestrogens act through nuclear estrogen receptors, ERα and ERβ, influencing transcription of their target genes. They exert effects on cellular processes that include proliferation, apoptosis and migration. The ERs can also be associated with the plasma membrane and cause rapid cytosolic signaling. Phytoestrogens also serve as ligands for the nonclassical membrane G-protein coupled estrogen receptor (GPR30) and induce estrogenic responses in cardiovascular and metabolic regulation through mitogen-activated protein kinases, phosphoinositide 3-kinase, adenylyl cyclase and phospholipase C signaling pathway (Govind and Thampan Citation2003; Prossnitz, Arterburn, and Sklar Citation2007).
The identification of ERβ in 1996 (Kuiper et al. Citation1996) and the subsequent demonstration that in comparison to ERα, soybean isoflavones preferentially bind to this newly discovered ER (Kuiper et al. Citation1998; Kuiper et al. Citation1997) changed the way in which isoflavones, as well as many other ER ligands are viewed. This is because in general, activation of ERα and ERβ are seen as exerting proliferative and anti-proliferative effects, respectively (Paruthiyil et al. Citation2004). As noted previously, the selectivity of isoflavones with respect to receptor binding, provided a molecular explanation for classifying isoflavones as SERMs (Oseni et al. Citation2008).
Typically, the relative potency of isoflavones in discussed in terms of relative binding affinity (RBA) and compared to 17β-estradiol, with the latter arbitrarily set at 100. Early results from Kuiper et al. (1998) show isoflavones are less potent than 17β-estradiol, that they bind with greater affinity to ERβ vs ERα and that genistein is more potent than daidzein (). Several years later, Hwang et al. (Citation2006) reported similar values for the isoflavones as did Jiang et al. () (Jiang et al. Citation2013).
Table 7. Relative binding affinity of isoflavones in comparison to estrogen (Kuiper et al. Citation1998).
Table 8. RBA of isoflavones for ERα and ERβ and comparison with E2 (Jiang et al. Citation2013).
Hwang et al. (Citation2006) also examined the ability of the isoflavones to regulate gene expression using a transiently transfected estrogen sensitive reporter gene in embryonic kidney cells and found genistein was nearly as potent as 17β-estradiol at stimulating gene expression (Hwang et al. Citation2006). In addition, they reported that in kidney cells isoflavones acted as estrogen agonists when cultured in media containing a low concentration of estrogen (∼1 × 10−11 M), but as estrogen antagonists in the presence of a high estrogen concentration (∼1 × 10−9 M)).
Earlier work had already demonstrated a biphasic effect of genistein on the growth of MCF-7 cells (an estrogen-dependent human breast cancer cell line) (Hsieh et al. Citation1998). At relatively low concentrations genistein stimulated growth, an effect dependent upon interaction with the ERs, whereas at higher concentrations (>10−5 M), growth was inhibited (Wang et al. Citation1996). The latter effect was independent of ERs, likely a result of the ability of genistein to inhibit the activity of enzymes overexpressed in cancer cells such as protein-tyrosine kinases or DNA topoisomerases (Constantinou and Huberman Citation1995; Constantinou et al. Citation1995). However, there is considerable doubt as to the biological relevance of these higher in vitro levels.
Methods
Search strategy
To identify relevant literature, a systematic search in Medline, EMBASE, and the Cochrane Library (from inception through January 2021), using the search strategy shown in was conducted. Manual searches of reference lists of review articles and included studies supplemented the electronic database searches. The electronic and manual searches were performed by one author (SBM). Articles found by experts in the field contributed to manual searches. Eligible for inclusion were clinical studies, observational studies and systematic reviews and meta-analyses (SRMAs) that investigated the effects or associations of soyfoods or soy/isoflavone supplements on outcomes of the endocrine human system. The search was restricted to humans. British Medical Journal (BMJ) study design search filters were used for clinical trials, observational studies and SRMAs (Anderson, Smith, and Washnock Citation1999). Duplicate articles, abstracts, reviews, in vitro and animal studies, as well as studies including soy as part of a dietary pattern were excluded. An assessment of study quality and certainty of evidence was not formally undertaken as doing so was beyond the scope of this technical review.
Table 9. Search strategy.
Results
shows the flow of the literature search and study selection. Of the 2038 reports identified, 1621 were excluded whereas 417 reports were included (229 observational studies, 157 clinical studies and 32 SRMAs).
Figure 1. Flow of the literature search and study selection. *1 report included both an SRMA and an observational study (Wei, Y.L., J. Guo, Y. Bian, Z. Gao, M. Du, H. Yang, L. Chen, Y. Zhang, X. Wang, T. Chen, J. Chen, Z. Yu, C. Huo, D. Li, L. China Kadoorie Biobank Collaborative, Group, Soy intake and breast cancer risk: a prospective study of 300,000 Chinese women and a dose-response meta-analysis. European Journal of Epidemiology, 2019. 21: p. 21.). Abbreviation: SRMA-systematic review and meta-analysis.
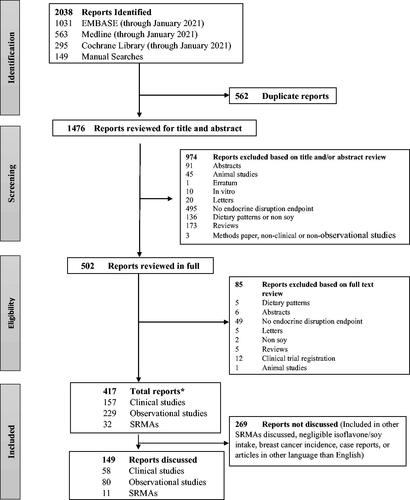
Individual observational and clinical studies with relevant endpoints are generally not specifically discussed if they were included in systematic reviews and meta-analyses that were cited. Further, individual studies published prior to the publication of a systematic review and/or meta-analysis that were not included in the review or meta-analysis were in general not discussed even if the endpoints were relevant, because it was assumed these studies were eliminated for failure to meet the established inclusion/exclusion criteria.
Thyroid function
The impact of soy on thyroid function has been investigated for nearly a century (McCarrison Citation1933) and for at least 30 years, soy has been labeled as a goitrogenic food in the peer-reviewed literature (Gaitan Citation1990). In the early 1960s, several cases of goiter were attributed to the use of SIF, although this issue was eliminated soon thereafter once the formula began to be fortified with iodine (van Wyk et al. Citation1959; Shepard et al. Citation1960; Pinchera et al. Citation1965). However, concern arose again several decades later based primarily on in vitro and animal studies involving isolated isoflavones (Divi, Chang, and Doerge Citation1997; Divi and Doerge Citation1996; Doerge and Chang Citation2002). Drawing attention to the thyroid issue were remarks about the goitrogenic effects of isoflavones submitted to the US FDA in 1999 during the open comment period in connection with the FDA’s evaluation of the evidence in support of the heart health claim for soyfoods (Food Labeling: Health Claims; Soy Protein and Coronary Heart Disease Citation1999).
Several mechanisms have been offered for the untoward effects of isoflavones on thyroid function (de Souza Dos Santos et al. Citation2011). For example, in vitro isoflavones inhibit the activity of thyroid peroxidase (TPO) and serve as an alternate substrate to tyrosine for iodination (Doerge and Chang Citation2002). TPO liberates iodine for addition onto tyrosine residues on thyroglobulin for the production of thyroxine (T4) and triiodothyronine (T3) (Divi and Doerge Citation1996). A variety of flavonoids inhibit TPO activity in vitro (Divi and Doerge Citation1996), although isoflavones are more potent than most (Divi, Chang, and Doerge Citation1997). High flavonoid intake reportedly contributed to the high prevalence of iodine deficiency disease among Indian children living in an iodine-deficient area (Brahmbhatt, Brahmbhatt, and Boyages Citation2000). Isoflavones were also shown in vitro to interfere with thyroxine binding to the transport protein transthyretin (Köhrle Citation2004; Radovic, Mentrup, and Kohrle Citation2006).
In 2004, a retrospective analysis of infants with congenital hypothyroidism that included eight fed SIF and 70 not fed SIF, led Conrad, Chiu, and Silverman (Citation2004) to conclude that SIF-fed infants had prolonged increases in levels of thyroid stimulating hormone (TSH). This increase was not attributed to a direct effect on the thyroid, but rather to an inhibitory effect of soy on the absorption of levothyroxine, a medication used for the treatment of hypothyroidism. Earlier work in rats showed a soy-containing diet caused greater T4 excretion than the control diet (Van Middlesworth Citation1957), an effect also reported in an infant who was refractory to thyroid hormone while on SIF (Pinchera et al. Citation1965). Thus, in the case of infants with congenital hypothyroidism, it may be prudent to avoid using SIF (Conrad, Chiu, and Silverman Citation2004; Fruzza, Demeterco-Berggren, and Jones Citation2012).
In hypothyroid adults, it is not clear as to whether soy warrants special consideration as food in general and many herbs, drugs and fiber and calcium supplements also inhibit levothyroxine absorption (Liel, Harman-Boehm, and Shany Citation1996; Chiu and Sherman Citation1998; Shakir et al. Citation1997; Liel, Sperber, and Shany Citation1994; Sperber and Liel Citation1992; Sherman, Tielens, and Ladenson Citation1994; Siraj, Gupta, and Reddy Citation2003; Rosenberg Citation1994; Harmon and Seifert Citation1991; Liwanpo and Hershman Citation2009; Garber et al. 2012). Recommendations do not call for soyfoods to be avoided by hypothyroid patients as one can opt to temporally separate ingestion of levothyroxine from soyfood ingestion. General recommendations are to consume levothyroxine 30–60 minutes before breakfast or 4 hours after the last meal (Garber et al. 2012). An alternate approach to temporal separation is to be consistent in medication administration and food (soy) consumption so that, if necessary, the levothyroxine dose can be appropriately titrated (Zeitler and Solberg Citation2010).
In 2006, a narrative review of 14 clinical trials concluded that there is “… little evidence that in euthyroid, iodine-replete individuals, soy foods, or isoflavones adversely affect thyroid function” (Messina and Redmond Citation2006). This conclusion is consistent with considerable subsequently published data. Notable in this regard is a 3-year randomized, double blind, placebo-controlled trial involving 138 postmenopausal women that intervened with 54 mg/d genistein (provided as aglycone). No effects were seen on T3, T4, and TSH, autoantibodies against TPO, thyroglobulin and thyroid microsomal antigen and thyroid hormone receptor and retinoid receptor expression from peripheral blood monocytes (Bitto et al. Citation2010). Other long-term trials (2–3 y) have also shown isoflavone exposure does not affect thyroid function (Alekel et al. Citation2015; Levis et al. Citation2011; Steinberg et al. Citation2011).
In 2019, the first meta-analysis to examine the effect of soy and isoflavones on thyroid hormones, which included 18 studies, found that soy had no effect on free T3 or free T4 (Otun et al. Citation2019). Studies mainly intervened with food supplements containing soy isoflavones, soy extracts, soy protein, daidzein-rich isoflavones and isolated genistein. The isoflavone dose ranged from 40 to 200 mg/d. Of the 18 studies, two included both men and women, three included only men, and 13 included only women. Two studies involved subclinical hypothyroid patients.
The 2006 review previously referenced noted that “… there remains a theoretical concern based on in vitro and animal data that in individuals with compromised thyroid function and/or whose iodine intake is marginal soy foods may increase risk of developing clinical hypothyroidism” (Messina and Redmond Citation2006). Both conditions have since been addressed. Regarding the latter, as noted previously, there is the potential for isoflavones to compete with the amino acid tyrosine for iodination (Doerge and Chang Citation2002). However, Sosvorova et al. (Sosvorova et al. Citation2012) found that daily supplementation with 80 mg isoflavones derived from red clover or soy for three months led to only negligible amounts (∼0.01%) of iodinated isoflavones in urine samples from study participants. Mean urinary genistein levels increased from 20 nmol/l at baseline to 121 nmol/l after 3 months of supplementation. These results suggest, although do not prove, that even in those whose iodine intake is marginal, isoflavones will not adversely affect thyroid function.
Data on the effect of soy in individuals whose thyroid function is suboptimal was published in 2011 (Sathyapalan et al. Citation2011) and 2018 (Sathyapalan et al. Citation2018). Sathyapalan et al. (Citation2011) found in a cross-over study that exposure to 30 g/d SPI for eight weeks that provided 16 mg isoflavones increased the likelihood of progressing from subclinical to overt hypothyroidism in comparison to the consumption of 30 g/d SPI that provided only 2 mg isoflavones. However, in 2018, a similarly-designed study conducted by the same research group found isoflavone exposure had no effect on the progression of subclinical hypothyroidism, even though a much larger dose of isoflavones (30 g/d SPI; 66 mg isoflavones vs 0 isoflavones) was used (Sathyapalan et al. Citation2018). These follow up results call into question the initial findings.
Two points about these two studies involving subclinical hypothyroidism warrant comment. Both studies used as a control a SPI from which the content of naturally occurring isoflavone had been almost totally eliminated. Eliminating isoflavones by alcohol extraction to such an extent can potentially disrupt the tertiary nature of the protein, thereby calling into question the suitability of the control protein (Nikolaidis, Andreadis, and Moschakis Citation2017). Also, in the 2011 study, the decrease in blood pressure, insulin resistance and inflammation was much greater than had previously been reported (Sathyapalan et al. Citation2011), which suggests that the study findings may not be generalizable.
In 2015, EFSA concluded that “… the administration of food supplements containing isoflavones is not associated with clinically relevant changes in thyroid function (hypo or hyperthyroidism) in the population of interest” that is, peri- and postmenopausal women (EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) 2015). Three years later, after extensively reviewing the literature, the SKLM concluded that isoflavone exposure does not adversely affect thyroid function in healthy women (men were not evaluated) (Hüser et al. Citation2018).
However, the SKLM also noted that special attention should be given to susceptible risk groups; for example, people who are iodine deficient and subclinical hypothyroid patients. In regard to the former, the study by Sosvorova et al. (Citation2012) was cited by the SKLM, but only as evidence that isoflavones are iodinated, not as evidence that they are iodinated to a negligible extent and therefore, unlikely to exacerbate marginal iodine status. In regard to subclinical hypothyroid patients, the report by the SKLM was published prior to the follow up study by Sathyapalan et al. (Citation2018), which as noted, found that in contrast to their initial study (Sathyapalan et al. Citation2011), isoflavone exposure did not affect the progression of subclinical hypothyroidism.
As noted previously, the meta-analysis of soy and thyroid hormones by Otun et al. (Citation2019) found no effects on free T4 or T3. However, there was a small, but statistically significant rise in TSH levels (weighted mean difference: 0.248 mIU/L, 95% CI: 0.001, 0.494; p = 0.049) (Otun et al. Citation2019). The authors concluded that the clinical significance of this finding, if any, is unclear. There is disagreement about that which constitutes the normal TSH reference range; however, the upper limit is thought to be <5mIU, although treatment is typically not recommended until levels reach 10 mIU (Biondi Citation2013). Thus, the increase noted by Otun et al. (Citation2019) would appear to be relatively modest. Nevertheless, it is consistent with a finding from a subset of participants of the Adventist Health Study-2, in which high soy/isoflavone intake was associated with elevated TSH (>5 mIU/l) among women (n = 548), although not among men (n = 295) (Tonstad et al. Citation2016). When comparing the 5th with the 1st isoflavone intake quintile, the odds ratios (OR, 95% confidence interval [CI]) for women and men were 4.17 (1.73, 10.06; p = 0.001) and 1.05 (0.27, 4.07; p = 0.9). However, in contrast to this observational study, no relationship between urinary isoflavone levels and serum TSH was noted among participants in the in the National Health and Nutrition Examination Survey (2007–2010) (Janulewicz et al. Citation2019), but given the low isoflavone intake of the general US population (Bai, Wang, and Ren Citation2014), this finding is of questionable value.
The increased TSH level reported by Otun et al. (Citation2019) was of marginal statistical significance; furthermore, the forest plot of the results shows that the increase was driven almost entirely by four of the 26 comparisons, all four of which were conducted by the same research group; two of these are the studies involving subclinical hypothyroid patients already discussed (Sathyapalan et al. Citation2011, Citation2018), another involved early menopausal women (Sathyapalan et al. Citation2017), and one men with type 2 diabetes mellitus and subclinical hypogonadism (Sathyapalan et al. Citation2016). In addition, in a two-year study not included in the meta-analysis involving postmenopausal women who were given either a placebo (n = 126) or 200 mg/d isoflavones (n = 122), neither TSH levels nor the number of women with positive thyroid peroxidase autoantibodies differed between groups (Levis et al. Citation2011).
Recently, the effects of isoflavone intake on reverse T3 (rT3) were published although they are derived from a post hoc analysis of two studies in which men or women consumed 15 g/d SPI lacking in isoflavones or that provided 66 mg isoflavones (Sathyapalan et al. Citation2018). In the study involving men, there was an increase rT3 in the isoflavone group compared to the control (0.45 vs 0.40 nmol/L; p < 0.001) over the 3-month study period (Sathyapalan et al. Citation2016) whereas in the study involving women there was an increase in the isoflavone group (0.33–0.37 nmol/L; p < 0.001) at the 3 month but not at the 6 month time point (0.33–0.31 nmol/l) (Sathyapalan et al. Citation2017). Although rT3 is a major endogenous T4 metabolite, it is probably devoid of major biological action in adults and its clinical significance has not been established (Schmidt et al. Citation2018; Gomes-Lima and Burman Citation2018).
One study was identified that examined the impact of isoflavone intake on thyroid function in patients with Hashimoto thyroiditis (Zhang et al. Citation2017), which is considered to be the most common autoimmune disease (Caturegli, De Remigis, and Rose Citation2014). The women in this study were randomized to either the placebo (n = 143) or genistein (n = 135) group. Women in the latter group consumed 600 mg/d as the purified soy extract. After one month of genistein treatment, T4 concentration increased from 9.53 ± 2.51 µg/dl to 12.69 ± 2.71 µg/dL; fT4 concentration increased from 0.92 ± 0.22 µg/dL .34 ± 0.31 µg/dl and serum TSH concentration decreased from 12.8 ± 3.1 nmU/L to 8.8 ± 2.3 mU/L. Genistein also decreased thyroperoxidase antibody (TPOAb) and thyroglobulin antibody (TgAb) levels. The authors of this study concluded that since levothyroxine dosage was unchanged, the results suggest genistein improved thyroid function. However, the genistein dose used for this study should be considered a pharmacological, not a physiological, dose.
Finally, there are the results of three observational studies that examined isoflavone intake in relation to thyroid function. One found no relationship between soyfood intake (n = 505) and urinary isoflavone levels (n = 95) and measures of thyroid function (serum free T4, TSH, and TPOAb levels and TPOAb-positive percentages) among pregnant women from Shenyang, China (Li et al. Citation2011). Another, which involved 139 girls and 129 boys aged 8–15 y from the Czech Republic, found positive associations between circulating isoflavone levels and several measures of thyroid function, but not between soyfood intake (soyfood consumed within the past 24 h, yes or no) and TSH and free T3 levels, although free T4 levels were higher in the soyfood consumers (16.48 vs 15.42 pmol/l, p = 0.0032) (Milerová et al. Citation2006). A third case-control study involving nearly 600 Iranian children ages 6 to 12 y found that the occurrence of goiter did not differ according to soy intake (Mousavi, Tavakoli, and Mardan Citation2006). Since the latter two studies involved low-soy-consuming populations, their relevance is of questionable value (Messina Citation2004). In the study from the Czech Republic, blood genistein and daidzein levels were below 1 nmol/l (Milerová et al. Citation2006). Even among those children who consumed soyfoods within the past 24 h, mean genistein and daidzein blood levels were only 1.4 and 1.1 nmol/l, respectively. And in the Iranian study, soy intake was expressed as the percentage of cases (goiter) and controls who had consumed soy within the past month, which illustrates soy intake is not common in Iran (Mousavi, Tavakoli, and Mardan Citation2006).
Conclusions
Concerns about goitrogenic effects of isoflavones are based primarily on the results of in vitro and animal studies. In contrast to this research, extensive clinical data show that isoflavones, even when intake greatly exceeds typical Japanese intake, do not adversely affect T4 or T3 in euthyroid individuals. Less research has focused on the effects of isoflavones in those with a compromised thyroid function such as subclinical hypothyroid patients and/or whose iodine intake is marginal, but the studies that have involved such individuals do not raise concerns. Still, more research involving subclinical hypothyroid patients is warranted.
Research involving men only
Feminization/hormone levels
The idea that soy isoflavones may affect hormone levels in men has been prompted by some studies but not corroborated by most others. Weber et al. (Citation2001) found that an isoflavone-rich diet lowered testosterone levels in adult male Sprague-Dawley rats. Three years earlier, Strauss et al. (Citation1998) found that genistein reduced serum and testicular testosterone concentrations and prostate weight in mice. Results from these rodent studies and others raised concern that isoflavones feminize men, a concern which coincided with rising apprehension that environmental estrogens play a role in the declining sperm count occurring among men worldwide (Sharpe and Skakkebaek Citation1993; Toppari et al. Citation1996; Skakkebaek, Rajpert-De Meyts, and Main Citation2001).
A few clinical studies have also reported decreases in testosterone levels in response to soy consumption (Goodin et al. 2007; Gardner-Thorpe et al. Citation2003). For example, Gardner-Thorpe et al. (Citation2003) found that in young men consuming 3 scones daily providing a total of 120 mg isoflavones, serum testosterone levels decreased over a 6 week period in a pre-post comparison from 19.30 to 18.20 nmol/l (p = 0.03). There was no decrease in the control group although data were not presented. Four years later, Goodin et al. (2007) found that in response to 56 g/d SPI, serum testosterone decreased 19% in healthy males during the 4-week supplementation period and increased within 2 weeks after discontinuation of soy protein. This study did not involve a control group and the isoflavone content of the SPI was not reported. The amount of soy protein taken by the study participants was approximately 5–6 times typical Japanese intake (Konishi et al. Citation2019).
Two additional publications can be credited with raising early concerns about male feminization. One was a US pilot case-control study by Chavarro et al. (Citation2008), which found that soy intake was associated with lower sperm concentration among 99 males in subfertile couples treated at a fertility center. Testosterone was not measured in this study (Chavarro et al. Citation2008). The other was a case-report by Martinez and Lewi (Martinez and Lewi Citation2008), which described a 60-y-old male who developed gynecomastia allegedly as a result of consuming 3 liters of soymilk per day that was estimated to provided 360 mg isoflavones (∼9 times Japanese intake). In this case, testosterone levels were normal but estrogen and estradiol levels were elevated (Martinez and Lewi Citation2008).
In contrast to this single case-report of gynecomastia, two clinical trials reported no such effects. In one, men consumed for 3 months either 15 g/d SPI that provided 66 mg isoflavones or 15 g SPI that was devoid of isoflavones (Sathyapalan et al. Citation2016). Breast ultrasounds were performed on each study participant at enrollment and study termination. No changes in breast tissue volume were noted in either group. In the other study, >300 men consumed a placebo or 40 g/d SPI that provided approximately 100 mg isoflavones for nearly 3 years (Fleshner et al. 2011). There were no differences in breast tenderness or swelling/enlargement between groups.
In contrast to the few reports of lowered testosterone, the first meta-analysis of clinical studies to examine the effects of soy on reproductive hormone levels in men, which was published in 2010, found no statistically significant effects of isoflavone exposure via supplements or foods on circulating levels of total testosterone, free testosterone, sex hormone binding globulin (SHBG) or the free androgen index, regardless of statistical model employed (Hamilton-Reeves et al. Citation2010). This analysis included 15 placebo-controlled treatment groups with baseline and ending measures and 32 reports involving 36 treatment groups. Study participants ranged in age from 21 to 74 y; study length ranged from 1 week to 4 y (although 1-y data were used in the analysis for 4-y study (Li et al. Citation2008)) and daily isoflavone and soy protein intake ranged from 20 to 900 mg and from 0 to 71 g, respectively.
Reports showing soy lowered testosterone have been published subsequent to the meta-analysis (Hamilton-Reeves et al. Citation2010). For example, a case-report by Siepmann et al. (Citation2011) described a 19-y-old vegan who developed hypogonadism and erectile dysfunction allegedly as a result of his soy consumption. Coincidentally, his isoflavone intake was estimated to be 360 mg/d, the same as in the previously cited case-report (Martinez and Lewi Citation2008). Also, in resistance-trained young men, supplementation with soy protein resulted in lower testosterone levels in comparison to whey protein and carbohydrate supplementation within 30 minutes after exercise performance (Kraemer et al. Citation2013), although evidence indicates that this acute decrease in testosterone level does not affect muscle protein synthesis (Morton et al. Citation2018). In support of this contention are the results of a recent meta-analysis showing that soy protein supplementation in men undergoing resistance exercise training led to gains in muscle mass and strength similar to those observed in men supplemented with whey protein or other animal proteins (Messina et al. Citation2018). In addition, a subsequently published study found no differences in increases in lean mass and strength in untrained participants engaged in a resistance exercise program who supplemented their diet with whey protein (19 g/d) or soy protein (26 g/d) matched for leucine content (Lynch et al. 2020).
In 2021, an update to the 2010 meta-analysis (Hamilton-Reeves et al. Citation2010) that included 41 clinical studies that measured circulating total testosterone (n = 1753) and/or free testosterone (n = 752) levels in men reached the same conclusion as the 2010 analysis, that is, isoflavone intake, even when exceeding 75 mg/d, did not affect hormone levels (Hamilton-Reeves et al. Citation2010; Reed et al. Citation2020). In addition to the lack of effect on testosterone, there were also no effects on circulating estradiol (n = 1000) or estrone levels (n = 239). These latter findings concur with a previously published narrative review that found soy/isoflavones have no effect on estrogen levels in men or other endpoints related to feminization (Messina Citation2010). Furthermore, subsequent to the updated meta-analysis an 18-month study was published in which older men were randomized to receive daily either approximately 19 g casein or 19 g soy protein containing approximately 41 mg isoflavones (Bosland et al. Citation2021). No differences between groups were found for circulating levels of free testosterone or estradiol. Finally, as somewhat of an aside because this research did not involve an intervention, a Taiwanese cross-sectional study found that a diet rich in preserved vegetables or processed meat or fish, deep-fried foods, innards organs, rice or flour products cooked in oil, and dipping sauce, but low in milk, dairy products, legumes, or beans (soy), and dark or leafy vegetables) was associated with poor testicular function (lower testosterone levels, lower sperm concentration and subpar sperm morphology) (Kurniawan et al. Citation2021).
Conclusions
The overwhelming amount of clinical evidence indicates neither soyfood nor isoflavone intake affect levels of total or free testosterone or estrogen or estradiol levels in men.
Fertility
Male fertility, like female fertility, has also become a soy-related topic of interest in part because estrogen is vital for the development, maintenance and function of the male reproductive system (Schulster, Bernie, and Ramasamy Citation2016). In addition, several animal studies raised concern about the adverse impact of isoflavone exposure on spermatogenesis. For example, Glover and Assinder (Citation2006) found that a high-isoflavone diet (465 μg/g) fed to rats for 3–25 days reduced fecundity, likely as a result of lipid peroxidation of epididymal sperm. A year later, these researchers showed that a high-isoflavone diet disrupted spermatogenesis and increased germ cell apoptosis in Wistar rats, an effect attributed to an estrogenic effect in the testis (Assinder et al. Citation2007). On the other hand, the authors of a case report suggested that isoflavones could be a treatment for low sperm concentration (Casini, Gerli, and Unfer Citation2006). The observational (Toshima et al. Citation2012; Xia et al. Citation2013; Yuan et al. Citation2019; Mínguez-Alarcón et al. Citation2015) and clinical (Mitchell et al. Citation2001; Beaton et al. Citation2010; Messina, Watanabe, and Setchell Citation2009) studies providing insight into the effects of isoflavones on spermatogenesis and fertility are discussed below.
Observational studies
A study involving 42 Japanese males (age, 36.8 ± 5.4) of couples who had an infertility consultation at a gynecology clinic in Tokyo found urinary daidzein was inversely related to sperm concentration (p ≤ 0.001) and equol (p ≤ 0.05) was inversely related to sperm motility (Toshima et al. Citation2012). As noted previously, urinary isoflavones are considered to be a good representative for exposure assessment (Chávez-Suárez et al. Citation2017; Franke et al. Citation2010; Franke et al. Citation2006). In contrast, there was no statistically significant relationship between soyfood intake and semen parameters based on data collected from a self-administered questionnaire. The questionnaire was used to collect information on smoking status and consumption frequency of some food items (vegetable, fruit, soy products, alcohol, coffee, and tea), but it is not clear whether the statistical analysis controlled for confounding variables.
In agreement with the Japanese study is a Chinese study involving 608 idiopathic infertile men (age, 28.89 ± 4.39) and 469 fertile controls (age, 29.86 ± 3.58), that found urinary daidzein and genistein levels were significantly associated with idiopathic male infertility, and with idiopathic male infertility with low sperm concentration and reduced motility (Xia et al. Citation2013). A questionnaire was used to collect information including personal background, lifestyle factors, occupational and environmental exposures, genetic risk factors, sexual and reproduction status, medical history and physical activity. However, soy intake was not reported in this study and other than age and abstinence time, it does not appear that the results were adjusted for possible confounders.
More recently, in a cross-sectional study involving 1319 reproductive-aged men (age, 32.2 ± 5.8 years) from Shenzhen, China, there were inverse associations between semen genistein and sperm counts (p = 0.02) and concentrations (p = 0.02) (Yuan et al. Citation2019). However, these relationships did not exist for daidzein. The results were adjusted for age, body mass index (BMI), abstinence time, and diet preference (meat lovers, vegetable lovers or vegetarians and balanced diets with meat and vegetables). This study also found statistically significant associations between semen concentrations of the lignan secoisolariciresinol and lower sperm concentrations, sperm counts and total motility. Finally, a Chinese study found no associations between the urinary daidzein metabolic rate and infertile risk (Qin et al. Citation2014). In this study, which involved 401 infertile Chinese men aged 30 (range, 20–44) and 600 cases aged 29 (19–51) that were recruited from the affiliated Hospitals of Nanjing Medical University, daidzein metabolic rate represented the urinary equol concentration divided by the concentration of daidzein plus equol.
The results of the three Asian studies described above are consistent with the previously mentioned US pilot case-control study, which found that soy intake was associated with lower sperm concentration (∼33%) among 99 male partners in subfertile couples who presented for semen analyses to a fertility center (Chavarro et al. Citation2008). However, it is notable that in this study about half of the decreased sperm concentration resulted from the higher ejaculate volume in the fourth (4.1 ml) compared to the first (3.5 ml) soyfood intake quartile. Total sperm count was only reduced by ∼10% when comparing extremes of soy intake, a decrease which was not statistically significant; nor was there an effect of soyfood intake on sperm motility or morphology. Since there is no biological basis for soy increasing ejaculate volume, the observed association between soy and sperm concentration lacks credibility. A closer look at the data supports this contention.
In multivariate analyses, men in the highest soyfood intake category (≥0.30 servings/d, median isoflavone intake, 13.5 mg/d) had on average, 41 million fewer sperm/ml than men who did not eat soyfoods (p = 0.02). Men in the second soyfoods intake category had 24 million fewer sperm/ml than men who did not eat soyfoods even though the intake category amount of 0.01–0.07 servings/d (median isoflavone intake, 0.85 mg/d) is highly unlikely to exert a biological effect. In any event, a follow up study by this research group involving 184 men from couples undergoing infertility treatment with in vitro fertilization, found that male partners’ intakes of soyfoods and soy isoflavones were unrelated to fertilization rates, proportions of poor quality embryos, accelerated or slow embryo cleavage rate, and implantation, clinical pregnancy and live birth (Mínguez-Alarcón et al. Citation2015).
Four additional observational studies examined the relationship between sperm and semen parameters and isoflavone exposure; however, all four involved low-soy-intake Western populations. In one, which involved 501 male partners of couples desiring pregnancy and discontinuing contraception, Mumford et al. (Citation2015) found that urinary genistein and daidzein were associated with a lower percentage of normal sperm and increased abnormalities in semen morphology after adjustment for age, research site, serum lipids, and cotinine. Isoflavone exposure was not associated with couple fecundity and the association between genistein and daidzein and sperm concentration and semen morphology was reduced as BMI increased. The BMI finding contrasts with a finding by Chavarro et al. (Citation2008), in that in their study, there was a suggestion that the association between soyfood intake and sperm concentration was more pronounced among overweight and obese men than among lean men (p for interaction, 0.10).
Chung et al. (Citation2019) examined the relationships between urine and serum concentrations of 128 chemicals classified as endocrine disruptors, including isoflavones, and 7 semen quality endpoints in a prospective cohort study comprising 473 men. None of the chemicals were associated with semen quality endpoints after adjusting for multiple tests. The referent study population were couples participating in the LIFE Study (Longitudinal Investigation of Fertility and the Environment), all of whom were discontinuing contraception for purposes of becoming pregnant. Participants were recruited between 2009 and 2012 from 16 counties in Michigan and Texas, USA. From this cohort 473 male partners provided semen samples representing the study cohort for analysis.
Song et al. (Citation2006) recruited 48 men from New York State with abnormal semen parameters who had been trying to conceive with their partners for at least one year. Controls were 10 men with normal semen analyses who had fathered a pregnancy within the previous year. Block food frequency questionnaires were used to estimate the dietary intake of isoflavones (genistein and daidzein) of each patient. Higher mean intake of genistein and daidzein were observed in fertile control men compared to infertile men (p < 0.05). Daidzein and genistein were also higher in men with good sperm DNA integrity compared to men with poor sperm DNA integrity. Regression analysis showed significant correlation between dietary intake of genistein and daidzein and semen parameters including sperm count, motility, progressive motility, and sperm DNA fragmentation index. However, this study exists only as an abstract. Furthermore, as expected isoflavone intake was extremely low (genistein, 527 ± 183 µg/d in infertile men vs. 1722 ± 714 ug/d in fertile controls; daidzein, 241 ± 84 µg/d vs. 788 ± 327 µg/d, p < 0.05).
Finally, there is a case-referent study involving the male partners of couples attempting conception with unprotected intercourse for 12 months or more without success, recruited from 14 UK assisted reproduction clinics (Povey et al. Citation2020). Food intake was estimated by a 65-item food frequency questionnaire (FFQ) covering the 12 months prior to recruitment. After adjustment for clustering and potential confounding, among the 1907 participants low motile sperm concentration was inversely related to daidzein intake (OR, 0.58; 95% CI: 0.42, 0.82)0.58 (0.42–0.82) but was unrelated to poor sperm morphology.
Clinical studies
Clinical data show isoflavone exposure is unrelated to sperm or semen parameters. Three such studies, two published in full manuscript form and one described in the proceedings from a scientific meeting, have been conducted. None of the studies reported any adverse effects. In one, healthy British volunteers aged 18–35, took a placebo or a daily supplement containing 40 mg isoflavones for two months (Mitchell et al. Citation2001). Mean plasma concentrations of genistein and daidzein increased to approximately 1 µM and 0.5 µM respectively, during supplementation. In addition to the lack of effect on sperm concentration, there was no effect on ejaculate or testicular volumes. In another study, which utilized a cross-over design, 32 healthy Canadian men (age, 27.5 ± 5.67 y) consumed diets in random order for 57 d which were supplemented with milk protein isolate or SPI that provided isoflavones at either 0.02 or 0.75 mg/kg bw per day (Beaton et al. Citation2010). Urinary isoflavones were significantly higher after consumption of the high isoflavone vs low isoflavone SPI and milk protein isolate. Semen parameters, including semen volume, sperm concentration, sperm count, sperm percent motility, total motile sperm count, and sperm morphology, were not significantly affected by consumption of either the low or high SPI compared to the milk protein isolate. In the third study, 20 Italian men were randomized to three different groups in which they were provided 60, 320 or 480 mg/d isoflavones for three months (Messina, Watanabe, and Setchell Citation2009). There were no statistically significant effects on any of the outcomes measured (ejaculate volume, sperm concentration, sperm count, and motility of spermatozoa).
Conclusions
Observational studies have found inconsistent associations between sperm/semen parameters and isoflavone intake. Three clinical studies that varied in duration from 2-3 months and in which men consumed from 40 to 480 mg/d isoflavones did not report any adverse effects, although one of these studies was not published as a complete manuscript.
Research involving women only
Reproductive hormones
In theory, isoflavones may influence estrogen action by virtue of effects on enzymes involved in steroid metabolism, such as aromatase (Rice, Mason, and Whitehead Citation2006), 17β-hydroxysteroid dehydrogenases, steroid sulfatases and sulfotransferases (Lacey et al. Citation2005), among others (Mesiano et al. Citation1999; Ohno et al. Citation2002). Isoflavones could also affect biologically active levels of hormones by affecting SHBG concentrations (Loukovaara et al. Citation1995).
Hooper et al. (Citation2009) published a systematic review and meta-analysis of clinical studies that evaluated the impact of isoflavone exposure via foods, soy protein and supplements on reproductive hormones in pre- and postmenopausal women. The meta-analysis included 11 studies involving 579 premenopausal women, 35 studies involving 1,165 postmenopausal women and 1 study involving 69 perimenopausal women. The number of women analyzed in these studies ranged in size from 10 to 304 (mean, 59). Thirty-two studies were parallel in design and 15 were crossover. Nineteen studies assessed the effect of an isoflavone supplement (vs control), nine compared an isoflavone-containing SPI with an isoflavone-depleted SPI, 13 compared SPI with a non-soy control, and nine compared whole soy or soyfoods with a control (some studies included more than one comparison). Studies ranged in length from 4 to 104 weeks: 29 were 4–12 weeks in duration; nine were 13–26 weeks; seven 27–52 weeks; and two were >1 year.
Soy and isoflavone consumption had no effect on circulating total estradiol, estrone or SHBG concentrations in premenopausal women (based on 6–11 studies per comparison). In postmenopausal women, there was a small increase (∼14%) in circulating total estradiol concentrations following soy isoflavone consumption (based on 21 studies involving 580 women), but this change was not statistically significant (p = 0.07). Soy isoflavones had no effect on circulating total estrone (7 studies, 152 in control) or SHBG (17 studies, 459 women in control groups) concentrations.
In premenopausal women, soy isoflavones significantly reduced circulating levels of follicle stimulating hormone (FSH) by ∼22% (p = 0.01) and luteinizing hormone (LH) by ∼24% (p = 0.05), based on 7 studies involving 73 participants using standardized mean differences, but not mean differences. However, in sensitivity analysis when only studies at low risk of bias were retained, the results were no longer statistically significant. Soy isoflavones had no statistically significant effects on progesterone or circulating free estradiol concentrations.
Hooper et al. (Citation2009) were unable to conclude whether the observed but tentative premenopausal changes in FSH and LH reflect an estrogenic or anti-estrogenic effect because these hormones were assessed in different studies at different points in the menstrual cycle. During the midcycle gonadotrophin surge, a decrease in LH is best construed as an anti-estrogenic effect, whereas during the luteal phase a decrease in LH may be an estrogenic effect. A surge in LH is required for ovulation, although there is a diversity of LH surges in terms of configuration, amplitude, and duration in cycles of normally fertile women (Direito et al. Citation2013).
In postmenopausal women, soy isoflavone intake had no effect on circulating FSH, LH, circulating estrone sulfate or free estradiol (where there were at least three studies and at least 50 women in combined control groups). In the one study involving perimenopausal women, there were no effects on circulating FSH, estrone or estradiol.
Subsequent to the meta-analysis by Hooper et al. (Citation2009), 10 studies were identified that examined the impact of isoflavone exposure on reproductive hormone levels in women. Seven of these involved postmenopausal women (Levis et al. Citation2011; Delmanto et al. Citation2013; Ye et al. Citation2012; Evans et al. Citation2011; Carmignani et al. Citation2015; Husain et al. Citation2015; Villa et al. Citation2009), one premenopausal women (Maskarinec et al. Citation2011) and two included pre- and postmenopausal women (Chung et al. Citation2019; Khan et al. Citation2012). Studies ranged in duration from 8 weeks (Husain et al. Citation2015) to 2 years (Levis et al. Citation2011), sample size in the isoflavone group from 20 (Carmignani et al. Citation2015) to 97 (Levis et al. Citation2011) and isoflavone dose from 30 mg/d (genistein only) (Evans et al. Citation2011) to 235 mg/d (Khan et al. Citation2012).
In the premenopausal study, no significant effects of 50 mg/d isoflavones from soyfoods were observed on estradiol, estrone or estradiol sulfate (Maskarinec et al. Citation2011). In the two studies that included pre- and postmenopausal women, there were no statistically significant effects on estradiol (Chung et al. Citation2019; Khan et al. Citation2012), and no effects on FSH and SHBG in the study that assessed these endpoints (Khan et al. Citation2012). In the other seven studies, all of which involved postmenopausal women, only one reported a statistically significant effect on estradiol or FSH or LH. In this one study, Ye et al. (Citation2012) found the percent decrease in estradiol in the high dose isoflavone group (126 mg/d) was less than the decrease in the placebo group whereas there was no difference between the placebo and the group consuming 84 mg/d isoflavones.
The following text consists of brief comments about the several of the studies published after the 2009 Hooper et al. meta-analysis (Hooper et al. Citation2009). The study by Ye et al. (Citation2012), was the only one that intervened with isoflavones derived from soy germ; consequently, genistein exposure was quite low (∼15% of total isoflavone intake). The study by Villa et al. (Citation2009) intervened with 54 mg/d genistein provided in aglycone form. The six-month study by Khan et al. (Khan et al. Citation2012), which involved pre and postmenopausal women, is notable for several reasons. One, all the participants were at an increased risk for breast cancer or had a history of unilateral minimal risk breast cancer. Two, of the total daily isoflavone dose of 235 mg taken by the participants, 150 mg was genistein. Three, the lack of effect of isoflavones on FSH (baseline mean 5.73, interquartile range [IQR]: 2.46–8.30; final mean, 6.01: IQR: 0.01–9.64 mIU/ml) in the 53 premenopausal women in this study, contrasts with the ∼22% decrease in FSH noted by Hooper et al., (Citation2009) in a total of 73 women from seven studies. In the study by Husain et al. (Citation2015), which involved postmenopausal women, although the difference was not statistically significant, in the control group estradiol increased approximately 10% whereas in the soy/isoflavone group it increased by about 50%. Finally, in the study by Carmignani et al. (Citation2015), final estradiol values increased 10 times more in the group given hormone therapy (1 mg estradiol + 0.5 mg norethisterone) than in women taking isoflavones (53 mg/d). One 12-week intervention study involving perimenopausal women that did not assess hormone levels found that vaginal bleeding occurred in three participants (12.5%) ingesting 100 mg/d isoflavones and in one participant (4.3%) ingesting 50 mg/d isoflavones (4.3%) whereas no bleeding occurred in the placebo group (Schneider et al. Citation2019). However, these findings were not statistically significant.
Finally, there are suggestive clinical data that isoflavones favorably affect conditions common among women with polycystic ovary syndrome (PCOS), which may affect as many as 25% of reproductive age women (Setji and Brown Citation2014). For example, among women with PCOS, Karamali et al. (Citation2018) found that a soy-containing diet lowered testosterone and insulin levels, findings which agree with those of Khani et al. (Citation2011), who reported that genistein (36 mg/d) decreased testosterone levels and Jamilian and Asemi (Citation2016), who reported that soy isoflavones (50 mg/d) significantly reduced the free androgen index among women with PCOS. In contrast, Romualdi et al. (Citation2008) reported that in a small pilot study (n = 12), genistein (36 mg/d) had no effect on androstenedione levels
Conclusions
Despite the in vitro identification of mechanisms whereby isoflavones can potentially impact hormone levels and some reports of isoflavone-induced hormonal disturbances noted in animal studies, a critical review of the clinical literature shows neither soy intake nor isoflavone exposure significantly affects reproductive hormone levels in women.
Menstrual cycle
Observational data
Four observational studies that have related soy intake or urinary isoflavone excretion to menstrual cycle length (MCL) have been conducted across different geographical regions including Japan (Nagata, Oba, and Shimizu Citation2006), Singapore (Jakes et al. Citation2001), England (Verkasalo et al. Citation2001) and the US (Levine et al. Citation2020). In the Japanese study, Nagata, Oba, and Shimizu (Citation2006) found that among 341 women aged 18–29 y, a higher habitual soy intake (median 1st and 5th soy intake (g/d) was 14.9 and 100.9, respectively) was unrelated to MCL. In contrast, a higher habitual intake of polyunsaturated fat and fiber was associated with shorter and longer MCL, respectively. Mean isoflavone intake is difficult to accurately estimate from the data reported, but from the information about soyfood consumption, a conservative estimate would be approximately 25 mg/d isoflavones (Messina, Nagata, and Wu Citation2006).
In contrast to the Japanese study, Jakes et al. (Citation2001) found that among Singaporean women, a higher habitual intake of soy protein tended to be associated with increased MCL based on self-report when assessed cross-sectionally and prospectively by recording three consecutive cycles, but the results did not reach statistical significance. More specifically, comparing the highest versus lowest quartile of self-reported soy protein (Q1, <3.3 g/d versus Q4 ≥ 8.7 g/d), MCL was 30.8 days in Q4 compared to 28.2 days in Q1 (p for trend, 0.052), while based on food diary data, MCL was 30.9 days in Q4 compared to 29.7 d in Q1 (p for trend, 0.16).
In another cross-sectional study involving predominantly premenopausal British vegetarian and vegan participants (n = 636), Verkasalo et al. (Citation2001) found a non-statistically significant trend toward a higher soymilk intake being associated with a shorter MCL (0.7 d; p = 0.086 for trend). Isoflavone intake in this study was estimated by Verkasalo et al. (Citation2001) to be 12.4 and 36.8 mg/d in the intermediate and high soymilk-consuming groups, respectively. Although this research involved a Western population, because it was comprised mostly of vegetarians (65%), it avoids the major limitation of Western populations cited previously (Messina Citation2004). On the other hand, of the various soy products consumed by British women, only the intake of soymilk was considered (Verkasalo et al. Citation2001).
Finally, there are the results of two US studies. In a population-based prospective cohort of 326 women aged 18–40 (followed until pregnancy or for 12 months of attempting) urinary isoflavone levels were not associated with MCL (Levine et al. Citation2020). However, it is evident that the women in this study consumed little soy given that mean urinary genistein levels in women whose cycles were <26 d, 26–35 d and >35 d were only 167, 104, and 152 nmol/l, respectively. There was no association with MCL; however, each 1 nmol/L increase in genistein was associated with menstrual cycle irregularity (OR 1.19, 95% CI 1.02, 1.38), although the authors concluded that the results were reassuring for women attempting pregnancy.
In the other study, which involved women with regular menstrual cycles (246 participants in final analysis), who were followed-up for two menstrual cycles, luteal phase deficiency (LPD) (which may be associated with an increased risk of infertility/early miscarriage) occurred in 8.9% of the menstrual cycles (Andrews et al. Citation2015). In multivariate analyses, LPD was associated with a higher Mediterranean diet score, higher intake of dietary fiber and isoflavones, and lower intake of selenium. Given the limited number of LPD cycles included in this exploratory study and the associations observed with quite low isoflavone intakes (7 mg/d), additional data are required to further interpret these findings. Also, given that the Mediterranean diet score and fiber were associated with LPD, it may be that the association with isoflavone intake simply reflects the consumption of a more plant-based diet.
Clinical data
The impact of soy on MCL was first reported in studies published by Cassidy, Bingham, and Setchell (Citation1994) and Cassidy, Bingham, and Setchell (Citation1995) both of which involved participants following carefully controlled diets while living in a metabolic ward (Franke, Custer, and Hundahl Citation2004; Franke et al. Citation2006). In 2009, with the inclusion of 10 intervention studies, Hooper et al. (Citation2009) published a systematic review and meta-analysis of the effects of soy/isoflavone exposure on MCL; they found that MCL was increased by 1.05 d (95% CI: 0.13, 1.97; 10 studies, n = 148 soy intervention; n = 153, control). In sensitivity analysis, removing studies not at low risk of bias, resulted in statistical significance being lost but this result was based on only two studies. Menstrual cycle function is suggested to be a sentinel of fecundity, irrespective of pregnancy intentions (Mumford et al. Citation2012; Vassena et al. Citation2014). However, short, but not long, menstrual cycles have been linked to 11–36% longer time to pregnancy (Crawford et al. Citation2017; Wesselink et al. Citation2016; Wise et al. Citation2011). Since the publication of this analysis (Hooper et al. Citation2009), no subsequently published trials evaluating MCL were identified.
Conclusions
The available data from the limited population-based studies provide little evidence for an association between habitual soy intake and MCL although levels of intake of soy and isoflavones were low in three of the four studies. Based on the most recent meta-analysis of clinical trials, soy/isoflavone intervention results in a small but significant increase in MCL.
Fertility
Observational studies
Several population-based studies have examined the associations between soy intake or urinary isoflavone levels and fertility. Although there are limited data in high soy-consumers, in studies with low habitual intakes, no relationship was found between isoflavone intake and fecundability among two web-based preconception cohorts, one involving 4880 women participating in the Pregnancy Study Online (PRESTO, North America) and the other involving 2898 women participating in the Snart Foraeldre (SF, Denmark) (Wesselink et al. Citation2020). In the PRESTO and SF, the cutoff for the ≥90th percentile isoflavone intake was only 3 and 2 mg/d, respectively. Study participants had been attempting conception for ≤6 cycles at study entry. There was some evidence of improved fecundability with increasing isoflavone intake among women age ≥30 y in the PRESTO (fecundability ratios: 1.12; 95% CI: 0.94, 1.34, for comparison of ≥ 90th with < 25th percentile intake) and SF (fecundability ratios: 1.19; 95% CI: 0.92, 1.55).
In a US study involving 18,555 women without a history of infertility who were followed as they attempted a pregnancy or became pregnant, replacing animal protein with vegetable protein was associated with a decreased risk of ovulatory infertility; however, tofu intake was unrelated to ovulatory infertility (Chavarro et al. Citation2008). And in another US study, which involved 315 women undergoing assisted reproductive technology, a higher soy isoflavone intake was positively related to live birth rates and in multivariable-adjusted models, the ORs of live birth (95% CI) for women in increasing categories of isoflavone intake were 1.32 (0.76–2.27) for women consuming 0.54–2.63 mg/d, 1.87 (1.12–3.14) for women consuming 2.64–7.55 mg/d, and 1.77 (1.03–3.03) for women consuming 7.56–27.89 mg/d (Vanegas et al. Citation2015).
To date, only two studies have examined the associations between urinary isoflavone concentrations and pregnancy related outcomes. In a study of 501 couples who were followed for 12 months or until pregnancy, urinary isoflavone concentrations in men and women were not associated with time to pregnancy (Mumford et al. Citation2014). Likewise, in a previously cited US population-based prospective cohort of 326 women ages 18–40 with self-reported cycles 21–42 days who were followed until pregnancy or for 12 months of attempting pregnancy, urinary isoflavone levels were not associated with MCL (Levine et al. Citation2020).
Only one study involving women with a high habitual soy intake suggests that isoflavones may be associated with a reduction in pregnancy rate. This cross-sectional study, which involved 11,688 US women aged 30–50 years, found that a higher habitual intake of isoflavones was associated with an increased risk of nulliparity and nulligravidity (Jacobsen et al. Citation2014). The mean isoflavone intake (17.9 mg/d) of this population (North American Seventh-day Adventists) is much higher than typical North American intake (<3 mg/d) (Bai, Wang, and Ren Citation2014). Only 6% of the women indicated no isoflavone intake.
After adjustment for age, marital status, and educational level, a higher isoflavone intake was associated with a lower likelihood of ever having become a mother. In women with high (≥40 mg/d) isoflavone intake (12% of the cohort), the adjusted lifetime probability of giving birth to a live child was reduced by approximately 3% compared to women with low (<10 mg/d) intake. No relationships were found between the isoflavone intake and parity or age at first delivery in parous women. A similar inverse relationship (p = 0.03) was found between isoflavone intake and the risk of nulligravidity with a 13% higher risk of never have been pregnant in women with high (≥40 mg/d) isoflavone intake. These relationships were found mainly in women who reported problems becoming pregnant. Although of more limited informative value, in the Shanghai Women’s Health Study, among 33,054 postmenopausal women, no relationship between soy intake and reproductive span (years between menses and menopause) or menopause onset was noted (Dorjgochoo et al. Citation2011).
Finally, Chavarro et al. (Citation2016) examined the interactions between environmental estrogens such as bisphenol A (BPA) and soy intake on pregnancy-related outcomes in a prospective study of women undergoing in vitro fertilization treatment. A higher habitual soyfood intake modified the association of urinary BPA concentration with live birth rates (P for interaction = 0.01). Among women who did not consume soyfoods, the adjusted live birth rates per initiated cycle in increasing quartiles of cycle-specific urinary BPA concentrations were 54%, 35%, 31%, and 17% (p for trend = 0.03). The corresponding live birth rates among women reporting pretreatment consumption of soyfoods were 38%, 42%, 47%, and 49% (p for trend = 0.35). A similar pattern was found for implantation (P for interaction = 0.02) and clinical pregnancy rates (p for interaction = 0.03) per initiated cycle, where urinary BPA was inversely related to these outcomes among women not consuming soyfoods, but unrelated to them among soy consumers. These data suggest significant interactions between soy and environmental estrogens, an area which warrants further research.
Clinical studies
The topic of isoflavones and female fertility has only peripherally been identified in two supplementation studies. In one, patients with unexplained infertility and recurrent clomiphene citrate (CC) induction failure, were randomized to group I (n = 60) or group II (n = 59). Both groups received CC (150 mg/d; days 3–7) (Shahin et al. Citation2008). Group I also received 120 mg/d phytoestrogens derived from Cimicifuga racemosa (C. racemosa) on days 1 to 12. Human chorionic gonadotrophin injection (10,000 IU i.m.) was given and timed intercourse was recommended when a leading follicle reached >17 mm and serum estradiol exceeded 200 (pg/ml). Although there was a trend toward a shortening of induction cycles in group I, it did not reach statistical significance. Estradiol and LH concentrations were higher in group I as was endometrial thickness, serum progesterone and clinical pregnancy rate (8.9 ± 1.4 mm versus 7.5 ± 1.3 mm, p < 0.001; 13.3 ± 3.1 ng/ml versus 9.3 ± 2.0 ng/ml, p < 0.01; 36.7% versus 13.6%, p < 0.01, respectively). These data suggest that the addition of C. racemosa extract to CC induction can improve the pregnancy rate and cycle outcomes. However, C. racemose does not contain isoflavones.
In the other study, infertile women (n = 134, aged 25–35 years with duration of infertility > 2 years) who had oligomenorrhea or amenorrhea were randomly assigned to CC (100 mg daily for 5 days) or CC (100 mg daily for 5 days) in combination with isoflavones (1500 mg daily for 10 days) (Unfer et al. Citation2004). Both treatments resulted in an increase in FSH, LH and 17β-estradiol plasma concentrations, but there were no differences between groups. In contrast, endometrial thickness was significantly increased in the isoflavone group, although no significant differences in the pulsatility index values or in the number of preovulatory follicles were noted. These data suggest that a high isoflavone dose can reverse the deleterious effects of CC on endometrial thickness and could contribute to higher pregnancy rates. However, because a pharmacological dose was used, the findings do not have bearing upon dietary exposure to isoflavones.
Conclusions
To date no clinical studies have directly examined the effect of soy on fertility, however,a small study that included the addition of a phytoestrogen supplement to standard fertility treatment supports its potential to improve endometrial thickness and pregnancy rate, but further robust trials are required. Furthermore, a pharmacological dose of isoflavones was used in this study (Unfer et al. Citation2004). In relation to population-based studies, specifically examining the impact of a dietary constituent on pregnancy is difficult, but the available data provide limited evidence to suggest soy intake has any impact on pregnancy related outcomes. Future studies should focus on high-soy-consuming populations.
Breast cancer
As noted at the onset, beginning in the late 1990s, concerns based on rodent research were raised that isoflavone intake, and more specifically, genistein intake, may increase the risk of high-risk women developing breast cancer and worsen the prognosis of breast cancer survivors (Hsieh et al. Citation1998). The research group responsible for much of this work continued to publish rodent research on this topic over the next two decades (Setchell et al. Citation2002; Ju et al. Citation2001; Ju et al. Citation2006; Santell, Kieu, and Helferich Citation2000; Ju et al. Citation2002; Du et al. Citation2012; Ju et al. Citation2008; Allred et al. Citation2001 2004; Zhang et al. Citation2017). It is worth noting however, that when Onoda et al. (Onoda et al. Citation2011) slightly tweaked the basic model used for this research, they did not find that genistein stimulated mammary tumor growth. Onoda et al. (Citation2011) suggested the reason for the discrepant findings is that in their model, prior to implantation, cancer cells are maintained in culture lacking estrogen whereas in the model in which genistein stimulates tumor growth, cells are cultured in medium containing 1 nM 17β-estradiol, which is approximately 1,000 fold higher than the unbound 17β-estradiol concentration in women. Onoda et al. (Citation2011) speculated that this supraphysiologic estrogen concentration enhances the estrogen dependence of the MCF-7 cells making them extremely sensitive to the effects of ER agonists.
It should be acknowledged that despite extensive research, a definitive understanding of the relationship between isoflavone intake and breast cancer prognosis has not been achieved. This is because the effects of soy intake on breast cancer recurrence and/or breast cancer-specific mortality among breast cancer survivors has not been examined in a randomized clinical trial. However, considerable observational and clinical evidence suggests that post-diagnosis soy intake is safe for breast cancer survivors. This conclusion is consistent with the position of the American Cancer Society (Rock et al. Citation2012), American Institute for Cancer Research (American Institute for Cancer Research 2021), World Cancer Research Fund International (World Cancer Research Fund International Citation2014) and the Canadian Cancer Society (Eating Well After Breast Cancer 2021).
EFSA has concluded from its review of 43 human studies and 62 animal studies that there is no indication for adverse effects of isoflavones on the mammary gland of postmenopausal women (EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) 2015). However, EFSA also noted that in the face of limited data, it was not possible to reach a conclusion about the risk of consuming isoflavone-based food-supplements in postmenopausal women with a current diagnosis or history of estrogen-dependent cancer. The review by EFSA focused on the possible health risks associated with the intake of isolated isoflavones in food supplements by peri- and postmenopausal women. Three years later, the SKLM concluded that “The available human studies do not indicate that an isoflavone exposure as reported in the thoroughly studied Asian population or as investigated in clinical studies (i.e., about 100 mg/day) negatively influences breast cancer risk …” (Hüser et al. Citation2018) However, as a precautionary measure, the SKLM recommended that high-risk groups abstain from the intake of isoflavone-containing supplements and that isoflavone intake via the consumption of soyfoods not exceed the median intake of Asian countries of about 50 mg/d.
Clinical studies
Primary studies that have examined the safety of soy on breast health have done so through measurement of biological markers such as mammographic density (Boyd et al. Citation2011; Rice et al. Citation2016). Hooper et al. (Citation2010) meta-analyzed clinical trials that examined the effect of isoflavone exposure (ranging from 40 to 120 mg/d) for at least 6 months on mammographic density in postmenopausal women (five studies), premenopausal women (five studies) or both (two studies). Interventions included red clover-based isoflavone supplements (two studies), soy-based isoflavone supplements (three studies; isolated genistein, soy germ based isoflavones and mixed soy isoflavones), additional soyfoods (one study) and soy protein powder compared with milk protein powder (two studies). Results showed no effect of isoflavones on mammographic density in all women combined [mean difference (MD) 0.69%, 95% CI: −0.78, 2.17] or postmenopausal women (MD −1.10%, 95% CI: −3.22, 1.03). However, there was a modest increase in mammographic density in premenopausal women (MD 1.83%, 95% CI: 0.25, 3.40) without heterogeneity, but this effect was lost in one of three sensitivity analyses. Hooper et al. (Citation2010) were unable to determine the potential clinical significance of a small increased mammographic density in premenopausal women.
Mammographic density was also examined in three subsequently published isoflavone intervention studies. In one, Wu et al. (Citation2015) found no evidence that 50 mg/d soy isoflavones significantly affected mammographic density or breast MRI fibroglandular tissue density in high-risk women and previously treated breast cancer patients over a one-year period. In another one-year study, which involved Greek postmenopausal women experiencing climacteric symptoms, a soy extract found no effect on mammographic density, although this was also true for low-dose hormone therapy (Labos et al. Citation2013). However, the isoflavone content of the intervention product, DT56a (Tofupill/Femarelle, Se-cure Pharmaceuticals, Dalton, Israel) was not indicated although it has been described as a “unique enzymatic isolate of soybeans” (Somjen et al. Citation2007). Finally, in a two-year study involving premenopausal women treated surgically for breast cancer, mammographic density significantly decreased in both the placebo group and in women ingesting 80 mg/d isoflavones (p < 0.0001), although there were no differences between groups (Ferraris et al. Citation2020).
In vivo breast cell proliferation is another commonly used biological marker that is a better predictor of breast cancer risk than mammographic density (Hanahan and Weinberg Citation2011). The most widely practiced measurement of proliferation involves immunohistochemical detection of the nuclear non-histone protein Ki67, which is thought to be involved in ribosomal RNA synthesis (Rahmanzadeh et al. Citation2007; Scholzen and Gerdes Citation2000). Combined hormone therapy (CHT, estrogen plus progestin) has been shown to increase proliferation four to 10-fold within just 12 weeks (Conner et al. Citation2003; Murkes et al. Citation2011; Conner Citation2007).
In contrast to the proliferative effects of CHT, none of the six studies that evaluated the effects of isoflavones on in vivo breast cell proliferation showed an increase () (Khan et al. Citation2012; Hargreaves et al. Citation1999; Sartippour et al. Citation2004; Palomares et al. Citation2004; Cheng et al. Citation2007; Shike et al. Citation2014). These studies included healthy women, women at high-risk of developing breast cancer and breast cancer survivors. The duration of these studies ranged from two weeks (Hargreaves et al. Citation1999) to one year (Palomares et al. Citation2004) and daily isoflavone intake (expressed in aglycone equivalents) ranged from 36 (Cheng et al. Citation2007) to 235 (Khan et al. Citation2012) mg. For comparison, one serving of a traditional soyfood (e.g. 100 g tofu or 250 ml soymilk) contains ∼25 mg isoflavones and as noted previously, mean daily isoflavone intake among older adults in Japan ranges from 30 to 50 mg (Messina, Nagata, and Wu Citation2006).
Table 10. Selected characteristics and results of intervention studies evaluating the effects of isoflavone exposure on breast cell proliferation.
In the study by Palomares et al. (Citation2004), which included breast cancer survivors, the contralateral breast was used to assess cell proliferation and in the study by Sartippour et al. (Citation2004), the ratio of the number of apoptotic to mitotic cells was used as an assessment of proliferation. It is noteworthy that in the studies by Shike et al. (Citation2014) and Khan et al. (Citation2012), women were exposed to approximately 62 and 150 mg/d genistein, which equates to amounts provided by approximately five and 12 servings of traditional soyfoods, respectively. Interestingly, three of the six studies found that gene expression was modified in a way suggestive of an increased breast cancer risk and similar to that which might be expected from exposure to estrogen (Khan et al. Citation2012; Hargreaves et al. Citation1999; Shike et al. Citation2014). Nevertheless, cell proliferation was unaffected even in response to pharmacologic doses of genistein.
Soy exposure has also been studied to determine whether it regulates gene expression in the breast through epigenetic mechanisms. Coussement et al. (Citation2018) reported no major general epigenetic reprogramming in the breast following 5 d of soymilk consumption. Participants in this exploratory study were randomized to consume 250 ml soymilk (approximately 25 mg isoflavones) at breakfast, lunch and dinner (N = 6) or their usual diet (control, N = 5) before their esthetic breast reduction surgery, after which the breast tissue was examined for global DNA methylation. However, the findings of Coussement et al. (Citation2018) contrast with an earlier study from Qin et al. (Citation2009), who found that after healthy premenopausal women consumed 40 or 140 mg/d supplemental isoflavones for one menstrual cycle, there was an increase in breast cancer-related gene RARβ2 hypermethylation that correlated with serum genistein in those receiving 140 mg isoflavones (r = 0.68, p = 0.021) and in both groups combined (r = 0.67, p = 0.0017). There was also an increase in breast cancer-related gene CCND2 (connective tissue growth factor) hypermethylation that was correlated with serum genistein in those receiving 40 mg (r = 0.79, p = 0.011) but not 140 mg isoflavones, although the authors questioned the biological significance of these methylation changes. The study also showed no changes in breast cytology and an inverse correlation between serum levels of the estrogenic marker complement (C)3 and the change in serum genistein (r = −0.76, p = 0.0045) in women consuming 40 mg, but not 140 mg isoflavones, suggesting an anti-estrogenic effect. Finally, there was also no change in nipple aspirate fluid C3 levels in response to isoflavone intake, indicating the lack of an estrogenic effect based on this one marker, which contrasts with the change Petrakis et al. (Citation1996) observed in nipple aspirate fluid in response to isoflavone-rich soy protein.
Observational studies (post-diagnosis intake)
Considerable evidence from observational studies supports the safety of soy consumption after a breast cancer diagnosis. The first such study was the Shanghai Breast Cancer Survival Study (SBCSS, N = 5033), which assessed dietary intake four times following a diagnosis of breast cancer (Shu et al. Citation2009). After an average follow up of 3.9 y, there were 444 deaths and 534 recurrences or breast cancer-related deaths. Mean isoflavone intake among all women was 45.9 mg/d. The hazard ratio (HR, 95% CI) for recurrence/breast cancer-specific mortality was 0.77 (0.60, 0.98) for women in the fourth isoflavone intake quartile (cutoff, > 62.7 mg/d; mean, 85.09 mg/d) compared to those in the first intake quartile (cutoff, < 20 mg/d; mean 11.5 mg/d). The HR was below 1.0 even at intakes approaching 25 g/d soy protein (Shu et al. Citation2009).
US observational studies have also examined soy isoflavone intake by breast cancer survivors, namely, two prospective studies published in 2009 (Guha et al. Citation2009) and 2011 (Caan et al. 2011) that were analyzed together with the SBCSS (Shu et al. Citation2009) in a pooled analysis that included 9514 breast cancer survivors (Nechuta et al. Citation2012). After an average of 7.4 y of follow up, there were 1171 deaths (881 from breast cancer) and 1348 recurrences. The HR (95% CI) for recurrence was 0.75 (0.61, 0.92) for all women in the highest vs the lowest isoflavone group with similar results among Chinese (HR, 0.69; 95% CI: 0.47, 1.01) and non-Asian US women (HR, 0.74; 95 CI: 0.56, 0.97) (Nechuta et al. Citation2012). Due to the relatively low isoflavone intakes of US women, the isoflavone intake cutoffs (mg/d) for the low, medium and high intake groups were <4.0, 4.0–9.99 and ≥10.0, respectively. This study also stratified the analysis by breast cancer subtype and menopausal status with results showing the HR (95% CI) for breast cancer recurrence as 0.81 (0.63, 1.01) for ER+, 0.64 (0.44, 0.94) for ER-, 0.93 (0.69, 1.26), for premenopausal women and 0.64 (0.48, 0.87) for postmenopausal women, all comparing the highest to the lowest isoflavone intakes.
As more observational studies that examined soy exposure in breast cancer survivors were published, Chi et al. (Citation2013) completed a meta-analysis that included the three studies in the aforementioned pooled analysis (Nechuta et al. Citation2012) plus two additional Chinese prospective studies (Kang et al. Citation2010; Zhang et al. Citation2012) to total 11,206 participants. The analysis found that the highest post-diagnosis soyfood intake was associated with decreased breast cancer recurrence (HR, 0.74; 95% CI: 0.64, 0.85) and mortality (HR, 0.84; 95% CI: 0.71, 0.99) when compared with the lowest post-diagnosis soyfood intake. This analysis, which also stratified by breast cancer subtype and menopausal status, found that when comparing highest versus lowest soyfood intake, the risk [HR + (95% CI)] for breast cancer recurrence was 0.81 (0.63, 1.04) for ER+, 0.64 (0.44, 0.94) for ER-, 0.91 (0.72, 1.14) for premenopausal women and 0.67 (0.56, 0.80) for postmenopausal women.
In 2019, a meta-analysis by Qiu and Jiang (Citation2019) included a focus on post-diagnosis soy intake by summarizing the pooled analysis of the three studies by Nechuta et al. (Citation2012) and an additional study by Zhang et al. (Citation2017) that involved an international Breast Cancer Family Registry with six sites from the United States, Canada, and Australia. Results showed when comparing highest versus lowest soyfoods intake, the HR (95% CI) for overall survival was 0.80 (0.62, 1.04) based on all four studies, and for breast cancer specific survival it was 0.83 (0.64, 1.07), based on the three studies from the Nechuta pooled analysis (Nechuta et al. Citation2012). More recently, when Micek et al. (Citation2021) examined the impact of isoflavone intake on breast cancer recurrence, they found the overall HR (95% CI) was 0.73 (0.64, 0.84), and ranged from 0.66 to 0.91 for the four subpopulations (pre- and postmenopausal, ER + and ER−) analyzed, but of those four, only the HR for postmenopausal women was statistically significant.
Finally, a recent prospective case-control study went beyond dietary intake to examine circulating levels of isoflavones in breast cancer survivors. The MARIE (Mamma Carcinoma Risk factor InvEstigation) was conducted in two German study regions, the Free and Hanseatic City of Hamburg (HH) and the Rhine–Neckar–Karlsruhe (RNK) region (Jaskulski et al. Citation2019). The study related baseline circulating isoflavones from 1,686 breast cancer survivors followed up for a median of 5.3 y at which time 142 (8.4%) women had died, 73 (51.4%) of which was due to breast cancer. Neither circulating genistein and daidzein was related to risk of overall or breast cancer specific mortality, but genistein (not daidzein) was positively related to risk of recurrence (HR 1.17, 95% CI: 1.01, 1.36, p = 0.04). It should be noted however that the mean genistein concentration was only 10 ng/ml (∼37 nmol/l), indicating extremely low isoflavone intake and raising doubt about the relevance of the findings. Parenthetically, circulating resveratrol was related to risk of recurrence (HR, 1.19; 95% CI: 1.02, 1.40, p = 0.03) and circulating luteolin to breast cancer specific mortality (HR, 1.96; 95% CI: 1.07, 3.58, p = 0.03).
Conclusions
In summary, the safety of soy intake related to breast cancer stems from concern that the phytoestrogenic isoflavones would exacerbate an estrogen-dependent tumor, a hypothesis confirmed in several animal model studies (Setchell, Brown, and Lydeking-Olsen Citation2002; Ju et al. Citation2001; Ju et al. Citation2006; Santell, Kieu, and Helferich Citation2000; Ju et al. Citation2002; Du et al. Citation2012; Ju et al. Citation2008; Allred et al. Citation2001; Allred et al. Citation2004; Zhang et al. Citation2017), although the human representativeness of these models has been questioned (Onoda et al. Citation2011). Human studies focused on biological markers of breast health have found no significant effects of soy isoflavones on mammographic density (Hooper et al. Citation2010; Wu et al. Citation2015; Labos et al. Citation2013) or breast cell proliferation (Khan et al. 2012; Hargreaves et al. Citation1999; Sartippour et al. Citation2004; Palomares et al. Citation2004; Cheng et al. Citation2007; Shike et al. Citation2014).
Exploratory studies have examined effects of soy on breast cancer-related DNA methylation with one finding no effect of soymilk on the human mammary gland epigenome (Coussement et al. Citation2018) and another finding positive associations between serum genistein and hypermethylated breast cancer related genes following isoflavone supplements, yet no changes in breast cytology and suggestions of an anti-estrogenic effect were noted. More direct, yet not causal, evidence from observational studies indicates that post-diagnosis soy intake is safe for breast cancer survivors with consumption associated with decreased risk of breast cancer recurrence and mortality (Nechuta et al. Citation2012; Chi et al. Citation2013; Qiu and Jiang Citation2019). It is important to again emphasize that there have not been any randomized clinical trials that have examined the effects of soy on recurrence or mortality of cancer survivors and therefore, although national and international organizations advise that breast cancer survivors can safely consume soy (Rock et al. Citation2012; American Institute for Cancer Research 2021; World Cancer Research Fund International Citation2014; Eating Well After Breast Cancer 2021), it is prudent to remain conservative and support continued research in this area.
Endometrium
Concern about the susceptibility of the endometrium to isoflavones is logical given their classification as phytoestrogens. The publication of several case reports supporting this concern emphasize the importance of evaluating the results from clinical studies. Regarding the former, Chandrareddy et al. (Citation2008) described three case reports involving an assortment of uterine/endometrial abnormalities, including leiomyoma, severe dysmenorrhea, uterine bleeding, and endometriosis, that were ascribed to excessive soy intake. In all three cases symptoms resolved upon discontinuation of soy consumption. One case involved a 56 y old women who reported an “… unusually high intake of soy milk, equivalent to 40 g of soy isoflavones every day for the last 3 years for control of her climacteric symptoms.” It should be noted, however, that it is not possible to consume gram quantities of isoflavones from soymilk. Another case was a 43 y old women who reported “… regularly consuming excessive amounts of soy (soy milk, tofu and baloney) as a health supplement over the last 5 years, every day.” Isoflavone intake was not reported. A third case was a 35 y old women who reported “… an extremely high intake of soy in various forms (milk, tofu, soy granules and soy protein concentrate) since the age of 14 years, every day.” Isoflavone intake was again not reported. It was noted that withholding soy intake resulted in a dramatic improvement in her symptoms and a subsequent pregnancy. Finally, there is a case report of a woman diagnosed with grade 1 endometrioid adenocarcinoma of the endometrium whose history was notable for extensive use of supplemental phytoestrogens although none were actually derived from soy (Johnson et al. Citation2001). These included dong quai, vitex berry, black cohosh root, licorice root and motherwort plus 24 other supplements. Given that these case reports are of individuals, there is a need to examine the role of soy in endometrial health more rigorously. The impact of soy on the endometrium has been studied regarding both endometriosis and endometrial cancer.
Endometriosis
Endometriosis is a common, benign gynecologic condition characterized by the presence of endometrial-like lesions in areas outside of the uterus. It affects approximately 10% of reproductive-age women and 20% of infertile women (Eskenazi and Warner Citation1997). In the US, endometriosis is the third leading cause of gynecologic hospitalizations after pelvic inflammatory disease and benign ovarian cysts (Velebil et al. Citation1995). The most well-accepted theory to explain the development and progression of peritoneal endometriotic lesions is retrograde menstruation (Halme et al. Citation1984). However, the fact that most women experience retrograde menstruation but only a small minority develop endometriosis indicates that other factors are involved in the pathogenesis of this disease.
Diet is one of the factors that has been identified as being involved in the pathology of endometriosis. For example, data from the Nurse’s Health Study II found red meat (more than >2 servings/d) was associated with a 56% increased risk of endometriosis (when compared with ≤1 serving red meat/week) (Yamamoto et al. Citation2018). Soy is another dietary factor that has been studied in relation to endometriosis. Some evidence (Miyazawa Citation1976; Arumugam and Templeton Citation1992; Sangi-Haghpeykar and Poindexter Citation1995), but not all (Missmer et al. Citation2004), indicates that Asian women have higher rates of endometriosis than Caucasian women.
Furthermore, use of SIF has been linked with increased risk of endometriosis. A US case-control study (n = 310 cases, n = 727 controls) found women who were regularly fed SIF as infants had more than twice the risk of endometriosis compared those that were not fed SIF (OR, 2.4; 95% CI: 1.2, 4.9) (Upson et al. Citation2015). In agreement, African-American women ever-fed SIF as infants were more likely to report ever use of hormonal contraception for menstrual pain (relative risk (RR), 1.4; 95% CI: 1.1, 1.9) and moderate/severe menstrual discomfort/pain with 'most periods', but not 'every period', during early adulthood (ages 18–22 when not using hormonal contraception) than those who were not fed SIF (Upson et al. Citation2019). However, another study that focused on moderate-severe menstrual pain (a common symptom of endometriosis) found that SIF use was not significantly associated with ever or current use of any medication for menstrual pain (DiVasta et al. Citation2018).an
Adult consumption of isoflavones has also been studied in relation to endometriosis. For example, a case-report which described a 75 y old women who had been taking supplements providing 75 mg/d isoflavones for five years concluded that there is the possibility that “…phytoestrogens at least in concentrated form may play a role not only in maintenance of endometriosis but also in its malignant transformation.” (Noel et al. Citation2006) However, in contrast to this case report and the aforementioned SIF studies, other studies do not provide support for a role of soy consumption in the etiology of endometriosis. One US study found that although rates of endometriosis were higher among Asian relative to non-Asian women, the connection to soy consumption was not clear since the comparison was true for Asian women of Filipino, Indian, Japanese and Korean origin, despite the fact that soyfoods are consumed by only the latter two groups (Yamamoto et al. Citation2017). Another cross-sectional study, this time focused on Japanese women, reported inverse associations between urinary genistein and daidzein and advanced endometriosis (genistein OR, 0.21; 95% CI: 0.06, 0.76 and daidzein OR, 0.29; 95% CI: 0.08, 1.03) as well as severity of endometriosis (p for trend = 0.01 for genistein and 0.07 for daidzein) (Tsuchiya et al. Citation2007). More recently, isoflavone intake was inversely related to endometriosis risk in a small Iranian case-control study, although the low intakes of this population question the utility of the results (Youseflu et al. Citation2020).
Finally, there is a Japanese prospective study involving 1,172 female participants of the Takayama Study who were premenopausal at enrollment (Nagata et al. Citation2001). During the six year follow up period, 31 women underwent premenopausal hysterectomies; since endometriosis is a prominent reason for hysterectomy (Treloar et al. Citation1999), its relation to isoflavone intake was examined. Results showed that the second isoflavone intake tertile (median, 32.8 mg/d) was significantly associated with a decreased risk of premenopausal hysterectomy compared to the lowest intake tertile (median, 20.4 mg/d) after controlling for age and total energy (rate ratio, 0.35, 95% CI: 0.13, 0.97), although the dose-response relationship was not statistically significant.
The conflicting evidence for the role of soy in endometriosis is also seen in rodent studies with some studies having implicated early soy exposure as a causative factor in experimental endometriosis (Mvondo et al. Citation2019) whereas other rodent models have shown isoflavones to have a protective role (Yavuz et al. Citation2007; Takaoka et al. Citation2018; Sutrisno et al. Citation2018). Overall, the human and animal work indicates conflicting results for the role of soy in the etiology of endometriosis. Since it is clear that endometriosis is estrogen-dependent (Anderson Citation2019) continued research in the role of soy isoflavones is warranted.
Endometrial cancer
Endometrial cancer (cancer of the corpus uteri) represents the most common gynecological malignancy in the industrialized world and is the seventh most common cancer among females, although incidence and mortality rates vary markedly among geographical regions and countries (Pisani, Bray, and Parkin Citation2002). The highest rates are in the US and Europe and the lowest in Asia and Africa (Parkin, Pisani, and Ferlay Citation1999). Migration data suggest that the international variation in endometrial cancer rates is due to environmental (lifestyle), not genetic factors (Liao et al. Citation2003). It is clear that estrogen contributes to the etiology of endometrial cancer. Ever-users of unopposed estrogen therapy are about two to three times more likely to develop endometrial cancer as never-users (Weiderpass et al. Citation1999; North American Menopause Society Citation2003; Grady et al. Citation1995). Consequently, there is concern that isoflavone-containing foods could increase risk of developing endometrial cancer and stimulate the growth of existing endometrial tumors.
Epidemiology
Several epidemiological studies have examined the role of soy isoflavones in endometrial cancer. These data were summarized in a meta-analysis of 10 observational studies (eight case-control, two prospective) that found soy intake was inversely associated with endometrial cancer risk with an overall risk estimate (RE) of 0.81 (95% CI: 0.72, 0.91) (Zhang et al. Citation2015). Although the cohort (RE, 0.80; 95% CI: 0.51, 1.26) and case-control (RE, 0.83; 95% CI: 0.73, 0.94) studies produced similar REs, only the latter was statistically significant. Subgroup analyses found statistically significant protective effects for both Asian (n = 3, RE, 0.79; 95% CI: 0.66, 0.95) and non-Asian (n = 7, RE, 0.83; 95% CI: 0.71, 0.96) populations. Of the three Asian studies, the Japanese case-control study evaluated only tofu intake (Hirose et al. Citation1996), the Chinese case-control study estimated total isoflavone intake (Xu et al. Citation2008) and the Japanese prospective study reported total and individual soyfood intake and intake of isoflavones (Budhathoki et al. Citation2015).
Clinical studies
The impact of soy isoflavone intake and endometrial cancer risk has also been examined in clinical studies that have used high-resolution transvaginal ultrasound (TVU) to measure endometrial thickness and screen for endometrial cancer risk (Smith-Bindman, Weiss, and Feldstein Citation2004). In general, the thickness of the menopausal endometrium is normally ≤5 mm, which is consistent with atrophy (Nalaboff et al. Citation2001). After menopause, increased endometrial thickness may indicate proliferative endometrium (Wolfman et al. Citation2010) and increased endometrial thickness even without bleeding is predictive of endometrial cancer. The relation of endometrial thickness to endometrial cancer was shown in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial that completed TVU in 1,018 postmenopausal women (55–74 y) and found that those with endometrial thickness ≥5.0 mm had a 5-fold increased risk of endometrial carcinoma (RR, 5.02; 95% CI: 0.96, 26.36) in models adjusted for menopausal hormone use and BMI when compared to women with baseline endometrial thickness of 1.0–2.99 mm (Felix et al. Citation2014). During the 12.5 y (range: 0.3–13.8 y) follow-up period, however, only 14 women developed endometrial carcinoma. More recently, a systematic review found that the risk of endometrial cancer/endometrial hyperplasia was 2.6 times greater in women with endometrial thickness ≥11 mm vs women with endometrial thickness 5–10 mm, although there was significant heterogeneity in estimates across studies (Alcázar et al. Citation2018).
In 2015, EFSA reviewed 25 clinical studies (n = 1484) that examined the effect of soy isoflavones on endometrial thickness (EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) 2015); no studies of isoflavones and endometrial cancer were identified. Results showed no statistically significant changes in endometrial thickness in any of the studies which included the use of food supplements containing soy isoflavones/soy extract (60–120 mg isoflavones/d, 3–36 months), soy protein (90–120 mg isoflavones/d, 4–36 months), daidzein-rich isoflavones (80–120 mg isoflavones/d, 24 months), glycitein-rich isoflavones (114 mg isoflavones/d, 3 months), genistein (30–54 mg/d, 3–36 months) and red clover extract (40–120 mg isoflavones/d, 12–36 months). A two-year trial not included in the EFSA review also found no effect on endometrial thickness; in this case postmenopausal women were randomized to receive either a placebo or 54 mg/d genistein (Atteritano et al. 2007). A two-year study published after the EFSA review also found no change in endometrial thickness in premenopausal women in response to 80 mg/d isoflavones derived from red clover (Ferraris et al. Citation2020).
EFSA also reviewed 9 studies (n = 677) that examined the effect of isoflavones on histo(patho)logical changes in the endometrium. These studies intervened with a variety of isoflavone-containing products including soy isoflavones/soy extract (60–120 mg/d, 3–6 months), soy protein (65–154 mg isoflavones/d, 3–36 months), daidzein-rich isoflavones (80–120 mg/d, 24 months), glycitein-rich isoflavones (114 mg/d isoflavones/d, 3 months) and red clover extract (50 mg/d isoflavones, 3 months duration). Results were again insignificant with only one study, which is discussed below, reporting significant histo(patho)logical changes in the uterus (Unfer et al. Citation2004). EFSA concluded that based on the human and animal studies, isoflavone exposure does not cause adverse effects on the uterus in postmenopausal women when taken in the doses and for the durations examined.
EFSA’s conclusion is consistent with a recent meta-analysis of clinical studies which found that when all 23 studies (n = 2,167) were included in the analysis there was no effect of isoflavones on endometrial thickness whereas when only the seven North American studies (n = 726) were analyzed, there was a statistically significant decrease in endometrial thickness (Liu et al. Citation2016). Thus, among North American women, the clinical data suggest that soy consumption may reduce risk of developing endometrial cancer. On the other hand, when the three Asian studies were analyzed, there was a small increase in endometrial thickness, although none of these studies intervened with isoflavones derived from soybeans. One of the studies in the meta-analysis, which was not cited in the EFSA review, is notable for its size (N = 224) and duration (3 y) (Quaas et al. Citation2013). This study randomized US postmenopausal women to consume either 25 g/d soy protein containing 91 mg isoflavones or 25 g/d milk protein and found that the rate of endometrial hyperplasia/malignancy was lower in the soy group (0% vs 14.3%); however, the difference was not statistically significant.
The only clinical study to find a significant effect of isoflavones on endometrial histo(patho)logical change is also notable for its size (N = 319) and duration (5 y) (Unfer et al. Citation2004). This Italian study conducted by Unfer et al. (Citation2004), randomized postmenopausal women to consume either a placebo or an isoflavone supplement (150 mg/d, unclear if value represents aglycone equivalent or glycoside weight) and examined endometrial histology from biopsies at baseline, 30 months, and 5 y after treatment. Results showed no cases of endometrial cancer over the 5 y period and no effects on endometrial tissue at 30 months; however, at 5 y, there were 5 women (3.2%) in the isoflavone group who developed simple hyperplasia and 1 (0.6%) who developed complex hyperplasia whereas none of the women in the placebo group developed hyperplasia. Simple endometrial hyperplasia is reversible and rarely progresses to endometrial cancer (Münstedt et al. Citation2004; Kurman, Kaminski, and Norris Citation1985).
The study by Unfer et al. (Citation2004), had several notable weaknesses. For example, as noted by Foth and Nawroth (Citation2005), women with inaccessible endometrium samples at baseline (∼25% of all participants) were not excluded for evaluation at future time points. Thus, it is unclear whether the endometrial hyperplasia found in the isoflavone group was also present at baseline, questioning whether it occurred because of isoflavone exposure. This point was also noted by the EFSA (EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) 2015) and the US National Toxicology Program (National Toxicology Program UDoHaHS, Center for the Evaluation of Risks to Human Reproduction Citation2006) in their evaluations of the safety of isoflavones. The study is also limited in its lack of information on endometrial thickness or bleeding patterns and lack of a biological measure (i.e. urinary or plasma isoflavones) or subjective (i.e. asking participants) of compliance to the isoflavone supplement. Finally, although not a design weakness, the results of this study warrant cautious interpretation since should even a few cases of endometrial hyperplasia in the placebo group have occurred, which would not have been unexpected, the differences between groups would likely not have been statistically significant. For comparison, in the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial, 1.6% of the women in the placebo group developed hyperplasia over a three-year period (The Writing Group for the PEPI Trial Citation1996). It is logical to speculate that this percentage would have been higher at 5 y.
Isoflavones have also been studied for their ability to inhibit the effects of agents, such as estrogen, that cause endometrial tissue proliferation. For example, a study by Bitto et al. (Bitto et al. Citation2010), found that 54 mg/d genistein consumed for 6 months by premenopausal women (n = 19) with non-atypical endometrial hyperplasia improved symptoms to approximately the same degree as the drug norethisterone acetate (n = 19), leading the authors to conclude that genistein might be useful for the management of endometrial hyperplasia in women who cannot be treated with progestin (Bitto et al. Citation2010).
Another study showed that SPI was able to attenuate the effect of estradiol on increasing endometrial thickness over 6 months. This study randomized 39 postmenopausal women to daily treatment of either estradiol at 2 doses (0.5 mg estradiol + placebo or 1.0 mg estradiol + placebo) or estradiol at the same 2 doses but combined with SPI (0.5 mg estradiol + 25 g ISP providing 120 mg isoflavones, or 1.0 mg estradiol + 25 g ISP with 120 mg isoflavones). Endometrial thickness increases over the six months were from 3.6 to 12.0 mm and from 3.6 to 11.6 mm in the 0.5 mg and 1.0 mg estradiol and placebo groups, respectively. This increase was attenuated, specifically from 3.0 to 6.8 mm and 3.0 to 7.1 mg in the 0.5 mg and 1.0 mg estradiol and SPI groups, respectively (comparison among all groups, p = 0.08) (Murray et al. Citation2003).
Finally, in a third study, Unfer et al. (Citation2004), which was previously cited, found that co-administration of isoflavones and CC reversed the deleterious effects of CC on endometrial thickness and contributed to higher pregnancy rates than CC alone. The 134 women in this study were aged 25–35 y, infertile for at least two years and had oligomenorrhea or amenorrhea associated with a positive menstrual response to the intramuscular progesterone-challenge test. In an accompanying editorial, Casper (Citation2004) attributed the likely mechanism of isoflavones to their ability to displace CC from ERs. However, a pharmacologic dose of isoflavones was used in this study.
Conclusions
Soy isoflavones have been studied in relation to endometrial health through their effects on endometriosis and endometrial cancer risk. Rationale for concern comes from the role of estrogen in the etiology of these conditions and adverse effects of excessively high intakes of soy isoflavones reported from individual case reports (Chandrareddy et al. Citation2008; Johnson et al. Citation2001; Noel et al. Citation2006). Building upon the higher rates of endometriosis among Asian women relative to Western women (Miyazawa Citation1976; Arumugam and Templeton Citation1992; Sangi-Haghpeykar and Poindexter Citation1995), some studies have suggested an increased risk of endometriosis with early soy exposure (SIF) (Upson et al. Citation2015; Upson et al. Citation2019). In contrast, studies focused on adult exposure have raised doubt about the connection between soy and endometriosis (Yamamoto Citation2017 #23084) or even found a protective association (Tsuchiya et al. Citation2007). With respect to endometrial cancer risk, results seem more consistent for a protective or null role for soy isoflavones with meta-analysis finding an inverse association between soy isoflavones and endometrial cancer risk (Zhang et al. Citation2015) and a review of clinical studies showing no adverse effects of soy isoflavones on endometrial thickness or histo(patho)logy (EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) 2015), although these effects may be ethnicity-specific (Liu et al. Citation2016). In addition to individual effects on the endometrium, there is evidence that soy isoflavones can attenuate the proliferative effects of estrogen on the endometrium (Unfer et al. Citation2004; Murray et al. Citation2003). The epidemiological and clinical studies do not corroborate the case reports and instead point to a protective or null effect with consideration required for core factors like estrogen exposure, timing of soy exposure and ethnicity.
Uterine fibroids
Uterine fibroids (UF), also called leiomyomata, are benign, hormonally-dependent tumors that grow in the walls of the uterus, and are detected in 70–80% of women by age 50 y (Taylor and Leppert Citation2012). UF are responsible for causing symptoms such as heavy and irregular menstrual periods, infertility, and spontaneous abortions and are listed as a leading risk factor for hysterectomy (Harding et al. Citation2008; Merrill Citation2008).
UF tend to shrink during menopause and often become asymptomatic, thus most such patients require no treatment (Segars et al. Citation2014; Sener et al. Citation1996). Specific therapy is reserved only for large fibroids or leiomyomas that continue to grow after menopause and, in this age group, usually result in total hysterectomy. Estrogen-containing contraceptives and menopausal hormone therapy have been associated with an increased risk of fibroids (Sabry and Al-Hendy Citation2012; Templeman et al. Citation2009; Pavone et al. Citation2018; Stewart et al. Citation2017; Wise and Laughlin-Tommaso Citation2016). However, Moro et al. (Citation2019) concluded that progestin, rather than estrogen component of menopausal hormone therapy, may be the more important risk factor.
Recently, Qin et al. (Citation2019) meta-analyzed the epidemiologic studies that examined both infant (n = 4) and adult (n = 7) soy/isoflavone exposure and risk of UF among premenopausal women. The infant studies, all of which were conducted in the US, included 2,908 cases. There was a non-statistically significant positive association between SIF consumption and risk of UF (OR, 1.19; 95% CI, 0.99-1.43; p = 0.06).
With respect to adults, there were a total of 3,136 cases; of the seven studies, five were case-control and one each was a cohort and cross-sectional study. Exposure in five studies was based on the ingestion of soyfoods whereas in two it was based on urinary isoflavone excretion. When all seven studies were considered in the analysis, there was a positive, but non-statistically significant relationship between soy exposure and UF risk (OR, 1.92; 95% CI: 0.92, 4.03; p = 0.08). However, the results showed high heterogeneity with an I2 of 94.3%.
Of the seven studies, three involved non-Asian, low-soy intake populations. When the three studies involving non-Asians were analyzed separately by Qin et al. (Citation2019), there was no relationship between exposure and risk (OR, 0.99; 95% CI: 0.78, 1.26; p = 0.94). In contrast, among the four Asian studies involving 940 cases, three of which were from China and one from Japan, there was a significant increased risk (OR, 2.50; 95% CI: 1.09, 5.74; p = 0.03). In addition, the dose-response analysis showed an accumulating positive association of soy isoflavone intake with the risk of UF. The pooled ORs (95% CIs) of UF risk for low vs occasional, moderate vs occasional, and high vs occasional soy isoflavone intake were 1.00 (0.87, 1.14), 1.08 (0.94, 1.24), and 1.23 (0.99, 1.53), respectively. However, in the high-consumption subgroup, the heterogeneity was high, with an I2 of 92.4%. Furthermore, in three of the four Asian studies, soy/isoflavone exposure was not comprehensively and/or not independently assessed.
In the study by Gao and Wang (Citation2018), frequent (four or more ≥ 4 times/wk) consumption of cow’s milk or soybean consumption was compared with infrequent (<4 times/wk) consumption. However, portion size was not considered and cow’s milk and soybean consumption were included in a combined category; these foods were not assessed independently (Gao and Wang Citation2018). In the study by He et al. (Citation2013), three soy intake categories were compared: low (never/less than one day per week), intermediate (two days per week) and high (more than three days per week) (He et al. Citation2013), but portion size again was not considered. In the study by Shen et al. (Citation2013), only the intake of soymilk was assessed and again without consideration of portion size; also, no definition of the intake categories (none, occasional and frequent) was provided. Only the Japanese study, which did not find a statistically significant association with isoflavones (high vs low isoflavone intake; OR, 1.82; 95% CI: 0.79, 4.17), comprehensively evaluated isoflavone intake (Nagata et al. Citation2009). Isoflavone intake categories (mean, mg/d) were low (21.2), medium (35.4) and high (61.1) (Nagata et al. Citation2009). Isoflavone intake in this study was almost certainly higher than in the Chinese studies given what is known about Chinese and Japanese soy intake (Messina, Nagata, and Wu Citation2006).
Another observational study that was previously discussed, did not include UF as an endpoint but still may be relevant to the discussion. The results of this Japanese prospective study involving 1,172 female participants of the Takayama Study who were aged 35–54 y and premenopausal at the time they were enrolled suggest that isoflavone intake is protective against the development of UF (Nagata et al. Citation2001). As noted previously, UF are a reason for hysterectomy (Treloar et al. Citation1999). The only clinical study identified that examined the impact of isoflavone exposure on UF was conducted by Steinberg et al. (Citation2011) In this two-year study involving 403 postmenopausal women who were randomized to consume either a placebo or 80 or 120 mg/d isoflavones, no effect on the development or growth of UF was observed. Two limitations of this study include the involvement of postmenopausal rather than premenopausal women and the use of isoflavones derived from soygerm, which is low in genistein.
Conclusions
Given the limited data and the limitations of the existing studies, it is not possible to reach conclusions about the impact of isoflavone exposure on the risk of developing UF. More research is warranted.
Research involving children
Puberty onset
The possible impact of soy on puberty onset is a relationship that has garnered attention in part because pubertal characteristics are occurring at an earlier age in children throughout the world as evidenced by a number of changes including the advance in the age at which menarche occurs (Euling et al. Citation2008; Biro et al. Citation2010; Junqueira Do Lago et al. Citation2003; Harris, Prior, and Koehoorn Citation2008; Hosokawa, Imazeki, and Mizunuma Citation2012; Cho et al. Citation2010; Morris et al. Citation2011; Cabanes et al. Citation2009; Herman-Giddens Citation2006; Himes Citation2006; Talma et al. Citation2013). In addition, children may absorb isoflavones to a greater extent than adults (Halm, Ashburn, and Franke Citation2007; Maskarinec et al. Citation2005). However, the trend toward earlier puberty onset is occurring in countries where soy is not a traditional part of the diet as well as in those where soy is (Messina et al. Citation2017). In comparison to girls, less evidence is available in support of a secular trend for earlier pubertal timing for boys although recently Ohlsson et al. (Citation2019) found that among Swedish boys born between 1947 and 1996, after adjusting for childhood BMI, age at peak height velocity was 1.2 months earlier per decade increase in birth year.
Among women, early menarche is associated with shorter stature, a relationship which has been shown in both high- and low-income countries (Kang et al. Citation2019; Onland-Moret et al. Citation2005). Earlier menarche has also been implicated in the etiology of breast and ovarian cancer so the trend toward earlier puberty is a health concern (Ruder et al. Citation2008; Barker et al. Citation2008) as well as a social concern (De Genna, Larkby, and Cornelius Citation2011; Graber Citation2013). On the other hand, older age at puberty may be associated with lower bone mineral density (Elhakeem et al. Citation2019). Many factors potentially contribute to the trend toward earlier puberty such as increasing adiposity (Biro et al. Citation2003) and xenoestrogen exposure (Wolff et al. Citation2010; Buck Louis et al. Citation2008). Diet may also play a role as epidemiological studies have found that both total protein and animal protein intake is associated with earlier menarche and earlier development pubertal characteristics (Rogers et al. Citation2010; Günther et al. Citation2010).
There has been only limited investigation of the effects of soy on pubertal development. This issue received some attention as a result of a case report from Brazil describing a nearly 5-y-old girl who presented with premature thelarche that was attributed to an intake of ∼40 mg/d isoflavones from soyfoods (Fortes et al. Citation2007). Blood isoflavone levels were not measured, however. The parents were advised to limit their daughter’s soy consumption to once per week. While this case report is certainly intriguing, its informative value is limited because no follow up examination is described until the patient is almost nine years of age, when it is said that the patient was at the same stage of pubertal development as her peers and maintained the growth rate and bone development compatible with chronological age.
More relevant are two Korean case-control studies which found urinary isoflavones in girls with precocious puberty were higher than in children serving as controls (Kim et al. Citation2011; Yum, Lee, and Kim Citation2013). In one, 108 girls (aged 8.6 ± 0.8 y) with central precocious puberty were compared with 91 age-matched controls (aged 8.5 ± 0.8 y) (Kim et al. Citation2011). Serum mean (± SD) total isoflavone concentrations (nmol/l) were significantly greater in the central precocious puberty group than in the control children (77.9 ± 57.2 vs 62.9 ± 40.2; p = 0.0009). However, the actual difference in means between groups was relatively small, especially considering the large standard deviations, which indicates there was considerable overlap. In addition to the differing means, the prevalence of precocious puberty was significantly higher in children with a serum isoflavone level ≥30 nmol/l than it was for those with a serum level <30 nmol/l (p = 0.0008). However, this cutoff was arbitrarily chosen by the researchers after the data had been tabulated. Also, when assessing the risk between central precocious puberty and serum isoflavones the only factors adjusted for were age, height, and body weight. Finally, neither estradiol nor peak FSH levels differed between normal girls and girls with precocious puberty although peak LH was higher in the latter group.
The other Korean study involved 150 girls (age, 8.91 ± 1.40 y) with precocious puberty and 90 control subjects (age, 8.50 ± 1.68 y) (Yum, Lee, and Kim Citation2013). The plasma genistein concentration (ng/ml) was significantly higher in the girls with precocious puberty compared with the control girls (8.12 ± 12.71 vs 3.04 ± 4.21, p = 0.0008), but there were no differences between groups for daidzein or the daidzein metabolite equol (Setchell and Clerici Citation2010). Further, it appears that no statistical adjustments were made for potentially confounding variables when comparing groups. It is noteworthy that the serum isoflavone levels among the children in this study (Kim et al. Citation2011) and the previous one (Yum, Lee, and Kim Citation2013) were very low relative to older Japanese adults, and much lower than infants consuming SIF (Setchell et al. Citation1997).
In Korea, there has been an ongoing trend for girls to begin menstruating at younger ages (Cho et al. Citation2010). One analysis identified a host of factors that appear to influence age of menses (AOM) onset in Korean girls (Cho et al. Citation2010). These included maternal menarcheal age, BMI, maternal age at birth, and diet. Importantly, since there is no evidence that soy consumption has increased over the past several decades in Korea, it would appear unlikely that soy intake is contributing to the trend in the AOM (Kim, Moon, and Popkin Citation2000).
In contrast to the two Korean studies (Kim et al. Citation2011; Yum, Lee, and Kim Citation2013), a study with a stronger prospective design involving 1,239 US girls aged 6-8 at enrollment who were followed for seven years found no relationship between pubertal development and urinary isoflavone excretion (Wolff et al. Citation2015). Another US study involving 192 healthy 9-year-old girls residing in New York City, but which utilized a cross-sectional design, found isoflavone exposure was associated with delayed breast development (Wolff et al. Citation2008). This finding agrees with the results of a German longitudinal study involving 227 girls (Cheng et al. Citation2010). Girls whose diet was in the highest dietary isoflavone intake tertile experienced Tanner stage 2 for breast development ∼0.7 y later and reached peak height velocity ∼0.6 y later than did girls whose diet was in the lowest isoflavone tertile adjusted for BMI z score and fiber intake. In boys, dietary isoflavones were not associated with pubertal markers. Finally, urinary isoflavone levels were not associated with pubertal markers in girls or boys. However, because of the low isoflavone intake of these Western populations (Wolff et al. Citation2008, Citation2015; Cheng et al. Citation2010), their informative value is limited.
One US cross-sectional study that avoids the limitations of the Western studies cited above involved Seventh-day Adventist (SDA) girls (N = 327; age range 12 to 18; mean age, 15 y) (Segovia-Siapco et al. Citation2014). Since ∼40% of SDAs practice some form of vegetarianism (Orlich et al. Citation2013), their soy consumption is much higher than the general US population (Rizzo et al. Citation2013). For this study, current soy intake was used as a surrogate for intake prior to menses onset, an approach used by other researchers (Cutler et al. Citation2009).
The mean number of servings of soyfoods among the adolescent girls was 12.9 per week and 21.1% of the girls consumed soyfoods ≥4x/d. The mean AOM of all girls in the study was 12.5 y. The consumption of total soyfoods (p = 0.77) and the intake of three specific types of soyfoods (meat alternatives, tofu/traditional soy, and soy beverages; p values = 0.838, 0.401, 0.759, respectively) was not significantly associated with AOM nor was total soy intake significantly associated with the odds of early (<12 y of age) or late (≥14 y of age) AOM (Segovia-Siapco et al. Citation2014).
A similarly designed study involving SDA boys was published four years later (Segovia-Siapco et al. Citation2018). In this case, the measure of puberty onset was the first onset of pubic hair (Tanner stage 2). Among the 248 boys aged 12-18 attending SDA schools, moderate and high total soy isoflavone intake was significantly associated with earlier adjusted median age at pubarche: 12.58 y [RR (95% CI): 1.58 (1.06, 2.36)] for moderate and 12.50 y [RR (95% CI): 1.63 (1.03, 2.60)] for high vs. 13.00 years for low soy consumers. However, no significant associations were noted between isoflavone intake and facial hair onset, which was used as a secondary measure of puberty onset. Also, it is notable that even among high-soy-consuming boys, puberty onset was much later than is typical for US boys (Herman-Giddens et al. Citation2012). To this point, Herman-Giddens et al. (Citation2012) reported that among 4,131 boys, puberty onset (Tanner stage 2) was 11.47, 10.25, and 11.43 y for non-Hispanic white, African American, and Hispanic boys, respectively. In these two cross-sectional analyses involving SDAs, soy protein contributed about 15% (∼10 g/day) of total protein intake. More specifically, among the 299 girls and 231 boys, mean (SD) soy protein was 10.8 ± 9.9 and 9.9 ± 10.1 g/d, respectively (Segovia-Siapco et al. Citation2019).
Indirectly arguing against soy intake advancing puberty onset in girls are animal (Lamartiniere et al. Citation1995; Lamartiniere, Zhao, and Fritz Citation2000; Peng et al. Citation2010) and observational (Shu et al. Citation2001; Wu et al. Citation2009; Korde et al. 2009; Baglia et al. Citation2016) data indicating soy consumption during childhood and/or adolescence reduces breast cancer risk later in life. As noted previously, earlier menarche is associated with an increased risk of breast cancer (Ruder et al. Citation2008; Barker et al. Citation2008), although not all data are supportive of this relationship (Schoemaker et al. Citation2017). Furthermore, an increased breast cancer risk may be more closely tied to earlier thelarche than to earlier AOM onset (Bodicoat et al. Citation2014). Isoflavone exposure early in life appears to cause breast cells in the developing breast to become permanently less likely to be transformed into cancer cells (Messina and Hilakivi-Clarke Citation2009). The effect of isoflavones may be similar to that of early pregnancy, which also reduces breast cancer risk (Russo et al. Citation2005). Proposed mechanisms for the protective effect of early isoflavone exposure include increasing cell differentiation (Russo et al. Citation2005; Brown et al. Citation2010) and increasing BRCA1 gene expression (de Assis et al. Citation2011) and ERβ levels (Rahal and Simmen Citation2011).
Finally, no relationship was identified in a prospective study between use of SIF and signs of early puberty involving 89 infants (29 SIF-fed, 60 controls) (Sinai et al. Citation2019). The use of soy products is common in young children with cow milk allergy (CMA). Infants who consumed only SIF were followed from birth until three years of age. Study participants were reevaluated between ages 7.8 and 10.5 y by an interview, nutritional intake by three-day food diaries, and height, weight, and pubertal signs by physical examination. The groups had comparable height and BMI z scores. No association was detected between puberty and infantile nutrition, after controlling for BMI and family data.
Conclusions
Current evidence suggests no clear relationship between a high habitual intake of soyfoods with advanced puberty onset. Although an early case-study raised concerns of precocious pubertal development linked to high soy isoflavone intake, subsequent cross-sectional, case-control, and prospective cohort studies have not shown a consistent association with early development in girls or boys. Other lines of evidence from observational studies and animal models linking soy to lower risk of breast cancer provide evidence that any potential effect is not associated with long-term harm. To address the uncertainties, more high-quality evidence is needed from prospective cohort studies that provide large ranges of soy exposure and randomized controlled trials of soy-based interventions in peri-pubertal children.
Hormones
Two clinical studies and one population-based study were identified that examined the impact of soy intake on hormone levels in children. The cross-sectional study of 230 Japanese boys and 198 Japanese girls aged 3-6 y, found that after adjusting for potential confounding, a higher soy intake was inversely related to urinary estrone (p for trend, 0.013) and estradiol (p for trend, 0.026) in boys and positively related to urinary testosterone (p for trend, 0.003) and androstenediol (p for trend, 0.027) in girls (Wada et al. Citation2011). Similar findings were reported for isoflavone intake. The highest (fourth quartile) median isoflavone intake in boys and girls was 26.5 and 24.0 mg/d, respectively.
In contrast to this cross-sectional study, no effects of isoflavone exposure were noted in the two clinical intervention studies, although both involved small numbers of participants. In one, Maskarinec et al. (Citation2005) enrolled 17 US girls aged 8–14 for eight weeks during which time they were instructed to consume one daily serving of soy (average isoflavone intake was ∼27 mg/d). They observed an increase in dehydroepiandrosterone but no change in levels of all other urinary hormones (5 androgens and 6 estrogens). The authors noted that in their study since sex steroid levels were associated with pubertal development based on Tanner stages, their results support the validity of the sex steroid measurements (Maskarinec et al. Citation2005).
The other intervention trial was a small 8-week randomized controlled trial with a cross-over design involving 12 hypercholesterolemic Israeli children (8 females) aged 5.3–11.2 y, who received in random order a placebo or 16 or 48 mg/d isoflavones in tablet form separated by a two-week washout period (Zung et al. Citation2010). Despite the high dose of isoflavones, there were no effects on serum levels of estradiol (measured in girls only), testosterone (measured in boys only), FSH, LH, TSH, free T4 or total T3 (Zung et al. Citation2010). Limitations of this study are the small sample size and that the data were not presented separately for boys and girls.
Data on thyroid hormones come from three studies including two observational studies and one intervention study. (Note that two of these studies were previously discussed in the section on thyroid hormones). In a study involving 139 girls and 129 boys aged 8–15 y from the Czech Republic, a very modest association was found between circulating isoflavone levels and measurements of thyroid function (Milerová et al. Citation2006). However, when children were divided into two groups (soyfood eaten within the past 24 h, yes or no), TSH and free T3 levels did not differ between groups, although free T4 levels were higher in the soyfood consumers. In the other observational study, which involved nearly 600 Iranian children ages 6–12 y, risk of goiter (thyroid hormones were not assessed) was unrelated to soy intake (Mousavi, Tavakoli, and Mardan Citation2006). Although the findings from these two epidemiologic studies are mentioned here, they are likely of limited utility because appreciable amounts of soy are not consumed in either the Czech Republic or Iran.
Finally, Helk and Widhalm (Citation2020) recently conducted a randomized controlled trial in pediatric age patients with heterozygous familial hypercholesterolemia. Children were assigned to consume a low saturated fat therapeutic diet (n = 13, 8.0 ± 3.46 y) or the same diet enriched with soy protein (n = 13, aged, 9.46 ± 4.05 y) for 13 weeks. Children in the soy group were instructed to consume 0.25 g/kg bw soy protein. In comparison to the therapeutic diet only group, low density lipoprotein-cholesterol was significantly reduced in the soy group. There were no clinically significant changes in thyroid hormones (TSH, T4 and T3) in any of the patients. Average isoflavone intake in the soy group was 0.341 mg/kg bw. In addition to this study, Mejia et al. (Mejia et al. Citation2019) examined the effects of a one-year soy protein intervention on bone formation markers, lipid profile and insulin-like growth factor-1 in Columbian children aged 2–9 y. No differences between groups were noted but the intervention provided only 7 g protein and minimal isoflavones (0.13 mg/kg bw/d). Because of the low intake, and the limited data on hormones, this study does not provide meaningful insight into the effects of isoflavones.
Conclusions
The available evidence does not support a meaningful relationship between soy intake and hormone levels in children. Although some weak evidence from cross-sectional studies have shown limited associations of soy intake with sex hormones and thyroid hormones, these have not been replicated in intervention studies of soy and soy isoflavones.
As the intervention studies have been small and of short duration, there remains a need for more high-quality evidence from prospective cohort studies that provide large ranges of soy exposure over long durations of follow up and randomized controlled trials of soy-based interventions. The difficulty of conducting such studies warrants mentions because differences in growth rates among similarly aged children will result in considerable variations in hormone levels which might mask any effects of an intervention, unless vary large numbers of participants are involved. Furthermore, in the case of intervention studies, compliance is also likely to be problematic in young people. Finally, better insight may be gained by focusing on more clinically relevant endpoints, such as puberty onset, rather than on hormone levels.
Fetal effects of maternal soy/isoflavone intake
In Asian countries, including Japan and China, soy is commonly consumed during pregnancy (Li et al. Citation2011; Miyake et al. Citation2005). Ishitsuka et al., (Citation2020) recently reported that among Japanese women, during pregnancy, pulse intake, which in Japan is represented primarily by soy products, is similar to the intake prior to pregnancy. Miyake et al. (Citation2005) reported that the genistein and daidzein intake (mean ± SD) of 1,002 pregnant Japanese women participating in the Japan Osaka Maternal and Child Health Study was 15.0 ± 10.1 mg/d and 9.0 ± 6.1 mg/d, respectively. These values are similar to the value of 21.7 ± 13.7 mg/d, which was the total isoflavone intake of 194 pregnant Japanese women reported by Nagata, Oba, and Shimizu (Citation2006) And also similar to the value of 26.4 mg/d, which was the quintile 3 mean intake among 84,948 Japanese singleton pregnant women (median gestational age 12 weeks) as recently reportedly by Dong et al. (Citation2021). For this particular study, dietary intake was recorded during the 12 months preceding study enrollment. However, although women may alter their diet because of vomiting and appetite change during early pregnancy, the food frequency questionnaire used for this study has been suggested as a useful instrument, regardless of nausea (Ogawa et al. Citation2017).
Despite the common practice among Asians of consuming soy while pregnant, concern has been raised that the resulting in utero isoflavone exposure could adversely impact the fetus (Yang et al. Citation2000; Shibayama et al. Citation2001). In some sense, this concern is predictable given the well-studied case of diethylstilbestrol (DES), a potent synthetic nonsteroidal estrogen that was taken by pregnant women from the 1940s to 1975 to prevent miscarriage and other complications (Hilakivi-Clarke, de Assis, and Warri Citation2013; Dieckmann et al. Citation1953), but which was eventually shown to cause harmful effects in the offspring (Herbst, Ulfelder, and Poskanzer Citation1971; Troisi et al. Citation2007; Herbst Citation1976; Troisi et al. Citation2013; Harris and Waring Citation2012; Titus-Ernstoff et al. Citation2008; Swan Citation2000). However, depending upon the assay, DES can be 1000-fold more estrogenic than genistein (Song, Hendrich, and Murphy Citation1999; Lewis et al. Citation2003).
Interest in the relationship between the in utero environment and chronic disease later in life was spurred by the work of Barker (Barker Citation1990, Citation1995), who in 1990 wrote that “The old model of adult degenerative disease was based on the interaction between genes and an adverse environment in adult life. The new model that is developing will include programming by the environment in fetal and infant life” (Barker Citation1990). Research has shown that prenatal exposure to famine is associated with increased adiposity (Stein et al. Citation2007), metabolic syndrome (Correia-Branco, Keating, and Martel Citation2015), hyperglycemia (Li et al. Citation2010) and hypertension (Stein et al. Citation2006) in adulthood.
In the early 2000s, rodent data led several researchers to express caution about in utero isoflavone exposure. For example, Shibayama et al. (Citation2001) concluded that “… it might be better for … pregnant women to refrain from ingesting large quantities of soy foods” and Yang et al. (Yang et al. Citation2000) concluded that “… the ingestion of soy during pregnancy … must be done with caution.” It is notable that these two research groups are from Japan.
Balakrishnan et al. (Citation2010) demonstrated in an ex-vivo human placental perfusion model that genistein can transfer across the human placenta at environmentally relevant concentrations. However, it was already known that maternal isoflavone exposure leads to quantifiable amounts of isoflavones in amniotic fluid. In 2002, after evaluating amniotic fluid isoflavone levels, Foster et al. (Foster et al. Citation2002) commented that “in utero exposure to dietary phyto-estrogens may be a risk factor for developmental abnormalities of the male reproductive tract.” Their concern was based on a British observational study (North and Golding Citation2000), which is discussed below, that found women who ate a vegetarian diet during pregnancy were more likely to have male infants affected by hypospadias than non-vegetarian women. However, after reviewing the literature, Tan, Zhao, and Wang (Citation2019) recently concluded that findings regarding the association between Asian women who consumed a vegetarian diet during pregnancy and hypospadias were inconclusive. The discussion below highlights studies that examined the relationship between soy intake during pregnancy and vegetarian diet during pregnancy and risk of hypospadias. Although vegetarian diet per se does not necessarily inform about the impact of soy, several of the studies reporting on vegetarianism also reported soy intake.
Hypospadias – background
Hypospadias is a common genitourinary anomaly in which the opening of the urethra is on the underside of the penis. Springer, van den Heijkant, and Baumann (Citation2016) concluded that hypospadias may be on the rise and cited prevalence rates ranging from 5 to 50/10,000 births with high rates in European and North America and low rates in China, Japan, South-East Asia, and South America. In the US, the mean prevalence rate is reported to be 34.2/10,000 (approximately 1 in 300 male children).
The etiology of hypospadias remains uncertain although some have argued that it is a component of a larger disorder referred to as testicular dysgenesis syndrome, which comprises a set of genitourinary malformations (including hypospadias, cryptorchidism, hypospermatogenesis, and testicular cancer) linked with genetic as well as environmental factors (Toppari et al. Citation2010; Main et al. Citation2010; Wohlfahrt-Veje, Main, and Skakkebaek Citation2009). Qiao et al. (Citation2012) concluded that aberrant estrogenic effects have a role in the etiology of hypospadia and Krysiak-Baltyn et al. (Krysiak-Baltyn et al. Citation2010) suggested that the higher rates of male reproductive disorders, including cryptorchidism and hypospadias in Denmark compared with Finland, might be due to the greater exposure to endocrine disrupting chemicals in the former. Other evidence implicates industrial chemical agents, some of which are classified as endocrine disruptors, as possible etiological factors in hypospadias (Nordenvall et al. Citation2014; Czeizel Citation1985; Paulozzi, Erickson, and Jackson Citation1997; Giordano et al. Citation2010; Nordkap et al. Citation2012; Botta, Cunha, and Baskin Citation2014).
One might expect that if isoflavone exposure increased risk of hypospadias, prevalence rates would be higher in Japan than in non-soyfood-consuming countries, but the opposite appears to be the case (Paulozzi Citation1999; Kurahashi et al. Citation2004). Furthermore, one report suggested Japanese rates had increased over a recent 20 y period (Paulozzi Citation1999), a time during which soy protein (and therefore isoflavone exposure) as a percentage of total protein intake decreased (Messina, Nagata, and Wu Citation2006). While noteworthy, since there are likely multiple factors involved in the etiology of hypospadias (Yiee and Baskin Citation2010), these types of epidemiologic observations are of only modest utility in addressing the concerns initial raised by the previously referenced British study (North and Golding Citation2000).
Regarding isoflavones, a review by Botta, Cunha, and Baskin (Citation2014) cited three rodent studies implicating genistein as a possible risk factor for hypospadias (Ross et al. Citation2011; Padilla-Banks et al. Citation2012; Vilela et al. Citation2007). However, there is reason for skepticism about the utility of animal models for studying this condition. More than 40 years ago, Robinson et al. (Robinson et al. Citation1977) highlighted the high concentrations of estrogen to which the human fetus is naturally exposed. And according to Witorsch (Citation2002), “… circulating levels of estrogen attained during pregnancy in the rat and mouse compared to human are vastly different, those of the latter far exceeding those of the former.” This difference in in utero estrogen concentration suggests that isoflavones are more likely to exert an estrogenic effect in utero in rodents compared to humans. Hollier et al. (Citation2014) commented that “Due to the many developmental differences between species (e.g., duration of pregnancy, maturity at birth, and susceptibility to a variety of environmental conditions), it is difficult to extrapolate animal model findings to human development.”
Observational data: soy/vegetarian diet and hypospadias
The previously cited British prospective study, which included 7928 boys born to mothers taking part in the Avon Longitudinal Study of Pregnancy and Childhood, was the first observational study to examine the relationship between maternal soy intake and risk of hypospadias (North and Golding Citation2000). Compared with omnivores who did not supplement their diet with iron, there was an approximate 5-fold increased risk (OR, 4.99; 95% CI: 2.10, 11.88) of hypospadias in sons of mothers who ate a vegetarian diet during pregnancy (North and Golding Citation2000). A total of 51 boys had hypospadias. These results agree with those of a case–control study by Akre et al. (Citation2008) that included 292 cases and 427 controls from Sweden and Denmark, which found that a diet during pregnancy lacking both fish and meat (4.8 and 1.9% of cases and controls, respectively, fell into this category) was associated with a more than 4-fold increased risk of hypospadias (OR, 4.6; 95% CI: 1.56, 13.07).
The study by Akre et al. (Citation2008) did not report soy intake but in the British study, mothers who drank soymilk [yes or no; OR, 3.67; 95% CI: 0.87, 15.44)] or who ate soy “meat” (≥1x/wk versus never; OR, 2.95; 95% CI: 0.90, 9.68) during pregnancy were about 3-fold more likely to give birth to boys with hypospadias (North and Golding Citation2000). However, these associations were not statistically significant; furthermore, ≤2% of the >6000 mothers enrolled in this study consumed soy during pregnancy (North and Golding Citation2000). Also, although the authors speculated that isoflavones might be responsible for the apparent association with soy intake, legume (dried peas, beans, lentils, and chick peas) intake was associated with a 7-fold increased risk of hypospadias (≥4x/wk versus never; OR, 7.56; 95% CI: 2.25, 25.42), despite non-soy legumes containing negligible amounts of isoflavones (Franke et al. Citation1998; Murphy et al. Citation1999). Soy meat analogs, which as noted previously are also typically low in isoflavones, were also associated with an increased risk (Murphy et al. Citation1999).
A Dutch case–control study nested within a cohort of 8,698 male births that included 313 controls and 78 and 56 cases of cryptorchidism (a condition in which one or both of the testes fail to descend from the abdomen into the scrotum) and hypospadias, respectively, found the ORs for cryptorchidism and hypospadias for the first (0 g/d), second (>0–20 g/d) and third (≥20 g/d) soy protein intake tertiles were 1.0, 1.1, and 0.6 and 1.0, and 1.1 and 1.0, respectively (Pierik et al. Citation2004). Thus, these results provide no evidence soy intake is linked to hypospadias. However, there is reason to question the findings. In this study, 16% of the controls fell into the third soy protein intake tertile, which had a cutoff of ≥20 g/d. This intake level, which is similar that found in Japan (Messina, Nagata, and Wu Citation2006), is inconsistent with several reports showing relatively little soy is consumed by the Dutch population (Boker et al. Citation2002; Kreijkamp-Kaspers et al. Citation2004).
Somewhat parenthetically, this Dutch study (Pierik et al. Citation2004) found no association between a vegetable-rich diet and risk of hypospadias, which concurs with the results of a Dutch study published three years later involving 583 cases and 251 controls; 10 of the mothers of cases and seven of the mothers of controls were vegetarian (Brouwers et al. Citation2007). Also in agreement, are the results of two case–control studies; one a British study involving 471 cases and 490 controls (Ormond et al. Citation2009) and the other, a US study (discussed below) involving 1250 cases and 3118 controls (Carmichael et al. Citation2012). In the former, there was no relationship between vegetarianism and hypospadias (OR, 0.85; 95% CI; 0.61, 1.19); of the total number of participants, 168 (17.5%) were classified as vegetarian or vegan. However, in a later report from this British cohort (Ormond et al. Citation2009), only about 3% of the participants were classified as vegetarian (de Kort, Nieuwenhuijsen, and Mendez Citation2011). This later analysis found that mothers consuming diets that were classified as ‘non-health conscious’ (low frequency of consumption of yogurt, cheese, eggs, fruit and vegetables, fish, beans and pulses, olive oil and organic food) had a higher risk of hypospadias (OR, 1.54; 95% CI: 1.06, 2.26) compared with ‘health conscious’ participants who frequently consumed fresh fruit and vegetables, dried fruit, fresh or frozen fish, beans, pulses, soy products, olive oil and organic food. Intakes of individual foods were not strongly associated with hypospadias. The percentage of participants consuming soy products ≥1x/mo in the health-conscious, mixed, and non-health-conscious categories was 32.9, 5.5 and 6.5, respectively (p < 0.0001) (de Kort, Nieuwenhuijsen, and Mendez Citation2011).
Two small Indian studies reported contrasting results regarding vegetarian diets and hypospadias as one (80 cases, 120 controls) (Shekharyadav et al. Citation2011) found no association whereas the other (101 cases, 110 controls) (Samtani et al. Citation2014) found a more than two-fold increased risk (OR 2.47; 95% CI: 1.4, 4.3). Ghosh et al. (Citation2016), who also studied this relationship in India, found a very high percentage of mothers with infants with hypospadias were vegetarians, but this work did not include a control group and no statistical model was used to evaluate the findings.
The most direct examination of a possible association between isoflavone intake and risk of hypospadias may be a nationwide birth cohort study, which recruited women as early in pregnancy as possible throughout Japan between 2011 and 2014 (Michikawa et al. Citation2019). Daily genistein intake (as the representative isoflavone) was estimated from the response to a self-administered food-frequency questionnaire. Information on cases that were diagnosed until the first month after birth was obtained from medical records. Among 41,578 mothers who delivered singleton live male births, 51 cases of hypospadias were identified, and the median genistein intake was 15.3 mg/d. Compared with mothers in the reference group (genistein intake 11th-89th percentiles), those in the low intake group (≤10th percentile) had an elevated risk of their sons having hypospadias (multivariable-adjusted OR, 2.8; 95% CI: 1.4, 5.8). Neither adverse nor beneficial effects of genistein on hypospadias were observed in the high intake group (≥90th percentile) (OR, 0.9, 95% CI: 0.4, 2.4). The authors concluded that low maternal isoflavone intake in early pregnancy was associated with an elevated risk of hypospadias. Low natto and tofu intake were each associated with about a 2-fold increased risk.
Finally, no relationship was found between meat intake and risk of hypospadias in the National Birth Defects Prevention Study, a US study which included 1250 cases with second- or third-degree hypospadias and 3118 male, liveborn, non-malformed controls (Carmichael et al. Citation2012). The authors concluded that their study “does not support an association of a vegetarian diet … with hypospadias.” This study did find that maternal intake of total phytoestrogens (isoflavones, lignans and coumestrol) was associated with a statistically significant 60% decrease in the risk of hypospadias although after adjustment for possible confounding factors (maternal age, parity, race/ethnicity, education, BMI, study center, folic acid-containing supplement intake, and energy intake) the association was no longer statistically significant (Carmichael et al. Citation2013). Similarly, isoflavone intake alone was associated with a statistically significant 60% decreased risk, but after adjustment, the 30% decreased risk was no longer significant. However, because the cutoff for the third isoflavone intake cutoff was ≥103.5 µg/d, the utility of this finding for informing about the health effects of isoflavones is doubtful.
Conclusions
Although limited, the existing evidence does not suggest that maternal isoflavone intake increases risk of hypospadias. Although a British study found a non-significant association with soy intake (North and Golding Citation2000), as highlighted there are reasons to question the findings. Furthermore, there was no association in three other non-Asian studies (Pierik et al. Citation2004; de Kort, Nieuwenhuijsen, and Mendez Citation2011; Carmichael et al. Citation2013), although the low soy intake of these populations limits the utility of the findings. More importantly, there was no association between isoflavone exposure and hypospadias in a Japanese study despite much higher maternal isoflavone intake (Michikawa et al. Citation2019). Also, rates of hypospadias are lower in Japan than in Europe and the US, despite Japanese women consuming soy during pregnancy (Springer et al. Citation2016; Paulozzi Citation1999; Kurahashi et al. Citation2004).
Outcomes other than hypospadias
Six studies were identified that assessed the impact of soy intake during pregnancy on offspring outcomes other than hypospadias. Three of these involved non-Asian populations (Marks et al. Citation2017; Schmitt, Dekant, and Stopper Citation2001; Starling et al. Citation2017), two involved a Chinese population (Liu et al. Citation2020; Chen et al. Citation2021) and one a Japanese population (Suzuki et al. Citation2012), although only the latter examined an outcome possibly related to hypospadias.
A British longitudinal study by Marks et al. (Citation2017) involving 367 mother-daughter dyads found no association between gestational urinary genistein, daidzein or equol levels and risk of early puberty. In contrast, urinary levels of O-desmethylangolensin (ODMA), an intestinally derived metabolite of the soybean isoflavone daidzein, were associated with an increased likelihood of girls entering puberty before the age of 11.5 years. In utero exposure to endocrine disruptors has been proposed as advancing the age of puberty onset in girls (Harley et al. Citation2019).
The link with ODMA, but not the other isoflavones, is inconsistent with a possible estrogenic effect since ODMA is considered to be less estrogenic than genistein, daidzein and equol (Schmitt, Dekant, and Stopper Citation2001). Marks et al. (Citation2017) suggested that earlier puberty may simply be the result of a specific phenotype, which is also characterized by the ability to make large amounts of ODMA. Although puberty onset in girls is a much different endpoint than hypospadias in boys, the lack of effect on the latter informs about the possible impact of isoflavones on the developing fetus. However, the low isoflavone intake of the British population raises doubts about the utility of this study. This limitation also applies to the next study.
A US study conducted between 1999 and 2005 involving 387 partners of pregnant women found that beef consumption during pregnancy of the mothers of the male partners was inversely related to sperm concentration and the proportion of men with sperm concentration <20 million/ml (Swan et al. Citation2007). In contrast, maternal consumption during pregnancy of fish, chicken, soy products and vegetables was unrelated to the son’s sperm concentration (all p values, 0.225–0.655).
And in another US study, which involved 764 mothers, a pattern characterized by starchy vegetables, eggs, non-whole grains, and a low intake of dairy, dark-green vegetables, whole grains, and soy was associated with greater maternal fasting glucose and greater newborn birth weight and adiposity (Starling et al. Citation2017). In a study from Northwest China, which used data from 7194 women from a large-scale cross-sectional survey, medium and high adherence to a dietary pattern that included soy products were less likely to give birth to low birth weight and small for gestational age (SGA) infants (Liu et al. Citation2020). Also from China is a study that found among 1188 mother-infant pairs, sometimes or frequent consumption of soy during weeks 12–16 weeks of pregnancy was associated with higher birth weight among girls, whereas no association between soy intake and birthweight among boys was found (Chen et al. Citation2021).
In research published in 2012, spot urine samples taken from 111 pregnant Japanese women were used to determine whether phthalate ester metabolites were related to birth outcomes and anogenital distance (AGD), the distance from the center of the anus to external genitalia. A recent meta-analysis found that boys with hypospadias or cryptorchidism have shorter AGD (Hua et al. Citation2018). In a multiple regression model, the log-transformed mono-2-ethylhexyl phthalate concentration (specific gravity-corrected) was negatively, and maternal smoking status, positively related, to the anogenital index (AGI, AGD expressed in mm ⁄ kg). Urinary daidzein (genistein was not measured) concentrations were unrelated to AGI (Suzuki et al. Citation2012). Urinary daidzein levels (corrected for creatinine) were similar to those reported by Nagata, Oba, and Shimizu (Citation2006) but about 50-fold higher than reported for US adults (Centers for Disease Control and Prevention Citation2019).
Finally, only one study was identified that intervened with soy protein in pregnant women. For this study, Indian women presenting with gestational diabetes were randomized to a high fiber complex carbohydrate diet (n = 30) or the same diet in which 25% of the cereal component of the high fiber, complex carbohydrate diet was replaced by soy (n = 32) (Sarathi et al. Citation2016). At the end of the intervention, patients in the non-soy group had significantly higher postprandial blood glucose levels whereas fasting pre-meal blood glucose values tended to be lower in the soy group. In addition, 12 (40%) women in the non-soy group failed to achieve the glycemic targets compared to four (12.5%) in the soy group. Thyroid function did not differ between groups. Regarding infants, there were no significant differences between groups in neonatal TSH levels; none of the neonates in either group had TSH >15 μIU/ml. While this study is unique, its findings are of somewhat limited value with respect to both the women and their infants because the intervention was only one week in duration and the amount of protein and isoflavones provided by soy products (beans, chunks, granules or flour) isn’t clear from the study description.
In utero isoflavone concentrations
Assessing the impact of maternal soy intake on birth outcomes is the most direct way of determining the extent to which, if any, in utero isoflavone exposure affects the fetus. However, by comparing fetal isoflavone exposure with fetal estrogen exposure, some insight is potentially gained about the likelihood of maternal soy intake having an impact on the fetus, assuming isoflavones exert an effect through an estrogen-related mechanism.
Amniotic samples provide an approximation of circulating fetal hormones by gauging the hormone levels that have entered the amniotic fluid via fetal urination or diffusion through fetal skin (Nagamani et al. Citation1979). While amniotic hormones are thought to relate to hormone levels in fetal blood, the strength of the relationship remains unclear. Currently, there is no “gold standard” approach to the measurement of prenatal hormone exposure (van de Beek et al. Citation2004). Umbilical cord blood is typically collected after delivery near term, and so cord plasma or serum hormone concentrations are thought to reflect the levels in the fetal circulation at late gestation (Keelan et al. Citation2012).
According to recent reviews, umbilical cord blood 17β-estradiol concentrations range from about 20 to 40 nmol/l (Hollier et al. Citation2014; Kuijper et al. Citation2013). In two of the studies in , one reported cord blood estriol levels of >7000 nmol/l (Nagata, Oba, and Shimizu Citation2006) and the other reported a level >3,000 nmol/l (Adlercreutz et al. Citation1999). Amniotic estradiol concentrations at mid pregnancy are about 1 nmol/l (van de Beek et al. Citation2004; Forest et al. Citation1980; van de Beek et al. Citation2009) and estrone levels are only about 20% of that (Forest et al. Citation1980). As to whether there are differences between Asian and non-Asian women, Lagiou et al. (Citation2011) found that cord blood levels of estradiol and estriol in US women were 129.9 and 1328.6 nM, respectively, and women from China, 292.5 and 1411.9 nM, respectively.
Table 11. Amniotic fluid and umbilical cord concentrations1 (mean or geometric mean or median) of isoflavones and estrogen.
When considering isoflavone levels in cord blood, it is important to recognize that the half-life of genistein and daidzein is approximately eight hours (Setchell et al. Citation2003). Thus, cord blood isoflavone levels will likely be impacted by the timing of the mother’s last meal containing isoflavones prior to delivery. To this point, Todaka et al. (Citation2005), found much higher blood isoflavone levels of Japanese volunteers who consumed a breakfast (isoflavone content not indicated) a few hours prior to sampling than maternal blood levels at delivery.
Jarrell, Foster, and Kinniburgh (Citation2012) showed that amniotic fluid genistein and daidzein levels were higher (∼3–5 fold) in daily vs weekly soyfood consumers, indicating levels respond to isoflavone intake. This point is also illustrated by the higher amniotic fluid isoflavone levels among Japanese (Adlercreutz et al. Citation1999) in comparison to US women (Foster et al. Citation2002; Engel et al. Citation2006), which also is true for cord levels as evidenced by the higher cord blood isoflavones levels in the Japanese studies (Nagata, Oba, and Shimizu Citation2006; Todaka et al. Citation2005) vs the Canadian study (Jarrell, Foster, and Kinniburgh Citation2012) listed in .
On the other hand, Mustafa et al. (Citation2007) commented that “Analysis of cord plasma and corresponding maternal plasma did not produce linear relationship” and that “no correlation on the relevance of mothers' diet to the phytoestrogens level in cord could be derived.” However, despite these comments, in their study involving 300 Malaysian women, total mean levels of soybean isoflavones plus coumestrol in maternal and cord plasma were 18.3 ng/ml and 18.6 ng/ml, respectively.
Todaka et al. (Citation2005) found a weak correlation between maternal and cord serum (genistein, r2 = 0.32; daidzein, r2 = 0.29; equol, r2 = 0.22) among 51 Japanese mothers who underwent a cesarean section. Adlercreutz et al. (1999) reported that cord plasma genistein levels from seven Japanese women were about twice as high as maternal plasma genistein levels (165 vs 89 nmol/l) whereas daidzein levels were more similar (59 vs 46 nmol/l). Food consumption based on three-day dietary records from 97 women in the area from which the pregnant women resided was reported to include 77 g of bean products, mainly soybeans, tofu, and miso. Dalais, Meliala, and Wahlqvist (Citation2000) reported very similar maternal and cord plasma levels among Indonesian women (maternal plasma genistein and daidzein, 83.1 ± 11.7 and 28.9 ± 6.49, respectively; cord plasma genistein and daidzein, 91.7 ± 12.5 and 33.9 ± 5.62 nmol/L, respectively).
If one considers only the three studies involving Japanese women (Nagata, Oba, and Shimizu Citation2006; Adlercreutz et al. Citation1999; Todaka et al. Citation2005), cord blood genistein and daidzein levels ranged from approximately 72 to 165 nmol/l and from approximately 20 to 39 nmol, respectively (). In the only study involving Japanese women that measured amniotic fluid levels, genistein, daidzein and equol levels ranged from 42 to 46 nmol/l (Adlercreutz et al. Citation1999). For the sake of simplicity, if one adds genistein and daidzein levels together (despite dissimilar potency) and takes an average of the three studies, cord blood isoflavone levels are approximately 3–6 times higher than estradiol levels. However, this comparison ignores the contribution of estriol and estrone. Even estriol is considerably more estrogenic than isoflavones (Dang et al. Citation2011).
If one arbitrarily doubles or triples isoflavone levels to account for higher steady state levels (vs levels at delivery), it would appear that the estrogenic influence of endogenously produced estrogens will dwarf the influence of isoflavones, assuming the fetus responds to isoflavones in a manner similar to adults. A direct comparison comes from Adlercreutz et al. (1999), who found that mean total estrogens in maternal plasma, cord plasma and amniotic fluid were 1093, 5579 and 3800 nmol/l, whereas mean total isoflavone levels in maternal plasma, cord plasma and amniotic fluid were 232, 299 and 233 nmol/l, respectively.
Finally, there is need for information on in utero α-fetoprotein protein (AFP) in relation to isoflavones. AFP is glycoprotein that is formed in the yolk sack and in the fetal liver that plays an important role in the regulation of fetal growth (Vakharia and Mizejewski Citation2000). Keel et al. (Citation1992) reported that human AFP does not bind estradiol; however, Vakharia and Mizejewski (Citation2000) showed that AFP peptides have an anti-estrogenic effect. Garreau et al. (Citation1991) found that in vitro AFP binds isoflavones. The interaction between AFP and estrogen and AFP and isoflavones could influence the potential for each of these compounds to impact fetal development.
Conclusions
Evidence indicates that maternal isoflavone intake results in exposure of the fetus to isoflavones. However, in utero isoflavone concentrations are markedly lower than estrogen concentrations. The difference in concentrations suggests, although does not prove, that isoflavones are unlikely to exert an estrogenic effect on the fetus.
Breastfeeding
In 1996, an editorial in Clinical Chemistry pondered whether phytoestrogens (isoflavones) in human milk might be another advantage of breast feeding (Slavin Citation1996). This question has not been addressed clinically or via observational studies. Nevertheless, as discussed, there is evidence upon which one can reasonably speculate as to whether breast-fed infants are likely to be impacted by maternal isoflavone intake.
As background, normal concentrations of estrogens and progesterone in breast milk exhibit a broad range of physiological functions ranging from modulation of bone density, promoting the conversion of linolenic acid to docosahexaenoic acid, improvement of brain function, improvement of the morphology and motility of cell types and the promotion of cholesterol mobilization (Shang Citation2006; Shang Citation2007; Burdge and Wootton Citation2002; Mulac-Jericevic et al. Citation2000; Piña-Medina et al. Citation2016; Barros, Tufik, and Andersen Citation2015). Conversely, high breast milk estrogen and progesterone concentrations may cause sexual precocity of children (Main et al. Citation2007).
Cruz et al. (Citation1994) were the first to demonstrate that infants are capable of absorbing isoflavones, but in their study the delivery vehicle was SIF, not human milk. Although isoflavones are detectable in breastfed infants (Cao et al. Citation2009), the bioavailability of isoflavones may differ between SIF and human milk because isoflavones are found as glucuronide conjugates in the latter (Franke and Custer Citation1996) whereas they are present mostly as glycosidic conjugates in the former (Coward et al. Citation1993).
More than 20 years ago, Huggett et al. (Citation1997) suggested isoflavones are biologically inactive in infants because their analysis revealed that none of the plasma samples from four infants fed SIF contained detectable “free” (unconjugated) isoflavones, even after continuous feeding for more than 4 weeks. However, in their pilot observational study, Adgent et al. (Citation2018) found that infants who consume SIF present with changes to tissue consistent with those seen with exogenous estrogen.
Several studies have determined the isoflavone concentration of human milk in women after consuming soy as well as in women from soyfood-consuming countries. In addition to reviewing these studies, the isoflavone concentration in human milk is compared to human milk estrogen concentrations and to the isoflavone content of SIF. Also compared are the circulating isoflavone concentrations in infants consuming human milk from mothers consuming soyfoods with isoflavone levels in infants consuming SIF.
Franke and Custer (Citation1996) were the first to report on the human milk isoflavone content following a soy intervention. For this pilot study, milk was collected from a Caucasian woman at 0, 24 and 72 h following the consumption of 5, 10, and 20 g of roasted soybeans containing approximately 10, 20 and 40 mg isoflavones, respectively. Milk from a Chinese woman consuming her usual diet was also collected at these times. In the Caucasian woman, human milk isoflavone concentration increased in a dose response fashion. Maximum milk concentrations were reached 10–14 h after soy intake and returned to baseline values two to four days later, depending upon the intake. The concentrations of genistein and daidzein in milk (30–50 and 80–110 nmol/L, respectively) from the Chinese woman consuming her usual diet were similar to those observed in the milk of the Caucasian women after challenge with roasted soybeans.
In contrast to the study by Franke and Custer (Citation1996), Zhou et al. (2020) found that after a Chinese woman consumed 500 ml soymilk, maximum isoflavone breastmilk concentrations were reached much earlier, after only six h. At this time point, mean genistein and daidzein concentrations were approximately 99 (270 nM) and 104 ug/kg (260 nM), respectively. These concentrations are similar to those reported by Min, Wang, and Liang (Citation2020) for Chinese women, as genistein and daidzein concentrations in breastmilk were 144 nmol/l (38.92 ug/l) and 52 nmol/l (13.09 ug/l), respectively. The urinary genistein and daidzein concentrations of the breastfed infants were 96 nmol/l and 44 nmol/l, respectively.
These values for Chinese women are similar to the human milk isoflavone concentrations in healthy Korean women (age 37 ± 4 y) as shown in . No information about the isoflavone intake of these women or the number of women sampled was reported, although the plasma values indicate soy was consumed (Choi et al. Citation2002). As can be seen, the isoflavone concentration of human milk is only approximately twice the concentration of 17β-estradiol whereas in plasma, isoflavone concentration is 10 to 40 times higher. In 32 Chinese women, Lu et al. (Citation2017) reported 17β-estradiol concentrations of 1.60 ± 0.96 μg/l, 0.83 ± 0.36 μg/l and 1.26 ± 0.48 μg/L in colostrum (day 1), transitional milk (day 14) and mature human milk (day 42), respectively. The concentrations of estriol were 2.09 ± 1.66 μg/L, 2.23 ± 1.74 μg/L and 4.64 ± 2.15 μg/L, respectively.
Table 12. Estrogen and isoflavone concentrations in breast milk and plasma of Korean women (Choi et al. Citation2002).
Morton et al. (Citation1998) reported that the genistein and daidzein concentration of breast milk from five women from Hong Kong was 4.8 (17.8 nmol/l) and 3.6 ng/ml (14.2 nmol/l), respectively, which is at the very low end of the range reported for the Korean women. However, this report was published in abstract form only and genistein and daidzein were detected in only four and three of the milks, respectively. In contrast to the concentrations in Asian women, Jarrell, Foster, and Kinniburgh (Citation2012) reported that breast milk genistein (n = 84) and daidzein (n = 0.25) concentrations from Canadian women were only 0.61 ng/ml (2.3 nmol/l) and 0.25 ng/ml (0.98 nmol/l), respectively. The difference between the Asian and non-Asian women supports the notion that habitual isoflavone intake impacts breast milk isoflavone concentrations.
Franke et al. (Citation2006) determined the breast milk isoflavone concentrations in seven mothers who consumed one daily serving (36.5 g) of a soy protein beverage for 2–4 d that contained approximately 55 mg isoflavones. Breast milk isoflavone concentrations increased from 5.1 ± 2.2 to 70.7 ± 19.2 nmol/L after the mothers consumed soy. The latter value is consistent with the previously cited studies (Franke and Custer Citation1996; Choi et al. Citation2002). For this study, after the soy beverage was consumed on the last day in the early morning, sample collection was performed that afternoon and was completed within 1–2 h. Mothers collected their milk and urine as well as the infant’s urine.
This study by Franke et al. (Citation2006) also provides data about the impact of isoflavone exposure via human milk on infant plasma isoflavone concentrations. The mean total isoflavone concentration in plasma obtained from 11 infants that were breastfed by mothers consuming soy daily was 19.7 ± 13.2 nmol/L. In contrast, in three children aged 9–25 months who ate 15–90 g tofu, mean plasma isoflavone value was 1048.6 nmol/L (median: 663.1 nmol/L; range: 629.1–1853.6 nmol/L). Blood was collected for 2-4 h after the infants consumed on average 44 g tofu (equivalent to 7.4 mg isoflavones). Franke et al. (Citation2006) also found that urinary isoflavone excretion per hour adjusted for dose per bw was 81% lower for breastfed infants and 24% higher for tofu-fed infants than for their mothers after eating soy.
Jochum et al. (Citation2017) determined breast milk isoflavone concentrations in 18 German women who were advised to consume one daily serving (250 mL) of a soy drink. The soy drink increased the average daily isoflavone (daidzein plus genistein) intake from 0.2 mg to 12.7 mg. Milk samples (>10 mL) were taken before intervention (day 1), during intervention (days 4 and 7), and after intervention (day 8) five min after the start of a given breastfeeding interval in the evening (about 10 h after study drink ingestion) using a breast pump. As can be seen from , isoflavones were not detected in breast milk prior to the intervention and increased to a peak of 4.3 nmol/l and 9.1 nmol/l for genistein and daidzein, respectively, on study day seven. These levels are much lower than the levels reported in the study by Franke et al. (Citation2006) of 70.7 ± 19.2 nmol/L, but in the latter, women consumed 55 mg/d isoflavones versus only 12.7 mg/d in the former.
Table 13. Isoflavone concentrations (nmol/L, mean ± SD) in human breast milk samples before (day 1), during (days 3 and 6), and after (day 7) intervention with soy drink (Jochum et al. Citation2017).
Jochum et al. (Citation2017) estimated that the isoflavone (genistein and daidzein) intake of an infant breast fed by the mothers consuming 250 ml soy drink (12.5 isoflavones) would be 11 nmol (2.8 ug), assuming a daily average breast milk volume of 800 mL for a 4-month old infant. In contrast, they noted that infants consuming SIF containing an isoflavone concentration of 32–47 mg/L (0.12–0.18 mmol/L) (Setchell et al. Citation1997) would result in about 1,000-fold higher intake (4.5–8.0 mg/kg bw/d) compared with breastmilk feeding. These values for infant intake are similar to those reported by Irvine, Fitzpatrick, and Alexander (Citation1998), who estimated that the daily dose of isoflavones (genistein plus daidzein) was 3.2 ± 0.2 mg/kg body weight in infants on SIF.
Finally, Setchell et al. (Citation1997) found that the mean ± SD plasma concentrations of genistein and daidzein in seven infants fed SIF were 684 ± 443 ng/mL (2.53 ± 1.64 µmol/l) and 295 ± 60 ng/mL (∼1.16 ± 0.23 µmol/l), respectively. These plasma values are roughly 200 times greater than the values reported Frank and Custer (Franke et al. Citation2006) in 11 infants who were breastfed by mothers consuming soy. Accordingly, Setchell et al. (Citation1997) concluded that there is “… little reason for concern about phyto-estrogens from human breast-milk, even when mothers consume soy during lactation.”
Conclusions
Maternal soy consumption increases breastmilk isoflavone concentrations in a dose-response fashion. However, the relatively low concentration, especially in comparison to SIF, suggests breastfed infants are unlikely to be affected by maternal soy consumption.
Kawasaki disease
Kawasaki disease (KD) is an acute, self-limited vasculitis that affects small- and medium-sized vessels that can lead to severe cardiac complications (Burns et al. Citation2000) that is responsible for more cases of acquired heart disease among children than any other condition (Newburger et al. Citation2004). KD most commonly affects children between ages six months and five years (peak incidence occurs between the ages of 6 and 11 months) (Nakamura et al. Citation2012). There are approximately 6000 new cases of KD each year in the US (Huang et al. Citation2013). The occurrence incidence of KD in the US is estimated to be between 17.5 and 20.8 per 100,000 children <5 y (Lin and Wu Citation2017).
Asian ethnicity appears to be an important risk factor for KD (Nakamura et al. Citation2012) and genetic studies have indicated a major role for Fcγ receptors (FcGRs) in KD pathogenesis (Shrestha et al. Citation2012; Shrestha et al. Citation2011). These receptors bind to antibodies that are attached to infected cells or invading pathogens. Based on the knowledge that in vitro, genistein inhibits FcGR function (Huang et al. Citation2006), the prevalence of KD in Japan and Asia is elevated relative to Western countries (Holman et al. Citation2010) and soy is a commonly consumed food among Japanese, Portman (Citation2013) hypothesized that soy consumption increases KD risk. A possible association with maternal soy consumption was first tentatively identified in a pilot observational study published more than 20 years ago (Ross Citation1998).
To examine this hypothesis, Portman et al. (Citation2016) evaluated soyfood intake and isoflavone consumption in nearly 200 US KD cases (age, 4.0 ± 3.7) and 200 age-matched controls (age, 5.2 ± 4.2) using a food frequency questionnaire for children and their mothers. The assessed intake period was three months prior to diagnosis. Maternal soy intake during pregnancy and nursing showed no significant differences in isoflavone consumption between groups. In contrast, isoflavone intake among children was associated with a two-fold increased risk of KD (OR, 2.33; 95% CI: 1.37, 3.96) when comparing high-soy consumers vs nonconsumers.
When the data were sub-analyzed, the increased risk associated with isoflavone intake was primarily found in Asian children. Risk among Asian-American children with the highest intake was increased nearly seven-fold (OR, 7.29; 95% CI: 1.73, 30.75) whereas among white children risk was not significantly increased (OR, 1.61; 95% CI: 0.86, 3.01). While these findings are certainly intriguing and warrant additional research, it is important to recognize that this study included only 51 Asian children. In addition, the low mean isoflavone intake (27.6 mg/week) even among the cases raises doubt about the biological plausibility of the findings. In fact, risk was increased nearly threefold (OR, 4.09; 95% CI: 0.46, 36.18), although non-significantly so, even among those whose isoflavone intake was <16.6 mg/week.
Furthermore, although Huang et al. (Citation2006) found that FcγRIIA-mediated phagocytosis is more sensitive to a reduction in tyrosine phosphorylation in response to genistein than is endocytosis, the genistein concentration showing even minor inhibitory effects (18.5 µmol/l) is far greater than can be reached in children in response to the consumption of soyfoods. Portman et al. (Portman Citation2013) also cited as evidence in support of a role for genistein a marked reduction in thymic mass in mice in vivo with plasma genistein levels that approximate those obtained in humans by dietary ingestion (Cimafranca et al. Citation2010). However, in this study (Cimafranca et al. Citation2010), genistein was administered neonatally (postnatal days 1–5). Also, although serum genistein levels were in the low micromolar range, which can be achieved in humans, the aglycone fraction in the serum of mice ranged from 20% to 40% of the total serum genistein concentration, whereas in humans, the aglycone fraction represents <2% of the total concentration (Setchell et al. Citation2011).
Finally, in Japan, KD prevalence rates (incidence/100,000 children age 0–4) have increased dramatically over the past 30 years, a time during which soy consumption has not increased and may have declined somewhat (Makino et al. Citation2015). Some evidence suggests that the causative agent of KD is a preformed windborne toxin or environmental agent rather than an organism requiring replication (Rodo et al. Citation2014; Ballester et al. Citation2019). Nagata recently concluded that among the numerous proposed etiologies, the most credible theory is that bacterial infection triggers KD, but the possibility of fungi and new types of viruses also playing a role was not excluded (Nagata Citation2019).
Conclusions
The notion that soy intake increases risk of KD has been investigated to only a limited extent and thus this hypothesis remains speculative. No conclusions about this hypothesis can be drawn at this time.
Summary and conclusions
Isoflavones and soyfoods have been rigorously investigated over the past 30 years. Early on, there was considerable excitement about the possible role of isoflavones in preventing and treating a variety of chronic diseases (Messina Citation1995). Some of that enthusiasm faded as subsequently published research produced equivocal findings (Balk et al. Citation2005). Nevertheless, research into the benefits of soy and isoflavones – with results often supportive of efficacy – continue to be published at an impressive rate (Man et al. Citation2021; Sansai et al. Citation2020; Cui et al. Citation2020; Li et al. Citation2020). However, concerns about the safety of isoflavones have led to these soybean constituents, and as a result, to soyfoods, becoming controversial. These concerns, are, for the most part, based on rodent studies.
The conclusion of the current technical review, which consists of a comprehensive evaluation of the clinical and observational literature, is that there is little evidence to suggest that isoflavones, when consumed at levels not exceeding Asian intake (≤100 mg/d), exert untoward effects in adults. This evidence includes a lack of significant effect of isoflavones on reproductive hormone levels in men and women, a possible modest effect on menstrual cycle length, and a lack of effect on thyroid function and on markers of breast cancer risk.
Furthermore, evidence does not support the notion that isoflavone exposure is contraindicated for breast cancer survivors; in fact, the observational data suggest the opposite may be the case. There is also evidence that soy may reduce the risk of developing endometrial cancer. However, because data related to the development of uterine fibroids are inconsistent, and limited, more research in this area is warranted. Overall, based on research in adults, the evidence does not warrant classifying isoflavones (or soyfoods) as endocrine disruptors.
Note that the upper intake figure of 100 mg/d isoflavones is not meant to imply that when intake exceeds this level, there is evidence of harm. In fact, adverse effects have not been reported in long-term trials in which study participants consumed >100 mg/d isoflavones. Rather, this figure is suggested because there is no historical precedent for habitual consumption higher than this amount. Besides, consuming >100 mg/d isoflavones from traditional soyfoods, requires the consumption of >4 servings/d. Consuming >4 servings/d of any food is inconsistent with the dietetic principles of moderation and variety.
Not unexpectedly, much less research on the effects of soy and isoflavone intake in children has been conducted. Because when expressed on a bw basis, there is the potential for isoflavone exposure to be higher in children than adults, and isoflavone absorption may be greater in children, more research involving children is certainly warranted. This research should not be limited to safety concerns but should also explore potential benefits. Regarding the latter, there is substantial observational evidence that soy intake early in life reduces later risk of developing breast cancer. More research aimed at evaluating this hypothesis is needed.
The existing data, although limited, are generally supportive of safety of isoflavone intake in children. No clinically relevant hormonal effects have been observed in young people consuming soyfoods or isoflavones. Regarding puberty onset, the data are mixed. The largest study to examine this issue, which involved SDAs, found no relationship between soyfood intake and AOM, although in boys, in one study, there was a modest effect on the age of onset of pubic hair (primary endpoint), but not on facial hair (secondary endpoint). The clinical implications, if any, of this effect, even if confirmed by future research, are unclear. Especially because puberty onset among the high-soy-consuming SDA boys was later than average for non-SDA US boys.
To address this issue more definitively requires data from large prospective studies, which would need to involve either Western vegetarians or Asians, to assure soy intake is sufficiently high. It is difficult to make intake recommendations, but in keeping with the previously mentioned dietetic principles, a reasonable upper isoflavone limit for younger people, is 50 mg/d. Again, this upper limit is not to suggest that when intake exceeds 50 mg/d, evidence of harm has been observed.
Finally, no clinical studies have evaluated the effects of isoflavone intake on the developing fetus or of infants exposed to isoflavones as a result of maternal soy consumption, but given the low concentration of isoflavones in breast milk and the high in utero estrogen concentrations, adverse effects would not be expected. These exposures periods are difficult to study prospectively because of the long follow up period and large sample size that would be required. Certainly though, the historical precedence of Asian women consuming soy during pregnancy and lactation provide a considerable measure of comfort.
In closing, the conclusion of this review of the observational and clinical studies covering a broad range of health outcomes likely to be affected by endocrine active substances is that neither soyfoods nor isoflavones warrant classification as endocrine disruptors. Future research exploring the biological effects of soy its constituent isoflavones should be encouraged.
Acknowledgements
Funds were provided by the Soy Nutrition Institute and the European Plant-based Food Association to MM and the Toronto 3D Knowledge Synthesis & Clinical Trials Foundation for work related to the development and writing of this paper.
Disclosure statement
Mark John Messina receives funding from the Soy Nutrition Institute as its Executive Director. Both Mindy Kurzer and John Sievenpiper are on the advisory board of the Soy Nutrition Institute. Ian Rowland is on the advisory board of the European Plant-based Foods Association. I have disclosed those interests fully to Taylor & Francis, and have in place an approved plan for managing any potential conflicts arising from these positions.
References
- Adams, N. R. 1995. Detection of the effects of phytoestrogens on sheep and cattle. Journal of Animal Science 73 (5):1509–15. doi: 10.2527/1995.7351509x.
- Adgent, M. A., D. M. Umbach, B. S. Zemel, A. Kelly, J. I. Schall, E. G. Ford, K. James, K. Darge, J. C. Botelho, H. W. Vesper, et al. 2018. A longitudinal study of estrogen-responsive tissues and hormone concentrations in infants fed soy formula. The Journal of Clinical Endocrinology and Metabolism 103 (5):1899–909. doi: 10.1210/jc.2017-02249.
- Adlercreutz, H., and T. Luukkainen. 1970. Identification and determination of oestrogens in various biological materials in pregnancy. Annals of Clinical Research 2 (4):365–80. https://pubmed.ncbi.nlm.nih.gov/5531219/.
- Adlercreutz, H., H. Markkanen, and S. Watanabe. 1993. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet (London, England) 342 (8881):1209–10. doi: 10.1016/0140-6736(93)92188-y.
- Adlercreutz, H., T. Yamada, K. Wähälä, and S. Watanabe. 1999. Maternal and neonatal phytoestrogens in Japanese women during birth. American Journal of Obstetrics and Gynecology 180 (3 Pt 1):737–43. doi: 10.1016/s0002-9378(99)70281-4.
- Akaza, H., N. Miyanaga, N. Takashima, S. Naito, Y. Hirao, T. Tsukamoto, T. Fujioka, M. Mori, W.-J. Kim, J. M. Song, et al. 2004. Comparisons of percent equol producers between prostate cancer patients and controls: Case-controlled studies of isoflavones in Japanese, Korean and American residents. Japanese Journal of Clinical Oncology 34 (2):86–9. doi: 10.1093/jjco/hyh015.
- Akre, O., H. A. Boyd, M. Ahlgren, K. Wilbrand, T. Westergaard, H. Hjalgrim, A. Nordenskjöld, A. Ekbom, and M. Melbye. 2008. Maternal and gestational risk factors for hypospadias. Environmental Health Perspectives 116 (8):1071–6. doi: 10.1289/ehp.10791.
- Alcázar, J. L., L. Bonilla, J. Marucco, A. I. Padilla, E. Chacón, N. Manzour, and A. Salas. 2018. Risk of endometrial cancer and endometrial hyperplasia with atypia in asymptomatic postmenopausal women with endometrial thickness >/=11 mm: A systematic review and meta-analysis. Journal of Clinical Ultrasound 46 (9):565–70. doi: 10.1002/jcu.22631.
- Alekel, D. L., M. D. Van Loan, K. J. Koehler, L. N. Hanson, J. W. Stewart, K. B. Hanson, M. S. Kurzer, and C. T. Peterson. 2010. The soy isoflavones for reducing bone loss (SIRBL) study: A 3-y randomized controlled trial in postmenopausal women. The American Journal of Clinical Nutrition 91 (1):218–30. doi: 10.3945/ajcn.2009.28306.
- Alekel, D. L., U. Genschel, K. J. Koehler, H. Hofmann, M. D. Van Loan, B. S. Beer, L. N. Hanson, C. T. Peterson, and M. S. Kurzer. 2015. Soy Isoflavones for Reducing Bone Loss study: Effects of a 3-year trial on hormones, adverse events, and endometrial thickness in postmenopausal women. Menopause (New York, N.Y.) 22 (2):185–97. doi: 10.1097/GME.0000000000000280.
- Allred, C. D., K. F. Allred, Y. H. Ju, et al. 2001. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res 61:5045–50. https://pubmed.ncbi.nlm.nih.gov/11431339/.
- Allred, C. D., K. F. Allred, Y. H. Ju, T. S. Goeppinger, D. R. Doerge, and W. G. Helferich. 2004. Soy processing influences growth of estrogen-dependent breast cancer tumors. Carcinogenesis 25 (9):1649–57. doi: 10.1093/carcin/bgh178.
- American Institute for Cancer Research. 2021. Soy is safe for breast cancer survivors. Accessed February 09, 2021. https://www.aicr.org/cancer-prevention/food-facts/soy/.
- Anderson, G. 2019. Endometriosis pathoetiology and pathophysiology: Roles of vitamin A, estrogen, immunity, adipocytes, gut microbiome and melatonergic pathway on mitochondria regulation. Biomolecular Concepts 10 (1):133–49. doi: 10.1515/bmc-2019-0017.
- Anderson, J. W., B. M. Smith, and C. S. Washnock. 1999. Cardiovascular and renal benefits of dry bean and soybean intake. The American Journal of Clinical Nutrition 70 (3 Suppl):464S–74. S. doi: 10.1093/ajcn/70.3.464s.
- Andrade, J. E., N. C. Twaddle, W. G. Helferich, and D. R. Doerge. 2010. Absolute bioavailability of isoflavones from soy protein isolate-containing food in female BALB/c mice. Journal of Agricultural and Food Chemistry 58 (7):4529–36. doi: 10.1021/jf9039843.
- Andrews, M. A., K. C. Schliep, J. Wactawski-Wende, J. B. Stanford, S. M. Zarek, R. G. Radin, L. A. Sjaarda, N. J. Perkins, R. A. Kalwerisky, A. O. Hammoud, et al. 2015. Dietary factors and luteal phase deficiency in healthy eumenorrheic women. Human Reproduction 30 (8):1942–51. doi: 10.1093/humrep/dev133.
- Arumugam, K., and A. A. Templeton. 1992. Endometriosis and race. The Australian & New Zealand Journal of Obstetrics & Gynaecology 32 (2):164–5. doi: 10.1111/j.1479-828x.1992.tb01932.x.
- Assinder, S., R. Davis, M. Fenwick, and A. Glover. 2007. Adult-only exposure of male rats to a diet of high phytoestrogen content increases apoptosis of meiotic and post-meiotic germ cells. Reproduction (Cambridge, England) 133 (1):11–9. DOI: 10.1677/joe.1.06709
- Atkinson, C., C. L. Frankenfeld, and J. W. Lampe. 2005. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Experimental Biology and Medicine (Maywood, N.J.) 230 (3):155–70. doi: 10.1177/153537020523000302.
- Atkinson, C., H. E. Skor, E. D. Fitzgibbons, et al. 2002. Overnight urinary isoflavone excretion in a population of women living in the United States, and its relationship to isoflavone intake. Cancer Epidemiology, Biomarkers & Prevention 11:253–60. https://pubmed.ncbi.nlm.nih.gov/11895874/.
- Atteritano, M., H. Marini, L. Minutoli, F. Polito, A. Bitto, D. Altavilla, S. Mazzaferro, R. D'Anna, M. L. Cannata, A. Gaudio, et al. 2007. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: A two-year randomized, double-blind, placebo-controlled study. The Journal of Clinical Endocrinology and Metabolism 92 (8):3068–75. doi: 10.1210/jc.2006-2295.
- Axelson, M., J. Sjövall, B. E. Gustafsson, and K. D. Setchell. 1984. Soya-a dietary source of the non-steroidal oestrogen equol in man and animals . The Journal of Endocrinology 102 (1):49–56. doi: 10.1677/joe.0.1020049.
- Badger, T. M., J. M. Gilchrist, R. T. Pivik, A. Andres, K. Shankar, J.-R. Chen, and M. J. Ronis. 2009. The health implications of soy infant formula. The American journal of clinical nutrition 89 (5):1668S–72. S. doi: 10.3945/ajcn.2009.26736U.
- Badger, T. M., M. J. J. Ronis, R. Hakkak, J. C. Rowlands, and S. Korourian. 2002. The health consequences of early soy consumption. The Journal of Nutrition 132 (3):559S–65. S. doi: 10.1093/jn/132.3.559S.
- Baglia, M. L., W. Zheng, H. Li, G. Yang, J. Gao, Y.-T. Gao, and X.-O. Shu. 2016. The association of soy food consumption with the risk of subtype of breast cancers defined by hormone receptor and HER2 status. International Journal of Cancer 139 (4):742–8. doi: 10.1002/ijc.30117.
- Bai, W., C. Wang, and C. Ren. 2014. Intakes of total and individual flavonoids by US adults. International Journal of Food Sciences and Nutrition 65 (1):9–20. doi: 10.3109/09637486.2013.832170.
- Balakrishnan, B., E. B. Thorstensen, A. P. Ponnampalam, and M. D. Mitchell. 2010. Transplacental transfer and biotransformation of genistein in human placenta. Placenta 31 (6):506–11. doi: 10.1016/j.placenta.2010.03.007.
- Balk, E., M. Chung, P. Chew, et al. 2005. Effects of soy on health outcomes. Evidence Report/Technology Assessment (Summary):1–8. https://www.ncbi.nlm.nih.gov/books/NBK11870/.
- Ballester, J., S. Borràs, R. Curcoll, A. Navarro-Gallinad, S. Pozdniakova, L. Cañas, J. C. Burns, and X. Rodó. 2019. On the interpretation of the atmospheric mechanism transporting the environmental trigger of Kawasaki Disease. PloS One 14 (12):e0226402. doi: 10.1371/journal.pone.0226402.
- Bar-El, D. S., and R. Reifen. 2010. Soy as an endocrine disruptor: Cause for caution? Journal of Pediatric Endocrinology & Metabolism: JPEM 23 (9):855–61. doi: 10.1515/jpem.2010.138.
- Barker, D. J. 1990. The fetal and infant origins of adult disease. BMJ (Clinical Research ed.) 301 (6761):1111 doi: 10.1136/bmj.301.6761.1111.
- Barker, D. J. 1995. Fetal origins of coronary heart disease. BMJ (Clinical Research ed.) 311 (6998):171–4. doi: 10.1136/bmj.311.6998.171.
- Barker, D. J. P., C. Osmond, K. L. Thornburg, E. Kajantie, and J. G. Eriksson. 2008. A possible link between the pubertal growth of girls and ovarian cancer in their daughters. American Journal of Human Biology: The Official Journal of the Human Biology Council 20 (6):659–62. doi: 10.1002/ajhb.20789.
- Barnes, S. 2010. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphatic Research and Biology 8 (1):89–98. doi: 10.1089/lrb.2009.0030.
- Barnes, S., C. C. Wang, M. Kirk, M. Smith-Johnson, L. Coward, N. C. Barnes, G. Vance, and B. Boersma. 2002. HPLC-mass spectrometry of isoflavonoids in soy and the American groundnut, Apios americana. Advances in Experimental Medicine and Biology 505:77–88. doi: 10.1007/978-1-4757-5235-9_7.
- Barnes, S., M. Kirk, and L. Coward. 1994. Isoflavones and their conjugates in soy foods: Extraction conditions and analysis by HPLC mass spectrometry. Journal of Agricultural and Food Chemistry 42 (11):2466–74. doi: 10.1021/jf00047a019.
- Barros, L. A., S. Tufik, and M. L. Andersen. 2015. The role of progesterone in memory: An overview of three decades. Neuroscience and Biobehavioral Reviews 49:193–204. doi: 10.1016/j.neubiorev.2014.11.015.
- Beaton, L. K., B. L. McVeigh, B. L. Dillingham, J. W. Lampe, and A. M. Duncan. 2010. Soy protein isolates of varying isoflavone content do not adversely affect semen quality in healthy young men. Fertility and Sterility 94 (5):1717–22. doi: 10.1016/j.fertnstert.2009.08.055.
- Beeson, W. L., P. K. Mills, R. L. Phillips, M. Andress, and G. E. Fraser. 1989. Chronic disease among Seventh-day Adventists, a low-risk group. Rationale, methodology, and description of the population. Cancer 64 (3):570–81. doi: 10.1002/1097-0142(19890801)64:3<570::aid-cncr2820640303>3.0.co;2-4.
- Bennetts, H. W., E. J. Underwood, and F. L. Shier. 1946. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Australian Veterinary Journal 22 (1):2–8. doi: 10.1111/j.1751-0813.1946.tb15473.x.
- Beszterda, M., and R. Frański. 2018. Endocrine disruptor compounds in environment: As a danger for children health. Pediatric Endocrinology, Diabetes, and Metabolism 24 (2):88–95. doi: 10.18544/PEDM-24.02.0107.
- Bhatia, J., and F. Greer. 2008. Use of soy protein-based formulas in infant feeding. Pediatrics 121 (5):1062–8. doi: 10.1542/peds.2008-0564.
- Biondi, B. 2013. The normal TSH reference range: What has changed in the last decade? The Journal of Clinical Endocrinology and Metabolism 98 (9):3584–7. doi: 10.1210/jc.2013-2760.
- Biro, F. M., A. W. Lucky, L. A. Simbartl, B. A. Barton, S. R. Daniels, R. Striegel-Moore, S. S. Kronsberg, and J. A. Morrison. 2003. Pubertal maturation in girls and the relationship to anthropometric changes: Pathways through puberty. The Journal of Pediatrics 142 (6):643–6. doi: 10.1067/mpd.2003.244.
- Biro, F. M., M. P. Galvez, L. C. Greenspan, P. A. Succop, N. Vangeepuram, S. M. Pinney, S. Teitelbaum, G. C. Windham, L. H. Kushi, and M. S. Wolff. 2010. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics 126 (3):e583–e590. doi: 10.1542/peds.2009-3079.
- Bitto, A., F. Polito, M. Atteritano, D. Altavilla, S. Mazzaferro, H. Marini, E. B. Adamo, R. D'Anna, R. Granese, F. Corrado, et al. 2010. Genistein aglycone does not affect thyroid function: Results from a three-year, randomized, double-blind, placebo-controlled trial. The Journal of Clinical Endocrinology and Metabolism 95 (6):3067–72.,doi: 10.1210/jc.2009-2779.
- Bitto, A., R. Granese, O. Triolo, D. Villari, D. Maisano, D. Giordano, D. Altavilla, H. Marini, E. B. Adamo, P. A. Nicotina, et al. 2010. Genistein aglycone: A new therapeutic approach to reduce endometrial hyperplasia. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 17 (11):844–50. doi: 10.1016/j.phymed.2010.03.024.
- Blair, H. C., S. E. Jordan, T. G. Peterson, and S. Barnes. 1996. Variable effects of tyrosine kinase inhibitors on avian osteoclastic activity and reduction of bone loss in ovariectomized rats. Journal of Cellular Biochemistry 61 (4):629–37. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C629::AID-JCB17%3E3.0.CO;2-A.
- Bobby, R., M. Akond, S. Kantartzi, K. Meksem, H. Herrera, C. Akbay, D. A. Lightfoot, and M. A. Kassem. 2014. Effect of row spacing on seed isoflavone contents in soybean [Glycine max (L.) Merr.]. American Journal Plant Sciences 5:4003–10. doi: 10.4236/ajps.2014.526418.
- Bodicoat, D. H., M. J. Schoemaker, M. E. Jones, E. McFadden, J. Griffin, A. Ashworth, and A. J. Swerdlow. 2014. Timing of pubertal stages and breast cancer risk: The Breakthrough Generations Study. Breast Cancer Research 16 (1):R18. doi: 10.1186/bcr3613.
- Boker, L. K., Y. T. Van der Schouw, M. J. J. De Kleijn, P. F. Jacques, D. E. Grobbee, and P. H. M. Peeters. 2002. Intake of dietary phytoestrogens by Dutch women. The Journal of Nutrition 132 (6):1319–28. doi: 10.1093/jn/132.6.1319.
- Bolca, S., M. Urpi-Sarda, P. Blondeel, N. Roche, L. Vanhaecke, S. Possemiers, N. Al-Maharik, N. Botting, D. De Keukeleire, M. Bracke, et al. 2010. Disposition of soy isoflavones in normal human breast tissue. The American Journal of Clinical Nutrition 91 (4):976–84. doi: 10.3945/ajcn.2009.28854.
- Bosland, M. C., I. Kato, A. Zeleniuch-Jacquotte, J. Schmoll, E. Enk Rueter, J. Melamed, M. X. Kong, V. Macias, A. Kajdacsy-Balla, L. H. Lumey, et al. 2013. Effect of soy protein isolate supplementation on biochemical recurrence of prostate cancer after radical prostatectomy: A randomized trial. JAMA 310 (2):170–8. doi: 10.1001/jama.2013.7842.
- Bosland, M. C., J. Huang, M. J. Schlicht, et al. 2021. Impact of 18-month soy protein supplementation on steroid hormones and serum biomarkers of angiogenesis, apoptosis, and the growth hormone/IGF-1 axis: Results of a randomized, placebo-controlled trial in males following prostatectomy. Nutrition and Cancer:1–12.
- Botta, S., G. R. Cunha, and L. S. Baskin. 2014. Do endocrine disruptors cause hypospadias? Translational Andrology and Urology 3 (4):330–9. doi: 10.3978/j.issn.2223-4683.2014.11.03.
- Boucher, B. A., M. Cotterchio, N. Kreiger, and L. U. Thompson. 2008. Soy formula and breast cancer risk. Epidemiology (Cambridge, Mass.) 19 (1):165–6. doi: 10.1097/EDE.0b013e31815c40ab.
- Boyd, N. F., L. J. Martin, M. J. Yaffe, and S. Minkin. 2011. Mammographic density and breast cancer risk: Current understanding and future prospects. Breast Cancer Research : BCR 13 (6):223. doi: 10.1186/bcr2942.
- Bradbury, R. B., and D. R. White. 1954. Estrogen and related substances in plants. In eds. R. S. Harris, G. F. Marrian, K. V. Thimann, 207–30. Vitam Horm. New York: Academic Press.
- Brahmbhatt, S., R. M. Brahmbhatt, and S. C. Boyages. 2000. Thyroid ultrasound is the best prevalence indicator for assessment of iodine deficiency disorders: A study in rural/tribal schoolchildren from Gujarat (Western India). European Journal of Endocrinology 143 (1):37–46. doi: 10.1530/eje.0.1430037.
- Brössner, C., K. Petritsch, K. Fink, M. Auprich, A. Ponholzer, S. Madersbacher, H. Adlercreutz, and P. Petritsch. 2006. Prostatic phyto-oestrogen tissue levels in different Austrian regions. Urologia Internationalis 76 (4):327–31. doi: 10.1159/000092056.
- Brouwers, M. M., W. F. J. Feitz, L. A. J. Roelofs, L. A. L. M. Kiemeney, R. P. E. de Gier, and N. Roeleveld. 2007. Risk factors for hypospadias. European Journal of Pediatrics 166 (7):671–8. doi: 10.1007/s00431-006-0304-z.
- Brown, N. M., C. A. Belles, S. L. Lindley, L. D. Zimmer-Nechemias, X. Zhao, D. P. Witte, M.-O. Kim, and K. D. R. Setchell. 2010. The chemopreventive action of equol enantiomers in a chemically induced animal model of breast cancer. Carcinogenesis 31 (5):886–93. doi: 10.1093/carcin/bgq025.
- Brzezinski, A., and A. Debi. 1999. Phytoestrogens: The "natural" selective estrogen receptor modulators? European Journal of Obstetrics, Gynecology, and Reproductive Biology 85 (1):47–51. doi: 10.1016/s0301-2115(98)00281-4.
- Buck Louis, G. M., L. E. Gray, M. Marcus, S. R. Ojeda, O. H. Pescovitz, S. F. Witchel, W. Sippell, D. H. Abbott, A. Soto, R. W. Tyl, et al. 2008. Environmental factors and puberty timing: Expert panel research needs. Pediatrics 121 (Suppl 3):S192–S207. doi: 10.1542/peds.1813E.
- Budhathoki, S., M. Iwasaki, N. Sawada, T. Yamaji, T. Shimazu, S. Sasazuki, M. Inoue, and S. Tsugane. 2015. Soy food and isoflavone intake and endometrial cancer risk: The Japan Public Health Center-based prospective study. BJOG : An International Journal of Obstetrics and Gynaecology 122 (3):304–11. doi: 10.1111/1471-0528.12853.
- Burdge, G. C., and S. A. Wootton. 2002. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. The British Journal of Nutrition 88 (4):411–20. doi: 10.1079/BJN2002689.
- Burnett, B. P., L. Pillai, A. Bitto, F. Squadrito, and R. M. Levy. 2011. Evaluation of CYP450 inhibitory effects and steady-state pharmacokinetics of genistein in combination with cholecalciferol and citrated zinc bisglycinate in postmenopausal women. International Journal of Women's Health 3:139–50. doi: 10.2147/IJWH.S19309.
- Burns, J. C., H. I. Kushner, J. F. Bastian, H. Shike, C. Shimizu, T. Matsubara, and C. L. Turner. 2000. Kawasaki disease: A brief history. Pediatrics 106 (2):E27–e27. doi: 10.1542/peds.106.2.e27.
- Busby, M. G., A. R. Jeffcoat, L. T. Bloedon, M. A. Koch, T. Black, K. J. Dix, W. D. Heizer, B. F. Thomas, J. M. Hill, J. A. Crowell, et al. 2002. Clinical characteristics and pharmacokinetics of purified soy isoflavones: Single-dose administration to healthy men. The American Journal of Clinical Nutrition 75 (1):126–36. doi: 10.1093/ajcn/75.1.126.
- Butler, T. L., G. E. Fraser, W. L. Beeson, S. F. Knutsen, R. P. Herring, J. Chan, J. Sabaté, S. Montgomery, E. Haddad, S. Preston-Martin, et al. 2008. Cohort profile: The Adventist Health Study-2 (AHS-2). International Journal of Epidemiology 37 (2):260–5. doi: 10.1093/ije/dym165.
- Caan, B. J., L. Natarajan, B. Parker, E. B. Gold, C. Thomson, V. Newman, C. L. Rock, M. Pu, W. Al-Delaimy, and J. P. Pierce. 2011. Soy food consumption and breast cancer prognosis. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 20 (5):854–8. doi: 10.1158/1055-9965.EPI-10-1041.
- Cabanes, A., N. Ascunce, E. Vidal, M. Ederra, A. Barcos, N. Erdozain, V. Lope, and M. Pollán. 2009. Decline in age at menarche among Spanish women born from 1925 to 1962. BMC Public Health 9:449. doi: 10.1186/1471-2458-9-449.
- Cao, Y., A. M. Calafat, D. R. Doerge, D. M. Umbach, J. C. Bernbaum, N. C. Twaddle, X. Ye, and W. J. Rogan. 2009. Isoflavones in urine, saliva, and blood of infants: Data from a pilot study on the estrogenic activity of soy formula. Journal of Exposure Science & Environmental Epidemiology 19 (2):223–34. doi: 10.1038/jes.2008.44.
- Carmichael, S. L., C. Ma, M. L. Feldkamp, R. G. Munger, R. S. Olney, L. D. Botto, G. M. Shaw, and A. Correa. 2012. Nutritional factors and hypospadias risks. Paediatric and Perinatal Epidemiology 26 (4):353–60. doi: 10.1111/j.1365-3016.2012.01272.x.
- Carmichael, S. L., M. E. Cogswell, C. Ma, A. Gonzalez-Feliciano, R. S. Olney, A. Correa, and G. M. Shaw. 2013. Hypospadias and maternal intake of phytoestrogens. American Journal of Epidemiology 178 (3):434–40. doi: 10.1093/aje/kws591.
- Carmignani, L. O., A. O. Pedro, E. B. Montemor, V. A. Arias, L. H. Costa-Paiva, and A. M. Pinto-Neto. 2015. Effects of a soy-based dietary supplement compared with low-dose hormone therapy on the urogenital system: A randomized, double-blind, controlled clinical trial. Menopause 22 (7):741–9. doi: 10.1097/GME.0000000000000380.
- Carter, M. W., G. Matrone, and W. W. G. J. Smart. 1955. Effect of genistin on reproduction of the mouse. The Journal of Nutrition 55 (4):639–45. doi: 10.1093/jn/55.4.639.
- Carter, M. W., W. W. G. Smart, Jr., and G. Matrone. 1953. Estimation of estrogenic activity of genistein obtained from soybean meal. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 84 (2):506–7. doi: 10.3181/00379727-84-20693.
- Casini, M. L., S. Gerli, and V. Unfer. 2006. An infertile couple suffering from oligospermia by partial sperm maturation arrest: Can phytoestrogens play a therapeutic role? A case report study. Gynecological Endocrinology: The Official Journal of the International Society of Gynecological Endocrinology 22 (7):399–401. doi: 10.1080/09513590600858691.
- Casper, R. F. 2004. Phytoestrogens, clomiphene, and the uterus. Journal of the Society for Gynecologic Investigation 11 (5):261–2. doi: 10.1016/j.jsgi.2004.03.001.
- Cassidy, A., J. E. Brown, A. Hawdon, M. S. Faughnan, L. J. King, J. Millward, L. Zimmer-Nechemias, B. Wolfe, and K. D. R. Setchell. 2006. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. The Journal of Nutrition 136 (1):45–51. doi: 10.1093/jn/136.1.45.
- Cassidy, A., S. Bingham, and K. D. Setchell. 1994. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. The American Journal of Clinical Nutrition 60 (3):333–40. doi: 10.1093/ajcn/60.3.333.
- Cassidy, A., S. Bingham, and K. Setchell. 1995. Biological effects of isoflavones in young women: Importance of the chemical composition of soyabean products. British Journal of Nutrition 74 (4):587–601. doi: 10.1079/BJN19950160.
- Caturegli, P., A. De Remigis, and N. R. Rose. 2014. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmunity Reviews 13 (4-5):391–7. doi: 10.1016/j.autrev.2014.01.007.
- Cederroth, C. R., C. Zimmermann, and S. Nef. 2012. Soy, phytoestrogens and their impact on reproductive health. Molecular and Cellular Endocrinology 355 (2):192–200. doi: 10.1016/j.mce.2011.05.049.
- Centers for Disease Control and Prevention (CDC). 2019. United States. Fourth National Report on Human Exposure to Environmental Chemicals Updated Tables, January Volume One. Accessed February 09, 2021. https://www.cdc.gov/exposurereport/.
- Chan, S. G., P. A. Murphy, S. C. Ho, N. Kreiger, G. Darlington, E. K. F. So, and P. Y. Y. Chong. 2009. Isoflavonoid content of Hong Kong soy foods. Journal of Agricultural and Food Chemistry 57 (12):5386–90. doi: 10.1021/jf803870k.
- Chandrareddy, A., O. Muneyyirci-Delale, S. I. McFarlane, and O. M. Murad. 2008. Adverse effects of phytoestrogens on reproductive health: A report of three cases. Complementary Therapies in Clinical Practice 14 (2):132–5. doi: 10.1016/j.ctcp.2008.01.002.
- Chavarro, J. E., J. W. Rich-Edwards, B. A. Rosner, et al. 2008. Protein intake and ovulatory infertility. American Journal of Obstetrics and Gynecology 198:210–7. e1- doi: 10.1016/j.ajog.2007.06.057.
- Chavarro, J. E., L. Mínguez-Alarcón, Y.-H. Chiu, A. J. Gaskins, I. Souter, P. L. Williams, A. M. Calafat, and R. Hauser. 2016. Soy intake modifies the relation between urinary bisphenol A concentrations and pregnancy outcomes among women undergoing assisted reproduction. The Journal of Clinical Endocrinology and Metabolism 101 (3):1082–90. doi: 10.1210/jc.2015-3473.
- Chavarro, J. E., T. L. Toth, S. M. Sadio, and R. Hauser. 2008. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Human Reproduction (Oxford, England) 23 (11):2584–90. doi: 10.1093/humrep/den243.
- Chávez-Suárez, K., M. Ortega-Vélez, A. Valenzuela-Quintanar, M. Galván-Portillo, L. López-Carrillo, J. Esparza-Romero, M. Saucedo-Tamayo, M. Robles-Burgueño, S. Palma-Durán, M. Gutiérrez-Coronado, et al. 2017. Phytoestrogen concentrations in human urine as biomarkers for dietary phytoestrogen intake in Mexican women. Nutrients 9 (10):1078. doi: 10.3390/nu910.
- Chen, Y., T. Li, H. Ji, X. Wang, X. Sun, M. Miao, Y. Wang, Q. Wu, H. Liang, and W. Yuan. 2021. Associations of maternal soy product consumption and urinary isoflavone concentrations with neonatal anthropometry: A prospective cohort study. Environmental Pollution 274:115752. doi: 10.1016/j.envpol.2020.115752.
- Cheng, E., C. D. Story, L. Yoder, W. H. Hale, and W. Burroughs. 1953. Estrogenic activity of isoflavone derivatives extracted and prepared from soybean oil meal. Science (New York, N.Y.) 118 (3058):164–5. doi: 10.1126/science.118.3058.164.
- Cheng, G., B. Wilczek, M. Warner, J.-A. Gustafsson, and B.-M. Landgren. 2007. Isoflavone treatment for acute menopausal symptoms. Menopause (New York, N.Y.) 14 (3 Pt 1):468–73. doi: 10.1097/GME.0b013e31802cc7d0.
- Cheng, G., T. Remer, R. Prinz-Langenohl, M. Blaszkewicz, G. H. Degen, and A. E. Buyken. 2010. Relation of isoflavones and fiber intake in childhood to the timing of puberty. The American Journal of Clinical Nutrition 92 (3):556–64. doi: 10.3945/ajcn.2010.29394.
- Chi, F., R. Wu, Y.-C. Zeng, R. Xing, Y. Liu, and Z.-G. Xu. 2013. Post-diagnosis soy food intake and breast cancer survival: A meta-analysis of cohort studies. Asian Pacific Journal of Cancer Prevention : APJCP 14 (4):2407–12. doi: 10.7314/apjcp.2013.14.4.2407.
- Chiu, A. C., and S. I. Sherman. 1998. Effects of pharmacological fiber supplements on levothyroxine absorption. Thyroid: Official Journal of the American Thyroid Association 8 (8):667–71. doi: 10.1089/thy.1998.8.667.
- Cho, G. J., H. T. Park, J. H. Shin, J. Y. Hur, Y. T. Kim, S. H. Kim, K. W. Lee, and T. Kim. 2010. Age at menarche in a Korean population: Secular trends and influencing factors. European Journal of Pediatrics 169 (1):89–94. doi: 10.1007/s00431-009-0993-1.
- Cho, S.-H., B. H. Jung, W.-Y. Lee, and B. C. Chung. 2006. Direct determination of estriol conjugates in amniotic fluid by capillary electrophoresis with electrospray tandem mass spectrometry. Rapid Communications in Mass Spectrometry : RCM 20 (19):2995–8. doi: 10.1002/rcm.2685.
- Choi, M. H., K.-R. Kim, J. K. Hong, S. J. Park, and B. C. Chung. 2002. Determination of non-steroidal estrogens in breast milk, plasma, urine and hair by gas chromatography/mass spectrometry. Rapid Communications in Mass Spectrometry : RCM 16 (24):2221–8. doi: 10.1002/rcm.845.
- Chun, J., G. M. Kim, K. W. Lee, I. D. Choi, G.-H. Kwon, J.-Y. Park, S.-J. Jeong, J.-S. Kim, and J. H. Kim. 2007. Conversion of isoflavone glucosides to aglycones in soymilk by fermentation with lactic acid bacteria. Journal of Food Science 72 (2):M39–44. doi: 10.1111/j.1750-3841.2007.00276.x.
- Chun, O. K., S. J. Chung, and W. O. Song. 2007. Estimated dietary flavonoid intake and major food sources of U.S. adults. The Journal of Nutrition 137 (5):1244–52. doi: 10.1093/jn/137.5.1244.
- Chung, M. K., G. M. Buck Louis, K. Kannan, and C. J. Patel. 2019. Exposome-wide association study of semen quality: Systematic discovery of endocrine disrupting chemical biomarkers in fertility require large sample sizes. Environment International 125:505–14. doi: 10.1016/j.envint.2018.11.037.
- Cimafranca, M. A., J. Davila, G. C. Ekman, R. N. Andrews, S. L. Neese, J. Peretz, K. A. Woodling, W. G. Helferich, J. Sarkar, J. A. Flaws, et al. 2010. Acute and chronic effects of oral genistein administration in neonatal mice. Biology of Reproduction 83 (1):114–21. doi: 10.1095/biolreprod.109.080549.
- Committee on Nutrition. 1983. Soy-protein formulas: Recommendations for use in infant feeding. Pediatrics 72:359–63.
- Conner, P. 2007. Breast response to menopausal hormone therapy-aspects on proliferation, apoptosis and mammographic density. Annals of Medicine 39 (1):28–41. doi: 10.1080/07853890601039842.
- Conner, P., G. Söderqvist, L. Skoog, T. Gräser, F. Walter, E. Tani, K. Carlström, and B. von Schoultz. 2003. Breast cell proliferation in postmenopausal women during HRT evaluated through fine needle aspiration cytology. Breast Cancer Research and Treatment 78 (2):159–65. doi: 10.1023/A:1022987618445.
- Conrad, S. C., H. Chiu, and B. L. Silverman. 2004. Soy formula complicates management of congenital hypothyroidism. Archives of Disease in Childhood 89 (1):37–40. doi: 10.1136/adc.2002.009365.
- Constantinou, A., and E. Huberman. 1995. Genistein as an inducer of tumor cell differentiation: Possible mechanisms of action. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 208 (1):109–15. doi: 10.3181/00379727-208-43841.
- Constantinou, A., R. Mehta, C. Runyan, K. Rao, A. Vaughan, and R. Moon. 1995. Flavonoids as DNA topoisomerase antagonists and poisons: Structure-activity relationships. Journal of Natural Products 58 (2):217–25. doi: 10.1021/np50116a009.
- Cooper, A. R. 2019. To eat soy or to not eat soy: The ongoing look at phytoestrogens and fertility. Fertility and Sterility 112 (5):825–6. doi: 10.1016/j.fertnstert.2019.07.016.
- Correia-Branco, A., E. Keating, and F. Martel. 2015. Maternal undernutrition and fetal developmental programming of obesity: The glucocorticoid connection. Reproductive Sciences 22 (2):138–45. doi: 10.1177/1933719114542012.
- Court, M. H., and D. J. Greenblatt. 2000. Molecular genetic basis for deficient acetaminophen glucuronidation by cats: UGT1A6 is a pseudogene, and evidence for reduced diversity of expressed hepatic UGT1A isoforms. Pharmacogenetics 10 (4):355–69. doi: 10.1097/00008571-200006000-00009.
- Coussement, L., S. Bolca, W. Van Criekinge, G. Trooskens, K. Mensaert, K. Poels, N. Roche, P. Blondeel, L. Godderis, H. Depypere, et al. 2018. Exploratory analysis of the human breast DNA methylation profile upon soymilk exposure. Scientific Reports 8 (1):13617. doi: 10.1038/s41598-018-31767-x.
- Coward, L., N. C. Barnes, K. D. R. Setchell, and S. Barnes. 1993. Genistein, daidzein, and their B-glycoside conjugates: Antitumor isoflavones in soybean foods from American and Asian diets. Journal of Agricultural and Food Chemistry 41 (11):1961–7. doi: 10.1021/jf00035a027.
- Crawford, N. M., D. A. Pritchard, A. H. Herring, and A. Z. Steiner. 2017. Prospective evaluation of luteal phase length and natural fertility. Fertility and Sterility 107 (3):749–55. doi: 10.1016/j.fertnstert.2016.11.022.
- Cruz, M. L. A., W. W. Wong, F. Mimouni, D. L. Hachey, K. D. R. Setchell, P. D. Klein, and R. C. Tsang. 1994. Effects of infant nutrition on cholesterol synthesis rates. Pediatric Research 35 (2):135–40. doi: 10.1203/00006450-199402000-00001.
- Cui, C., R. L. Birru, B. E. Snitz, M. Ihara, C. Kakuta, B. J. Lopresti, H. J. Aizenstein, O. L. Lopez, C. A. Mathis, Y. Miyamoto, et al. 2020. Effects of soy isoflavones on cognitive function: A systematic review and meta-analysis of randomized controlled trials. Nutrition Reviews 78 (2):134–44. doi: 10.1093/nutrit/nuz050.
- Cutler, G. J., A. Flood, P. Hannan, and D. Neumark-Sztainer. 2009. Major patterns of dietary intake in adolescents and their stability over time. The Journal of Nutrition 139 (2):323–8. doi: 10.3945/jn.108.090928.
- Czeizel, A. 1985. Increasing trends in congenital malformations of male external genitalia. The Lancet 325 (8426):462–3. doi: 10.1016/S0140-6736(85)91185-7.
- Dakora, F. D., and D. A. Phillips. 1996. Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiological and Molecular Plant Pathology 49 (1):1–20. doi: 10.1006/pmpp.1996.0035.
- Dalais, F. S., A. Meliala, and M. L. Wahlqvist. 2000. Maternal and cord blood phytoestrogen levels in Indonesian women. Journal of Nutrition 130:684S. doi: 10.1093/jn/130.3.680S.
- Dang, Z., S. Ru, W. Wang, E. Rorije, B. Hakkert, and T. Vermeire. 2011. Comparison of chemical-induced transcriptional activation of fish and human estrogen receptors: Regulatory implications. Toxicology Letters 201 (2):152–75. doi: 10.1016/j.toxlet.2010.12.020.
- Davey, G. K., E. A. Spencer, P. N. Appleby, N. E. Allen, K. H. Knox, and T. J. Key. 2003. EPIC-Oxford: Lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutrition 6 (3):259–69. doi: 10.1079/PHN2002430.
- Day, A. J., F. J. Cañada, J. C. Díaz, P. A. Kroon, R. Mclauchlan, C. B. Faulds, G. W. Plumb, M. R. Morgan, and G. Williamson. 2000. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Letters 468 (2-3):166–70. doi: 10.1016/s0014-5793(00)01211-4.
- de Assis, S., A. Warri, C. Benitez, W. Helferich, and L. Hilakivi-Clarke. 2011. Protective effects of prepubertal genistein exposure on mammary tumorigenesis are dependent on BRCA1 expression. Cancer Prevention Research 4 (9):1436–48. doi: 10.1158/1940-6207.CAPR-10-0346.
- De Genna, N. M., C. Larkby, and M. D. Cornelius. 2011. Pubertal timing and early sexual intercourse in the offspring of teenage mothers. Journal of Youth and Adolescence 40 (10):1315–28. doi: 10.1007/s10964-010-9609-3.
- de Kort, C. A., M. J. Nieuwenhuijsen, and M. A. Mendez. 2011. Relationship between maternal dietary patterns and hypospadias. Paediatric and Perinatal Epidemiology 25 (3):255–64. doi: 10.1111/j.1365-3016.2011.01194.x.
- de Souza Dos Santos, M. C., C. F. L. Gonçalves, M. Vaisman, A. C. F. Ferreira, and D. P. de Carvalho. 2011. Impact of flavonoids on thyroid function. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 49 (10):2495–502. doi: 10.1016/j.fct.2011.06.074.
- Decroos, K., S. Vanhemmens, S. Cattoir, N. Boon, and W. Verstraete. 2005. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Archives of Microbiology 183 (1):45–55. doi: 10.1007/s00203-004-0747-4.
- Delmanto, A., J. Nahas-Neto, P. Traiman, G. Uemura, E. C. Pessoa, and E. A. P. Nahas. 2013. Effects of soy isoflavones on mammographic density and breast parenchyma in postmenopausal women: A randomized, double-blind, placebo-controlled clinical trial. Menopause (New York, N.Y.) 20 (10):1049–54. doi: 10.1097/GME.0b013e3182850270.
- Department of Health and Human Services. 2017. Food Labeling: Health Claims; Soy Protein and Coronary Heart Disease. Federal Register 82:50324–46.
- Dieckmann, W. J., M. E. Davis, L. M. Rynkiewicz, and R. E. Pottinger. 1953. Does the administration of diethylstilbestrol during pregnancy have therapeutic value? American Journal of Obstetrics and Gynecology 66 (5):1062–81. doi: 10.1016/S0002-9378(16)38617-3.
- Direito, A., S. Bailly, A. Mariani, and R. Ecochard. 2013. Relationships between the luteinizing hormone surge and other characteristics of the menstrual cycle in normally ovulating women. Fertility and Sterility 99 (1):279–85. doi: 10.1016/j.fertnstert.2012.08.047.
- DiVasta, A. D., A. F. Vitonis, M. R. Laufer, and S. A. Missmer. 2018. Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs adulthood. American Journal of Obstetrics and Gynecology 218:324 e1–e11. doi: 10.1016/j.ajog.2017.12.007.
- Divi, R. L., and D. R. Doerge. 1996. Inhibition of thyroid peroxidase by dietary flavonoids. Chemical Research in Toxicology 9 (1):16–23. doi: 10.1021/tx950076m.
- Divi, R. L., H. C. Chang, and D. R. Doerge. 1997. Anti-thyroid isoflavones from soybean: Isolation, characterization, and mechanisms of action. Biochemical Pharmacology 54 (10):1087–96. doi: 10.1016/s0006-2952(97)00301-8.
- Dixon, R. A., and L. W. Sumner. 2003. Legume natural products: Understanding and manipulating complex pathways for human and animal health. Plant Physiology 131 (3):878–85. doi: 10.1104/pp.102.017319.
- Doerge, D., and H. Chang. 2002. Inactivation of thyroid peroxidase by soy isoflavones, in vitro and in vivo. Journal of Chromatography B 777 (1-2):269–79. doi: 10.1016/S1570-0232(02)00214-3.
- Dong, J.-Y., T. Kimura, S. Ikehara, M. Cui, Y. Kawanishi, T. Kimura, K. Ueda, and H. Iso. 2021. Soy consumption and incidence of gestational diabetes mellitus: The Japan Environment and Children’s Study. European Journal of Nutrition 60 (2):897–904. doi: 10.1007/s00394-020-02294-1.
- Dorjgochoo, T., K. Gu, Y. Zheng, A. Kallianpur, Z. Chen, W. Zheng, W. Lu, and X. O. Shu. 2011. Soy intake in association with menopausal symptoms during the first 6 and 36 months after breast cancer diagnosis. Breast Cancer Research and Treatment 130 (3):879–89. doi: 10.1007/s10549-010-1096-4.
- Du, M., X. Yang, J. A. Hartman, P. S. Cooke, D. R. Doerge, Y. H. Ju, and W. G. Helferich. 2012. Low-dose dietary genistein negates the therapeutic effect of tamoxifen in athymic nude mice. Carcinogenesis 33 (4):895–901. doi: 10.1093/carcin/bgs017.
- Eating Well After Breast Cancer. Accessed February 08, 2021. https://www.cancer.ca/en/cancer-information/cancer-type/breast/supportive-care/eating-well-after-breast-cancer/?region=on.)
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food). 2015. Scientific opinion on the risk assessment for peri- and post-menopausal women taking food supplements containing isolated isoflavones. EFSA Journal 13 (4246):342.
- Eldridge, A. C., and W. F. Kwolek. 1983. Soybean isoflavones: Effect of environment and variety on composition. Journal of Agricultural and Food Chemistry 31 (2):394–6. doi: 10.1021/jf00116a052.
- Elhakeem, A., M. Frysz, K. Tilling, J. H. Tobias, and D. A. Lawlor. 2019. Association between age at puberty and bone accrual from 10 to 25 years of age. JAMA Network Open 2 (8):e198918. doi: 10.1001/jamanetworkopen.2019.8918.
- Engel, S. M., B. Levy, Z. Liu, D. Kaplan, and M. S. Wolff. 2006. Xenobiotic phenols in early pregnancy amniotic fluid. Reproductive Toxicology 21 (1):110–2. doi: 10.1016/j.reprotox.2005.07.007.
- Esaki, H., S. Kawakishi, Y. Morimitsu, and T. Osawa. 1999. New potent antioxidative o-dihydroxyisoflavones in fermented Japanese soybean products. Bioscience, Biotechnology, and Biochemistry 63 (9):1637–9. doi: 10.1271/bbb.63.1637.
- Eskenazi, B., and M. L. Warner. 1997. Epidemiology of endometriosis. Obstetrics and Gynecology Clinics of North America 24 (2):235–58. doi: 10.1016/S0889-8545(05)70302-8.
- Euling, S. Y., M. E. Herman-Giddens, P. A. Lee, S. G. Selevan, A. Juul, T. I. A. Sørensen, L. Dunkel, J. H. Himes, G. Teilmann, and S. H. Swan. 2008. Examination of US puberty-timing data from 1940 to 1994 for secular trends: Panel findings. Pediatrics 121 Suppl 3 (Suppl 3):S172–S91. doi: 10.1542/peds.2007-1813D.
- European Food Safety Authority. 2010. Scientific report of the endocrine active substances task force. EFSA Journal 8:1–59. .
- Evans, M., J. G. Elliott, P. Sharma, R. Berman, and N. Guthrie. 2011. The effect of synthetic genistein on menopause symptom management in healthy postmenopausal women: A multi-center, randomized, placebo-controlled study. Maturitas 68 (2):189–96. doi: 10.1016/j.maturitas.2010.11.012.
- Fang, N., S. Yu, and T. M. Badger. 2004. Comprehensive phytochemical profile of soy protein isolate. Journal of Agricultural and Food Chemistry 52 (12):4012–20. doi: 10.1021/jf049842y.
- Fanti, P., B. P. Sawaya, L. J. Custer, et al. 1999. Serum levels and metabolic clearance of the isoflavones genistein and daidzein in hemodialysis patients. J Am Soc Nephrol 10:864–71. https://pubmed.ncbi.nlm.nih.gov/10203372/.
- Faupel-Badger, J. M., R. N. Hoover, N. Potischman, J. M. Roberts, and R. Troisi. 2009. Pregnancy weight gain is not associated with maternal or mixed umbilical cord estrogen and androgen concentrations. Cancer Causes & Control : CCC 20 (2):263–7. doi: 10.1007/s10552-008-9235-5.
- Felix, A. S., J. L. Weissfeld, R. M. Pfeiffer, F. Modugno, A. Black, L. M. Hill, J. Martin, A. S. Sit, M. E. Sherman, and L. A. Brinton. 2014. Endometrial thickness and risk of breast and endometrial carcinomas in the prostate, lung, colorectal and ovarian cancer screening trial. International Journal of Cancer 134 (4):954–60. doi: 10.1002/ijc.28404.
- Fernandez-Lopez, A., V. Lamothe, M. Delample, M. Denayrolles, and C. Bennetau-Pelissero. 2016. Removing isoflavones from modern soyfood: Why and how? Food Chemistry 210:286–94. doi: 10.1016/j.foodchem.2016.04.126.
- Ferraris, C., B. Ballestra, C. Listorti, V. Cappelletti, C. Reduzzi, G. P. Scaperrotta, I. Pulice, E. G. A. Ferrari, S. Folli, L. Mariani, et al. 2020. Red clover and lifestyle changes to contrast menopausal symptoms in premenopausal patients with hormone-sensitive breast cancer receiving tamoxifen. Breast Cancer Research and Treatment 180 (1):157–65. doi: 10.1007/s10549-020-05534-4.
- Fleshner, N. E., L. Kapusta, B. Donnelly, S. Tanguay, J. Chin, K. Hersey, A. Farley, K. Jansz, D. R. Siemens, K. Trpkov, et al. 2011. Progression from high-grade prostatic intraepithelial neoplasia to cancer: A randomized trial of combination vitamin-E, soy, and selenium. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 29 (17):2386–90. doi: 10.1200/JCO.2010.32.0994.
- Folman, Y., and G. S. Pope. 1966. The interaction in the immature mouse of potent oestrogens with coumestrol, genistein and other utero-vaginotrophic compounds of low potency. The Journal of Endocrinology 34 (2):215–25. doi: 10.1677/joe.0.0340215.
- Folman, Y., and G. S. Pope. 1969. Effect of norethisterone acetate, dimethylstilboestrol, genistein and coumestrol on uptake of [3H]oestradiol by uterus, vagina and skeletal muscle of immature mice. The Journal of Endocrinology 44 (2):213–8. doi: 10.1677/joe.0.0440213.
- Food Labeling: Health Claims; Soy Protein and Coronary Heart Disease. 1999. Food and Drug Administration, HHS. Final rule. Federal Register 64:57700–33. https://pubmed.ncbi.nlm.nih.gov/11010706/.
- Forest, M. G., E. DE Peretti, A. Lecoq, E. Cadillon, M.-T. Zabot, and J.-M. Thoulon. 1980. Concentration of 14 steroid hormones in human amniotic fluid of midpregnancy. Journal of Clinical Endocrinology and Metabolism 51 (4):816–22. doi: 10.1210/jcem-51-4-816.
- Fortes, E. M., M. I. Malerba, P. D. Luchini, E. K. Sugawara, L. Sumodjo, L. M. Ribeiro Neto, and I. T. N. Verreschi. 2007. [High intake of phytoestrogens and precocious thelarche: Case report with a possible correlation.]. Arquivos Brasileiros de Endocrinologia e Metabologia 51 (3):500–3. doi: 10.1590/s0004-27302007000300021.
- Foster, W. G., S. Chan, L. Platt, and C. L. Hughes. 2002. Detection of phytoestrogens in samples of second trimester human amniotic fluid. Toxicology Letters 129 (3):199–205. doi: 10.1016/s0378-4274(02)00018-8.
- Foth, D., and F. Nawroth. 2005. Effect of phytoestrogens on the endometrium? Fertility and Sterility 83 (1):256–7. doi: 10.1016/j.fertnstert.2004.10.021.
- Franke, A. A., and L. J. Custer. 1996. Daidzein and genistein concentrations in human milk after soy consumption. Clinical Chemistry 42 (6 Pt 1):955–64. doi: 10.1093/clinchem/42.6.955.
- Franke, A. A., B. M. Halm, L. J. Custer, Y. Tatsumura, and S. Hebshi. 2006. Isoflavones in breastfed infants after mothers consume soy. The American Journal of Clinical Nutrition 84 (2):406–13. doi: 10.1093/ajcn/84.1.406.
- Franke, A. A., J. F. Lai, and B. M. Halm. 2014. Absorption, distribution, metabolism, and excretion of isoflavonoids after soy intake. Archives of Biochemistry and Biophysics 559:24–8. doi: 10.1016/j.abb.2014.06.007.
- Franke, A. A., L. J. Custer, and S. A. Hundahl. 2004. Determinants for urinary and plasma isoflavones in humans after soy intake. Nutrition and Cancer 50 (2):141–54. doi: 10.1207/s15327914nc5002_3.
- Franke, A. A., L. J. Custer, W. Wang, and C. Y. Shi. 1998. HPLC analysis of isoflavonoids and other phenolic agents from foods and from human fluids. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 217 (3):263–73. doi: 10.3181/00379727-217-44231.
- Franke, A. A., M. C. Yu, G. Maskarinec, P. Fanti, W. Zheng, and L. J. Custer. 1999. Phytoestrogens in human biomatrices including breast milk. Biochemical Society Transactions 27 (2):308–18. doi: 10.1042/bst0270308.
- Franke, A. A., S. M. Hebshi, I. Pagano, N. Kono, W. J. Mack, and H. N. Hodis. 2010. Urine accurately reflects circulating isoflavonoids and ascertains compliance during soy intervention. Cancer Epidemiology Biomarkers & Prevention 19 (7):1775–83. doi: 10.1158/1055-9965.EPI-10-0116.
- Franke, A. A., Y. Morimoto, L. M. Yeh, and G. Maskarinec. 2006. Urinary isoflavonoids as a dietary compliance measure among premenopausal women. Asia Pacific Journal of Clinical Nutrition 15 (1):88–94. https://pubmed.ncbi.nlm.nih.gov/16500883/.
- Fruzza, A. G., C. Demeterco-Berggren, and K. L. Jones. 2012. Unawareness of the effects of soy intake on the management of congenital hypothyroidism. Pediatrics 130 (3):e699-702–e702. doi: 10.1542/peds.2011-3350.
- Fujimoto, K., M. Tanaka, Y. Hirao, Y. Nagata, M. Mori, N. Miyanaga, H. Akaza, and W.-J. Kim. 2008. Age-stratified serum levels of isoflavones and proportion of equol producers in Japanese and Korean healthy men. Prostate Cancer and Prostatic Diseases 11 (3):252–7. doi: 10.1038/sj.pcan.4501030.
- Fukutake, M., M. Takahashi, K. Ishida, H. Kawamura, T. Sugimura, and K. Wakabayashi. 1996. Quantification of genistein and genistin in soybeans and soybean products. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 34 (5):457–61. doi: 10.1016/0278-6915(96)87355-8.
- Gaitan, E. 1990. Goitrogens in food and water. Annual Review of Nutrition 10:21–39. doi: 10.1146/annurev.nu.10.070190.000321.
- Gao, M., and H. Wang. 2018. Frequent milk and soybean consumption are high risks for uterine leiomyoma: A prospective cohort study. Medicine 97 (41):e12009. doi: 10.1097/MD.0000000000012009.
- Garber, J. R., R. H. Cobin, H. Gharib, J. V. Hennessey, I. Klein, J. I. Mechanick, R. Pessah-Pollack, P. A. Singer, and K. A. Woeber. 2012. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid: Official Journal of the American Thyroid Association 22 (12):1200–35. doi: 10.4158/EP12280.GL.
- Gardner, C. D., B. Oelrich, J. P. Liu, D. Feldman, A. A. Franke, and J. D. Brooks. 2009. Prostatic soy isoflavone concentrations exceed serum levels after dietary supplementation. The Prostate 69 (7):719–26. doi: 10.1002/pros.20922.
- Gardner-Thorpe, D., C. O'Hagen, I. Young, and S. J. Lewis. 2003. Dietary supplements of soya flour lower serum testosterone concentrations and improve markers of oxidative stress in men. European Journal of Clinical Nutrition 57 (1):100–6. doi: 10.1038/sj.ejcn.1601495.
- Garreau, B., G. Vallette, H. Adlercreutz, K. Wähälä, T. Mäkelä, C. Benassayag, and E. A. Nunez. 1991. Phytoestrogens: New ligands for rat and human alpha-fetoprotein. Biochimica et Biophysica Acta 1094 (3):339–45. doi: 10.1016/0167-4889(91)90095-f.
- Ghosh, S., R. Shukla, V. S. Mujalde, V. Chaturvedi, and D. K. Barolia. 2016. Maternal vegetarian diet in pregnancy, a predisposition to hypospadias? International Journal of Research in Medical Sciences 5 (1):344–5. doi: 10.18203/2320-6012.ijrms20164575.
- Ginsburg, J. 1996. Tackling environmental endocrine disrupters. Lancet (London, England) 347 (9014):1501–2. doi: 10.1016/s0140-6736(96)90667-4.
- Giordano, F., A. Abballe, E. De Felip, A. di Domenico, F. Ferro, P. Grammatico, A. M. Ingelido, V. Marra, G. Marrocco, S. Vallasciani, et al. 2010. Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Research. Part A, Clinical and Molecular Teratology 88 (4):241–50. doi: 10.1002/bdra.20657.
- Glover, A., and S. J. Assinder. 2006. Acute exposure of adult male rats to dietary phytoestrogens reduces fecundity and alters epididymal steroid hormone receptor expression. The Journal of Endocrinology 189 (3):565–73. doi: 10.1677/joe.1.06709.
- Gomes-Lima, C., and K. D. Burman. 2018. Reverse T3 or perverse T3? Still puzzling after 40 years. Cleveland Clinic Journal of Medicine 85 (6):450–5. doi: 10.3949/ccjm.85a.17079.
- Goodin, S., F. Shen, W. J. Shih, N. Dave, M. P. Kane, P. Medina, G. H. Lambert, J. Aisner, M. Gallo, and R. S. DiPaola. 2007. Clinical and biological activity of soy protein powder supplementation in healthy male volunteers. Cancer Epidemiology, Biomarkers & Prevention: a Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 16 (4):829–33. doi: 10.1158/1055-9965.EPI-06-0882.
- Gore, A. C., V. A. Chappell, S. E. Fenton, J. A. Flaws, A. Nadal, G. S. Prins, J. Toppari, and R. T. Zoeller. 2015. EDC-2: The endocrine society's second scientific statement on endocrine-disrupting chemicals. Endocrine Reviews 36 (6):E1–E150. doi: 10.1210/er.2015-1010.
- Govind, A. P., and R. V. Thampan. 2003. Membrane associated estrogen receptors and related proteins: Localization at the plasma membrane and the endoplasmic reticulum. Molecular and Cellular Biochemistry 253 (1-2):233–40. doi: 10.1023/a:1026068017309.
- Graber, J. A. 2013. Pubertal timing and the development of psychopathology in adolescence and beyond. Hormones and Behavior 64 (2):262–9. doi: 10.1016/j.yhbeh.2013.04.003.
- Grace, P. B., J. I. Taylor, Y. L. Low, et al. 2004. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiology, Biomarkers & Prevention 13:698–708. https://pubmed.ncbi.nlm.nih.gov/15159299/.
- Grady, D., T. Gebretsadik, K. Kerlikowske, V. Ernster, and D. Petitti. 1995. Hormone replacement therapy and endometrial cancer risk: A meta-analysis. Obstetrics and Gynecology 85 (2):304–13. doi: 10.1016/0029-7844(94)00383-O.
- Gramec Skledar, D., V. Tvrdý, M. Kenda, A. Zega, M. Pour, P. Horký, P. Mladěnka, M. Sollner Dolenc, and L. Peterlin Mašič. 2020. Applicability of the OECD 455 in-vitro assay for determination of hERa agonistic activity of isoflavonoids. Toxicology and Applied Pharmacology 386:114831. doi: 10.1016/j.taap.2019.114831.
- Gu, D., J. He, X. Duan, et al. 2006. Body weight and mortality among men and women in China. JAMA 295 (7):776–83. doi: 10.1001/jama.295.7.776.
- Gu, L., S. E. House, R. L. Prior, N. Fang, M. J. J. Ronis, T. B. Clarkson, M. E. Wilson, and T. M. Badger. 2006. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. The Journal of Nutrition 136 (5):1215–21. doi: 10.1093/jn/136.5.1215.
- Guha, N., M. L. Kwan, C. P. Quesenberry, E. K. Weltzien, A. L. Castillo, and B. J. Caan. 2009. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: The life after cancer epidemiology study. Breast Cancer Research and Treatment 118 (2):395–405. doi: 10.1007/s10549-009-0321-5.
- Günther, A. L. B., N. Karaolis-Danckert, A. Kroke, T. Remer, and A. E. Buyken. 2010. Dietary protein intake throughout childhood is associated with the timing of puberty. The Journal of Nutrition 140 (3):565–71. doi: 10.3945/jn.109.114934.
- Halm, B. M., L. A. Ashburn, and A. A. Franke. 2007. Isoflavones from soya foods are more bioavailable in children than adults. British Journal of Nutrition 98 (5):998–1005. doi: 10.1017/S0007114507771866.
- Halme, J., M. G. Hammond, J. F. Hulka, S. G. Raj, and L. M. Talbert. 1984. Retrograde menstruation in healthy women and in patients with endometriosis. Obstetrics and Gynecology 64 (2):151–4. https://pubmed.ncbi.nlm.nih.gov/6234483/.
- Hamilton-Reeves, J. M., G. Vazquez, S. J. Duval, W. R. Phipps, M. S. Kurzer, and M. J. Messina. 2010. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: Results of a meta-analysis. Fertility and Sterility 94 (3):997–1007. doi: 10.1016/j.fertnstert.2009.04.038.
- Hanahan, D., and R. A. Weinberg. 2011. Hallmarks of cancer: The next generation. Cell 144 (5):646–74. doi: 10.1016/j.cell.2011.02.013.
- Hara, A., S. Sasazuki, M. Inoue, M. Iwasaki, T. Shimazu, N. Sawada, T. Yamaji, and S. Tsugane. 2012. Isoflavone intake and risk of gastric cancer: A population-based prospective cohort study in Japan. The American Journal of Clinical Nutrition 95 (1):147–54. doi: 10.3945/ajcn.111.020479.
- Hara, A., S. Sasazuki, M. Inoue, T. Miura, M. Iwasaki, N. Sawada, T. Shimazu, T. Yamaji, and S. Tsugane. 2013. Plasma isoflavone concentrations are not associated with gastric cancer risk among Japanese men and women. The Journal of Nutrition 143 (8):1293–8. doi: 10.3945/jn.113.175505.
- Harding, G., K. S. Coyne, C. L. Thompson, and J. B. Spies. 2008. The responsiveness of the uterine fibroid symptom and health-related quality of life questionnaire (UFS-QOL). Health and Quality of Life Outcomes 6 (1):99. doi: 10.1186/1477-7525-6-99.
- Hargreaves, D. F., C. S. Potten, C. Harding, et al. 1999. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. Journal of Clinical Endocrinology and Metabolism 84 (11):4017–24. doi: 10.1210/jcem.84.11.6152.
- Harley, K. G., K. P. Berger, K. Kogut, K. Parra, R. H. Lustig, L. C. Greenspan, A. M. Calafat, X. Ye, and B. Eskenazi. 2019. Association of phthalates, parabens and phenols found in personal care products with pubertal timing in girls and boys. Human Reproduction (Oxford, England) 34 (1):109–17. doi: 10.1093/humrep/dey337.
- Harmon, S. M., and C. F. Seifert. 1991. Levothyroxine-cholestyramine interaction reemphasized. Annals of Internal Medicine 115 (8):658–9. doi: 10.7326/0003-4819-115-8-658_2.
- Harrigan, G. G., W. P. Ridley, S. G. Riordan, M. A. Nemeth, R. Sorbet, W. A. Trujillo, M. L. Breeze, and R. W. Schneider. 2007. Chemical composition of glyphosate-tolerant soybean 40-3-2 grown in Europe remains equivalent with that of conventional soybean (Glycine max L.). Journal of Agricultural and Food Chemistry 55 (15):6160–8. doi: 10.1021/jf0704920.
- Harris, M. A., J. C. Prior, and M. Koehoorn. 2008. Age at menarche in the Canadian population: secular trends and relationship to adulthood BMI. The Journal of Adolescent Health: Official Publication of the Society for Adolescent Medicine 43 (6):548–54. doi: 10.1016/j.jadohealth.2008.07.017.
- Harris, R. M., and R. H. Waring. 2012. Diethylstilboestrol-a long-term legacy. Maturitas 72 (2):108–12. doi: 10.1016/j.maturitas.2012.03.002.
- Hasanah, Y., T. C. Nisa, H. Armidin, and H. Hanum. 2015. Isoflavone content of soybean [Glycine max (L). Merr.] cultivars with different nitrogen sources and growing season under dry land conditions. JAEID 109:5–17. doi: 10.12895/jaeid.20151.216.
- Hatena Blog. How much is it in Tokyo? Hatena Blog. Accessed February 09, 2021. http://nbakki.hatenablog.com/entry/Kids_Average_Height_and_Weight_by_Age_in_Japan.
- He, Y., Q. Zeng, S. Dong, L. Qin, G. Li, and P. Wang. 2013. Associations between uterine fibroids and lifestyles including diet, physical activity and stress: A case-control study in China. Asia Pacific Journal of Clinical Nutrition 22 (1):109–17. doi: 10.6133/apjcn.2013.22.1.07.
- Hedlund, T. E., P. D. Maroni, P. G. Ferucci, R. Dayton, S. Barnes, K. Jones, R. Moore, L. G. Ogden, K. WäHäLä, H. M. Sackett, et al. 2005. Long-term dietary habits affect soy isoflavone metabolism and accumulation in prostatic fluid in Caucasian men. The Journal of Nutrition 135 (6):1400–6. doi: 10.1093/jn/135.6.1400.
- Helk, O., and K. Widhalm. 2020. Effects of a low-fat dietary regimen enriched with soy in children affected with heterozygous familial hypercholesterolemia. Clinical Nutrition Espen 36:150–6. doi: 10.1016/j.clnesp.2019.09.009.
- Herbst, A. L. 1976. Summary of the changes in the human female genital tract as a consequence of maternal diethylstilbestrol therapy. Journal of Toxicology and Environmental Health. Supplement 1:13–20. https://pubmed.ncbi.nlm.nih.gov/994230/.
- Herbst, A. L., H. Ulfelder, and D. C. Poskanzer. 1971. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. The New England Journal of Medicine 284 (15):878–81. doi: 10.1056/NEJM197104222841604.
- Herman-Giddens, M. E. 2006. Recent data on pubertal milestones in United States children: The secular trend toward earlier development. International Journal of Andrology 29 (1):241–6. doi: 10.1111/j.1365-2605.2005.00575.x.
- Herman-Giddens, M. E., J. Steffes, D. Harris, E. Slora, M. Hussey, S. A. Dowshen, R. Wasserman, J. R. Serwint, L. Smitherman, and E. O. Reiter. 2012. Secondary sexual characteristics in boys: Data from the Pediatric Research in Office Settings Network. Pediatrics 130 (5):e1058-68–e1068. doi: 10.1542/peds.2011-3291.
- Hilakivi-Clarke, L., S. de Assis, and A. Warri. 2013. Exposures to synthetic estrogens at different times during the life, and their effect on breast cancer risk. Journal of Mammary Gland Biology and Neoplasia 18 (1):25–42. doi: 10.1007/s10911-013-9274-8.
- Hill, M., A. Parízek, R. Kancheva, M. Dusková, M. Velíková, L. Kríz, M. Klímková, A. Pasková, Z. Zizka, P. Matucha, et al. 2010. Steroid metabolome in plasma from the umbilical artery, umbilical vein, maternal cubital vein and in amniotic fluid in normal and preterm labor. The Journal of Steroid Biochemistry and Molecular Biology 121 (3-5):594–610. doi: 10.1016/j.jsbmb.2009.10.012.
- Himes, J. H. 2006. Examining the evidence for recent secular changes in the timing of puberty in US children in light of increases in the prevalence of obesity. Molecular and Cellular Endocrinology 254-255:13–21. doi: 10.1016/j.mce.2006.04.013.
- Hirose, K., K. Tajima, N. Hamajima, T. Takezaki, M. Inoue, T. Kuroishi, K. Kuzuya, S. Nakamura, and S. Tokudome. 1996. Subsite (cervix/endometrium)-specific risk and protective factors in uterus cancer. Japanese Journal of Cancer Research 87 (9):1001–9. doi: 10.1111/j.1349-7006.1996.tb02132.x.
- Ho, S. C., J. Woo, S. Lam, Y. Chen, A. Sham, and J. Lau. 2003. Soy protein consumption and bone mass in early postmenopausal Chinese women. Osteoporosis International 14 (10):835–42. doi: 10.1007/s00198-003-1453-9.
- Hodis, H. N., W. J. Mack, N. Kono, S. P. Azen, D. Shoupe, J. Hwang-Levine, D. Petitti, L. Whitfield-Maxwell, M. Yan, A. A. Franke, et al. 2011. Isoflavone soy protein supplementation and atherosclerosis progression in healthy postmenopausal women: A randomized controlled trial. Stroke 42 (11):3168–75. doi: 10.1161/STROKEAHA.111.620831.
- Hollier, L. P., J. A. Keelan, M. Hickey, M. T. Maybery, and A. J. O. Whitehouse. 2014. Measurement of androgen and estrogen concentrations in cord blood: Accuracy, biological interpretation, and applications to understanding human behavioral development. Frontiers in Endocrinology 5:64. doi: 10.3389/fendo.2014.00064.
- Holman, R. C., E. D. Belay, K. Y. Christensen, A. M. Folkema, C. A. Steiner, and L. B. Schonberger. 2010. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatric Infectious Disease Journal 29 (6):483–8. doi: 10.1097/INF.0b013e3181cf8705.
- Hong, S. J., S. I. Kim, S. M. Kwon, J. R. Lee, and B. C. Chung. 2002. Comparative study of concentration of isoflavones and lignans in plasma and prostatic tissues of normal control and benign prostatic hyperplasia. Yonsei Medical Journal 43 (2):236–41. doi: 10.3349/ymj.2002.43.2.236.
- Hooper, L., G. Madhavan, J. A. Tice, S. J. Leinster, and A. Cassidy. 2010. Effects of isoflavones on breast density in pre- and post-menopausal women: A systematic review and meta-analysis of randomized controlled trials. Human Reproduction Update 16 (6):745–60. doi: 10.1093/humupd/dmq011.
- Hooper, L., J. J. Ryder, M. S. Kurzer, J. W. Lampe, M. J. Messina, W. R. Phipps, and A. Cassidy. 2009. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: A systematic review and meta-analysis. Human Reproduction Update 15 (4):423–40. doi: 10.1093/humupd/dmp010.
- Hosoda, K., T. Furuta, A. Yokokawa, and K. Ishii. 2010. Identification and quantification of daidzein-7-glucuronide-4'-sulfate, genistein-7-glucuronide-4'-sulfate and genistein-4',7-diglucuronide as major metabolites in human plasma after administration of kinako. Analytical and Bioanalytical Chemistry 397 (4):1563–72. doi: 10.1007/s00216-010-3714-8.
- Hosokawa, M., S. Imazeki, and H. Mizunuma. 2012. Secular trends in age at menarche and time to establish regular menstrual cycling in Japanese women born between 1930 and 1985. BMC Womens Health 12:19. doi: 10.1186/1472-6874-12-19.
- Hozawa, A., Y. Sugawara, Y. Tomata, M. Kakizaki, T. Tsuboya, K. Ohmori-Matsuda, N. Nakaya, S. Kuriyama, A. Fukao, I. Tsuji, et al. 2013. Relationship between serum isoflavone levels and disability-free survival among community-dwelling elderly individuals: Nested case-control study of the Tsurugaya project. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 68 (4):465–72. doi: 10.1093/gerona/gls198.
- Hsiao, A. K.-F., and P. M. Lyons-Wall. 2000. Soy consumption in Taiwanese children in Taipei. Journal of Nutrition 130:705S. doi: 10.1093/jn/130.3.680S.
- Hsieh, C. Y., R. C. Santell, S. Z. Haslam, and W. G. Helferich. 1998. Estrogenic effects of genistein on the growth of estrogen receptor- positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Research 58 (17):3833–8. https://pubmed.ncbi.nlm.nih.gov/9731492/.
- Hu, X. J., W. R. Song, L. Y. Gao, S. P. Nie, G. Eisenbrand, and M. Y. Xie. 2014. Assessment of dietary phytoestrogen intake via plant-derived foods in China. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment 31 (8):1325–35. doi: 10.1080/19440049.2014.930562.
- Hua, X.-G., R. Hu, C.-Y. Hu, F.-L. Li, W. Jiang, and X.-J. Zhang. 2018. Associations between hypospadias, cryptorchidism and anogenital distance: Systematic review and meta-analysis. Andrologia 50 (10):e13152 doi: 10.1111/and.13152.
- Huang, S.-K., M.-T. Lin, H.-C. Chen, S.-C. Huang, and M.-H. Wu. 2013. Epidemiology of Kawasaki disease: Prevalence from national database and future trends projection by system dynamics modeling. Journal of Pediatrics 163 (1):126–31 e1. doi: 10.1016/j.jpeds.2012.12.011.
- Huang, Z.-Y., D. R. Barreda, R. G. Worth, Z. K. Indik, M.-K. Kim, P. Chien, and A. D. Schreiber. 2006. Differential kinase requirements in human and mouse Fc-gamma receptor phagocytosis and endocytosis. Journal of Leukocyte Biology 80 (6):1553–62. doi: 10.1189/jlb.0106019.
- Huggett, A. C., S. Pridmore, A. Malnoë, F. Haschke, and E. A. Offord. 1997. Phyto-oestrogens in soy-based infant formula. Lancet (London, England) 350 (9080):815–6. doi: 10.1016/s0140-6736(05)62613-x.
- Hur, H. G., J. O. Lay, R. D. Beger, J. P. Freeman, and F. Rafii. 2000. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Archives of Microbiology 174 (6):422–8. doi: 10.1007/s002030000222.
- Husain, D., K. Khanna, S. Puri, and M. Haghighizadeh. 2015. Supplementation of soy isoflavones improved sex hormones, blood pressure, and postmenopausal symptoms. Journal of the American College of Nutrition. 34 (1):42–8. doi: 10.1080/07315724.2013.875434.
- Hüser, S., S. Guth, H. G. Joost, S. T. Soukup, J. Köhrle, L. Kreienbrock, P. Diel, D. W. Lachenmeier, G. Eisenbrand, G. Vollmer, et al. 2018. Effects of isoflavones on breast tissue and the thyroid hormone system in humans: A comprehensive safety evaluation. Archives of Toxicology 92 (9):2703–48. doi: 10.1007/s00204-018-2279-8.
- Hutchins, A. M., J. L. Slavin, and J. W. Lampe. 1995. Urinary isoflavonoid phytoestrogen and lignan excretion after consumption of fermented and unfermented soy products. Journal of the American Dietetic Association 95 (5):545–51. doi: 10.1016/S0002-8223(95)00149-2.
- Hwang, C. S., H. S. Kwak, H. J. Lim, S. H. Lee, Y. S. Kang, T. B. Choe, H. G. Hur, and K. O. Han. 2006. Isoflavone metabolites and their in vitro dual functions: They can act as an estrogenic agonist or antagonist depending on the estrogen concentration. The Journal of Steroid Biochemistry and Molecular Biology 101 (4-5):246–53. doi: 10.1016/j.jsbmb.2006.06.020.
- Ichige, M., E. Fukuda, S. Miida, J-i Hattan, N. Misawa, S. Saito, T. Fujimaki, M. Imoto, and K. Shindo. 2013. Novel isoflavone glucosides in groundnut (Apios americana Medik) and their antiandrogenic activities. Journal of Agricultural and Food Chemistry 61 (9):2183–7. doi: 10.1021/jf305233t.
- ILSI range is from ILSI Crop Composition Database. 2019. Accessed February 9, 2020 (ILSI).
- Irvine, C. H., M. G. Fitzpatrick, and S. L. Alexander. 1998. Phytoestrogens in soy-based infant foods: Concentrations, daily intake, and possible biological effects. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 217 (3):247–53. DOI: 10.1073/pnas.73.7.2275
- Irvine, C. H., N. Shand, M. G. Fitzpatrick, and S. L. Alexander. 1998. Daily intake and urinary excretion of genistein and daidzein by infants fed soy- or dairy-based infant formulas. The American Journal of Clinical Nutrition 68 (6):1462S–5S. doi: 10.1093/ajcn/68.6.1462S.
- Irvine, C., M. Fitzpatrick, I. Robertson, and D. Woodhams. 1995. The potential adverse effects of soybean phytoestrogens in infant feeding. The New Zealand Medical Journal 108 (1000):208–9. https://pubmed.ncbi.nlm.nih.gov/7783996/.
- Ishitsuka, K., S. Sasaki, K. Yamamoto-Hanada, H. Mezawa, M. Konishi, and Y. Ohya. 2020. Changes in dietary intake in pregnant women from periconception to pregnancy in the Japan Environment and Children's Study: A nationwide Japanese birth cohort study. Maternal and Child Health Journal 24 (3):389–400. doi: 10.1007/s10995-019-02835-z.
- Islam, M. A., R. Bekele, J. H. J. Vanden Berg, Y. Kuswanti, O. Thapa, S. Soltani, F. X. R. van Leeuwen, I. M. C. M. Rietjens, and A. J. Murk. 2015. Deconjugation of soy isoflavone glucuronides needed for estrogenic activity. Toxicology in Vitro: An International Journal Published in Association with BIBRA 29 (4):706–15. doi: 10.1016/j.tiv.2015.01.013.
- Iwasaki, M., M. Inoue, T. Otani, S. Sasazuki, N. Kurahashi, T. Miura, S. Yamamoto, and S. Tsugane. 2008. Plasma isoflavone level and subsequent risk of breast cancer among Japanese women: A nested case-control study from the Japan Public Health Center-based prospective study group. Journal of Clinical Oncology : official Journal of the American Society of Clinical Oncology 26 (10):1677–83. doi: 10.1200/JCO.2007.13.9964.
- Izumi, T., M. K. Piskula, S. Osawa, A. Obata, K. Tobe, M. Saito, S. Kataoka, Y. Kubota, and M. Kikuchi. 2000. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. The Journal of Nutrition 130 (7):1695–9. doi: 10.1093/jn/130.7.1695.
- Jacobsen, B. K., K. Jaceldo-Siegl, S. F. Knutsen, et al. 2014. Soy isoflavone intake and the likelihood of ever becoming a mother: The Adventist Health Study-2. International Journal of Women's Health 6:377–84. doi: 10.2147/IJWH.S57137.
- Jakes, R. W., L. Alexander, S. W. Duffy, J. Leong, L. H. Chen, and W. H. Lee. 2001. Dietary intake of soybean protein and menstrual cycle length in pre-menopausal Singapore Chinese women. Public Health Nutrition 4 (2):191–6. doi: 10.1079/phn200063.
- Jamilian, M., and Z. Asemi. 2016. The effects of soy isoflavones on metabolic status of patients with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 101 (9):3386–94. doi: 10.1210/jc.2016-1762.
- Jang, C. H., C. S. Park, J. K. Lim, et al. 2008. Metabolism of isoflavone derivatives during the manufacturing of traditional meju and doenjang. Food Science and Biotechnology17:442–5. https://www.koreascience.or.kr/article/JAKO200833338360467.page.
- Jang, H.-H., H. Noh, H.-W. Kim, S.-Y. Cho, H.-J. Kim, S.-H. Lee, S.-H. Lee, M. J. Gunter, P. Ferrari, A. Scalbert, et al. 2020. Metabolic tracking of isoflavones in soybean products and biosamples from healthy adults after fermented soybean consumption. Food Chemistry 330:127317. doi: 10.1016/j.foodchem.2020.127317.
- Janulewicz, P. A., J. M. Carlson, A. K. Wesselink, L. A. Wise, E. E. Hatch, L. M. Edwards, and J. L. Peters. 2019. Urinary isoflavones levels in relation to serum thyroid hormone concentrations in female and male adults in the U.S. general population. International Journal of Environmental Health Research :1–12. doi: 10.1080/09603123.2019.1663497.
- Jarrell, J., W. G. Foster, and D. W. Kinniburgh. 2012. Phytoestrogens in human pregnancy. Obstetrics and Gynecology International 2012:850313. doi: 10.1155/2012/850313.
- Jaskulski, S., A. Y. Jung, M. Huebner, G. Poschet, R. Hell, A. Hüsing, S. Gonzalez-Maldonado, S. Behrens, N. Obi, H. Becher, and J. Chang-Claude. 2019. Prognostic associations of circulating phytoestrogens and biomarker changes in long-term survivors of postmenopausal breast cancer. Nutrition and Cancer :1–15.
- Jefferson, W. N., H. B. Patisaul, and C. J. Williams. 2012. Reproductive consequences of developmental phytoestrogen exposure. Reproduction (Cambridge, England) 143 (3):247–60. doi: 10.1530/REP-11-0369.
- Jiang, Y., P. Gong, Z. Madak-Erdogan, T. Martin, M. Jeyakumar, K. Carlson, I. Khan, T. J. Smillie, A. G. Chittiboyina, S. C. K. Rotte, et al. 2013. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 27 (11):4406–18.,doi: 10.1096/fj.13-234617.
- Jochum, F., B. Alteheld, P. Meinardus, N. Dahlinger, A. Nomayo, and P. Stehle. 2017. Mothers' consumption of soy drink but not black tea increases the flavonoid content of term breast milk: A pilot randomized, controlled intervention study. Annals of Nutrition & Metabolism 70 (2):147–53. doi: 10.1159/000471857.
- Jocsak, G., E. Ioja, D. S. Kiss, I. Toth, Z. Barany, T. Bartha, L. V. Frenyo, and A. Zsarnovszky. 2019. Endocrine disruptors induced distinct expression of thyroid and estrogen receptors in rat versus mouse primary cerebellar cell cultures. Brain Sciences 9 (12):359. doi: 10.3390/brainsci9120359.
- Johnson, E. B., M. G. Muto, E. H. Yanushpolsky, and G. L. Mutter. 2001. Phytoestrogen supplementation and endometrial cancer. Obstetrics and Gynecology 98 (5 Pt 2):947–50. doi: 10.1016/s0029-7844(01)01542-3.
- Ju, Y. H., C. D. Allred, K. F. Allred, K. L. Karko, D. R. Doerge, and W. G. Helferich. 2001. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. The Journal of Nutrition 131 (11):2957–62. doi: 10.1093/jn/131.11.2957.
- Ju, Y. H., D. R. Doerge, K. A. Woodling, J. A. Hartman, J. Kwak, and W. G. Helferich. 2008. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis 29 (11):2162–8. doi: 10.1093/carcin/bgn161.
- Ju, Y. H., D. R. Doerge, K. F. Allred, C. D. Allred, and W. G. Helferich. 2002. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Research 62 (9):2474–7. https://pubmed.ncbi.nlm.nih.gov/11980635/.
- Ju, Y. H., J. Fultz, K. F. Allred, D. R. Doerge, and W. G. Helferich. 2006. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis 27 (4):856–63. doi: 10.1093/carcin/bgi320.
- Junqueira Do Lago, M., E. Faerstein, C. De Souza Lopes, and G. L. Werneck. 2003. Family socio-economic background modified secular trends in age at menarche: Evidence from the Pró-Saúde Study (Rio de Janeiro, Brazil) . Ann Hum Biol 30 (3):347–52. ). doi: 10.1080/0301446031000091783.
- Kang, J., T. M. Badger, M. J. J. Ronis, and X. Wu. 2010. Non-isoflavone phytochemicals in soy and their health effects. Journal of Agricultural and Food Chemistry 58 (14):8119–33. doi: 10.1021/jf100901b.
- Kang, S., Y. M. Kim, J. A. Lee, D. H. Kim, and J. S. Lim. 2019. Early menarche is a risk factor for short stature in young Korean females: An epidemiologic study. Journal of Clinical Research in Pediatric Endocrinology 11 (3):234–9. doi: 10.4274/jcrpe.galenos.2018.2018.0274.
- Kang, X., Q. Zhang, S. Wang, X. Huang, and S. Jin. 2010. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. Canadian Medical Association Journal 182 (17):1857–62. doi: 10.7314/apjcp.2013.14.4.2407.
- Kano, M., T. Takayanagi, K. Harada, S. Sawada, and F. Ishikawa. 2006. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. The Journal of Nutrition 136 (9):2291–6. doi: 10.1093/jn/136.9.2291.
- Karamali, M., M. Kashanian, S. Alaeinasab, and Z. Asemi. 2018. The effect of dietary soy intake on weight loss, glycaemic control, lipid profiles and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: A randomised clinical trial. Journal of Human Nutrition and Dietetics 31 (4):533–43. Aug doi: 10.1111/jhn.12545.
- Kato, N., H. Takimoto, T. Yokoyama, S. Yokoya, T. Tanaka, and H. Tada. 2014. Updated Japanese growth references for infants and preschool children, based on historical, ethnic and environmental characteristics. Acta Paediatrica ( Paediatrica 103 (6):e251–e61. doi: 10.1111/apa.12587.
- Kavlock, R. J., G. P. Daston, C. DeRosa, P. Fenner-Crisp, L. E. Gray, S. Kaattari, G. Lucier, M. Luster, M. J. Mac, C. Maczka, et al. 1996. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: A report of the U.S. EPA-sponsored workshop. Environmental Health Perspectives 104:715–40. doi: 10.1289/ehp.96104s4715.
- Keel, B. A., K. B. Eddy, S. Cho, B. K. Gangrade, and J. V. May. 1992. Purified human alpha fetoprotein inhibits growth factor-stimulated estradiol production by porcine granulosa cells in monolayer culture. Endocrinology 130 (6):3715–7. doi: 10.1210/endo.130.6.1375908.
- Keelan, J. A., E. Mattes, H. Wei Tan, A. Dinan, J. P. Newnham, A. J. O. Whitehouse, P. Jacoby, and M. Hickey. 2012. Androgen concentrations in umbilical cord blood and their association with maternal, fetal and obstetric factors. PloS One 7 (8):e42827. doi: 10.1371/journal.pone.0042827.
- Khan, S. A., R. T. Chatterton, N. Michel, M. Bryk, O. Lee, D. Ivancic, R. Heinz, C. M. Zalles, I. B. Helenowski, B. D. Jovanovic, et al. 2012. Soy isoflavone supplementation for breast cancer risk reduction: A randomized phase II trial. Cancer Prevention Research (Philadelphia, Pa.) 5 (2):309–19. doi: 10.1158/1940-6207.CAPR-11-0251.
- Khani, B., F. Mehrabian, E. Khalesi, et al. 2011. Effect of soy phytoestrogen on metabolic and hormonal disturbance of women with polycystic ovary syndrome. Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences 16:297–302.
- Kim, J. K., E.-H. Kim, I. Park, B.-R. Yu, J. D. Lim, Y.-S. Lee, J.-H. Lee, S.-H. Kim, and I.-M. Chung. 2014. Isoflavones profiling of soybean [Glycine max (L.) Merrill] germplasms and their correlations with metabolic pathways. Food Chemistry. 153:258–64. doi: 10.1016/j.foodchem.2013.12.066.
- Kim, J., S. Kim, K. Huh, Y. Kim, H. Joung, and M. Park. 2011. High serum isoflavone concentrations are associated with the risk of precocious puberty in Korean girls. Clinical Endocrinology 75 (6):831–5. doi: 10.1111/j.1365-2265.2011.04127.x.
- Kim, S., S. Moon, and B. M. Popkin. 2000. The nutrition transition in South Korea. The American Journal of Clinical Nutrition 71 (1):44–53. doi: 10.1093/ajcn/71.1.44.
- Kim, Y. J., M. Y. Park, N. Chang, and O. Kwon. 2015. Intake and major sources of dietary flavonoid in Korean adults: Korean National Health and Nutrition Examination Survey 2010-2012. Asia Pacific Journal of Clinical Nutrition 24 (3):456–63. doi: 10.6133/apjcn.2015.24.3.04.
- King, R. A., and D. B. Bursill. 1998. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. The American Journal of Clinical Nutrition 67 (5):867–72. doi: 10.1093/ajcn/67.5.867.
- King, R. A., J. L. Broadbent, and R. J. Head. 1996. Absorption and excretion of the soy isoflavone genistein in rats. The Journal of Nutrition 126 (1):176–82. doi: 10.1093/jn/126.1.176.
- Koh, W.-P., J.-M. Yuan, C.-L. Sun, H.-P. Lee, and M. C. Yu. 2005. Middle-aged and older Chinese men and women in Singapore who smoke have less healthy diets and lifestyles than nonsmokers. The Journal of Nutrition 135 (10):2473–7. doi: 10.1093/jn/135.10.2473.
- Köhrle, J. 2004. Low dose competition of flavonoids with endogenous thyroid transport proteins: Potential relevance to the thyroid hormone axis. In: Functional food: Safety aspects, symposium Forschungsberichte (DFG) Deutsche Forschungsgemeinschaft, ed. W.-V. Verlag, 112–37.
- Konishi, K., K. Wada, M. Yamakawa, Y. Goto, F. Mizuta, S. Koda, T. Uji, M. Tsuji, and C. Nagata. 2019. Dietary soy intake is inversely associated with risk of type 2 diabetes in Japanese women but not in men. The Journal of Nutrition 149 (7):1208–14. doi: 10.1093/jn/nxz047.
- Korde, L. A., A. H. Wu, T. Fears, A. M. Y. Nomura, D. W. West, L. N. Kolonel, M. C. Pike, R. N. Hoover, and R. G. Ziegler. 2009. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 18 (4):1050–9. doi: 10.1158/1055-9965.EPI-08-0405.
- Kraemer, W. J., G. Solomon-Hill, B. M. Volk, B. R. Kupchak, D. P. Looney, C. Dunn-Lewis, B. A. Comstock, T. K. Szivak, D. R. Hooper, S. D. Flanagan, et al. 2013. The effects of soy and whey protein supplementation on acute hormonal reponses to resistance exercise in men. Journal of the American College of Nutrition 32 (1):66–74. doi: 10.1080/07315724.2013.770648.
- Kreijkamp-Kaspers, S., L. Kok, M. L. Bots, D. E. Grobbee, and Y. T. van der Schouw. 2004. Dietary phytoestrogens and vascular function in postmenopausal women: A cross-sectional study. Journal of Hypertension 22 (7):1381–8. doi: 10.1097/01.hjh.0000125435.28861.d2.
- Krysiak-Baltyn, K., J. Toppari, N. E. Skakkebaek, T. S. Jensen, H. E. Virtanen, K.-W. Schramm, H. Shen, T. Vartiainen, H. Kiviranta, O. Taboureau, et al. 2010. Country-specific chemical signatures of persistent environmental compounds in breast milk. International Journal of Andrology 33 (2):270–8. doi: 10.1111/j.1365-2605.2009.00996.x.
- Kudou, S., Y. Fleury, D. Welti, D. Magnolato, T. Uchida, K. Kitamura, and K. Okubo. 1991. Malonyl isoflavone glycosides in soybean seeds (Glycine max MERRILL). Agricultural and Biological Chemistry 55 (9):2227–33. doi: 10.1080/00021369.1991.10870966.
- Kuijper, E. A. M., J. C. F. Ket, M. R. Caanen, and C. B. Lambalk. 2013. Reproductive hormone concentrations in pregnancy and neonates: A systematic review. Reproductive Biomedicine Online 27 (1):33–63. doi: 10.1016/j.rbmo.2013.03.009.
- Kuiper, G. G., B. Carlsson, K. Grandien, E. Enmark, J. Häggblad, S. Nilsson, and J. A. Gustafsson. 1997. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138 (3):863–70. doi: 10.1210/endo.138.3.4979.
- Kuiper, G. G., E. Enmark, M. Pelto-Huikko, S. Nilsson, and J. A. Gustafsson. 1996. Cloning of a novel receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences of the United States of America 93 (12):5925–30. doi: 10.1073/pnas.93.12.5925.
- Kuiper, G. G., J. G. Lemmen, B. Carlsson, J. C. Corton, S. H. Safe, P. T. van der Saag, B. van der Burg, and J. A. Gustafsson. 1998. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139 (10):4252–63. doi: 10.1210/endo.139.10.6216.
- Kuo, L.-C., W.-Y. Cheng, R.-Y. Wu, C.-J. Huang, and K.-T. Lee. 2006. Hydrolysis of black soybean isoflavone glycosides by Bacillus subtilis natto. Applied Microbiology and Biotechnology 73 (2):314–20. doi: 10.1007/s00253-006-0474-7.
- Kurahashi, N., M. Iwasaki, M. Inoue, S. Sasazuki, and S. Tsugane. 2008. Plasma isoflavones and subsequent risk of prostate cancer in a nested case-control study: The Japan Public Health Center. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 26 (36):5923–9. doi: 10.1200/JCO.2008.16.8807.
- Kurahashi, N., M. Murakumo, H. Kakizaki, K. Nonomura, T. Koyanagi, S. Kasai, F. Sata, and R. Kishi. 2004. The estimated prevalence of hypospadias in Hokkaido, Japan. Journal of Epidemiology 14 (3):73–7. doi: 10.2188/jea.14.73.
- Kurman, R. J., P. F. Kaminski, and H. J. Norris. 1985. The behavior of endometrial hyperplasia. A long-term study of "untreated" hyperplasia in 170 patients. Cancer 56 (2):403–12. doi: 10.1002/1097-0142(19850715)56:2 < 403::aid-cncr2820560233 > 3.0.co;2-x.
- Kurniawan, A.-L., C.-Y. Hsu, J. C.-J. Chao, R. Paramastri, H.-A. Lee, P.-C. Lai, N.-C. Hsieh, and S.-F V. Wu. 2021. Association of testosterone-related dietary pattern with testicular function among adult men: A cross-sectional health screening study in Taiwan. Nutrients 13 (1):259. doi: 10.3390/nu13010.
- Kwack, S. J., K.-B. Kim, H. S. Kim, K. S. Yoon, and B. M. Lee. 2009. Risk assessment of soybean-based phytoestrogens. Journal of Toxicology and Environmental Health, Part A 72 (21-22):1254–61. doi: 10.1080/15287390903212212.
- Labos, G., E. Trakakis, P. Pliatsika, A. Augoulea, V. Vaggopoulos, G. Basios, G. Simeonidis, M. Creatsa, A. Alexandrou, Z. Iliodromiti, et al. 2013. Efficacy and safety of DT56a compared to hormone therapy in Greek post-menopausal women. Journal of Endocrinological Investigation 36:521–6. doi: 10.3275/8926.
- Lacey, M., J. Bohday, S. M. R. Fonseka, A. I. Ullah, and S. A. Whitehead. 2005. Dose-response effects of phytoestrogens on the activity and expression of 3beta-hydroxysteroid dehydrogenase and aromatase in human granulosa-luteal cells. The Journal of Steroid Biochemistry and Molecular Biology 96 (3-4):279–86. doi: 10.1016/j.jsbmb.2005.03.006. [InsertedFromOnline[pubmedMismatch]]
- Lagiou, P., E. Samoli, W. Okulicz, B. Xu, A. Lagiou, L. Lipworth, C. Georgila, L. Vatten, H. O. Adami, D. Trichopoulos, et al. 2011. Maternal and cord blood hormone levels in the United States and China and the intrauterine origin of breast cancer. Annals of Oncology 22 (5):1102–8. doi: 10.1093/annonc/mdq565.
- Lamartiniere, C. A., J. Moore, M. Holland, and S. Barnes. 1995. Neonatal genistein chemoprevents mammary cancer. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 208 (1):120–3. doi: 10.3181/00379727-208-43843.
- Lamartiniere, C. A., Y. X. Zhao, and W. A. Fritz. 2000. Genistein: Mammary cancer chemoprevention, in vivo mechanisms of action, potential for toxicity and bioavailability in rats. Journal of Women's Cancer 2:11–9.
- Lampe, J. W., D. R. Gustafson, A. M. Hutchins, M. C. Martini, S. Li, K. Wähälä, G. A. Grandits, J. D. Potter, and J. L. Slavin. 1999. Urinary isoflavonoid and lignan excretion on a Western diet: Relation to soy, vegetable, and fruit intake. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 8 (8):699–707. https://pubmed.ncbi.nlm.nih.gov/10744130/.
- Lappé, M. A., E. B. Bailey, C. Childress, and K. D. R. Setchell. 1998. Alterations in clinically important phytoestrogens in genetically modified, herbicide-tolerant soybeans. Journal of Medicinal Food 1 (4):241–5. doi: 10.1089/jmf.1998.1.241.
- Lee, A., L. Beaubernard, V. Lamothe, and C. Bennetau-Pelissero. 2019. New evaluation of isoflavone exposure in the French population. Nutrients 11 (10):2308. doi: 10.3390/nu11102308.
- Lee, M. J., and J. H. Kim. 2007. Estimated dietary isoflavone intake among Korean adults. Nutrition Research and Practice 1 (3):206–11. doi: 10.4162/nrp.2007.1.3.206.
- Lee, S.-A., W. Wen, Y.-B. Xiang, S. Barnes, D. Liu, Q. Cai, W. Zheng, and X. O. Shu. 2007. Assessment of dietary isoflavone intake among middle-aged Chinese men. The Journal of Nutrition 137 (4):1011–6. doi: 10.1093/jn/137.4.1011.
- Lepping, M. D., R. A. Herman, and B. L. Potts. 2013. Compositional equivalence of DAS-444Ø6-6 (AAD-12 + 2mEPSPS + PAT) herbicide-tolerant soybean and nontransgenic soybean . Journal of Agricultural and Food Chemistry 61 (46):11180–90. doi: 10.1021/jf403775d.
- Levine, L. D., K. Kim, A. Purdue-Smithe, R. Sundaram, E. F. Schisterman, M. Connell, E. A. Devilbiss, Z. Alkhalaf, J. G. Radoc, G. M. Buck Louis, et al. 2020. Urinary phytoestrogens and relationship to menstrual cycle length and variability among healthy, eumenorrheic women. Journal of the Endocrine Society 4 (2):bvz003. doi: 10.1210/jendso/bvz003.
- Levis, S., N. Strickman-Stein, P. Ganjei-Azar, P. Xu, D. R. Doerge, and J. Krischer. 2011. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: A randomized, double-blind trial. Archives of Internal Medicine 171 (15):1363–9. doi: 10.1001/archinternmed.2011.330.
- Lewis, R. W., N. Brooks, G. M. Milburn, A. Soames, S. Stone, M. Hall, and J. Ashby. 2003. The effects of the phytoestrogen genistein on the postnatal development of the rat. Toxicological Sciences : An Official Journal of the Society of Toxicology 71 (1):74–83. doi: 10.1093/toxsci/71.1.74.
- Li, J., X. Teng, W. Wang, Y. Chen, X. Yu, S. Wang, J. Li, L. Zhu, C. Li, C. Fan, et al. 2011. Effects of dietary soy intake on maternal thyroid functions and serum anti-thyroperoxidase antibody level during early pregnancy. Journal of Medicinal Food 14 (5):543–50. doi: 10.1089/jmf.2010.1078.
- Li, N., X. Wu, W. Zhuang, L. Xia, Y. Chen, R. Zhao, M. Yi, Q. Wan, L. Du, Y. Zhou, et al. 2020. Soy and isoflavone consumption and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomized trials in humans. Molecular Nutrition & Food Research 64 (4):e1900751 doi: 10.1002/mnfr.201900751.
- Li, Y., Y. He, L. Qi, V. W. Jaddoe, E. J. M. Feskens, X. Yang, G. Ma, and F. B. Hu. 2010. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes 59 (10):2400–6. doi: 10.2337/db10-0385.
- Li, Z., W. J. Aronson, J. R. Arteaga, K. Hong, G. Thames, S. M. Henning, W. Liu, R. Elashoff, J. M. Ashley, and D. Heber. 2008. Feasibility of a low-fat/high-fiber diet intervention with soy supplementation in prostate cancer patients after prostatectomy. European Journal of Clinical Nutrition 62 (4):526–36. doi: 10.1038/sj.ejcn.1602743.
- Liao, C. K., K. A. Rosenblatt, S. M. Schwartz, and N. S. Weiss. 2003. Endometrial cancer in Asian migrants to the United States and their descendants. Cancer Causes & Control : CCC 14 (4):357–60. doi: 10.1023/a:1023925010837. [12846367]
- Liel, Y., A. D. Sperber, and S. Shany. 1994. Nonspecific intestinal adsorption of levothyroxine by aluminum hydroxide. The American Journal of Medicine 97 (4):363–5. doi: 10.1016/0002-9343(94)90303-4.
- Liel, Y., I. Harman-Boehm, and S. Shany. 1996. Evidence for a clinically important adverse effect of fiber-enriched diet on the bioavailability of levothyroxine in adult hypothyroid patients. The Journal of Clinical Endocrinology and Metabolism 81 (2):857–9. doi: 10.1210/jcem.81.2.8636317.
- Lin, M. T., and M. H. Wu. 2017. The global epidemiology of Kawasaki disease: Review and future perspectives. Global Cardiology Science & Practice 2017 (3):e201720. doi: 10.21542/gcsp.2017.20.
- Liu, D., Y. Cheng, B. Mi, L. Zeng, P. Qu, S. Li, R. Zhang, Q. Qi, C. Wu, X. Gao, et al. 2020. Maternal dietary patterns during pregnancy derived by reduced-rank regression and birth weight in the Chinese population. British Journal of Nutrition 123 (10):1176–86. doi: 10.1017/S0007114520000392.
- Liu, J., F. Yuan, J. Gao, B. Shan, Y. Ren, H. Wang, and Y. Gao. 2016. Oral isoflavone supplementation on endometrial thickness: A meta-analysis of randomized placebo-controlled trials. Oncotarget 7 (14):17369–79. doi: 10.18632/oncotarget.7959.
- Liu, Z., W. Li, J. Sun, et al. 2004. Intake of soy foods and soy isoflavones by rural adult women in China. Asia Pacific Journal of Clinical Nutrition 13:204–9. https://pubmed.ncbi.nlm.nih.gov/15228989/.
- Liwanpo, L., and J. M. Hershman. 2009. Conditions and drugs interfering with thyroxine absorption. Best Practice & Research. Clinical Endocrinology & Metabolism 23 (6):781–92. doi: 10.1016/j.beem.2009.06.006.
- Loukovaara, M., M. Carson, A. Palotie, and H. Adlercreutz. 1995. Regulation of sex hormone-binding globulin production by isoflavonoids and patterns of isoflavonoid conjugation in HepG2 cell cultures. Steroids 60 (9):656–61. doi: 10.1016/0039-128X(95)00089-9.
- Lu, M., H. Xiao, K. Li, J. Jiang, K. Wu, and D. Li. 2017. Concentrations of estrogen and progesterone in breast milk and their relationship with the mother's diet. Food & Function 8 (9):3306–10. doi: 10.1039/C7FO00324B.
- Lundh, T. J. O., H. I. Pettersson, and K. A. Martinsson. 1990. Comparative levels of free and conjugated plant estrogens in blood plasma of sheep and cattle fed estrogenic silage. Journal of Agricultural and Food Chemistry 38 (7):1530–4. doi: 10.1021/jf00097a022.
- Lynch, H. M., M. P. Buman, J. M. Dickinson, L. B. Ransdell, C. S. Johnston, and C. M. Wharton. 2020. No significant differences in muscle growth and strength development when consuming soy and whey protein supplements matched for leucine following a 12 week resistance training program in men and women: A randomized trial. International Journal of Environmental Research and Public Health 17 (11):3871. doi: 10.3390/ijerph17113871.
- Ma, X., A. Beeghly-Fadiel, X.-O. Shu, H. Li, G. Yang, Y.-T. Gao, and W. Zheng. 2013. Anthropometric measures and epithelial ovarian cancer risk among Chinese women: Results from the Shanghai Women's Health Study. British Journal of Cancer 109 (3):751–5. doi: 10.1038/bjc.2013.384.
- Main, K. M., H. Kiviranta, H. E. Virtanen, E. Sundqvist, J. T. Tuomisto, J. Tuomisto, T. Vartiainen, N. E. Skakkebaek, and J. Toppari. 2007. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environmental Health Perspectives 115 (10):1519–26. doi: 10.1289/ehp.9924.
- Main, K. M., N. E. Skakkebaek, H. E. Virtanen, and J. Toppari. 2010. Genital anomalies in boys and the environment. Best Practice & Research. Clinical Endocrinology & Metabolism 24 (2):279–89. doi: 10.1016/j.beem.2009.10.003.
- Makino, N., Y. Nakamura, M. Yashiro, R. Ae, S. Tsuboi, Y. Aoyama, T. Kojo, R. Uehara, K. Kotani, and H. Yanagawa. 2015. Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: From the results of the 22nd nationwide survey. Journal of Epidemiology 25 (3):239–45. doi: 10.2188/jea.JE20140089.
- Man, B., C. Cui, X. Zhang, D. Sugiyama, E. Barinas-Mitchell, and A. Sekikawa. 2021. The effect of soy isoflavones on arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. European Journal of Nutrition 60 (2):603–14. . doi: 10.1007/s00394-020-02300-6.
- Marks, K. J., T. J. Hartman, E. V. Taylor, M. E. Rybak, K. Northstone, and M. Marcus. 2017. Exposure to phytoestrogens in utero and age at menarche in a contemporary British cohort. Environmental Research 155:287–93. doi: 10.1016/j.envres.2017.02.030.
- Martinez, J., and J. E. Lewi. 2008. An unusual case of gynecomastia associated with soy product consumption. Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 14 (4):415–8. doi: 10.4158/EP.14.4.415.
- Maskarinec, G., C. Oshiro, Y. Morimoto, S. Hebshi, R. Novotny, and A. A. Franke. 2005. Urinary isoflavone excretion as a compliance measure in a soy intervention among young girls: A pilot study. European Journal of Clinical Nutrition 59 (3):369–75. doi: 10.1038/sj.ejcn.1602083.
- Maskarinec, G., K. Watts, J. Kagihara, S. M. Hebshi, and A. A. Franke. 2008. Urinary isoflavonoid excretion is similar after consuming soya milk and miso soup in Japanese-American women. The British Journal of Nutrition 100 (2):424–9. doi: 10.1017/S0007114508898686.
- Maskarinec, G., N. J. Ollberding, S. M. Conroy, Y. Morimoto, I. S. Pagano, A. A. Franke, E. Gentzschein, and F. Z. Stanczyk. 2011. Estrogen levels in nipple aspirate fluid and serum during a randomized soy trial. Cancer Epidemiology Biomarkers & Prevention 20 (9):1815–21. doi: 10.1158/1055-9965.EPI-11-0363.
- Maskarinec, G., Y. Morimoto, R. Novotny, F. J. Nordt, F. Z. Stanczyk, and A. A. Franke. 2005. Urinary sex steroid excretion levels during a soy intervention among young girls: A pilot study. Nutrition and Cancer 52 (1):22–8. doi: 10.1207/s15327914nc5201_3.
- Mathey, J., V. Lamothe, V. Coxam, M. Potier, P. Sauvant, and C. Bennetau-Pelissero. 2006. Concentrations of isoflavones in plasma and urine of post-menopausal women chronically ingesting high quantities of soy isoflavones. Journal of Pharmaceutical and Biomedical Analysis 41 (3):957–65. doi: 10.1016/j.jpba.2006.01.051.
- Matsumura, A., A. Ghosh, G. S. Pope, and P. D. Darbre. 2005. Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells. The Journal of Steroid Biochemistry and Molecular Biology 94 (5):431–43. doi: 10.1016/j.jsbmb.2004.12.041.
- Matsushita, Y., Y. Takahashi, T. Mizoue, M. Inoue, M. Noda, and S. Tsugane. 2008. Overweight and obesity trends among Japanese adults: A 10-year follow-up of the JPHC Study. International Journal of Obesity (2005) 32 (12):1861–7. doi: 10.1038/ijo.2008.188.
- McCann, M. C., K. Liu, W. A. Trujillo, and R. C. Dobert. 2005. Glyphosate-tolerant soybeans remain compositionally equivalent to conventional soybeans (Glycine max L.) during three years of field testing. Journal of Agricultural and Food Chemistry 53 (13):5331–5. doi: 10.1021/jf0504317.
- McCarrison, R. 1933. The goitrogenic action of soya-bean and ground-nut. Ind J Med Res XXI:179–81.
- McCarver, G., J. Bhatia, C. Chambers, R. Clarke, R. Etzel, W. Foster, P. Hoyer, J. S. Leeder, J. M. Peters, E. Rissman, et al. 2011. NTP-CERHR expert panel report on the developmental toxicity of soy infant formula. Birth Defects Research. Part B, Developmental and Reproductive Toxicology 92 (5):421–68. doi: 10.1002/bdrb.20314.
- McMichael-Phillips, D. F., C. Harding, M. Morton, S. A. Roberts, A. Howell, C. S. Potten, and N. J. Bundred. 1998. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. The American Journal of Clinical Nutrition 68 (6):1431S–5S. doi: 10.1093/ajcn/68.6.1431S.
- Meezan, E., E. M. Meezan, K. Jones, R. Moore, S. Barnes, and J. K. Prasain. 2005. Contrasting effects of puerarin and daidzin on glucose homeostasis in mice. Journal of Agricultural and Food Chemistry 53 (22):8760–7. doi: 10.1021/jf058105e. [InsertedFromOnline[pubmedMismatch]]
- Mejia, W., D. Cordoba, P. Duran, Y. Chacón, and D. Rosselli. 2019. Effect of daily exposure to an isolated soy protein supplement on body composition, energy and macronutrient intake, bone formation markers, and lipid profile in children in Colombia. Journal of Dietary Supplements 16:1–13. doi: 10.1080/19390211.2017.1409851.
- Merrill, R. M. 2008. Hysterectomy surveillance in the United States, 1997 through 2005. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 14 (1):CR24–31. https://pubmed.ncbi.nlm.nih.gov/18160941/.
- Merritt, R. J., and B. H. Jenks. 2004. Safety of soy-based infant formulas containing isoflavones: The clinical evidence. The Journal of Nutrition 134 (5):1220S–4S. doi: 10.1093/jn/134.5.1220S.
- Mesiano, S., S. L. Katz, J. Y. Lee, and R. B. Jaffe. 1999. Phytoestrogens alter adrenocortical function: Genistein and daidzein suppress glucocorticoid and stimulate androgen production by cultured adrenal cortical cells. The Journal of Clinical Endocrinology and Metabolism 84 (7):2443–8. doi: 10.1210/jcem.84.7.5839.
- Messina, M. 1995. Modern applications for an ancient bean: Soybeans and the prevention and treatment of chronic disease. Journal of Nutrition 125 (3 Suppl):567S–9S. doi: 10.1093/jn/125.3_Suppl.567S.
- Messina, M. 2004. Western soy intake is too low to produce health effects. The American Journal of Clinical Nutrition 80 (2):528–9. doi: 10.1093/ajcn/80.2.528.
- Messina, M. 2010. Soybean isoflavone exposure does not have feminizing effects on men: A critical examination of the clinical evidence. Fertility and Sterility 93 (7):2095–104. doi: 10.1016/j.fertnstert.2010.03.002.
- Messina, M. 2011. Evidence does not support the conclusion that soy is an endocrine disruptor. Journal of Pediatric Endocrinology & Metabolism: JPEM 24 (9-10):859–60. doi: 10.1515/JPEM.2011.271.
- Messina, M., and G. Redmond. 2006. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: A review of the relevant literature. Thyroid: Official Journal of the American Thyroid Association 16 (3):249–58. doi: 10.1089/thy.2006.16.249.
- Messina, M., and L. Hilakivi-Clarke. 2009. Early intake appears to be the key to the proposed protective effects of soy intake against breast cancer. Nutrition and Cancer 61 (6):792–8. doi: 10.1080/01635580903285015.
- Messina, M., and S. Barnes. 1991. The role of soy products in reducing risk of cancer. Journal of the National Cancer Institute 83 (8):541–6. doi: 10.1093/jnci/83.8.541.
- Messina, M., C. Nagata, and A. H. Wu. 2006. Estimated Asian adult soy protein and isoflavone intakes. Nutrition and Cancer 55 (1):1–12. doi: 10.1207/s15327914nc5501_1.
- Messina, M., H. Lynch, J. M. Dickinson, and K. E. Reed. 2018. No difference between the effects of supplementing with soy protein versus animal protein on gains in muscle mass and strength in response to resistance exercise. International Journal of Sport Nutrition and Exercise Metabolism 28 (6):674–85. doi: 10.1123/ijsnem.2018-0071.
- Messina, M., M. M. Rogero, M. Fisberg, and D. Waitzberg. 2017. Health impact of childhood and adolescent soy consumption. Nutrition Reviews 75 (7):500–15. doi: 10.1093/nutrit/nux016.
- Messina, M., S. Watanabe, and K. D. Setchell. 2009. Report on the 8th International symposium on the role of soy in health promotion and chronic disease prevention and treatment. The Journal of Nutrition 139 (4):796S–802S. doi: 10.3945/jn.108.104182.
- Micek, A., J. Godos, T. Brzostek, A. Gniadek, C. Favari, P. Mena, M. Libra, D. Del Rio, F. Galvano, and G. Grosso. 2021. Dietary phytoestrogens and biomarkers of their intake in relation to cancer survival and recurrence: A comprehensive systematic review with meta-analysis. Nutrition Reviews 79 (1):42–65. doi: 10.1093/nutrit/nuaa043.
- Michikawa, T., M. Inoue, N. Sawada, Y. Tanaka, T. Yamaji, M. Iwasaki, T. Shimazu, S. Sasazuki, M. Mizokami, S. Tsugane, et al. 2015. Plasma isoflavones and risk of primary liver cancer in Japanese women and men with hepatitis virus infection: A nested case-control study. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 24 (3):532–7. doi: 10.1158/1055-9965.EPI-14-1118.
- Michikawa, T., S. Yamazaki, M. Ono, T. Kuroda, S. F. Nakayama, E. Suda, T. Isobe, M. Iwai-Shimada, Y. Kobayashi, J. Yonemoto, et al. 2019. Isoflavone intake in early pregnancy and hypospadias in the Japan Environment and Children's Study. Urology 124:229–36. doi: 10.1016/j.urology.2018.11.008.
- Milerová, J., J. Cerovská, V. Zamrazil, R. Bílek, O. Lapcík, and R. Hampl. 2006. Actual levels of soy phytoestrogens in children correlate with thyroid laboratory parameters. Clinical Chemistry and Laboratory Medicine 44 (2):171–4. doi: 10.1515/CCLM.2006.031.
- Min, J., Z. Wang, and C. Liang. 2020. Detection of phytoestrogen metabolites in breastfed infants' urine and the corresponding breast milk by LC-MS/MS. Journal of Agricultural and Food Chemistry. doi: 10.1021/acs.jafc.9b08107.
- Minatoya, M., G. Kutomi, S. Asakura, S. Otokozawa, Y. Sugiyama, H. Ohnishi, H. Akasaka, T. Miura, M. Mori, K. Hirata, et al. 2015. Relationship of serum isoflavone, insulin and adiponectin levels with breast cancer risk. Breast Cancer (Tokyo, Japan) 22 (5):452–61. doi: 10.1007/s12282-013-0502-2.
- Mínguez-Alarcón, L., M. C. Afeiche, Y.-H. Chiu, J. C. Vanegas, P. L. Williams, C. Tanrikut, T. L. Toth, R. Hauser, and J. E. Chavarro. 2015. Male soy food intake was not associated with in vitro fertilization outcomes among couples attending a fertility center. Andrology 3 (4):702–8. doi: 10.1111/andr.12046.
- Missmer, S. A., S. E. Hankinson, D. Spiegelman, R. L. Barbieri, L. M. Marshall, and D. J Hunter. 2004. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. American Journal of Epidemiology 160 (8):784–96. doi: 10.1093/aje/kwh275.
- Mitchell, J. H., E. Cawood, D. Kinniburgh, A. Provan, A. R. Collins, and D. S. Irvine. 2001. Effect of a phytoestrogen food supplement on reproductive health in normal males. Clinical Science (London, England: 1979) 100 (6):613–8. doi: 10.1042/CS20000212.
- Miyake, Y., S. Sasaki, Y. Ohya, S. Miyamoto, I. Matsunaga, T. Yoshida, Y. Hirota, and H. Oda. 2005. Soy, isoflavones, and prevalence of allergic rhinitis in Japanese women: The Osaka Maternal and Child Health Study. The Journal of Allergy and Clinical Immunology 115 (6):1176–83. doi: 10.1016/j.jaci.2005.02.016.
- Miyazawa, K. 1976. Incidence of endometriosis among Japanese women. Obstet Gynecol. 48:407–9. https://pubmed.ncbi.nlm.nih.gov/967375/
- Moro, E., E. Degli Esposti, G. Borghese, F. Manzara, M. Zanello, D. Raimondo, G. Gava, A. Arena, P. Casadio, M. C. Meriggiola, et al. 2019. The impact of hormonal replacement treatment in postmenopausal women with uterine fibroids: A state-of-the-art review of the literature. Medicina ( Medicina 55 (9):549. doi: 10.3390/medicina55090549.
- Morris, D. H., M. E. Jones, M. J. Schoemaker, A. Ashworth, and A. J. Swerdlow. 2011. Secular trends in age at menarche in women in the UK born 1908-93: Results from the Breakthrough Generations Study. Paediatric and Perinatal Epidemiology 25 (4):394–400. doi: 10.1111/j.1365-3016.2011.01202.x.
- Morton, M. S., O. Arisaka, N. Miyake, L. D. Morgan, and B. A. J. Evans. 2002. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. The Journal of Nutrition 132 (10):3168–71. doi: 10.1093/jn/131.10.3168.
- Morton, M. S., S. S. F. Leung, D. P. Davies, et al. 1998. Determination of isoflavonoids and lignans in human breast milk from British and Chinese women by gas chromatography-mass spectrometry. Am J Clinical Nutr 68. 1537S.
- Morton, R. W., K. Sato, M. P. B. Gallaugher, S. Y. Oikawa, P. D. McNicholas, S. Fujita, and S. M. Phillips. 2018. Muscle androgen receptor content but not systemic hormones is associated with resistance training-induced skeletal muscle hypertrophy in healthy, young men. Frontiers in Physiology 9 (1373). doi: 10.3389/fphys.2018.01373.
- Mousavi, S. M., N. Tavakoli, and F. Mardan. 2006. Risk factors for goiter in primary school girls in Qom city of Iran. European Journal of Clinical Nutrition 60 (3):426–33. doi: 10.1038/sj.ejcn.1602335.
- Mueller, N. T., A. O. Odegaard, M. D. Gross, W.-P. Koh, M. C. Yu, J.-M. Yuan, and M. A. Pereira. 2012. Soy intake and risk of type 2 diabetes in Chinese Singaporeans [corrected]. European Journal of Nutrition 51 (8):1033–40. doi: 10.1007/s00394-011-0276-2.
- Mulac-Jericevic, B., R. A. Mullinax, F. J. DeMayo, J. P. Lydon, and O. M. Conneely. 2000. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science (New York, N.Y.) 289 (5485):1751–4. doi: 10.1126/science.289.5485.1751.
- Mumford, S. L., A. Z. Steiner, A. Z. Pollack, N. J. Perkins, A. C. Filiberto, P. S. Albert, D. R. Mattison, J. Wactawski-Wende, and E. F. Schisterman. 2012. The utility of menstrual cycle length as an indicator of cumulative hormonal exposure. The Journal of Clinical Endocrinology & Metabolism 97 (10):E1871–9. doi: 10.1210/jc.2012-1350.
- Mumford, S. L., R. Sundaram, E. F. Schisterman, A. M. Sweeney, D. B. Barr, M. E. Rybak, J. M. Maisog, D. L. Parker, C. M. Pfeiffer, and G. M. B. Louis. 2014. Higher urinary lignan concentrations in women but not men are positively associated with shorter time to pregnancy. The Journal of Nutrition 144 (3):352–8. doi: 10.3945/jn.113.184820.
- Mumford, S. L., S. Kim, Z. Chen, D. B. Barr, and G. M. B. Louis. 2015. Urinary phytoestrogens are associated with subtle indicators of semen quality among male partners of couples desiring pregnancy. The Journal of Nutrition 145 (11):2535–41. doi: 10.3945/jn.115.214973.
- Münstedt, K., P. Grant, J. Woenckhaus, G. Roth, and H.-R. Tinneberg. 2004. Cancer of the endometrium: Current aspects of diagnostics and treatment. World Journal of Surgical Oncology 2:24. doi: 10.1186/1477-7819-2-24.
- Murkes, D., P. Conner, K. Leifland, E. Tani, A. Beliard, E. Lundström, and G. Söderqvist. 2011. Effects of percutaneous estradiol-oral progesterone versus oral conjugated equine estrogens-medroxyprogesterone acetate on breast cell proliferation and bcl-2 protein in healthy women. Fertility and Sterility 95 (3):1188–91. doi: 10.1016/j.fertnstert.2010.09.062.
- Murkies, A. L., C. Lombard, B. J. G. Strauss, G. Wilcox, H. G. Burger, and M. S. Morton. 1995. Dietary flour supplementation decreases post-menopausal hot flushes: Effect of soy and wheat. Maturitas 21 (3):189–95. doi: 10.1016/0378-5122(95)00899-V.
- Murphy, P. A., K. Barua, and C. C. Hauck. 2002. Solvent extraction selection in the determination of isoflavones in soy foods. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 777 (1-2):129–38. doi: 10.1016/s1570-0232(02)00342-2.
- Murphy, P. A., T. Song, G. Buseman, K. Barua, G. R. Beecher, D. Trainer, and J. Holden. 1999. Isoflavones in retail and institutional soy foods. Journal of Agricultural and Food Chemistry 47 (7):2697–704. doi: 10.1021/jf981144o.
- Murray, M. J., W. R. Meyer, B. A. Lessey, R. H. Oi, R. E. DeWire, and M. A. Fritz. 2003. Soy protein isolate with isoflavones does not prevent estradiol-induced endometrial hyperplasia in postmenopausal women: a pilot trial. Menopause 10 (5):456–64. D1 doi: 10.1097/01.GME.0000063567.84134.D1.
- Mustafa, A. M., N. T. Malintan, S. Seelan, Z. Zhan, Z. Mohamed, J. Hassan, R. Pendek, R. Hussain, and N. Ito. 2007. Phytoestrogens levels determination in the cord blood from Malaysia rural and urban populations. Toxicology and Applied Pharmacology 222 (1):25–32. doi: 10.1016/j.taap.2007.03.014.
- Mvondo, M. A., J. D. Ekenfack, S. Minko Essono, H. Saah Namekong, C. F. Awounfack, M. W. Laschke, and D. Njamen. 2019. Soy intake since the prepubertal age may contribute to the pathogenesis of endometriosis in adulthood. Journal of Medicinal Food. 22 (6):631–8. doi: 10.1089/jmf.2018.0160.
- Nagamani, M., P. G. McDonough, J. O. Ellegood, and V. B. Mahesh. 1979. Maternal and amniotic fluid steroids throughout human pregnancy. American Journal of Obstetrics and Gynecology 134 (6):674–80. doi: 10.1016/0002-9378(79)90649-5.
- Nagata, C., H. Shimizu, R. Takami, M. Hayashi, N. Takeda, and K. Yasuda. 2002. Soy product intake and serum isoflavonoid and estradiol concentrations in relation to bone mineral density in postmenopausal Japanese women. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 13 (3):200–4. doi: 10.1007/s001980200014.
- Nagata, C., K. Nakamura, S. Oba, M. Hayashi, N. Takeda, and K. Yasuda. 2009. Association of intakes of fat, dietary fibre, soya isoflavones and alcohol with uterine fibroids in Japanese women. The British Journal of Nutrition 101 (10):1427–31. doi: 10.1017/s0007114508083566.
- Nagata, C., N. Takatsuka, N. Kawakami, and H. Shimizu. 2001. Soy product intake and premenopausal hysterectomy in a follow-up study of Japanese women. European Journal of Clinical Nutrition 55 (9):773–7. doi: 10.1038/sj.ejcn.1601223.
- Nagata, C., S. Iwasa, M. Shiraki, T. Ueno, S. Uchiyama, K. Urata, Y. Sahashi, and H. Shimizu. 2006. Associations among maternal soy intake, isoflavone levels in urine and blood samples, and maternal and umbilical hormone concentrations (Japan). Cancer Causes & Control : CCC 17 (9):1107–13. doi: 10.1007/s10552-006-0044-4.
- Nagata, C., S. Oba, and H. Shimizu. 2006. Associations of menstrual cycle length with intake of soy, fat, and dietary fiber in Japanese women. Nutrition and Cancer 54 (2):166–70. doi: 10.1207/s15327914nc5402_2.
- Nagata, S. 2019. Causes of Kawasaki Disease-From past to present. Front Pediatr 7:18. doi: 10.3389/fped.2019.00018.
- Nagata, Y., Y. Sugiyama, F. Fukuta, A. Takayanagi, N. Masumori, T. Tsukamoto, H. Akasaka, H. Ohnishi, S. Saitoh, T. Miura, et al. 2016. Relationship of serum levels and dietary intake of isoflavone, and the novel bacterium Slackia sp. strain NATTS with the risk of prostate cancer: A case-control study among Japanese men. International Urology and Nephrology 48 (9):1453–60. doi: 10.1007/s11255-016-1335-7.
- Nakamura, Y., M. Yashiro, R. Uehara, A. Sadakane, S. Tsuboi, Y. Aoyama, K. Kotani, E.-O. Tsogzolbaatar, and H. Yanagawa. 2012. Epidemiologic features of Kawasaki disease in Japan: Results of the 2009-2010 nationwide survey. Journal of Epidemiology 22 (3):216–21. doi: 10.2188/jea.je20110126.
- Nalaboff, K. M., J. S. Pellerito, and E. Ben-Levi. 2001. Imaging the endometrium: Disease and normal variants. Radiographics: A Review Publication of the Radiological Society of North America, Inc 21 (6):1409–24. doi: 10.1148/radiographics.21.6.g01nv211409.
- National Toxicology Program UDoHaHS, Center for the Evaluation of Risks to Human Reproduction. 2006. NTP-CERHR Expert Panel Report on the Reproductive and Developmental Toxicity of Soy Formula. doi: 10.1002/bdrb.20087.
- Nechuta, S. J., B. J. Caan, W. Y. Chen, W. Lu, Z. Chen, M. L. Kwan, S. W. Flatt, Y. Zheng, W. Zheng, J. P. Pierce, et al. 2012. Soy food intake after diagnosis of breast cancer and survival: An in-depth analysis of combined evidence from cohort studies of US and Chinese women. The American Journal of Clinical Nutrition 96 (1):123–32. doi: 10.3945/ajcn.112.035972.
- Németh, K., G. W. Plumb, J.-G. Berrin, N. Juge, R. Jacob, H. Y. Naim, G. Williamson, D. M. Swallow, and P. A. Kroon. 2003. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. European Journal of Nutrition 42 (1):29–42. doi: 10.1007/s00394-003-0397-3.
- Newburger, J. W., M. Takahashi, M. A. Gerber, et al. 2004. Diagnosis, treatment, and long-term management of Kawasaki disease: A statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young. American Heart Association. Circulation 110:2747–71. https://pubmed.ncbi.nlm.nih.gov/15505111/.
- Nikolaidis, A., M. Andreadis, and T. Moschakis. 2017. Effect of heat, pH, ultrasonication and ethanol on the denaturation of whey protein isolate using a newly developed approach in the analysis of difference-UV spectra. Food Chemistry 232:425–33. doi: 10.1016/j.foodchem.2017.04.022.
- Noel, J.-C., V. Anaf, I. Fayt, and E. Wespes. 2006. Ureteral mullerian carcinosarcoma (mixed mullerian tumor) associated with endometriosis occurring in a patient with a concentrated soy isoflavones supplementation. Archives of Gynecology and Obstetrics 274 (6):389–92. doi: 10.1007/s00404-006-0188-1.
- Nordenvall, A. S., L. Frisén, A. Nordenström, P. Lichtenstein, and A. Nordenskjöld. 2014. Population based nationwide study of hypospadias in Sweden, 1973 to 2009: Incidence and risk factors. The Journal of Urology 191 (3):783–9. doi: 10.1016/j.juro.2013.09.058.
- Nordkap, L., U. N. Joensen, M. Blomberg Jensen, and N. Jørgensen. 2012. Regional differences and temporal trends in male reproductive health disorders: Semen quality may be a sensitive marker of environmental exposures. Molecular and Cellular Endocrinology 355 (2):221–30. doi: 10.1016/j.mce.2011.05.048.
- North, K., and J. Golding. 2000. A maternal vegetarian diet in pregnancy is associated with hypospadias. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. BJU International 85 (1):107–13. doi: 10.1046/j.1464-410x.2000.00436.x.
- North American Menopause Society. 2003. Role of progestogen in hormone therapy for postmenopausal women: Position statement of The North American Menopause Society. Menopause 10:113–32. doi: 10.1097/00042192-200310020-00003.
- Novak, W. K., and A. G. Haslberger. 2000. Substantial equivalence of antinutrients and inherent plant toxins in genetically modified novel foods. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 38 (6):473–83. doi: 10.1016/s0278-6915(00)00040-5.
- Ogawa, K., S.-C. Jwa, M. Kobayashi, N. Morisaki, H. Sago, and T. Fujiwara. 2017. Validation of a food frequency questionnaire for Japanese pregnant women with and without nausea and vomiting in early pregnancy. Journal of Epidemiology 27 (5):201–8. doi: 10.1016/j.je.2016.06.004.
- Ohlsson, C., M. Bygdell, J. Celind, A. Sondén, A. Tidblad, L. Sävendahl, and J. M. Kindblom. 2019. Secular trends in pubertal growth acceleration in Swedish boys born from 1947 to 1996. JAMA Pediatrics 173 (9):860–5. doi: 10.1001/jamapediatrics.2019.2315.
- Ohno, S., S. Shinoda, S. Toyoshima, H. Nakazawa, T. Makino, and S. Nakajin. 2002. Effects of flavonoid phytochemicals on cortisol production and on activities of steroidogenic enzymes in human adrenocortical H295R cells. The Journal of Steroid Biochemistry and Molecular Biology 80 (3):355–63. doi: 10.1016/S0960-0760(02)00021-3.
- Onland-Moret, N. C., P. H. M. Peeters, C. H. van Gils, F. Clavel-Chapelon, T. Key, A. Tjønneland, A. Trichopoulou, R. Kaaks, J. Manjer, S. Panico, et al. 2005. Age at menarche in relation to adult height: The EPIC study. American Journal of Epidemiology 162 (7):623–32. doi: 10.1093/aje/kwi260.
- Onoda, A., T. Ueno, S. Uchiyama, S.-I. Hayashi, K. Kato, and N. Wake. 2011. Effects of S-equol and natural S-equol supplement (SE5-OH) on the growth of MCF-7 in vitro and as tumors implanted into ovariectomized athymic mice. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 49 (9):2279–84. doi: 10.1016/j.fct.2011.06.027.
- Orlich, M. J., P. N. Singh, J. Sabaté, K. Jaceldo-Siegl, J. Fan, S. Knutsen, W. L. Beeson, and G. E. Fraser. 2013. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Internal Medicine 173 (13):1230–8. doi: 10.1001/jamainternmed.2013.6473.
- Ormond, G., M. J. Nieuwenhuijsen, P. Nelson, M. B. Toledano, N. Iszatt, S. Geneletti, and P. Elliott. 2009. Endocrine disruptors in the workplace, hair spray, folate supplementation, and risk of hypospadias: Case-control study. Environmental Health Perspectives 117 (2):303–7. doi: 10.1289/ehp.11933.
- Oseni, T., R. Patel, J. Pyle, and V. C. Jordan. 2008. Selective estrogen receptor modulators and phytoestrogens. Planta Medica 74 (13):1656–65. doi: 10.1055/s-0028-1088304.
- Otokozawa, S., R. Tanaka, H. Akasaka, E. Ito, S. Asakura, H. Ohnishi, S. Saito, T. Miura, T. Saito, M. Mori, et al. 2015. Associations of serum isoflavone, adiponectin and insulin levels with risk for epithelial ovarian cancer: Results of a case-control study. Asian Pacific Journal of Cancer Prevention 16 (12):4987–91. doi: 10.7314/APJCP.2015.16.12.4987.
- Otun, J., A. Sahebkar, L. Östlundh, S. L. Atkin, and T. Sathyapalan. 2019. Systematic review and meta-analysis on the effect of soy on thyroid function. Scientific Reports 9 (1):3964. doi: 10.1038/s41598-019-40647-x.
- Ozasa, K., M. Nakao, Y. Watanabe, K. Hayashi, T. Miki, K. Mikami, M. Mori, F. Sakauchi, M. Washio, Y. Ito, et al. 2004. Serum phytoestrogens and prostate cancer risk in a nested case-control study among Japanese men. Cancer Science 95 (1):65–71. doi: 10.1111/j.1349-7006.2004.tb03172.x.
- Padgette, S. R., N. B. Taylor, D. L. Nida, M. R. Bailey, J. MacDonald, L. R. Holden, and R. L. Fuchs. 1996. The composition of glyphosate-tolerant soybean seeds is equivalent to that of conventional soybeans. The Journal of Nutrition 126 (3):702–16. doi: 10.1093/jn/126.3.702.
- Padilla-Banks, E., W. N. Jefferson, P. H. Myers, D. R. Goulding, and C. J. Williams. 2012. Neonatal phytoestrogen exposure causes hypospadias in female mice. Molecular Reproduction and Development 79 (1):3 doi: 10.1002/mrd.21395.
- Palomares, M. R., L. Hopper, L. Goldstein, et al. 2004. Effect of soy isoflavones on breast proliferation in postmenopausal breast cancer survivors. Breast Cancer Res Treatment 88 (1):4002.
- Pan, Y., M. Anthony, and T. B. Clarkson. 1999a. Effect of estradiol and soy phytoestrogens on choline acetyltransferase and nerve growth factor mRNAs in the frontal cortex and hippocampus of female rats. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 221 (2):118–25. doi: 10.1046/j.1525-1373.1999.d01-64.x.
- Pan, Y., M. Anthony, and T. B. Clarkson. 1999b. Evidence for up-regulation of brain-derived neurotrophic factor mRNA by soy phytoestrogens in the frontal cortex of retired breeder female rats. Neuroscience Letters 261 (1-2):17–20. doi: 10.1016/S0304-3940(98)00994-X.
- Parkin, D. M., P. Pisani, and J. Ferlay. 1999. Estimates of the worldwide incidence of 25 major cancers in 1990. International Journal of Cancer 80 (6):827–41. doi: 10.1002/ijc.1571.
- Paruthiyil, S., H. Parmar, V. Kerekatte, G. R. Cunha, G. L. Firestone, and D. C. Leitman. 2004. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Research 64 (1):423–8. doi: 10.1158/0008-5472.can-03-2446.
- Patisaul, H. B. 2017. Endocrine disruption by dietary phyto-oestrogens: Impact on dimorphic sexual systems and behaviours. The Proceedings of the Nutrition Society 76 (2):130–44. doi: 10.1017/S0029665116000677.
- Patisaul, H. B., and W. Jefferson. 2010. The pros and cons of phytoestrogens. Frontiers in Neuroendocrinology 31 (4):400–19. doi: 10.1016/j.yfrne.2010.03.003.
- Patisaul, H. B., S. E. Fenton, and D. Aylor. 2018. Animal models of endocrine disruption. Best Practice & Research. Clinical Endocrinology & Metabolism 32 (3):283–97. doi: 10.1016/j.beem.2018.03.011.
- Paul, B., K. J. Royston, Y. Li, M. L. Stoll, C. F. Skibola, L. S. Wilson, S. Barnes, C. D. Morrow, and T. O. Tollefsbol. 2017. Impact of genistein on the gut microbiome of humanized mice and its role in breast tumor inhibition. PloS One 12 (12):e0189756. doi: 10.1371/journal.pone.0189756.
- Paulozzi, L. J. 1999. International trends in rates of hypospadias and cryptorchidism. Environmental Health Perspectives 107 (4):297–302. doi: 10.1289/ehp.99107297.
- Paulozzi, L. J., J. D. Erickson, and R. J. Jackson. 1997. Hypospadias trends in two US surveillance systems. Pediatrics 100 (5):831–4. doi: 10.1542/peds.100.5.831.
- Pavone, D., S. Clemenza, F. Sorbi, M. Fambrini, and F. Petraglia. 2018. Epidemiology and risk factors of uterine fibroids. Best Practice & Research. Clinical Obstetrics & Gynaecology 46:3–11. doi: 10.1016/j.bpobgyn.2017.09.004.
- Peeters, P. H. M., N. Slimani, Y. T. van der Schouw, P. B. Grace, C. Navarro, A. Tjonneland, A. Olsen, F. Clavel-Chapelon, M. Touillaud, M.-C. Boutron-Ruault, et al. 2007. Variations in plasma phytoestrogen concentrations in European adults. The Journal of Nutrition 137 (5):1294–300. doi: 10.1093/jn/137.5.1294.
- Peng, J.-H., J.-D. Zhu, M.-T. Mi, F.-J. Li, L. Cai, J.-Z. Dong, H.-X. Zhang, Y. Zhao, and R.-L. Xue. 2010. Prepubertal genistein exposure affects erbB2/Akt signal and reduces rat mammary tumorigenesis. European Journal of Cancer Prevention: The Official Journal of the European Cancer Prevention Organisation (ECP) 19 (2):110–9. doi: 10.1097/CEJ.0b013e3283362a3e.
- Petrakis, N. L., S. Barnes, E. B. King, J. Lowenstein, J. Wiencke, M. M. Lee, R. Miike, M. Kirk, and L. Coward. 1996. Stimulatory influence of soy protein isolate on breast secretion in pre- and postmenopausal women. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 5 (10):785–94. https://pubmed.ncbi.nlm.nih.gov/8896889/.
- Pierik, F. H., A. Burdorf, J. A. Deddens, R. E. Juttmann, and R. F. A. Weber. 2004. Maternal and paternal risk factors for cryptorchidism and hypospadias: A case-control study in newborn boys. Environmental Health Perspectives 112 (15):1570–6. doi: 10.1289/ehp.7243.
- Piña-Medina, A. G., V. Hansberg-Pastor, A. González-Arenas, M. Cerbón, and I. Camacho-Arroyo. 2016. Progesterone promotes cell migration, invasion and cofilin activation in human astrocytoma cells. Steroids 105:19–25. doi: 10.1016/j.steroids.2015.11.008.
- Pinchera, A., M. H. Macgillivray, J. D. Crawford, and A. G. Freeman. 1965. Thyroid refractoriness in an athyreotic cretin fed soybean formula. The New England Journal of Medicine 273:83–7. doi: 10.1056/NEJM196507082730205.
- Pisani, P., F. Bray, and D. M. Parkin. 2002. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. International Journal of Cancer 97 (1):72–81. doi: 10.1002/ijc.1571.
- Portman, M. A. 2013. Kawasaki disease and soy: Potential role for isoflavone interaction with Fcγ receptors. Pediatric Research 73 (2):130–4. DOI: 10.1097/INF.0b013e3181cf8705
- Portman, M. A., S. L. Navarro, M. E. Bruce, and J. W. Lampe. 2016. Soy isoflavone intake is associated with risk of Kawasaki disease. Nutrition Research (New York, N.Y.) 36 (8):827–34. doi: 10.1016/j.nutres.2016.04.002.
- Potter, S. M., J. A. Baum, H. Teng, R. J. Stillman, N. F. Shay, and J. W. Erdman. 1998. Soy protein and isoflavones: Their effects on blood lipids and bone density in postmenopausal women. The American Journal of Clinical Nutrition 68 (6):1375S–9S. doi: 10.1093/ajcn/68.6.1375S.
- Povey, A. C., J. A. Clyma, R. McNamee, H. D. Moore, H. Baillie, A. A. Pacey, J. E. Cade, and N. M. Cherry. 2020. Phytoestrogen intake and other dietary risk factors for low motile sperm count and poor sperm morphology. Andrology 8 (6):1805–14. Nov doi: 10.1111/andr.12858.
- Prasain, J. K., N. Peng, E. Acosta, R. Moore, A. Arabshahi, E. Meezan, S. Barnes, and J. M. Wyss. 2007. Pharmacokinetic study of puerarin in rat serum by liquid chromatography tandem mass spectrometry. Biomedical Chromatography: BMC 21 (4):410–4. doi: 10.1002/bmc.772.
- Prossnitz, E. R., J. B. Arterburn, and L. A. Sklar. 2007. GPR30: A G protein-coupled receptor for estrogen. Molecular and Cellular Endocrinology 265-266:138–42. doi: 10.1016/j.mce.2006.12.010.
- Pumford, S. L., M. M. Morton, A. Turkes, and K. Griffiths. 2002. Determination of the isoflavonoids genistein and daidzein in biological samples by gas chromatography-mass spectrometry. Annals of Clinical Biochemistry 39 (Pt 3):281–92. doi: 10.1258/0004563021901982.
- Qiao, L., E. Rodriguez, D. A. Weiss, M. Ferretti, G. Risbridger, G. R. Cunha, and L. S. Baskin. 2012. Expression of estrogen receptor alpha and beta is decreased in hypospadias. Journal of Urology 187 (4):1427–33. doi: 10.1016/j.juro.2011.12.008.
- Qin, H., Z. Lin, E. Vásquez, X. Luan, F. Guo, and L. Xu. 2019. High soy isoflavone or soy-based food intake during infancy and in adulthood is associated with an increased risk of uterine fibroids in premenopausal women: a meta-analysis. Nutrition Research (New York, N.Y.) 71:30–42. doi: 10.1016/j.nutres.2019.06.002.
- Qin, W., W. Zhu, H. Shi, J. E. Hewett, R. L. Ruhlen, R. S. MacDonald, G. E. Rottinghaus, Y.-C. Chen, and E. R. Sauter. 2009. Soy isoflavones have an antiestrogenic effect and alter mammary promoter hypermethylation in healthy premenopausal women. Nutrition and Cancer 61 (2):238–44. doi: 10.1080/01635580802404196.
- Qin, Y., G. Du, M. Chen, W. Hu, C. Lu, W. Wu, B. Hang, Z. Zhou, X. Wang, and Y. Xia. 2014. Combined effects of urinary phytoestrogens metabolites and polymorphisms in metabolic enzyme gene on idiopathic male infertility. Archives of Toxicology 88 (8):1527–36. doi: 10.1007/s00204-014-1205-y.
- Qiu, S., and C. Jiang. 2019. Soy and isoflavones consumption and breast cancer survival and recurrence: A systematic review and meta-analysis. European Journal of Nutrition 58 (8):3079–90. doi: 10.1007/s00394-018-1853-4.
- Quaas, A. M., N. Kono, W. J. Mack, H. N. Hodis, J. C. Felix, R. J. Paulson, and D. Shoupe. 2013. Effect of isoflavone soy protein supplementation on endometrial thickness, hyperplasia, and endometrial cancer risk in postmenopausal women: A randomized controlled trial. Menopause (New York, N.Y.) 20 (8):840–4. doi: 10.1097/GME.0b013e3182804353.
- Radovic, B., B. Mentrup, and J. Kohrle. 2006. Genistein and other soya isoflavones are potent ligands for transthyretin in serum and cerebrospinal fluid. The British Journal of Nutrition 95 (6):1171–6. doi: 10.1079/bjn20061779.
- Rahal, O. M., and R. C. Simmen. 2011. Paracrine-acting adiponectin promotes mammary epithelial differentiation and synergizes with genistein to enhance transcriptional response to estrogen receptor β signaling. Endocrinology 152 (9):3409–21. doi: 10.1210/en.2011-1085.
- Rahmanzadeh, R., G. Hüttmann, J. Gerdes, and T. Scholzen. 2007. Chromophore-assisted light inactivation of pKi-67 leads to inhibition of ribosomal RNA synthesis. Cell Proliferation 40 (3):422–30. doi: 10.1111/j.1365-2184.2007.00433.x.
- Rannikko, A., A. Petas, S. Rannikko, and H. Adlercreutz. 2006. Plasma and prostate phytoestrogen concentrations in prostate cancer patients after oral phytoestogen supplementation. The Prostate 66 (1):82–7. doi: 10.1002/pros.20315.
- Reed, K. E., J. Camargo, J. Hamilton-Reeves, M. Kurzer, and M. Messina. 2020. Neither soy nor isoflavone intake affects male reproductive hormones: An expanded and updated meta-analysis of clinical studies. Reproductive Toxicology (Elmsford, N.Y.) 100:60–7. doi: 10.1016/j.reprotox.2020.12.019.
- Rice, M. S., K. A. Bertrand, T. J. VanderWeele, B. A. Rosner, X. Liao, H.-O. Adami, and R. M. Tamimi. 2016. Mammographic density and breast cancer risk: A mediation analysis. Breast Cancer Research 18 (1):94. doi: 10.1186/s13058-016-0750-0.
- Rice, S., H. D. Mason, and S. A. Whitehead. 2006. Phytoestrogens and their low dose combinations inhibit mRNA expression and activity of aromatase in human granulosa-luteal cells. Journal of Steroid Biochemistry and Molecular Biology 101 (4-5):216–25. doi: 10.1016/j.jsbmb.2006.06.021.
- Richelle, M., S. Pridmore-Merten, S. Bodenstab, M. Enslen, and E. A. Offord. 2002. Hydrolysis of isoflavone glycosides to aglycones by beta-glycosidase does not alter plasma and urine isoflavone pharmacokinetics in postmenopausal women. The Journal of Nutrition 132 (9):2587–92. doi: 10.1093/jn/132.9.2587.
- Rietjens, I., J. Louisse, and K. Beekmann. 2017. The potential health effects of dietary phytoestrogens. British Journal of Pharmacology 174 (11):1263–80. doi: 10.1111/bph.13622.
- Rípodas, C., V. D. Via, O. M. Aguilar, M. E. Zanetti, and F. A. Blanco. 2013. Knock-down of a member of the isoflavone reductase gene family impairs plant growth and nodulation in Phaseolus vulgaris. Plant Physiology and Biochemistry: PPB 68:81–9. doi: 10.1016/j.plaphy.2013.04.003.
- Rivera-Sagredo, A., F. J. Cañada, O. Nieto, J. Jimenez-Barbero, and M. Martín-Lomas. 1992. Substrate specificity of small-intestinal lactase. Assessment of the role of the substrate hydroxyl groups. European Journal of Biochemistry 209 (1):415–22. doi: 10.1111/j.1432-1033.1992.tb17304.x.
- Rizzo, N. S., K. Jaceldo-Siegl, J. Sabate, and G. E. Fraser. 2013. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. Journal of the Academy of Nutrition and Dietetics 113 (12):1610–9. doi: 10.1016/j.jand.2013.06.349.
- Robinson, J. D., H. L. Judd, P. E. Young, O. W. Jones, and S. S. Yen. 1977. Amniotic fluid androgens and estrogens in midgestation. The Journal of Clinical Endocrinology and Metabolism 45 (4):755–61. doi: 10.1210/jcem-45-4-755.
- Rock, C. L., C. Doyle, W. Demark-Wahnefried, J. Meyerhardt, K. S. Courneya, A. L. Schwartz, E. V. Bandera, K. K. Hamilton, B. Grant, M. McCullough, et al. 2012. Nutrition and physical activity guidelines for cancer survivors. CA: A Cancer Journal for Clinicians 62 (4):242–74. doi: 10.3322/caac.21142.
- Rodo, X., R. Curcoll, M. Robinson, J. Ballester, J. C. Burns, D. R. Cayan, W. I. Lipkin, B. L. Williams, M. Couto-Rodriguez, Y. Nakamura, et al. 2014. Tropospheric winds from northeastern China carry the etiologic agent of Kawasaki disease from its source to Japan. Proceedings of the National Academy of Sciences 111 (22):7952–7. doi: 10.1073/pnas.1400380111.
- Rogers, I. S., K. Northstone, D. B. Dunger, A. R. Cooper, A. R. Ness, and P. M. Emmett. 2010. Diet throughout childhood and age at menarche in a contemporary cohort of British girls. Public Health Nutrition 13 (12):2052–63. doi: 10.1017/S1368980010001461.
- Romualdi, D., B. Costantini, G. Campagna, A. Lanzone, and M. Guido. 2008. Is there a role for soy isoflavones in the therapeutic approach to polycystic ovary syndrome? Results from a pilot study. Fertility and Sterility 90 (5):1826–33. doi: 10.1016/j.fertnstert.2007.09.020.
- Ronis, M. J., J. M. Little, G. W. Barone, G. Chen, A. Radominska-Pandya, and T. M. Badger. 2006. Sulfation of the isoflavones genistein and daidzein in human and rat liver and gastrointestinal tract. Journal of Medicinal Food 9 (3):348–55. doi: 10.1089/jmf.2006.9.348.
- Rosenberg, R. 1994. Malabsorption of thyroid hormone with cholestyramine administration. Conn Med 58 (109) https://pubmed.ncbi.nlm.nih.gov/8004946/.
- Ross, A. E., L. Marchionni, T. M. Phillips, R. M. Miller, P. J. Hurley, B. W. Simons, A. H. Salmasi, A. J. Schaeffer, J. P. Gearhart, and E. M. Schaeffer. 2011. Molecular effects of genistein on male urethral development. Journal of Urology 185 (5):1894–8. doi: 10.1016/j.juro.2010.12.095.
- Ross, J. A. 1998. Maternal diet and infant leukemia: A role for DNA topoisomerase II inhibitors? International Journal of Cancer. Supplement = Journal International du Cancer. Supplement 11:26–8. doi: 10.1002/(SICI)1097-0215(1998)78:11+<26::AID-IJC8>3.0.CO;2-M.
- Rowland, I. R., H. Wiseman, T. A. B. Sanders, H. Adlercreutz, and E. A. Bowey. 2000. Interindividual variation in metabolism of soy isoflavones and lignans: Influence of habitual diet on equol production by the gut microflora. Nutrition and Cancer 36 (1):27–32. doi: 10.1207/S15327914NC3601_5.
- Ruder, E. H., J. F. Dorgan, S. Kranz, P. M. Kris-Etherton, and T. J. Hartman. 2008. Examining breast cancer growth and lifestyle risk factors: Early life, childhood, and adolescence. Clinical Breast Cancer 8 (4):334–42. doi: 10.3816/CBC.2008.n.038.
- Russo, J., D. Mailo, Y. F. Hu, G. Balogh, F. Sheriff, and I. H. Russo. 2005. Breast differentiation and its implication in cancer prevention. Clinical Cancer Research 11:931s–6s. https://pubmed.ncbi.nlm.nih.gov/15701889/.
- Sabry, M., and A. Al-Hendy. 2012. Medical treatment of uterine leiomyoma. Reproductive Sciences (Thousand Oaks, Calif.) 19 (4):339–53. doi: 10.1177/1933719111432867.
- Salsano, S., S. Pérez-Debén, A. Quiñonero, R. González-Martín, and F. Domínguez. 2019. Phytoestrogen exposure alters endometrial stromal cells and interferes with decidualization signaling. Fertility and Sterility 112 (5):947–58 e3. doi: 10.1016/j.fertnstert.2019.06.014.
- Samtani, R., M. Bajpai, P. K. Ghosh, and K. N. Saraswathy. 2014. Hypospadias risk among North-Indian children. Annals of Health and Health Sciences 1 (1):61–4. doi: 10.5958/j.2322-0422.1.1.012.
- Sangi-Haghpeykar, H., and A. N. Poindexter. 1995. 3rd. Epidemiology of endometriosis among parous women. Obstetrics & Gynaecology 85:983–92. doi: 10.1016/0029-7844(95)00074-2.
- Sansai, K., M. Na Takuathung, R. Khatsri, S. Teekachunhatean, N. Hanprasertpong, and N. Koonrungsesomboon. 2020. Effects of isoflavone interventions on bone mineral density in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Osteoporosis International 31 (10):1853–64. oi: . doi: 10.1007/s00198-020-05476-z.
- Santell, R. C., N. Kieu, and W. G. Helferich. 2000. Genistein inhibits growth of estrogen-independent human breast cancer cells in culture but not in athymic mice. The Journal of Nutrition 130 (7):1665–9. doi: 10.1093/jn/130.7.1665.
- Sarathi, V., A. Kolly, H. B. Chaithanya, and C. S. Dwarakanath. 2016. Effect of soya based protein rich diet on glycaemic parameters and thyroid function tests in women with gestational diabetes mellitus. Romanian Journal of Diabetes Nutrition and Metabolic Diseases 23 (2):201–8. doi: 10.1515/rjdnmd-2016-0024.
- Sartippour, M. R., J. Y. Rao, S. Apple, D. Wu, S. Henning, H. Wang, R. Elashoff, R. Rubio, D. Heber, and M. N. Brooks. 2004. A pilot clinical study of short-term isoflavone supplements in breast cancer patients. Nutrition and Cancer 49 (1):59–65. doi: 10.1207/s15327914nc4901_8.
- Sathyapalan, T., A. J. Dawson, A. S. Rigby, N. J. Thatcher, E. S. Kilpatrick, and S. L. Atkin. 2018. The effect of phytoestrogen on thyroid in subclinical hypothyroidism: Randomized, double blind, crossover study. Frontiers of Endocrinology (Lausanne) 9:531. doi: 10.3389/fendo.2018.00531.
- Sathyapalan, T., A. M. Manuchehri, N. J. Thatcher, A. S. Rigby, T. Chapman, E. S. Kilpatrick, and S. L. Atkin. 2011. The effect of soy phytoestrogen supplementation on thyroid status and cardiovascular risk markers in patients with subclinical hypothyroidism: A randomized, double-blind, crossover study. Journal of Clinical Endocrinology and Metabolism 96 (5):1442–9. doi: 10.1210/jc.2010-2255.
- Sathyapalan, T., A. S. Rigby, S. Bhasin, N. J. Thatcher, E. S. Kilpatrick, and S. L. Atkin. 2016. Effect of soy in men with type 2 diabetes mellitus and subclinical hypogonadism: A randomized controlled study. Journal of Clinical Endocrinology and Metabolism 102:425–33. doi: 10.1210/jc.2016-2875.
- Sathyapalan, T., J. Köhrle, E. Rijntjes, A. S. Rigby, S. R. Dargham, E. S. Kilpatrick, and S. L. Atkin. 2018. The effect of high dose isoflavone supplementation on serum reverse T3 in euthyroid men with type 2 diabetes and post-menopausal women. Frontiers in Endocrinology 9:698. doi: 10.3389/fendo.2018.00698.
- Sathyapalan, T., M. Aye, A. S. Rigby, W. D. Fraser, N. J. Thatcher, E. S. Kilpatrick, and S. L. Atkin. 2017. Soy reduces bone turnover markers in women during early menopause: A randomized controlled trial. Journal of Bone and Mineral Research 32 (1):157–64. doi: 10.1002/jbmr.2927.
- Savarese, T. M., W. C. Strohsnitter, H. P. Low, Q. Liu, I. Baik, W. Okulicz, D. P. Chelmow, P. Lagiou, P. J. Quesenberry, K. L. Noller, et al. 2007. Correlation of umbilical cord blood hormones and growth factors with stem cell potential: Implications for the prenatal origin of breast cancer hypothesis. Breast Cancer Research 9 (3):R29. doi: 10.1186/bcr1674.
- Schmidt, R. L., J. S. LoPresti, M. T. McDermott, S. M. Zick, and J. A. Straseski. 2018. Does reverse triiodothyronine testing have clinical utility? An analysis of practice variation Based on order data from a national reference laboratory. Thyroid: Official Journal of the American Thyroid Association 28 (7):842–8. doi: 10.1089/thy.2017.0645.
- Schmitt, E., W. Dekant, and H. Stopper. 2001. Assaying the estrogenicity of phytoestrogens in cells of different estrogen sensitive tissues. Toxicology in Vitro 15 (4-5):433–9. doi: 10.1016/S0887-2333(01)00048-0.
- Schneider, L. S., G. Hernandez, L. Zhao, A. A. Franke, Y.-L. Chen, S. Pawluczyk, W. J. Mack, and R. D. Brinton. 2019. Safety and feasibility of estrogen receptor-β targeted phytoSERM formulation for menopausal symptoms: phase 1b/2a randomized clinical trial . Menopause (New York, N.Y.) 26 (8):874–84. doi: 10.1097/GME.0000000000001325.
- Schoemaker, M. J., M. E. Jones, S. Allen, J. Hoare, A. Ashworth, M. Dowsett, and A. J. Swerdlow. 2017. Childhood body size and pubertal timing in relation to adult mammographic density phenotype. Breast Cancer Research 19 (1):13. doi: 10.1186/s13058-017-0804-y.
- Scholzen, T., and J. Gerdes. 2000. The Ki-67 protein: From the known and the unknown. Journal of Cellular Physiology 182 (3):311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3 < 311::AID-JCP1 > 3.0.CO;2-9.
- Schulster, M., A. M. Bernie, and R. Ramasamy. 2016. The role of estradiol in male reproductive function. Asian Journal of Andrology 18 (3):435–40. doi: 10.4103/1008-682X.173932.
- Scilewski da Costa Zanatta, T., R. Manica-Berto, C. D. Ferreira, M. M. C. Cardozo, C. V. Rombaldi, R. C. Zambiazi, and Á. R. G. Dias. 2017. Phosphate fertilizer and growing environment change the phytochemicals, oil quality, and nutritional composition of Roundup Ready genetically modified and conventional soybean. Journal of Agricultural and Food Chemistry 65 (13):2661–9. doi: 10.1021/acs.jafc.6b05499.
- Sebastian, R. S., C. Wilkinson Enns, J. D. Goldman, C. L. Martin, L. C. Steinfeldt, T. Murayi, and A. J. Moshfegh. 2015. A new database facilitates characterization of flavonoid intake, sources, and positive associations with diet among US adults. The Journal of Nutrition 145 (6):1239–48. doi: 10.3945/jn.115.213025.
- Segars, J. H., E. C. Parrott, J. D. Nagel, X. C. Guo, X. Gao, L. S. Birnbaum, V. W. Pinn, and D. Dixon. 2014. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: Comprehensive review, conference summary and future recommendations. Human Reproduction Update 20 (3):309–33. doi: 10.1093/humupd/dmt058.
- Segovia-Siapco, G., G. Khayef, P. Pribis, K. Oda, E. Haddad, and J. Sabaté. 2019. Animal protein intake is associated with general adiposity in adolescents: The Teen Food and Development Study. Nutrients 12 (1):110. doi: 10.3390/nu12010.
- Segovia-Siapco, G., P. Pribis, K. Oda, and J. Sabaté. 2018. Soy isoflavone consumption and age at pubarche in adolescent males. European Journal of Nutrition 57 (6):2287–94. DOI: 10.3945/jn.108.090928
- Segovia-Siapco, G., P. Pribis, M. Messina, K. Oda, and J. Sabaté. 2014. Is soy intake related to age at onset of menarche? A cross-sectional study among adolescents with a wide range of soy food consumption. Nutrition Journal 13:54 doi: 10.1186/1475-2891-13-54.
- Sener, A. B., N. C. Seçkin, S. Ozmen, O. Gökmen, N. Doğu, and E. Ekici. 1996. The effects of hormone replacement therapy on uterine fibroids in postmenopausal women. Fertility and Sterility 65 (2):354–7. doi: 10.1016/s0015-0282(16)58098-4.
- Setchell, K. D. 2000. Absorption and metabolism of soy isoflavones-from food to dietary supplements and adults to infants. The Journal of Nutrition 130 (3):654S–5S. doi: 10.1093/jn/130.3.654S.
- Setchell, K. D. R., M. S. Faughnan, T. Avades, L. Zimmer-Nechemias, N. M. Brown, B. E. Wolfe, W. T. Brashear, P. Desai, M. F. Oldfield, N. P. Botting, et al. 2003. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. The American Journal of Clinical Nutrition 77 (2):411–9. doi: 10.1093/ajcn/77.2.411.
- Setchell, K. D. R., N. M. Brown, L. Zimmer-Nechemias, W. T. Brashear, B. E. Wolfe, A. S. Kirschner, and J. E. Heubi. 2002. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. The American Journal of Clinical Nutrition 76 (2):447–53. doi: 10.1093/ajcn/76.2.447.
- Setchell, K. D., and C. Clerici. 2010. Equol: History, chemistry, and formation. The Journal of Nutrition 140 (7):1355S–62S. doi: 10.3945/jn.109.119776.
- Setchell, K. D., L. Zimmer-Nechemias, J. Cai, and J. E. Heubi. 1997. Exposure of infants to phyto-oestrogens from soy-based infant formula. The Lancet 350 (9070):23–7. doi: 10.1016/S0140-6736(96)09480-9.
- Setchell, K. D., L. Zimmer-Nechemias, J. Cai, and J. E. Heubi. 1998. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. The American Journal of Clinical Nutrition 68 (6):1453S–61. doi: 10.1093/ajcn/68.6.1453S.
- Setchell, K. D., N. M. Brown, and E. Lydeking-Olsen. 2002. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. The Journal of Nutrition 132 (12):3577–84. doi: 10.1093/jn/132.12.3577.
- Setchell, K. D., N. M. Brown, P. Desai, L. Zimmer-Nechemias, B. E. Wolfe, W. T. Brashear, A. S. Kirschner, A. Cassidy, and J. E. Heubi. 2001. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. The Journal of Nutrition 131 (4 Suppl):1362S–75S. doi: 10.1093/jn/131.4.1362S.
- Setchell, K. D., N. M. Brown, X. Zhao, S. L. Lindley, J. E. Heubi, E. C. King, and M. J. Messina. 2011. Soy isoflavone phase II metabolism differs between rodents and humans: Implications for the effect on breast cancer risk. The American Journal of Clinical Nutrition 94 (5):1284–94. doi: 10.3945/ajcn.111.019638.
- Setchell, K. D., S. J. Gosselin, M. B. Welsh, J. O. Johnston, W. F. Balistreri, L. W. Kramer, B. L. Dresser, and M. J. Tarr. 1987. Dietary estrogens–a probable cause of infertility and liver disease in captive cheetahs. Gastroenterology 93 (2):225–33. doi: 10.1016/0016-5085(87)91006-7.
- Setchell, K. D., S. P. Borriello, P. Hulme, D. N. Kirk, and M. Axelson. 1984. Nonsteroidal estrogens of dietary origin: Possible roles in hormone-dependent disease. The American Journal of Clinical Nutrition 40 (3):569–78. doi: 10.1093/ajcn/40.3.569.
- Setji, T. L., and A. J. Brown. 2014. Polycystic ovary syndrome: Update on diagnosis and treatment. The American Journal of Medicine 127 (10):912–9. doi: 10.1016/j.amjmed.2014.04.017.
- Sfakianos, J., L. Coward, M. Kirk, and S. Barnes. 1997. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. The Journal of Nutrition 127 (7):1260–8. doi: 10.1093/jn/127.7.1260.
- Shahin, A. Y., A. M. Ismail, K. M. Zahran, and A. M. Makhlouf. 2008. Adding phytoestrogens to clomiphene induction in unexplained infertility patients–a randomized trial. Reproductive Biomedicine Online 16 (4):580–8. doi: 10.1016/S1472-6483(10)60465-8.
- Shakir, K. M., J. P. Chute, B. S. Aprill, and A. A. Lazarus. 1997. Ferrous sulfate-induced increase in requirement for thyroxine in a patient with primary hypothyroidism. Southern Medical Journal 90 (6):637–9. doi: 10.1097/00007611-199706000-00011.
- Shang, Y. 2006. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nature Reviews. Cancer 6 (5):360–8. doi: 10.1038/nrc1879.
- Shang, Y. 2007. Hormones and cancer. Cell Research 17 (4):277–9. doi: 10.1038/cr.2007.26.
- Sharpe, R. M., and N. E. Skakkebaek. 1993. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet (London, England) 341 (8857):1392–5. doi: 10.1016/0140-6736(93)90953-e.
- Shekharyadav, C., M. Bajpai, V. Kumar, R. S. Ahmed, P. Gupta, and B. D. Banerjee. 2011. Polymorphism in CYP1A1, GSTMI, GSTT1 genes and organochlorine pesticides in the etiology of ogy of hypospadias. Human & Experimental Toxicology 30 (10):1464–74. doi: 10.1177/0960327110392402.
- Shelnutt, S. R., C. O. Cimino, P. A. Wiggins, M. J. J. Ronis, and T. M. Badger. 2002. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. The American Journal of Clinical Nutrition 76 (3):588–94. doi: 10.1093/ajcn/76.3.588.
- Shen, Y., Q. Xu, J. Xu, M. L. Ren, and Y. L. Cai. 2013. Environmental exposure and risk of uterine leiomyoma: An epidemiologic survey. European Review for Medical and Pharmacological Sciences 17:3249–56. https://pubmed.ncbi.nlm.nih.gov/24338469/.
- Shepard, T. H., G. E. Pyne, J. F. Kirschvink, and M. McLean. 1960. Soybean goiter. New England Journal of Medicine 262 (22):1099–103. doi: 10.1056/NEJM196006022622201.
- Sherman, S. I., E. T. Tielens, and P. W. Ladenson. 1994. Sucralfate causes malabsorption of L-thyroxine. The American Journal of Medicine 96 (6):531–5. doi: 10.1016/0002-9343(94)90093-0.
- Shibata, A., D. T. Harris, and P. R. Billings. 2002. Concentrations of estrogens and IGFs in umbilical cord blood plasma: a comparison among Caucasian, Hispanic, and Asian-American females. Journal of Clinical Endocrinology and Metabolism 87 (2):810–5. doi: 10.1210/jcem.87.2.8227.
- Shibayama, T., H. Fukata, K. Sakurai, T. Adachi, M. Komiyama, T. Iguchi, and C. Mori. 2001. Neonatal exposure to genistein reduces expression of estrogen receptor alpha and androgen receptor in testes of adult mice. Endocrine Journal 48 (6):655–63. doi: 10.1507/endocrj.48.655.
- Shike, M., A. S. Doane, L. Russo, R. Cabal, J. Reis-Filo, W. Gerald, H. Cody, R. Khanin, J. Bromberg, and L. Norton. 2014. The effects of soy supplementation on gene expression in breast cancer: A randomized placebo-controlled study. JNCI Journal of the National Cancer Institute 106 (9):dju189–dju189. doi: 10.1093/jnci/dju189.
- Shimazu, T., M. Inoue, S. Sasazuki, M. Iwasaki, N. Sawada, T. Yamaji, and S. Tsugane. 2011. Plasma isoflavones and the risk of lung cancer in women: A nested case-control study in Japan. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 20 (3):419–27. doi: 10.1158/1055-9965.EPI-10-1025.
- Shrestha, S., H. W. Wiener, A. K. Olson, J. C. Edberg, N. E. Bowles, H. Patel, and M. A. Portman. 2011. Functional FCGR2B gene variants influence intravenous immunoglobulin response in patients with Kawasaki disease. The Journal of Allergy and Clinical Immunology 128 (3):677–80. doi: 10.1016/j.jaci.2011.04.027.
- Shrestha, S., H. Wiener, A. Shendre, R. A. Kaslow, J. Wu, A. Olson, N. E. Bowles, H. Patel, J. C. Edberg, and M. A. Portman. 2012. Role of activating FcγR gene polymorphisms in Kawasaki disease susceptibility and intravenous immunoglobulin response. Circulation: Cardiovascular Genetics 5 (3):309–16. doi: 10.1161/CIRCGENETICS.111.962464.
- Shu, X. O., F. Jin, Q. Dai, et al. 2001. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiology, Biomarkers & Prevention 10:483–8. https://pubmed.ncbi.nlm.nih.gov/11352858/
- Shu, X. O., Y. Zheng, H. Cai, K. Gu, Z. Chen, W. Zheng, and W. Lu. 2009. Soy food intake and breast cancer survival. JAMA 302 (22):2437–43. doi: 10.1001/jama.2009.1783.
- Shu, X.-O., H. Li, G. Yang, J. Gao, H. Cai, Y. Takata, W. Zheng, and Y.-B. Xiang. 2015. Cohort Profile: The Shanghai Men's Health Study. International Journal of Epidemiology 44 (3):810–8. doi: 10.1093/ije/dyv013.
- Siepmann, T., J. Roofeh, F. W. Kiefer, and D. G. Edelson. 2011. Hypogonadism and erectile dysfunction associated with soy product consumption. Nutrition (Burbank, Los Angeles County, Calif.) 27 (7-8):859–62. doi: 10.1016/j.nut.2010.10.018.
- Silva, F., T. C. C. Lemos, D. Sandôra, M. Monteiro, and D. Perrone. 2020. Fermentation of soybean meal improves isoflavone metabolism after soy biscuit consumption by adults. Journal of the Science of Food and Agriculture 100 (7):2991–8. doi: 10.1002/jsfa.10328.
- Sinai, T., S. Ben-Avraham, I. Guelmann-Mizrahi, M. R. Goldberg, L. Naugolni, G. Askapa, Y. Katz, and M. Rachmiel. 2019. Consumption of soy-based infant formula is not associated with early onset of puberty. European Journal of Nutrition 58 (2):681–7. doi: 10.1007/s00394-018-1668-3.
- Siraj, E. S., M. K. Gupta, and S. S. Reddy. 2003. Raloxifene causing malabsorption of levothyroxine. Archives of Internal Medicine 163 (11):1367–70. doi: 10.1001/archinte.163.11.1367.
- Skakkebaek, N. E., E. Rajpert-De Meyts, and K. M. Main. 2001. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects. Human Reproduction (Oxford, England) 16 (5):972–8. doi: 10.1093/humrep/16.5.972.
- Slavin, J. L. 1996. Phytoestrogens in breast milk–another advantage of breast-feeding? Clinical Chemistry 42 (6 Pt 1):841–2. doi: 10.1093/clinchem/42.6.841.
- Smith-Bindman, R., E. Weiss, and V. Feldstein. 2004. How thick is too thick? When endometrial thickness should prompt biopsy in postmenopausal women without vaginal bleeding. Ultrasound in Obstetrics and Gynecology 24 (5):558–65. doi: 10.1002/uog.1704.
- Solecki, R., A. Kortenkamp, Å. Bergman, I. Chahoud, G. H. Degen, D. Dietrich, H. Greim, H. Håkansson, U. Hass, T. Husoy, et al. 2017. Scientific principles for the identification of endocrine-disrupting chemicals: A consensus statement. Archives of Toxicology 91 (2):1001–6. doi: 10.1007/s00204-016-1866-9.
- Somjen, D., S. Katzburg, E. Knoll, D. Hendel, N. Stern, A. M. Kaye, and I. Yoles. 2007. DT56a (Femarelle): A natural selective estrogen receptor modulator (SERM). The Journal of Steroid Biochemistry and Molecular Biology 104 (3-5):252–8. doi: 10.1016/j.jsbmb.2007.03.00
- Song, G., L. Kochman, E. Andolina, R. C. Herko, K. J. Brewer, and V. Lewis. 2006. Beneficial effects of dietary intake of plant phytoestrogens on semen parameters and sperm DNA integrity in infertile men. Fertility and Sterility 86 (3):S49. Abstract 0-115). doi: 10.1016/j.fertnstert.2006.07.134.
- Song, T. T., S. Hendrich, and P. A. Murphy. 1999. Estrogenic activity of glycitein, a soy isoflavone. Journal of Agricultural and Food Chemistry 47 (4):1607–10. doi: 10.1021/jf981054j.
- Sosvorova, L., P. Miksatkova, M. Bicikova, N. Kaňová, and O. Lapčík. 2012. The presence of monoiodinated derivates of daidzein and genistein in human urine and its effect on thyroid gland function. Food and Chemical Toxicology 50:2774–9. doi: 10.1016/j.fct.2012.05.037.
- Soukup, S. T., J. Helppi, D. R. Müller, O. Zierau, B. Watzl, G. Vollmer, P. Diel, A. Bub, and S. E. Kulling. 2016. Phase II metabolism of the soy isoflavones genistein and daidzein in humans, rats and mice: A cross-species and sex comparison. Archives of Toxicology 90 (6):1335–47. doi: 10.1007/s00204-016-1663-5.
- Soy Infant Formula. 2019. National Institute of Environmental Health Sciences. Accessed November 17, 2019. https://www.niehs.nih.gov/health/topics/agents/sya-soy-formula/.
- Sperber, A. D., and Y. Liel. 1992. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Archives of Internal Medicine 152 (1):183–4. doi: 10.1001/archinte.1992.00400130181024.
- Springer, A., M. van den Heijkant, and S. Baumann. 2016. Worldwide prevalence of hypospadias. Journal of Pediatric Urology 12 (3):152 e1-7–152.e7. doi: 10.1016/j.jpurol.2015.12.002.
- Starling, A. P., K. A. Sauder, J. L. Kaar, A. L. Shapiro, A. M. Siega-Riz, and D. Dabelea. 2017. Maternal dietary patterns during pregnancy are associated with newborn body composition. The Journal of Nutrition 147 (7):1334–9. doi: 10.3945/jn.117.248948.
- Stein, A. D., H. S. Kahn, A. Rundle, P. A. Zybert, K. van der Pal–de Bruin, and L. H. Lumey. 2007. Anthropometric measures in middle age after exposure to famine during gestation: Evidence from the Dutch famine. The American Journal of Clinical Nutrition 85 (3):869–76. doi: 10.1093/ajcn/85.3.869.
- Stein, A. D., P. A. Zybert, K. van der Pal-de Bruin, and L. H. Lumey. 2006. Exposure to famine during gestation, size at birth, and blood pressure at age 59 y: Evidence from the Dutch Famine. European Journal of Epidemiology 21 (10):759–65. doi: 10.1007/s10654-006-9065-2.
- Steinberg, F. M., M. J. Murray, R. D. Lewis, M. A. Cramer, P. Amato, R. L. Young, S. Barnes, K. L. Konzelmann, J. G. Fischer, K. J. Ellis, et al. 2011. Clinical outcomes of a 2-y soy isoflavone supplementation in menopausal women. The American Journal of Clinical Nutrition 93 (2):356–67. doi: 10.3945/ajcn.110.008359.
- Stevens, N. 2017. Soy-based infant formulas: A review of developmental and nutritional considerations. Journal of Pediatrics & Neonatal Care 7 (3) doi: 10.15406/jpnc.2017.07.00287.
- Stewart, A. J., B. R. Westley, and F. E. May. 1992. Modulation of the proliferative response of breast cancer cells to growth factors by oestrogen. British Journal of Cancer 66 (4):640–8. doi: 10.1038/bjc.1992.330.
- Stewart, E. A., C. L. Cookson, R. A. Gandolfo, and R. Schulze-Rath. 2017. Epidemiology of uterine fibroids: A systematic review. BJOG : An International Journal of Obstetrics and Gynaecology 124 (10):1501–12. doi: 10.1111/1471-0528.14640.
- Strauss, L., S. Mäkelä, S. Joshi, I. Huhtaniemi, and R. Santti. 1998. Genistein exerts estrogen-like effects in male mouse reproductive tract. Molecular and Cellular Endocrinology 144 (1-2):83–93. doi: 10.1016/s0303-7207(98)00152-x.
- Subramanian, S., G. Stacey, and O. Yu. 2006. Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. The Plant Journal : For Cell and Molecular Biology 48 (2):261–73. doi: 10.1111/j.1365-313X.2006.02874.x.
- Sugiyama, Y., Y. Nagata, F. Fukuta, A. Takayanagi, N. Masumori, T. Tsukamoto, H. Akasaka, H. Ohnishi, S. Saito, T. Miura, et al. 2014. Counts of Slackia sp. strain NATTS in intestinal flora are correlated to serum concentrations of equol both in prostate cancer cases and controls in Japanese men. Asian Pacific Journal of Cancer Prevention 15 (6):2693–7. doi: 10.7314/APJCP.2014.15.6.2693.
- Surh, J., M.-J. Kim, E. Koh, Y.-K L. Kim, and H. Kwon. 2006. Estimated intakes of isoflavones and coumestrol in Korean population. International Journal of Food Sciences and Nutrition 57 (5-6):325–44. doi: 10.1080/09637480600802348.
- Sutrisno, S., H. Aprina, H. M. Simanungkalit, A. Andriyani, W. Barlianto, H. Sujuti, S. Santoso, P. M. Dwijayasa, E. S. Wahyuni, and E. Mustofa. 2018. Genistein modulates the estrogen receptor and suppresses angiogenesis and inflammation in the murine model of peritoneal endometriosis. Journal of Traditional and Complementary Medicine 8 (2):278–81. doi: 10.1016/j.jtcme.2017.03.002.
- Suzuki, Y., J. Yoshinaga, Y. Mizumoto, S. Serizawa, and H. Shiraishi. 2012. Foetal exposure to phthalate esters and anogenital distance in male newborns. International Journal of Andrology 35 (3):236–44. doi: 10.1111/j.1365-2605.2011.01190.x.
- Swan, S. H. 2000. Intrauterine exposure to diethylstilbestrol: Long-term effects in humans. APMIS: Acta Pathologica, Microbiologica, et Immunologica Scandinavica 108 (12):793–804. DOI: 10.1097/EDE.0b013e318163152a
- Swan, S. H., F. Liu, J. W. Overstreet, C. Brazil, and N. E. Skakkebaek. 2007. Semen quality of fertile US males in relation to their mothers' beef consumption during pregnancy. Human Reproduction (Oxford, England) 22 (6):1497–502. doi: 10.1093/humrep/dem068.
- Tai, T. Y., K. S. Tsai, S. T. Tu, J. S. Wu, C. I. Chang, C. L. Chen, N. S. Shaw, H. Y. Peng, S. Y. Wang, and C. H. Wu. 2012. The effect of soy isoflavone on bone mineral density in postmenopausal Taiwanese women with bone loss: A 2-year randomized double-blind placebo-controlled study. Osteoporosis International 23 (5):1571–80. doi: 10.1007/s00198-011-1750-7.
- Takaoka, O., T. Mori, F. Ito, H. Okimura, H. Kataoka, Y. Tanaka, A. Koshiba, I. Kusuki, S. Shigehiro, T. Amami, et al. 2018. Daidzein-rich isoflavone aglycones inhibit cell growth and inflammation in endometriosis. Journal of Steroid Biochemistry and Molecular Biology. 181:125–32. doi: 10.1016/j.jsbmb.2018.04.004.
- Takashima, N., N. Miyanaga, K. Komiya, M. More, and H. Akaza. 2004. Blood isoflavone levels during intake of a controlled hospital diet. Journal of Nutritional Science and Vitaminology 50 (4):246–52. doi: 10.3177/jnsv.50.246.
- Takimoto, C. H., K. Glover, X. Huang, S. A. Hayes, L. Gallot, M. Quinn, B. D. Jovanovic, A. Shapiro, L. Hernandez, A. Goetz, et al. 2003. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 12 (11 Pt 1):1213–21. https://pubmed.ncbi.nlm.nih.gov/14652284/.
- Talaei, M., W.-P. Koh, R. M. van Dam, J.-M. Yuan, and A. Pan. 2014. Dietary soy intake is not associated with risk of cardiovascular disease mortality in Singapore Chinese adults. The Journal of Nutrition 144 (6):921–8. doi: 10.3945/jn.114.190454.
- Talma, H., Y. Schönbeck, P. van Dommelen, B. Bakker, S. van Buuren, and R. A. Hirasing. 2013. Trends in menarcheal age between 1955 and 2009 in the Netherlands. PloS One 8 (4):e60056. doi: 10.1371/journal.pone.0060056.
- Tan, C., Y. Zhao, and S. Wang. 2019. Is a vegetarian diet safe to follow during pregnancy? A systematic review and meta-analysis of observational studies. Critical Reviews in Food Science and Nutrition 59 (16):2586–96. doi: 10.1080/10408398.2018.1461062.
- Taylor, D. K., and P. C. Leppert. 2012. Treatment for uterine fibroids: Searching for effective drug therapies. Drug Discovery Today. Therapeutic Strategies 9 (1):e41–e9. doi: 10.1016/j.ddstr.2012.06.001.
- Taylor, M., A. Bickel, R. Mannion, E. Bell, and G. G. Harrigan. 2017. Dicamba-tolerant soybeans (Glycine max L.) MON 87708 and MON 87708 × MON 89788 are compositionally equivalent to conventional soybean. Journal of Agricultural and Food Chemistry 65 (36):8037–45. doi: 10.1021/acs.jafc.7b03844.
- Templeman, C., S. F. Marshall, C. A. Clarke, K. DeLellis Henderson, J. Largent, S. Neuhausen, P. Reynolds, G. Ursin, and L. Bernstein. 2009. Risk factors for surgically removed fibroids in a large cohort of teachers. Fertility and Sterility 92 (4):1436–46. doi: 10.1016/j.fertnstert.2008.08.074.
- Testa, I., C. Salvatori, G. Di Cara, A. Latini, F. Frati, S. Troiani, N. Principi, and S. Esposito. 2018. Soy-based infant formula: Are phyto-oestrogens still in doubt? Frontiers in Nutrition 5:110. doi: 10.3389/fnut.2018.00110.
- The Writing Group for the PEPI Trial. 1996. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA 275:370–5. doi: 10.1001/jama.1996.03530290040035.
- Thompson, L. U., B. A. Boucher, Z. Liu, M. Cotterchio, and N. Kreiger. 2006. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutrition and Cancer 54 (2):184–201. doi: 10.1207/s15327914nc5402_5.
- Thrane, M., P. V. Paulsen, M. W. Orcutt, et al. 2017. Soy protein: Impacts, production, and applications. In: Sustainable protein sources, eds. S. R. Nadathur, J. P. D. Wanasundara, and L. Scanlin, 23–46. United Kingdom: Academic Press.
- Titus-Ernstoff, L., R. Troisi, E. E. Hatch, M. Hyer, L. A. Wise, J. R. Palmer, R. Kaufman, E. Adam, K. Noller, A. L. Herbst, et al. 2008. Offspring of women exposed in utero to diethylstilbestrol (DES): A preliminary report of benign and malignant pathology in the third generation. Epidemiology (Cambridge, Mass.) 19 (2):251–7. doi: 10.1097/EDE.0b013e318163152a.
- Todaka, E., K. Sakurai, H. Fukata, H. Miyagawa, M. Uzuki, M. Omori, H. Osada, Y. Ikezuki, O. Tsutsumi, T. Iguchi, et al. 2005. Fetal exposure to phytoestrogens-the difference in phytoestrogen status between mother and fetus. Environmental Research 99 (2):195–203. doi: 10.1016/j.envres.2004.11.006.
- Tonstad, S., K. Jaceldo-Siegl, M. Messina, E. Haddad, and G. E. Fraser. 2016. The association between soya consumption and serum thyroid-stimulating hormone concentrations in the Adventist Health Study-2. Public Health Nutrition 19 (8):1464–70. doi: 10.1017/S1368980015002943.
- Toppari, J., H. E. Virtanen, K. M. Main, and N. E. Skakkebaek. 2010. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): Environmental connection. Birth Defects Research. Part A, Clinical and Molecular Teratology 88 (10):910–9. doi: 10.1002/bdra.20707.
- Toppari, J., J. C. Larsen, P. Christiansen, A. Giwercman, P. Grandjean, L. J. Guillette, B. Jégou, T. K. Jensen, P. Jouannet, N. Keiding, et al. 1996. Male reproductive health and environmental xenoestrogens. Environmental Health Perspectives 104 Suppl 4 (Suppl 4):741–803. doi: 10.1289/ehp.96104s4741.
- Toshima, H., Y. Suzuki, K. Imai, J. Yoshinaga, H. Shiraishi, Y. Mizumoto, S. Hatakeyama, C. Onohara, and S. Tokuoka. 2012. Endocrine disrupting chemicals in urine of Japanese male partners of subfertile couples: A pilot study on exposure and semen quality. International Journal of Hygiene and Environmental Health 215 (5):502–6. doi: 10.1016/j.ijheh.2011.09.00
- Treloar, S. A., K. A. Do, V. M. O'Connor, D. T. O'Connor, M. A. Yeo, and N. G. Martin. 1999. Predictors of hysterectomy: An Australian study. American Journal of Obstetrics and Gynecology 180 (4):945–54. doi: 10.1016/S0002-9378(99)70666-6.
- Troisi, R., E. E. Hatch, L. Titus-Ernstoff, M. Hyer, J. R. Palmer, S. J. Robboy, W. C. Strohsnitter, R. Kaufman, A. L. Herbst, and R. N. Hoover. 2007. Cancer risk in women prenatally exposed to diethylstilbestrol. International Journal of Cancer 121 (2):356–60. doi: 10.1002/ijc.22631.
- Troisi, R., M. Hyer, E. E. Hatch, L. Titus-Ernstoff, J. R. Palmer, W. C. Strohsnitter, A. L. Herbst, E. Adam, and R. N. Hoover. 2013. Medical conditions among adult offspring prenatally exposed to diethylstilbestrol. Epidemiology (Cambridge, Mass.) 24 (3):430–8. doi: 10.1097/EDE.0b013e318289bdf7.
- Troisi, R., N. Potischman, C. N. Johnson, J. M. Roberts, D. Lykins, G. Harger, N. Markovic, P. Siiteri, and R. N. Hoover. 2003. Estrogen and androgen concentrations are not lower in the umbilical cord serum of pre-eclamptic pregnancies. Cancer Epidemiology, Biomarkers & Prevention 12:1268–70. https://pubmed.ncbi.nlm.nih.gov/14652293/.
- Troisi, R., N. Potischman, J. Roberts, P. Siiteri, A. Daftary, C. Sims, and R. N. Hoover. 2003. Associations of maternal and umbilical cord hormone concentrations with maternal, gestational and neonatal factors (United States). Cancer Causes & Control : CCC 14 (4):347–55. doi: 10.1023/a:1023934518975.
- Troisi, R., P. Lagiou, D. Trichopoulos, B. Xu, L. Chie, F. Z. Stanczyk, N. Potischman, H.-O. Adami, R. N. Hoover, C.-C. Hsieh, et al. 2008. Cord serum estrogens, androgens, insulin-like growth factor-I, and insulin-like growth factor binding protein-3 in Chinese and U.S. Caucasian neonates. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 17 (1):224–31. doi: 10.1158/1055-9965.EPI-07-0536.
- Tsangalis, D., G. Wilcox, N. P. Shah, and L. Stojanovska. 2005. Bioavailability of isoflavone phytoestrogens in postmenopausal women consuming soya milk fermented with probiotic bifidobacteria. British Journal of Nutrition 93 (6):867–77. doi: 10.1079/BJN20041299.
- Tsuchiya, M., T. Miura, T. Hanaoka, M. Iwasaki, H. Sasaki, T. Tanaka, H. Nakao, T. Katoh, T. Ikenoue, M. Kabuto, et al. 2007. Effect of soy isoflavones on endometriosis: Interaction with estrogen receptor 2 gene polymorphism. Epidemiology (Cambridge, Mass.) 18 (3):402–8. doi: 10.1097/01.ede.0000257571.01358.f9.
- Tsunoda, N., S. Pomeroy, and P. Nestel. 2002. Absorption in humans of isoflavones from soy and red clover is similar. The Journal of Nutrition 132 (8):2199–201. doi: 10.1093/jn/132.8.2199.
- Unfer, V., M. L. Casini, L. Costabile, M. Mignosa, S. Gerli, and G. C. Di Renzo. 2004. High dose of phytoestrogens can reverse the antiestrogenic effects of clomiphene citrate on the endometrium in patients undergoing intrauterine insemination: A randomized trial. Journal of the Society for Gynecologic Investigation 11 (5):323–8. doi: 10.1016/j.jsgi.2003.12.007.
- Unfer, V., M. L. Casini, L. Costabile, M. Mignosa, S. Gerli, and G. C. Di Renzo. 2004. Endometrial effects of long-term treatment with phytoestrogens: A randomized, double-blind, placebo-controlled study. Fertility and Sterility 82 (1):145–8. doi: 10.1016/j.fertnstert.2003.11.041.
- Upson, K., M. A. Adgent, G. Wegienka, and D. D. Baird. 2019. Soy-based infant formula feeding and menstrual pain in a cohort of women aged 23-35 years. Human Reproduction (Oxford, England) 34 (1):148–54. doi: 10.1093/humrep/dey303.
- Upson, K., S. Sathyanarayana, D. Scholes, and V. L. Holt. 2015. Early-life factors and endometriosis risk. Fertility and Sterility 104 (4):964–71 e5. doi: 10.1016/j.fertnstert.2015.06.040.
- Vakharia, D., and G. J. Mizejewski. 2000. Human alpha-fetoprotein peptides bind estrogen receptor and estradiol, and suppress breast cancer. Breast Cancer Research and Treatment 63 (1):41–52. doi: 10.1023/A:1006484223325.
- van de Beek, C., J. H. H. Thijssen, P. T. Cohen-Kettenis, S. H. M. van Goozen, and J. K. Buitelaar. 2004. Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: What is the best source of information to investigate the effects of fetal hormonal exposure? Hormones and Behavior 46 (5):663–9. doi: 10.1016/j.yhbeh.2004.06.010.
- van de Beek, C., S. H. M. van Goozen, J. K. Buitelaar, and P. T. Cohen-Kettenis. 2009. Prenatal sex hormones (maternal and amniotic fluid) and gender-related play behavior in 13-month-old Infants. Archives of Sexual Behavior 38 (1):6–15. doi: 10.1007/s10508-007-9291-z.
- van der Velpen, V., P. C. Hollman, M. van Nielen, E. G. Schouten, M. Mensink, P. Van't Veer, and A. Geelen. 2014. Large inter-individual variation in isoflavone plasma concentration limits use of isoflavone intake data for risk assessment. European Journal of Clinical Nutrition 68 (10):1141–7. doi: 10.1038/ejcn.2014.108.
- Van Middlesworth, L. 1957. Thyroxine excretion, a possible cause of goiter. Endocrinology 61 (5):570–3. doi: 10.1210/endo-61-5-570.
- van Wyk, J. J., M. B. Arnold, J. Wynn, and F. Pepper. 1959. The effects of a soybean product on thyroid function in humans. Pediatrics 24:752–60.
- Vandenplas, Y., P. G. Castrellon, R. Rivas, C. J. Gutiérrez, L. D. Garcia, J. E. Jimenez, A. Anzo, B. Hegar, and P. Alarcon. 2014. Safety of soya-based infant formulas in children. The British Journal of Nutrition 111 (8):1340–60. doi: 10.1017/S0007114513003942.
- Vanegas, J. C., M. C. Afeiche, A. J. Gaskins, L. Mínguez-Alarcón, P. L. Williams, D. L. Wright, T. L. Toth, R. Hauser, and J. E. Chavarro. 2015. Soy food intake and treatment outcomes of women undergoing assisted reproductive technology. Fertility and Sterility 103 (3):749–55 e2. doi: 10.1016/j.fertnstert.2014.12.104.
- Vassena, R., R. Vidal, O. Coll, and V. Vernaeve. 2014. Menstrual cycle length in reproductive age women is an indicator of oocyte quality and a candidate marker of ovarian reserve. European Journal of Obstetrics & Gynecology and Reproductive Biology 177:130–4. doi: 10.1016/j.ejogrb.2014.03.027.
- Velebil, P., P. A. Wingo, Z. Xia, L. S. Wilcox, and H. B. Peterson. 1995. Rate of hospitalization for gynecologic disorders among reproductive-age women in the United States. Obstetrics and Gynecology 86 (5):764–9. doi: 10.1016/0029-7844(95)00252-M.
- Verkasalo, P. K., P. N. Appleby, G. K. Davey, and T. J. Key. 2001. Soy milk intake and plasma sex hormones: A cross-sectional study in pre- and postmenopausal women (EPIC-Oxford). Nutrition and Cancer 40 (2):79–86. doi: 10.1207/S15327914NC402_1.
- Verkasalo, P. K., P. N. Appleby, N. E. Allen, G. Davey, H. Adlercreutz, and T. J. Key. 2001. Soya intake and plasma concentrations of daidzein and genistein: Validity of dietary assessment among eighty British women (Oxford arm of the European Prospective Investigation into Cancer and Nutrition)). British Journal of Nutrition 86 (3):415–21. doi: 10.1079/bjn2001424.
- Vieux, F., M. Maillot, and C. D. Rehm. 2020. Flavonoid intakes in the US diet are linked to higher socioeconomic status and to tea consumption: Analyses of NHANES 2011–16 data. Journal of Nutrition. doi: 10.1093/jn/nxaa145.
- Vilela, M. L. B., E. Willingham, J. Buckley, B. C. Liu, K. Agras, Y. Shiroyanagi, and L. S. Baskin. 2007. Endocrine disruptors and hypospadias: Role of genistein and the fungicide vinclozolin. Urology 70 (3):618–21. doi: 10.1016/j.urology.2007.05.004.
- Villa, P., B. Costantini, R. Suriano, C. Perri, F. Macrì, L. Ricciardi, S. Panunzi, and A. Lanzone. 2009. The differential effect of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women: Relationship with the metabolic status. The Journal of Clinical Endocrinology and Metabolism 94 (2):552–8. doi: 10.1210/jc.2008-0735.
- Wada, K., K. Nakamura, T. Masue, Y. Sahashi, K. Ando, and C. Nagata. 2011. Soy intake and urinary sex hormone levels in preschool Japanese children. American Journal of Epidemiology 173 (9):998–1003. doi: 10.1093/aje/kwr006.
- Wang, T. T., N. Sathyamoorthy, and J. M. Phang. 1996. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis 17 (2):271–5. doi: 10.1093/carcin/17.2.271.
- Watanabe, S., M. Yamaguchi, T. Sobue, T. Takahashi, T. Miura, Y. Arai, W. Mazur, K. Wähälä, and H. Adlercreutz. 1998. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako). The Journal of Nutrition 128 (10):1710–5. doi: 10.1093/jn/128.10.1710.
- Weber, K. S., K. D. Setchell, D. M. Stocco, and E. D. Lephart. 2001. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5alpha-reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. The Journal of Endocrinology 170 (3):591–9. doi: 10.1677/joe.0.1700591.
- Wei, Q.-K., T.-R. Chen, and J.-T. Chen. 2008. Use of Bacillus subtilis to enrich isoflavone aglycones in fermented natto. Journal of the Science of Food and Agriculture 88 (6):1007–11. doi: 10.1002/jsfa.3181.
- Weiderpass, E., H. O. Adami, J. A. Baron, C. Magnusson, R. Bergström, A. Lindgren, N. Correia, and I. Persson. 1999. Risk of endometrial cancer following estrogen replacement with and without progestins. Journal of the National Cancer Institute 91 (13):1131–7. doi: 10.1093/jnci/91.13.1131.
- Wesselink, A. K., E. E. Hatch, E. M. Mikkelsen, E. Trolle, S. K. Willis, S. E. McCann, L. Valsta, A. Lundqvist, K. L. Tucker, K. J. Rothman, et al. 2020. Dietary phytoestrogen intakes of adult women are not strongly related to fecundability in 2 preconception cohort studies. The Journal of Nutrition 150 (5):1240–51. May 1 doi: 10.1093/jn/nxz335.
- Wesselink, A. K., L. A. Wise, E. E. Hatch, K. J. Rothman, E. M. Mikkelsen, J. B. Stanford, C. J. McKinnon, and S. Mahalingaiah. 2016. Menstrual cycle characteristics and fecundability in a North American preconception cohort. Annals of Epidemiology 26 (7):482–7 e1. doi: 10.1016/j.annepidem.2016.05.006.
- West, M. C. L., L. Anderson, N. McClure, and S. E. M. Lewis. 2005. Dietary oestrogens and male fertility potential. Human Fertility (Cambridge, England) 8 (3):197–207. doi: 10.1080/14647270500030266.
- White, L. R., H. Petrovitch, G. W. Ross, K. Masaki, J. Hardman, J. Nelson, D. Davis, and W. Markesbery. 2000. Brain aging and midlife tofu consumption. Journal of the American College of Nutrition 19 (2):242–55. doi: 10.1080/07315724.2000.10718923.
- Whitehouse-Tedd, K. M., N. J. Cave, C. E. Ugarte, L. A. Waldron, J. K. Prasain, A. Arabshahi, S. Barnes, and D. G. Thomas. 2011. Dietary isoflavone absorption, excretion, and metabolism in captive cheetahs (Acinonyx jubatus). Journal of Zoo and Wildlife Medicine: official Publication of the American Association of Zoo Veterinarians 42 (4):658–70. doi: 10.1638/2011-0060.1.
- WHO & UNEP. 2013. State of the Science of Endocrine Disrupting Chemicals — 2012 (World Health Organization).
- Wise, L. A., and S. K. Laughlin-Tommaso. 2016. Epidemiology of uterine fibroids: From menarche to menopause. Clinical Obstetrics and Gynecology 59 (1):2–24. doi: 10.1097/GRF.0000000000000164.
- Wise, L. A., E. M. Mikkelsen, K. J. Rothman, A. H. Riis, H. T. Sørensen, K. F. Huybrechts, and E. E. Hatch. 2011. A prospective cohort study of menstrual characteristics and time to pregnancy. American Journal of Epidemiology 174 (6):701–9. doi: 10.1093/aje/kwr130.
- Wiseman, H., K. Casey, E. A. Bowey, R. Duffy, M. Davies, I. R. Rowland, A. S. Lloyd, A. Murray, R. Thompson, and D. B. Clarke. 2004. Influence of 10 wk of soy consumption on plasma concentrations and excretion of isoflavonoids and on gut microflora metabolism in healthy adults. The American Journal of Clinical Nutrition 80 (3):692–9. doi: 10.1093/ajcn/80.3.692.
- Witorsch, R. J. 2002. Low-dose in utero effects of xenoestrogens in mice and their relevance to humans: An analytical review of the literature. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 40 (7):905–12. doi: 10.1016/s0278-6915(02)00069-8.
- Wohlfahrt-Veje, C., K. M. Main, and N. E. Skakkebaek. 2009. Testicular dysgenesis syndrome: Foetal origin of adult reproductive problems. Clinical Endocrinology 71 (4):459–65. doi: 10.1111/j.1365-2265.2009.03545.x.
- Wolff, M. S., J. A. Britton, L. Boguski, S. Hochman, N. Maloney, N. Serra, Z. Liu, G. Berkowitz, S. Larson, and J. Forman. 2008. Environmental exposures and puberty in inner-city girls. Environmental Research 107 (3):393–400. doi: 10.1016/j.envres.2008.03.006.
- Wolff, M. S., S. L. Teitelbaum, K. McGovern, S. M. Pinney, G. C. Windham, M. Galvez, A. Pajak, M. Rybak, A. M. Calafat, L. H. Kushi, et al. 2015. Environmental phenols and pubertal development in girls. Environment International 84:174–80. doi: 10.1016/j.envint.2015.08.008.
- Wolff, M. S., S. L. Teitelbaum, S. M. Pinney, G. Windham, L. Liao, F. Biro, L. H. Kushi, C. Erdmann, R. A. Hiatt, M. E. Rybak, et al. 2010. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environmental Health Perspectives 118 (7):1039–46. doi: 10.1289/ehp.0901690.
- Wolfman, W., N. Leyland, W. Wolfman, M. Heywood, S. S. Singh, D. A. Rittenberg, R. Soucy, C. Allaire, A. Awadalla, C. Best, et al. 2010. Asymptomatic endometrial thickening. Journal of Obstetrics and Gynaecology Canada 32 (10):990–9. doi: 10.1016/S1701-2163(16)34690-4.
- World Cancer Research Fund International. 2014. Continuous Update Project Report: Diet, Nutrition, Physical Activity, and Breast Cancer Survivors. Accessed February 08, 2021. www.wcrf.org/sites/default/files/Breast-Cancer-Survivors-2014-Report.pdf.
- Wu, A. H., D. Spicer, A. Garcia, C.-C. Tseng, L. Hovanessian-Larsen, P. Sheth, S. E. Martin, D. Hawes, C. Russell, H. MacDonald, et al. 2015. Double-blind randomized 12-month soy intervention had no effects on breast MRI fibroglandular tissue density or mammographic density. Cancer Prevention Research (Philadelphia, Pa.) 8 (10):942–51. doi: 10.1158/1940-6207.CAPR-15-0125.
- Wu, A. H., M. C. Yu, C. C. Tseng, et al. 2003. Plasma isoflavone levels versus self-reported soy isoflavone levels in Asian-American women in Los Angeles County. Carcinogenesis 25 (1):77–81. doi: 10.1093/carcin/bgg189.
- Wu, A. H., M. C. Yu, C.-C. Tseng, F. Z. Stanczyk, and M. C. Pike. 2009. Dietary patterns and breast cancer risk in Asian American women. The American Journal of Clinical Nutrition 89 (4):1145–54. doi: 10.3945/ajcn.2008.26915.
- Xia, Y., M. Chen, P. Zhu, C. Lu, G. Fu, X. Zhou, D. Chen, H. Wang, B. Hang, S. Wang, et al. 2013. Urinary phytoestrogen levels related to idiopathic male infertility in Chinese men. Environment International 59:161–7. doi: 10.1016/j.envint.2013.06.009.
- Xiao, Y., S. Zhang, H. Tong, and S. Shi. 2018. Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytotherapy Research: PTR 32 (3):384–94. doi: 10.1002/ptr.5966.
- Xu, W. H., W. Zheng, Q. Cai, J.-R. Cheng, H. Cai, Y.-B. Xiang, and X. O. Shu. 2008. The Asp(327)Asn polymorphism in the sex hormone-binding globulin gene modifies the association of soy food and tea intake with endometrial cancer risk. Nutrition and Cancer 60 (6):736–43. doi: 10.1080/01635580802192833.
- Xu, X., H. J. Wang, P. A. Murphy, and S. Hendrich. 2000. Neither background diet nor type of soy food affects short-term isoflavone bioavailability in women. The Journal of Nutrition 130 (4):798–801. doi: 10.1093/jn/130.4.798.
- Yamamoto, A., E. B. Johnstone, M. S. Bloom, H. G. Huddleston, and V. Y. Fujimoto. 2017. A higher prevalence of endometriosis among Asian women does not contribute to poorer IVF outcomes. Journal of Assisted Reproduction and Genetics 34 (6):765–74. doi: 10.1007/s10815-017-0919-1.
- Yamamoto, A., H. R. Harris, A. F. Vitonis, et al. 2018. A prospective cohort study of meat and fish consumption and endometriosis risk. Am J Obstet Gynecol 219:178. e1- e10. doi: 10.1016/j.ajog.2018.05.034.
- Yamamoto, S., T. Sobue, S. Sasaki, M. Kobayashi, Y. Arai, M. Uehara, H. Adlercreutz, S. Watanabe, T. Takahashi, Y. Iitoi, et al. 2001. Validity and reproducibility of a self-administered food-frequency questionnaire to assess isoflavone intake in a Japanese population in comparison with dietary records and blood and urine isoflavones. The Journal of Nutrition 131 (10):2741–7. doi: 10.1093/jn/131.10.2741.
- Yang, G., X.-O. Shu, F. Jin, X. Zhang, H.-L. Li, Q. Li, Y.-T. Gao, and W. Zheng. 2005. Longitudinal study of soy food intake and blood pressure among middle-aged and elderly Chinese women. The American Journal of Clinical Nutrition 81 (5):1012–7. doi: 10.1093/ajcn/81.5.1012.
- Yang, J., H. Nakagawa, K. Tsuta, and A. Tsubura. 2000. Influence of perinatal genistein exposure on the development of MNU-induced mammary carcinoma in female Sprague-Dawley rats. Cancer Letters 149 (1-2):171–9. doi: 10.1016/s0304-3835(99)00357-2.
- Yavuz, E., M. Oktem, I. Esinler, S. A. Toru, and H. B. Zeyneloglu. 2007. Genistein causes regression of endometriotic implants in the rat model. Fertility and Sterility 88 (4 Suppl):1129–34. doi: 10.1016/j.fertnstert.2007.01.010.
- Ye, Y.-b., Z.-l. Wang, S.-y. Zhuo, W. Lu, H.-f. Liao, M. A. Verbruggen, S. Fang, H.-y. Mai, Y.-m. Chen, and Y.-x. Su. 2012. Soy germ isoflavones improve menopausal symptoms but have no effect on blood lipids in early postmenopausal Chinese women: A randomized placebo-controlled trial. Menopause 19 (7):791–8. doi: 10.1097/gme.0b013e31823dbeda.
- Yiee, J. H., and L. S. Baskin. 2010. Environmental factors in genitourinary development. The Journal of Urology 184 (1):34–41. doi: 10.1016/j.juro.2010.03.051.
- Yilmaz, B., H. Terekeci, S. Sandal, and F. Kelestimur. 2020. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Reviews in Endocrine & Metabolic Disorders 21 (1):127–47. doi: 10.1007/s11154-019-09521-z.
- Youseflu, S., S. H. Jahanian Sadatmahalleh, A. Mottaghi, and A. Kazemnejad. 2020. Dietary phytoestrogen intake and the risk of endometriosis in Iranian women: A case-control study. International Journal of Fertility & Sterility 13 (4):296–300. doi: 10.22074/ijfs.2020.5806.
- Yu, D., X. Zhang, Y.-B. Xiang, G. Yang, H. Li, S. Fazio, MRae Linton, Q. Cai, W. Zheng, Y.-T. Gao, et al. 2014. Association of soy food intake with risk and biomarkers of coronary heart disease in Chinese men. International Journal of Cardiology 172 (2):e285–e287. doi: 10.1016/j.ijcard.2013.12.200.
- Yuan, B., L. Wang, Y. Jin, H. Zhen, P. Xu, Y. Xu, C. Li, and H. Xu. 2012. Role of metabolism in the effects of genistein and its phase II conjugates on the growth of human breast cell lines. The AAPS Journal 14 (2):329–44. doi: 10.1208/s12248-012-9338-5.
- Yuan, G., Y. Liu, G. Liu, L. Wei, Y. Wen, S. Huang, Y. Guo, F. Zou, and J. Cheng. 2019. Associations between semen phytoestrogens concentrations and semen quality in Chinese men. Environment International 129:136–44. doi: 10.1016/j.envint.2019.04.076.
- Yum, T., S. Lee, and Y. Kim. 2013. Association between precocious puberty and some endocrine disruptors in human plasma. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering 48 (8):912–7. doi: 10.1080/10934529.2013.762734.
- Zamora-Ros, R., P. Ferrari, C. A. González, A. Tjønneland, A. Olsen, L. Bredsdorff, K. Overvad, M. Touillaud, F. Perquier, G. Fagherazzi, et al. 2013. Dietary flavonoid and lignan intake and breast cancer risk according to menopause and hormone receptor status in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Breast Cancer Research and Treatment 139 (1):163–76. doi: 10.1007/s10549-013-2483-4.
- Zeitler, P., and P. Solberg. 2010. Food and levothyroxine administration in infants and children. The Journal of Pediatrics 157 (1):13–4. doi: 10.1016/j.jpeds.2010.05.025.
- Zhang, F. F., D. E. Haslam, M. B. Terry, J. A. Knight, I. L. Andrulis, M. B. Daly, S. S. Buys, and E. M. John. 2017. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: The Breast Cancer Family Registry. Cancer 123 (11):2070–9. doi: 10.1002/cncr.30615.
- Zhang, G.-Q., J.-L. Chen, Q. Liu, Y. Zhang, H. Zeng, and Y. Zhao. 2015. Soy intake is associated with lower endometrial cancer risk: A systematic review and meta-analysis of observational studies. Medicine 94 (50):e2281 doi: 10.1097/MD.0000000000002281.
- Zhang, K., Y. Wang, W. Ma, Z. Hu, and P. Zhao. 2017. Genistein improves thyroid function in Hashimoto's thyroiditis patients through regulating Th1 cytokines. Immunobiology 222 (2):183–7. doi: 10.1016/j.imbio.2016.10.004.
- Zhang, X., K. L. Cook, A. Warri, I. M. Cruz, M. Rosim, J. Riskin, W. Helferich, D. Doerge, R. Clarke, and L. Hilakivi-Clarke. 2017. Lifetime genistein intake increases the response of mammary tumors to tamoxifen in rats. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research 23 (3):814–24. doi: 10.1158/1078-0432.CCR-16-1735.
- Zhang, Y.-F., H.-B. Kang, B.-L. Li, and R.-M. Zhang. 2012. Positive effects of soy isoflavone food on survival of breast cancer patients in China. Asian Pacific Journal of Cancer Prevention: APJCP 13 (2):479–82. doi: 10.7314/apjcp.2012.13.2.479.
- Zhao, J. H., S. J. Sun, Y. Arao, E. Oguma, K. Yamada, H. Horiguchi, and F. Kayama. 2006. Identification of equol producers in a Japanese population by high-performance liquid chromatography with coulometric array for determining serum isoflavones. Phytomedicine : international Journal of Phytotherapy and Phytopharmacology 13 (5):304–9. doi: 10.1016/j.phymed.2005.09.013.
- Zhou, W., H. Wu, Q. Wang, X. Zhou, Y. Zhang, W. Wu, Y. Wang, Z. Ren, H. Li, Y. Ling, et al. 2020. Simultaneous determination of formononetin, biochanin A and their active metabolites in human breast milk, saliva and urine using salting-out assisted liquid-liquid extraction and ultra high performance liquid chromatography-electrospray ionization tandem mass spectrum. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 1145:122108. doi: 10.1016/j.jchromb.2020.122108.
- Ziauddeen, N., A. Rosi, D. Del Rio, B. Amoutzopoulos, S. Nicholson, P. Page, F. Scazzina, F. Brighenti, S. Ray, and P. Mena. 2019. Dietary intake of (poly)phenols in children and adults: Cross-sectional analysis of UK National Diet and Nutrition Survey Rolling Programme (2008-2014). European Journal of Nutrition 58 (8):3183–98. doi: 10.1007/s00394-018-1862-3.
- Zubik, L., and M. Meydani. 2003. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. The American Journal of Clinical Nutrition 77 (6):1459–65. doi: 10.1093/ajcn/77.6.1459.
- Zung, A., S. Shachar, Z. Zadik, and Z. Kerem. 2010. Soy-derived isoflavones treatment in children with hypercholesterolemia: A pilot study. Journal of Pediatric Endocrinology & Metabolism: JPEM 23 (1-2):133–41. doi: 10.1515/jpem.2010.23.1-2.133.

