ABSTRACT
Apigenin (4,’5,7-trihydroxyflavone) is one of the most studied flavonoids with low toxicity and abundantly present phenolic compound in the plant kingdom. The main sources of apigenin are fruits, vegetables, nuts, herbs, honey, and plant-based drinks like tea. Numerous plants produce apigenin as a secondary metabolite and its intake is strongly related to its anti-inflammatory propensities. The purpose of present review was to wade through the literature on the anti-inflammatory mechanisms of this metabolite in various diseases and summarize the key objectives as they appear. Existing literature reported that apigenin is a promising candidate in managing a panoply of inflammatory-related diseases including cancer, diabetes, obesity, depression, insomnia, infection, and respiratory, cardiovascular, hepatoprotective, neurodegenerative, and skin diseases. Studies showed that apigenin significantly decreases the secretion of various proinflammatory cytokines specifically tumor necrosis factor (TNF)-α, IL-1β, IL-6, and IL-10. Moreover, apigenin effectively blocks the nitric oxide-mediated cyclooxygenase-2 expression and monocyte attachment and Prostaglandin by lowering iNOS and COX-2 in both microglial and macrophage mouse cells. Apigenin can slow the course of nonalcoholic fatty liver disease (NAFLD) in vivo by reducing high fat diet (HFD)-induced deposition of lipids and oxidative stress. It also controlled redox imbalances, suppress neuronal death and showed memory enhancement/learning skills and a reduction of fibrillar amyloid deposits with lowered insoluble Aβ concentrations in vivo. Taken together, it can be argued that apigenin can wane inflammation and thus offers a promising future in slowing down the development of chronic diseases and associated complications.
Introduction
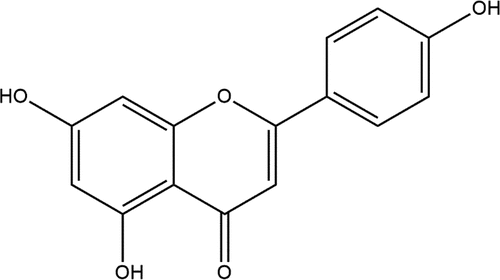
Apigenin (4,’5,7-trihydroxyflavone) belongs to the flavonoid group and is abundantly present in fruits, vegetables, nuts, and herbs (). The apigenin contents were reported high in celery and parsley with amounts of 19 and 215 mg per 100 g, respectively.[Citation1] Other sources rich in this bioactive molecule include wheat sprouts, oranges, rutabagas, tea, onion, chamomile, and cilantro. The daily intake of apigenin differs among countries across the globe. The results of a study in Netherlands found that 16 mg of apigenin per day is sufficient to intake on a regular basis,[Citation2] whereas in the Dutch diet, an intake of 1–1.5 mg per day is recommended.[Citation3]
Inflammation is a complex but natural response triggered by a variety of harmful factors, namely, physical trauma, infections, and toxins[Citation4] leading to pathological progression of organ damage. Inflammation is a common pathogenesis of many common chronic diseases and may cause acute or chronic inflammatory reactions in vital organs of the body leading to tissue damages and onset of diabetes, cancer, cardiovascular, bowel diseases, arthritis, and or neurodegenerative disorders.[Citation5] It has been argued that inflammatory responses depend on the specific nature of the initial stimulus and its location in the body, they all have a unifying mechanism, which encompass: i) cell surface pattern receptors recognition of stimuli; ii) activiation of inflammatory pathways; iii) release of inflammatory markers; and iv) recruitment of inflammatory cells.
The three main pathways, NF-κB, MAPK, and JAK-STAT, play major roles in inflammation. A dysregulation of such pathways may culminate to inflammation-associated disease. For instance, in an injured tissue, macrophages, monocytes, and neutrophils are activated and proinflammatory cytokines are produced hastily, particularly interleukins including tumor necrosis factor (TNF)-α, IL-1β, IL-6, and IL-10.[Citation6] These responses cause emergence and expansion of inflammatory and autoimmune complications.[Citation7] Many reports highlighted that apigenin is a biologically active molecule and has potent anti-inflammatory activities than other flavonoids, namely, quercetin, luteolin, tectorigenin, wogonin, and kaempferol. Apigenin has a potential to inhibit transcriptional activation of inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase-2 (COX-2) in RAW 264.7 cell lines that are activated by lipopolysaccharide (LPS), and resultantly lessens the production of NO.[Citation8,Citation9] It is reported that apigenin can suppress the production of NO in C6 astrocyte cells that were stimulated by LPS/gamma-interferon (IFN-γ) in dose-dependent manner resulting in an IC50 < 10–3 M.[Citation10]
Apigenin has attracted the interest of numerous researchers due to its multiple valuable bioactivities. A large set of published reviews geared toward the anti-cancer property of apigenin,[Citation11–16] on the general therapeutic potential of the flavone[Citation17] and a few elaborating on the pharmacokinetics and bioavailability of the molecule[Citation18–20] have been published. Only some piecemeal reviews are available so far on the inflammatory-reducing propensities of apigenin and there is a dearth of studies on the anti-inflammatory properties of this flavone. Therefore, present review is schemed to deliver pharmacokinetics, detailed break-down on useful effects on inflammatory-related diseases and other therapeutic benefits of apigenin by collecting and presenting as much research data as possible under one single review.
Food sources of apigenin
The richest food sources of apigenin includes celery, parsley, chamomile, vine-spinach, artichokes, and oregano in dried form. According to reports, dried parsley contains highest concentration of apigenin (45,035 μg/g). The dried chamomile flowers contain 3,000 to 5,000 μg/g of apigenin. The seeds of celery contain 786.5 μg/g while the Chinese celery contains 240.2 μg/g of apigenin. The vine spinach contains 622 μg/g of apigenin.
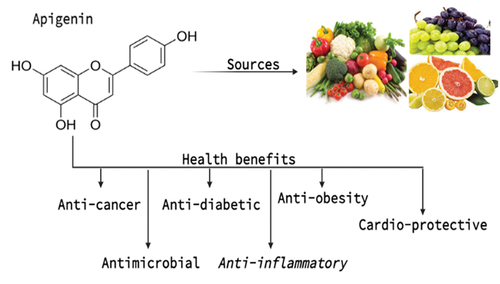
Common fruits like grapefruit, oranges, beverages made from plants, vegetables like onions, chamomile, parsley etc., wheat sprouts, tea, and various seasonings all contain a lot of apigenin. The dried flowers of the chamomile plant, Matricaria chamomilla, are also source of apigenin used in herbal teas.[Citation21] Other species, most of which are used in traditional herbal or alternative medicines, have also been found to contain apigenin as an active bioactive molecule, including propolis, Combretum erythrophyllum, Gentiana veitchiorum, Marrubium globosum, and Portulaca oleracea L. The richest sources of apigenin are the corresponding dried forms of celery, oregano, and parsley. Apigenin can be found in various plants as an aglycone or as a number of apigenin glycosides. Apigenin-7-O-glucoside, apigenin-6-C-glucoside (isovitexin), apigenin-8-C-glucoside (vitexin), apigenin-7-O-neohesperidoside (rhoifolin), and apigenin-6-C-glucoside-8-C-arabinoside are the common apigenin glycosides.[Citation22] Natural sources and potential health benefits of apigenin are shown in .
Pharmacokinetics: absorption, distribution, metabolism, and excretion
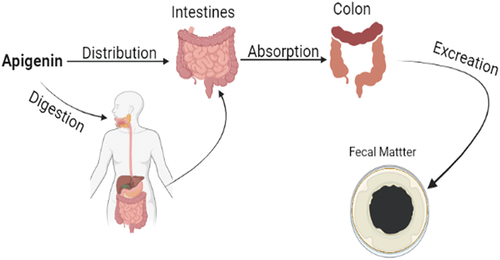
Apigenin exists naturally in glycoside conjugate form as apigenin-7-O-glucoside and different acylated derived forms.[Citation21] The glycoside forms and by-products has high solubility in water than apigenin. The solubility, bioavailability, and absorption are primarily dependent on the structure whereby a maximum bioavailability is reported as apigenin is linked with β-glycosides.[Citation23] The main site for the absorption of polyphenols is the small intestine where 5–10% of total polyphenols absorption occur in the form of monomers and dimers. Therefore, 90–95% of the total polyphenol reaches the colon region in its actual form.[Citation24] A study conducted on rats concluded that 28.6% of consumed apigenin is defecated within 72 h after oral administration.[Citation25] A 10-day rat trial concluded that 9.4 to 12% of radiolabelled apigenin administered orally was found in the intestine and feces, respectively. It can be concluded from the above studies that a large quantity of apigenin uses intestinal rote to pass through where it has strong interactions with gut microbiota.[Citation26shown in .
Bio-distribution and circulating levels of apigenin depends on its molecular size in the tissues. The net absorption is reduced due to the back secretion of glucuronides into the intestinal mucosa. The conversion of apigenin to bigger molecules in tissues has resulted reduced circulation level of this molecule and subsequent decrease in bio-distribution or bio-availability. For instance, glucuronides may be discharged back into the gut’s lumen after their production from intestinal mucosa and reduced the amount of nutrients that are actually absorbed. Cancer patients may have major changes in the distribution of the efflux transporters multi-drug resistance protein-1 (also known as ABCB1, P-gp, CD-243) and multi-drug resistance-associated protein-2 (also known as CMOAT and ABCC2). These transporters may also help with the movement of these conjugated flavonoids. Additionally, apigenin can undergo glucuronidation, sulfation, and methylation, all of which have an impact on its bioactivity and distribution.[Citation27–29]
To understand the breakdown of apigenin in the intestine, caco-2 cell monolayer system was used in study. During glucuronidation, apigenin acts as a substrate in epithelial cells of the intestine for 5′-diphospho-glucuronosyl transferase.[Citation30] Intravenous administration of scutellarin and oral administration of chrysanthemum to rats showed that most of the flavonoid metabolism occurred in the intestinal mucosa with minimum presystemic hepatic elimination.[Citation31,Citation32] However, some studies suggested that glucuronidation and sulfation of apigenin occurred in the hepatic cells at subcellular fraction level, which means that it is the type of flavonoid which determine the precise kinetics.[Citation33] Bio-transformation of various flavonoid groups depends on plant enzymes and microbes and in some cases, it is similar to the mammalian plant metabolism. These findings provide an helpful testimonial about the effects of human gut microflora on flavonoids.[Citation34] Poor bioavailability of flavonoids is mainly due to the UDP-glucuronosyltransferases (UGTs) metabolism. The metabolism of apigenin is very dynamic in the intestine than to liver because of UGTs. Apigenin excretion in Winstar rats was found to be lower compared to Gunn rats (deficient in UGT1A), signifying the upregulation of UGT isoforms in Gunn rats. It is the hepatic anion efflux transporters and upregulation of intestinal UGT2B in the UGT1A deficient Gunn rats that result in an efficient metabolism of flavonoids.[Citation35] Generalized models for the absorption and distribution of apigenin in mammals suggested that it underwent two steps of metabolism. In phase I, monohydroxy derivatives, i.e., luteolin, scutellarein, and iso-scutellarein are produced, while in phase II, the derivatives underwent conjugation as proved by both in vivo and ex vivo studies.[Citation36] An in vitro study proposed that during the metabolism of apigenin, one methyl conjugate, two sulphoconjugates, and four monoglucurono conjugates are formed. Contrary to the in vitro study, no metabolites from phase I were observed in group of rats perfused with apigenin. However, one sulfo and two monoglucurono conjugates were found in the 2nd phase of the metabolism.[Citation37] In another study, radio-labeled apigenin was administrated orally in a single dose. It was found that 51% of the radioactive traces were present in urine, 9.4–12% in the intestine, 1.2% in the blood and liver, 0.4% in the kidney and the rest of the body contained 24.8% in 10 days trial period. It was also observed that a higher level of monoglucurono conjugate of apigenin was found in the immature female and male rats’ excretion (10.0–31.6% versus 2.0–3.6%, respectively) compared to monosulphoconjugate of apigenin. Kinetics revealed the half-life of apigenin in the blood were 91.8 h, with 259 mL of distribution volume and 1.95 mL/h of plasmatic clearance. From the above-mentioned facts, it can be said that apigenin is slowly engaged and removed in the body.[Citation26] Chrysanthemum morifolium Ramat. extract containing apigenin has shown efficient absorption in the intestinal mucosa and slow elimination rate in serum compared to luteolin. After apigenin dosing, the maximum peak was observed at 1.1 and 3.9 h with total recovery of 16.6% in the urine and 28.6% in the feces. A randomized trial was performed on 14 volunteers to define the effect of parsley on urinary excretion of flavones. The trial was based on two weeks interventional study. In the first week, the subjects were given a basic diet and in the second week parsley supplemented diet (3.73–4.49 mg apigenin/MJ) was given. Results showed that 1.59–409.9 µg/MJ apigenin was excreted per 24 h via urine in the second week (supplemented diet), compared to excretion of 0–112.27 µg/MJ of apigenin per 24 h in the first week (basic diet).[Citation38]
In light of the studies highlighted above, it can be concluded that apigenin is a bioactive molecule with poor solubility and bioavailability. It is either promptly metabolized after absorption or expelled in the urine or feces unabsorbed. Bioavailability is one of the most important properties in drug design and development, therefore it is important to improve this property of apigenin to make it a good drug candidate.
Health perspectives
Anticancer
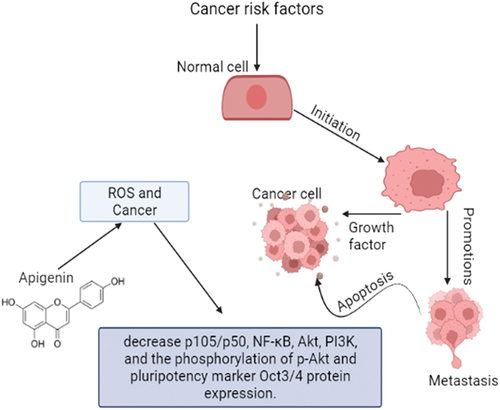
Apigenin’s anticancer properties are a result of cell growth arrest and apoptosis by modulating various signaling pathways in lungs, breast, skin, liver, colon, blood, pancreatic, prostate, oral, cervical, and stomach tumors. Upregulation in the mRNA expression of TNF-α, caspases-3, is activated by apigenin, which causes apoptosis via extrinsic caspase-dependent route. Apigenin also caused an intrinsic apoptotic pathway, as seen by increased levels of caspase-3, cytochrome c, and Bax, while the levels of B-cell lymphoma 2, caspase 8, and TNF remained the same in PC3 cells of human prostate cancer. Furthermore, apigenin also inhibited the invasion of tumor factors, decreased the p105/p50, NF-κB, Akt, PI3K, expression of pluripotency marker Oct3/4 protein and phosphorylation of p-Akt (). In addition, reduction of phosphorylation of p-Akt is linked with downregulation of NF-κB/Akt/PI3K signaling.[Citation12] In another recent study conducted on HepG2 cells of experimental subjects, apigenin in combination with sorafenib showed preventive effect on cell viability than a single treatment. The combined effect of both compounds has been found to enhance apoptotic cell death, reduce migration and invasion, exhibit cell cycle arrest, and increase the apoptotic gene expressions.[Citation16] Different researchers and investigators have worked on the preventive role of apigenin in different hepatic cancer cell lines such as Huh-7, HepG2, and SMMC-7721 etc., dose-dependently and observed that apigenin has the ability to arrest G1 phase of cell cycle and up regulate the cyclinD1 while down regulate the expressions of CDK4.[Citation15,Citation39]
A study conducted by Hamadou and colleagues revealed that apigenin exhibited antiproliferative effects in the wild type colon colorectal cancer cells, HCT116 p53, by stimulating p53 response pathway, reducing MAP kinase signaling, and modulating poly-ADp-ribose polymerase (PARP) cleavage.[Citation40] In another recent study conducted on mouse models subcutaneously xenografted with human esophagus cancer cells (eca-109) reported anti-tumor activity of apigenin. Flavones remarkably impeded proliferation of cells and triggered apoptosis by stimulating caspase-8 expression and cleaved PARP.[Citation41] In an in vitro study, apigenin was applied to C6 glioma cells in cultures of isolated rats found multiple changes such as enhancement in activated M1 profile markers iNOS and OX-42, modulation of IL-10 and tumor necrosis factor release, reduction in cell viability, tumor migration and M2 profile marker CD206 expressions, respectively.[Citation14,Citation42] In colorectal cancer cell lines (SW480), apigenin lowered cell growth, senescence, induces apoptosis, and affects the proapoptotic and antiapoptotic proteins.[Citation43]
In vitro analysis showed that apigenin significantly reduced cell proliferation, invasion, and migration during prostate cancer (PCa), and suppressed the development of tumor and metastasis in vivo.[Citation44] Interestingly, the rates of survival of the animals were extended post-apigenin treatment. Studies reported that apigenin treatment caused the downregulation of kazal-like domains proteoglycan 1 (SPOCK1), followed the decreased expression of mesenchymal markers and consequently reduced the harmful effect of PCa cells. In PCa xenografts, overexpression of SPOCK1 led to substantial tumor development and relieved apigenin-induced anticancer effects. In clinical trials, higher levels of SPOCK1 were reported in tumor tissues in contrast to non-tumor tissues, which was attributed to a reduced disease-free survival in PCa patients.[Citation45] A study showed that apigenin can efficiently inhibit colon cancer cell proliferation due to autophagy and apoptosis induction.[Citation46] In another study, two different pancreatic cancer cell lines (Panc1 and PaCa44) were used to investigate cytotoxic effects of apigenin, whereby a stronger effect was recorded against Panc1 compared to PaCa44 cells. Such high cytotoxic effect was attributed to ample presence of intracellular ROS, reduction of HSP90 and mutant (mut) p53 expression, and inhibition of mTORC1. Importantly, contact with HSP90 stabilizes (mut) p53, which then triggers a +ve feedback loop between p62 and NRF2.[Citation47] Moreover, apigenin treatment significantly increased caspase-9 activity during G2/M phase of cell cycle, up-regulated the COX2 (inflammation-associated gene) and BCL2 (apoptosis-associated gene) transcripts, and suppressed cell development in dose-dependent fashion in different lung fibroblast cell line (WI-38), ovarian adenocarcinoma cell line (SKOV-3), and skin fibroblast cell line (CCD-986Sk).[Citation48]
When apigenin was linked with gefitinib, numerous oncogenic drivers including epidermal growth factor receptor (EGFR), c-Myc, and HIF-1α, were inhibited, the expressions MCT1 and Gluts protein were reduced, and the signaling of 5’ adenosine monophosphate-activated protein kinase (AMPK) was deactivated. Consequently, glucose uptake was controlled and energy metabolism is balanced that leads to reduction in utilization of energy in EGFR of H1975 lung cancer cells that are L858R-T790M-mutated. Post treatment with apigenin and gefitinib, dysregulation and apoptosis were reported in H1975 cells. This combined treatment with apigenin and gefitinib presented an interesting approach that can be implemented as an alternative treatment to resist EGFR-TKIs in non-small-cell ling carcinoma (NSCLC).[Citation49] Snails are considered as important epithelial-mesenchymal transition (EMT) transcription factors for predicting colon cancer. According to a study, in NF-KB/snail pathway, the activity of these snails was inhibited effectively by apigenin.[Citation50] In SKOV3 ovarian cancer cell lines, apigenin caused induction of apoptosis via forming ROS and reducing mitochondrial membrane potential and modulating the B-cell lymphoma 2 (Bcl-2) and its associated X protein expression.[Citation51]
In TNBC cells, apigenin reduced the activity of YAP/TAZ and influenced the target genes to express particularly CYR61 and CTGF genes. The study also revealed that YAP/TAZ-TEAD protein–protein interaction was effectively disrupted after treatment with apigenin and TAZ sensitized TNBC cells expression reduced considerably.[Citation52] Apigenin and curcumin displayed growth-suppressive and proapoptotic effects in malignant melanoma. Flavonoids, including apigenin, have the potential to remarkably inhibit upregulation of IFN-γ-induced PD-L1 and simultaneously phosphorylation of STAT1. It was also observed that A375 cells treated with apigenin were highly sensitive to T cell-mediated death. The results of an in vivo study highlighted that apigenin has a significant role in inhibition of growth of A375 melanoma xenograft with improved infiltration of T cells into tumor tissues. Apigenin successfully reduced expression of PD-L1 in dendritic cells, which enhanced the cytokine-induced killer cells cytotoxicity against melanoma cells. The flavonoid prevented the growth of melanomas via several mechanisms, one of which is the decline in PD-L1 expression, which had a dual impact as it controls antigen-presenting cells as well as tumor formation.[Citation53]
Therefore, apigenin exert anticancer properties via several mechanisms. It has the ability to upregulate miR-101 and miR-520b in numerous cancerous cells, which consequently suppress the development of tumors. Furthermore, when combined with miR-138 mimics or miR-423-5p inhibitors, the apigenin-induced apoptotic rate was considerably greater.[Citation54] Several experimental evidences have demonstrated the effectiveness of apigenin in apoptosis induction, cell cycle arrest and inhibition of cellular proliferation, in different cancer cell lines such as hepatic, prostate, colon, lung, skin, and TNBC. Taken together, apigenin alone or in combination with other inhibitors such as curcumin, sorafenib, or gefitinib has the potential to manage highly aggressive cancers. In concusion, apigenin has showed promising in vitro and in vivo anticancer properties that warrants further investigation via clinical studies.
Anti-diabetes
Administration of apigenin proved to be beneficial by ameliorating the resistance to insulin, relieving liver injury, and preventing modification of lipid contents in mice fed with high amount of fructose. Additionally, apigenin has successfully assisted in the accruing of Nrf2 nuclear translocation followed by rise in NQO1 and HO-1 proteins expression, which are in charge of reducing oxidative stress. Findings from molecular docking using Keap1 protein showed that apigenin has strong interaction with the Nrf2-binding site of this protein.[Citation55] A study was conducted in streptozotocin (STZ)-induced diabetic rats to investigate whether apigenin has the ability to halt the development and progression of diabetic nephropathy. The results showed that oxidative stress and fibrosis appreciably arrest after treatment of apigenin (20 mg/kg), which subsequently enhanced renal damage caused by diabetic nephropathy.[Citation56]
By inhibiting key hydrolyzing enzymes such as α-glucosidase activity, postprandial hyperglycemia may be suppressed. The results of kinetic investigation showed that α-glucosidase activity was reversibly inhibited by apigenin, with IC50 value of 10.5 × 10−6 mol/L and was a noncompetitive type of inhibition. In silico studies demonstrated that α-glucosidase activity was due to apigenin as it forms interactions at a site close to active site, which may prompt the closure of channels that ultimately inhibits or blocks substrate access. The relations between myricetin and apigenin by isobolographic investigation revealed that at subtle concentrations, these displayed synergistic outcomes while at greater level, both exhibited additive or antagonistic interactions.[Citation57]
In numerous in vitro and in vivo research, apigenin has been the subject to examine its anti-diabetic, anti-inflammatory, and anti-obesity properties, but the long-term additional effects of flavonoids on obesity at low concentrations are still unknown. Therefore, Jung et al.[Citation58] used HFD – induced obese mice to determine protective benefits of apigenin against transcriptional and metabolic responses related to obesity and associated metabolic abnormalities. In high-fat fed mice, apigenin enhance hepatic steatosis while decrease in plasma levels of total cholesterol, free fatty acids (FFAs), markers of hepatic dysfunction, and apolipoprotein B was observed. Apigenin had no impact on food intake or adiposity. The observed activities were partially attributed to the upregulation expression of genes, monitoring oxidative phosphorylation, cholesterol homeostasis, fatty acid oxidation, electron transport chain, and tricarboxylic acid cycle, as well as the downregulation in expression of lipolytic and lipogenic genes and the suppression of certain enzymes necessary for synthesis of triglycerides and cholesterol esters in the liver.[Citation58] A study conducted by Ren and colleagues[Citation59] showed that administrated apigenin at the rate of 50 or 100 mg/kg to high fat diet induced diabetic rats caused a momentous reduction in blood glucose, serum lipid, malonaldehyde, ICAM-1 levels and insulin resistance index and boost antioxidant enzymes like superoxide dismutase, and impaired glucose tolerance. In aortic tissues, apigenin also showed restoration of phenylephrine-mediated contractions and acetylcholine. Additionally, it also suppressed NF-κB activation and ICAM-1 mRNA expression and exhibited improvements in NO production in insulin presence using in vitro PA-treated endothelial cells.[Citation59] A study of Cazarolli et al.[Citation60] observed diabetic rats to investigate impact of apigenin-6-C-b-L-fucopyranoside on glucose and insulin levels in serum samples. Findings showed that the compound exhibited glucose lowering effects in diabetic rats and enhanced the production of glucose-induced insulin. Inductions of alloxan has been found to enhance hepatic LPO, serum cholesterol; lower antioxidant enzymes, thyroid hormones, levels of serum insulin; increase activity of hepatic glucose-6-phospatase (G-6-Pase), enhance glucose concentration and amounts of triiodothyronine (T3) and thyroxine (T4) whereas administration of apigenin (0.78 mg/kg) reverted these changes in diabetic rats.[Citation61] In conclusion, apigenin, possess promising antidiabetic properties via inhition of key carbohydrate enzymes that impact on postprandial hyperglycemia.
Anti-obesity
Apigenin has the potential to reduce body weight and visceral adipose tissues (VAT) but not epididymal adipose tissue (EAT) or subcutaneous adipose tissue (SAT) in obese mice fed with HFD. Studies revealed that whereas HFD did not affect SAT and EAT, it did enhance STAT3 phosphorylation in VAT. Other research revealed that apigenin bound to non-phosphorylated STAT3, reduced phosphorylation of STAT3 and VAT transcription activity, which therefore caused reduction in STAT3 target genes expression for differentiation 36 (CD36). Due to decreased CD36 expression, peroxisome proliferator-activated receptor gamma (PPAR-γ), a crucial component of adipogenesis, was expressed at lower levels in adipocytes.[Citation62]
Obesity is associated with several inflammatory responses, including IL-1 activation, mitochondrial dysfunction, and interference with adipocyte browning. In primary human adipocytes, apigenin provides protection against browning of IL-1β induced by dibutyryl-cAMP, as observed by an increase in oxygen consumption, mitochondrial content, and brown-specific markers. Furthermore, apigenin impeded NF-κB activation and functioning of inflammatory markers in these adipocytes incurred due to IL-1β. Interestingly, in response to treatment of IL-1β, apigenin stimulated prostaglandin E2 (PGE2) and COX2 expression. In IL-1-treated cells, pharmacological suppression of COX2 or RNA silencing reduces apigenin’s beneficial impact on adipocyte browning. In addition, due to blockage of PGE2 receptor 4 (EP4), apigenin-mediated browning of adipocyte is reduced. In IL-1β-treated adipocytes, adipocyte browning is accompanied by rise in intracellular Ca2+ level partially due to TRPV1/4 receptor activation after administration of apigenin.[Citation63] In human mesenchymal stem cells, different doses of apigenin (1, 10, and 25 µM) reduced TG accumulation, increased mRNA levels of ATG, and lowered FASN expression.[Citation64] In obese rats, apigenin prevents skeletal muscle atrophy brought on by a high-fat diet. Without affecting body weight, it showed decreased fat pad development and lower levels of inflammatory cytokines. It increased skeletal muscle mass while expression of atrophic genes including Atrogin-1 and MuRF1 decreased noticeably. However, it also boosted exercise ability. Apigenin improved performance and biogenesis of mitochondria. Additionally, apigenin prevented the mitochondrial dysfunction and muscular atrophy that palmitic acid-induced in C2C12 cell culture. Additionally, it stimulated AMPK in the muscle and C2C12 cells in HFD fed obese mice.[Citation65]
Apigenin substantially inhibited differentiation in 3T3-L1 cells, reduced the levels of peroxisome proliferator-activated receptor γ and CCAAT/enhancer binding protein (C/EBP) α. No clonal expansion was observed in 3T3-L1 cells treated with apigenin during primary phases of adipocyte differentiation. Apigenin prevented the progression of cell cycle at the G0/G1 phase. The simultaneous and sustained synthesis of p27 (Kip1) and a considerable decrease in the expression of cyclin-dependent kinase 4 and cyclin D1 were associated with this reaction. Apigenin enhanced the C/EBP inhibitors expression including the phospho-liver-enriched inhibitory protein isoform of liver-enriched activating protein C/EBPβ and C/EBP homologous protein while reduced the 35 kDa isoform of C/EBPβ expression. This reduced the ability of C/EBPβ to bind DNA in differentiating 3T3-L1 cells.[Citation66]
Hepatoprotective role
Globally, the prime factor in liver-related morbidity and mortality is nonalcoholic fatty liver disease (NAFLD). However, there is currently no proven pharmaceutical treatment for NAFLD. In a study, a HFD-induced NAFLD model was used to assess hepatoprotective effects of apigenin and probable mechanism of action to establish its potential as a molecular candidate to treat NAFLD. The findings suggested that gavage administration of apigenin may lessen the harm that the HFD-fed mice’s livers experienced as well as increase insulin sensitivity and considerably reduce liver fat accumulation. Furthermore, apigenin therapy mice showed recovered hepatic steatosis and macrophage recruitment when compared to mice receiving HFD alone. Importantly, apigenin may suppress xanthine oxidase (XO) activity, reduce uric acid and ROS, and reverse the activation of the NLRP3 inflammasome induced by HFD. Due to these outcomes, formation of pro-inflammatory cytokines including IL-1 and IL-18 will promptly control. Apigenin’s pharmacological function was validated further utilizing a FFA driven cellular NAFLD model.[Citation67,Citation68] A possible mechanism for acute liver damage might be due to oxidative stress caused by H2O2 in HepG2 cells and carbon tetrachloride (CCl4) in mice. Apigenin substantially declined activity of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in mice serum confronted with CCl4, as well as lipid peroxidation as evidenced by decreased malondialdehyde (MDA), catalase (CAT), glutathione (GSH), and glutathione peroxidases (GSH-Px) and increased superoxide dismutase (SOD). Apigenin prevented the rise in AST and ALT levels as a result of H2O2-induced oxidative stress, altered the equilibrium between GSH, SOD activity, production of ROS, and elevated the IL-6 and of TNF-* expression. Additionally, the results of the real-time qPCR, Western blot, and immunofluorescence assays demonstrated that apigenin could also increase the activity of cellular inhibitor of apoptosis protein (c-IAP) 1, TNF receptor-associated factor (TRAF) 2/3, moderate the nuclear translocation of hepatic nuclear factor-B (NF-B), and ameliorate NF-B-inducing kinase (NIK), thereby inhibiting the non-canonical NF-B.[Citation69] Apigenin also inhibited the rise in malondialdehyde (MDA) caused by paracetamol. The activity of both glutathione reductase (GR) and catalase (CAT) enzymes were considerably elevated in liver homogenates after a toxic dose of paracetamol was administered.[Citation70] In Male rats, hepatotoxicity was produced by addition of 0.1 mg/ml of N-nitrosodiethylamine in drinking water, but apigenin (10, 20, or 40 mg/ml) exhibited a substantial dose-dependent reduction of the enzymes including LDH, SGPT ALP, and SGOT in blood. It also reduced the carbonyl concentration of proteins and lipid peroxidation. The results of the comet experiment in bone marrow cells, blood lymphocytes, and rat hepatocytes revealed a substantial dose-dependently reduction in mean length of tail.[Citation71] Acetaminophen caused hepatotoxicity in male Wistar albino rats, but apigenin (50 mg/kg, PO) mitigated the damage, as evidenced by lower serum aspartate aminotransferase and alanine aminotransferase activity and a substantial reduction in histological damages. Apigenin inhibited the inflammatory responses by lowering signal transducer and interleukin-1 levels and activating expressions of transcription 3, as well as impairing oxidative stress, which is evinced by momentous decline in nitrite/nitrate and malondialdehyde levels in the liver, as well as hepatic contents restitution with reduced activity of superoxide dismutase and glutathione. Likewise, It inhibited apoptosis by lowering levels of caspase-3 expression.[Citation72] Intraperitoneal administration of apigenin prevented liver injuries incurred due to ischemia/reperfusion (I/R) by lowering BCL-2 levels and ICAM-1 levels.[Citation73]
The possible molecular mechanisms of apigenin’s protective action against mice liver injury caused by lipopolysaccharide (LPS)/d-galactosamine (D-GalN) are being researched. The aspartate aminotransferase and alanine aminotransferase levels in blood serum were dramatically lowered after 100–200 mg/kg oral administration of apigenin for 7 days. In contrast, apigenin pretreatment decreased tumor necrosis factor expression and NF-B protein expression and while improved PPAR and hepatic nuclear factor erythroid 2-related factor 2 (Nrf-2) protein expression, as well as increased the activities of glutathione reductase, glutathione S-transferase, catalase, and superoxide dismutase activities.[Citation74] Apigenin was given to mice orally at the dosage of 150 mg/kg and 300 mg/kg and the group of mice receiving dose of 300 mg/kg in particular showed a rise in levels of glutathione S-transferase, glutathione peroxidase, glutathione reductase, and hepatic reduced glutathione. Nuclear factor kappa B and hepatic cytochrome P450 2E1 (CYP2E1) expression were both reduced by apigenin. The 300 mg/kg group significantly lessened hepatic steatosis. In the liver, pretreatment with apigenin may suppress the expression of sterol regulatory element binding protein-1c, diacylglycerol acyltransferase, the proteins fatty acid synthase while increase the expression of PPAR and the proteins carnitine palmitoyltransferase-1.[Citation75]
NAFLD is also a key risk factor for onset of several metabolic syndromes, mostly begins due to oxidative stress in the liver and irregularities related to lipid metabolism. In HFD induced model, apigenin has the ability to minimize in vivo oxidative stress and lipid accumulation imposed on by the HFD and halt the progression of NAFLD. As a PPARM, flavone was unable to boost PPARγ expression despite apigenin significantly inhibits PPARγ target genes expression that encode the enzyme related to fat metabolism. It is important to note that apigenin endorsed entry of Nrf2 into the nucleus, greatly stimulating it to suppress genes associated to fat metabolism and increase genes linked to oxidative stress. Furthermore, Nrf2 activity was required for apigenin to modulate PPAR target genes, according to research on Nrf2 knockdown/knockout and overexpression.[Citation76] In the liver tissues of rats with nonalcoholic steatohepatitis, the levels of PPAR protein expression are affected by the dosages of apigenin. In a HFD-induced rat model, apigenin showed improved insulin sensitivity, decreased fasting blood glucose, fasting insulin, triglycerides, total cholesterol, low-density lipoproteins, aspartate aminotransferase, and alanine aminotransferase. It also showed higher levels of protein and mRNA of PPARα and PPARγ and increased high-density lipoprotein.[Citation77] In Sprague-Dawley rats, renal injury was induced by 3-chloro-1, 2-propanediol (3-MCPD) and explored that apigenin (20, 40 mg/kg body weight/day) exerted a renal protective activities by modulating oxidative phosphorylation especially due to reduction in cytochrome c release, reestablishment of mitochondrial membrane potential (MMP), relieving the increase of Bax/Bcl2 ratio, and upregulation of ATP6 and ATP8 expressions, thus inhibiting the Caspase 3 and Caspase 9 activation.[Citation78] In a study on group of rats with hepatic I/R, apigenin was administrated intraperitoneally and lowered the Fas and FasL genes expressions.[Citation79] The acute liver failure is commonly due to dacetaminophen (APAP) overdose. In the presence of APAP, apigenin activated the NRF2 pathway, boosted APAP-induced autophagy, and decreased nuclear p65 transcriptional activity. EX-527, a SIRT1 inhibitor, decreased the apigenin’s ability to provide protection against APAP-induced damages to liver. The outcomes of molecular docking point out an interaction between apigenin and SIRT1 that may occur. Apigenin provides protection to the liver from APAP-induced damages by reducing APAP-induced inflammatory reactions, enhancing APAP-induced autophagy, altering the SIRT1-p53 axis, and curtailing the oxidative stress damages.[Citation80]
Cardiovascular role
Doxorubicin drug has been found to cause cardiotoxicity through increasing cardiac and liver injury markers such as malondialdehyde, apoptotic proteins (Casp3, Bax), percentage of cardiac fibrosis, and lowering the antiapoptotic proteins (Bcl2) expressions. Contrary to this, apigenin treated with experimental subjects caused substantial reductions in MDA levels, apoptotic proteins, and enhancement in antiapoptotic proteins.[Citation81] A study on myocardial injury model induced by lipopolysaccharide reported that apigenin can enhance cardiac functions and ameliorate cardiac injury, apoptosis, and tissue damages. Moreover, the study revealed that this flavonoid could ease myocardial injury induced due to endotoxin by regulating inflammatory cytokines (IL-1β, MIP-1α, MIP-2, and TNF-α) and oxidative stress predominantly related to nitro tyrosine and protein carbonyl. Hence, apigenin can be a promising bioactive molecule in treating myocardial oxidative and/or inflammatory-induced injury.[Citation82]
In apoE-/- mice and RAW264.7 macrophages, apigenin reduced LPS-induced inflammation and ABCA1-mediated cholesterol efflux in cells. Apigenin significantly enhanced ABCA1 expression time- and dose-dependently by suppressing miR-33. Quercetin treatment of macrophage-derived foam cells may boost ABCA1-mediated cholesterol efflux while decreasing total cholesterol, FC, and CE levels. When treating LPS-treated macrophage cells with apigenin, p-IκB-α, MyD88, TLR-4, and nuclear NF-κB p65 expression levels were reduced successfully. Furthermore, significant decline in the secretion of numerous proinflammatory cytokines due to apigenin was monitored. The administration of Apigenin also increased the ABCA1 expression in LPS-challenged apoE-/- mice, improved plasma lipid profile, decreased levels of NF-B p65, TLR-4, and miR-33, reduced the macrophages contents and smooth muscle cellular mass in atherosclerotic lesions, and alleviated inflammation, resulting in smaller atherosclerotic lesions.[Citation83,Citation84] Apigenin inhibits apoptosis in H9C2 rat cardiomyocytes by inhibiting ROS generation, oxidative stress, and apoptotic capacity of MI/R-induced H9C2 cells. Apigenin also enhanced MMP levels in these cells. Furthermore, apigenin regulated expression of certain proteins related to apoptosis and RAC serine/threonine protein kinase (Akt) signalling/phosphatidylinositol 3’-kinase (PI3K) in MI/R driven H9C2 cells. The apigenin’s anti-apoptotic activity was abolished by treatment with LY294002.[Citation85] In lipoteichoic acid treated embryonic heart cells of mouse (H9c2), apigenin in dose-dependent manner blocked the activation of c-Jun N-terminal kinase (JNK), p38, and extracellular signal-regulated kinase (ERK)1/2 in cardiomyocytes. Moreover, apigenin blocked IκB degradation and NF-κB translocation.[Citation86]
Apigenin was observed to prevent hemodynamic perturbations, reestablish the function of the left ventricle, and maintain a controlled redox status in the rat model. The compound also protected the rats against myocardial infarction by weakening myonecrosis, edema, apoptosis and oxidative stress.[Citation87] Apigenin remarkably enhanced heart function, overcoming endoplasmic reticulum stress by triggering AMPK signaling pathway, alleviated myocardial injury, and increased cell viability and decreased cell death.[Citation88] Findings gathered from in vitro experiments showed that apoptosis of oxidized low-density lipoprotein-loaded murine peritoneal macrophages could be induced by apigenin. The plasminogen activator inhibitor 2 (PAI-2) expression was also reported to be reduced by this flavonoid.[Citation89] Apigenin therapy can reduce miR-15b expression, enhance the activity of the JAK2-STAT3 pathway, increase the expression of JAK2 and reduce myocardial cell death and ROS generation. In a rat model of I-R injury, apigenin lessen myocardial I-R injuries. It decreased and increased miR-15b and expression of JAK2, as well as the activity of the JAK2-STAT3 pathway, decreasing cardiomyocyte cell death, myocardial I-R damage, and ROS generation.[Citation90]
Apigenin caused mitochondria-dependent cell death in hypoxic PASMCs to provide protection against pulmonary hypertension (PH) in male Sprague-Dawley rats. In both in vitro and in vivo investigations, apigenin ability was established to undo the hypoxia-induced suppression of KV1.5 expression. In hypoxic PASMCs, apigenin-induced mitochondrial-dependent apoptosis was inhibited by diphenyl phosphine oxide-1 (DPO-1) which is actually an inhibitor KV1.5, demonstrating that KV1.5 is implicated in this process. Additionally, functional studies revealed that apigenin activated hypoxia-induced factor 1 (HIF-1) signaling to promote mitochondria-dependent apoptosis.[Citation91–93] Apigenin (50 and 100 mg/kg) reduced blood levels of lactate dehydrogenase (LDH), creatine kinase (CK), TNF-, IL-6, and IL-1 in a lipopolysaccharide-induced endotoxemic rat model. In the heart, Bcl-2, Bax, cleaved caspase-3, and cleaved caspase-9 were all found using the Western blot method. The heart-derived myogenic cell line H9c2 in rat embryo was investigated in vitro to see if apigenin could counteract the effects of LPS. The LDH, IL-1β, IL-6, TNF-α, and CK-MB levels in serum were all reduced by apigenin. In addition, it was also observed in vitro study that apigenin inhibited SphK1/S1P signaling system, the MAPK pathway, and intracellular calcium passage. It also inhibited Bax cleaved caspase-3 and −9, and proteins in the heart. Apigenin exerted notable cardioprotective activity in rats subjected to LPS. By blocking the SphK1/S1P signaling pathway, the observed action was linked to the decrease of myocardial apoptosis and inflammation.[Citation94]
Apigenin protected myocardial against I/R injury by dramatically improving contractile functionality of rat heart, decreasing infarct size, apoptotic rate, and LDH release, upregulating expressions of caspase-3 and Bcl-2 and downregulating expression of cleaved caspase-3 after A/R. Furthermore, apigenin improved cell survival while decreasing LDH release, ROS generation, apoptosis, and mitochondrial cytochrome c flow into the cytosol following A/R in the culture of H9c2 cardiomyocytes. In addition, ABT-737 inhibited Bcl-2 activity, which dramatically decreased apigenin’s protective impact on A/R-induced myocardial damage.[Citation95] In experimental rats, triphenyltetrazolium chloride induces myocardial I/R injury and caused a significant increment in lactate dehydrogenase, creatine kinase, MDA level, inhibited the activity of SOD, enhanced the phosphorylation of p38 MAPK. On the other side, apigenin treatment to experimental subjects has been found to revert these changes.[Citation96] In myoblastic H9c2 cells, apigenin inhibited apoptosis and pyroptosis caused by I/H damage. Furthermore, pro-inflammatory cytokines (IL-1 and IL-18) were used to observe functions of apigenin in I/H-induced myocardial damage and found that treatment of I/H-induced H9C2 cells with apigenin significantly reduced a rise in levels of IL-8 and IL-1.[Citation97]
Antimicrobial
Apigenin showed antimicrobial activities against numerous pathogens, including Enterobacter aerogenes, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, and Salmonella typhimurium.[Citation98] The structure of cell membrane is disrupted by apigenin as this molecule change order and orientation of lipids in membrane, resulting in leakage of vesicles.[Citation99] According to several studies, flavonoids’ antibacterial activities mostly comprised inhibition in synthesis of nucleic acid, inhibition of cytoplasmic membrane function to impact permeability, biofilm growth, porins, and interactions with various important enzymes.[Citation100] Apigenin has been demonstrated to inhibit Escherichia coli DNA gyrase,[Citation101] and to prevent the production of E. coli biofilm.[Citation102] In a recent study, liposomal formulation of apigenin was explored. When apigenin liposomes interacted with the membranes of the studied bacteria, it increased its antibacterial activities, resulting in bacterial cell lysis. As findings were reviewed, liposomal apigenin was shown to be considerably more effective against both Gram +ve and Gram -ve bacteria, such as E. coli (MIC = 16 g/mL), P. aeruginosa (MIC = 64 g/mL), S. aureus (MIC = 8 g/mL), and B. subtilis (MIC = 4 g/mL).[Citation103]
Neuroprotective
Alzheimer’s disease (AD) and Parkinson’s disease (PD) are associated with progressive nerve cells deterioration affecting a large portion of the cerebral cortex and the cognitive ability. Apigenin has been shown to reduce the production of neurotoxic agents such as ROS and accompanying inflammatory cascades.[Citation104] AD is the most common neurodegenerative disease (ND) as existing therapeutic measures are insufficient owing to the disease’s complicated etiology. ND are defined by the permanent loss of nerve cells. In case of familial and sporadic AD induced in human pluripotent stem cells (iPSCs) model, apigenin exhibits strong anti-inflammatory effects, with the potential to increase cell viability and preservation of neurites and by inducing a downregulation in the production of cytokines and NO. Furthermore, apigenin can preserve iPSC-derived AD neurons in several ways, including substantially decrease in caspase-3/7-mediated death and lowering the spontaneous Ca2+ signals frequency.[Citation105,Citation106] The peripheral nerve degeneration is also protected by apigenin by inhibition of four important phenotypes such as myelin fragmentation, trans-dedifferentiation, axonal deterioration, and Schwann cell proliferation via Krox20- and extracellular signal-regulated kinase-independent mechanisms.[Citation107] The apigenin (117, 234, and 351/kg) pretreatment intragastrically improves the aberrant neuronal morphology generated by ACN and lowers the autonomic activity in rat models with acrylonitrile gavage (46 mg/kg) was given to enhance oxidative damages and to induce inflammation-related disease, such as AD. Additionally, apigenin may lower levels of oxidative stress, TNF-, and IL-6, downregulate the TLR4/NF-B signaling pathway, and block mitochondria-mediated neuron death.[Citation108] Apigenin prevented A burden in an AD model by downregulating BACE1 and -CTF levels, relieving A deposition, and decreasing insoluble A levels. Apigenin also boosted the antioxidant enzymes activity including superoxide dismutase and glutathione peroxidase and hindered superoxide anion scavenging. Additionally, apigenin stimulated the cerebral cortex’s neurotrophic ERK/CREB/BDNF pathway.[Citation109]
Apigenin inhibited the microglial activation, as seen by modification in morphology of microglia (Iba-1 + cells) and suppression of proliferation (BrdU+ cells), as well as lowered the M1 inflammatory marker CD68 expression. Furthermore, inflammatory stimuli generated by IL-1 decreased the mRNA expression of IL-10 while enhanced the mRNA expression of CCL5, IL-1, and IL-6, as revealed by RT-qPCR. In contrast, decrease in expression of gp130, IL-6, and OX42, as well as modification in inflammatory cytokine mRNA expression was observed after treatment with apigenin in inflammatory stimuli (IL-1 or LPS). Furthermore, apigenin improved the production of brain-derived neurotrophic factor (BDNF) alone or in response to IL-1 stimulated inflammation a result that have direct association with neuroprotection and anti-inflammatory responses.[Citation110]
It was reported in a study that apigenin has the potential to preserve neurons and the integrity of astrocytes by β-tubulin III and GFAP, accordingly. Findings from the same study demonstrated that the flavonoid was non-neurotoxic and exhibited neuroprotective activity against inflammatory damage. Taken together, results from the study showed that apigenin represents a promising neuroimmunomodulator agent for managing neurodegenerative complications.[Citation110] Although research suggests that apigenin may have protective properties that might be interrelated with reduction of toxicity caused by amyloid-peptidepeptides (A) although, the mechanism behind this action remains unknown. In an AD cell model, apigenin’s in vitro neuroprotective effect was linked to mental equilibrium and amyloid toxicity and its possible signal transduction was investigated. Apigenin keeps neurons in protection against copper-induced A-mediated toxicity, which was characterized by lessened neuronal nuclear condensation, increased neuronal survival, and reduced mitochondrial membrane dissipation. However, it was shown that apigenin could not decrease expression of β-amyloid precursor protein (AβPP) and could not lower the secretion of Aβ (1–42). Apigenin substantially improved spatial working memory in AD patients. In the hilus region, it also substantially decreased the amount of degenerative neurons. Interestingly, apigenin completely inhibited the release of caspase 9 and cytochrome c in hilus.[Citation111] Apigenin (3, 6, and 12 M) pretreatment to rat adrenal pheochromocytoma cells (PC12 cells) caused significant elevation in mitochondrial membrane potential, increase in cell viability, decrease in the level of ROS and inhibition in lactate dehydrogenase release. Additionally, it significantly prevented the PC12 cells’ MPP (+) induced high rate of apoptosis and decreased Bcl 2 to Bax ratio.[Citation112] In rotenone-induced PD, apigenin (10 and 20 mg/kg) decreased expression of NF-B gene and safeguarded substantia nigra pars compacta (SNpc) region from neuroinflammation. It also reduced the activity of pro-inflammatory enzyme iNOS-1, which was stimulated by ROT and production of proinflammatory cytokines IL-6 and TNF-. Furthermore, in ROT-lesioned rats, apigenin reduced the downregulation in mRNA expression of neurotrophic factors GDNF and BDNF. Apigenin also increased expression of dopamine D2 receptor (D2R) and TH protein while reduced synuclein aggregation in ROT-lesioned rats.[Citation113] Probenecid and MPTP (250 mg/kg and 25 mg/kg, respectively) were given to mice to induce parkinsonism. Apigenin was given orally to mice for 5 days as pretreatment and continue the therapy for 26 days. The results showed that apigenin treatment ameliorated the muscular and locomotor activities in mice treated with MPTP. It is assumed that the protective effect exerted by apigenin on dopaminergic neurons was probably due to the reduced microglial activation, neuroinflammation, and oxidative damage, and due to an enhanced neurotrophic potential.[Citation114]
Anti-inflammatory role
Numerous investigations have discovered that the activation of an inflammatory response involves both MAPKs and NF-B signaling pathways.[Citation115,Citation116] Thus, a promising target for inflammatory therapy has been identified based on blocking the LPS-stimulated signal transduction cascades. The results of in vivo experiment reported that apigenin inhibited LPS-induced inflammation by deactivating NF-B and dephosphorylating Ser536 in the p65 subunit. Moreover, In human THP-1 macrophages, apigenin greatly reduced NF-B activation and LPS-induced ERK1/2.[Citation117]
Apigenin exhibited anti-inflammatory activity in human periodontal ligament (hPDL) cells that are stimulated by LPS and nicotine. Apigenin (10–40 M) retarded the production and activity of HO-1 proteins produced by LPS and nicotine. Additionally, the substance significantly decreased the production of IL-1 β, IL-6, IL-12, TNF-α, prostaglandin E2, and nitric oxide, as well as COX-2 and iNOS and in hPDL cells when nicotine and LPS were present. Hemin, a selective inducer of HO-1, eradicated suppression of LPS- and nicotine-induced cytokine production, PGE2, and NO mediated by apigenin. In cells exposed to nicotine and LPS, various inhibitors including JNK, MAPKs, p38, and phosphoinositide 3-kinase as well as a protein kinase C inhibitor prevented the anti-inflammatory effects when treated with apigenin.[Citation118] To assess the effectiveness of apigenin and underlying mechanism, mouse J774A.1 macrophage cells and human THP-1-induced macrophages were used to determine LPS-induced inflammatory response. Data revealed that apigenin has a pivotal role in inhibition of LPS-induced proinflammatory cytokines production, namely TNF-α, IL-1β, and IL-6, via regulating several intracellular signaling pathways in macrophage cells.[Citation119]
Like other flavonoids, apigenin inhibits TNF-α by either reducing its synthesis or impairing its activity. The chronic inflammatory disorders often appear due to activation of NF-B by malfunctioning or overproduction of TNF. This is accomplished by ligating the transmembrane TNF- receptor, which activates the IĸBα kinase via stimulating cellular pathways.[Citation120] Additionally, the decrease in TNF-gene expression helps to partially explain how apigenin acts as an anti-inflammatory. The NOS and COX-2 genes are inactivated as a result of inhibition of NF-ĸB activity, that is critically essential for cell-cycle arrest and the suppression of inflammation.[Citation121] Conclusively, apigenin has a vital role to inhibit inflammatory processes by targeting and downregulating certain factors particularly NF-ĸB and TNF-α.[Citation122–124]
Reproductive role
In a study, the cryoprotective effect of apigenin on boar sperm during cryopreservation was examined. These sperms are highly sensitive to oxidative stress and to conserve sperm quality, suitable antioxidants are required. Apigenin is a highly efficient antioxidant and results from the study showed that supplementing the freezer extenders with apigenin demonstrated a positive effect on frozen-thawed boar sperm.[Citation125] In male Sprague-Dawley rats, apigenin exerted a protective effect against sub-chronic sperm and testis injury induced by acrylonitrile (ACN). Apigenin enhanced mitochondrial membrane potential (MMP), sperm concentration and motility, but ACN decreased these parameters. Apigenin, on the other hand, significantly decreased ROS and malondialdehyde (MDA). This flavonoid also reduced ACN-induced damage to the ultrastructure of sperm. Apigenin inhibited ACN-induced pathological damage and spermatogenic cell death in rat testes. Additionally, apigenin decreased MDA level while enhanced glutathione peroxidase activity. Finally, apigenin decreases ACN-induced sperm quality by inhibiting oxidative stress and inflammation.[Citation126,Citation127] Dang and colleagues determined the preventive role of apigenin against psoriasis through several pathways, namely, reduction in IL-1β, IL-6, and TNF-α expression, enhancement of sorbitol dehydrogenase and lactate dehydrogenase isozymes, reduction in ROS development, suppression of phosphorylation of the inhibitory κBα, upregulation of cleaved caspase-3, downregulation of NF-κB levels, and induction of apoptosis.[Citation126]
Respiratory role
Apigenin (20 mg/kg/day) treatment prevented asthmatic mice from developing AHR when exposed to PM 2.5 and elicited a mixed IL-17/T helper (Th) 2 cell response. In ovalbumin (OVA)-sensitized and PM2.5-exposed mice, the treatment significantly reduced AHR, the proportion of neutrophil, and eosinophils infiltration in the lung tissues and bronchoalveolar lavage fluid (BALF). In addition, IL-4, IL-13 (Th2-related cytokines) and IL-17 (Th17-related cytokines) and total serum immunoglobulin E (IgE) in BALF, all dropped significantly. In the asthmatic mice, apigenin therapy also lowered the quantity of NF-B p65 expression in lung tissues.[Citation128]
Apigenin administered orally at the dosage of 10, 15, and 20 mg/kg/day prevented the fibrotic process in rat lung tissues. These therapies may stop the rise in TNF-* and TGF-* levels, MPO activity, hydroxyproline content, and decrease SOD activity caused by bleomycin. Additionally, apigenin therapy reduced excessive collagen deposition.[Citation129] Apigenin greatly suppressed immune activity in the lung both in vitro and in vivo experimentation using LPS-induced NO production in RAW264.7 macrophages and acute lung injury in mice respectively. It also showed anti-proliferative actions via the inhibition of NF-κB and MAPK pathways.[Citation130] When compared to the ovalbumin group, apigenin reduced the airway hyperresponsiveness, total IgE levels and infiltration ability of inflammatory cells. Additionally, immune response due to allergens is shifted toward Th1 by apigenin.[Citation131] In OVA-induced asthma model mice, apigenin reduced infiltration of inflammatory cells into the airways and blood vessels surrounding the lungs, airways luminal constriction, eosinophils count in BALF, and the development of airway hyperresponsiveness.[Citation132] In OVA-induced asthma model of BALB/c mice, a higher dose of apigenin (20 mg/kg) significantly decreased eosinophil percentage in BALF, cell count, airway inflammation, airway hyperreactivity, serum IgE concentrations and IL-13, and IL-4 levels in BALF and GATA-3 protein expression in lung tissue.[Citation133]
Depression and insomnia
A study showed that apigenin injected in rats could reduce locomotor activity without hampering anticonvulsant, myorelaxant, or anxiolytic activities. The inhibitory role of apigenin on the locomotor behavior of rats cannot be attributed to the interaction between the flavonoid and GABA(A)-benzodiazepine receptor as failure in blockage with Ro 15–1788 was observed which proposed that it may interact with other neurotransmission systems.[Citation134] Weng et al. studied depressed mice to examine the effect of apigenin. They found that apigenin administration in corticosterone-treated mice could regulate BDNF and serum corticosterone levels. The study confirmed the anti-depressive effect of apigenin and the mechanism behind these effects is partly upregulation of BDNF levels in the cerebral cortex.[Citation135] Nakazawa et al. confirmed the anti-depressive effect of apigenin in mice by observing alterations in dopamine and norepinephrine turnover in the hypothalamus and amygdala.[Citation136] Han et al. evaluated apigenin effectiveness to inhibit monoamine oxidase (MAO). Apigenin dynamically inhibited MAO-A (IC50 values of 1.7 µM) and MAO-B (IC50 values of 12.8 µM).[Citation137] Chaurasiya et al. revealed that the MAO-A inhibition by apigenin was 1.7-fold more selective than MAO-B. In an isolated rat atria model, apigenin enhanced noradrenalin activity and simultaneously inhibited MAO activity.[Citation138] The effects of apigenin on the platelet adenylyl cyclase activity, hypothalamic – pituitary–adrenal (HPA) axis, and monoaminergic neurotransmitter system was studied by Yi et al., using stressed mice. The results showed that the serum concentration was regulated and the platelet adenylyl cyclase activity was depressed in the rat models. They suggested that the observed activity could be due to a combined biochemical effect.[Citation139] Li and coworkers developed LPS-induced rat models to determine apigenin potential to reduce depressive-like behavior. Tested animals were pretreated with apigenin for 7 days with dosage of 25 or 50 mg/kg/day. Apigenin decrease the production of proinflammatory cytokines (TNF-α and IL-1β) and impeded the LPS-induced unusual behavior. Interestingly, they also noted that apigenin inhibited the production of COX-2 and iNOS. Apigenin (50 mg/kg) efficiently restored depressive behavior induced by TNF with no adverse effects on locomotor activities. In LPS-treated mice, apigenin with anti-inflammatory qualities had antidepressant-like effects.[Citation140] Insomnia is a common sleep condition that can have serious consequences for a person’s well-being and health.[Citation141] The preliminary efficacy and safety of apigenin was evaluated in chronic insomnia patients by obtaining extract from chamomile flower that contained more than 2.5 mg of apigenin and evaluated for its effectiveness in improving sleep and daytime symptoms. Thirty-four primary insomnia patients were enrolled (aged between 18 to 65 years) by Zick et al..[Citation142] No significant differences were observed in different groups in terms of sleep efficiency, sleep quality, total sleep time, wakefulness following the onset of sleep, sleep latency, or several awakenings. The utilization of apigenin extract caused modest betterment in daytime functioning and sleep parameters.
Skin diseases
A study reported that apigenin stimulated nucleotide excision repair genes, which subsequently results in the protection of skin keratinocytes from ultraviolet B (UVB) and genetic mutations.[Citation143] Choi et al. reported that a topical cream containing apigenin can effectively cause an increment in dermal density, ameliorate skin elasticity, maintain skin tone evenness, regulate trans-epidermal loss of water, minimize the formation of fine lines and wrinkles, and stability in moisture contents. It was also well established norm that apigenin can become a potential anti-aging molecule for cosmetic industries.[Citation144] The study of Tong et al. revealed that the flavonoid possessed thrombospondin-1 restoration ability in skin keratinocytes exposed to UVB and renormalize cell growth and synthesize more blood vessels in skin highly exposed to UVB. The findings gathered in the study suggested that apigenin can control UVB-induced carcinogenesis.[Citation145] summarizes the health perspectives with basic mechanisms of action of the bioactive compound apigenin in inflammatory-related diseases.
Table 1. Health perspectives and basic mechanisms of action of apigenin.
Conclusion
The current review presented a detailed break-down on the main medicinal propensities of apigenin, a common component in human diets, on inflammatory-related diseases. The promising bioactive plant component, apigenin, has being researched for possible health advantages. Its impact on the immune system, sleep, anxiety, testosterone production, blood sugar levels, and cancer are just a few of these possible advantages. According to research on rheumatoid arthritis, apigenin reduced activity of collagenase and stopped producing lipopolysaccharide-induced nitric oxide in RAW 264.7 macrophage cells dose-dependently. It was shown that apigenin can successfully influence redox homeostasis, and showed potential to regulate autophagy which may be responsible for its anti-inflammatory effects. According to recent studies, flavonoids can reduce oxidative stress and inflammatory cytokines, which can help to prevent cardiac injury brought on by endotoxins. Several accounts have supported the fact that apigenin can improve an effective and novel treatment against myocardial injury. Interestingly, experimental data revealed the positive effect of apigenin on depression and insomnia by upregulating brain-derived neurotrophic factor levels. Apigenin also has ability to manage respiratory problems caused by inflammation. In this review, it was observed that apigenin has garnered a fair amount of researchers’ attention toward its anticancer properties as evidenced by the large number of publications. Several other inflammatory-related diseases in which apigenin could play a fundamental role in their treatment strategies are cutaneous problems, obesity, diabetes, infections, reproductive, hepatoprotective, and neurodegenerative complications. Apigenin has the ability to impede or delay the onset of numerous chronic diseases. The potent inhibitory effect of that bioactive compound on inflammatory responses demonstrated its usefulness as a promising functional food or an integrative medicine for treating oxidative and/or inflammatory related diseases.
Acknowledgement
The authors are thankful to the Government College University Faisalabad Pakistan, University of Mauritius, Réduit 80837, Mauritius and Agricultural Extension Directorate, MAAR, Damascus, Syria for providing library facilities for data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bhagwat, S.; Haytowitz, D. B.; Holden, J. M. USDA Database for the Flavonoid Content of Selected Foods Release 3.1. 2014. https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav (Accessed 10 January 2021).
- Lee, W.-J.; Chen, W.-K.; Wang, C.-J.; Lin, W.-L.; Tseng, T.-H. Apigenin Inhibits HGF-Promoted Invasive Growth and Metastasis Involving Blocking PI3K/Akt Pathway and β4 Integrin Function in MDA-MB-231 Breast Cancer Cells. Toxicol. Appl. Pharmacol. 2008, 226(2), 178–191. DOI: 10.1016/j.taap.2007.09.013.
- Mullie, P.; Clarys, P.; Deriemaeker, P.; Hebbelinck, M. Estimation of Daily Human Intake of Food Flavonoids. Plant Foods Hum. Nutr. 2007, 62(3), 93–98. DOI: 10.1007/s11130-007-0047-7.
- Villagómez-Rodríguez, A.; Pérez-Ramos, J.; Esquivel-Campos, A. L.; Pérez-González, C.; Soto-Peredo, C. A.; Pérez-Gutiérrez, S. Anti-Inflammatory Activity of Jefea Gnaphalioides (A. Gray), Astereaceae. BMC Compl. Alternative Med. 2019, 19(1), 1–8. DOI: 10.1186/s12906-019-2654-x.
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget. 2018, 9(6), 7204. DOI: 10.18632/oncotarget.23208.
- Capelari-Oliveira, P.; Paula, C. A. D.; Rezende, S. A.; Campos, F. T.; Grabe-Guimarães, A.; Lombardi, J. A.; Saúde-Guimarães, D. Anti-Inflammatory Activity of Lychnophora Passerina, Asteraceae (Brazilian “Arnica”). J. Ethnopharmacol. 2011, 135(2), 393–398. DOI: 10.1016/j.jep.2011.03.034.
- Edwards, J. C.; Cambridge, G. B-Cell Targeting in Rheumatoid Arthritis and Other Autoimmune Diseases. Nat. Rev. Immunol. 2006, 6(5), 394–403. DOI: 10.1038/nri1838.
- Kim, H. K.; Cheon, B. S.; Kim, Y. H.; Kim, S. Y.; Kim, H. P. Effects of Naturally Occurring Flavonoids on Nitric Oxide Production in the Macrophage Cell Line RAW 264.7 and Their Structure–Activity Relationships. Biochem. Pharmacol. 1999, 58(5), 759–765. DOI: 10.1016/S0006-2952(99)00160-4.
- Liang, Y.-C.; Huang, Y.-T.; Tsai, S.-H.; Lin-Shiau, S.-Y.; Chen, C.-F.; Lin, J.-K. Suppression of Inducible Cyclooxygenase and Inducible Nitric Oxide Synthase by Apigenin and Related Flavonoids in Mouse Macrophages. Carcinogenesis. 1999, 20(10), 1945–1952. DOI: 10.1093/carcin/20.10.1945.
- Surh, Y.-J.; Chun, K.-S.; Cha, H.-H.; Han, S. S.; Keum, Y.-S.; Park, K.-K.; Lee, S. S. Molecular Mechanisms Underlying Chemopreventive Activities of Anti-Inflammatory Phytochemicals: Down-Regulation of COX-2 and iNOS Through Suppression of NF-Κb Activation. Mutat. Res. 2001, 480, 243–268. DOI: 10.1016/S0027-5107(01)00183-X.
- Bhattacharya, S.; Mondal, L.; Mukherjee, B.; Dutta, L.; Ehsan, I.; Debnath, M. C.; Gaonkar, R. H.; Pal, M. M.; Majumdar, S. Apigenin Loaded Nanoparticle Delayed Development of Hepatocellular Carcinoma in Rats. Nanomed. NBM. 2018, 14(6), 1905–1917. DOI: 10.1016/j.nano.2018.05.011.
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi‐Rad, J. Apigenin as an Anticancer Agent. Phytother. Res. 2020, 34(8), 1812–1828. DOI: 10.1002/ptr.6647.
- Salmani, J. M. M.; Zhang, X.-P.; Jacob, J. A.; Bao-An, C. Apigenin’s Anticancer Properties and Molecular Mechanisms of Action: Recent Advances and Future Prospectives. Chin. J. Nat. Med. 2017, 15(5), 321–329. DOI: 10.1016/S1875-5364(17)30052-3.
- Sharma, A.; Ghani, A.; Sak, K.; Tuli, H. S.; Sharma, A. K.; Setzer, W. N.; Sharma, S.; Das, A. K. Probing into Therapeutic Anti-Cancer Potential of Apigenin: Recent Trends and Future Directions. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13(2), 124–133. DOI: 10.2174/1872213X13666190816160240.
- Singh, D.; Khan, M. A.; Siddique, H. R. Apigenin, a Plant Flavone Playing Noble Roles in Cancer Prevention via Modulation of Key Cell Signaling Networks. Recent Pat. Anticancer Drug Discov. 2019, 14(4), 298–311. DOI: 10.2174/1574892814666191026095728.
- Şirin, N.; Elmas, L.; Seçme, M.; Dodurga, Y. Investigation of Possible Effects of Apigenin, Sorafenib and Combined Applications on Apoptosis and Cell Cycle in Hepatocellular Cancer Cells. Gene. 2020, 737, 144428. DOI: 10.1016/j.gene.2020.144428.
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E. B.; Novellino, E., et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20(6), 1305. DOI: 10.3390/ijms20061305.
- Meyer, H.; Bolarinwa, A.; Wolfram, G.; Linseisen, J. Bioavailability of Apigenin from Apiin-Rich Parsley in Humans. Ann. Nutr. Metab. 2006, 50(3), 167–172. DOI: 10.1159/000090736.
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic Properties and Drug Interactions of Apigenin, a Natural Flavone. Expert. Opin. Drug. Metab Toxicol. 2017, 13(3), 323–330. DOI: 10.1080/17425255.2017.1251903.
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed Res. Int. 2019, 2019, 1–18. DOI: 10.1155/2019/7010467.
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant Flavone Apigenin: An Emerging Anticancer Agent. Curr. Pharmacol. Rep. 2017, 3(6), 423–446. DOI: 10.1007/s40495-017-0113-2.
- Thomas, S. D.; Jha, N. K.; Jha, S. K.; Sadek, B.; Ojha, S. Pharmacological and Molecular Insight on the Cardioprotective Role of Apigenin. Nutrients. 2023, 15(2), 385. DOI: 10.3390/nu15020385.
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and Cancer Chemoprevention: Progress, Potential and Promise. Int. J. Oncol. 2007, 30(1), 233–245. DOI: 10.3892/ijo.30.1.233.
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F. J.; Queipo-Ortuño, M. I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr Biochem. 2013, 24(8), 1415–1422. DOI: 10.1016/j.jnutbio.2013.05.001.
- Chen, T.; Li, L.-P.; Lu, X.-Y.; Jiang, H.-D.; Zeng, S. Absorption and Excretion of Luteolin and Apigenin in Rats After Oral Administration of Chrysanthemum Morifolium Extract. J. Agric. Food. Chem. 2007, 55(2), 273–277. DOI: 10.1021/jf062088r.
- Gradolatto, A.; Basly, J.-P.; Berges, R.; Teyssier, C.; Chagnon, M.-C.; Siess, M.-H.; Canivenc-Lavier, M.-C. Pharmacokinetics and Metabolism of Apigenin in Female and Male Rats After a Single Oral Administration. Drug Metab. Dispos. 2005, 33(1), 49–54. DOI: 10.1124/dmd.104.000893.
- Andlauer, W.; Kolb, J.; Fürst, P. Absorption and Metabolism of Genistin in the Isolated Rat Small Intestine. FEBS. Lett. 2000, 475(2), 127–130. DOI: 10.1016/S0014-5793(00)01642-2.
- Kool, M.; de Haas, M.; Scheffer, G. L.; Scheper, R. J.; van Eijk, M. J.; Juijn, J. A.; Baas, F.; Borst, P. Analysis of Expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, Homologues of the Multidrug Resistance-Associated Protein Gene (MRP1), in Human Cancer Cell Lines. Cancer. Res. 1997, 57(16), 3537–3547.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79(5), 727–747. DOI: 10.1093/ajcn/79.5.727.
- Ng, S. P.; Wong, K. Y.; Zhang, L.; Zuo, Z.; Lin, G. Evaluation of the First-Pass Glucuronidation of Selected Flavones in Gut by Caco-2 Monolayer Model. J. Pharm. Pharm. Sci. 2004, 8(1), 1–9.
- Chen, Z.; Kong, S.; Song, F.; Li, L.; Jiang, H. Pharmacokinetic Study of Luteolin, Apigenin, Chrysoeriol and Diosmetin After Oral Administration of Flos Chrysanthemi Extract in Rats. Fitoterapia. 2012, 83(8), 1616–1622. DOI: 10.1016/j.fitote.2012.09.011.
- Hao, X.; Cheng, G.; Yu, J.; He, Y.; An, F.; Sun, J.; Cui, F. Study on the Role of Hepatic First-Pass Elimination in the Low Oral Bioavailability of Scutellarin in Rats. Pharmazie. 2005, 60(6), 477–478.
- Cai, H.; Boocock, D. J.; Steward, W. P.; Gescher, A. J. Tissue Distribution in Mice and Metabolism in Murine and Human Liver of Apigenin and Tricin, Flavones with Putative Cancer Chemopreventive Properties. Cancer Chemother. Pharmacol. 2007, 60(2), 257–266. DOI: 10.1007/s00280-006-0368-5.
- Das, S.; Rosazza, J. P. Microbial and Enzymatic Transformations of Flavonoids. J. Nat. Prod. 2006, 69(3), 499–508. DOI: 10.1021/np0504659.
- Wang, S. W.; Kulkarni, K. H.; Tang, L.; Wang, J. R.; Yin, T.; Daidoji, T.; Yokota, H.; Hu, M. Disposition of Flavonoids via Enteric Recycling: UDP-Glucuronosyltransferase (UGT) 1As Deficiency in Gunn Rats is Compensated by Increases in UGT2Bs Activities. J. Pharmacol. Exp. Ther. 2009, 329(3), 1023–1031. DOI: 10.1124/jpet.108.147371.
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130(8), 2073S–2085S. DOI: 10.1093/jn/130.8.2073S.
- Gradolatto, A.; Canivenc-Lavier, M.-C.; Basly, J.-P.; Siess, M.-H.; Teyssier, C. Metabolism of Apigenin by Rat Liver Phase I and Phase II Enzymes and by Isolated Perfused Rat Liver. Drug Metab. Dispos. 2004, 32(1), 58–65. DOI: 10.1124/dmd.32.1.58.
- Nielsen, S.; Young, J.; Daneshvar, B.; Lauridsen, S.; Knuthsen, P.; Sandström, B.; Dragsted, L. O. Effect of Parsley (Petroselinum Crispum) Intake on Urinary Apigenin Excretion, Blood Antioxidant Enzymes and Biomarkers for Oxidative Stress in Human Subjects. Br. J. Nutr. 1999, 81(6), 447–455. DOI: 10.1017/S000711459900080X.
- Bauer, D.; Mazzio, E.; Soliman, K. F. Whole Transcriptomic Analysis of Apigenin on TNFα Immuno-Activated MDA-MB-231 Breast Cancer Cells. Cancer Genom. Proteom. 2019, 16(6), 421–431. DOI: 10.21873/cgp.20146.
- Hamadou, M. H.; Kerkatou, M.; Gatto, P.; Pancher, M.; Bisio, A.; Inga, A.; Menad, A.; Benayache, S.; Benayache, F.; Ameddah, S. Apigenin Rich-Limonium Duriusculum (de Girard) Kuntze Promotes Apoptosis in HCT116 Cancer Cells. Nat. Prod. Res. 2019, 35(17), 1–5. DOI: 10.1080/14786419.2019.1672070.
- Qiu, J.-G.; Wang, L.; Liu, W.-J.; Wang, J.-F.; Zhao, E.-J.; Zhou, F.-M.; Ji, X.-B.; Wang, L.-H.; Xia, Z.-K.; Wang, W. Apigenin Inhibits IL-6 Transcription and Suppresses Esophageal Carcinogenesis. Front. Pharmacol. 2019, 10, 1002. DOI: 10.3389/fphar.2019.01002.
- Coelho, P. L.; Amparo, J. A.; da Silva, A. B.; da Silva, K. C.; Braga‐de‐Souza, S.; Barbosa, P. R.; Lopes, G. P. D. F.; Costa, S. L. Apigenin from Croton Betulaster Müll Restores the Immune Profile of Microglia Against Glioma Cells. Phytother. Res. 2019, 33(12), 3191–3202. DOI: 10.1002/ptr.6491.
- Zohreh, B.; Masoumeh, V.; Fakhraddin, N.; Omrani, G. H. Apigenin-Mediated Alterations in Viability and Senescence of SW480 Colorectal Cancer Cells Persist in the Presence of L-Thyroxine. Anti Cancer Agents Med. Chem. 2019, 19(12), 1535–1542. DOI: 10.2174/1871520619666190704102708.
- Lee, H. H.; Jung, J.; Moon, A.; Kang, H.; Cho, H. Antitumor and Anti-Invasive Effect of Apigenin on Human Breast Carcinoma Through Suppression of IL-6 Expression. Int. J. Mol. Sci. 2019, 20(13), 3143. DOI: 10.3390/ijms20133143.
- Chien, M.-H.; Lin, Y.-W.; Wen, Y.-C.; Yang, Y.-C.; Hsiao, M.; Chang, J.-L.; Huang, H.-C.; Lee, W.-J. Targeting the SPOCK1-Snail/slug Axis-Mediated Epithelial-To-Mesenchymal Transition by Apigenin Contributes to Repression of Prostate Cancer Metastasis. J. Exp. Clin. Cancer Res. 2019, 38(1), 1–17. DOI: 10.1186/s13046-019-1247-3.
- Chen, X.; Xu, H.; Yu, X.; Wang, X.; Zhu, X.; Xu, X. Apigenin Inhibits in vitro and in vivo Tumorigenesis in Cisplatin-Resistant Colon Cancer Cells by Inducing Autophagy, Programmed Cell Death and Targeting M-TOR/PI3K/Akt Signalling Pathway. J. Buon. 2019, 24(2), 488–493.
- Gilardini Montani, M. S.; Cecere, N.; Granato, M.; Romeo, M. A.; Falcinelli, L.; Ciciarelli, U.; D’orazi, G.; Faggioni, A.; Cirone, M. Mutant p53, Stabilized by Its Interplay with HSP90, Activates a Positive Feed-Back Loop Between NRF2 and p62 That Induces Chemo-Resistance to Apigenin in Pancreatic Cancer Cells. Cancers. 2019, 11(5), 703. DOI: 10.3390/cancers11050703.
- Ittiudomrak, T.; Puthong, S.; Roytrakul, S.; Chanchao, C. α-Mangostin and Apigenin Induced Cell Cycle Arrest and Programmed Cell Death in SKOV-3 Ovarian Cancer Cells. Toxicol. Res. 2019, 35(2), 167–179. DOI: 10.5487/TR.2019.35.2.167.
- Chen, Z.; Tian, D.; Liao, X.; Zhang, Y.; Xiao, J.; Chen, W.; Liu, Q.; Chen, Y.; Li, D.; Zhu, L. Apigenin Combined with Gefitinib Blocks Autophagy Flux and Induces Apoptotic Cell Death Through Inhibition of HIF-1α, C-Myc, P-EGFR, and Glucose Metabolism in EGFR L858R+ T790M-Mutated H1975 Cells. Front. Pharmacol. 2019, 10, 260. DOI: 10.3389/fphar.2019.00260.
- Tong, J.; Shen, Y.; Zhang, Z.; Hu, Y.; Zhang, X.; Han, L. Apigenin Inhibits Epithelial-Mesenchymal Transition of Human Colon Cancer Cells Through NF-Κb/snail Signaling Pathway. Biosci. Rep. 2019, 39(5), 5. DOI: 10.1042/BSR20190452.
- Qi, Y.; Ding, Z.; Yao, Y.; Ma, D.; Ren, F.; Yang, H.; Chen, A. Novel Triazole Analogs of Apigenin‑7‑Methyl Ether Exhibit Potent Antitumor Activity Against Ovarian Carcinoma Cells via the Induction of Mitochondrial‑Mediated Apoptosis. Exp. Ther. Med. 2019, 17(3), 1670–1676. DOI: 10.3892/etm.2018.7138.
- Li, Y.-W.; Xu, J.; Zhu, G.-Y.; Huang, Z.-J.; Lu, Y.; Li, X.-Q.; Wang, N.; Zhang, F.-X. Apigenin Suppresses the Stem Cell-Like Properties of Triple-Negative Breast Cancer Cells by Inhibiting YAP/TAZ Activity. Cell. Death Discov. 2018, 4(1), 1–9. DOI: 10.1038/s41420-018-0124-8.
- Xu, L.; Zhang, Y.; Tian, K.; Chen, X.; Zhang, R.; Mu, X.; Wu, Y.; Wang, D.; Wang, S.; Liu, F. Apigenin Suppresses PD-L1 Expression in Melanoma and Host Dendritic Cells to Elicit Synergistic Therapeutic Effects. J. Exp. Clin. Cancer Res. 2018, 37(1), 1–15. DOI: 10.1186/s13046-018-0929-6.
- Ozbey, U.; Attar, R.; Romero, M. A.; Alhewairini, S. S.; Afshar, B.; Sabitaliyevich, U. Y.; Hanna‐Wakim, L.; Ozcelik, B.; Farooqi, A. A. Apigenin as an Effective Anticancer Natural Product: Spotlight on TRAIL, WNT/β‐Catenin, JAK‐STAT Pathways, and microRnas. J. Cell. Biochem. 2019, 120(2), 1060–1067. DOI: 10.1002/jcb.27575.
- Yang, M.; Jiang, Z.-H.; Li, C.-G.; Zhu, Y.-J.; Li, Z.; Tang, Y.-Z.; Ni, C.-L. Apigenin Prevents Metabolic Syndrome in High-Fructose Diet-Fed Mice by Keap1-Nrf2 Pathway. Biomed. Pharmacother. 2018, 105, 1283–1290. DOI: 10.1016/j.biopha.2018.06.108.
- Malik, S.; Suchal, K.; Khan, S. I.; Bhatia, J.; Kishore, K.; Dinda, A. K.; Arya, D. S. Apigenin Ameliorates Streptozotocin-Induced Diabetic Nephropathy in Rats via MAPK-NF-κB-TNF-α and TGF-β1-MAPK-Fibronectin Pathways. Am. J. Physiol. Renal Physiol. 2017, 313(2), F414–F422. DOI: 10.1152/ajprenal.00393.2016.
- Zeng, L.; Zhang, G.; Lin, S.; Gong, D. Inhibitory Mechanism of Apigenin on α-Glucosidase and Synergy Analysis of Flavonoids. J. Agric. Food. Chem. 2016, 64(37), 6939–6949. DOI: 10.1021/acs.jafc.6b02314.
- Jung, U. J.; Cho, Y.-Y.; Choi, M.-S. Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice. Nutrients. 2016, 8(5), 305. DOI: 10.3390/nu8050305.
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and Naringenin Regulate Glucose and Lipid Metabolism, and Ameliorate Vascular Dysfunction in Type 2 Diabetic Rats. Eur. J. Pharmacol. 2016, 773, 13–23. DOI: 10.1016/j.ejphar.2016.01.002.
- Cazarolli, L. H.; Folador, P.; Moresco, H. H.; Brighente, I. M. C.; Pizzolatti, M. G.; Silva, F. R. M. B. Stimulatory Effect of Apigenin-6-C-β-L-Fucopyranoside on Insulin Secretion and Glycogen Synthesis. Eur. J. Med. Chem. 2009, 44(11), 4668–4673. DOI: 10.1016/j.ejmech.2009.07.001.
- Panda, S.; Kar, A. Apigenin (4 ‘, 5, 7‐Trihydroxyflavone) Regulates Hyperglycaemia, Thyroid Dysfunction and Lipid Peroxidation in Alloxan‐Induced Diabetic Mice. J. Pharm. Pharmacol. 2007, 59(11), 1543–1548. DOI: 10.1211/jpp.59.11.0012.
- Su, T.; Huang, C.; Yang, C.; Jiang, T.; Su, J.; Chen, M.; Fatima, S.; Gong, R.; Hu, X.; Bian, Z. Apigenin Inhibits STAT3/CD36 Signaling Axis and Reduces Visceral Obesity. Pharmacol. Res. 2020, 152, 104586. DOI: 10.1016/j.phrs.2019.104586.
- Okla, M.; Al Madani, J. O.; Chung, S.; Alfayez, M. Apigenin Reverses Interleukin‐1β‐Induced Suppression of Adipocyte Browning via COX2/PGE2 Signaling Pathway in Human Adipocytes. Mol. Nutr Food Res. 2020, 64(1), 1900925. DOI: 10.1002/mnfr.201900925.
- Gomez-Zorita, S.; Lasa, A.; Abendaño, N.; Fernandez-Quintela, A.; Mosqueda-Solís, A.; Garcia-Sobreviela, M. P.; Arbonés-Mainar, J. M.; Portillo, M. P. Phenolic Compounds Apigenin, Hesperidin and Kaempferol Reduce in vitro Lipid Accumulation in Human Adipocytes. J. Transl. Med. 2017, 15(1), 1–10. DOI: 10.1186/s12967-017-1343-0.
- Choi, W. H.; Son, H. J.; Jang, Y. J.; Ahn, J.; Jung, C. H.; Ha, T. Y. Apigenin Ameliorates the Obesity‐Induced Skeletal Muscle Atrophy by Attenuating Mitochondrial Dysfunction in the Muscle of Obese Mice. Mol. Nutr Food Res. 2017, 61(12), 1700218. DOI: 10.1002/mnfr.201700218.
- Kim, M.-A.; Kang, K.; Lee, H.-J.; Kim, M.; Kim, C. Y.; Nho, C. W. Apigenin Isolated from Daphne Genkwa Siebold Et Zucc. Inhibits 3T3-L1 Preadipocyte Differentiation Through a Modulation of Mitotic Clonal Expansion. Life. sci. 2014, 101(1–2), 64–72. DOI: 10.1016/j.lfs.2014.02.012.
- Lv, Y.; Gao, X.; Shen, W.; Fan, T.; Yao, C.; Ding, M.; Yan, S.; Song, L.; Yan, L. Apigenin Ameliorates HFD-Induced NAFLD Through Regulation of the XO/NLRP3 Pathways. J. Nutr Biochem. 2019, 71, 110–121. DOI: 10.1016/j.jnutbio.2019.05.015.
- Moharram, F. A.; El Dib, R. A. E. M.; Marzouk, M. S.; El-Shenawy, S. M.; Ibrahim, H. A. New Apigenin Glycoside, Polyphenolic Constituents, Anti-Inflammatory and Hepatoprotective Activities of Gaillardia Grandiflora and Gaillardia pulchella Aerial Parts. Phcog. Mag. 2017, 13(Suppl 2), S244. DOI: 10.4103/pm.pm_344_16.
- Yue, S.; Xue, N.; Li, H.; Huang, B.; Chen, Z.; Wang, X. Hepatoprotective Effect of Apigenin Against Liver Injury via the Non-Canonical NF-Κb Pathway in vivo and in vitro. Inflammation. 2020, 43(5), 1634–1648. DOI: 10.1007/s10753-020-01238-5.
- Rašković, A.; Gigov, S.; Čapo, I.; Kusturica, M. P.; Milijašević, B.; Kojić-Damjanov, S.; Martić, N. Antioxidative and Protective Actions of Apigenin in a Paracetamol-Induced Hepatotoxicity Rat Model. Eur. J. Drug Metab. Pharmacokinet. 2017, 42(5), 849–856. DOI: 10.1007/s13318-017-0407-0.
- Ali, F.; Jyoti, F.; Naz, S.; Siddique, Y. H.; Siddique, Y. H. Protective Effect of Apigenin Against N-Nitrosodiethylamine (NDEA)-Induced Hepatotoxicity in Albino Rats. Mutat. Res. 2014, 767, 13–20. DOI: 10.1016/j.mrgentox.2014.04.006.
- Mohamed, W. R.; Kotb, A. S.; Abd El‐Raouf, O. M.; Mohammad Fikry, E. Apigenin Alleviated Acetaminophen‐Induced Hepatotoxicity in Low Protein‐Fed Rats: Targeting Oxidative Stress, STAT3, and Apoptosis Signals. J. Biochem. Mol. Toxicol. 2020, 34(5), e22472. DOI: 10.1002/jbt.22472.
- Tsaroucha, A. K.; Tsiaousidou, A.; Ouzounidis, N.; Tsalkidou, E.; Lambropoulou, M.; Giakoustidis, D.; Chatzaki, E.; Simopoulos, C. Intraperitoneal Administration of Apigenin in Liver Ischemia/Reperfusion Injury Protective Effects. Saudi J. Gastroenterol. 2016, 22(6), 415. DOI: 10.4103/1319-3767.195556.
- Zhou, R.-J.; Ye, H.; Wang, F.; Wang, J.-L.; Xie, M.-L. Apigenin Inhibits D-Galactosamine/lps-Induced Liver Injury Through Upregulation of Hepatic Nrf-2 and PPARγ Expressions in Mice. Biochem. Biophys. Res. Commun. 2017, 493(1), 625–630. DOI: 10.1016/j.bbrc.2017.08.141.
- Wang, F.; Liu, J.-C.; Zhou, R.-J.; Zhao, X.; Liu, M.; Ye, H.; Xie, M.-L. Apigenin Protects Against Alcohol-Induced Liver Injury in Mice by Regulating Hepatic CYP2E1-Mediated Oxidative Stress and PPARα-Mediated Lipogenic Gene Expression. Chem. Biol. Interact. 2017, 275, 171–177. DOI: 10.1016/j.cbi.2017.08.006.
- Feng, X.; Yu, W.; Li, X.; Zhou, F.; Zhang, W.; Shen, Q.; Li, J.; Zhang, C.; Shen, P. Apigenin, a Modulator of PPARγ, Attenuates HFD-Induced NAFLD by Regulating Hepatocyte Lipid Metabolism and Oxidative Stress via Nrf2 Activation. Biochem. Pharmacol. 2017, 136, 136–149. DOI: 10.1016/j.bcp.2017.04.014.
- Shi, T.; Zhuang, R.; Zhou, H.; Wang, F.; Shao, Y.; Cai, Z. Effect of Apigenin on Protein Expressions of PPARs in Liver Tissues of Rats with Nonalcoholic Steatohepatitis. Zhonghua. gan zang bing za zhi. 2015, 23(2), 124–129. DOI: 10.3760/cma.j.issn.1007-3418.2015.02.010.
- Zhong, Y.; Jin, C.; Wang, X.; Li, X.; Han, J.; Xue, W.; Wu, P.; Peng, X.; Xia, X. Protective Effects of Apigenin Against 3-MCPD-Induced Renal Injury in Rat. Chem. Biol. Interact. 2018, 296, 9–17. DOI: 10.1016/j.cbi.2018.08.005.
- Tsalkidou, E. G.; Tsaroucha, A. K.; Chatzaki, E.; Lambropoulou, M.; Papachristou, F.; Trypsianis, G.; Pitiakoudis, M.; Vaos, G.; Simopoulos, C. The Effects of Apigenin on the Expression of Fas/FasL Apoptotic Pathway in Warm Liver Ischemia-Reperfusion Injury in Rats. Biomed Res. Int. 2014, 2014, 1–7. DOI: 10.1155/2014/157216.
- Zhao, L.; Zhang, J.; Hu, C.; Wang, T.; Lu, J.; Wu, C.; Chen, L.; Jin, M.; Ji, G.; Cao, Q. Apigenin Prevents Acetaminophen-Induced Liver Injury by Activating the SIRT1 Pathway. Front. Pharmacol. 2020, 11, 11. DOI: 10.3389/fphar.2020.00514.
- Zare, M. F. R.; Rakhshan, K.; Aboutaleb, N.; Nikbakht, F.; Naderi, N.; Bakhshesh, M.; Azizi, Y. Apigenin Attenuates Doxorubicin Induced Cardiotoxicity via Reducing Oxidative Stress and Apoptosis in Male Rats. Life. sci. 2019, 232, 116623. DOI: 10.1016/j.lfs.2019.116623.
- Li, F.; Lang, F.; Zhang, H.; Xu, L.; Wang, Y.; Zhai, C.; Hao, E. Apigenin Alleviates Endotoxin-Induced Myocardial Toxicity by Modulating Inflammation, Oxidative Stress, and Autophagy. OXID. MED. CELL LONGEV. 2017, 2017, 1–10. DOI: 10.1155/2017/2302896.
- Liu, H.-J.; Fan, Y.-L.; Liao, H.-H.; Liu, Y.; Chen, S.; Ma, Z.-G.; Zhang, N.; Yang, Z.; Deng, W.; Tang, Q.-Z. Apigenin Alleviates STZ-Induced Diabetic Cardiomyopathy. Mol. Cell. Biochem. 2017, 428(1–2), 9–21. DOI: 10.1007/s11010-016-2913-9.
- Ren, K.; Jiang, T.; Zhou, H.-F.; Liang, Y.; Zhao, G.-J. Apigenin Retards Atherogenesis by Promoting ABCA1-Mediated Cholesterol Efflux and Suppressing Inflammation. Cell. Physiol. Biochem. 2018, 47(5), 2170–2184. DOI: 10.1159/000491528.
- Zhou, Z.; Zhang, Y.; Lin, L.; Zhou, J. Apigenin Suppresses the Apoptosis of H9C2 Rat Cardiomyocytes Subjected to Myocardial Ischemia‑Reperfusion Injury via Upregulation of the PI3K/Akt Pathway. Mol. Med. Rep. 2018, 18(2), 1560–1570. DOI: 10.3892/mmr.2018.9115.
- Gutiérrez-Venegas, G.; González-Rosas, Z. Apigenin Reduce Lipoteichoic Acid-Induced Inflammatory Response in Rat Cardiomyoblast Cells. Arch. Pharm. Res. 2017, 40(2), 240–249. DOI: 10.1007/s12272-016-0756-2.
- Mahajan, U. B.; Chandrayan, G.; Patil, C. R.; Arya, D. S.; Suchal, K.; Agrawal, Y. O.; Ojha, S.; Goyal, S. N. The Protective Effect of Apigenin on Myocardial Injury in Diabetic Rats Mediating Activation of the PPAR-γ Pathway. Int. J. Mol. Sci. 2017, 18(4), 756. DOI: 10.3390/ijms18040756.
- Feng, Y.; Lu, Y.; Liu, D.; Zhang, W.; Liu, J.; Tang, H.; Zhu, Y. Apigenin-7-O-β-D-(-6 ″-P-Coumaroyl)-Glucopyranoside Pretreatment Attenuates Myocardial Ischemia/Reperfusion Injury via Activating AMPK Signaling. Life. sci. 2018, 203, 246–254. DOI: 10.1016/j.lfs.2018.04.048.
- Zeng, P.; Liu, B.; Wang, Q.; Fan, Q.; Diao, J.-X.; Tang, J.; Fu, X.-Q.; Sun, X.-G. Apigenin Attenuates Atherogenesis Through Inducing Macrophage Apoptosis via Inhibition of AKT Ser473 Phosphorylation and Downregulation of Plasminogen Activator Inhibitor-2. OXID. MED. CELL LONGEV. 2015, 2015, 1–12. DOI: 10.1155/2015/379538.
- Wang, P.; Sun, J.; Lv, S.; Xie, T.; Wang, X. Apigenin Alleviates Myocardial Reperfusion Injury in Rats by Downregulating MiR-15b. Med. Sci. Monit. 2019, 25, 2764. DOI: 10.12659/MSM.912014.
- He, Y.; Fang, X.; Shi, J.; Li, X.; Xie, M.; Liu, X. Apigenin Attenuates Pulmonary Hypertension by Inducing Mitochondria-Dependent Apoptosis of PASMCs via Inhibiting the Hypoxia Inducible Factor 1α–KV1. 5 Channel Pathway. Chem. Biol. Interact. 2020, 317, 108942. DOI: 10.1016/j.cbi.2020.108942.
- Jing, Y.; Chen, R.; Dong, M.; Liu, Y.; Hou, X.; Guo, P.; Li, W.; Lv, J.; Zhang, M. Apigenin Relaxes Rat Intrarenal Arteries, Depresses Ca2±activated Cl− Currents and Augments Voltage-Dependent K+ Currents of the Arterial Smooth Muscle Cells. Biomed. Pharmacother. 2019, 115, 108926. DOI: 10.1016/j.biopha.2019.108926.
- Yao, H.; Jia, Y.; Xue, Z.; Guo, M.; Lyu, J. Effects of Apigenin on Lipopolysaccharide Induced Proliferation of Rat Aortic Vascular Smooth Muscle Cells. Zhonghua. Xin Xue Guan Bing Za Zhi. 2017, 45(4), 323–328. DOI: 10.3760/cma.j.issn.0253-3758.2017.04.013.
- Zhang, T.; Yan, T.; Du, J.; Wang, S.; Yang, H. Apigenin Attenuates Heart Injury in Lipopolysaccharide-Induced Endotoxemic Model by Suppressing Sphingosine Kinase 1/Sphingosine 1-Phosphate Signaling Pathway. Chem. Biol. Interact. 2015, 233, 46–55. DOI: 10.1016/j.cbi.2014.12.021.
- Chen, C.; He, H.; Luo, Y.; Zhou, M.; Yin, D.; He, M. Involvement of Bcl-2 Signal Pathway in the Protective Effects of Apigenin on Anoxia/reoxygenation-Induced Myocardium Injury. J. Cardiovasc. Pharmacol. 2016, 67(2), 152–163. DOI: 10.1097/FJC.0000000000000331.
- Yang, X.; Yang, J.; Hu, J.; Li, X.; Zhang, X.; Li, Z. Apigenin Attenuates Myocardial Ischemia/Reperfusion Injury via the Inactivation of p38 Mitogen‑Activated Protein Kinase. Mol. Med. Rep. 2015, 12(5), 6873–6878. DOI: 10.3892/mmr.2015.4293.
- Li, W.; Chen, L.; Xiao, Y. Apigenin Protects Against Ischemia-/hypoxia-Induced Myocardial Injury by Mediating Pyroptosis and Apoptosis. Vitro Cell. Dev. Biol. Animal. 2020, 56(4), 307–312. DOI: 10.1007/s11626-020-00434-9.
- Nayaka, H. B.; Londonkar, R. L.; Umesh, M. K.; Tukappa, A. Antibacterial Attributes of Apigenin, Isolated from Portulaca oleracea L. Int. J. Bacteriol. 2014, 2014, 175851. DOI: 10.1155/2014/175851.
- Ollila, F.; Halling, K.; Vuorela, P.; Vuorela, H.; Slotte, J. P. Characterization of Flavonoid–Biomembrane Interactions. Arch. Biochem. Biophys. 2002, 399(1), 103–108. DOI: 10.1006/abbi.2001.2759.
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18(1), 241–272. DOI: 10.1007/s11101-018-9591-z.
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2015, 22(1), 132–149. DOI: 10.2174/0929867321666140916113443.
- Lee, J.-H.; Regmi, S. C.; Kim, J.-A.; Cho, M. H.; Yun, H.; Lee, C.-S.; Lee, J. Apple Flavonoid Phloretin Inhibits Escherichia coli O157: H7 Biofilm Formation and Ameliorates Colon Inflammation in Rats. Infect. Immun. 2011, 79(12), 4819–4827. DOI: 10.1128/IAI.05580-11.
- Banerjee, K.; Banerjee, S.; Das, S.; Mandal, M. Probing the Potential of Apigenin Liposomes in Enhancing Bacterial Membrane Perturbation and Integrity Loss. J. Colloid. Interface. Sci. 2015, 453, 48–59. DOI: 10.1016/j.jcis.2015.04.030.
- Nabavi, S. F.; Khan, H.; D’onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A. R.; Argüelles, S.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as Neuroprotective Agent: Of Mice and Men. Pharmacol. Res. 2018, 128, 359–365. DOI: 10.1016/j.phrs.2017.10.008.
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S. S.; Lum, J. S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M. Neuroprotective Effects of Apigenin Against Inflammation, Neuronal Excitability and Apoptosis in an Induced Pluripotent Stem Cell Model of Alzheimer’s Disease. Sci. Rep. 2016, 6(1), 1–16. DOI: 10.1038/srep31450.
- Sang, Z.; Wang, K.; Shi, J.; Cheng, X.; Zhu, G.; Wei, R.; Ma, Q.; Yu, L.; Zhao, Y.; Tan, Z. Apigenin-Rivastigmine Hybrids as Multi-Target-Directed Liagnds for the Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2020, 187, 111958. DOI: 10.1016/j.ejmech.2019.111958.
- Kim, M.; Jung, J.; Jeong, N. Y.; Chung, H.-J. The Natural Plant Flavonoid Apigenin is a Strong Antioxidant That Effectively Delays Peripheral Neurodegenerative Processes. Anat. Sci. Int. 2019, 94(4), 285–294. DOI: 10.1007/s12565-019-00486-2.
- Zhao, F.; Dang, Y.; Zhang, R.; Jing, G.; Liang, W.; Xie, L.; Li, Z. Apigenin Attenuates Acrylonitrile-Induced Neuro-Inflammation in Rats: Involved of Inactivation of the TLR4/NF-Κb Signaling Pathway. Int. Immunopharmacol. 2019, 75, 105697. DOI: 10.1016/j.intimp.2019.105697.
- Zhao, L.; Wang, J.-L.; Liu, R.; Li, X.-X.; Li, J.-F.; Zhang, L. Neuroprotective, Anti-Amyloidogenic and Neurotrophic Effects of Apigenin in an Alzheimer’s Disease Mouse Model. Molecules. 2013, 18(8), 9949–9965. DOI: 10.3390/molecules18089949.
- Dourado, N. S.; dos Santos Souza, C.; de Almeida, M. M. A.; da Silva, A. B.; Dos Santos, B. L.; Silva, V. D. A.; De Assis, A. M.; da Silva, J. S.; Souza, D. O.; Costa, M. D. F. D. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated with Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 12. DOI: 10.3389/fnagi.2020.00119.
- Nikbakht, F.; Khadem, Y.; Haghani, S.; Hoseininia, H.; Sadat, A. M.; Heshemi, P.; Jamali, N. Protective Role of Apigenin Against Aβ25-35 Toxicity via Inhibition of Mitochondrial Cytochrome C Release. Basic Clin. Neurosci. J. 2019, 10(6), 557. DOI: 10.32598/bcn.9.10.385.
- Liu, W.; Kong, S.; Xie, Q.; Su, J.; Li, W.; Guo, H.; Li, S.; Feng, X.; Su, Z.; Xu, Y., et al. Protective Effects of Apigenin Against 1-Methyl-4-Phenylpyridinium Ion‑Induced Neurotoxicity in PC12 Cells. Int. J. Mol. Med. 2015, 35(3), 739–746. DOI: 10.3892/ijmm.2014.2056.
- Anusha, C.; Sumathi, T.; Joseph, L. D. Protective Role of Apigenin on Rotenone Induced Rat Model of Parkinson’s Disease: Suppression of Neuroinflammation and Oxidative Stress Mediated Apoptosis. Chem. Biol. Interact. 2017, 269, 67–79. DOI: 10.1016/j.cbi.2017.03.016.
- Patil, S. P.; Jain, P. D.; Sancheti, J. S.; Ghumatkar, P. J.; Tambe, R.; Sathaye, S. RETRACTED: Neuroprotective and Neurotrophic Effects of Apigenin and Luteolin in MPTP Induced Parkinsonism in Mice. Neuropharmacology. 2014, 86, 192–202. DOI: 10.1016/j.neuropharm.2014.07.012.
- Izzi, V.; Masuelli, L.; Tresoldi, I.; Sacchetti, P.; Modesti, A.; Galvano, F.; Bei, R. The Effects of Dietary Flavonoids on the Regulation of Redox Inflammatory Networks. Front. Biosci. 2012, 17(7), 2396–2418. DOI: 10.2741/4061.
- Kim, S.; Joo, Y.-E. Theaflavin Inhibits LPS-Induced IL-6, MCP-1, and ICAM-1 Expression in Bone Marrow-Derived Macrophages Through the Blockade of NF-Κb and MAPK Signaling Pathways. Chonnam Med. J. 2011, 47(2), 104. DOI: 10.4068/cmj.2011.47.2.104.
- Nicholas, C.; Batra, S.; Vargo, M. A.; Voss, O. H.; Gavrilin, M. A.; Wewers, M. D.; Guttridge, D. C.; Grotewold, E.; Doseff, A. I. Apigenin Blocks Lipopolysaccharide-Induced Lethality in vivo and Proinflammatory Cytokines Expression by Inactivating NF-Κb Through the Suppression of p65 Phosphorylation. J. Immunol. 2007, 179(10), 7121–7127. DOI: 10.4049/jimmunol.179.10.7121.
- Jeong, G.-S.; Lee, S.-H.; Jeong, S.-N.; Kim, Y.-C.; Kim, E.-C. Anti-Inflammatory Effects of Apigenin on Nicotine-And Lipopolysaccharide-Stimulated Human Periodontal Ligament Cells via Heme Oxygenase-1. Int. Immunopharmacol. 2009, 9(12), 1374–1380. DOI: 10.1016/j.intimp.2009.08.015.
- Zhang, X.; Wang, G.; Gurley, E. C.; Zhou, H. Flavonoid Apigenin Inhibits Lipopolysaccharide-Induced Inflammatory Response Through Multiple Mechanisms in Macrophages. Plos One. 2014, 9(9), e107072. DOI: 10.1371/journal.pone.0107072.
- Aggarwal, B. B.; Vijayalekshmi, R.; Sung, B. Targeting Inflammatory Pathways for Prevention and Therapy of Cancer: Short-Term Friend, Long-Term Foe. Clin. Cancer Res. 2009, 15(2), 425–430. DOI: 10.1158/1078-0432.CCR-08-0149.
- Woo, E.-R.; Pokharel, Y. R.; Yang, J. W.; Lee, S. Y.; Kang, K. W. Inhibition of Nuclear Factor-Κb Activation by 2′, 8 ″-Biapigenin. Biol. Pharm. Bull. 2006, 29(5), 976–980. DOI: 10.1248/bpb.29.976.
- Kowalski, J.; Samojedny, A.; Paul, M.; Pietsz, G.; Wilczok, T. Effect of Apigenin, Kaempferol and Resveratrol on the Expression of Interleukin-1beta and Tumor Necrosis Factor-Alpha Genes in J774. 2 Macrophages. Pharmacol. Rep. 2005, 57(3), 390–394.
- Seo, H.-S.; Sikder, M. A.; Lee, H. J.; Ryu, J.; Lee, C. J. Apigenin Inhibits Tumor Necrosis Factor-α-Induced Production and Gene Expression of Mucin Through Regulating Nuclear Factor-Kappa B Signaling Pathway in Airway Epithelial Cells. Biomol. Ther. 2014, 22(6), 525. DOI: 10.4062/biomolther.2014.094.
- Xu, C.; Shen, G.; Chen, C.; Gélinas, C.; Kong, A. N. Suppression of NF-kappaB and NF-kappaB-Regulated Gene Expression by Sulforaphane and PEITC Through IkappaBalpha, IKK Pathway in Human Prostate Cancer PC-3 Cells. Oncogene. 2005, 24(28), 4486–4495. DOI: 10.1038/sj.onc.1208656.
- Pei, Y.; Yang, L.; Wu, L.; He, H.; Geng, G.; Xu, D.; Chen, H.; Li, Q. Combined Effect of Apigenin and Ferulic Acid on Frozen‐Thawed Boar Sperm Quality. Anim. Sci. J. 2018, 89(7), 956–965. DOI: 10.1111/asj.13009.
- Dang, Y.; Li, Z.; Luo, B.; Pan, L.; Wei, Q.; Zhang, Y. Protective Effects of Apigenin Against Acrylonitrile-Induced Subchronic Sperm Injury in Rats. Food. Chem. Toxicol. 2017, 109, 517–525. DOI: 10.1016/j.fct.2017.09.025.
- Shi, X.; Liu, S.; Chen, Y.; Li, F.; Xue, H.; Dang, Y.; Li, Z. Apigenin Affects Semen Parameters in Male Mice. Zhonghua. nan ke xue. 2010, 16(9), 778–782.
- Pang, L.; Zou, S.; Shi, Y.; Mao, Q.; Chen, Y. Apigenin Attenuates PM2. 5-Induced Airway Hyperresponsiveness and Inflammation by Down-Regulating NF-Κb in Murine Model of Asthma. Int. J. Clin. Exp. Pathol. 2019, 12(10)), 3700.
- Chen, L.; Zhao, W. Apigenin Protects Against Bleomycin‑Induced Lung Fibrosis in Rats. Exp. Ther. Med. 2016, 11(1), 230–234. DOI: 10.3892/etm.2015.2885.
- Li, K.-C.; Ho, Y.-L.; Hsieh, W.-T.; Huang, S.-S.; Chang, Y.-S.; Huang, G.-J. Apigenin-7-Glycoside Prevents LPS-Induced Acute Lung Injury via Downregulation of Oxidative Enzyme Expression and Protein Activation Through Inhibition of MAPK Phosphorylation. Int. J. Mol. Sci. 2015, 16(1), 1736–1754. DOI: 10.3390/ijms16011736.
- Li, R.-R.; Pang, L.-L.; Du, Q.; Shi, Y.; Dai, W.-J.; Yin, K.-S. Apigenin Inhibits Allergen-Induced Airway Inflammation and Switches Immune Response in a Murine Model of Asthma. Immunopharmacol. Immunotoxicol. 2010, 32(3), 364–370. DOI: 10.3109/08923970903420566.
- Choi, J.-R.; Lee, C.-M.; Jung, I. D.; Lee, J. S.; Jeong, Y.-I.; Chang, J. H.; Park, H.-J.; Choi, I.-W.; Kim, J.-S.; Shin, Y. K., et al. Apigenin Protects Ovalbumin-Induced Asthma Through the Regulation of GATA-3 Gene. Int. Immunopharmacol. 2009, 9(7–8), 918–924. DOI: 10.1016/j.intimp.2009.03.018.
- Pang, L.; Li, R.; Zhou, L. Inhibitory Effects of Apigenin on the Expression of GATA-3 and Th2 Cytokines in Asthmatic Mice. Zhongguo. Zhong Xi Yi Jie He Za Zhi. 2010, 30(4), 383–387.
- Avallone, R.; Zanoli, P.; Puia, G.; Kleinschnitz, M.; Schreier, P.; Baraldi, M. Pharmacological Profile of Apigenin, a Flavonoid Isolated from Matricaria Chamomilla. Biochem. Pharmacol. 2000, 59(11), 1387–1394. DOI: 10.1016/S0006-2952(00)00264-1.
- Weng, L.; Guo, X.; Li, Y.; Yang, X.; Han, Y. Apigenin Reverses Depression-Like Behavior Induced by Chronic Corticosterone Treatment in Mice. Eur. J. Pharmacol. 2016, 774, 50–54. DOI: 10.1016/j.ejphar.2016.01.015.
- Nakazawa, T.; Yasuda, T.; Ueda, J.; Ohsawa, K. Antidepressant-Like Effects of Apigenin and 2, 4, 5-Trimethoxycinnamic Acid from Perilla Frutescens in the Forced Swimming Test. Biol. Pharm. Bull. 2003, 26(4), 474–480. DOI: 10.1248/bpb.26.474.
- Han, X. H.; Hong, S. S.; Hwang, J. S.; Lee, M. K.; Hwang, B. Y.; Ro, J. S. Monoamine Oxidase Inhibitory Components from Cayratia Japonica. Arch. Pharm. Res. 2007, 30(1), 13–17. DOI: 10.1007/BF02977772.
- Chaurasiya, N. D.; Ibrahim, M. A.; Muhammad, I.; Walker, L. A.; Tekwani, B. L. Monoamine Oxidase Inhibitory Constituents of Propolis: Kinetics and Mechanism of Inhibition of Recombinant Human MAO-A and MAO-B. Molecules. 2014, 19(11), 18936–18952. DOI: 10.3390/molecules191118936.
- Yi, L.-T.; Li, J.-M.; Li, Y.-C.; Pan, Y.; Xu, Q.; Kong, L.-D. Antidepressant-Like Behavioral and Neurochemical Effects of the Citrus-Associated Chemical Apigenin. Life. sci. 2008, 82(13–14), 741–751. DOI: 10.1016/j.lfs.2008.01.007.
- Li, R.; Zhao, D.; Qu, R.; Fu, Q.; Ma, S. The Effects of Apigenin on Lipopolysaccharide-Induced Depressive-Like Behavior in Mice. Neurosci. Lett. 2015, 594, 17–22. DOI: 10.1016/j.neulet.2015.03.040.
- Leach, M. J.; Page, A. T. Herbal Medicine for Insomnia: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2015, 24, 1–12. DOI: 10.1016/j.smrv.2014.12.003.
- Zick, S. M.; Wright, B. D.; Sen, A.; Arnedt, J. T. Preliminary Examination of the Efficacy and Safety of a Standardized Chamomile Extract for Chronic Primary Insomnia: A Randomized Placebo-Controlled Pilot Study. BMC Compl. Alternative Med. 2011, 11(1), 1–8. DOI: 10.1186/1472-6882-11-78.
- Das, S.; Das, J.; Paul, A.; Samadder, A.; Khuda-Bukhsh, A. R. Apigenin, a Bioactive Flavonoid from Lycopodium Clavatum, Stimulates Nucleotide Excision Repair Genes to Protect Skin Keratinocytes from Ultraviolet B-Induced Reactive Oxygen Species and DNA Damage. J. Acupunct. Meridian Stud. 2013, 6(5), 252–262. DOI: 10.1016/j.jams.2013.07.002.
- Choi, S.; Youn, J.; Kim, K.; Joo, D. H.; Shin, S.; Lee, J.; Lee, H. K.; An, I.-S.; Kwon, S.; Youn, H. J. Apigenin Inhibits UVA-Induced Cytotoxicity in vitro and Prevents Signs of Skin Aging in vivo. Int. J. Mol. Med. 2016, 38(2), 627–634. DOI: 10.3892/ijmm.2016.2626.
- Tong, X.; Mirzoeva, S.; Veliceasa, D.; Bridgeman, B. B.; Fitchev, P.; Cornwell, M. L.; Crawford, S. E.; Pelling, J. C.; Volpert, O. V. Chemopreventive Apigenin Controls UVB-Induced Cutaneous Proliferation and Angiogenesis Through HuR and Thrombospondin-1. Oncotarget. 2014, 5(22), 11413. DOI: 10.18632/oncotarget.2551.
- Sun, Y.-S.; Qu, W. Dietary Apigenin Promotes Lipid Catabolism, Thermogenesis, and Browning in Adipose Tissues of HFD-Fed Mice. Food. Chem. Toxicol. 2019, 133, 110780. DOI: 10.1016/j.fct.2019.110780.