ABSTRACT
Mycobiota are inseparable from human health, shaking up the unique position held by bacteria among microorganisms. What is surprising is that this seemingly small species can trigger huge changes in the human body. Dysbiosis and invasion of mycobiota are confirmed to cause disease in different parts of the body. Meanwhile, our body also produces corresponding immune changes upon mycobiota infection. Several recent studies have made a connection between intestinal mycobiota and the human immune system. In this review, we focus on questions related to mycobiota, starting with an introduction of select species, then we summarize the typical diseases caused by mycobiota in different parts of the human body. Moreover, we constructed a framework for the human anti-fungal immune system based on genetics and immunology. Finally, the progression of fungal detection methods is also reviewed.
Introduction
The mycobiota, a general designation of fungal species, evolved from the branch of a single-celled marine organism and then underwent further evolution consistent with that of animals, the common ancestor is the organism with a single flagellumCitation1, which indicated the close connection between two species. Mycobiota play a role in maintaining stability in both terrestrial and aquatic ecological environments, acting as a decomposer, pathogen, and mycorrhizae.Citation2
Research on human microorganisms has been extensively concentrated on bacterial species. However, the growing number of mycobiota-related research papers in recent years indicates an expansion in the field of the mycobiota. Accumulating evidences show that many diseases are inextricably linked to mycobiota.Citation3-Citation5 Meanwhile, numerous achievements have been made in the research of organism fungal immunityCitation6,Citation7 (). It is suggested that its latent role in human health is growing recognized. In particular, the latest researchesCitation8,Citation9 reveal the mechanism of intestinal mycobiota in colorectal carcinogenesis, which is of great significance for the diagnosis and treatment of colorectal tumor. Thus, overviewing mycobiota in the human body is necessary for further breakthroughs.
Figure 1. Time line of clinical progress of mycobiota and related diseases in various parts of the human body.
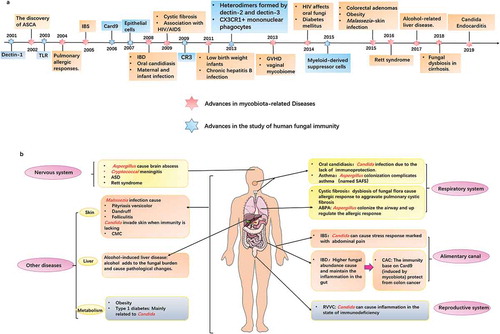
In this review, we systematically describe the colonization of mycobiota in healthy people and summarize their association with pathology and immunity. In addition to a comprehensive understanding of the relationship between mycobiota and disease, we also conclude methods for detecting mycobiota to provide guidance for relevant researchers.
Mycobiota at healthy state
Advanced detection techniques give us an opportunity to understand the colonization of mycobiota in the healthy human body. The numerical inferiority of the mycobiota have no effect on its irreplaceable role.Citation10,Citation11 The colonization of mycobiota in different body sites of a healthy human is characteristic, in general, Candida, Malassezia, Aspergillus, Epicoccum, Saccharomyces, Alternaria, and Cladosporium are common species.Citation7 As an essential part of the human microbiome, mycobiota are widely distributed in the healthy human body, mainly exist on the skin and nearly all the mucosal surfaces, such as the gastrointestinal tract,Citation12 oral cavity,Citation13 skin,Citation14 and vagina.Citation15 They interact with the host as commensalism and contribute greatly to the maintenance of healthy homeostasis in the human body, which depends on the host, environmental and fungal factors.Citation16
Mycobiota in the gastrointestinal tract(GI)
Using high-throughput sequencing to explore, we realized the abundance of the mycobiota in the intestine.Citation17,Citation18 There are nearly 70 genera and more than 184 species of mycobiota colonized in the human gut, with Candida, Saccharomyces, and Cladosporium species being major. Among the Candida species, Candida albicans(C.albicans), Candida glabrata, Candida dubliniensis, and Candida parapsilosis are the most common species in the gut. Mycobiota are at great risk of instability due to the influence of other factors in the gut. The antibiotic therapy can change the bacterial community, which in turn promoted the colonization of C.albicans in the GI of mice. Additionally, these mice were more likely to be attacked by Aspergillus fumigatus and developed the allergic response.Citation19 The stomach and gut are tasked with digestion and absorption of food, so that diet can change the composition of intestinal mycobiota to absorb nutrients better. It is significant to have abundant human diet habits and adapt to a new diet quickly.Citation20 Age and sex can also cause differences in the gastrointestinal fungal population.Citation21 The abundance of mycobiota in infantile gut is much higher than that of adults, it could be attributed to the weak bacterial competition. Due to the role of sex hormones, there are a larger number of mycobiota in the body of female compared to male subjects.
Many studies have discussed the protective benefits of commensal bacteria. Jiang and colleagues emphasized that intestinal mycobiota are also beneficial for human health.Citation22 Mycobiota can protect people from mucosal injury and reduce the risk of diseases such as colitis and influenza. The role of mycobiota such as C.albicans or Saccharomyces cerevisiae is shown during the bacterial depletion caused by the use of antibiotics, which suggests the substitution of mycobiota to bacteria.
Mycobiota in the oral cavity
The oral fungal community of healthy people is quite complex, Up to date, the groundbreaking research by Ghannoum and colleaguesCitation13 have shown that the number of species of mycobiota is considerable. The most common is Candida; therefore, Candida is commonly used as a standard for cavity cleaning in healthy individuals. Cladosporium, Aureobasidium, and Aspergillus also occur in the oral cavity. The difference is obvious in the colonization of mycobiota among individuals. Dupuy and colleagues made the supplement in 2014.Citation23 Their team firstly identifies the existence of Malassezia in the oral fungal community. Due to their large cell bodies, hyphae and other characteristics, mycobiota have an unexpected impact on oral health by the interaction of bacteria.Citation24 For example, C.albicans can cause dental caries couple with Streptococcus mutans or Streptococcus oralis.
Mycobiota in the respiratory tract
In the past, it was widely accepted that the respiratory tract in a healthy state was aseptic. With further researches, scientists found that there is a microbial community on the bronchus.Citation25 But the result only shown the colonization of bacteria. As the matter of fact, the fungal colonization in the bronchoalveolar lavage of healthy people is cut no figure.Citation26 The few fungal abundance in distrinct segments of the respiratory tract exists with significant differences, the part near the mouth are more affected by the environment factors,Citation27 such as Davidiellaceae, Aspergillus, and Cladosporium.
Mycobiota of the skin and vagina
Skin is the first part of human body to touch environmental microorganisms, with a tremendous community of bacteria and mycobiota.Citation14 Mycobiota mainly conclude Malassezia, Penicillium, and Aspergillus. Malassezia dominates skin mycobiota in different sites of the healthy human body. By contrast, the foot sites such as toenail and plantar heel shown the highest abundance of mycobiota, which suggested that the foot is easier to suffer a fungal infection. The reason is that a moist skin environment and rich protein provide favorable conditions for fungal colonization.Citation28 With the quantitative analysis, Malassezia represents 53% to 80% of total mycobiota on the human skinCitation29 and impact the health of our skin greatly. When the skin is damaged, the mycobiota fills the wound and slows down the healing.Citation30
Vaginal mycobiota are similar to those in the oral area; C. albicans, C. glabrata and C. krusei, three subtypes of Candida, are the major colonizing mycobiota.Citation31 Mycobiota are generally recognized as healthy microbes in the vagina, but C.albicans can colonize in the vagina without causing any symptoms and can be contagious.Citation32 Bacteria act as inhibitors against fungal invasion,Citation33 Lactobacilli is commensal bacteria in the female vagina and have the ability to prevent the adhesion of Candida.
Mycobiota and disease
Commensal mycobiota are beneficial for human health, but they are also opportunistic pathogens. Mycobiota cause human disease for two reasons, dysbiosis and infection. Here, we provide a summary of mycobiota-related diseases based on the parts of the human body ().
Mycobiota-related diseases in the gastrointestinal tract
Inflammatory bowel disease (IBD)
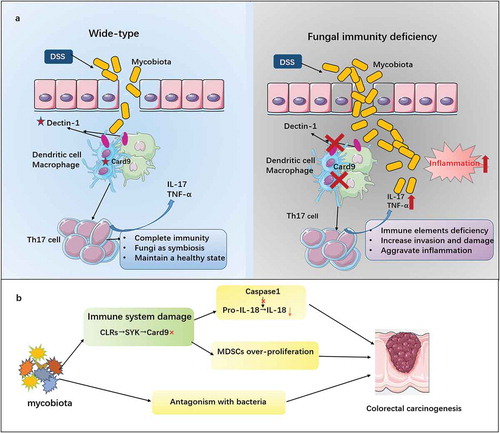
IBD contains two common forms, Crohn’s disease, and ulcerative colitis. The main characteristic pathological change, mucosal inflammation, is proved that associated with the decreased abundance of gastrointestinal microbiome.Citation34 With the accumulation of correlative researches, people have begun to realize the effect of mycobiota on IBD.Citation35 The detection and analysis showed more intuitively that the intestinal fungal abundance of patients with IBD altered greatly compared with healthy individuals,Citation36 with an increased Basidiomycota/Ascomycota ratio and incremental C.albicans. It is might because the absence of bacteria provide a more favorable environment for mycobiota. Additionally, the raising of Malassezia was also identified as the supplement.Citation37 As early as 1990, researchers used immunological methods to test patients with Crohn’s disease and an antibody against fungal cell wall components was found in their serum. The antibody is known as Anti-S. cerevisiae antibodies (ASCA).Citation38 Later, studies provide evidence that intestinal mycobiota trigger the production of systemic antibodies, such as IgG and IgA ASCA, could be used as serological markers of IBD to diagnose and predict the progression of the disease.Citation39-Citation41 Dectin-1 and Card9 play a key role in the antifungal immunity. Study by Iliev and colleaguesCitation42 showed that the deficiency of dectin-1 limited the antifungal immunity of dendritic cells and increased the colonization of C.tropicalis, which raised the susceptibility of induced colitis. Furthermore, the use of antifungals could allay the inflammation. The results supported powerfully that mycobiota involved in the process of IBD dynamically. However, mycobiota are only closely related to the occurrence and maintenance of inflammation, rather than a directly induced factor of IBD. The previous research of our group clearly identified that the stimulation of C. tropicalalis could lead to the lack of NF-kB signaling pathway, resulting in reduced secretion of IL-6 and thus affecting the repair of intestinal epithelial cells.Citation43 On the other hand, taking the genetic mechanism into consideration, the mutation of mycobiota-sensing related genes, Card9 and dectin-1, were associated with the increasing susceptibility of IBD.Citation44 ().
Table 1. Genetic polymorphisms related diseases.
Colorectal carcinogenesis
Crohn’s disease and ulcerative colitis can increase the risk of colorectal cancer,Citation70 which is collectively known as colitis-associated colorectal cancer (CAC). The involved mechanism concludes genes, inflammatory molecules, gastrointestinal tract microbiota and extracellular matrix. The effect of intestinal bacteria had been identified.Citation71 After that, researchers found five fungi phyla in the intestine of patients with adenomas, which shown the advantage of abundance compared with the two phyla from patients with IBD.Citation72 Moreover, they also identified that Fusarium genus of advanced adenomas was much richer than that of non-advanced subjects. It indicated that fungal colonization contribute the progression of adenoma. Subsequently, Gao and colleaguesCitation73 researched the mycobiota of polyp and CAC patients and complemented the contrast of healthy subjects. They verified the higher abundance of mycobiota in polyp and CAC patients with the comparsion of control group, characterized with the enrichment of Malassezia, Talaromyces, and Trametes. In addition, the difference of fungal structure in three groups was also shown in the result. Coker and colleaguesCitation74 creatively demonstrated that patients with colorectal cancer were characterized with the enhancive Basidiomycota:Ascomycota ratio, the changes in the abundance of Saccharomycetes, Pneumocystidomycetes, and Malasseziomycetes classes. Moreover, the antagonistic effects of mycobiota and bacteria in the intestine contribute to the development of colorectal tumors ().
How mycobiota affect the development of CAC? Two studies published on Immunity found a link between Card9 and the development of colorectal carcinogenesis, which marked the entry of the mycobiota into the stage of colon carcinogenesis. Malik and colleaguesCitation8 aimed at understanding the role of the Syk-Card9 signal axis in colorectal cancer. Learning from comparative experiments on wild-type and Card9-deficient mice, we known that the inflammatory reaction and the maturation of IL-18 induced by Syk-Card9 signaling provide strong support for the protective effects of mycobiota against colitis and colon cancer. Meanwhile, due to the phenomenon that Card9-deficient mice may take on a higher risk of colon cancer, Wang and colleaguesCitation9 provided evidence that the impaired anti-fungal immunity of Card9-deficient mice results in increased mycobiota, especially C.tropicalis, which increase the proliferation of myeloid-derived suppressor cells (MDSCs) and promote the risk of colon cancer. Similar results from two researches suggest that mycobiota induces a series of response to protect against colon cancer (). One interesting thing is that Bergmann and colleaguesCitation75 found a conclusion inconsistent with the above results. They proved that Card9 induces the production of IL-1β, thus exacerbating colon cancer. In this study, Card9-deficient mice and wild-type mice were co-housed for 3 weeks before the experiment, which weakens the influence of fungal factors on tumor development. In conclusion, fungal dysbiosis is closely linked to the deterioration of colitis to cancer, which reminds us that regulating the state of intestinal mycobiota can be a way to treat colorectal cancer.Citation76
Irritable bowel syndrome (IBS)
IBS has a great impact on human health. The connection with mycobiota was described when it was discovered that the yeast Candida can make allergic patients suffer from IBS. In recent years, increasing research has been conducted on this disease.Citation77 It is a type of disease that is characterized by abdominal pain caused by stress allergies. Botschuijve and colleaguesCitation78 provided further information, indicating that differences in mycobiota existed between patients with IBS and healthy people; in other words, fungal dysbiosis was related to IBS. The study suggests the mechanism of the disease: in the first phase, after the stress reaction, corticotropin releasing factor was activated, and then mast cells released histamine, which damaged the barrier function. In the second stage, mycobiota became pathogenic, and the body initiated the immune response depending on dectin-1/Syk, which is the main cause of abdominal pain. Meanwhile, barrier dysfunction and hypersensitivity continued, causing a vicious circle.
Mycobiota-related diseases in the respiratory system
Oropharyngeal candidiasis (OPC)
As mentioned, the dominant fungal species in the mouth is C.albicans, which causes OPC (also known as thrush) in the absence of immunity.Citation79 In the core oral mycobiome of Human Immunodeficiency Virus (HIV) patients and healthy individuals, the researcher identify the enrichment of Candida in HIV patients, leading to a higher risk of oral candidiasis caused by an opportunistic fungal infection.Citation80 Apart from Candida, Epicoccum, and Alternaria were also common genera in HIV-infected patients. The occurrence is based on the imbalance between fungal invasiveness and the immune reaction.Citation81 HIV blocks the function of antigen-presenting cells and CD4 + T cells. The resistance of oral mucosa is also destroyed. In addition, at the gene level, it was found that the transcription of SAPs in HIV-positive mice was higher than that in the negative control group. In this way, the starting point of prevention and treatment of oral candidiasis should be the enhancement of immunityCitation82,Citation83 and resistance to mycobiota. However, the abuse of antifungals could resulted in the drug-resistance of Candida.Citation84
Cystic fibrosis and asthma
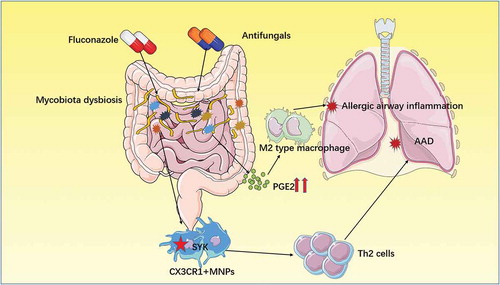
The lungs of healthy people have very little fungal colonization, which is affected by the environmental and oral mycobiota. When pathological changes occur in the lungs, such as cystic fibrosis mycobiota (mainly Aspergillus fumigatusCitation85), overpopulate due to a mutation of the transcriptional repressor Nrg1.Citation86 Notably, the fungal dysbiosis is insensitive to antibiotic therapyCitation87and can lead to allergic bronchopulmonary mycosis (ABPM) (Box1). This allergic reaction is a complication of cystic fibrosis, increasing the severity of the disease.Citation88 There are many serum markers that can be used to diagnose cystic fibrosis complicated with allergic diseases,Citation89,Citation90 such as thymus- and activation-regulated chemokine, CD203c, IgE, and so on. Studies have shown that vitamin D3 can be used to prevent allergic airway disease in cystic fibrosis, hindering further health deterioration.Citation91 Several studies explained that intestinal mycobiota can cause allergic airway disease in the lung through the entero-pulmonary axis mechanismCitation92,Citation93 (). Allergic airway diseases also account for the severity of asthma.Citation94 The term for this type of disease is severe asthma with fungal sensitization (SAFS) (Box1). The treatment of SAFS mainly include anti-IgE monoclonal antibodies (omalizumab) and high-dose intravenous corticosteroids rather than conventional treatments.Citation95
Graft-versus-host disease (GVHD)
According to the result of multivariate analysis, Candida spp colonization induce the Th17/IL-23 response through pattern-recognition receptors, which promotes the development of GVHD.Citation103 The success of lung transplantation is mainly affected by infectious complications. Using bronchoalveolar lavage (BAL) and oropharyngeal wash (OW) to sample lung transplant patients and healthy people,Citation26 the result clearly show that both BAL and OW samples from patients contain Candida at higher abundance than healthy individuals, while Aspergillus is also dominant in BAL samples. This provides good evidence that mycobiota threaten lung transplantation by causing infection.
Mycobiota-related metabolic diseases
Intestinal bacteria have been recognized as pathogenic factors affecting obesity and diabetes.Citation104,Citation105 Mar Rodriguez and colleaguesCitation106 emphasized the composition changes of the fungal community in obese subjects for the first time. They studied the mycobiota in fecal samples from obese subjects and control groups. The difference of fungal abundance between two groups was unconspicuous; however, the common genus in obese patients include Candida, Nakaseomyces, and Penicillium while Mucor, Candida and Penicillium were most frequently detected in non-obese individuals. Significantly, It is discovered that phylum Ascomycota et al. were associated with abnormal metabolism and phylum Zygomycota et al. contributed to the defense of metabolic disturbance conversely, which indicates that mycobiota will become therapeutic sites for metabolic diseases. Gosiewski and colleaguesCitation107 used Quantitative real-time PCR(qPCR) to analyze gut mycobiota, such as Candida, in patients with type 1 diabetes and concluded that the high prevalence of C.albicans is one of the characteristics of diabetes.
Other mycobiota-related diseases
Malassezia, the main mycobiota in the skin, can cause different skin infections. Pityriasis versicolor is a form of skin tinea that has a definite connection with Malassezia.Citation108 The hyphae of Malassezia invades the skin, and its metabolites form small-scale pigmented plaques in lipid spills. Balaji and colleaguesCitation109 detected that cross-reactivity between fungal thioredoxin and human thioredoxin may related to the inflammation in the patients with atopic dermatitis. In addition, Malassezia also contributes to dandruffCitation110 and folliculitis,Citation111which bring stubborn trouble to the patient. Candida can also cause skin infection, named chronic mucocutaneous candidiasis (CMC),Citation112 marked with the deficiency of IL-17. In the patient’s body, mutations in STAT1 prevent T cells from differentiating into Th17 cells and thus fail to secrete immune effectors such as IL-17, which are the key to skin resistance to Candida infection.Citation65,Citation68,Citation113 Moreover, patients with autoimmune disease produce antibodies to IL-17, which impair immunity and can also trigger CMC.Citation62
Recent study by Yang and colleagues shows that intestinal mycobiota contributed to the aggravation of alcohol-induced liver disease.Citation114 After drinking alcohol, the burden of intestinal mycobiota in mice increased, with the majority being Humicola species, Fusarium and Aspergillus, which led to an increase of mortality. Moreover, the development of liver disease depends on dectin-1, the signal induces the production of IL-1β from Kupffer cells and promotes liver inflammation. These findings suggest that the intestinal mycobiota may become the therapeutic target for alcohol-related liver disease.Citation115
Recurrent vulvovaginal candidiasis(RVVC) is a mucosal fungal infection that seriously affects the health of women.Citation116 C.albicans can act as a symbiont in the vagina of most healthy women, causing inflammation through promoting epithelial cells to produce cytokines and chemokinesCitation117 when the body is compromised, such as with HIV infection, pregnancy, diabetes, antibiotic use, and so on. In addition, genetic factors can also increase susceptibility, which is linked with genetic polymorphisms of immune molecular-related gene, such as dectin-1.Citation118 Antifungal drugs such as fluconazole are commonly used in clinic,Citation54 but there is a risk associated with their use in pregnant women.Citation119 Therefore, probiotics and vaccinesCitation54,Citation120are hopeful use instead of to anti-fungal therapy.
Multiple fungal species can be associated with the central nervous system when the body’s immune function is defective, mainly Aspergillus and Cryptococcus.Citation121 For example, brain abscesses are caused by Aspergillus infection in leukemia patients because of neutropenia. Additionally, cryptococcal meningitis is related to HIV infection, which is a great threat to human life.Citation122 Meningitis usually originates from a lung infection, and there is evidence that antifungal treatment for meningitis may exacerbate immune reconstitution inflammatory syndrome.Citation123 Intestinal mycobiota may be involved in nervous system diseases, such as autism spectrum disordersCitation124 and Rett syndrome.Citation125
Host immunity and mycobiota
We have long known the damage caused by mycobiota to the human body and the severity of the damage warrants vigilance. Furthermore, the polymorphisms caused by fungal immunity-related gene mutations impact the immune response, which is associated with the susceptibility to fungal genetic diseases or infectious diseases (). Healthy people can live normally in environments with an abundance of mycobiota or harbor mycobiota in their body thanks to a strong immune system interaction with the commensal mycobiota to achieve balance. This process includes the immune defense against infection and the reaction of mycobiota to the immune system.
Immunity of organisms to fungal infection and dysbiosis
The skin and mucosa of the human body are the most exposed to mycobiota. Innate immunity begins with the perception and recognition of fungal invasion at these sites (). After that, the downstream signaling pathway begins, with transduction in the nucleus, triggering a series of immune effects.
Pattern recognition receptors
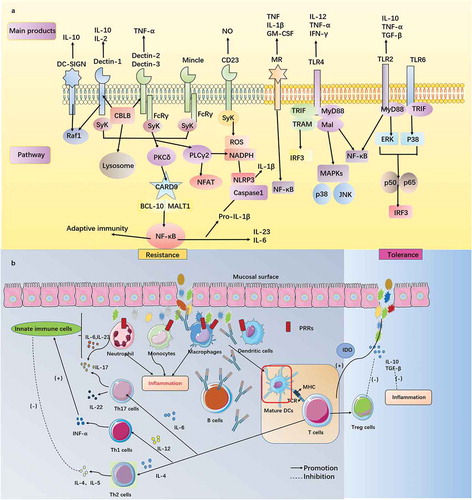
Pattern recognition receptors (PRRs) in the human body consist of C-type lectin receptors (CLRs), toll-like receptors (TLRs), NOD-like receptors (NLRs) and RIG-I-like receptors (RLRs). Research on RLRs is still insufficient, but we are aware of a function in the recognition of Candida. PRRs recognize fungal pathogen-associated molecular patterns (PAMPs) in particular ().
CLRs dominate fungal perception and immunity, they can activate the Syk-Card9 pathway through immune receptor tyrosine-based activation motifs (ITAMs) or FcRγ(relate to ITAMs), which in turn activates nuclear factor kappa B(NF-kB) signaling and a series of inflammatory reactions.Citation126 However, there are differences among receptors (), dectin-1 (also known as Clec7a) is a CLR that is expressed by many immunological cells. This molecule recognizes β-glucan and then promotes the production of cytokines to induce inflammation, thus controlling Candida infection mainly.Citation127 The deficiency of dectin-1 may cause the reduction of IL-17, the patient may be more likely to develop recurrent vaginal candidiasis and onychomycosis.Citation46 However, the results of subsequent experiments are contradictory. Saijo and colleaguesCitation128 suggested that the function of neutrophils in killing C. albicans was not impaired when dectin-1 was lacking, which indicated that dectin-1 had no obvious effect on invasive fungal infection. This led to speculation until studies found that the response of dectin-1 was species specific, only involving C.albicans yeast but not filaments, depending on the nature of the β-glucan in the cell wall of the yeast.Citation129 Apart from the isolation of infection, experimental evidence has shown the role of dectin-1 in fungal dysbiosis. Researchers have shown that dectin-1 inhibits the excessive growth of Candida in the gastrointestinal tract to coordinate the ecological environment of the intestine and avoid colitis.Citation42 Interestingly, Iliev and colleaguesCitation130 found the role of dectin-1 was explained in the opposite way. They demonstrated that dectin-1 signals, while resistant to mycobiota, also inhibited the growth of Lactobacillus in the gastrointestinal tract and affected the infiltration of Treg cells, which undermined the body’s ability to protect against colitis. In addition, dectin-1 also assists the function of TLRs(TLR2, TLR4).Citation131 Dectin-2, dectin-3, and Mincle have a secondary role in fungal immunity and connect to the downstream Sky-Card9-Bcl10-Malt1 pathway through FcRγ. Unlike dectin-1, dectin-2 can reduce systemic Candida infection by stimulating the differentiation of Th17 cells.Citation132 Additionally, the recognition of dectin-2 by α-mannan is essential to trigger the immune response of the human body against Malassezia, which is also associated with Mincle via the recognition of lipophilic components in vitro.Citation133 The effect of dectin-3 is similar to that of dectin-2, which enhances the immune control of fungal invasion. Dectin-2 and dectin-3 cooperate with each other to form heterodimers, which promote the production of proinflammatory factors (IL-1β, IL-6). Research has shown that this type of complex improves the ability to sense mycobiota pathogens.Citation134 Mincle could kill mycobiota by promoting phagocytosis and TNF production, although this function is not widely accepted.Citation135 A recent study provided more information about dectin-3, scholars found that the composition of the gut mycobiota is altered in model mice with dectin-3 deletion and the dysbiosis causes a higher risk of colitis induced by dextran sodium sulfate. This finding indicates the role of dectin-3 in maintaining the homeostasis of the colon microenvironment.Citation43 Mannose receptor(MR) recognizes α-mannan, mediating the sensing of mycobiota on the surface of a macrophage.Citation136 A characteristic of MR is that it can build a bridge for the immune effect of Th17 cells induced by C.albicans in human peripheral blood mononuclear cells without anti-CD3 or anti-CD28 antibodies, which assist IL-17 in fungal resistance.Citation137 Importantly, MR requires the assistance of other CLRs, such as dectin-1 and TLR2. It is worth emphasizing that the absence of MR leads to the susceptibility to C.neoformans and is ineffective against infection by C.albicans and P. carinii.Citation138 CD23 is a newly discovered CLR, JNK1 (also known as MAPK8) can down-regulate the body’s antifungal immunity. According to an experiment with mice, we concluded that inactivating the activity of JNK1 could increase the level of CD23 and promote the production of nitric oxide, which contributes to stronger immunity against fungal pathogens.Citation139 Therefore, we expect that the JNK1 inhibitor may bring hope for antifungal therapy. Later, an article revealed the mechanism of antifungal immunity of CD23: it connects the NF-κB signal pathway through the FcRg subunit and upregulates the production of nitric oxide from macrophages. Notably, this applies to defense against C. albicans and A. fumigatus, but not C.neoformans.Citation140
TLRs such as TLR2, TLR4, and TLR9 are essential parts of PRRs that participate in sensing mycobiota. In addition, the polymorphisms of TLR4 and TLR9 could affect human susceptibility to mycobiota (). After the recognition of fungal PAMPs, protease-activated receptors (PARs) are activated, and then PARs and TLRs influence each other and impact fungal immunity. The results of studies have shown that PAR1 enhances immune inflammation in Candida infection with the help of TLR2, while PAR2 cooperates with TLR4, downregulating the response to Aspergillus.Citation141 TLR2 can also maintain the balance between Th17 cells and Treg cells, with the depression of Th17 cells.Citation142 The downstream of Toll-like receptor is linked with MYD88. Using in vivo experiments, we found that the IL-1 R/MyD88 pathway is essential for the defense against C.albicans and that the TLR4/MyD88 pathway protects us from succumbing to Aspergillus fumigatus.
The function of NLRs is shown in the NLRP3 inflammasome. The signal transduction of fungal infection is dependent on Sky, which can endow NLRP3 activity and produce the precursor of IL-1β. Then, the role of NLRP3 becomes relevant, which activates caspase1 for processing IL-1β. This process is significant for the defense against mycobiota.Citation143 NLRC4, which controls the activity of IL-17 and IL-1β, is also required in resisting mucosal candida infection.Citation144
The role of cells and molecules
The roles of cells and molecules are divided into two parts: innate immunity and adaptive immunity. These two parts work together to build harmonious and unified anti-fungal immune homeostasis ().
Neutrophil is the most powerful phagocyte, and this type of cell has the ability to kill mycobiota through oxidative and nonoxidative mechanisms. For oxidative methods, neutrophils can first product mycobiotacidal peroxide through NADPH oxidase and myeloperoxidase, which is known as a respiratory burst.Citation145 The deficiency of protection from NADPH oxidase has been proven to be linked with chronic granulomatous disease, a hereditary disease marked by chronic inflammation and lethal fungal infection. Secondly, another system is based on reactive nitrogen intermediates, which are guided by inducible nitric oxide synthase (iNOS or NOS2).Citation145 For the nonoxidative system, nuclear members are functional proteins or polypeptides, including antimicrobial peptides and hydrolases. All of these pose a powerful threat to fungal invasion. Additionally, some proteases in neutrophils have been reported to have a role in neutrophil extracellular traps, which are able to catch mycobiota. Otherwise, the neutrophils can also provide a source of IL-17, assisted by IL-6 and IL-23.Citation146
The cytotoxicity of epithelial cells is not strong and mainly functions as a barrier. Themacrophage is not able to kill fungal spores but can control their growth and help pathogens become exposed to anti-fungal drugs. Due to the experiments on CX3CR1 deficient mice, we underline the potent protection from renal resident macrophages in the early stage of systemic Candida infection, which relies on CX3CR1.Citation67 The latest study found that CX3CR1+ mononuclear phagocytes (MNPs) have an impact on antifungal immunity. CX3CR1+ MNPs could trigger patient defenses such as Th17 cells and antibodies to reduce fungal colonization during bowel disease depending on CLR signaling pathways, and even better, these cells can regulate the intestinal fungal community and prevent imbalances.Citation147 Monocytes play a major assisting role; they can kill fungal spores or transport them into lymph nodes in the form of dendritic cells, triggering adaptive immunity. In addition, mononuclear cells can also provide assistance to neutrophils in preventing the progression of disseminated candida infection, but overreaction can lead to immune-related pathology in the kidneys.Citation148
In the innate anti-fungal immunity, except for the effects of the abovementioned cells, the role of other cells and molecules should not be ignored. Innate immune cells include epithelial cells, natural killer cells and dendritic cells, while molecules contain chemokine, complement, and newly discovered Casitas B lymphoma-b (CBLB) molecules ().
Table 2. Other contributors of anti-fungal immunity.
Adaptive immunity against mycobiota is supported by T lymphocytes and antibodies produced from B lymphocytes. T lymphocytes include CD4+ and CD8 + T cells. Some studies have authenticated that CD8 + T cells are able to supplement CD4 + T cells’ functional deficiency in the case of immunodeficiencyCitation153 and other antifungal activities.Citation154 Here, we only focus on CD4 + T cells, such as Th1, Th2, and Th17 cells.
It has been reported that T cells of different subtypes have specific functions. Th1 cells can be activated by cytokines (IL-12) produced downstream of PRRs, although the guidance of dendritic cells(DCs) is found to have an effect when the body suffers from systemic infection,Citation155 then performing functions such as the production of IFNγ and enhancing the anti-fungal ability of innate immune cells like phagocytes. To employ a different role, the function of Th2 cells is more likely to soften the immune system. These cells can be regarded as the target of negative regulation in an organism’s anti-fungal immunity, maintaining a balance. Thus, the activation of the Th2 cell response requires Th1 cell inhibition as a cost, which causes susceptibility to Candida infection.Citation156 IL-4 is a trigger factor of the Th2-type response, and this upregulated response of Th2 cells may cause people to suffer from diseases.Citation91
Th17 cells are the main force of specific immunity against fungal infection in organisms. Th17 cells can receive stimulatory signals from multiple cytokines (IL-23, IL-6, and IL-1β), with an article highlighting the role of IL-6 produced by the Langerhans cell combined with CLRs.Citation155 The upstream events may lead back to a few signaling pathways that were previously discussed; the well-known mechanism is combined with Card9, which is the center of anti-fungal immunity.Citation157,Citation158 For example, in the response to Aspergillus fumigatus infection, researchers found that Card9 accepts the signal from dectin-1 and impels CD4 + T cells toward the direction of Th17 cell differentiation, which is based on the inhibition of Th1 cell formation.Citation159 After activation, Th17 cells can produce cytokines based on IL-22 and IL-17. Accumulating evidence has shown that IL-17 predominates the protective responseCitation160 with the second role of IL-22 when the body encounters OPC and RVVC. It suggests that Th17 cells have a role in the immunity of mucosal fungal infection specifically.Citation79
Treg cells are another important subtype of T cells. The effects of Treg cells and monoclonal antibodies have complemented the antifungal adaptive immunity so that the organism can mount a better response to fungal-induced lesions ().
Effects of mycobiota on organism immunity
The evasive fungal pathogen response to the immune system
The recognition of PAMPs is the basis for the immune system to monitor fungal pathogens. Therefore, many mycobiota utilize this point for immune escape. For example, the polysaccharide layer of Cryptococcus neoformans and the protein layer from Aspergillus fumigatus can hide PAMPs.Citation145 Additionally, the morphology of mycobiota may play the same role,Citation161 such as the hyphae of Candida. Damage to recognition is also realized by other pathways, including the activation of complements and the inhibition of phagocyte function. After being identified, mycobiota still struggle to survive by various methods, including the destruction of the mature of phagocytes, escape,Citation162 and the resistance to unfavorable environments, which are used by different species of mycobiota.Citation145
Furthermore, fungal pathogen Pneumocystis carinii causes infection and initiates the Syk pathway triggered by Dectin-1. This response promotes MR shedding, which gives functionality to MR (in the form of sMR) based on metalloproteinase. Soluble MR (sMR) is widely thought to help mycobiota avoid clearance by host immunity.Citation163 Interestingly, the Mincle receptor is a powerful weapon for mycobiota, but toxic factors of mycobiota could reduce the activation of Interferon Regulatory Factor (IRF1), which is associated with the pathway induced by E3 ligase Mdm2. The alteration could damage the ability to resist fungal infection by blocking the production of IL-12, which weakens Th1 cells activity but increases that of Th2 cells.Citation164
Impact of mycobiota on the immune system
More and more studies show that mycobiota have the effect on human immune system. In early times, researchers found the benefits of Saccharomyces boulardii, a type of probiotic mycobiota, which can regulate the immune system against the invasion of C. difficile and relieve intestinal inflammation.Citation148 C.albicans was found to affect immune components such as TLR4, Dectin-1 and build patient defense in the gut and lung.Citation165 There is also evidence that mycobiota regulate lymphocyte recirculation. Fungal flora can induce Raldh+ dendritic cells gathering in peripheral lymph nodes. Without this process, lymphocyte adhesion molecules (necessary in recirculation) would have no response. To survive under the surveillance of the immune system, apart from the help from Treg cells mentioned above, mycobiota could also accommodate immune sensitivity. C. albicans can activate the tolerance of macrophage and DCs increase the impression of indoleamine-2,3-dioxygenase. It is widely accepted that it can induce the enrichment of Treg cells and the depletion of Th17 cells. All of these may help mycobiota adapt to immunity better. In addition, the contribution of cytokines cannot be ignored, and TGF-β and IL-10 are used by Malassezia to avoid excessive inflammatory responses.Citation156
MDSCs are usually believed to promote tumor formation depending on the nature of hyper proliferation.Citation166 According to further study, people began to realize the effect they play in the interaction between fungal pathogens and hosts. A murine study further provides evidence that the recognition between fungal pathogens and dectin-1 drives the neutrophilic subtype of MDSCs to obstruct the activity of Natural killer cells and Th17 cells through cooperation with caspase-8, IL-1β and ROS, thereby preventing the human body from suffering severe inflammation. It is also provedCitation9 that C.tropicalis induce MDSCs differentiation from bone marrow cells, which promote the development of CAC. In short, MDSCs can be used by mycobiota as a medium to achieve the regulation of the immune system.
Except for the primary immune response, some researchers found that the innate immune system that has been exposed to fungal pathogens can provide a faster and stronger defense against reinfection, which is called ‘trained immunity’ (Box2) for fungal pathogens. This revolutionizes our perception of innate immunity. It was found that C.albicans can enhance the resistance of monocytes to secondary infection in the body by means of dectin-1 and it’s epigenetic modification.Citation167 Recently, Tso and colleagues had shown that the mechanism of fungal pathogen evolution involves a symbiotic relationship with the host gastrointestinal tract. The use of antibodies selects a fungal strain with hyphal deficiency, which has low toxicity and high immunogenicity. This fungal pathogen’s adaptation to the intestinal tract enhances the protective power depending on the innate immune system. The effect is impermanent, but it can relieve a crisis of adaptive immune deficiency, such as Acquired immune deficiency syndrome (AIDS).Citation168 It can also give us an advantage when searching for proper vaccines for infection protection, for example, Bacillus Calmette-Guerin is a well-known anti-tuberculosis vaccine that also has protective effects on fatal systemic fungal infections, which has been demonstrated to have an association with the change of metabolic pathways.Citation169
Methods for detecting mycobiota
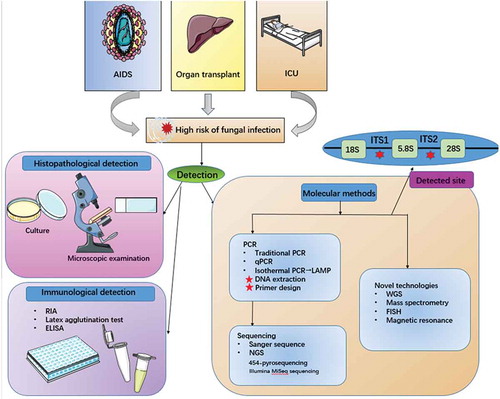
Hospital AIDSCitation172 and transplant patientsCitation173 as well as intensive care patientsCitation174 have a high risk of fungal infection. In addition, drug-resistance due to inappropriate anti-fungal treatment can also lead to a challenging disaster for humans.Citation175 To realize more accurate and fast detection, we must rely on convenient and advanced methods and techniques().
Histopathological and immunological methods
Traditional diagnostic methods for fungal diseases depend on histopathological examination (selective culture, microscopic examination, biochemical detection). This method provides a reliable standard for Candida infection but more damage to the patient due to the length of the detection time, including disease deterioration and economic losses.Citation176 There are differences in detection methods for different types of fungal infections;Citation177 however, false positivity, confusion, and invalidation may occur in the morphological diagnosis of fungal infection.Citation178
The immunological method is a technique for detecting fungal antigens, mainly components of the fungal cell wall, such as β-d-glucan.Citation179 Some laboratory researchers used radioimmunoassay to detect fungal mannan, which has diagnostic significance for systemic candidiasis.Citation180 The Cand-Tec latex agglutination test also has a related application.Citation181 Enzyme-linked immunosorbent assay (ELISA) is used in the diagnosis of penicilliosis by detecting the antibody Mp1p.Citation182 It has been proven that ELISA is better than the latex agglutination test in aspergillus infection identification.Citation183 Nevertheless, antigen detection is influenced by many factors, such as immune complexes and detection thresholds, which are limited; therefore, prospective improvements are required.Citation184
Molecular biological methods
The above methods cannot address the complexity of the fungal community. Therefore, molecular biological methods have entered the stage of fungal detection.Citation185 Polymerase chain reaction (PCR) is a basic technology related to fungal DNA detection. Although traditional PCR technology has advantages of being quick and intuitive, the low DNA content of fungal pathogens has led to the fact that this technique cannot meet the required sensitivity.Citation186 Thus, some improved technologies were born. qPCR allowed us to access real-time detection, enhancing sensitivity and specificity.Citation187 This instant feedback signal was accomplished by fluorescent probes such as Taqman. Isothermal PCR eliminates the thermal cycle step, simplifying the PCR technology greatly. Loop-mediated isothermal amplification has an absolute advantage.Citation188 Use of PCR necessitates attention to some points: DNA extraction should be performed with attention to DNA quality and should avoid contamination;Citation189 in regard to the design of various primers,Citation4 the target is usually directed to the fungal rDNA operon region.Citation190 Researchers have harmonized the biomarkers used to identify fungal species. Based on the considerable sequences and analyses, The taxonomic and phylogenetic classification based on sequence analysis of the ITS genomic region has become a crucial component of fungal ecology and diversity studies.Citation191
After using PCR to amplify specific regions, sequencing is the next step. Currently, metagenome sequencing opens the door to a new world for fungal detection.Citation74 Next-generation sequencing (NGS) is the second generation high-throughput sequencing technology,Citation192 replacing Sanger sequencing.Citation193 NGS is supported by high-throughput sequencing platforms such as 454-pyrosequencing technologyCitation194 and the Illumina MiSeq sequencing platform.Citation195 The sequence results should be compared according to a common database (like GenBank). Although there are some shortcomings with searching, it has provided an effective method for the identification of mycobiota.
The methods of DNA analysis for mycobiota help us to familiarize ourselves with fungal colony changes associated with related diseases. For example, the microscopic pathological changes of patients with ulcerative colitis were studied by denatured gradient gel electrophoresis analysis.Citation18 Moreover, clinical studies have shown that microarray technology can be used in the pathological analysis of mycosis.Citation193 Because the structures and characteristics of mycobiota are diverse, we often need to use different methods for specific fungal species to achieve accurate judgments, except for common approaches such as β-glucan assays. For example, it is most appropriate to detect germ tube antibodies for Candida spp. while detecting galactomannan for Aspergillus spp.Citation177
Over time, the rapid development of new molecular identification methods has opened the understanding of the fungal world. Whole Genome Sequencing is applied in human oral mycobiota.Citation13 Other technologies such as Mass spectrometryCitation196 Fluorescence in situ HybridizationCitation197 and Magnetic resonance198 help us have a more thorough understanding of mycobiota.
Conclusions
Earlier, when it came to the microbiome of the human body, the first thing people considered was bacterial species. However, in recent years, the role of the mycobiota has gradually appeared, reflecting a position that cannot be ignored. Through reviewing these studies, we have realized that the effects of mycobiota have penetrated all aspects of human pathology, with a gradual expansion of scope. This tells us that the door to the new world of mycobiota has opened and that more novel problems are in need of our solutions: how to transfer the model of colon cancer into clinical practice; whether the functional similarity between bacteria and symbiotic mycobiota can be extended to other aspects; what should we do to preserve the benefits of mycobiota for the immune system and minimize the damage?
The rapid rise of fungal research also provides a new approach to the treatment of clinical-related diseases. It is worthwhile to note that we should consider fungal species in the internal microenvironment rather than in isolation. Only in this way can we systemically understand the responses and apply them in actual disease treatments for the benefit of more people.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Wainright PO, Hinkle G, Sogin ML, Stickel SK. Monophyletic origins of the metazoa: an evolutionary link with fungi. Science. 1993;260:340–342. doi:10.1126/science.8469985.
- Peay KG, Kennedy PG, Talbot JM. Dimensions of biodiversity in the earth mycobiome. Nat Rev Microbiol. 2016;14:434–447. doi:10.1038/nrmicro.2016.59.
- Limon JJ, Skalski JH, Underhill DM. Commensal fungi in health and disease. Cell Host Microbe. 2017;22:156–165. doi:10.1016/j.chom.2017.07.002.
- Huseyin CE, O’Toole PW, Cotter PD, Scanlan PD. Forgotten fungi-the gut mycobiome in human health and disease. FEMS Microbiol Rev. 2017;41:479–511. doi:10.1093/femsre/fuw047.
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv13. doi:10.1126/scitranslmed.3004404.
- Iliev ID, Leonardi I. Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat Rev Immunol. 2017;17:635–646. doi:10.1038/nri.2017.55.
- Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol. 2014;14:405–416. doi:10.1038/nri3684.
- Malik A, Sharma D, Malireddi RKS, Guy CS, Chang TC, Olsen SR, Neale G, Vogel P, Kanneganti TD. SYK-CARD9 signaling axis promotes gut fungi-mediated inflammasome activation to restrict colitis and colon cancer. Immunity. 2018;49:515–30 e5. doi:10.1016/j.immuni.2018.08.024.
- Wang T, Fan C, Yao A, Xu X, Zheng G, You Y, Jiang C, Zhao X, Hou Y, Hung MC, et al. The adaptor protein CARD9 protects against colon cancer by restricting mycobiota-mediated expansion of myeloid-derived suppressor cells. Immunity. 2018;49:504–14 e4. doi:10.1016/j.immuni.2018.08.018.
- Kumamoto CA. The fungal mycobiota: small numbers, large impacts. Cell Host Microbe. 2016;19:750–751. doi:10.1016/j.chom.2016.05.018.
- Huffnagle GB, Noverr MC. The emerging world of the fungal microbiome. Trends Microbiol. 2013;21:334–341. doi:10.1016/j.tim.2013.04.002.
- Hallen-Adams HE, Suhr MJ. Fungi in the healthy human gastrointestinal tract. Virulence. 2017;8:352–358. doi:10.1080/21505594.2016.1247140.
- Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, Gillevet PM. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6:e1000713. doi:10.1371/journal.ppat.1000713.
- Intramural Sequencing Center Comparative Sequencing; Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, Schoenfeld D, Nomicos E, Park M, Program NIH, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature. 2013;498:367–370. doi:10.1038/nature12171.
- Drell T, Lillsaar T, Tummeleht L, Simm J, Aaspollu A, Vain E, Saarma I, Salumets A, Donders GG, Metsis M. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS One. 2013;8:e54379. doi:10.1371/journal.pone.0054379.
- Hall RA, Noverr MC. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr Opin Microbiol. 2017;40:58–64. doi:10.1016/j.mib.2017.10.020.
- Scanlan PD, Marchesi JR. Micro-eukaryotic diversity of the human distal gut microbiota: qualitative assessment using culture-dependent and -independent analysis of faeces. Isme J. 2008;2:1183–1193. doi:10.1038/ismej.2008.76.
- Ott SJ, Kuhbacher T, Musfeldt M, Rosenstiel P, Hellmig S, Rehman A, Drews O, Weichert W, Timmis KN, Schreiber S. Fungi and inflammatory bowel diseases: alterations of composition and diversity. Scand J Gastroenterol. 2008;43:831–841. doi:10.1080/00365520801935434.
- Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun. 2004;72:4996–5003. doi:10.1128/IAI.72.9.4996-5003.2004.
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi:10.1038/nature12820.
- Strati F, Di Paola M, Stefanini I, Albanese D, Rizzetto L, Lionetti P, Calabro A, Jousson O, Donati C, Cavalieri D, et al. Age and gender affect the composition of fungal population of the human gastrointestinal tract. Front Microbiol. 2016;7:1227. doi:10.3389/fmicb.2016.01227.
- Jiang TT, Shao TY, Ang WXG, Kinder JM, Turner LH, Pham G, Whitt J, Alenghat T, Way SS. Commensal fungi recapitulate the protective benefits of intestinal bacteria. Cell Host Microbe. 2017;22:809–16 e4. doi:10.1016/j.chom.2017.10.013.
- Dupuy AK, David MS, Li L, Heider TN, Peterson JD, Montano EA, Dongari-Bagtzoglou A, Diaz PI, Strausbaugh LD. Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: discovery of Malassezia as a prominent commensal. PLoS One. 2014;9:e90899. doi:10.1371/journal.pone.0090899.
- Baker JL, Bor B, Agnello M, Shi W, He X. Ecology of the oral microbiome: beyond bacteria. Trends Microbiol. 2017;25:362–374. doi:10.1016/j.tim.2016.12.012.
- Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi:10.1371/journal.pone.0008578.
- Charlson ES, Diamond JM, Bittinger K, Fitzgerald AS, Yadav A, Haas AR, Bushman FD, Collman RG. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med. 2012;186:536–545. doi:10.1164/rccm.201204-0693OC.
- Burge HA. An update on pollen and fungal spore aerobiology. J Allergy Clin Immunol. 2002;110:544–552. doi:10.1067/mai.2002.128674.
- Oh J, Byrd AL, Park M, Kong HH, Segre JA; ; . Temporal stability of the human skin microbiome. Cell. 2016;165:854–866. doi:10.1016/j.cell.2016.04.008.
- Gao Z, Perez-Perez GI, Chen Y, Blaser MJ. Quantitation of major human cutaneous bacterial and fungal populations. J Clin Microbiol. 2010;48:3575–3581. doi:10.1128/JCM.00597-10.
- Kalan L, Loesche M, Hodkinson BP, Heilmann K, Ruthel G, Gardner SE, Grice EA. Redefining the chronic-wound microbiome: fungal communities are prevalent, dynamic, and associated with delayed healing. MBio. 2016;7:e01058–16.
- Merenstein D, Hu H, Wang C, Hamilton P, Blackmon M, Chen H, Calderone R, Li D. Colonization by Candida species of the oral and vaginal mucosa in HIV-infected and noninfected women. AIDS Res Hum Retroviruses. 2013;29:30–34. doi:10.1089/aid.2012.0269.
- Bradford LL, Ravel J. The vaginal mycobiome: A contemporary perspective on fungi in women’s health and diseases. Virulence. 2017;8:342–351. doi:10.1080/21505594.2016.1237332.
- Parolin C, Marangoni A, Laghi L, Foschi C, Nahui Palomino RA, Calonghi N, Cevenini R, Vitali B. Isolation of vaginal lactobacilli and characterization of anti-candida activity. PLoS One. 2015;10:e0131220. doi:10.1371/journal.pone.0131220.
- Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Folsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi:10.1136/gut.2003.025403.
- Moyes DL, Naglik JR. The mycobiome: influencing IBD severity. Cell Host Microbe. 2012;11:551–552. doi:10.1016/j.chom.2012.05.009.
- Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, Cohen D, Liguori G, Bourrier A, Nion-Larmurier I, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66:1039–1048. doi:10.1136/gutjnl-2015-310746.
- Limon JJ, Tang J, Li DL, Wolf AJ, Michelsen KS, Funari V, Gargus M, Nguyen C, Sharma P, Maymi VI, et al. Malassezia is associated with crohn’s disease and exacerbates colitis in mouse models. Cell Host Microbe. 2019;25:377-+. doi:10.1016/j.chom.2019.01.007.
- McKenzie H, Main J, Pennington CR, Parratt D. Antibody to selected strains of Saccharomyces cerevisiae (baker’s and brewer’s yeast) and Candida albicans in Crohn’s disease. Gut. 1990;31:536–538. doi:10.1136/gut.31.5.536.
- Quinton JF, Sendid B, Reumaux D, Duthilleul P, Cortot A, Grandbastien B, Charrier G, Targan SR, Colombel JF, Poulain D. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–791. doi:10.1136/gut.42.6.788.
- Standaert-Vitse A, Jouault T, Vandewalle P, Mille C, Seddik M, Sendid B, Mallet JM, Colombel JF, Poulain D. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn’s disease. Gastroenterology. 2006;130:1764–1775. doi:10.1053/j.gastro.2006.02.009.
- Standaert-Vitse A, Sendid B, Joossens M, Francois N, Vandewalle-El Khoury P, Branche J, Van Kruiningen H, Jouault T, Rutgeerts P, Gower-Rousseau C, et al. Candida albicans colonization and ASCA in familial Crohn’s disease. Am J Gastroenterol. 2009;104:1745–1753. doi:10.1038/ajg.2009.225.
- Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science (New York, NY). 2012;336:1314–1317. doi:10.1126/science.1221789.
- Wang T, Pan D, Zhou Z, You Y, Jiang C, Zhao X, Lin X. Dectin-3 deficiency promotes colitis development due to impaired antifungal innate immune responses in the gut. PLoS Pathog. 2016;12:e1005662. doi:10.1371/journal.ppat.1005662.
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi:10.1038/nature11582.
- Underhill DM, Pearlman E. Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity. 2015;43:845–858. doi:10.1016/j.immuni.2015.10.023.
- Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–1767. doi:10.1056/NEJMoa0901053.
- Plantinga TS, van der Velden WJ, Ferwerda B, van Spriel AB, Adema G, Feuth T, Donnelly JP, Brown GD, Kullberg BJ, Blijlevens NM, et al. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:724–732. doi:10.1086/604714.
- Jurevic RJ, Bai M, Chadwick RB, White TC, Dale BA. Single-nucleotide polymorphisms (SNPs) in human beta-defensin 1: high-throughput SNP assays and association with Candida carriage in type I diabetics and nondiabetic controls. J Clin Microbiol. 2003;41:90–96. doi:10.1128/JCM.41.1.90-96.2003.
- Babula O, Lazdane G, Kroica J, Linhares IM, Ledger WJ, Witkin SS. Frequency of interleukin-4 (IL-4) −589 gene polymorphism and vaginal concentrations of IL-4, nitric oxide, and mannose-binding lectin in women with recurrent vulvovaginal candidiasis. Clin Infect Dis. 2005;40:1258–1262. doi:10.1086/429246.
- Seo KW, Kim DH, Sohn SK, Lee NY, Chang HH, Kim SW, Jeon SB, Baek JH, Kim JG, Suh JS, et al. Protective role of interleukin-10 promoter gene polymorphism in the pathogenesis of invasive pulmonary aspergillosis after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;36:1089–1095. doi:10.1038/sj.bmt.1705181.
- Johnson MD, Plantinga TS, van de Vosse E, Velez Edwards DR, Smith PB, Alexander BD, Yang JC, Kremer D, Laird GM, Oosting M, et al. Cytokine gene polymorphisms and the outcome of invasive candidiasis: a prospective cohort study. Clin Infect Dis. 2012;54:502–510. doi:10.1093/cid/cir827.
- Ouederni M, Sanal O, Ikinciogullari A, Tezcan I, Dogu F, Sologuren I, Pedraza-Sánchez S, Keser M, Tanir G, Nieuwhof C, et al. Clinical features of Candidiasis in patients with inherited interleukin 12 receptor β1 deficiency. Clin Infect Dis. 2014;58:204–213. doi:10.1093/cid/cit722.
- Giraldo PC, Babula O, Goncalves AK, Linhares IM, Amaral RL, Ledger WJ, Witkin SS. Mannose-binding lectin gene polymorphism, vulvovaginal candidiasis, and bacterial vaginosis. Obstet Gynecol. 2007;109:1123–1128. doi:10.1097/01.AOG.0000260386.17555.a5.
- Palmeira-de-Oliveira R, Palmeira-de-Oliveira A, Martinez-de-Oliveira J. New strategies for local treatment of vaginal infections. Adv Drug Deliv Rev. 2015;92:105–122. doi:10.1016/j.addr.2015.06.008.
- Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–1735. doi:10.1056/NEJMoa0810719.
- Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi:10.1038/nature10209.
- Lev-Sagie A, Prus D, Linhares IM, Lavy Y, Ledger WJ, Witkin SS. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2009;200:303 e1–6. doi:10.1016/j.ajog.2008.10.039.
- Plantinga TS, Johnson MD, Scott WK, van de Vosse E, Velez Edwards DR, Smith PB, Alexander BD, Yang JC, Kremer D, Laird GM, et al. Toll-like receptor 1 polymorphisms increase susceptibility to candidemia. J Infect Dis. 2012;205:934–943. doi:10.1093/infdis/jir867.
- Nahum A, Dadi H, Bates A, Roifman CM. The biological significance of TLR3 variant, L412F, in conferring susceptibility to cutaneous candidiasis, CMV and autoimmunity. Autoimmun Rev. 2012;11:341–347. doi:10.1016/j.autrev.2011.10.007.
- Bochud PY, Chien JW, Marr KA, Leisenring WM, Upton A, Janer M, Rodrigues SD, Li S, Hansen JA, Zhao LP, et al. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med. 2008;359:1766–1777. doi:10.1056/NEJMoa0802629.
- Carvalho A, Pasqualotto AC, Pitzurra L, Romani L, Denning DW, Rodrigues F. Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J Infect Dis. 2008;197:618–621. doi:10.1086/587088.
- Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi:10.1084/jem.20091983.
- Oh J, Freeman AF, Park M, Sokolic R, Candotti F, Holland SM, Segre JA, Kong HH; ; . The altered landscape of the human skin microbiome in patients with primary immunodeficiencies. Genome Res. 2013;23:2103–2114. doi:10.1101/gr.159467.113.
- Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, Chen A, Kim HS, Lloret MG, Schulze I, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124:1289–302 e4. doi:10.1016/j.jaci.2009.10.038.
- Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, Toubiana J, Itan Y, Audry M, Nitschke P, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–1648. doi:10.1084/jem.20110958.
- Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi:10.1038/nature06096.
- Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, Green NM, Masedunskas A, Weigert R, Mikelis C, et al. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J Clin Invest. 2013;123:5035–5051. doi:10.1172/JCI71307.
- van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, Arts P, Rosentul DC, Carmichael AJ, Smits-van der Graaf CA, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365:54–61. doi:10.1056/NEJMoa1100102.
- Vaid M, Kaur S, Sambatakou H, Madan T, Denning DW, Sarma PU. Distinct alleles of mannose-binding lectin (MBL) and surfactant proteins A (SP-A) in patients with chronic cavitary pulmonary aspergillosis and allergic bronchopulmonary aspergillosis. Clin Chem Lab Med. 2007;45:183–186. doi:10.1515/CCLM.2007.033.
- Goel GA, Kandiel A, Achkar JP, Lashner B. Molecular pathways underlying IBD-associated colorectal neoplasia: therapeutic implications. Am J Gastroenterol. 2011;106:719–730. doi:10.1038/ajg.2011.51.
- Liu Z, Cao AT, Cong Y. Microbiota regulation of inflammatory bowel disease and colorectal cancer. Semin Cancer Biol. 2013;23:543–552. doi:10.1016/j.semcancer.2013.09.002.
- Luan C, Xie L, Yang X, Miao H, Lv N, Zhang R, Xiao X, Hu Y, Liu Y, Wu N, et al. Dysbiosis of fungal microbiota in the intestinal mucosa of patients with colorectal adenomas. Sci Rep. 2015;5:7980. doi:10.1038/srep07980.
- Gao R, Kong C, Li H, Huang L, Qu X, Qin N, Qin H. Dysbiosis signature of mycobiota in colon polyp and colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;36:2457–2468. doi:10.1007/s10096-017-3085-6.
- Coker OO, Nakatsu G, Dai RZ, Wu WKK, Wong SH, Ng SC, Chan FKL, Sung JJY, Yu J. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut. 2019;68:654–662. doi:10.1136/gutjnl-2018-317178.
- Bergmann H, Roth S, Pechloff K, Kiss EA, Kuhn S, Heikenwälder M, Diefenbach A, Greten FR, Ruland J. Card9-dependent IL-1β regulates IL-22 production from group 3 innate lymphoid cells and promotes colitis-associated cancer. Eur J Immunol. 2017;47:1342–1353. doi:10.1002/eji.v47.8.
- Conche C, Greten FR. Fungi enter the stage of colon carcinogenesis. Immunity. 2018;49:384–386. doi:10.1016/j.immuni.2018.09.002.
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949–958. doi:10.1001/jama.2015.0954.
- Botschuijver S, Roeselers G, Levin E, Jonkers DM, Welting O, Heinsbroek SEM, de Weerd HH, Boekhout T, Fornai M, Masclee AA, et al. Intestinal fungal dysbiosis is associated with visceral hypersensitivity in patients with irritable bowel syndrome and rats. Gastroenterology. 2017;153:1026–1039. doi:10.1053/j.gastro.2017.06.004.
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi:10.1084/jem.20081463.
- Mukherjee PK, Chandra J, Retuerto M, Sikaroodi M, Brown RE, Jurevic R, Salata RA, Lederman MM, Gillevet PM, Ghannoum MA. Oral mycobiome analysis of HIV-infected patients: identification of Pichia as an antagonist of opportunistic fungi. PLoS Pathog. 2014;10:e1003996. doi:10.1371/journal.ppat.1003996.
- de Repentigny L, Lewandowski D, Jolicoeur P. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev. 2004;17:729–759. table of contents. doi:10.1128/CMR.17.4.729-759.2004.
- Conti HR, Bruno VM, Childs EE, Daugherty S, Hunter JP, Mengesha BG, Saevig DL, Hendricks MR, Coleman BM, Brane L, et al. IL-17 receptor signaling in oral epithelial cells is critical for protection against oropharyngeal candidiasis. Cell Host Microbe. 2016;20:606–617. doi:10.1016/j.chom.2016.10.001.
- Conti HR, Peterson AC, Brane L, Huppler AR, Hernandez-Santos N, Whibley N, Garg AV, Simpson-Abelson MR, Gibson GA, Mamo AJ, et al. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J Exp Med. 2014;211:2075–2084. doi:10.1084/jem.20130877.
- Sanglard D, Odds FC. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect Dis. 2002;2:73–85. doi:10.1016/S1473-3099(02)00181-0.
- Amin R, Dupuis A, Aaron SD, Ratjen F. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest. 2010;137:171–176. doi:10.1378/chest.09-1103.
- Kim SH, Clark ST, Surendra A, Copeland JK, Wang PW, Ammar R, Collins C, Tullis DE, Nislow C, Hwang DM, et al. Global analysis of the fungal microbiome in cystic fibrosis patients reveals loss of function of the transcriptional repressor Nrg1 as a mechanism of pathogen adaptation. PLoS Pathog. 2015;11:e1005308. doi:10.1371/journal.ppat.1005308.
- Willger SD, Grim SL, Dolben EL, Shipunova A, Hampton TH, Morrison HG, Filkins LM, O’Toole GA, Moulton LA, Ashare A, et al. Characterization and quantification of the fungal microbiome in serial samples from individuals with cystic fibrosis. Microbiome. 2014;2:40. doi:10.1186/2049-2618-2-40.
- Greenberger PA. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 2002;110:685–692. doi:10.1067/mai.2002.130179.
- Latzin P, Hartl D, Regamey N, Frey U, Schoeni MH, Casaulta C. Comparison of serum markers for allergic bronchopulmonary aspergillosis in cystic fibrosis. Eur Respir J. 2008;31:36–42. doi:10.1183/09031936.00078107.
- Mirkovic B, Lavelle GM, Azim AA, Helma K, Gargoum FS, Molloy K, Gernez Y, Dunne K, Renwick J, Murphy P, et al. The basophil surface marker CD203c identifies Aspergillus species sensitization in patients with cystic fibrosis. J Allergy Clin Immunol. 2016;137:436–43 e9. doi:10.1016/j.jaci.2015.07.045.
- Kreindler JL, Steele C, Nguyen N, Chan YR, Pilewski JM, Alcorn JF, Vyas YM, Aujla SJ, Finelli P, Blanchard M, et al. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J Clin Invest. 2010;120:3242–3254. doi:10.1172/JCI42388.
- Li X, Leonardi I, Semon A, Doron I, Gao IH, Putzel GG, Kim Y, Kabata H, Artis D, Fiers WD, et al. Response to fungal dysbiosis by gut-resident CX3CR1(+) mononuclear phagocytes aggravates allergic airway disease. Cell Host Microbe. 2018;24:847–56 e4. doi:10.1016/j.chom.2018.11.003.
- Kim YG, Udayanga KG, Totsuka N, Weinberg JB, Nunez G, Shibuya A. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE(2). Cell Host Microbe. 2014;15:95–102. doi:10.1016/j.chom.2013.12.010.
- Maurya V, Gugnani HC, Sarma PU, Madan T, Shah A. Sensitization to Aspergillus antigens and occurrence of allergic bronchopulmonary aspergillosis in patients with asthma. Chest. 2005;127:1252–1259. doi:10.1378/chest.127.4.1252.
- Moss RB. Treatment options in severe fungal asthma and allergic bronchopulmonary aspergillosis. Eur Respir J. 2014;43:1487–1500. doi:10.1183/09031936.00139513.
- Knutsen AP, Bush RK, Demain JG, Denning DW, Dixit A, Fairs A, Greenberger PA, Kariuki B, Kita H, Kurup VP, et al. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol. 2012;129:280–91;quiz 92–3. doi:10.1016/j.jaci.2011.12.970.
- Muniz VS, Silva JC, Braga YAV, Melo RCN, Ueki S, Takeda M, Hebisawa A, Asano K, Figueiredo RT, Neves JS. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J Allergy Clin Immunol. 2018;141:571–85 e7. doi:10.1016/j.jaci.2017.07.048.
- Gago S, Overton NLD, Ben-Ghazzi N, Novak-Frazer L, Read ND, Denning DW, Bowyer P. Lung colonization by Aspergillus fumigatus is controlled by ZNF77. Nat Commun. 2018;9:3835. doi:10.1038/s41467-018-06148-7.
- Xu X, Xu JF, Zheng GX, Lu HW, Duan JL, Rui W, Guan JH, Cheng LQ, Yang DD, Wang MC, et al. CARD9(S12N) facilitates the production of IL-5 by alveolar macrophages for the induction of type 2 immune responses. Nat Immunol. 2018;19:547-+. doi:10.1038/s41590-018-0112-4.
- Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006;27:615–626. doi:10.1183/09031936.06.00074705.
- Fraczek MG, Chishimba L, Niven RM, Bromley M, Simpson A, Smyth L, Denning DW, Bowyer P. Corticosteroid treatment is associated with increased filamentous fungal burden in allergic fungal disease. J Allergy Clin Immun. 2018;142:407–414. doi:10.1016/j.jaci.2017.09.039.
- Zhang Z, Biagini Myers JM, Brandt EB, Ryan PH, Lindsey M, Mintz-Cole RA, Reponen T, Vesper SJ, Forde F, Ruff B, et al. β-Glucan exacerbates allergic asthma independent of fungal sensitization and promotes steroid-resistant T2/T17 responses. J Allergy Clin Immunol. 2017;139:54–65.e8. doi:10.1016/j.jaci.2016.02.031.
- van der Velden WJ, Netea MG, de Haan AF, Huls GA, Donnelly JP, Blijlevens NM. Role of the mycobiome in human acute graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:329–332. doi:10.1016/j.bbmt.2012.11.008.
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi:10.1038/nature05414.
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi:10.1038/nature11450.
- Mar Rodriguez M, Perez D, Javier Chaves F, Esteve E, Marin-Garcia P, Xifra G, Vendrell J, Jove M, Pamplona R, Ricart W, et al. Obesity changes the human gut mycobiome. Sci Rep. 2015;5:14600. doi:10.1038/srep14600.
- Gosiewski T, Salamon D, Szopa M, Sroka A, Malecki MT, Bulanda M. Quantitative evaluation of fungi of the genus Candida in the feces of adult patients with type 1 and 2 diabetes - a pilot study. Gut Pathog. 2014;6:43. doi:10.1186/s13099-014-0043-z.
- Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173–1182. doi:10.1016/S0140-6736(04)17107-9.
- Balaji H, Heratizadeh A, Wichmann K, Niebuhr M, Crameri R, Scheynius A, Werfel T. Malassezia sympodialis thioredoxin-specific T cells are highly cross-reactive to human thioredoxin in atopic dermatitis. J Allergy Clin Immunol. 2011;128:92–9 e4. doi:10.1016/j.jaci.2011.02.043.
- Park HK, Ha MH, Park SG, Kim MN, Kim BJ, Kim W. Characterization of the fungal microbiota (mycobiome) in healthy and dandruff-afflicted human scalps. PLoS One. 2012;7:e32847. doi:10.1371/journal.pone.0032847.
- Ashbee HR, Evans EG. Immunology of diseases associated with Malassezia species. Clin Microbiol Rev. 2002;15:21–57. doi:10.1128/CMR.15.1.21-57.2002.
- Ling Y, Cypowyj S, Aytekin C, Galicchio M, Camcioglu Y, Nepesov S, Ikinciogullari A, Dogu F, Belkadi A, Levy R, et al. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med. 2015;212:619–631. doi:10.1084/jem.20141065.
- Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi:10.1126/science.1200439.
- Yang AM, Inamine T, Hochrath K, Chen P, Wang L, Llorente C, Bluemel S, Hartmann P, Xu J, Koyama Y, et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest. 2017;127:2829–2841. doi:10.1172/JCI90562.
- Sarin SK, Pande A, Schnabl B. Microbiome as a therapeutic target in alcohol-related liver disease. J Hepatol. 2019;70:260–272. doi:10.1016/j.jhep.2018.10.019.
- Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis. 2018;18:e339–e47. doi:10.1016/S1473-3099(18)30103-8.
- Steele C, Fidel PL. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect Immun. 2002;70:577–583. doi:10.1128/IAI.70.2.577-583.2002.
- Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–1767. doi:10.1056/NEJMoa0901053.
- Mølgaard-Nielsen D, Svanström H, Melbye M, Hviid A, Pasternak B. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58–67. doi:10.1001/jama.2015.17844.
- Edwards JE, Schwartz MM, Schmidt CS, Sobel JD, Nyirjesy P, Schodel F, Marchus E, Lizakowski M, DeMontigny EA, Hoeg J, et al. A fungal immunotherapeutic vaccine (NDV-3A) for treatment of recurrent vulvovaginal candidiasis-A phase 2 randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2018;66:1928–1936. doi:10.1093/cid/ciy185.
- Schwartz S, Kontoyiannis DP, Harrison T, Ruhnke M. Advances in the diagnosis and treatment of fungal infections of the CNS. Lancet Neurol. 2018;17:362–372. doi:10.1016/S1474-4422(18)30030-9.
- Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17:873–881. doi:10.1016/S1473-3099(17)30243-8.
- Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, Harrison TS. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol. 2017;13:13–24. doi:10.1038/nrneurol.2016.167.
- Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, Jousson O, Leoncini S, Renzi D, Calabro A, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5:24. doi:10.1186/s40168-017-0242-1.
- Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, Jousson O, Leoncini S, Pindo M, Renzi D, et al. Altered gut microbiota in Rett syndrome. Microbiome. 2016;4:41. doi:10.1186/s40168-016-0185-y.
- Hou H, Guo Y, Chang Q, Luo T, Wu X, Zhao X. C-type lectin receptor: old friend and new player. Med Chem. 2017;13:536–543. doi:10.2174/1573406413666170510103030.
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi:10.1038/ni1408.
- Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi:10.1038/ni1425.
- Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. Embo J. 2005;24:1277–1286. doi:10.1038/sj.emboj.7600594.
- Iliev ID. Dectin-1 exerts dual control in the gut. Cell Host Microbe. 2015;18:139–141. doi:10.1016/j.chom.2015.07.010.
- Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi:10.1084/jem.20021787.
- Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi:10.1016/j.immuni.2010.05.001.
- Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T, Gonoi T, Saito T, Okawa Y, Shibata N, Miyamoto T, et al. Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe. 2013;13:477–488. doi:10.1016/j.chom.2013.03.008.
- Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY, Jia XM, Lin X. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity. 2013;39:324–334. doi:10.1016/j.immuni.2013.05.017.
- Plato A, Hardison SE, Brown GD. Pattern recognition receptors in antifungal immunity. Semin Immunopathol. 2015;37:97–106. doi:10.1007/s00281-014-0462-4.
- Cambi A, Gijzen K, de Vries LJ, Torensma R, Joosten B, Adema GJ, Netea MG, Kullberg BJ, Romani L, Figdor CG. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol. 2003;33:532–538. doi:10.1002/immu.200310029.
- van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, van den Berg WB, Williams DL, van der Meer JW, Joosten LA, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–340. doi:10.1016/j.chom.2009.02.006.
- Dan JM, Kelly RM, Lee CK, Levitz SM. Role of the mannose receptor in a murine model of Cryptococcus neoformans infection. Infect Immun. 2008;76:2362–2367. doi:10.1128/IAI.00095-08.
- Zhao X, Guo Y, Jiang C, Chang Q, Zhang S, Luo T, Zhang B, Jia X, Hung MC, Dong C, et al. JNK1 negatively controls antifungal innate immunity by suppressing CD23 expression. Nat Med. 2017;23:337–346. doi:10.1038/nm.4260.
- Guo Y, Chang Q, Cheng L, Xiong S, Jia X, Lin X, Zhao X. C-type lectin receptor CD23 is required for host defense against Candida albicans and Aspergillus fumigatus Infection. J Immunol. 2018;201:2427–2440. doi:10.4049/jimmunol.1800620.
- Moretti S, Bellocchio S, Bonifazi P, Bozza S, Zelante T, Bistoni F, Romani L. The contribution of PARs to inflammation and immunity to fungi. Mucosal Immunol. 2008;1:156–168. doi:10.1038/mi.2007.13.
- Loures FV, Pina A, Felonato M, Calich VL. TLR2 is a negative regulator of Th17 cells and tissue pathology in a pulmonary model of fungal infection. J Immuno (Baltimore, Md: 1950). 2009;183:1279–1290. doi:10.4049/jimmunol.0801599.
- Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi:10.1038/nature07965.
- Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, Fitzgerald KA, Hise AG. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 2011;7:e1002379. doi:10.1371/journal.ppat.1002379.
- Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011;29:1–21. doi:10.1146/annurev-immunol-030409-101229.
- Becker KL, Ifrim DC, Quintin J, Netea MG, van de Veerdonk FL. Antifungal innate immunity: recognition and inflammatory networks. Semin Immunopathol. 2015;37:107–116. doi:10.1007/s00281-014-0467-z.
- Leonardi I, Li X, Semon A, Li D, Doron I, Putzel G, Bar A, Prieto D, Rescigno M, McGovern DPB, et al. CX3CR1(+) mononuclear phagocytes control immunity to intestinal fungi. Science. 2018;359:232–236. doi:10.1126/science.aao1503.
- Wheeler ML, Limon JJ, Underhill DM. Immunity to commensal fungi: detente and disease. Annu Rev Pathol. 2017;12:359–385. doi:10.1146/annurev-pathol-052016-100342.
- Bar E, Whitney PG, Moor K, Reis E Sousa C, LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity. 2014;40:117–127. doi:10.1016/j.immuni.2013.12.002.
- Romani L, Puccetti P. Protective tolerance to fungi: the role of IL-10 and tryptophan catabolism. Trends Microbiol. 2006;14:183–189. doi:10.1016/j.tim.2006.02.003.
- Wuthrich M, Deepe GS Jr., Klein B. Adaptive immunity to fungi. Annu Rev Immunol. 2012;30:115–148. doi:10.1146/annurev-immunol-020711-074958.
- Xiao Y, Tang J, Guo H, Zhao Y, Tang R, Ouyang S, Zeng Q, Rappleye CA, Rajaram MV, Schlesinger LS, et al. Targeting CBLB as a potential therapeutic approach for disseminated candidiasis. Nat Med. 2016;22:906–914. doi:10.1038/nm.4141.
- Wuthrich M, Filutowicz HI, Warner T, Deepe GS Jr., Klein BS. Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts. J Exp Med. 2003;197:1405–1416. doi:10.1084/jem.20030109.
- Kumaresan PR, da Silva TA, Kontoyiannis DP. Methods of controlling invasive fungal infections using CD8(+) T cells. Front Immunol. 2017;8:1939. doi:10.3389/fimmu.2017.01939.
- Kashem SW, Igyarto BZ, Gerami-Nejad M, Kumamoto Y, Mohammed JA, Jarrett E, Drummond RA, Zurawski SM, Zurawski G, Berman J, et al. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity. 2015;42:356–366. doi:10.1016/j.immuni.2015.01.008.
- Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11:275–288. doi:10.1038/nri2939.
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi:10.1038/ni1460.
- Bishu S, Hernandez-Santos N, Simpson-Abelson MR, Huppler AR, Conti HR, Ghilardi N, Mamo AJ, Gaffen SL. The adaptor CARD9 is required for adaptive but not innate immunity to oral mucosal Candida albicans infections. Infect Immun. 2014;82:1173–1180. doi:10.1128/IAI.01335-13.
- Rivera A. Hohl T. M., Collins N., Leiner I., Gallegos A., Saijo S., Coward J. W., Iwakura Y., Pamer E. G. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J Exp Med. 2011;208:369–381. doi:10.1084/jem.20100906.
- Conti HR, Gaffen SL. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J Immunol. 2015;195:780–788. doi:10.4049/jimmunol.1500909.
- O’Meara TR, Veri AO, Ketela T, Jiang B, Roemer T, Cowen LE. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat Commun. 2015;6:6741. doi:10.1038/ncomms7741.
- Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16:2161–2165. doi:10.1016/j.cub.2006.09.061.
- Gazi U, Rosas M, Singh S, Heinsbroek S, Haq I, Johnson S, Brown GD, Williams DL, Taylor PR, Martinez-Pomares L. Fungal recognition enhances mannose receptor shedding through dectin-1 engagement. J Biol Chem. 2011;286:7822–7829. doi:10.1074/jbc.M110.185025.
- Wevers BA, Kaptein TM, Zijlstra-Willems EM, Theelen B, Boekhout T, Geijtenbeek TB, Gringhuis SI. Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host Microbe. 2014;15:494–505. doi:10.1016/j.chom.2014.03.008.
- Hernandez-Santos N, Klein BS. Through the scope darkly: the gut mycobiome comes into focus. Cell Host Microbe. 2017;22:728–729. doi:10.1016/j.chom.2017.11.013.
- Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi:10.1038/nrc3581.
- Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg BJ, Wijmenga C, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12:223–232. doi:10.1016/j.chom.2012.06.006.
- Tso GHW, Reales-Calderon JA, Tan ASM, Sem X, Le GTT, Tan TG, Lai GC, Srinivasan KG, Yurieva M, Liao W, et al. Experimental evolution of a fungal pathogen into a gut symbiont. Science. 2018;362:589–595. doi:10.1126/science.aat0537.
- Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, Kleinnijenhuis J, Lachmandas E, Goncalves LG, Belinha A, et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 2016;17:2562–2571. doi:10.1016/j.celrep.2016.11.011.
- Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–361. doi:10.1016/j.chom.2011.04.006.
- Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O’Neill LA, Xavier RJ. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi:10.1126/science.aaf1098.
- Armstrong-James D, Meintjes G, Brown GD. A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol. 2014;22:120–127. doi:10.1016/j.tim.2014.01.001.
- Fukuda T, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG, Martin PJ, Storb RF, Marr KA. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102:827–833. doi:10.1182/blood-2003-02-0456.
- Bassetti M, Garnacho-Montero J, Calandra T, Kullberg B, Dimopoulos G, Azoulay E, Chakrabarti A, Kett D, Leon C, Ostrosky-Zeichner L, et al. Intensive care medicine research agenda on invasive fungal infection in critically ill patients. Intensive Care Med. 2017;43:1225–1238. doi:10.1007/s00134-017-4731-2.
- Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science. 2018;360:739–742. doi:10.1126/science.aap7999.
- Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25–31. doi:10.1086/cid.2006.43.issue-1.
- Arvanitis M, Anagnostou T, Fuchs BB, Caliendo AM, Mylonakis E. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin Microbiol Rev. 2014;27:490–526. doi:10.1128/CMR.00091-13.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247–280. doi:10.1128/CMR.00053-10.
- Ostrosky-Zeichner L, Alexander BD, Kett DH, Vazquez J, Pappas PG, Saeki F, Ketchum PA, Wingard J, Schiff R, Tamura H, et al. Multicenter clinical evaluation of the (1–>3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654–659. doi:10.1086/432470.
- Weiner MH, Coats-Stephen M. Immunodiagnosis of systemic candidiasis: mannan antigenemia detected by radioimmunoassay in experimental and human infections. J Infect Dis. 1979;140:989–993. doi:10.1093/infdis/140.6.989.
- Bailey JW, Sada E, Brass C, Bennett JE. Diagnosis of systemic candidiasis by latex agglutination for serum antigen. J Clin Microbiol. 1985;21:749–752. doi:10.1128/JCM.21.5.749-752.1985.
- Cao L, Chen DL, Lee C, Chan CM, Chan KM, Vanittanakom N, Tsang DN, Yuen KY. Detection of specific antibodies to an antigenic mannoprotein for diagnosis of Penicillium marneffei penicilliosis. J Clin Microbiol. 1998;36:3028–3031.
- Stynen D, Goris A, Sarfati J, Latge JP. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J Clin Microbiol. 1995;33:497–500. doi:10.1128/JCM.33.2.497-500.1995.
- Yeo SF, Wong B. Current status of nonculture methods for diagnosis of invasive fungal infections. Clin Microbiol Rev. 2002;15:465–484. doi:10.1128/CMR.15.3.465-484.2002.
- Wickes BL, Wiederhold NP. Molecular diagnostics in medical mycology. Nat Commun. 2018;9:5135. doi:10.1038/s41467-018-07556-5.
- Alanio A, Bretagne S. Difficulties with molecular diagnostic tests for mould and yeast infections: where do we stand? Clin Microbiol Infect. 2014;20(Suppl 6):36–41. doi:10.1111/1469-0691.12617.
- Klingspor L, Jalal S. Molecular detection and identification of Candida and Aspergillus spp. from clinical samples using real-time PCR. Clin Microbiol Infect. 2006;12:745–753.
- Scheel CM, Zhou Y, Theodoro RC, Abrams B, Balajee SA, Litvintseva AP. Development of a loop-mediated isothermal amplification method for detection of Histoplasma capsulatum DNA in clinical samples. J Clin Microbiol. 2014;52:483–488. doi:10.1128/JCM.02739-13.
- Huseyin CE, Rubio RC, O’Sullivan O, Cotter PD, Scanlan PD. The fungal frontier: a comparative analysis of methods used in the study of the human gut mycobiome. Front Microbiol. 2017;8:1432. doi:10.3389/fmicb.2017.01432.
- Iwen PC, Hinrichs SH, Rupp ME. Utilization of the internal transcribed spacer regions as molecular targets to detect and identify human fungal pathogens. Med Mycol. 2002;40:87–109. doi:10.1080/mmy.40.1.87.109.
- Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Fungal Barcoding Consortium, Fungal Barcoding Consortium Author List. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci U S A. 2012;109:6241–6246. doi:10.1073/pnas.1117018109.
- Bittinger K, Charlson ES, Loy E, Shirley DJ, Haas AR, Laughlin A, Yi Y, Wu GD, Lewis JD, Frank I, et al. Improved characterization of medically relevant fungi in the human respiratory tract using next-generation sequencing. Genome Biol. 2014;15:487.
- Procop GW. Molecular diagnostics for the detection and characterization of microbial pathogens. Clin Infect Dis. 2007;45(Suppl 2):S99–S111. doi:10.1086/519259.
- Lindahl BD, Nilsson RH, Tedersoo L, Abarenkov K, Carlsen T, Kjøller R, Kõljalg U, Pennanen T, Rosendahl S, Stenlid J, et al. Fungal community analysis by high-throughput sequencing of amplified markers–a user’s guide. New Phytol. 2013;199:288–299. doi:10.1111/nph.12243.
- Heisel T, Podgorski H, Staley CM, Knights D, Sadowsky MJ, Gale CA. Complementary amplicon-based genomic approaches for the study of fungal communities in humans. PLoS One. 2015;10:e0116705. doi:10.1371/journal.pone.0116705.
- Buchan BW, Ledeboer NA. Emerging technologies for the clinical microbiology laboratory. Clin Microbiol Rev. 2014;27:783–822. doi:10.1128/CMR.00003-14.
- Rigby S. Procop G. W., Haase G., Wilson D., Hall G., Kurtzman C., Oliveira K., Von Oy S., Hyldig-Nielsen J. J., Coull J., Et Al. Fluorescence in Situ Hybridization with Peptide Nucleic Acid Probes for Rapid Identification of Candida Albicans Directly from Blood Culture Bottles. J Clin Microbiol. 2002;40:2182–2186.
- Neely LA, Audeh M, Phung NA, Min M, Suchocki A, Plourde D, Blanco M, Demas V, Skewis LR, Anagnostou T, et al. T2 magnetic resonance enables nanoparticle-mediated rapid detection of candidemia in whole blood. Sci Transl Med. 2013;5:182ra54. doi:10.1126/scitranslmed.3005377.