Abstract
The antioxidant capacity and phenolic content of three varieties of Phoenix dactylifera leaves, namely Deglet Nour, Medjhoul, and Barhee, were studied. The antioxidant activities of extracts of different leaf varieties obtained with solvents of different polarity were investigated using assays of 2, 2-diphenyl-2-picrymhydrasyl hydrate radical-scavenging activity, total phenolics and flavonoids amount, condensed tannins, reducing power, and total antioxidant capacity. The results showed that all the extracts exhibited antioxidant and radical-scavenging activities at different magnitudes and potency. The decreasing order of antioxidant and radical-scavenging activities among the extracts assayed were found to be methanol (MeOH) fraction > ethyl acetate fraction > hexane fraction > water extract. Correlation analysis indicated that there is a linear relationship between antioxidant potency, free radical-scavenging ability, and the content of phenolic and flavonoids compounds of Phoenix dactylifera leaf extracts. These results showed that Phoenix dactylifera leaf extracts are a valuable natural antioxidant, which can be applied in both healthy medicine and food industry and biotechnology.
INTRODUCTION
The date palm (Phoenix dactylifera L.) is a tropical and subtropical tree that belongs to the palmaceae (Arecaceae) family and plays an essential ecological role in Arabian countries. In Tunisia, oases cover almost 40,000 ha and represent an original form of human development in very harsh climatic conditions.[Citation1] Actually, each year after the fruit harvesting, the upkeep of the date palm trees results in the production of a huge renewable amount of palm leaves and floral stems supporting date fruits. These renewable resources are essentially used as biofuel for domestic purposes, animal feed, as well as shelter building.[Citation2] Chemical composition of some varieties of date palm roots and fruits[Citation3,Citation4] and its important antioxidant activities have been extensively investigated[Citation5,Citation6] due to the presence of water-soluble compounds with potent free radical-scavenging effects. Based on our knowledge, no studies have been done on the determination of phenolic content and antioxidant capacity of Phoenix dactylifera leaves. However, a thorough investigation of the chemical composition of this abundant renewable resource can lead to a new exploitation for medicine, food applications, and biotechnology.
In this context, phytochemicals extracted from natural plants were reported to have antioxidant and radical-scavenging activities.[Citation7,Citation8] Furthermore, enzymatic oxidation as well as auto-oxidation of lipids during storage and processing is the major reaction responsible for the deterioration of food quality affecting the colour, flavour, texture, and nutritive value of the foods. Antioxidants are often added to foods to prevent the radical chain reactions of oxidation by inhibiting the initiation and propagation step leading to the termination of the reaction and a delay in the oxidation process. However, the commonly used synthetic antioxidants, such as butylated hydroxyanisole and butylated hydroxy toluene (BHT), are restricted by legislative rules because they are suspected to have some toxic effects and possible carcinogens.[Citation9] Therefore, there has been a considerable interest by the industry and a growing trend in consumer preferences for natural antioxidants over synthetic compounds, and elimination of synthetic antioxidants in food applications has given more impetus to explore natural source of antioxidants. Thus, antioxidants are of interest to both food scientists and health professionals and there has been a convergence of interest among researchers in these fields as the role of antioxidants in the diet and their impact on human health has come under attention.
Furthermore, the effect of genotype imposes one of the greatest constraints in the process of date palm regeneration by tissue culture technique. This effect is largely dependent on the richness of tissues in phenolic compounds, which once emitted and oxidized in the culture media, become toxic, which blocks the cell proliferation process and impedes the strain establishment. The determination of tissue richness in antioxidants and phenolic compounds before any approach of multiplication allows to modulate at time the nature and the amount of organic additives (adsorbent, antioxidant) to add to the culture media so that cell proliferation can take place and the formation of new tissue can be ensured. Hence, a good knowledge of the phenolic compounds/antioxidants balance before any attempt of date palm regeneration via tissue culture technique is of interest.
The main objectives of this study were (i) to determine the polyphenolic content (total phenolics, total flavonoid, and condensed tannin), (ii) to evaluate their antioxidant capacity (ascorbic acid equivalent antioxidant capacity, reducing power and scavenging ability on 2, 2-diphenyl-2-picrymhydrazyl hydrate (DPPH) radicals), and (iii) to examine the efficiency of different solvent systems for the extraction of polyphenols from three varieties of Phoenix dactylifera palm leaves.
MATERIAL AND METHODS
Plant Material
Fresh leaves of three Phoenix dactylifera varieties (Deglet Nour, Medjhoul, and Barhee) were harvested in March from the garden of Sciences Faculty, Sfax, Tunisia. Collected leaves were washed with distilled water and allowed to air dry at room temperature for 15–20 days. After complete drying, the leaves were ground to a fine powder using a laboratory grinder and kept at 4°C until further use.
Extraction of Antioxidants from Phoenix dactylifera Leaves
The dried leaves of Phoenix dactylifera varieties (10 g) were extracted sequentially (by a maceration method) by adding solvents of increasing polarity: hexane, ethyl acetate, methanol, and water. The powder was first extracted by stirring with 100 mL of hexane at room temperature for 24 h. The extract was filtered through Whatman No. 1 filter paper in a Buchner funnel. The filtrate was concentrated to dryness with a rotary evaporator at 40°C. The remaining residues were successively extracted with ethyl acetate, methanol, and water under the same conditions. The water extract was freeze dried. All dried samples of each extract were kept in the dark at 4°C, after determining their yield prior to analysis.The dried sample of each extract was weighed and the yield of soluble constituents was calculated from the equation below:
where W 1 was the weight of extract after evaporation of different solvents and W 2 was the dry weight of the fresh plant sample.
Determination of Antioxidant Contents in Phoenix dactylifera Leaf Extracts
Estimation of total phenolic contents
Total phenols were determined according to the method described by Julkunen-Titto.[Citation10] An aliquot (50 μl) of each extract or standard solution was mixed with 1 ml of dd H2O and 0.5 ml of Folin–Ciocalteu's phenol reagent. Afterwards 2.5 ml of 20% Na2CO3 solution were added to the mixture followed by incubating at ambient temperature in the dark for 20 min. The absorbance against a blank was measured at 735 nm (Jenway 3305, Essex, England). Caffeic acid was used to prepare a standard curve (0.05–0.5 mg/ml; y = 2.5904x − 0.0091; r 2 = 0.998; where y is the absorbance and x is the standard concentration). The results were expressed as mg caffeic acid equivalents (CAE/g extract).
Estimation of total flavonoid contents
Flavonoid contents were determined according to the method of Zhishen et al.[Citation11] An aliquot (250 μl) of each extract or standard solution was mixed with 1.25 ml of dd H2O and 75 μl of 5% NaNO2 solution. After 6 min, 150 μl of 10% AlCl3 solution was added. An aliquot of 0.5 ml of NaOH (1 M) solution was added 5 min later and then the total volume was made up to 2.5 ml with dd H2O. Quercetin was used for standard curve construction (0.05–0.5 mg/ml; y = 0.752x + 0.0023; r 2 = 0.99; where y is the absorbance and x is the standard concentration). The results were expressed as mg quercetin equivalents (QE)/g extract.
Estimation of condensed tannin contents
Condensed tannins were determined according to the method of Julkunen-Titto.[Citation10] An aliquot (50 μl) of each extract or standard solution was mixed with 1.5 ml of 4% vanillin (prepared with MeOH) and then 750 μl of HCl (12 M) were added. The well-mixed solution was incubated at ambient temperature in the dark for 20 min. The absorbance against blank was determined at 500 nm. Catechin was used to make the standard curve (0.05–0.5 mg/ml; y = 0.5825x; r 2 = 0.9277; where y is the absorbance and x is the standard concentration). The results were expressed as mg catechin equivalents (CE)/g extract.
Antioxidant Capacity Assay of the Phoenix dactylifera Leaf Extracts
It is important to select and employ a stable and rapid method to determine antioxidant activity of many samples in time of consuming. Several methods have been developed to assay free radical scavenging capacity and total antioxidant activity of plant extracts. The most common and reliable method involves the determination of the disappearance of free radicals using a spectrophotometer. In this work, three methods were used: (i) 1,1-diphenyl picrylhydrazyl (DPPH) scavenging activity for estimation the free radical scavenging properties, (ii) the reducing power, and (iii) phosphomolybdenum method used for measuring the total antioxidant capacity.
Scavenging ability toward DPPH radical (DPPH• assay)
The DPPH• assay was performed as described by Shirwaikar et al.[Citation12] This method depends on the reduction of purple DPPH• to a yellow-coloured diphenyl picrylhydrazine and the remaining DPPH•, which showed maximum absorption at 517 nm, was measured. About 2 ml of various concentrations of each extract was added to 2 ml solution of 0.1 mM DPPH•. An equal amount of methanol and DPPH• served as the control. After 20 min of incubation at 37°C in the dark, the absorbance was recorded at 517 nm. The experiment was performed in triplicate. The DPPH radical scavenging activity was calculated according to the following equation:
where A sample and A control are absorbance of sample and control. The concentration of sample required to scavenge 50% of DPPH• (IC50) were determined. Decreasing of the DPPH• solution absorbance indicates an increase of the DPPH radical scavenging activity. Gallic acid was utilised for comparison.
Reducing power
Reducing power was determined according to the method of Oyaizu.[Citation13] An aliquot of each sample or standard solution prepared with MeOH (250 μl) was mixed with 250 μl of sodium phosphate buffer (0.2 M, pH 6.6) and 250 μl of 1% K3Fe (CN)6 incubated at 50°C for 20 min. After adding 250 μl of 10% trichloroacetic acid, the mixture was centrifuged at 3750 g for 10 min. The supernatant (100 μl) was then taken out and immediately mixed with 100 μl of MeOH and 25 μl of 0.1% ferric chloride. After incubation for 10 min, the absorbance against blank was determined at 700 nm. The EC50 value is the concentration at which the absorbance is 0.5. Ascorbic acid was used for comparison.
Determination of total antioxidant capacity by phosphomolybdenum method
The total antioxidant capacity of the extracts was evaluated by the phosphomolybdenum method as described by Prieto et al.[Citation14] The assay is based on the reduction of Mo(VI) to Mo(V) by the extract and subsequent formation of a green phosphate/Mo(V) complex at acid pH. Each sample solution (0.3 ml) and ascorbic acid (100 μg/ml) were combined with 3 ml of reagent (0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). A typical blank solution contained 3 ml of reagent solution and the appropriate volume of the same solvent used for the sample. All tubes were capped and incubated in a boiling water bath at 95°C for 90 min. After the samples had been cooled to room temperature, the absorbance of the solution of each sample was measured at 695 nm against the blank using a UV–Vis spectrophotometer. The experiment was performed in triplicate. The antioxidant activity is expressed as the number of equivalents of ascorbic acid/g of extract.
Statistical Analysis
The data were analysed using the statistical package program Stat view 5 Soft Ware for Windows (SAS Institute, Berkley, CA, USA). Statistical analysis was performed using one-way analysis of variance (ANOVA). All values were executed in triplicate and the mean values were calculated. Correlation analysis of the antioxidant capacity versus the total phenolic and flavonoid contents were carried out using the correlation and regression coefficients, which were calculated using the correlation matrix and regression analysis by the Stat view program. Differences were considered significant if p < 0.05.
RESULTS AND DISCUSSION
Extractable Total Phenols, Flavonoids, and Anthocyanins Content
It is well known that plant phenolics, in general, are highly effective free radical scavengers and antioxidants. Consequently, the antioxidant activities of plant/herb extracts are often explained with respect to their total phenolic and flavonoid contents, with good correlation. presents polyphenols, flavonoids, and condensed tannins contents obtained for all the leaf extracts. The content phenolic compounds in extracts, determined from regression equation of calibration curve (y = 2.5904x − 0.0091; r 2 = 0.998) and expressed in caffeic acid equivalents (CAE), varied between 1.41 mg and 146.46 mg/g plant extract. These amounts were comparable with results described in the literature for other extracts of plant products.[Citation15,Citation16] The highest content of total phenolic compounds was found in extracts obtained with methanol for the three varieties: Deglet Nour, Medjhoul, and Barhee (69.06 ± 0.41, 79.71 ± 2.95, and 146.46 ± 2.61 mg/g plant extract, respectively), whereas the contents obtained with ethyl acetate were much smaller for the three varieties cited above (10.69 ± 0.09, 6.51 ± 1.70, and 4.03 ± 0.47 mg/g plant extract, respectively). These results are in agreement with Skerget et al.'s[Citation17] study. Overall, the order of the phenolic contents of the test samples was methanol extract > ethyl acetate extract > water extract > hexane extract.
Table 1 Total amount of phenolic, flavonoid, and condensed tannin contents of leaf extracts from different solvent extraction systems
The content of flavonoids (mg/g plant extract) in the different extracts, determined from regression equation of calibration curve (y = 0.752x + 0.0023; r 2 = 0.99) and expressed in quercetin equivalents, varied from 22.84 to 321.71 mg/g plant extract. The results given in showed that ethyl acetate and methanol extracts contained high levels for all varieties (Deglet Nour, Medjhoul, and Barhee) indicating high correlation between total phenolics and flavonoids contents. Our results are in agreement with other studies,[Citation18,Citation19] which demonstrated that both flavonoid and phenolic compounds from plant leaves are known to have diverse biological activities and may also be responsible for the pharmacological actions. Moreover, our data indicate that high flavonoids and phenolic compounds in methanol and ethyl acetate extracts may account for their strong antioxidant activities compared with hexane and water extracts.
DPPH Radical Scavenging Activity
The bleaching of DPPH absorption (517 nm) by a test compound is representative of its capacity to scavenge free radicals, generated independent of any enzymatic or transition metal-based systems. illustrates a significant (p < 0.05) decrease in the concentration of DPPH radical due to the scavenging ability of the extracts gallic acid, as a positive compound, which presents the highest activity at all concentrations. The IC50 values (the concentration with scavenging activity of 50%) of scavenging activities on DPPH radical in methanol extract of three varieties (Deglet Nour, Medjhoul, and Barhee) were found to be 2.08, 2.11, and 2.14 mg/ml, respectively (). The DPPH radical-scavenging activity was found to be in the order of Barhee > Medjhoul > Deglet Nour. The water and hexane extracts showed the most potent activity and indicated that compounds have the strongest radical-scavenging activity in these varieties. This activity of extracts could be related to the nature of phenolics, thus, contributing to their electron transfer/hydrogen donating ability. Similar results were reported by others.[Citation20]
Figure 1 DPPH scavenging activity of leaf extracts from different solvent extraction systems: (a) Deglet Nour variety; (b) Medjhoul variety; (c) Barhee variety. Each value is expressed as mean ±SD (n = 3).
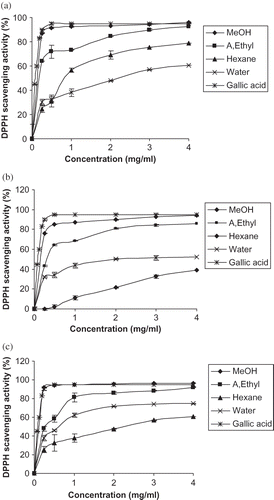
Table 2 Yield and free radical scavenging activity of leaf extract from different solvent extraction systems
Total Antioxidant Capacity
The phosphomolybdenum method is based on the reduction of Mo(VI) to Mo(V) by the antioxidant compound and the formation of a green phosphate/Mo(V) complex with a maximal absorption at 695 nm. Increase of the absorbance indicated the increase of the total antioxidant capacity. The antioxidant capacities of the extracts of Phoenix dactylifera were measured spectrophotometrically through this method and BHT was used as a positive compound. Potent antioxidant capacity has been found in all samples. The results showed that methanol extract exhibited the highest activity in the three varieties of Phoenix dactylifera: Deglet Nour, Medjhoul, and Barhee (244.79 ± 6.37, 285.65 ± 13.75, and 249.82 ± 34.46 mg Eq ascorbic acid/g extract). Published reports on the total antioxidant activity of these varieties of leaf extracts are not available. However, a total antioxidant activity of 245–376 mg ascorbic acid/g extract has been reported in higher plant extracts of Phillanthus species.[Citation21] Higher activity in methanol extract may be due to the interferences of other compounds present in this extract, and it has also been reported that solvents used for extraction have a dramatic effect on the chemical species.[Citation22]
Reducing Power
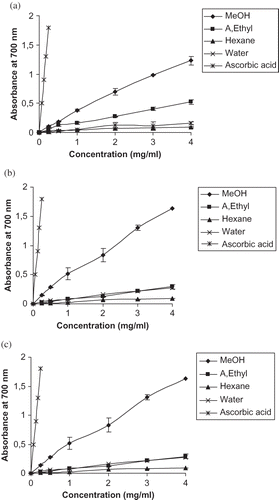
Fe (III) reduction is often used as an indicator of electron-donating activity, which is an important mechanism of phenolic antioxidant action, and can be strongly correlated with other antioxidant properties.[Citation23] The reducing power of the extracts was measured by direct electron donation in the reduction of [Fe(CN)6]3− to [Fe(CN)6]4−. The production was visualized by the addition of free Fe3+ ions after the reduction reaction, by forming the intense Prussian blue colour complex (Fe3+)4[Fe2+(CN−)6]3 and quantified by an absorbance measurement at 700 nm. Increased absorbance of the reaction mixture indicates increased reducing power.
shows the dose-response curves for the reducing power of the extracts from three varieties of Phoenix dactylifera leaves. The reducing power of the methanol extracts (Deglet Nour, Medjhoul, Barhee) increased from 0.105 ± 0.002, 0.145 ± 0.01, and 0.129 ± 0.01 at 0.25 mg/ml, respectively, to 1.23 ± 0.004, 1.63 ± 0.044, and 1.71 ± 0.040 at 4 mg/ml, respectively. The reducing power of ethyl acetate, hexane, and water extracts of the three varieties was slightly increased at a concentration of 0.25–4.00 mg/ml. For better comparison of reducing power among extracts from various leaves, the results were expressed as EC50 values, which is the effective concentration at which the absorbance was 0.5 and was obtained from interpolation from linear regression analysis. The EC50 value was very high for the methanol extract of the three varieties: Deglet Nour, Medjhoul, and Barhee (1.62 ± 0.01, 1.22 ± 0.03, and 1.16 ± 0.05 mg/ml, respectively), compared with those of ethyl acetate, hexane, and water extracts. EC50 values of the extracts in reducing power were significantly different (p < 0.05) with the EC50 values obtained for ascorbic acid (0.07 ± 0.003 mg/ml). Our results were in accordance with others who have reported that antioxidant properties are concomitant with the development of reducing power.[Citation16,Citation24]
Relationship between the Total Antioxidant Capacity and the Total Phenolic and Flavonoid Contents in Leaf Extracts
The correlation between the total content of phenolics and the antioxidant capacity were studied by many authors. Several studies established a linear correlation between the total phenolic content and the antioxidant capacity.[Citation19,Citation25] Others reported that there was no correlation.[Citation26] The correlation coefficient between the total antioxidant capacity monitored by phosphomolybdenum method and the total phenolic and flavonoid contents of the methanol and ethyl acetate leaf extracts of the Phoenix dactylifera varieties were determined (). A linear correlation appeared between the total antioxidant capacity and the total phenolic and flavonoid contents of the methanol extract with excellent correlation coefficient of the three varieties of date palm (R 2 = 0.687 for total phenolics and R 2 = 0.773 for total flavonoids). Furthermore, a linear correlation appeared between the total antioxidant capacity and the total phenolic and flavonoid contents of the ethyl acetate leaf extract with good correlation coefficient (R 2 = 0.713 for total phenolics and R 2 = 0.712 for total flavonoids). The results are in good accordance with previous studies, which showed that high total phenolic content increases the antioxidant activity.[Citation25,Citation27]
Table 3 Correlations between the antioxidant activity IC50/EC50/TAS values and the antioxidant components (total phenolics, flavonoids, and condensed tannins) of the methanol and ethyl acetate leaf extracts of three active Phoenix dactylifera varieties
Importance of the Determination of Polyphenol Content and Antioxidant Capacity in In Vitro Culture
The data presented in and show that the DN variety is significantly more rich in antioxidant amounts than the Barhee one with values of 353 and 304 (mg Eq ascorbic acid/g extract), respectively. However, the two varieties have very similar flavonoid contents but they differ in other classes of polyphenols. The Barhee variety displays a double dose of phenolic substances when compared to the DN variety, whereas it contains 5.5 times more in tannins. Although, for more antioxidants that are present in DN, there are much higher values of polyphenols in Barhee. Therefore, the balance between antioxidants and polyphenols is far in favor of the DN variety, which can explain the better behavior of this variety in in vitro culture.[Citation28] Date palm tissue browning is the consequence of the phenolic compounds oxidation and quinines formation, which are toxic to the tissues.[Citation29,Citation30] This study can be considered of high interest in the prevention of explants browning via the preliminary determination of their content in polyphenol compounds and in antioxidant capacity. Consequently, more conduction of the in vitro cultures for many recalcitrant species especially for some date palm cultivars is related to the significant reduction of the emissions and of the polyphenol oxidations in the culture media. That is why the previous determination of antioxidant/polyphenols balance would be very useful. In fact, based on the obtained results and to assure a better tissue culture process by reducing explant browning, it can be evaluated to choose either the addition of appropriate adsorbent doses to the culture media (33), or the treatment of the explants with an antioxidant solution[Citation31–33 or else the combination of both protocols when it is necessary.[Citation34]
Table 4 Reducing power (EC50) and total antioxidant capacity of leaf extract from different solvent extraction systems
CONCLUSION
The results obtained in this study clearly demonstrated that all the tested extracts of the three varieties of Phoenix dactylifera showed antioxidant and radical-scavenging activities at different magnitude and potency. The decreasing order of antioxidant and radical-scavenging activities among the extracts essayed through all methods is similar to the total phenolics and flavonoids contents of the extracts. Extract of Phoenix dactylifera leaves might be a valuable natural antioxidant source and seemed to be applicable in healthy medicine, the food industry, and biotechnology. However, the components responsible for the antioxidant activities of its extracts are currently unclear. Further work should be performed on the isolation and identification of the components in the extracts, especially in the MeOH extract. In addition, the in vivo safety needs to be thoroughly investigated in experimental rodent models prior to its possible application.
Notes
Hamadi Fetoui and Mohamed Makni contributed equally to this study.
REFERENCES
- Baaziz , M. , Majourhat , K. and Bendiab , K. Date palm culture in the Maghreb countries: Constraints and scientific researches . Proceedings of the Date Palm International Symposium . Windhoek , Namibia. February 22–25 . pp. 306 – 311 .
- Barreveld, W.H. Date palm products. FAO Agriculture Services Bulletin 1993, No. 101, 268 p. http://http//www.fao.org/docrep/t0681E/t0861e00.htm (http://http//www.fao.org/docrep/t0681E/t0861e00.htm)
- Al-Shahib , W. and Marshal , R.J. 2003 . The fruit of the date palm: Its possible use as the best food for the future . International Journal of the Food Science and Nutrition , 54 : 247 – 259 .
- Besbes , S. , Blecker , C. , Deroanne , C. , Drira , N. and Attia , H. 2004 . Date seeds: Chemical composition and characteristic profiles of the lipid fraction . Food Chemistry , 84 : 577 – 584 .
- Biglari , F. , AlKarkhi Abbas , F.M. and Easa , A.M. 2008 . Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran . Food Chemistry , 107 : 1636 – 1641 .
- Al-Farsi , M. , Alasalvar , C. , Morris , A. , Baron , M. and Shahidi , F. 2005 . Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman . Journal of Agricultural and Food Chemistry , 53 : 7592 – 7599 .
- Antonio , C. , Marino , B.A. and Arnao . 2005 . Hydrophilic and lipophilic antioxidant activity in different leaves of three lettuce varieties . International Journal of Food Properties , 8 ( 3 ) : 521 – 528 .
- Linda , G.R. , Saeedeh , A.D. and Asna , U. 2010 . Antioxidant efficacy of mulberry (Morus indica L.) leaves extract and powder in edible oil . International Journal of Food Properties , 13 ( 1 ) : 1 – 9 .
- Senevirathne , M. , Kim , S. , Siriwardhana , N. , Ha , J. , Lee , K. and Jeon , Y. 2006 . Antioxidant potential of Ecklonia cava on reactive oxygen species scavenging metal chelating, reducing power and lipid peroxidation inhibition . Food Science Technology International , 12 ( 1 ) : 27 – 38 .
- Julkunen-Titto , R. 1985 . Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics . Journal of Agricultural and Food Chemistry , 33 : 213 – 217 .
- Zhishen , J. , Mengcheng , T. and Jianming , W. 1999 . The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals . Food Chemistry , 57 : 307 – 316 .
- Shirwaikar , A. , Rajendran , K. and Punithaa , I.S. 2006 . In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine . Biological and Pharmaceutical Bulletin , 29 : 1906 – 1910 .
- Oyaizu , M. 1986 . Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine . Japanese Journal of Nutrition , 44 : 307 – 315 .
- Prieto , P. , Pineda , M. and Aguilar , M. 1999 . Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E . Analytical Biochemistry , 269 : 337 – 341 .
- Abdille , M.H. , Singh , R.P. , Jayaprakasha , G.K. and Jena , B.S. 2005 . Antioxidant activity of the extracts from Dillenia indica fruits . Food Chemistry , 90 : 891 – 896 .
- Chung , Y.C. , Chen , S.J. , Hsu , C.K. , Chang , C.T. and Chou , S.T. 2005 . Studies on the antioxidative activity of Graptopetalum paraguayense E . Walther. Food Chemistry , 91 : 419 – 424 .
- Skerget , M. , Kotnik , P. , Hadolin , M. , Hras , A.R. , Simonic , M. and Knez , Z. 2005 . Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities . Food Chemistry , 89 : 191 – 198 .
- Gimeno , M. , Castellote , A.I. , Lamuela-Raventós , R.M. , De la Torre , M.C. and López Sabater , M.C. 2002 . The effects of harvest and extraction methods on the antioxidant content (phenolics, α-tocopherol, and β-carotene) in virgin olive oil . Food Chemistry , 78 : 207 – 211 .
- Cai , Y. , Luo , Q. , Sun , M. and Corke , H. 2004 . Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer . Life Sciences , 74 : 157 – 184 .
- Singh , R. , Singh , S. , Kumar , S. and Arora , S. 2007 . Evaluation of antioxidant potential of ethyl acetate extract/fractions of Acacia auriculiformis A . Cunn. Food Chemistry Toxicology , 45 : 1216 – 1223 .
- Kumaran , A. and Karunakaran , R.J. 2007 . In vitro antioxidant properties of methanol extracts of five Phillanthus species from India . LWT–Food Science and Technology , 40 : 344 – 352 .
- Yuan , Y.V. , Bone , D.E. and Carrington , M.F. 2005 . Antioxidant activity of dulse (Palmaria palmate) extract evaluated in vitro . Food Chemistry , 91 : 485 – 494 .
- Dorman , H.J.D. , Peltoketo , A. , Hiltunen , R. and Tikkanen , M.J. 2003 . Characterization of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs . Food Chemistry , 83 : 255 – 262 .
- Kanatt , S.R. , Chander , R. and Sharma , A. 2007 . Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat . Food Chemistry , 100 : 451 – 458 .
- Kumaran , A. and Karunakaran , R.J. 2006 . Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus . Food Chemistry , 97 : 109 – 114 .
- Yu , L. , Haley , S. , Perret , J. , Harris , M. , Wilson , J. and Qian , M. 2002 . Free radical scavenging properties of wheat extracts . Journal of Agricultural and Food Chemistry , 50 : 1619 – 1624 .
- Wong , C. , Li , H. , Cheng , K. and Chen , F. 2006 . A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay . Food Chemistry , 97 : 705 – 711 .
- Fki , L. , Masmoudi , R. , Drira , N. and Rival , A. 2003 . An optimised protocol for plant regeneration from embryogenic suspension cultures of date palm, Phoenix dactylifera L., cv. Deglet Nour . Plant Cell Report , 21 : 517 – 524 .
- Zaid , A. 1987 . In vitro browning of tissue and media with special emphasis to date palm cultures—A review . Acta Horticulture , 212 : 561 – 566 .
- El hadrami , I. , Baaziz , M. , Cheikh , R. , Coumans , M. and Machex , J.J. 1996 . L'embryogenèse somatique chez Phoenix dactylifera L.: Importance des composés phénoliques dans le brunissement et l'acquisition des potentialités embryogènes . Options méditerranéennes , A 28 : 173 – 174 .
- Zaid , A. and Tisserat , B. 1983a . In vitro shoot tip differentiation in (Phoenix dactylifera L.) . Date Palm Journal , 2 ( 2 ) : 163 – 182 .
- Zaid , A. and Tisserat , B. 1983b . Morphogenetic responses obtained from a variety of somatic explant tissue of date palm . Botanical Magazine Tokyo , 96 : 67 – 73 .
- Sharma , D.R. , Kumari , R. and Chowdhury , J.B. 1980 . In vitro culture of female date palm (Phoenix dactylifera L.) tissues . Euphytica , 29 : 169 – 174 .
- Othmani , A. , Bayoudh , C. , Drira , N. and Trifi , M. 2009 . In vitro cloning of date palm (Phoenix dactylifera L.), cv. Deglet bey by using embryogenic suspension and temporary immersion bioreactor (TIB) . Biotechnology & Biotechnological Equipment , 23 : 1181 – 1188 .