Abstract
The management of osteoarthritis (OA) is a clinical challenge due to the particular avascular, dense, and occluded tissue structure. Despite numerous clinical reports and animal studies, the pathogenesis and progression of OA are still not fully understood. On the basis of traditional drugs, a large number of new drugs have been continuously developed. Intra-articular (IA) administration for OA hastens the development of targeted drug delivery systems (DDS). OA drugs modification and the synthesis of bioadaptive carriers contribute to a qualitative leap in the efficacy of IA treatment. Nanoparticles (NPs) are demonstrated credible improvement of drug penetration and retention in OA. Targeted nanomaterial delivery systems show the prominent biocompatibility and drug loading-release ability. This article reviews different drugs and nanomaterial delivery systems for IA treatment of OA, in an attempt to resolve the inconsonance between in vitro and in vivo release, and explore more interactions between drugs and nanocarriers, so as to open up new horizons for the treatment of OA.
1. Introduction
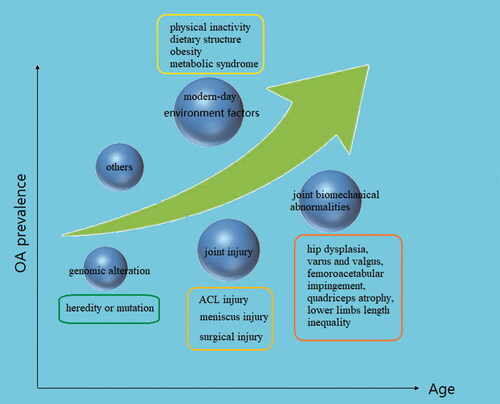
As life expectancy has increased over the past half-century, the prevalence of Osteoarthritis (OA) has been increasing prominently. In Europe, the United States and other developed countries, 10%−15% of adults over 60 years old suffer from OA due to the severity of the aging population, with a significantly higher prevalence rate in older women than older men (Bijlsma et al., Citation2011; Prieto-Alhambra et al., Citation2014; Wallace et al., Citation2017). Substantial evidence indicates that age, as the strongest risk factor for OA, interacts with multiple risk factors throughout the pathological process. Obesity is another non-negligible risk factor for OA, increasing the risk by more than three times (Blagojevic et al., Citation2010; Silverwood et al., Citation2015; Reyes et al., Citation2016). Obesity increases the load on the large joints of lower limbs (hip, knee and ankle) and further affects the biomechanics of the joints. Meanwhile, the increase of adipokines and inflammatory cytokines caused by obesity promotes the development of OA (Kulkarni et al., Citation2016; Wang & He, Citation2018; Liu et al., Citation2019; Misra et al., Citation2019). Different types of joint injury are an important basis for the pathogenesis of OA. Long-term high-intensity exercise training is one of the susceptibility factors of OA and post-traumatic osteoarthritis (PTOA) is a representative type (Bodkin et al., Citation2020; Rothrauff et al., Citation2020). Injuries of the anterior cruciate ligament (ACL) and meniscus are the most common causes of PTOA (Ajuied et al., Citation2014; Li et al., Citation2019; Wang et al., Citation2020). Knee ligament, meniscus, muscle, bone, and tendon injuries or surgery increase the risk of knee arthritis by at least four times (Muthuri et al., Citation2011; Poulsen et al., Citation2019). The joint biomechanical abnormalities caused by congenital or acquired joint anatomical dysfunction may lead to the occurrence of OA under the influence of self and environmental factors. Hip dysplasia, varus and valgus, femoroacetabular impingement, quadriceps atrophy, lower limbs length inequality will affect the pathological process of OA in varying degrees (Nishida et al., Citation2017; Wyles et al., Citation2017; Kim et al., Citation2018; Lynch et al., Citation2019; Hernandez et al., Citation2020; Springer et al., Citation2020; Xu et al., Citation2020). Genomic studies of OA patients and their families have revealed new biogenetics insights of OA pathogenesis and multiple gene loci were found relevant to the pathogenesis of OA (Zeggini et al., Citation2012). Similar to aging, changes in modern lifestyle also affect the incidence of OA. Physical inactivity and changes in dietary structure contribute to obesity and metabolic syndrome which result in abnormal regulation of bone metabolic factors such as dyslipidemia, impaired glucose tolerance and hypertension (Berenbaum et al., Citation2018) ().
2. Pathogenesis of OA
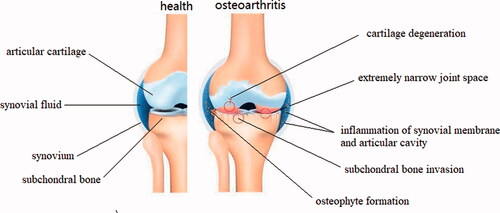
OA is the most common degenerative disease of the whole joint, progressively affecting the articular cartilage, synovium, subchondral bone, and periarticular tissues like ligaments, capsule, and periarticular muscles (Glyn-Jones et al., Citation2015; Martel-Pelletier et al., Citation2016; Sharma, Citation2021). The main pathological manifestations are degeneration of articular cartilage, thinning of subchondral bone, osteophyte formation around the joint, meniscal alterations, synovial fluid inflammation, ligament injury, and joint capsule hypertrophy (Hügle & Geurts, Citation2017; Roseti et al., Citation2019). Cardinal symptoms include pain, swelling or even deformity of the joints, stiffness (especially severe and transient morning stiffness), popping or crepitus during joint motion, and mobility disorder (Fu et al., Citation2018; Bacon et al., Citation2020; He et al., Citation2020). Traditionally, OA is regarded as a passive degenerative disease or injury caused by long-term wear and tear. However, new insights suggest that OA is actually an active dynamic process arising from imbalance of joint damage and repair. Initially, erosion begins on the surface of the cartilage and gradually deepens into the calcified cartilage area. During this process, chondrocytes attempt to repair the damage by enhancing proliferation and differentiation, but the accompanying inflammatory response inhibits chondrocyte function. Then the subchondral bone proliferates pathologically and erodes the cartilage layer. The endochondral pathologic enhancement of osteogenesis results in the formation of osteophytes around the joint margins ().
3. Management of OA
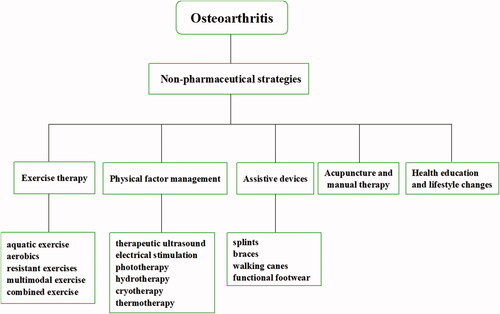
3.1. Non-pharmaceutical strategies
Almost all guidelines recommend regular and individualized exercise in the different pathological stages of OA. The most common exercise for OA treatment includes aquatic exercise, aerobics, resistant exercises, multimodal and combined exercise (Luan et al., Citation2019). However, the effect of exercise intensity on the outcome of OA rehabilitation has not been fully elucidated, especially in the acute stage of OA. Inappropriate exercise prescription may aggravate the development of the disease.
Physical therapy (PT) has a prominent therapeutic effect on OA including therapeutic ultrasound, electrical stimulation, phototherapy, hydrotherapy, magnet therapy, cryotherapy and thermotherapy. PT has a significant relief effect on the symptoms of osteoarthritis, including pain, edema, and joint motion disorders, which is suitable for emergency management in the acute phase (de Oliveira Melo et al., Citation2016; Aciksoz et al., Citation2017; Rothenberg et al., Citation2017; Langella et al., Citation2018). Physical factors are also effective triggers for stimuli-responsive NPs for controllably releasing agents in IA treatment and this will be introduced blew.
OA patients usually require assistive devices to compensate for decreased strength, impaired balance, pain during movement. Common devices include splints, braces, walking canes, functional footwear and other training equipment. Splint shows remarkable improvement of pain relief for base-of-thumb osteoarthritis with time dependence of efficacy (Rannou et al., Citation2009; Gomes Carreira et al., Citation2010; Becker et al., Citation2013). Daily cane use can diminish pain and maintain a normal gait which is crucial for OA patients to preserve joint function and muscle strength in the early stage of rehabilitation (Jones et al., Citation2012; Moe et al., Citation2012). Although clinical studies have yielded some positive results, questions remain about the necessity for assistive devices and their long-term safety.
Acupuncture is a non-pharmaceutical treatment of traditional Chinese medicine (TCM). Acupuncture plays a certain role in relieving pain and restoring function for OA treatment. The therapeutic effect of acupuncture may come from the regulation of inflammatory factors (Lin et al., Citation2020; Shi et al., Citation2020). However, evidence showed incertitude of acupuncture in treating OA, in particular the obvious difference between electroacupuncture and manual acupuncture (Wang et al., Citation2020; Tu et al., Citation2021), and in a small sample study, no difference was observed in the eight-week (three sessions per week) acupuncture intervention (Lin et al., Citation2018). In addition, non-pharmaceutical strategies include health education, lifestyle changes such as diet, physical activity, and weight control, and Self-management is an important measure to prevent OA ().
3.2. Pharmacological management
Considering that many patients with OA are unable to identify independent risk factors for intervention and there is still uncertainty about the efficacy and adaptability of non-pharmacological treatment. Pharmaceutical drugs remain the primary treatment for OA including topical, oral and injectable intervention. First-line drugs include non-steroidal anti-inflammatory drugs (NSAIDs), paracetamol, analgesics, capsaicin, and glucocorticoids and show remarkable efficacy in symptom control (Hochberg et al., Citation2012; Bannuru et al., Citation2019; Kolasinski et al., Citation2020). However, both topical and oral drug use have certain limitations in that topical drugs have lower systemic drug levels than oral, but they are limited by drug penetration, retention and high-frequency medication. In addition, long-term use of NSAIDs and cyclooxygenase 2 (COX2) is an inconvenient risk of gastrointestinal and cardiovascular systems (Nissen et al., Citation2016; Chan et al., Citation2017; Solomon et al., Citation2017). With the deepening of research, an increasing number of drugs are being developed for the treatment of OA.
Another class of commonly used drugs is referred to as cartilage protectors including glucosamine and chondroitin sulfate products. Although some studies have shown the anti-inflammatory and analgesic effects of chondroitin and glucosamine in OA treatment, thus alleviating clinical symptoms and delaying disease progression, the effect is only better than that of a placebo, lacking favorable evidence and relevancy (Bruyère et al., Citation2014; Sharma, Citation2021). Hyaluronic acid is a high-molecular-weight GAG in synovial fluid and cartilage which is widely used as a viscous supplement to lubricate joints and absorb impact. A large number of meta-analysis results showed that the clinical efficacy and outcome correlation of hyaluronic acid was not explicit, and the new treatment guidelines no longer recommend hyaluronic acid for OA treatment (Rutjes et al., Citation2012; McAlindon et al., Citation2014).
3.3. Surgical management
Surgical treatment is suitable for severe OA caused by some specific etiological factors like trauma, congenital joint deformity and dysplasia, osteonecrosis. Common techniques include arthroscopic debridement and lavage, cartilage transplantation, meniscus resection and arthroplasty. Arthroscopic debridement shows obvious clinical results in elbow OA, and joint foreign body and impingement are considered potential indications (MacLean et al., Citation2013; Carlier et al., Citation2019; French Arthroscopic Society, 2019). Some studies showed that arthroscopic debridement and lavage are also effective in the improvement of symptoms in shoulder, knee and thumb OA (Furia, Citation2010; Bexkens et al., Citation2018; Lo Presti et al., Citation2020). However, some clinical trials and the systematic review showed unsatisfactory results for debridement and lavage procedures that the outcomes after surgery are temporary or no different from placebo procedures (Moseley et al., Citation2002; Kirkley et al., Citation2008; Skelley et al., Citation2015). Although some minimally invasive procedures are well established, surgical treatment is also different degrees of trauma to the joint.
Osteochondral allograft transplantation (OCA) shows dependable improvement of pain, function and symptom scores in chondral lesions. In multiple follow-up studies over 10 to 20 years, OCA shows significant prognostic effects, greatly delayed the time of arthroplasty, and reduced reoperation rate (Frank et al., Citation2017; Pascual-Garrido et al., Citation2017; Stone et al., Citation2017; Ekman et al., Citation2018; Frank et al., Citation2018). Nonetheless, OCA shows definitely cost-effective which means the clinical outcomes are largely dependent on the overall cost of the operation, particularly the cost of the graft, which will increase the financial pressure on the patients (Mistry et al., Citation2019). OCA can help athletes return to competition, but there is a high probability of reoperation, requiring debridement and removal of the free body (Crawford et al., Citation2019). In addition, the study found an intractable relationship between the thickness of the graft and the prognosis that thin grafts may result in high risk of subchondral cysts and thicker grafts may delay osseous graft integration after surgery (Ackermann et al., Citation2019).
4. Targeted nanomaterial drug delivery systems
4.1. DDS targeting the inflammation of synovium and cartilage
Lornoxicam (Lnx) is a thienothiazine derivative NSAIDs of oxicam class with properties of anti-inflammatory, analgesic and joint repair (Berry et al., Citation1992; Kidd & Frenzel, Citation1996; Hall et al., Citation2009). However, due to poor aqueous solubility and upper digestive tract absorption, oral administration and simple interarticular injection have many restrictions, such as gastrointestinal reactions, low absorption rate, and rapid clearance rate (Hamza & Aburahma, Citation2009). Zhang (Zhang & Huang, Citation2012) et al evaluated the biocompatibility, systemic toxicity, retention time and anti-inflammatory effect of Lnx-loaded PLGA microspheres (Lnx-MS) in papain-induced OA rats. Lnx-MS showed remarkable retention time in joint tissue and persistent low level in plasma during 96 hours after injection. Lnx suspensions distribution in plasma peaked in 24 hours and they were cleared within 24 hours in joint tissue. Lnx-MS was verified to be biodegradable and safe to proliferate and differentiate chondrocytes. Pharmacodynamics showed a reduction in joint swelling and proteoglycan loss. Researchers used chitosan/tripolyphosphate MS carrying Lnx to intervene monosodium iodoacetic acid (MIA) induced OA rats. Compared to suspensions, chitosan MS prolonged drug delivery time to 8 days with a long-lasting anti-inflammatory effect (Abd-Allah et al., Citation2016). The optimized formula showed remarkable improvement in joint tissues and inflammatory cytokine inhibition.
Meloxicam (Mlx) is also a hydrophobic and lipophilic NSAID. Compared to other NSAIDs, Mlx is a preferential inhibitor of COX2 thus resulting in slight gastrointestinal reactions, but poor aqueous solubility limits the absorption and bioavailability. Shohreh Fattahpour (Fattahpour et al., Citation2020) et al compared two Mlx delivery systems that carboxymethyl chitosan-methylcellulose-pluronic hydrogel (CMC-MC-P hydrogels) containing Mlx NPs showed prominent biocompatibility, low degradation and swelling in comparison to hydrogel containing Mlx solution. The drug release studies showed that 85%-97% of Mlx in NPs released in 37 days while hydrogel-containing solution release almost 100% after 20 days. Chondrocytes have better proliferation and adhesion in NPs system and they are affected by the concentration of NPs and Mlx solution.
Celecoxib (Clx) is a kind of NSAID working as a COX-2 selective inhibitor and has significant anti-inflammatory and analgesic effects for OA (Davies et al., Citation2000; Hochberg et al., Citation2016; Puljak et al., Citation2017). Compared to nonselective NSAIDs, Clx shows certain drug safety in gastrointestinal symptoms and renal adverse events at an appropriate dose (Chan et al., Citation2010; Solomon et al., Citation2018; Yeomans et al., Citation2018). However, long-term use of aspirin can attenuate the gastrointestinal safety of Clx and cardiovascular safety is also a potential risk for oral administration of Clx (Silverstein et al., Citation2000; Nissen et al., Citation2016; Reed et al., Citation2018). Audrey Petit (Petit et al., Citation2014) et al evaluated the biocompatibility of Clx-loaded hydrogel carried on an acetyl-capped PCLA-PEG-PCLA triblock copolymer in vitro and in vivo. The copolymer is detected thermoset that it stays in a sol state at room temperature and turns into immobile gels at 37 °C. The study showed sustained topical Clx release both in vitro and in vivo (90 days and 4-8 weeks) based on polymer dissolution. No damage was observed in articular cartilage of healthy rats after subcutaneous injection of encapsulated Clx polymer thus revealing this Clx-loaded polymer as a safe DDS for OA treatment. Another formulation used endcapped PCLA-PEG-PCLA copolymer loaded with Clx showed similar results with acetyl-capped copolymer but longer release time (van Midwoud et al., Citation2018). These results suggest the potential of PCLA-PEG-PCLA for OA targeted injection treatment.
Polyesteramide (PEA) MS is another high-profile Clx-loaded NPs. Maarten Janssen (Janssen et al., Citation2016) et al reported that Clx was released sustainedly up to 80 days in vitro and inflammation responsive release was observed in the Hl-60 cell line. In OA-induced (ACLT + pMMx) rats, degradation of PEA MS was higher than health rats which verified inflammation responsive release again. Regrettably, no difference was observed in cartilage degeneration changes between 0.9% NaCl, single MS and Clx-loaded MS. A later similar study showed that Clx-loaded PEA MS reduced the osteophytes, subchondral ossifying, bone cysts, and synovial inflammation in surgery-induced OA rats (Tellegen et al., Citation2018). Ian J Villamagna (Villamagna et al., Citation2019) et al optimized the structure of PEA MS and demonstrated different toxicity in vitro and in vivo.
Recently, poly (D, L-lactic acid) (PDLLA) microparticles (MP) and hyaluronan nanocapsules were developed to load Clx for rats OA model research. Two different kinds of PDLLA MP (drug in solution MP and nano-drug embedded MP) showed good biocompatibility, drug loadings rate, entrapment efficiencies and long action sustained release of Clx. PGE2 decreased in IL-1β induced human articular synoviocytes after two MP interventions (Salgado et al., Citation2020). Clx-loaded hyaluronan nanocapsules showed remarkable entrapment efficiency and release time in vitro. In addition, nanocapsules improved knee joint swelling, morphology, histomorphology and inflammation in MIA-induced rats OA model (El-Gogary et al., Citation2020).
Diclofenac (Dcf) is also commonly used in the targeted treatment of OA by loading nanomaterials. Bryan B Hsu (Hsu et al., Citation2014) et al first reported a new polymer–Dcf conjugate system of biodegradable thin films using a layer-by-layer (LBL) self-assembly process and achieved sustaining small molecule release. Dcf is firstly activated with triethylene glycol (TriEG) to form a TriEG-Dcf prodrug conjugate and then conjugated to poly (l-glutamic acid) to complete integral PGA-TriEG-Dcf formulation assembly. After conjugating to poly(l-lysine) to form PLL/PGA-TriEG-Dcf, the release time increased significantly, exceeding several months. PLL/PGA-TriEG- Dcf showed a prominent anti-inflammatory effect by COX inhibition and no deleterious effects appeared in synthetic procedures. Adrian Sulistio et al. (Sulistio et al., Citation2017) developed a new polymer‐Dcf conjugate (PDCs) and found that PDCs provide high drug loading and a sustained steady release of Dcf. Notably, by regulating the feed ratio of PEG co‐monomers and the amount of PDCs, Dcf loading and release kinetics can be continuously optimized to achieve precise control. The hydrogel films of poly(n-vinylcaprolactam) NPs (νPVCL) were reported temperature-responsive on the basis of high drug loading by the LBL assembly technique (Zavgorodnya et al., Citation2017). After being loaded with Dcf, different layers of νPVCL show different performance of drug loading and release, and when (νPVCL)30 is in the artificial skin film at 30 °C (average skin temperature), the cumulative drug release in 24 hours is 12 times that of 22 °C. Kuan Zhang (Zhang et al., Citation2020) et al reported a dual-functional nanospheres PNIPAM-PMPC loaded with Dcf prepared by emulsion polymerization. PNIPAM-PMPC nanospheres show higher drug release at 37 °C than 22 °C. Meanwhile, it has prominent lubrication by forming a compact hydration layer outside. Dcf-loaded PNIPAM-PMPC nanospheres have good biocompatibility, which increase anabolic genes and inhibit catabolic genes of chondrocytes. Toshio Kawanami (Kawanami et al., Citation2020) et al developed a novel Dcf-hydrogel conjugate system produced by 2-pyridylamino-substituted 1-phenylethanol (PAPE) which reduced the production of the lactam in regular ester conjugates of Dcf. Besides, this hydrogel conjugate has an optimizable release rate regulated by physiological microenvironment. Recently, inartificial clay mineral attapulgite (ATP) was used to produce an enhanced supramolecular hydrogel by cyclodextrin pseudopolyrotaxane (PPR) system (Ha et al., Citation2021). This ATP hybrid hydrogel appears to sustained release of Dcf, good biocompatibility and remarkable anti-inflammatory in vivo test.
Etoricoxib (Ecx) is a highly hydrophobic selective inhibitor of COX-2 and is commonly used for acute pain caused by rheumatoid arthritis and OA. However, due to low solubility, severe pH dependence, and cardiovascular risk, oral or systemically administration of Ecx remains many challenges (Okumu et al., Citation2009). Polycaprolactone (PCL) MP is early used for Ecx-loaded targeted delivery. PCL MP showed satisfactory biocompatibility, hypotoxicity and long-term sustained release in vivo and in vitro tests (Arunkumar et al., Citation2016a). Loading PCL MP with chitosan gel to form novel injectable gel MP can enhance the duration of Ecx in synovial (Arunkumar et al., Citation2016b). Pingju Liu (Liu et al., Citation2019) et al developed novel PLGA-PEG-PLGA copolymer NPs loaded with Ecx. NPs showed sustained release in vitro and significant anti-inflammatory effects in subchondral bone, synovium, and cartilage in vivo. Alaa H Salama (Salama et al., Citation2020) et al reported Ecx-loaded PLA-CS NPs synthesized from polylactic acid and chitosan hydrochloride. By adjusting the ratio of surfactant, the formula with the smallest particle size and the most obvious slow-release effect was optimized. PLA-CS NPs showed cytocompatibility and enhanced ALP activity in vitro test.
In addition to chemically synthesized nanomaterials, organometallic materials are also used for drug-loaded targeted therapy. A UiO-66 metal-organic framework (MOF) was used as DDS for ketoprofen by introducing functional groups (NH2, NO2) (Li et al., Citation2019). Ketoprofen-loaded UiO-66-NH2 showed good biosafety and sustained drug release. NSAIDs are relatively mature in delivery systems research ().
Table 1. NSAIDs and delivery system in OA treatment.
4.2. DDS targeting cartilage protection and regeneration
Diacerein (Dcn) is a chondroprotective agent metabolized by acetyl esterases and it exerts anti-inflammatory and cartilage protective effects by metabolizing rhein (Jain et al., Citation2015; Lohberger et al., Citation2019). Dcn does not affect the production of prostaglandins, and there are few reports of gastrointestinal disorders, so it is recommended as a first-line drug for OA, especially for patients contraindicated to NSAIDs (Bartels et al., Citation2010; Pavelka et al., Citation2016). However, low water-solubility limits the oral bioavailability of both diazepine and rhein.
Achint Jain (Jain et al., Citation2013) et al developed Dcn-loaded solid lipid NPs (Dcn-SLN) by ultrasonication technique and characterized its physicochemical properties. Dcn-SLN shows sustained drug release in vitro and high bioavailability of oral management in a rat model. Mubashar Rehman (Rehman et al., Citation2015) et al optimized Dcn-SLN by mixing proportionally of solid and liquid lipids to form binary SLNs. This novel formula demonstrates not only sustained Dcn release but, more importantly, rapid release at high temperatures. This thermoresponsive release property makes it possible to combine it with OA thermal therapy. In subsequent studies, Dcn-loaded niosomes and self-nano emulsifying gel based on GLC and TPGS were successively developed (El-Say et al., Citation2016; Eltobshi et al., Citation2018). Both DDS showed good drug release performance in vitro and anti-inflammatory effect in vivo after optimization. Dcn or rhein-loaded PLGA NPs showed excellent biocompatibility and inflammatory inhibition in vitro (Gómez-Gaete et al., Citation2017; Jung et al., Citation2020). Dcn-loaded PLGA NPs could effectively protect the cartilage injury and inhibit the progression of inflammation after interarticular injection in vivo. Diana E Aziz (Aziz et al., Citation2018) et al developed a novel Dcn delivery system using elastosomes for transdermal delivery thereby avoiding oral adverse effects ().
Table 2. Cartilage protector and delivery system in OA treatment.
Chondroitin sulfate (CS) is a sulfated GAG and an important component of the cartilage extracellular matrix (Alessio et al., Citation2021). It is widely used in the adjuvant therapy of OA due to its anti-inflammatory, anti-oxidative and anti-apoptotic effects (Henrotin et al., Citation2010). Studies showed that polymer-modified CS significantly increased the inter-tissue retention time. CS-encapsulated PLGA copolymers with different lactide and glycolide ratios showed different CS burst releases, and this may be potential for controlled drug release (Jiang et al., Citation2011). Priyanka Dwivedi (Dwivedi et al., Citation2015) et al combined gold NPs with CS to reinforce drug delivery. AuNps-CS was demonstrated to enhance chondrocyte proliferation and promote ECM production in vitro. Besides, a novel polymer agent formulated by CS-cysteine conjugate showed remarkable bioadhesive properties and low biotoxicity in rat primary chondrocytes (Suchaoin et al., Citation2016).
4.3. DDS targeting GAG loss and ROS of chondrocyte
Glucocorticoids have obvious anti-inflammatory effects, but long-term repeated use of large doses can lead to a variety of local or systemic side effects. Dexamethasone (Dex) is the most commonly used glucocorticoid for OA treatment. In order to improve the efficiency of drug use and reduce side effects, a variety of DDS has been developed. Bajpayee (Bajpayee et al., Citation2016; Citation2017) et al conjugated avidin nano-carriers with Dex by two linkers (ester and hydrazone). Ester linker had faster drug release than hydrazone linker and avidin-Dex rescued IL-1-induced GAG loss with does dependence. Subsequent animal experiments confirmed that the avidin-Dex could penetrate cartilage and retain for 3 weeks, improve morphology and inhibit the formation of osteophytes, but increased Dex load was needed to further reduce the loss of GAG. Dex-carbon nanotubes were developed to restrain TNF-α induced inflammation in synovial fibroblasts (Lee et al., Citation2017). Nanotubes showed higher Dex uptake by caveolin-dependent endocytosis and efficient intracellular release to inhibited ROS production by targeting mitochondria.
Stefano Perni (Perni & Prokopovich, Citation2020) et al greatly increased the drug uptake of Dex-poly-beta-amino-esters (PBAEs) by continuously optimizing the polymer structure. Dex-PBAEs inhibited GAG loss induced by IL-1α in cartilage explants cultured and improved the chondrocyte activity. Dex-loaded PLGA MP showed significant sustained release, pro-anabolic and anti-inflammatory factor effects in vitro and in vivo (Stefani et al., Citation2020). Tengfei He (He et al., Citation2020) et al developed a novel multi-arm avidin NPs, which greatly increased the drug load with crosslinkers. These multi-arm avidin NPs showed remarkable control of drug release and cartilaginous permeability. In vitro tests indicated that it inhibited the generation of ROS, protected the activity of chondrocytes, and reduced the loss of GAG and collagen.
The glucocorticoid-loaded DDS shows great potential for OA intraarticular targeted treatment. At the same time, some studies confirmed that the biological effects of the NPs DDS are affected by the dynamic changes of the joint environment, such as the changes of proteins, hyaluronic acid and phospholipids in the synovial fluid (Magri et al., Citation2019).
4.4. Novel drug molecules targeting osteanagenesis
In recent years, some new drug molecules introduced to different DDS showed remarkable therapeutic effects of OA in experiments, which has laid a foundation for clinical application (). Human stromal cell-derived factor 1α (rhSDF-1α) is a significant chemokine facilitating stem cell migration and homing to injured tissue and promoting tissue repair (Hattori et al., Citation2001). rhSDF-1α-loaded fibrin/HA hydrogel was used to filled chondral defects and it recruited chondrogenic progenitor cells to chondral defects, which improved the morphology, proteoglycan density and cartilage ultrastructure (Yu et al., Citation2015).
Table 3. Novel drug molecules and delivery system in OA treatment.
Anabolic growth factors are efficient for OA treatment by enhancing chondrocyte activity and promoting matrix production. An earlier study found that insulin-like growth factor 1 (IGF-1) fused to heparin-binding domain had a distinct prolongation of intraarticular retention and rescued cartilage degeneration in the rat OA model (Loffredo et al., Citation2014). Brett C Geiger (Geiger et al., Citation2018) et al loaded IGF-1 on positively charged PEGylated polyamidoamine (PAMAM) dendrimers and this dendrimer-IGF-1 presented prominent performance of drug absorption, cartilage penetration, drug retention and biocompatibility in vitro. The nanocarriers enhanced the treatment effects of alleviating cartilage degeneration and osteophyte formation in vivo. BMP2 is an important excitoanabolic factor in bone metabolism. BMP2 adsorbed onto graphene oxide (GO) flakes showed remarkable biocompatibility and sustained slow release in vitro. In the OA rat model, GO-adsorbed BMP2 had a better histological appearance after intra-articular intervention (Zhong et al., Citation2017). GO-loaded TGF-β3 3 D nano-scaffold is a new progress of cartilage engineering. Culture of hMSCs encapsulated in 3 D GO scaffold-adsorbed TGF-β3 hydrogel improved chondrogenesis and ECM production. This novel 3 D GO scaffold demonstrated excellent drug delivery, low cytotoxicity and sustaining drug activity (Zhou et al., Citation2019).
Yung-Hsin Cheng (Cheng et al., Citation2017) et al developed a glutathione-loaded chitosan hydrogel and used it in Cisd2 deficiency-induced rat chondrocytes injury. The hydrogel showed thermosensitively and sustained drug release in chondrocytes without obvious cytotoxicity. Glutathione rescued the inflammation, apoptosis and oxidative stress in Cisd2–/– chondrocytes by restraining H2O2 activity.
Kartogenin (KGN) is found an important activator of the CBFβ-RUNX1 signaling pathway which promotes chondrogenesis and chondroprotection (Zhao et al., Citation2020). KGN was first conjugated to the head or end group of PEG-PAMAM to form KGN- PEG-PAMAM (KPP) or PEG-PAMAM-KGN (PPK) dendrimer. KPP showed the more prominent effect of CBFβ and chondrogenic markers activation. In vivo test showed prolonged retention of drug in a rat model (Hu et al., Citation2017). Pierre Maudens et al. (Maudens et al., Citation2018) introduced KGN nanocrystals acquiring by wet milling to PLA nanocrystal–polymer particles to form KGN-NPPs. The NPPs system presented commendable drug loading, sustained drug release and biocompatibility in vitro test. The KGN-loaded NPPs improved chondrohistology and osteophyte size in vivo test. In the past two years, tri-copolymer scaffolds structured by gelatin-chondroitin-hyaluronan and engineered exosomes were used to deliver KGN to chondrocytes and showed potential application future (Chen et al., Citation2021; Xu et al., Citation2021).
Nano-sized liposomes conjugated to MAbCII were synthesized to encapsulate TPCA-1, a selective inhibitor of NF-κB pathway, and showed distinguished improvement of inflammation, oxidative stress and cell apoptosis in TNF-α-treated chondrocytes (Bhatti et al., Citation2019). The advantage of liposomes is the sustained drug release properties but once the release is activated, it does not extend the clearance time. Xiuling Liu (Liu et al., Citation2019) et al conjugated adenosine to biodegradable PLA-b-PEG NPs in different binding sites. In vitro studies showed that adenosine-loaded NPs can significantly increase intracellular cAMP and inhibit a variety of inflammatory factors. Early injection of adenosine-loaded NPs into rat joints can effectively prevent traumatic OA.
Haimin Chen (Chen et al., Citation2019) et al optimized the MMP/pH-responsive DDS by synthesizing ferritin nanocages (CMFn) loaded with hydroxychloroquine (HCQ). The fluorescence intensity of CMFn can reflect the severity of OA and HCQ can be sustained released for 14 days in an acidic pH microenvironment. This dual sensitive DDS has great application prospects for precision OA diagnosis and treatment.
4.5. Chinese herb extracts and targeted DDS
Curcumin is extracted from the Chinese herb Curcuma longa which is commonly used in OA for inflammation and pain relief. Combined injection of curcumin and bone marrow mesenchymal stem cells (BMSCs) into the OA rat model can enhance the migration and proliferation of chondrocytes, improve the level of anabolic factors and promote chondrogenesis (Zhang et al., Citation2021). Regardless of oral, direct joint injection or transdermal administration, Curcumin shows obvious anti-OA effects and the potential mechanism is related to inhibition of oxidative stress, promotion of anabolism, and anti-inflammatory apoptosis (Nicoliche et al., Citation2020; Zhou et al., Citation2020). However, the traditional administration still has drawbacks such as multiple dosing and gastrointestinal side effects, although the study showed better tolerance of curcumin than that of Dcf (Shep et al., Citation2019). Some studies used gelatin/silk fibroin MPs and synthetic NPs loaded with curcumin and showed good drug penetration and sustained release (Zhang et al., Citation2016; Ratanavaraporn et al., Citation2017).
Qiumei Lan (Lan et al., Citation2020) et al developed another MMP/pH-responsive DDS loading psoralidin (PSO) which is extracted from pPoralea corylifolia. The MRC-PPL NPs are designed to target cartilage and respond to MMP-13. MRC-PPL@PSO showed remarkable anti-inflammatory and cartilage repair effects in vitro and in vivo test by regulating PI3K/AKT, MAPK and NF-κB signaling. Zhengxiao Ouyang et al. (Ouyang et al., Citation2019) introduced hesperetin (extracted from citrus fruit) to Gd2(CO3)3-based NPs with a cartilage-targeting peptide. The NPs displayed excellent biocompatibility and magnetic resonance suitability. Hesperetin showed remarkable chondrocytes protection by inhibiting the TLR-2/NF-κB/Akt pathway.
Salvianolic acid A (SAA) is extracted from Salvia miltiorrhiza Bunge and it can inhibit chondrocyte apoptosis and ECM degradation in the OA rat model by restraining NF-κB signaling and activating TIMP-1 and TIMP-2 to inhibit MMPs (Xu et al., Citation2017; Wu et al., Citation2020). Artesunate (ART) is derived from artemisinin which is an extract of Chinese herb used to treat malaria. ART can relieve OA rat inflammation and improve cartilage pathological by regulating AK/STAT signaling (Zhao et al., Citation2017). Ethanol extract of Agkistrodon acutus can alleviate the apoptosis of chondrocytes and correct the abnormal expression of MMPs and Col2in OA rats (Wang et al., Citation2019). These Chinese herb extracts demonstrate remarkable effects in OA, and they have tremendous potential to work with appropriate DDS in the clinical application ().
Table 4. Chinese herb extracts and gene delivery in OA treatment.
4.6. Gene delivery system
Transfering exogenous nucleic acids to intracellular compartments is an effective method for OA treatment. Gene therapy includes DNA, RNA and no-coding RNA that are specific to different diseases and tissues. Nanomaterial non-viral vectors can avoid the immunogenicity, oncogenic effects and other lethality of traditional viral vectors and become a promising gene therapy DDS (). Cristiano Sacchetti et al. (Sacchetti et al., Citation2014) used single-walled carbon nanotubes (SWCNTs) to load morpholino antisense oligonucleotides (mASOs) modified by anti-green fluorescent protein (GFP). Intra-articular injection of PEG-SWCNT-anti-GFP mASOs can effectively penetrate the ECM, deliver drugs to the chondrocytes with a prolonged retention time and inhibit GFP expression.
Indian Hedgehog (Ihh) is a non-collagen related marker which is closely related to chondrocyte injury and the development of OA (Zhang et al., Citation2012; Thompson et al., Citation2015). Lipid NPs were used to load Ihh siRNA into chondrocytes with 100% transfection efficiency. Lipid NPs-Ihh siRNA was found to accumulate in the ECM instead of the synovium. In addition, it showed remarkable excito-anabolic and anti-catabolism effects and effectively alleviated cartilage degeneration in the OA rat model (Wang et al., Citation2018). The fibrin/HA hydrogel and self-assembling peptidic NPs were also used for carrying antimiR-221 and NF-κB p65 siRNA, respectively. These DDS showed excellent targeting delivery, gene silencing and OA therapeutic effects (Lolli et al., Citation2019; Yan et al., Citation2019).
4.7. Multidrug delivery system
Some formulations use targeted delivery of combination drugs, which makes the therapeutic effect more comprehensive. Mamta Bishnoi et al. (Bishnoi et al., Citation2014) conjugated aceclofenac-loaded CS to SLN and the SLNs showed sustained drug release over 24 hours in vitro test. When administered subcutaneously, SLNs presented high concentrations in the joints but no significant accumulation in vital organs and reduced edema induced by MIA. KGN and Dcf were conjugated to thermoresponsive NPs outside and inside, respectively (Kang et al., Citation2016). The therapeutic effects of both drugs were fully demonstrated in the formula, and the drugs could be released with precise control in cold temperatures. Similarly, indomethalin and glucosamine were jointly loaded on PLGA nano-micelles and observably improved inflammatory response and histopathology in the OA rat model (Kamel et al., Citation2016).
Xu Chen (Chen et al., Citation2019) et al developed a photothermal-triggered nanogenerator which loaded NO and Notch1-siRNA on PLGA-PEG NPs. With the synergistic effect of phototherapy, this formula achieved NO anti-inflammatory effect, Notch1 gene silencing, and alleviation of cartilage erosion. The combination of multidrug delivery and physical factor therapy is a promising approach for OA treatment.
5. Conclusions and future prospects
The constant development of DDS displays a great avenue for targeted therapy of OA. Traditional drugs fully release the therapeutic potential and greatly reduce the drawbacks and side effects accompanied by systematic administration. Many emergent drug molecules present great therapeutic potential in vitro and in vivo studies, whether in inflammation suppression, chondrocyte protection, extracellular matrix generation, or the relief of corresponding symptoms. The future application of these drugs still needs more explorations to confirm and optimize the property, and help reveal the internal mechanism of the occurrence and development of OA.
Nano-DDS is an important optimization of OA topical administration, which realizes biological functions such as drug penetration, long-term retention and sustained release. By modulating DDS structure, the encapsulation efficiency of the drug is greatly improved, which avoid large dose or frequent administration. The penetration of chondrocyte ECM in DDS has made some progress, but it is still one of the difficult problems to be solved in the later period. Multi-structure integration of NPs can be loaded with different types of drugs, which can help enrich therapeutic strategies according to the specific condition of the disease. Responsive nano DDS opens a new horizon for the precise treatment of OA. Drug release controlled by joint microenvironment changes can help better grasp the disease progress, while the physical factor response system takes full advantage of physiotherapy on the basis of precision treatment.
Nanotechnology has revealed the great plasticity of new materials in the medical field and these findings will catalyze new breakthroughs of DDS. In future studies, it is necessary to more clearly reveal the pathogenesis of OA and find more drugs with definite efficacy to enrich treatment strategies. At present, many studies get remarkable results in vitro or ex vivo, but there is still a long way to go before clinical application. Each drug delivery strategy needs to be reevaluated for safety, consistency, and clinic efficiency over long-term clinical studies. The drug load is an important translation challenge due to the differences in drug requirements for human OA and animal studies. Biocompatibility, low biotoxicity, and biodegradability remain the primary concerns for the development of DDS. The nanomaterials need to be further optimized to achieve good human adaptability, and the pharmacokinetics of the drug loading system need to be clarified. In addition, the structure-function relationship between nanomaterials and different stages of OA (changes in synovial fluid, cartilage, subchondral bone, muscle, ligament and other tissues as well as the intraarticular environment) is also important challenge for clinical conversion.
Acknowledgments
We would like to thank all authors for contributing to this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abd-Allah H, Kamel AO, Sammour OA. (2016). Injectable long acting chitosan/tripolyphosphate microspheres for the intra-articular delivery of lornoxicam: optimization and in vivo evaluation. Carbohydr Polym 149:263–73.
- Aciksoz S, Akyuz A, Tunay S. (2017). The effect of self-administered superficial local hot and cold application methods on pain, functional status and quality of life in primary knee osteoarthritis patients. J Clin Nurs 26:5179–90.
- Ackermann J, Merkely G, Shah N, Gomoll AH. (2019). Decreased graft thickness is associated with subchondral cyst formation after osteochondral allograft transplantation in the knee. Am J Sports Med 47:2123–9.
- Ajuied A, Wong F, Smith C, et al. (2014). Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med 42:2242–52.
- Alessio N, Stellavato A, Aprile D, et al. (2021). Timely supplementation of hydrogels containing sulfated or unsulfated chondroitin and hyaluronic acid affects mesenchymal stromal cells commitment toward chondrogenic differentiation. Front Cell Dev Biol 9:641529.
- Arunkumar P, Indulekha S, Vijayalakshmi S, Srivastava R. (2016a). Synthesis, characterizations, in vitro and in vivo evaluation of etoricoxib-loaded poly (caprolactone) microparticles – a potential intra-articular drug delivery system for the treatment of osteoarthritis. J Biomater Sci Polym Ed 27:303–16.
- Arunkumar P, Indulekha S, Vijayalakshmi S, Srivastava R. (2016b). Poly (caprolactone) microparticles and chitosan thermogels based injectable formulation of etoricoxib for the potential treatment of osteoarthritis. Mater Sci Eng C Mater Biol Appl 61:534–44.
- Aziz DE, Abdelbary AA, Elassasy AI. (2018). Fabrication of novel elastosomes for boosting the transdermal delivery of diacerein: statistical optimization, ex-vivo permeation, in-vivo skin deposition and pharmacokinetic assessment compared to oral formulation. Drug Deliv 25:815–26.
- Bacon K, LaValley MP, Jafarzadeh SR, Felson D. (2020). Does cartilage loss cause pain in osteoarthritis and if so, how much? Ann Rheum Dis 79:1105–10.
- Bajpayee AG, De la Vega RE, Scheu M, et al. (2017). Sustained intra-cartilage delivery of low dose dexamethasone using a cationic carrier for treatment of post traumatic osteoarthritis. Eur Cell Mater 34:341–64.
- Bajpayee AG, Quadir MA, Hammond PT, Grodzinsky AJ. (2016). Charge based intra-cartilage delivery of single dose dexamethasone using avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthr Cartil 24:71–81.
- Bannuru RR, Osani MC, Vaysbrot EE, et al. (2019). OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil 27:1578–89.
- Bartels EM, Bliddal H, Schøndorff PK, et al. (2010). Symptomatic efficacy and safety of diacerein in the treatment of osteoarthritis: a meta-analysis of randomized placebo-controlled trials. Osteoarthr Cartil 18:289–96.
- Becker SJ, Bot AG, Curley SE, et al. (2013). A prospective randomized comparison of neoprene vs. thermoplast hand-based thumb spica splinting for trapeziometacarpal arthrosis. Osteoarthr Cartil 21:668–75.
- Berenbaum F, Wallace IJ, Lieberman DE, Felson DT. (2018). Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat Rev Rheumatol 14:674–81.
- Berry H, Bird HA, Black C, et al. (1992). A double blind, multicentre, placebo controlled trial of lornoxicam in patients with osteoarthritis of the hip and knee. Ann Rheum Dis 51:238–42.
- Bexkens R, van Bergen CJA, van den Bekerom MPJ, et al. (2018). Decreased defect size and partial restoration of subchondral bone on computed tomography after arthroscopic debridement and microfracture for osteochondritis dissecans of the capitellum. Am J Sports Med 46:2954–9.
- Bhatti FUR, Hasty KA, Cho H. (2019). Anti-inflammatory role of TPCA-1 encapsulated nanosomes in porcine chondrocytes against TNF-α stimulation. Inflammopharmacology 27:1011–9.
- Bijlsma JW, Berenbaum F, Lafeber FP. (2011). Osteoarthritis: an update with relevance for clinical practice. Lancet 377:2115–26.
- Bishnoi M, Jain A, Hurkat P, Jain SK. (2014). Aceclofenac-loaded chondroitin sulfate conjugated SLNs for effective management of osteoarthritis. J Drug Target 22:805–12.
- Blagojevic M, Jinks C, Jeffery A, Jordan KP. (2010). Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthr Cartil 18:24–33.
- Bodkin SG, Werner BC, Slater LV, Hart JM. (2020). Post-traumatic osteoarthritis diagnosed within 5 years following ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 28:790–6.
- Bruyère O, Cooper C, Pelletier JP, et al. (2014). An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 44:253–63.
- Carlier Y, Desmoineaux P, Lenoir H, Vidil A. (2019). Prospective comparative analysis of arthroscopic debridement for primary and post-traumatic elbow osteoarthritis. Orthop Traumatol Surg Res 105:S217–s220.
- Carlier Y, Lenoir H, Rouleau DM, et al. (2019). Arthroscopic debridement for osteoarthritis of the elbow: results and analysis of predictive factors. Orthop Traumatol Sur Res 105:S221–S7.
- Chan FK, Lanas A, Scheiman J, et al. (2010). Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet 376:173–9.
- Chan FKL, Ching JYL, Tse YK, et al. (2017). Gastrointestinal safety of celecoxib versus naproxen in patients with cardiothrombotic diseases and arthritis after upper gastrointestinal bleeding (CONCERN): an industry-independent, double-blind, double-dummy, randomised trial. Lancet 389:2375–82.
- Chen CY, Li C, Ke CJ, et al. (2021). Kartogenin enhances chondrogenic differentiation of MSCs in 3D tri-copolymer scaffolds and the self-designed bioreactor system. Biomolecules 11.
- Chen H, Qin Z, Zhao J, et al. (2019). Cartilage-targeting and dual MMP-13/pH responsive theranostic nanoprobes for osteoarthritis imaging and precision therapy. Biomaterials 225:119520.
- Chen X, Liu Y, Wen Y, et al. (2019). A photothermal-triggered nitric oxide nanogenerator combined with siRNA for precise therapy of osteoarthritis by suppressing macrophage inflammation. Nanoscale 11:6693–709.
- Cheng YH, Chavez E, Tsai KL, et al. (2017). Effects of thermosensitive chitosan-gelatin based hydrogel containing glutathione on Cisd2-deficient chondrocytes under oxidative stress. Carbohydr Polym 173:17–27.
- Crawford ZT, Schumaier AP, Glogovac G, Grawe BM. (2019). Return to sport and sports-specific outcomes after osteochondral allograft transplantation in the knee: a systematic review of studies with at least 2 years’ mean follow-up. Arthroscopy 35:1880–9.
- Davies NM, McLachlan AJ, Day RO, Williams KM. (2000). Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet 38:225–42.
- de Oliveira Melo M, Pompeo KD, Baroni BM, Vaz MA. (2016). Effects of neuromuscular electrical stimulation and low-level laser therapy on neuromuscular parameters and health status in elderly women with knee osteoarthritis: a randomized trial. J Rehabil Med 48:293–9.
- Dwivedi P, Nayak V, Kowshik M. (2015). Role of gold nanoparticles as drug delivery vehicles for chondroitin sulfate in the treatment of osteoarthritis. Biotechnol Prog 31:1416–22.
- Ekman E, Mäkelä K, Kohonen I, et al. (2018). Favourable long-term functional and radiographical outcome after osteoautograft transplantation surgery of the knee: a minimum 10-year follow-up. Knee Surg Sports Traumatol Arthrosc 26:3560–5.
- El-Gogary RI, Khattab MA, Abd-Allah H. (2020). Intra-articular multifunctional celecoxib loaded hyaluronan nanocapsules for the suppression of inflammation in an osteoarthritic rat model. Int J Pharm 583:119378.
- El-Say KM, Abd-Allah FI, Lila AE, et al. (2016). Diacerein niosomal gel for topical delivery: development, in vitro and in vivo assessment. J Liposome Res 26:57–68.
- Eltobshi AA, Mohamed EA, Abdelghani GM, Nouh AT. (2018). Self-nanoemulsifying drug-delivery systems for potentiated anti-inflammatory activity of diacerein. IJN 13:6585–602.
- Fattahpour S, Shamanian M, Tavakoli N, et al. (2020). An injectable carboxymethyl chitosan-methylcellulose-pluronic hydrogel for the encapsulation of meloxicam loaded nanoparticles. Int J Biol Macromol 151:220–9.
- Frank RM, Cotter EJ, Lee S, et al. (2018). Do outcomes of osteochondral allograft transplantation differ based on age and sex? A comparative matched group analysis. Am J Sports Med 46:181–91.
- Frank RM, Lee S, Levy D, et al. (2017). Osteochondral allograft transplantation of the knee: analysis of failures at 5 years. Am J Sports Med 45:864–74.
- Fu K, Robbins SR, McDougall JJ. (2018). Osteoarthritis: the genesis of pain. Rheumatology 57:iv43–50.
- Furia JP. (2010). Arthroscopic debridement and synovectomy for treating basal joint arthritis. Arthroscopy 26:34–40.
- Geiger BC, Wang S, Padera RF, Jr, et al. (2018). Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci Transl Med 10:eaat8800.
- Glyn-Jones S, Palmer AJ, Agricola R, et al. (2015). Osteoarthritis. Lancet 386:376–87.
- Gomes Carreira AC, Jones A, Natour J. (2010). Assessment of the effectiveness of a functional splint for osteoarthritis of the trapeziometacarpal joint on the dominant hand: a randomized controlled study. J Rehabil Med 42:469–74.
- Gómez-Gaete C, Retamal M, Chávez C, et al. (2017). Development, characterization and in vitro evaluation of biodegradable rhein-loaded microparticles for treatment of osteoarthritis. Eur J Pharm Sci 96:390–7.
- Ha W, Wang ZH, Zhao XB, Shi YP. (2021). Reinforced supramolecular hydrogels from attapulgite and cyclodextrin pseudopolyrotaxane for sustained intra-articular drug delivery. Macromol Biosci 21:e2000299.
- Hall PE, Derry S, Moore RA, McQuay HJ. (2009). Single dose oral lornoxicam for acute postoperative pain in adults. Cochrane Database Syst Rev 2009:CD007441.
- Hamza YE-S, Aburahma MH. (2009). Design and in vitro evaluation of novel sustained-release double-layer tablets of lornoxicam: utility of cyclodextrin and xanthan gum combination. AAPS PharmSciTech 10:1357–67.
- Hattori K, Heissig B, Tashiro K, et al. (2001). Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood 97:3354–60.
- Henrotin Y, Mathy M, Sanchez C, Lambert C. (2010). Chondroitin sulfate in the treatment of osteoarthritis: from in vitro studies to clinical recommendations. Ther Adv Musculoskelet Dis 2:335–48.
- Hernandez PA, Wells J, Usheva E, et al. (2020). Early-onset osteoarthritis originates at the chondrocyte level in hip dysplasia. Sci Rep 10:627.
- He T, Zhang C, Vedadghavami A, et al. (2020). Multi-arm avidin nano-construct for intra-cartilage delivery of small molecule drugs. J Controlled Release 318:109–23.
- He Y, Wu Z, Xu L, et al. (2020). The role of SIRT3-mediated mitochondrial homeostasis in osteoarthritis. Cell Mol Life Sci 77:3729–43.
- Hochberg MC, Martel-Pelletier J, Monfort J, et al. (2016). Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis 75:37–44.
- Hochberg MC, Altman RD, April KT, et al. (2012). American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 64:465–74.
- Hsu BB, Park MH, Hagerman SR, Hammond PT. (2014). Multimonth controlled small molecule release from biodegradable thin films. Proc Natl Acad Sci U S A 111:12175–80.
- Hu Q, Ding B, Yan X, et al. (2017). Polyethylene glycol modified PAMAM dendrimer delivery of kartogenin to induce chondrogenic differentiation of mesenchymal stem cells. Nanomedicine 13:2189–98.
- Hügle T, Geurts J. (2017). What drives osteoarthritis? Synovial versus subchondral bone pathology. Rheumatology 56:1461–71.
- Jain A, Singh R, Singh S, Singh S. (2015). Diacerein protects against iodoacetate-induced osteoarthritis in the femorotibial joints of rats. J Biomed Res 29:405–13.
- Jain A, Singh SK, Singh Y, Singh S. (2013). Development of lipid nanoparticles of diacerein, an antiosteoarthritic drug for enhancement in bioavailability and reduction in its side effects. J Biomed Nanotechnol 9:891–900.
- Janssen M, Timur UT, Woike N, et al. (2016). Celecoxib-loaded PEA microspheres as an auto regulatory drug-delivery system after intra-articular injection. J Control Release 244:30–40.
- Jiang T, Petersen RR, Call G, et al. (2011). Development of chondroitin sulfate encapsulated PLGA microsphere delivery systems with controllable multiple burst releases for treating osteoarthritis. J Biomed Mater Res B Appl Biomater 97:355–63.
- Jones A, Silva PG, Silva AC, et al. (2012). Impact of cane use on pain, function, general health and energy expenditure during gait in patients with knee osteoarthritis: a randomised controlled trial. Ann Rheum Dis 71:172–9.
- Jung JH, Kim SE, Kim HJ, et al. (2020). A comparative pilot study of oral diacerein and locally treated diacerein-loaded nanoparticles in a model of osteoarthritis. Int J Pharm 581:119249.
- Kamel R, Salama AH, Mahmoud AA. (2016). Development and optimization of self-assembling nanosystem for intra-articular delivery of indomethacin. Int J Pharm 515:657–68.
- Kang ML, Kim JE, Im GI. (2016). Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater 39:65–78.
- Kawanami T, LaBonte LR, Amin J, et al. (2020). A novel diclofenac-hydrogel conjugate system for intraarticular sustained release: development of 2-pyridylamino-substituted 1-phenylethanol (PAPE) and its derivatives as tunable traceless linkers. Int J Pharm 585:119519.
- Kidd B, Frenzel W. (1996). A multicenter, randomized, double blind study comparing lornoxicam with diclofenac in osteoarthritis. J Rheumatol 23:1605–11.
- Kim C, Nevitt M, Guermazi A, et al. (2018). Brief report: leg length inequality and hip osteoarthritis in the multicenter osteoarthritis study and the osteoarthritis initiative. Arthritis Rheumatol 70:1572–6.
- Kirkley A, Birmingham TB, Litchfield RB, et al. (2008). A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 359:1097–107.
- Kolasinski SL, Neogi T, Hochberg MC, et al. (2020). 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol 72:220–33.
- Kulkarni K, Karssiens T, Kumar V, Pandit H. (2016). Obesity and osteoarthritis. Maturitas 89:22–8.
- Lan Q, Lu R, Chen H, et al. (2020). MMP-13 enzyme and pH responsive theranostic nanoplatform for osteoarthritis. J Nanobiotechnology 18:117.
- Langella LG, Casalechi HL, Tomazoni SS, et al. (2018). Photobiomodulation therapy (PBMT) on acute pain and inflammation in patients who underwent total hip arthroplasty – a randomized, triple-blind, placebo-controlled clinical trial. Lasers Med Sci 33:1933–40.
- Lee YK, Kim SW, Park JY, et al. (2017). Suppression of human arthritis synovial fibroblasts inflammation using dexamethasone-carbon nanotubes via increasing caveolin-dependent endocytosis and recovering mitochondrial membrane potential. Int J Nanomedicine 12:5761–79.
- Li L, Yang X, Yang L, et al. (2019). Biomechanical analysis of the effect of medial meniscus degenerative and traumatic lesions on the knee joint. Am J Transl Res 11:542–56.
- Li Z, Zhao S, Wang H, et al. (2019). Functional groups influence and mechanism research of UiO-66-type metal-organic frameworks for ketoprofen delivery. Colloids Surf B Biointerfaces 178:1–7.
- Lin LL, Li YT, Tu JF, et al. (2018). Effectiveness and feasibility of acupuncture for knee osteoarthritis: a pilot randomized controlled trial. Clin Rehabil 32:1666–75.
- Lin LL, Tu JF, Wang LQ, et al. (2020). Acupuncture of different treatment frequencies in knee osteoarthritis: a pilot randomised controlled trial. Pain 161:2532–8.
- Liu P, Gu L, Ren L, et al. (2019). Intra-articular injection of etoricoxib-loaded PLGA-PEG-PLGA triblock copolymeric nanoparticles attenuates osteoarthritis progression. Am J Transl Res 11:6775–89.
- Liu X, Corciulo C, Arabagian S, et al. (2019). Adenosine-Functionalized Biodegradable PLA-b-PEG nanoparticles ameliorate osteoarthritis in rats. Sci Rep 9:7430.
- Liu Y, Ding W, Wang HL, et al. (2019). Gut microbiota and obesity-associated osteoarthritis. Osteoarthr Cartil 27:1257–65.
- Lo Presti M, Costa GG, Grassi A, et al. (2020). Graft-preserving arthroscopic debridement with hardware removal is effective for septic arthritis after anterior cruciate ligament reconstruction: a clinical, arthrometric, and magnetic resonance imaging evaluation. Am J Sports Med 48:1907–15.
- Loffredo FS, Pancoast JR, Cai L, et al. (2014). Targeted delivery to cartilage is critical for in vivo efficacy of insulin-like growth factor 1 in a rat model of osteoarthritis. Arthr Rheumatol 66:1247–55.
- Lohberger B, Kaltenegger H, Weigl L, et al. (2019). Mechanical exposure and diacerein treatment modulates integrin-FAK-MAPKs mechanotransduction in human osteoarthritis chondrocytes. Cell Signal 56:23–30.
- Lolli A, Sivasubramaniyan K, Vainieri ML, et al. (2019). Hydrogel-based delivery of antimiR-221 enhances cartilage regeneration by endogenous cells. J Control Release 309:220–30.
- Luan X, Tian X, Zhang H, et al. (2019). Exercise as a prescription for patients with various diseases. J Sport Health Sci 8:422–41.
- Lynch TS, O’Connor M, Minkara AA, et al. (2019). Biomarkers for femoroacetabular impingement and hip osteoarthritis: a systematic review and meta-analysis. Am J Sports Med 47:2242–50.
- MacLean SB, Oni T, Crawford LA, Deshmukh SC. (2013). Medium-term results of arthroscopic debridement and capsulectomy for the treatment of elbow osteoarthritis. J Shoulder Elbow Surg 22:653–7.
- Magri G, Selmin F, Cilurzo F, Fotaki N. (2019). Biorelevant release testing of biodegradable microspheres intended for intra-articular administration. Eur J Pharm Biopharm 139:115–22.
- Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. (2016). Osteoarthritis. Nat Rev Dis Primers 2:16072.
- Maudens P, Seemayer CA, Thauvin C, et al. (2018). Nanocrystal-polymer particles: extended delivery carriers for osteoarthritis treatment. Small 14.
- McAlindon TE, Bannuru RR, Sullivan MC, et al. (2014). OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil 22:363–88.
- Misra D, Fielding RA, Felson DT, et al. (2019). Risk of knee osteoarthritis with obesity, sarcopenic obesity, and sarcopenia. Arthritis Rheumatol 71:232–7.
- Mistry H, Metcalfe A, Smith N, et al. (2019). The cost-effectiveness of osteochondral allograft transplantation in the knee. Knee Surg Sports Traumatol Arthrosc 27:1739–53.
- Moe RH, Fernandes L, Osterås N. (2012). Daily use of a cane for two months reduced pain and improved function in patients with knee osteoarthritis. J Physiother 58:128.
- Moseley JB, O’Malley K, Petersen NJ, et al. (2002). A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 347:81–8.
- Muthuri SG, McWilliams DF, Doherty M, Zhang W. (2011). History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthr Cartil 19:1286–93.
- Nicoliche T, Maldonado DC, Faber J, Silva M. (2020). Evaluation of the articular cartilage in the knees of rats with induced arthritis treated with curcumin. PLOS One 15:e0230228.
- Nishida K, Matsumoto T, Takayama K, et al. (2017). Remaining mild varus limb alignment leads to better clinical outcome in total knee arthroplasty for varus osteoarthritis. Knee Surg Sports Traumatol Arthrosc 25:3488–94.
- Nissen SE, Yeomans ND, Solomon DH, et al. (2016). Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med 375:2519–29.
- Okumu A, DiMaso M, Löbenberg R. (2009). Computer simulations using GastroPlus to justify a biowaiver for etoricoxib solid oral drug products. Eur J Pharm Biopharm 72:91–8.
- Ouyang Z, Tan T, Liu C, et al. (2019). Targeted delivery of hesperetin to cartilage attenuates osteoarthritis by bimodal imaging with Gd2(CO3)3@PDA nanoparticles via TLR-2/NF-κB/Akt signaling. Biomaterials 205:50–63.
- Pascual-Garrido C, Daley E, Verma NN, Cole BJ. (2017). A comparison of the outcomes for cartilage defects of the knee treated with biologic resurfacing versus focal metallic implants. Arthroscopy 33:364–73.
- Pavelka K, Bruyère O, Cooper C, et al. (2016). Diacerein: benefits, risks and place in the management of osteoarthritis. an opinion-based report from the ESCEO. Drugs Aging 33:75–85.
- Perni S, Prokopovich P. (2020). Optimisation and feature selection of poly-beta-amino-ester as a drug delivery system for cartilage. J Mater Chem B 8:5096–108.
- Petit A, Sandker M, Müller B, et al. (2014). Release behavior and intra-articular biocompatibility of celecoxib-loaded acetyl-capped PCLA-PEG-PCLA thermogels. Biomaterials 35:7919–28.
- Poulsen E, Goncalves GH, Bricca A, et al. (2019). Knee osteoarthritis risk is increased 4-6 fold after knee injury – a systematic review and meta-analysis. Br J Sports Med 53:1454–63.
- Prieto-Alhambra D, Judge A, Javaid MK, et al. (2014). Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis 73:1659–64.
- Puljak L, Marin A, Vrdoljak D, et al. (2017). Celecoxib for osteoarthritis. Cochrane Database Syst Rev 5:CD009865.
- Rannou F, Dimet J, Boutron I, et al. (2009). Splint for base-of-thumb osteoarthritis: a randomized trial. Ann Intern Med 150:661–9.
- Ratanavaraporn J, Soontornvipart K, Shuangshoti S, et al. (2017). Localized delivery of curcumin from injectable gelatin/Thai silk fibroin microspheres for anti-inflammatory treatment of osteoarthritis in a rat model. Inflammopharmacology 25:211–21.
- Reed GW, Abdallah MS, Shao M, et al. (2018). Effect of aspirin coadministration on the safety of celecoxib, naproxen, or ibuprofen. J Am Coll Cardiol 71:1741–51.
- Rehman M, Madni A, Ihsan A, et al. (2015). Solid and liquid lipid-based binary solid lipid nanoparticles of diacerein: in vitro evaluation of sustained release, simultaneous loading of gold nanoparticles, and potential thermoresponsive behavior. Int J Nanomed 10:2805–14.
- Reyes C, Leyland KM, Peat G, et al. (2016). Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: a population-based cohort study. Arthritis Rheumatol 68:1869–75.
- Roseti L, Desando G, Cavallo C, et al. (2019). Articular cartilage regeneration in osteoarthritis. Cells 8:1305.
- Rothenberg JB, Jayaram P, Naqvi U, et al. (2017). The role of low-intensity pulsed ultrasound on cartilage healing in knee osteoarthritis: a review. PM R 9:1268–77.
- Rothrauff BB, Jorge A, de Sa D, et al. (2020). Anatomic ACL reconstruction reduces risk of post-traumatic osteoarthritis: a systematic review with minimum 10-year follow-up. Knee Surg Sports Traumatol Arthrosc 28:1072–84.
- Rutjes AW, Jüni P, da Costa BR, et al. (2012). Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med 157:180–91.
- Sacchetti C, Liu-Bryan R, Magrini A, et al. (2014). Polyethylene-glycol-modified single-walled carbon nanotubes for intra-articular delivery to chondrocytes. ACS Nano 8:12280–91.
- Salama AH, Abdelkhalek AA, Elkasabgy NA. (2020). Etoricoxib-loaded bio-adhesive hybridized polylactic acid-based nanoparticles as an intra-articular injection for the treatment of osteoarthritis. Int J Pharm 578:119081.
- Salgado C, Guénée L, Černý R, et al. (2020). Nano wet milled celecoxib extended release microparticles for local management of chronic inflammation. Int J Pharm 589:119783.
- Sharma L. (2021). Osteoarthritis of the knee. N Engl J Med 384:51–9.
- Shep D, Khanwelkar C, Gade P, Karad S. (2019). Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: a randomized open-label parallel-arm study. Trials 20:214.
- Shi GX, Tu JF, Wang TQ, et al. (2020). Effect of electro-acupuncture (EA) and manual acupuncture (MA) on markers of inflammation in knee osteoarthritis. JPR 13:2171–9.
- Silverstein FE, Faich G, Goldstein JL, et al. (2000). Gastrointestinal toxicity with celecoxib vs. nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib long-term arthritis safety study. JAMA 284:1247–55.
- Silverwood V, Blagojevic-Bucknall M, Jinks C, et al. (2015). Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthr Cartil 23:507–15.
- Skelley NW, Namdari S, Chamberlain AM, et al. (2015). Arthroscopic debridement and capsular release for the treatment of shoulder osteoarthritis. Arthroscopy 31:494–500.
- Solomon DH, Husni ME, Libby PA, et al. (2017). The risk of major NSAID toxicity with celecoxib, ibuprofen, or naproxen: a secondary analysis of the PRECISION trial. Am J Med 130:1415–22.e4.
- Solomon DH, Husni ME, Wolski KE, et al. (2018). Differences in safety of nonsteroidal antiinflammatory drugs in patients with osteoarthritis and patients with rheumatoid arthritis: a randomized clinical trial. Arthritis Rheumatol 70:537–46.
- Springer B, Bechler U, Waldstein W, et al. (2020). The influence of femoral and tibial bony anatomy on valgus OA of the knee. Knee Surg Sports Traumatol Arthrosc 28:2998–3006.
- Stefani RM, Lee AJ, Tan AR, et al. (2020). Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair. Acta Biomater 102:326–40.
- Stone KR, Pelsis JR, Na K, et al. (2017). Articular cartilage paste graft for severe osteochondral lesions of the knee: a 10- to 23-year follow-up study. Knee Surg Sports Traumatol Arthrosc 25:3824–33.
- Suchaoin W, Bonengel S, Griessinger JA, et al. (2016). Novel bioadhesive polymers as intra-articular agents: chondroitin sulfate-cysteine conjugates. Eur J Pharm Biopharm 101:25–32.
- Sulistio A, Reyes-Ortega F, D’Souza AM, et al. (2017). Precise control of drug loading and release of an NSAID-polymer conjugate for long term osteoarthritis intra-articular drug delivery. J Mater Chem B 5:6221–6.
- Tellegen AR, Rudnik-Jansen I, Pouran B, et al. (2018). Controlled release of celecoxib inhibits inflammation, bone cysts and osteophyte formation in a preclinical model of osteoarthritis. Drug Deliv 25:1438–47.
- Thompson CL, Patel R, Kelly TA, et al. (2015). Hedgehog signalling does not stimulate cartilage catabolism and is inhibited by Interleukin-1β. Arthritis Res Ther 17:373.
- Tu JF, Yang JW, Shi GX, et al. (2021). Efficacy of intensive acupuncture versus sham acupuncture in knee osteoarthritis: a randomized controlled trial. Arthritis Rheumatol 73:448–58.
- van Midwoud PM, Sandker M, Hennink WE, et al. (2018). In vivo pharmacokinetics of celecoxib loaded endcapped PCLA-PEG-PCLA thermogels in rats after subcutaneous administration. Eur J Pharm Biopharm 131:170–7.
- Villamagna IJ, Gordon TN, Hurtig MB, et al. (2019). Poly(ester amide) particles for controlled delivery of celecoxib. J Biomed Mater Res A 107:1235–43.
- Wallace IJ, Worthington S, Felson DT, et al. (2017). Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci U S A 114:9332–6.
- Wang C, Yan L, Yan B, et al. (2019). Agkistrodon ameliorates pain response and prevents cartilage degradation in monosodium iodoacetate-induced osteoarthritic rats by inhibiting chondrocyte hypertrophy and apoptosis. J Ethnopharmacol 231:545–54.
- Wang LJ, Zeng N, Yan ZP, et al. (2020). Post-traumatic osteoarthritis following ACL injury. Arthritis Res Ther 22:57.
- Wang S, Wei X, Sun X, et al. (2018). A novel therapeutic strategy for cartilage diseases based on lipid nanoparticle-RNAi delivery system. Int J Nanomedicine 13:617–31.
- Wang T, He C. (2018). Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev 44:38–50.
- Wang TQ, Li YT, Wang LQ, et al. (2020). Electroacupuncture versus manual acupuncture for knee osteoarthritis: a randomized controlled pilot trial. Acupunct Med 38:291–300.
- Wu Y, Wang Z, Lin Z, et al. (2020). Salvianolic acid a has anti-osteoarthritis effect in vitro and in vivo. Front Pharmacol 11:682.
- Wyles CC, Heidenreich MJ, Jeng J, et al. (2017). The John Charnley award: redefining the natural history of osteoarthritis in patients with hip dysplasia and impingement. Clin Orthop Related Res 475:336–50.
- Xu J, She G, Gui T, et al. (2020). Knee muscle atrophy is a risk factor for development of knee osteoarthritis in a rat model. J Orthop Translat 22:67–72.
- Xu X, Liang Y, Li X, et al. (2021). Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 269:120539.
- Xu X, Lv H, Li X, et al. (2017). Danshen attenuates osteoarthritis-related cartilage degeneration through inhibition of NF-κB signaling pathway in vivo and in vitro. Biochem Cell Biol 95:644–51.
- Yan H, Duan X, Pan H, et al. (2019). Development of a peptide-siRNA nanocomplex targeting NF- κB for efficient cartilage delivery. Sci Rep 9:442.
- Yeomans ND, Graham DY, Husni ME, et al. (2018). Randomised clinical trial: gastrointestinal events in arthritis patients treated with celecoxib, ibuprofen or naproxen in the PRECISION trial. Aliment Pharmacol Ther 47:1453–63.
- Yu Y, Brouillette MJ, Seol D, et al. (2015). Use of recombinant human stromal cell-derived factor 1α-loaded fibrin/hyaluronic acid hydrogel networks to achieve functional repair of full-thickness bovine articular cartilage via homing of chondrogenic progenitor cells. Arthritis Rheumatol 67:1274–85.
- Zavgorodnya O, Carmona-Moran CA, Kozlovskaya V, et al. (2017). Temperature-responsive nanogel multilayers of poly(N-vinylcaprolactam) for topical drug delivery. J Colloid Interface Sci 506:589–602.
- Zeggini E, Panoutsopoulou K, Southam L, et al. (2012). Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. Lancet 380:815–23.
- Zhang K, Yang J, Sun Y, et al. (2020). Thermo-sensitive dual-functional nanospheres with enhanced lubrication and drug delivery for the treatment of osteoarthritis. Chemistry 26:10564–74.
- Zhang R, Zhang Q, Zou Z, et al. (2021). Curcumin supplementation enhances bone marrow mesenchymal stem cells to promote the anabolism of articular chondrocytes and cartilage repair. Cell Transplant 30:963689721993776.
- Zhang R, Fang H, Chen Y, et al. (2012). Gene expression analyses of subchondral bone in early experimental osteoarthritis by microarray. PLOS One 7:e32356.
- Zhang Z, Huang G. (2012). Intra-articular lornoxicam loaded PLGA microspheres: enhanced therapeutic efficiency and decreased systemic toxicity in the treatment of osteoarthritis. Drug Deliv 19:255–63.
- Zhang Z, Leong DJ, Xu L, et al. (2016). Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res Ther 18:128.
- Zhao C, Liu Q, Wang K. (2017). Artesunate attenuates ACLT-induced osteoarthritis by suppressing osteoclastogenesis and aberrant angiogenesis. Biomed Pharmacother 96:410–6.
- Zhao Y, Teng B, Sun X, et al. (2020). Synergistic effects of kartogenin and transforming growth factor-β3 on chondrogenesis of human umbilical cord mesenchymal stem cells in vitro. Orthop Surg 12:938–45.
- Zhong C, Feng J, Lin X, Bao Q. (2017). Continuous release of bone morphogenetic protein-2 through nano-graphene oxide-based delivery influences the activation of the NF-κB signal transduction pathway. IJN Volume 12:1215–26.
- Zhou M, Lozano N, Wychowaniec JK, et al. (2019). Graphene oxide: a growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomater 96:271–80.
- Zhou Y, Ming J, Deng M, et al. (2020). Chemically modified curcumin (CMC2.24) alleviates osteoarthritis progression by restoring cartilage homeostasis and inhibiting chondrocyte apoptosis via the NF-κB/HIF-2α axis. J Mol Med 98:1479–91.