?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Fresh kidney beans (Phaseolus vulgaris L.) are common green vegetable that are widely cultured and consumed worldwide. The benefits of fresh kidney beans are highly substantial, but at the same time, toxicity caused by phytohemagglutinin (PHA) has raised concerns. PHA from fresh kidney beans accounted for 40% of poisoning events induced by poisonous plants in China from 2004 to 2013. However, an understanding of PHA levels in fresh kidney beans remains elusive. An analysis method for PHA based on agglutination of PHA to erythrocyte cell was established. We investigated the effect of cultivar, bean part, maturity, and cooking method on PHA content in fresh kidney beans. We demonstrated significant differences in the distribution of PHA among various predominant, fresh Chinese kidney bean cultivars and parts. One major Chinese strain, Zihuayoudou, was found to contain the highest amount of PHA, which was abundant in fresh kidney bean seeds. Moreover, PHA concentrations were found to decrease with the maturity of the fresh product. Further, cooking methods had a significant effect on PHA levels in fresh kidney beans. PHA could be effectively removed when stir-frying for more than 18 min, or braising for more than 10 min. These findings provide insight into the PHA content of fresh kidney beans in China and will be helpful to guide food safety measures.
Introduction
Fresh kidney beans (Phaseolus vulgaris L.) are one of the most common green vegetables in the world. It is widely cultured in different countries such as China, Italy, Spain, Mexico, Brazil, the United States, Argentina, and others.[Citation1] Further, there are many cultivars of kidney bean. Kidney beans are known as a good source of protein, carbohydrates, dietary fiber, some vitamins and minerals,[Citation2] and are beneficial to human health based on their effects on preventing and controlling various metabolic diseases, such as diabetes mellitus, coronary heart disease, and colon cancer.[Citation3] However, when considering the adverse effects induced by food itself, kidney beans are raising increasing concern.[Citation4] Food-borne health complications induced by kidney bean consumption represent a global problem. As such, there are several reports of poisoning incidents that have been attributed to kidney beans.
Phytohemagglutinins (PHAs) are types of plant lectins, which are carbohydrate-binding proteins that can interact with membrane receptors that agglutinates cells.[Citation5,Citation6] The first PHA was discovered in semen ricini extract.[Citation7–Citation9] To date, they have been found in many plants, and especially leguminous plants. More than 70 types of PHAs have been separated and purified from leguminous plants.[Citation10] Further, PHAs can exert anti-nutritive effects[Citation11,Citation12] and affect parameters related to animal growth and metabolism, such as small intestine growth[Citation13–Citation15] and intestinal microflora ecology.[Citation16–Citation18]
PHAs are abundant in many kidney bean cultivars. Among associated risk factors, PHAs in kidney beans were found to be responsible for most human toxicologic manifestations, such as gastroenteritis, nausea, and diarrhea. Further, toxicity caused by PHA is very common in the United Kingdom. Due to the high prevalence of PHA toxicity, safety guidelines have been recommended by the public health laboratory services (PHLS), Colindale, UK regarding kidney bean consumption.[Citation19] Moreover, the import of some kidney beans, for example red kidney bean, has been prohibited in South Africa because of potential human toxicity caused by PHA.[Citation4,Citation20] In addition, toxicity caused by the PHA in fresh kidney beans is common in China. Specifically, there were 124 poisoning events, affecting 7,526 individuals, related to fresh kidney beans between 2004 and 2013, and with respect to poisonous plants, this accounted for 40% of national poisoning events and 64% of poisoned individuals. These types of poisoning events mostly occurred in collective canteens throughout the year, and especially during harvest season.
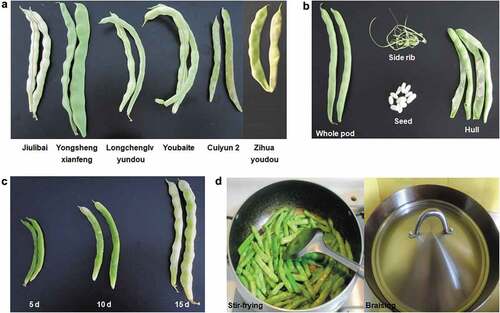
In China, fresh kidney beans are widely planted and consumed. Six strains of fresh kidney beans, namely Jiulibai, Yongshengxianfeng, Longchenglvyundou, Youbaite, Cuiyun 2, and Zihuayoudou, are major cultivars in China. The former five cultivars are mainly produced in Shandong Province. The last cultivar is mainly produced in Jilin Province. Poisoning events induced by fresh kidney beans often occur in Shandong Province, Jilin Province, and other areas.
Previous studies have mainly focused on PHA in the seeds of dry beans, rather than in fresh kidney beans.[Citation21,Citation22] In the present study, we established an analysis method for PHA based on agglutination of PHA to erythrocyte cell. We investigated the distribution of PHA in various cultivars and parts of fresh kidney beans. We also analyzed the effect of harvest maturity and cooking methods on PHA content in fresh kidney beans. Our results will increase the understanding of PHA in fresh kidney beans in China, which will be helpful to guide food safety efforts.
Materials and methods
Materials
PHA standard sample was purchased from Aladdin Science and Technology Co. Ltd (Shanghai, China). Rabbit erythrocyte cell suspension was purchased from Bersee Science and Technology Co. Ltd (Beijing, China). All other chemicals were purchased from Sigma (St. Louis, MO, USA). All reagents were of analytical grade and were used directly.
Collection of fresh kidney bean
Plant material: Six cultivars of fresh kidney bean (Jiulibai, Yongshengxianfeng, Longchenglvyundou, Youbaite, Cuiyun 2, and Zihuayoudou) were used in this study. This study did not involve endangered or protected species or locations. The mature fresh kidney beans (15 days after flowering) were sampled from leading fresh kidney bean-producing provinces. The former five cultivars were sampled from Weifang Experiment Station (119.149962 E, 36.719581 N) in Shandong Province on 21 July 2015. The major fresh kidney bean cultivar Jiulibai was sampled based on different harvest maturities (5, 10, or 15 days after flowering). The last cultivar, Zihuayoudou, was sampled from Gongzhuling Experiment Station (124.846017 E, 43.500374 N) in Jilin Province on 5 August 2015. These sampling locations are publicly available.
Extraction of PHA
Fresh kidney bean (250 g) was smashed and the slurry was homogenized with normal saline (500 mL). The mixture was placed in refrigerator at 4°C for 12 h and then centrifuged at 10,000 rounds per minute for 15 min. The supernatant was filtered with a hydrophilic PTFE syringe filter (0.45 μm), and the filtrate was used to determine hemagglutinating activity.
Different parts (whole pod, side rib, hull, and seed) of the six fresh kidney bean cultivars were separated and PHA was extracted. Further, different cooking treatments (stir-frying for 3, 6, 9, 12, 15, and 18 min and braising for 5, 10, 15, 20, 25, and 30 min) were applied to the fresh kidney bean cultivar Zihuayoudou and the PHA was extracted.
Establish of PHA analysis method
We established an analysis method for PHA based on agglutination of PHA to erythrocyte cell. PHA standardized stock solution (1,000 μg/mL) and standard working solutions (500, 400, 300, 200, and 100 μg/mL) were prepared with normal saline. Normal saline (25 μL) was added to all wells of a hemagglutination plate (96-well). The PHA sample (25 μL) was added to the first row of the plate, mixed well, and the mixture (25 μL) was pipetted into the second row. Then, the mixture in the second row was mixed and the liquid (25 μL) was pipetted into the third row. Remaining dilutions were performed in the same manner. Next, the rabbit erythrocyte cell suspension (25 μL) was added to each well and the agglutination effect was observed after 1.5 h.
Data analyses
To establish a method to determine PHA concentrations, standard samples comprising five different PHA concentrations were evaluated for hemagglutinating activity. The relationship between hemagglutinating activity (Y) and PHA concentration (X) was established based on the following equation (correlation coefficient, R2 = 0.999), which indicated that the method could be used for the determination of PHA concentrations.
The PHA concentration of tested samples (C) was calculated using the following equation:
where S0 and H0 were the PHA concentration and hemagglutinating activity of the standard sample, respectively and H, V, and M were the hemagglutinating activity, volume, and weight of the tested sample, respectively.
Results and discussion
The presence of anti-nutritional factors (toxic proteins, toxic alkaloids, etc.) decreases the food nutrient absorption rate, affecting human and animal health.[Citation23–Citation25] They represent a kind of risk factors. For example, trypsin inhibitors are responsible for the mal-absorption of proteins, loss of body weight, and increased serum cholecystokinin levels in the experimental model.[Citation26,Citation27] Caffeine is a risk factor for breast cancer and some reports suggested a positive hazard ratio for its association with prostatecancer. Risks for development of rheumatoid arthritis and osteoporosis increase with increased consumption of coffee on a regular basis.[Citation24] The World Health Organization International Agency for Research on Cancer published a list of carcinogens for preliminary reference. Caffeine is listed in three categories of carcinogens. PHAs are the most important risk factors[Citation28–Citation34] and are widely found in many plants. Pusztai et al. found that the extensive binding of PHA to intestinal epithelial cells can modify the glycosylation structure on the surface of epithelial cells.[Citation35] They also found that PHA can cause intestinal mast cells to degranulate and increase vascular permeability, thus allowing serum protein to infiltrate into the intestinal cavity.[Citation36] Grant et al. showed that PHA could cause significant changes in the structure of jejunal mucosa in vitro experiment[Citation37] and found that feeding PHA increased body fat loss and reduced glycogen in mice.[Citation38] They considered that PHA agglutinin primarily affects body fat metabolism in animals. Bardocz found that PHA could decrease body weight and the lipid weight proportion, depress rat growth.[Citation12] Linderoth found that enteral exposure to PHA in suckling rats causes mucosal disarrangement and functional impediment of the gut.[Citation39] Moreover, PHA was found to be responsible for most human toxicologic manifestations, such as gastroenteritis, nausea, and diarrhea. However, an understanding of PHA levels in fresh kidney beans remains elusive. Here, we established an analysis method for PHA based on agglutination of PHA to erythrocyte cell. We investigated the effect of cultivar, bean part, maturity, and cooking method on PHA content in fresh kidney beans.
Establish of PHA analysis method
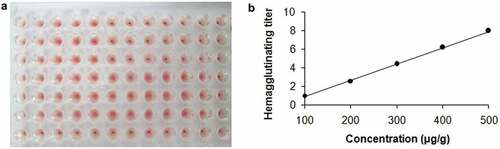
We established an agglutinin titer method for PHA analysis based on agglutination of PHA to erythrocyte cell (). The standard curve was shown in . This method detection limit was 200 μg/g. The recovery rate, average relative error, average relative deviation, and coefficient of variation were 92–101%, 1.0–2.5%, 1.2–2.2%, and 1.9–3.1%, respectively. From this point of view, the recovery rate, precision and accuracy were very good, indicating that the agglutinin titer method for PHA analysis is reliable.
Figure 1. (A) Representative traces from agglutination of PHA to rabbit erythrocyte cell suspension. (B) Standard curve of agglutination of PHA to rabbit erythrocyte cell suspension
The analysis methods for PHA are various. According to the properties of PHA binding specifically to sugar, glycoprotein or glycosylated protein, it can be detected by radioisotope labeled glycoconjugates. It is also possible to quantify PHA by rocket immunoelectrophoresis, enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay (RIA) with specific antibodies against PHA by immunological methods. However, immunological methods take months to prepare antibodies, and radioisotope-labeled glycoconjugates are limited in practice because of special equipment and protective measures. In this research, our established method for PHA analysis based on agglutination of PHA to erythrocyte cell is semi-quantitative. Compared with other methods, the established method is very simple and quick. In addition, it can be used for the detection of multiple samples in a short time. However, the detection limit of the established method was found to be 200 μg/g, which is relatively high. Advanced testing methods should be explored to obtain a lower detection limit.[Citation40–Citation44]
Distribution of PHA in various fresh kidney bean cultivars
PHA concentrations in fresh kidney bean cultivars were determined by hemagglutinating activity assays (). The standard curve is shown in (). The detection limit of this method was 200 μg/g. Results indicated a significant difference in the distribution of PHA among six fresh kidney bean cultivars (Jiulibai, Yongshengxianfeng, Longchenglvyundou, Youbaite, Cuiyun 2, and Zihuayoudou; ). PHA levels in Jiulibai, Youbaite, and Longchenglvyundou could not be detected, and therefore they were determined to be less than 200 μg/g. However, PHA concentrations in Yongshengxianfeng, Cuiyun 2, and Zihuayoudou were 800, 25,600, and 51,200 μg/g, respectively. Zihuayoudou was found to contain the highest amounts of PHA. This cultivar is widely consumed in three Northeast Provinces of China, and especially in Jilin Province. Our results indicated that PHA was of wide concentration range among different fresh kidney bean cultivars.
Distribution of PHA in various parts of the fresh kidney bean
In China, it is generally considered that PHA is abundant in the side rib of the fresh kidney bean. In this study, different parts (whole pod, side rib, hull, and seed) of the six fresh kidney bean cultivars were separated () and PHA concentrations in the different parts were then measured. Results showed significant differences in the distribution of PHA among the side rib, hull, and seed (). For example, PHA concentrations in the Zihuayoudou whole pod, side rib, hull, and seed were 51,200, 400, 400, and 102,400 μg/g, respectively. This indicates that PHA is abundant in the fresh kidney bean seed, and not in side rib. The result is reasonable because proteins are usually stored in seeds. It is helpful to understand PHA distributions within the fresh kidney bean. In previous research, one cultivar from Chongqing Province was published and its PHA concentrations in seed and hull were 7,415 and 965 μg/g, respectively.[Citation45] The report was consistent with our findings.
Table 1. Distributions of PHA in various parts of fresh kidney bean
Effect of harvest maturity on PHA content in fresh kidney beans
This food product is usually harvested 15 days after flowering, when it is mature. However, it is sometimes picked 5–10 days after flowering to acquire a tender texture. To date, the effect of harvest maturity on PHA content in fresh kidney beans had not been studied. Herein, the major fresh kidney bean cultivar, Jiulibai, was sampled based on different harvest maturities (5, 10, and 15 days after flowering; ), and PHA concentrations were tested. In the Jiulibai strain, levels were 400, 200, and less than 200 μg/g, respectively, 5, 10, and 15 d after flowering. This indicates a significant effect of harvest maturity on PHA content in fresh kidney beans. Moreover, PHA concentrations appeared to decrease with maturity. PHA is a kind of natural toxins that can protect plant itself against insect damage. PHA concentration is usually high during plant growth period. Therefore, our result is reasonable.
Effect of cooking methods on PHA content in fresh kidney beans
Different cooking methods (stir-frying for 3, 6, 9, 12, 15, and 18 min and braising for 5, 10, 15, 20, 25, and 30 min) using the fresh kidney beans of cultivar Zihuayoudou were tested () and PHA concentrations in the cooked kidney bean were measured. Results are shown in , and indicate that cooking methods can have significant effects on PHA levels in fresh kidney beans. Specifically, the PHA concentrations decreased with increasing cooking times. Moreover, when the Zihuayoudou cultivar was stir-fried for 18 min or braised for 10 min, PHA concentrations were lower than the detection limit (200 μg/g). Protein denaturation need enough temperature and time. Stir-frying need more time to remove PHA than braising. The reason may be that fresh kidney bean cann’t be uniform heated in stir-frying as good as that in braising. Our result indicated that stir-frying or braising for sufficient amounts of time can effectively remove PHA. Although the Zihuayoudou cultivar was found to contain high levels of PHA, it could be safe when cooked completely.
Table 2. PHA contents of Zihuayoudou treated by different cooking method
There have been many studies on the safety of PHA using experimental animals. For example, the safe level of PHA when fed to rats is 1,250 μg/g.[Citation10] However, there are few published reports on the safety profile of PHA with respect to humans. Thus, for safety evaluations and risk assessments regarding the consumption of PHA in daily human life, it is necessary to determine safe levels in future studies.
Conclusion
In conclusion, we provide important information regarding the content of PHA in fresh kidney beans in China, including the distribution of PHA in various cultivars and in various parts of the fresh kidney bean, as well as the effect of harvest maturity and cooking methods on the levels of this compound. This will be helpful to understand how dietary PHA from fresh kidney beans can be controlled, which will enhance food safety in China.
Additional information
Funding
References
- Food and Agriculture Organization of the United Nations. FAOSTAT, 2010. http://faostat.fao.org (accessed May 21, 2012).
- Osorio-Diaz, P.; Bello-Perez, L. A.; Sayago-Ayerdi, S. G.; Benitez-Reyes, M. D.; Tovar, J.; Paredes-Lopez, O. Effect of Processing and Storage Time on in Vitro Digestibility and Resistant Starch Content of Two Bean (Phaseolus Vulgaris) Varieties. J. Sci. Food Agr. 2010, 83, 1283–1288. DOI: 10.1002/jsfa.1413.
- Tharanathan, R. N.; Mahadevamma, S. Grain Legumes–A Boon to Human Nutrition. Trends Food Sci. Tech. 2003, 14, 507–518. DOI: 10.1016/j.tifs.2003.07.002.
- Kumar, S.; Verma, A. K.; Das, M.; Jain, S. K.; Dwivedi, P. D. Clinical Complications of Kidney Bean (Phaseolus Vulgaris L.) Consumption. Nutrition. 2013, 29, 821–827. DOI: 10.1016/j.nut.2012.11.010.
- Banwell, J. G.; Boldt, D. H.; Meyers, J.; Weber, F. L. Phytohemagglutinin Derived from Red Kidney Bean (Phaseolus Vulgaris): A Cause for Intestinal Malabsorption Associated with Bacterial Overgrowth in the Rat. Gastroenterology. 1983, 84, 506–515.
- Imran, M.; Anjum, F. M.; Butt, M. S.; Siddiq, M.; Sheikh, M. A. Reduction of Cyanogenic Compounds in Flaxseed (Linum Usitatissimum L.) Meal Using Thermal Treatment. Int. J. Food Prop. 2013, 16, 1809–1818. DOI: 10.1080/10942912.2011.608914.
- Stillmark, H.; Ueber, R. ein giftiges Ferment aus den Samen von Ricinus comm. L. und einigen anderen Euphorbiaceen: Inaugural-Dissertation. Schnakenburg’s Buchdruckerei, 1888.
- Barondes, S. H. Bifunctional Properties of Lectins: Lectins Redefined. Trends Biochem. Sci. 1988, 13, 480–482. DOI: 10.1016/0968-0004(88)90235-6.
- Peumans, W. J.; Van Damme, E. J. M. Lectins as Plant Defense Proteins. Plant Physiol. 1995, 109, 347–352.
- Dai, D. Z. Study on Quantification and Inactivation of Lectins in Feeds; Inaugural-Dissertation. Zhejiang University, 2003.
- Grant, G.; Oliveira, J. T. A.; Rubio L. A. Lectins: Biology, Biochemistry, Clinical Biochemistry. Proceedings of Iub Symposium, International Lectin Meeting. In van Driessche, E., Rouge, P., Beeckmans, S., Bog-Hansen, T. C., Eds.; Textop, 1996; pp 215–219.
- Bardocz, S.; Grant, G.; Pusztai, A.; Franklin, M. F.; Carvalho, A. D. F. The Effect of Phytohaemagglutinin at Different Dietary Concentrations on the Growth, Body Composition and Plasma Insulin of the Rat. Br. J. Nutr. 1996, 76, 613–626. DOI: 10.1079/BJN19960067.
- Bardocz S., Ewen S. W. B., Grant G.; Pusztai, A. Lectins as Growth Factors for the Small Intestine and the Gut. Lectins: Biomedical Perspectives. In Pusztai, A., Bardocz, S., Eds.; Taylor & Francis, 1995; pp 103–116.
- Pusztai, A.; Watt, W. B.; Stewart, J. C. Comprehensive Scheme for the Isolation of Trypsin Inhibitors and the Agglutinin from Soybean Seeds. J. Agr. Food Chem. 1991, 39, 862–866. DOI: 10.1021/jf00005a009.
- Pusztai, A.; Grant, G.; Brown D. S. Lectins: Biomedical Perspectives. In Pusztai, A., Bardocz, S., Eds.; Taylor & Francis, 1995; pp 141–154.
- Pusztai, A.; Grant, G.; Spencer, R. J.; Duguid, T. J.; Brown, D. S.; Ewen, S. W. B.; Peumans, W. J.; Van Damme, E. J. M.; Bardocz, S. Kidney Bean Lectin–Induced Escherichia Coli Overgrowth in the Small Intestine Is Blocked by GNA, a Mannose–Specific Lectin. J. Appl. Bact. 1993, 75, 360–368. DOI: 10.1111/j.1365-2672.1993.tb02788.x.
- Pusztai, A.; Gelencser, E.; Grant, G.; Bardocz, S. Recent Advances in Animal Nutrition. In Darmsworthy, P. C., Wiseman, J., Eds.; Nottingham University Press, 1997; pp 29–43.
- Pusztai, A.;. Transport of Proteins through the Membranes of the Adult Gastrointestinal Tract. A Potential for Drug Delivery? Drug Deliv. Res. 1989, 3, 215–228. DOI: 10.1016/0169-409X(89)90011-2.
- Bad Bug Book FDA. Food Borne Pathogenic Microorganisms and Natural Toxins Handbook; International Medical Publishing, Inc: McLean, VA, 2004.
- Venter, F. S.; Thiel, P. G. Red Kidney Beans-To Eat or Not to Eat? S. Afr. Med. J. 1995, 85, 250–252.
- Badari Nath, A. R. S.; Sivaramakrishna, A.; Marimuthu, K. M.; Saraswathy, R. A Comparative Study of Phytohaemagglutinin and Extract of Phaseolus Vulgaris Seeds by Characterization Andcytogenetics. Spectrochim. Acta A. 2015, 134, 143–147. DOI: 10.1016/j.saa.2014.05.086.
- Vijayakumari, K.; Siddhuraju, P.; Janardhanan, K. Effects of Various Water or Hydrothermal Treatments on Certain Antinutritional Compounds in the Seeds of the Tribal Pulse, Dolichos Lablab Var. Vulgaris L. Plant Foods Hum. Nutr. 1995, 48, 17–29. DOI: 10.1007/BF01089196.
- Sharmila, G. R.; Halami, P. M.; Venkateswaran, G. Identification and Characterization of a Calcium Dependent Bacillopeptidase from Bacillus Subtilis Cfr5 with Novel Kunitz Trypsin Inhibitor Degradation Activity. Food Res. Int. 2018, 103, 263–272. DOI: 10.1016/j.foodres.2017.10.023.
- Butt, M. S.; Sultan, M. T. Coffee and Its Consumption: Benefits and Risks. Crit. Rev. Food Sci. Nutr.. 2011, 51, 363–373. DOI: 10.1080/10408390903586412.
- Jen, H. C.; Nguyen, A. T.; Wu, Y. J.; Hoang, T.; Arakawa, O.; Lin, W. F.; Hwang, D. F. Tetrodotoxin and Paralytic Shellfish Poisons in Gastropod Species from Vietnam Analyzed by High-Performance Liquid Chromatography and Liquid Chromatography–Tandem Mass Spectrometry. J. Food Drug Anal. 2014, 22, 178–188. DOI: 10.1016/j.jfda.2013.09.005.
- Komarnytsky, S.; Cook, A.; Raskin, I. Potato Protease Inhibitors Inhibit Food Intake and Increase Circulating Cholecystokinin Levels by a Trypsin-Dependent Mechanism. Int. J. Obesity. 2011, 35, 236–243. DOI: 10.1038/ijo.2010.192.
- Gilani, G. M.; Xiao, C. W.; Cockell, K. A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108, S315–S332. DOI: 10.1017/S000711451200027X.
- Akande, K. E.; Doma, U. D.; Agu, H. O.; Adamu, H. M. Major Antinutrients Found in Plant Protein Sources: Their Effect on Nutrition. Pakistan J. Nutr. 2010, 9, 827–832. DOI: 10.3923/pjn.2010.827.832.
- Kala, B. K.; Mohan, V. R. Nutritional and Anti-Nutritional Potential of Three Accessions of Itching Bean (Mucuna Pruriens (L.) DC Var. Pruriens): An Under-Utilized Tribal Pulse. Int. J. Food Sci. Nutr. 2010, 61, 497–511. DOI: 10.3109/09637480903482801.
- Campion, B.; Perrone, D.; Galasso, I.; Bollini, R. Common Bean (Phaseolus Vulgaris L.) Lines Devoid of Major Lectin Proteins. Plant Breed. 2009, 128, 199–204. DOI: 10.1111/pbr.2009.128.issue-2.
- Vadivel, V.; Janardhanan, K. The Nutritional and Antinutritional Attributes of Sword Bean [Canavalia Gladiata (Jacq.) DC.]: An Under‐Utilized Tribal Pulse from South India. Int. J. Food Sci. Tech. 2004, 39, 917–926. DOI: 10.1111/ifs.2004.39.issue-9.
- Kalpanadevi, V.; Mohan, V. R. Nutritional and Antinutritional Assessment of under Utilized Legume D. Lablab Var. Vulgaris L. Bangladesh J. Sci. Ind. Res. 2013, 48, 119–130. DOI: 10.3329/bjsir.v48i2.15743.
- Siddhuraju, P.; Vijayakumari, K.; Janardhanan, K. Studies on the Underexploited Legumes, Indigofera Linifolia and Sesbania Bispinosa: Nutrient Composition and Antinutritional Factors. Int. J. Food Sci. Nutr. 1995, 46, 195–203. DOI: 10.3109/09637489509012549.
- Kordás, K.; Burghardt, B.; Kisfalvi, K.; Bardocz, S.; Pusztai, Á.; Varga, G. Diverse Effects of Phytohaemagglutinin on Gastrointestinal Secretions in Rats. J. Physiol. Paris. 2000, 94, 31–36. DOI: 10.1016/S0928-4257(99)00106-0.
- Pusztai, A.; Ewen, S. W.; Grant, G. Lectins and Also Bacteria Modify the Glycosylation of Gut Surface Receptors in the Rat. Glycoconjugate J. 1995, 12, 22–25. DOI: 10.1007/BF00731865.
- Pusztai, A.; Bardocz, S. Lectins Binding to the Gut Wall are Growth Factors for the Pancreas: Nutritional Implications for the Transgenic Plants. In Lectin Biomedical Perspective; Pusztai, A., Bardocz, S., Eds.; Bristol, London; Taylor, PA, Francis; Chapma, New York, 1997; pp 103–117.
- Grant, G.; Ewen, S. W. B. Local and Systemic Responses of Rats to Dietary Soybean Proteins; Pudoc Wageningen: The Netherlands, 1989.
- Grant, G.; Watt, W. B.; Stewared, J. C. S.; Pusztai, A. Effects of Dietary Soybean Lectin and Trypsin Inhibitors upon the Pancreas of Rat. Med. Sci. Res. 1987, 15, 197–1198.
- Linderoth, A.; Prykhod’ko, O.; Ahrén, B.; Fåk, F.; Pierzynowski, S. G.; Weström, B. R. Binding and the Effect of the Red Kidney Bean Lectin, Phytohaemagglutinin, in the Gastrointestinal Tract of Suckling Rats. Br. J. Nutr. 2006, 95, 105–115.
- Wang, H.; Wang, J. L.; Yang, S. X.; Tian, H. Y.; Liu, Y. G.; Sun, B. G. Highly Selective and Rapidly Responsive Fluorescent Probe for Hydrogen Sulfide Detection in Wine. Food Chem. 2018, 257, 150–154. DOI: 10.1016/j.foodchem.2018.02.130.
- Wang, J. L.; Hao, Y. F.; Wang, H.; Yang, S. X.; Tian, H. Y.; Sun, B. G.; Liu, Y. G. Rapidly Responsive and Highly Selective Fluorescent Probe for Bisulfite Detection in Food. J. Agric. Food Chem. 2017, 65, 2883–2887. DOI: 10.1021/acs.jafc.7b00353.
- Wang, J. L.; Wang, H.; Hao, Y. F.; Yang, S. X.; Tian, H. Y.; Sun, B. G.; Liu, Y. G. A Novel Reaction-Based Fluorescent Probe for the Detection of Cysteine in Milk and Water Samples. Food Chem. 2018, 262, 67–71. DOI: 10.1016/j.foodchem.2018.04.084.
- Wu, X. M.; Wang, H.; Yang, S. X.; Tian, H. Y.; Liu, Y. G.; Sun, B. G. A Novel Coumarin-Based Fluorescent Probe for Sensitive Detection of Copper (II) in Wine. Food Chem. 2019, 284, 23–27. DOI: 10.1016/j.foodchem.2019.01.090.
- Wang, H.; Wu, X. M.; Yang, S. X.; Tian, H. Y.; Liu, Y. G.; Sun, B. G. A Rapid and Visible Colorimetric Fluorescent Probe for Benzenethiol Flavor Detection. Food Chem. 2019, 286, 322–328. DOI: 10.1016/j.foodchem.2019.02.033.
- Zhang, C. J.; Chen, C. B. Preparation of Lectin from Seed and Bean Clip of Phaseolus Vulgaris. J. Chongqing Teachers Coll. (Natural Science Edition). 1993, 10, 77–79.