ABSTRACT
There is now strong evidence to support the interest in using lactic acid bacteria (LAB)in particular, strains of lactococci and lactobacilli, as well as bifidobacteria, for the development of new live vectors for human and animal health purposes. LAB are Gram-positive bacteria that have been used for millennia in the production of fermented foods. In addition, numerous studies have shown that genetically modified LAB and bifodobacteria can induce a systemic and mucosal immune response against certain antigens when administered mucosally. They are therefore good candidates for the development of new mucosal delivery strategies and are attractive alternatives to vaccines based on attenuated pathogenic bacteria whose use presents health risks. This article reviews the most recent research and advances in the use of LAB and bifidobacteria as live delivery vectors for human and animal health.
Introduction
Lactic acid bacteria (LAB) are Gram-positive bacteria that produce lactic acid as the main end-product of glucose fermentation. LAB are classified according to their phenotypic and metabolic characteristics such as cellular morphology, growth conditions (as temperature and sugar utilization) and the production of substances to inhibit the proliferation of other microorganisms during the fermentation process. LAB are members of the phylum Firmicutes, and order LactobacillalesCitation1. Concerning bifidobacteria, even if some of their morphological and metabolic characteristics (as the production of lactic acid during fermentation), led them to be classified initially as members of LAB, currently are recognized as part of an independent genus, Bifidobacterium, that belongs to Actinobacteria phylum and the Bifidobacteriales order.Citation2,Citation3 However, it would not be surprising that within a few years this classification will face considerable changes. Indeed, as Hugenholtz et al. point out in their recent review article, the increasing number of genomic sequences derived from unculturable prokaryotes makes taxonomic classification a major challenge for consensus and adaptation.Citation4 An important number of species belonging to both LAB and Bifidobacterium are widely used in industrial food fermentation processes and some genera are inhabitants of the intestinal microbiota.Citation5,Citation6 In addition, some of these species, when ingested in appropriate quantities, can survive passage through the digestive tract and exert different beneficial actions (e.g., improving fiber digestion, stimulating the immune system, and preventing or treating diarrhea); moreover, as mentioned above they are classified as GRAS and QPS microorganisms and several strains are considered as probiotics. Probiotics have been defined by the World Health Organization (WHO) in 2011, but a group of experts reexamined the concept in 2014 to reach a consensus definition as follows: “live microorganisms that, when administered in adequate amounts, confer a health benefit to the host”.Citation7
For all these reasons, the use of LAB and Bifidobacterium as vectors for biologically active molecules is a strategy that has aroused great interest, notably on the development of new live mucosal delivery vectors. In addition, mucosal vectors (e.g., administered orally, intranasally, vaginally, etc.), are more convenient than classical systemic routes of administration, because they are easier to administer and relatively cheaper to produce. For instance, the human mucosa (including the gastrointestinal, respiratory, and urogenital tracts) represents a major contact surface estimated at approximately 400 m2,Citation2,Citation8 besides containing a highly developed immune system: the Mucosa Associated Lymphoid Tissue (MALT) consisting of about 80% of the body’s immune cells and considered the most important lymphoid system in human.Citation9–11
LAB and Bifidobacterium as new live delivery vectors
The constant need to develop safer, easier to administer and cheaper vectors, such as vaccines, has led to intensive research on the possible use of live genetically modified microorganisms (GMM) as carriers of proteins of interest, such as protective antigens, especially for in situ administration. Attention has therefore turned to the use of Gram-positive and commensal LAB as protein delivery vectors. In this sense, the genetic and molecular studies carried out in the last years, mainly in Lactoccoccus lactis and some species of lactobacilli, have demonstrated that these bacteria can be used for this purpose in the prevention and treatment of diseases (). Different studies have revealed the potential of these bacteria to be used as drug carriers or to produce therapeutic molecules due to their intrinsic (adjuvant and immunomodulatory) natural properties.Citation12 These bacteria are also of interest from a technological point of view because some strains are resistant to low pH and therefore can survive the passage through the gastrointestinal tract and can adhere to the intestinal epithelium which makes them interesting for oral vaccine. Even today, other strategies have been developed to preserve bacterial cells during their passage through the stomach, such as encapsulation in microparticles or liposomes, which further improves their adhesion to the mucosa. Another alternative is the use of liquid carriers with protective properties such as trehalose.Citation13 In addition, they do not need to be stored at low temperatures because they can be lyophilized.Citation14 Their complete safety due to generally recognized as safe (GRAS status, FDA, USA)Citation15 “GRAS” status and with qualified presumption of safety (QPS, EFSA, Europe), combined with the ability of some of them to colonize external body cavities, makes
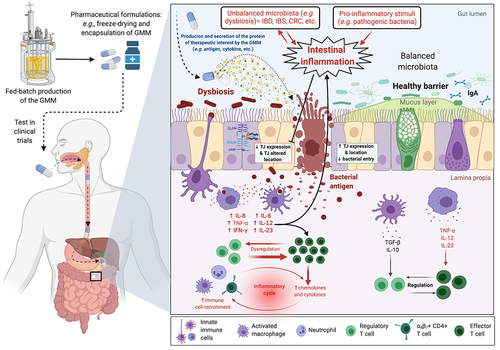
Figure 1. Schematic overview of the production (e.g., fed-batch production) of a genetically modified microorganism (GMM) to deliver a therapeutic molecule. Example of the in situ production and of a protein with anti-inflammatory properties by a GM L. lactis strain in the context of intestinal inflammation.
LAB, in particular lactobacilli, the candidates of choice to deliver vaccine antigens to the mucosa. These bacteria have the additional advantage of being easily administered orally or locally. Thus, the use of LAB as antigen vectors is a safer and less expensive strategy. In addition, these bacteria have already been used to express and deliver several proteins of medical interest.
Genetic engineering of LAB and Bifidobacteria strains Use of Lactococcus lactis as a live delivery vector
Lactis is a Gram-positive bacterium widely used in the manufacture of dairy products, especially cheese. In addition, this bacterium can synthesize bacteriocins,Citation16 which generally prevents the development of undesirable microorganisms in dairy products and helps to preserve the hygienic quality of the products. The numerous studies carried out on this bacterium have made it possible to characterize essential genes or genes of technological interest (genes involved in metabolism, stress resistance, growth, etc.), and to elucidate their expression mechanisms.Citation17 Increasingly efficient study tools and techniques have been developed such as mutagenesis and chromosomal integration systems for genes of interest, cloning and constitutive or inducible expression vectors, systems for targeting heterologous proteins in different compartments of the bacterium.Citation18,Citation19 In addition, the genomes of L. lactis subsp. lactis IL1403 and cremoris MG1363 have been sequenced.Citation20,Citation21 Thanks to this work, L. lactis is considered as the model LAB and is one of the best characterized bacteria along with E. coli and Bacillus subtilis. The development of knowledge on its capacities to produce and secret heterologous proteins makes it a good candidate for the secretion of proteins of therapeutic interest.
L. lactis has been widely studied and manipulated in recent years for the production of heterologous proteins such as several viral or bacterial antigens, as well as biologically active molecules (cytokines, hormones, etc.).Citation19,Citation22 In this context, our team has developed several tools to optimize the production of heterologous proteins in this bacterium, which we describe in the next paragraphs.
Vectors for heterologous protein expression in L. lactis
Currently, a wide range of constitutive or inducible expression systems have been described in L. lactis.Citation19,Citation22,Citation23 Based on these studies, we have developed in our laboratory a system for the production-export of heterologous proteins in L. lactis using a very stable and well-characterized model secreted protein, Staphylococcus aureus nuclease (Nuc).Citation24 This system is composed of a family of vectors that allow controlled targeting of protein expression inside the cell, secreted into the external environment or anchored to the cell-wall, pCYT, pSEC, and pCWA, respectively, under the control of the nisin-inducible promoter (PnisA)Citation25–28 (). In addition, these vectors are functional in a wide range of LAB and bifidobacteria, as will be shown below, and have been used successfully for the production of numerous heterologous proteins in L. lactis ().
Figure 2. Family of vectors that allow controlled expression and export of proteins in L. lactis. (A) Schematic structures of different expression cassettes (left) under the control of the lactococcal PnisA promoter for the indicated specific bacterial cellular localization and carried by the specified plasmids. For details of the plasmid constructions see the text. Stems topped with circles indicate the tryptophan transcriptional terminator (trpA). Not to scale. (B) Graphical representation on the production of the desired protein by using the plasmid indicated for the different bacterial localization of interest in L. lactis. pCYT: to obtain the expression of a protein in the cytoplasm, the gene of interest is fused only to the PnisA promoter. pSEC: in which the secretion pathway used is the Sec-dependent pathway. It recognizes proteins synthesized with an N-terminal signal peptide (SP) and ensures their export and translocation. It is worth highlighting that the nature of the SP used to secrete a protein can greatly influence the secretory efficiency of the protein. Thus, one of the most efficient SP for secreting heterologous proteins in L. lactis is that of the Usp45 protein (i.e. SPUsp45), which is the majority protein secreted by L. lactis 26. Indeed, this SPUsp45 has been used to export many heterologous proteins in L. lactis 27. pCWA: to obtain a protein anchored to the bacterial wall, the gene of interest is fused to SPUsp45 and the anchoring domain of the S. pyogenes M6 protein (CWAM6). This domain contains the necessary signals for wall anchoring 28. This figure was created with Biorender.com (accessed date: 9th June 2022).
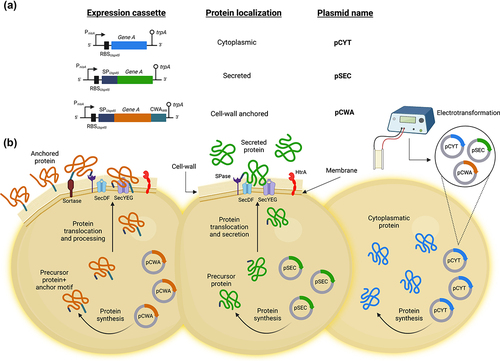
Table 1. Some examples of heterologous protein production in L. lactis for vaccination purposes.
The Nisin Induced Controlled Expression (NICE) system
Nisin is a bacteriocin from L. lactis widely used as an antimicrobial substance in the food industry. Eleven adjacent chromosomal genes (nisABTCIPRKFEG) are encoded for nisin biosynthesis and immunity.Citation46 The nisA gene corresponds to the structural gene for nisin and the nisRK genes constitute the two-component system responsible for the induction of other genes in the cluster. The study of nisin biosynthesis regulation has revealed the process by which extracellular nisin binds to the
NisK protein which autophosphorylates and becomes capable of activating the NisR regulator by phosphorylation. NisR then activates the transcription of the nisABTCIP and nisFEG operons. Characterization of the regulation of this system has led to the development of an inducible expression system in L. lactis using the nisA gene promoter and the nisRK genes.Citation47 The gene of interest is cloned downstream of the PnisA promoter and the plasmid is introduced into a strain possessing the nisRK genes (i.e. L. lactis NZ9000). The addition of sub-inhibitory amounts of nisin in the culture medium triggers the expression of the gene of interest in proportion to the amount of nisin present.
This versatile system is now widely used to express heterologous proteins not only in L. lactis but also in other LAB.Citation47 However, it requires the presence of regulatory genes (see above), which limits the choice of appropriate production conditions in both biotechnological and laboratory applications, and the induction of GM strain cultures prior to their use in vivo.
An alternative is the use of expression systems that require neither the presence of regulatory genes nor the pre-induction of the cultures prior to their use. In fact, in recent years several regulated expression systems have been described for LAB, in which gene expression can be controlled by an inducer, a repressor or by environmental factors, such as pH, sugar, temperature, or ion concentrations.Citation48 In the following paragraphs, we will present two new systems developed by our group and collaborators in which we exploit both stress- and sugar-inducible systems for the production of heterologous proteins in L. lactis.
The Stress-Inducible Controlled Expression (SICE) system
Heat-shock proteins are a conserved group of proteins (among which are the groESL and DnaKJ-GrpE chaperone complexes) synthesized in response to different stress stimuli such as heat-shock, low pH, UV irradiation, or salts stress. The SICE system is based on the use of the groESL heat shock protein operon promoter from L. lactis. This episomal system is composed of a vector that carries an expression cassette under the transcriptional control of a stress-inducible promoter.Citation49 In this system, the expression of the protein of interest is induced after administration to the host, since the GM bacterium finds different conditions than those of the culture and suffers different types of stress. Heat stress due to the body temperature of the host higher than the optimal growth temperature of the bacteria; and in the case of oral administration, the acid stress during passage through the stomach added to biliary stress in the duodenum are examples of stresses able to induce expression in this system, and allow the in situ production of the molecule of interest. The main advantage of this system is that it does not require the presence of regulatory genes or the induction in cultures before use.Citation50,Citation51
Xylose-Inducible Expression System (XIES)
This is a controlled production system that allows target heterologous proteins to cytoplasm or extracellular medium. It was described for L. lactis NCDO2118 and uses a xylose-inducible lactococcal promoter, PxylTCitation52(Miyoshi, 2004). The system was tested using the Staphylococcus aureus nuclease gene (nuc) to produce cytoplasmic and secreted proteins when they are fused or not to the SPUsp45 (see above ). Nuc is considered a good reporter protein because its activity is very easily detected in vivo by a staining test on bacterial colonies, in liquid culture or extracted from the digestive tract. This expression system can be switched on or off by adding either xylose or glucose, respectively, and was also used for the production of proteins of interest in L. lactis.Citation53,Citation54
Other vectors and cellular factors for improved production and secretion of heterologous proteins in L. lactis
In addition, another expression vector was further developed containing a synthetic propeptide (LEISSTCDA), which has been identified as an enhancer of production and secretion in L. lactis.Citation55,Citation56 Moreover, considering host factors affecting production-secretion, the ybdD gene was identified; and the inactivation of this gene resulted in overproduction of only secreted proteins by a mechanism not yet elucidated. L. lactis secretion machinery was also complemented with SecDF from Bacillus subtilis and it was observed that the resulting strain improved production and secretion.Citation57 Finally, we have also developed in our team to complete our toolbox of heterologous protein expression in L. lactis, a strain that produces neither the unique extracellular protease HtrACitation58 nor the major intracellular protease ClpP.Citation59 This double mutant strain allows a controlled and stable production of different heterologous proteins that are otherwise highly degraded in the wild-type strain.Citation60
In summary, numerous studies currently support the use of GM strains of L. lactis to induce both mucosal and systemic immune response.Citation19,Citation22 In this context, the first attempt to evaluate the potential of L. lactis as a mucosal vaccine, more than 30 years ago, was performed with a GM strain of this bacterium producing an anchored form of the protective antigen (PAc) of Streptococcus (S.) mutans.Citation61 This study shows for the first time that L. lactis can be used as a live mucosal vector to efficiently deliver an antigen to the immune system. Then, Wells et al. reported that the use of a live GM L. lactis strain producing tetanus toxin fragment C (TTFC) as a model antigen was able to protect mice against a lethal challenge with tetanus toxin after subcutaneous administration of this strain.Citation62 Later, the same group evaluated the effect of oral or nasal administration of GM TTFC-producing strains of lactococci in mice.Citation63,Citation64 Oral immunization in mice with these GM lactococci results in a lower humoral response (i.e., TTFC-specific serum IgG and mucosal IgA antibodies) than intranasal administration, but the measured protective efficacy (challenge with tetanus toxin) was the same.
Following these pioneering studies, several works have evaluated the expression of numerous heterologous proteins of viral, bacterial, or eukaryotic origin in L. lactis (). The immunogenicity of the resulting GM strains was also evaluated in animal models with very promising results. In particular, our team succeeded (as mentioned above) in producing about 40 heterologous proteins in L. lactis (). Since our main goal was to induce an immune response at the host mucosal level, our efforts were mainly focused on the presentation of medically relevant proteins such as cytokines, antigens, allergens, and antioxidants by LAB.
Altogether, our results together with findings from other teams confirm the potential of GM strains of L. lactis as live mucosal vectors of proteins of health interest and open the way for such GM LAB strains to be approved to bring new solutions in the future for disease prevention and treatment.
Lactobacilli as a live delivery vector
Unlike L. lactis, some lactobacilli species can colonize certain regions of the mucosa and induce a local immune response, which is an advantage in vaccine development. Furthermore, the possible use of certain probiotic strains of lactobacilli as a mucosal delivery vehicle is an additional advantage.Citation65 However, the biodiversity of this genus makes its use as a vector for live delivery vector more complex than that of L. lactis, where only one strain (MG1363) has been used. Indeed, this genus is widespread and contains more than 60 species that differ in their biochemical, ecological, and immunological properties. However, the ability of the genus Lactobacillus to produce antigens has also been demonstrated. Nonetheless, the ability of the Lactobacillus genus to produce antigens has also been demonstrated; in fact, different studies have shown that vaccines based on lactobacilli are capable of inducing a strong humoral and cellular immune response in both blood and mucosa when administered orally or intranasally (Yu et al., 2013).
Production of heterologous proteins in Lactobacillus spp.
In the late 1990s and early 2000s, several laboratories reported the use of GM strains of lactobacilli as live vectors to deliver proteins of medical interest to the mucosal surface.Citation23,Citation65 Thus, we decided to transfer all our knowledge on the production of heterologous proteins acquired in L. lactis to Lactobacillus strains (more specifically to Lactobacillus casei and Lactiplantibacillus plantarum strains), which present the double interest of transiting slower in the digestive tract and display interesting adjuvant properties.
The Lactobacilli In Vivo Expression (LIVE) system
This system consists of a plasmid that allows the production and secretion of inducible heterologous proteins in vivo by several strains of lactobacilli.Citation66 Indeed, for the construction of this plasmid, called pLB210, the stress-inducible promoter Plp_07755 from L. plantarum WCFS1 was cloned into the plasmid pLB141Citation48 and then used to transform the lactobacillus strains. Thus, it was confirmed that this LIVE system is an in vivo-regulated expression system that is mainly induced by stress conditions such as exposure to high temperature or high concentrations of bile salts.Citation66
The LIVE system was used for the expression of the anti-inflammatory cytokine IL-10 as a therapy against intestinal inflammation. To this end, a DNA fragment coding for murine IL-10 was cloned into the plasmid pLB210 and used to transform different species of lactobacilli. The GM bacterium was then evaluated in a murine model of IBD and the results demonstrated the efficacy of the LIVE system to produce and deliver the therapeutic molecule in the mucosal surface, since an increase in the ratio of anti-inflammatory/pro-inflammatory cytokines was evidenced at intestinal level.Citation66
In addition, the LIVE system was also evaluated in an immunization model against a bacterial pathogen, evaluating the antigen GbpB production and stimulation of the immune response against a cariogenic strain S. mutans with the aim of combating dental caries. Similarly, the GbpB gene from Streptococcus mutans was cloned into the plasmid pLB210, which was used to transform the lactobacillus strain. The GM strain expressing the GbpB antigen was used to immunize mice and the results showed that the LIVE system allowed the expression of the antigen and stimulation of the host’s immune response.Citation66–68
This system has advantages over to expression systems based on preinduction prior to oral administration or genetic mutation on chromosomal bacterial DNA.
Production of heterologous proteins in Lactobacillus sppSimilar
To L. lactis, several works have reported the expression of a variety of heterologous proteins of viral, bacterial, or eukaryotic origin in lactobacilli (). For instance, the use of GM lactobacilli to produce heterologous proteins and to develop a new generation of mucosal vaccines was first proposed in the early 1990s, but most of our current knowledge on their use as a live vaccine was obtained with the model antigen TTFC.Citation81,Citation82 Indeed, taking into account the positive results obtained with the expression of tetanus toxin fragment C (TTFC) in L. lactis, similar tools were applied in lactobacilli. In the first study, cell extracts of the GM Lactobacillus casei expressing TTFC were used to parenterally immunize mice and to evaluate the immune response. In addition, in another study, GM Lactobacillus strains producing TTFC in three different cellular locations: cytoplasm, secreted, or anchored to the cell-surface were used to immunize mice by subcutaneous, intranasal, and intragastrical routes. In both studies, the induction of the immune response against TTFC was observed.Citation83,Citation84
Table 2. Some examples of heterologous protein production in lactobacilli for vaccination purposes.
Our team also explored the immunogenicity of a GM strain of L. plantarum producing the HPV-16 E7 antigen in animal models with very promising results.Citation85 We have also developed GM L. casei strains producing antioxidant enzymes such as catalases or superoxide dismutase. The beneficial effects of oral administration of lactobacilli strains with antioxidant properties in a murine model of inflammation were evaluated in order to reproduce Inflammatory Bowel Disease (IBD) syndromes. IBD is a disease caused by abnormal inflammation of the intestinal tract that leads to gastrointestinal dysfunction. These diseases, often disabling and of long duration, are characterized by an excess of active oxygenated derivatives, accompanied by decreased capacities of the antioxidant systems as well as an imbalance between pro- and anti-inflammatory cytokines. First, a manganese-dependent catalase (MnKaT) from L. plantarumCitation86 was expressed in L. casei BL23 and its anti-inflammatory effect was evaluated in a mouse model of colitis induced by DSS (Dextran Sulfate Sodium). The results showed that a daily oral administration of both wild-type and MnKat catalase-producing L. casei strains significantly limited inflammation in the cecum and colon, in contrast to control mice treated with PBS alone, in which diarrhea and mucosal lesions were observed.Citation86 Then, in order to improve the antioxidant potential of these strains, the L. lactis soda gene
was expressed in the L. casei BL23 strain, and tested for the manganese superoxide dismutase (MnSOD) activity as well as an antioxidant and anti-inflammatory effect.Citation87
In another study, Chang et al. constructed a GM strain of L. jensenii, a commensal vaginal bacterium, to express and secrete a domain of the human immunodeficiency virus (HIV) gp120 binding protein, CD4, and demonstrated that co-incubation of this GM bacterium with an HIV virus carrying the luciferase reporter gene (e.g., HIV-1HxB2) results in a significant decrease in the entry of this virus into HeLa cells (expressing the CD4-CXCR4-CCR5 receptor) in vitro.Citation88
It has also been reported that the immune response obtained is related to the different origins of the strains. Two GM strains LA4356-pH and DLD17-pH that express the foreign HPAI virus protein hemaglutinin 1 (HA1) were constructed and orally administered to mice in which mucosal and systemic immune responses were assessed. It was observed that both GM strains were able to increase the anti-HA1 IgA antibodies’ level in the mucosa and the anti-HA1 IgG level in serum. However, DLD17-pH induced a mucosal immune response in both the digestive and respiratory tracts while LA4356-pH only in the digestive tract. This difference was attributed to the different origin of the strains, since DLD17 was isolated from chicken gut and therefore DLD17-pH could better adapt and survive in the intestine and persistently stimulate the immune response, while LA4356 was isolated from human pharynx and therefore the adhesion to intestine of this strain could be weak, resulting in a lower mucosal immune response.Citation89 Later, studies showed that in addition to the origin of the strain used for the delivery to the host, other variables can influence the immune response obtained such as the different expression systems used that can affect antigen expression levelsCitation90 or even the chosen immunization route.Citation91 The mode of presentation of the antigen (cytoplasmic, secreted, or associated with the cell surface) is also a factor that influences the immune response.Citation92
In conclusion, different studies have shown that it is possible to enhance the immune response induced by lactobacilli vaccine vectors against pathogens. Furthermore, besides their application as vaccines, lactobacilli can also be used to deliver anti-infective molecules or antimicrobial products in situ. An example is the use of GM strains of lactobacilli to prevent dental caries in an animal model.Citation93
Bifidobacterium spp. as live mucosal delivery vector
The bifidobacteria genus is included within the Actinobacteria phylum and constitutes one of the dominant populations of the human intestinal microbiota, especially in infants. Some strains of bifidobacteria showed beneficial effects in the prevention and treatment of diseases and are therefore considered probiotic microorganisms.Citation94 Bifidobacterium spp. has advantages over lactobacilli and lactococci for its use as live vector for protein delivery. Certain strains have been shown to reside longer in the digestive tract than strains of lactobacilli and lacotococci (which do not colonize the intestine). On the other hand, bifidobacteria have low resistance to antibiotics (intrinsic and acquired), being safe for use in humans.Citation95 There are only few studies about the use of bifidobacteria as vectors for the delivery of heterologous proteins due to considering them complex to manipulate genetically and only some strains have been used for this purpose. GM bifidobacteria has been used for cancer therapy,Citation96,Citation97 and as live vaccine to express antigens of pathogenic bacteria.Citation98–100
Bifidobacteria Expression SysTem (BEST)
This system, similar to NICE and SICE systems for lactococci and LIVE system for lactobacilli, is a controlled expression system for the delivery of heterologous proteins in bifodobacteria. This system has three constituents: a broad-host range plasmid pWV01, a stress inducible dnaK promoter from Bifidobacterium (B.) longum dnaK operon, and two different signal peptides (SPs): one issued from L. lactis (SPExp4) and one from B. longum (SPBL1181).Citation95
The BEST system was used to express the murine anti-inflammatory cytokine IL-10 in B. bifidum. For the construction of the plasmid, the promoter of the DnaK operon of B. longum was cloned into the plasmid pLB270 resulting in pBESTExp4:Nuc. The PnisA promoter was replaced by PDnaK since the dnak operon encodes for heat-shock proteins and the transcription of these proteins is increased by exposure to bile salts and different pH. The nuc genes were replaced by the murine IL-10 gene obtaining the plasmid pBESTExp4:IL-10 which was established in B. bifidum BS42. In order to increase the levels of secreted IL-10, SPExp4 from L. lactis was replaced by SPBL1181 from B. longum. The in vivo results showed that the GM bacteria were capable of delivering IL-10 at intestinal level with better results for B. bifidum harboring pBESTBL1181:IL10 plasmid than for B. bifidum harboring pBESTExp4:IL-10 plasmid, and this cytokine was capable of exerting its anti-inflammatory effect in an IBD murine model.Citation95
Applications of GM LAB in health
Virus infections. HPV-16 is one of the viruses with oncogenic potential found (along with type 18) in more than 90% of cervical cancers (300,000 deaths per year worldwide).Citation101,Citation102 Current strategies to prevent or treat the infection with this virus are promising but expensive, limiting their use in developing countries where there are about 80% of HPV-related cancer deaths. Prophylactic vaccines based on virus-like particles (VLPs) have recently induced significant reductions in HPV-16 and HPV-18 infections and associated cancers in human clinical trials. L. lactis was GM to deliver two proteins: i) the HPV-16 E7 antigen, a protein consistently found in carcinomas caused by HPV infections and one of the candidate antigens for the development of anti-HPV therapy, and ii) interleukin-12 (IL-12), a stimulatory molecule of the cellular immune response during infections.Citation103
A GM strain of L. lactis was constructed to secret the E7 antigen, which is described as a very labile protein.Citation104 E7 was also targeted to the expression in three different locations (i.e. cytoplasm, wall and extracellular medium), and it was demonstrated an E7-specific immune response in mice following nasal administration with the three GM strains expressing E7. It was also observed in an increased immunogenicity of the anchored form of E7.Citation105
Recently, GM LAB strains have been also constructed to fight against respiratory virus infection. Severe Acute Respiratory Syndrome Coronavirus −2 (SARS-CoV-2) is a novel member of beta-coronavirus that causes a severe respiratory syndrome called coronavirus disease-19 (COVID-19). The COVID-19 pandemic was declared by the WHO due to the rapid spread of the virus worldwide, which was associated with high morbidity and mortality.Citation106 Vaccines and therapeutic agents are still being studied with the aim of preventing the spread of the virus and achieving mass immunity in order to restore social and economic activities.Citation107 A mucosal vaccine was developed using a GL L. plantarum expressing on its surface the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. The immune response evaluated in mice after intranasal administration of the GM strain showed an induction of the humoral immune response at respiratory and gastrointestinal mucosal levels.Citation80
In addition, oral immunization of mice with cell extracts from a GM strain of L. lactis expressing SARS-CoV-2 Spike Protein RBD S1 subunit was also studied. The results showed the induction of the mucosal and systemic immune response with the production of specific antibodies, showing that this strategy could be used to develop oral viral vaccines.Citation44
Other strains of LAB have also been studied as live vaccines against other respiratory viruses. A GM strain of Enterococcus faecium L3 expressing either HA2 hemagglutinin subunit of H1N1 influenza virus or its conserved part, long alpha helix (LAH) antigen, in combination with four conserved extracellular domain of the matrix protein 2 (M2e) epitopes was used to generate two mucosal vaccines against influenza virus. Oral immunization of mice demonstrated the induction of systemic humoral immune response.Citation108 The same strain expressing the antigens of chimeric protein PSPF (Pneumococcus Surface Proteins and Flagellin) was previously studied as a mucosal vaccine against the S. pneumoniae showing the versatility of the probiotic vaccine against a range of respiratory pathogens.Citation109
Regarding bifidobacteria, a study reported the cloning and expression of enterovirus 71 capsid protein 1 (EV71-VP1) in a strain of B. pseudocatenulatum M115 showing the possibility of using bifidobacteria for the expression of genes encoding virulence factors.Citation110
GM LAB have also been studied as vectors for the production of heterologous proteins of animal viruses. Porcine epidemic diarrhea virus (PEDV) is a member of alphacoronavirus that causes an intestinal disease which have caused economic losses around the world.Citation111 A GM strain of L. casei has been used to express N antigen protein of PEDV, and administered oral and intranasally to pregnant sow and mice. This strain was able to both induce mucosal and systemic immune response.Citation112 The same strain was GM to express the protein S of the PEDV virus and the GM L. casei was administered to mice observing an induction of cellular, humoral, and mucosal immunity.Citation113 A bivalent oral vaccine against PDEV and TGEV (porcine transmissible gastroenteritis virus) was also developed using a strain of L. casei which was GM to express TGEV S protein D antigen and PEDV S protein-neutralizing antigen epitope region COE as immunogens. Immunized mice showed an induction of humoral and mucosal immune response against TGEV and PDEV, evidencing the potential of this vaccine to prevent both infections.Citation114 In addition, other systems have been developed for the expression of proteins using L. lactis as a vector for the prevention of avian influenza virus infection and infection bursal disease in chicken.Citation115,Citation116
Bacterial infections. Gastroenteritis of bacterial origin is a disease characterized mainly by episodes of diarrhea caused by pathogens such as E. coli, Vibrio cholerae, Campylobacter spp., Salmonella spp., Shigella, Aeromonas spp., and Yersinia enterocolitica, among others.Citation117 Different studies showed the potential of using LAB as mucosal vaccines against these intestinal pathogens. In these senses, a GM L. lactis expressing HCP (Hemolysin co-regulated protein) of Campylobacter jejuni T6SS was administered to mice, and the results showed the induction of specific neutralizing antibodies and the prevention of pathogen colonization.Citation118 In addition, a GM L. lactis expressing the binding domain of heat-labile toxin (LBT) from enterotoxigenic E. coli (ETEC) was used to immunize rabbits and was able to induce the production of antibodies at the intestinal mucosa level.Citation119
LAB have also been studied as mucosal vaccines against respiratory pathogens. A vaccine against tuberculosis was developed by constructing a GM strain of L. Lactis expressing two antigens of Mycobacterium tuberculosis: Early Secreted Antigenic Target (ESAT-6) and the antigen 85 complex (Ag85A). The GM strain was used to immunize mice in which induced humoral and cellular immune responses.Citation120 In another study, an oral vaccine against S. aureus was developed. For this purpose, a strain of lactobacilli synthesizing S. aureus nontoxic mutated-hemolysins (HlaH35L) was constructed, and the GM strain was able to induce the mucosal immune response in mice and protected against pulmonary and skin infection.Citation77 This study demonstrated the potential of lactobacilli to be used as a delivery vector in the development of oral vaccines against bacterial pathogens.
Parasites infections
Trichinellosis is a disease caused by the parasitic nematode Trichinella. Infection in humans is caused by the consumption of larvae present in raw or undercooked meat.Citation121 A vaccine against Trichinella (T.) spiralis was developed using a strain of L. plantarum coexpressing the T. spiralis cathepsin F-like protease 1 gene (TsCPF1) and murine IL-4 (mIL-4). After immunization, mice showed the production of specific antibodies which protected against T. spiralis infection.Citation69
Leishmaniasis is a disease caused by more than 30 species of the Leishmania parasite and is transmitted by the female sandfly vector to humans.Citation122 Different alternatives have been evaluated with the aim of developing a live oral vaccine for this human parasite. A strain of L. lactis co- expressing the protective Leishmania homologue of activated C kinase (LACK) and mouse IL-12 induced an antigen-specific mucosal immune response in protected mice.Citation123 Another study used a GM strain of L. lactis to express the protein PpSP15 an immunogenic component of saliva from the sand fly Phlebotomus papatasi. The strain was evaluated to immunize mice and it was described the induction of a strong immune response with a long-term protection against Leishmania major.Citation41
Malaria is a disease caused by different species of the Plasmodium parasite and transmitted by the female Anopheles mosquito.Citation124 Different vaccines have been studied with the aim of producing specific antibodies against proteins expressed during the development of the parasite in the mosquito. In this sense, L lactis has been used to express the cysteine-rich Pfs48/45 protein, exposed on the surface of sexual stages of the parasiteCitation125,Citation126 or the Circumsporozoite protein (PfCSP), a sporozoite surface protein essential for its development in the mosquito and cell invasion in the mammalian host.Citation40 The results showed the induction of high levels of functional antibodies in rodents. The expression in L. lactis of the fusion protein Pfs230-Pfs48/45 was also studied and the final product elicited high levels of functional antibodies in mice.Citation127
Chagas disease is an infectious disease caused by the parasite Trypanoma cruzi.Citation128 A mucosal vaccine was designed using a GM L. lactis co-producing the antigen (a fragment of the trans-sialidase (TScf) enzyme from the Trypanosoma cruzi parasite) and the mucosal adjuvant 3’ 5’- cyclic di adenosine monophosphate(c-di-AMP). Immunization of mice with the engineered bacteria induced a specific immune response against the antigen.Citation129
In addition, other vaccines have been developed using L. lactis as a vector to deliver antigens from other parasites such as Taenia solium and Echinococcus granulosus; these strains were able to stimulate in mice the immune response against these diseases that affect both animals and humans.Citation39,Citation130
Cancer. The cytokine IL-12 has already successfully used in immunotherapy and cancer therapy. A GM strain of L. lactis secreting a native heterodimeric form of IL-12 was constructed. The biological activity of IL-12 produced by L. lactis was then confirmed in vitro in mouse spleen cells and in vivo by intranasal administration to mice and as adjuvant by combining them with the L. lactis strain producing the anchored form of the E7 antigen.Citation131 In order to evaluate the preventive and curative capabilities of the combination of these two lactococci, a mouse model was developed in which tumors were induced by subcutaneous implantation of tumor cells expressing HPV-16 antigen E7 (TC-1), and their progression was measured following intranasal administration of the strains.Citation132 The preventive and curative effects of intranasal co-administration of E7- and IL-12-producing lactococcal strains in mice were evaluated on TC-1 tumor development. The results demonstrated that preventive administration of lactococci, before tumor cell injection, induced the absence of tumor development in 50% of the immunized animals. In addition, a significant adjuvant effect of IL-12 co-delivered with the E7 antigen was found; in the absence of the IL-12 producing strain, the absence of tumors was observed in only 25% of immunized mice. Moreover, mice immunized with LL-E7 and LL-IL12 were able to resist a second challenge (2 months after the first immunization) suggesting that the induced immunity is durable.Citation132 Therapeutic use of these strains in mice with already implanted tumors resulted in complete regression of tumors in 35% of treated animals. These anti-tumor effects were the consequence of a cytotoxic response dependent on CD4+ and CD8 + T lymphocytes. These results in mice constitute the first evidence of a preventive and curative effect against cervical cancer by mucosal vaccination with GM lactococcal strains.
Allergy, Inflammation, and autoimmune disease
Allergic disease is a chronic inflammatory disorder characterized by a dysregulated immune response to allergens. The most common allergies include allergic asthma, allergic rhinitis, atopic dermatitis, and food allergies. In most cases, patients do not respond to conventional treatments. In this sense, LAB-based mucosal vaccines are an attractive option for the prevention and treatment of allergic diseases.Citation133–136 A mucosal vaccine based on L. lactis expressing major dust mite allergen Der p2 was developed, and its prophylactic effect was evaluated in a Der p2-sensitized mouse model. The results showed that the GM LAB was able to prevent the development of allergen-induced airway inflammation primarily by the induction of specific mucosal immune tolerance with reduction of inflammatory parameters. L. lactis has been also used as a vector to express Ara h 2.02 (one of the two isoforms of the Ara h 2 major peanut allergen) and administered to allergen-sensitized and -challenged mice. Animals that received the GM bacteria showed an alleviation of the Th2-associated responses.
L. lactis strains have also been used to treat other inflammatory pathologies. A GM strain was evaluated for secreting bioactive hemeoxygenase-1 (HO-1) in a model of hyperoxia-induced lung injury in rat pups. It was observed that the intranasal administration of the GM bacteria was able to prevent pulmonary inflammation through the attenuation of inflammatory parameters 136. L. lactis expressing therapeutic proteins has been studied in different models of intestinal inflammation. One study used a GM strain to deliver IL-10 in a mouse model of chronic irritable bowel syndrome and it was demonstrated a beneficial effect.Citation137 In another study, L. lactis was also used to express IL-27, an immunosuppressive cytokine, and attenuation of colitis in mice was observed.Citation138 A strain of L. lactis engineered to express human pancreatitis-associated protein I (PAP) was also used to prevent intestinal mucositis in mice.Citation139
The anti-inflammatory effect associated with the administration of milks fermented by strains of L. lactis that express IL-10 under the control of the XIES was evaluated using a TNBS-induced colitis murine model.Citation140 Milks fermented by strains producing IL-10 in the cytoplasm (Cyt strain) or secreted (Sec strain) showed decreased inflammation in their large intestines with a regulated immune response. In another study, considering that reactive oxygen species are involved in the intestinal inflammation, L. casei BL23 strains producing either catalase (CAT) or superoxide dismutase (SOD) were evaluated in mice before and after intrarectal administration of TNBS. These strains were associated with faster recovery of initial weight loss, and decrease of intestinal inflammation.Citation141,Citation142
Type 1 diabetes is a chronic autoimmune disease characterized by a destruction of the insulin-producing β cells of the pancreas due to attack by autoreactive T cells resulting in hyperglycemia. L. lactis has been studied as a vehicle for oral vaccines in the treatment of different autoimmune diseases. Oral immunization with a GM strain of L. lactis expressing the heat shock protein 65 and tandemly repeated IA2P2 (HSP65-6IA2P2) in mice was studied. It was observed that the GM strain was capable of efficiently delivering the antigen at the mucosal level, inducing immunotolerance and preventing the appearance of type 1 diabetes in animals.Citation143 A genetically modified L. lactis strain was also used as a strategy to administer proinsulin and IL-10 combined with low dose of anti-CD3 (aCD3) and it was observed a restoration of β-cell tolerance and glucose homeostasis in diabetic mice.Citation144 In addition, a GM strain of L. lactis expressing IL-4 and IL-10 was able to protect against type 1 diabetes in mice by preventing hyperglycemia and reducing pancreatic cell destruction.Citation145
Multiple sclerosis is an autoimmune neurological disease that results in destruction of the central nervous system white matter.Citation146 Current treatments are only useful to reduce symptoms and slow the progression of the disease, and can even have severe adverse effects; therefore, new therapies are being studied. L. lactis has been used as vector for the expression of heat shock protein (Hsp65) and specific epitopes of the three main myelin proteins (myelin oligodendrocyte glycoprotein MOG, myelin basic protein MBP, and proteolipid protein PLP). GM LAB were evaluated in models of experimental autoimmune encephalomyelitis (EAE) in mice and rats, respectively, and were able to prevent the development of the disease or reduce the clinical symptoms.Citation147–149
Rheumatoid arthritis is a chronic autoimmune disease that mainly affects cartilage and bone.Citation150 Two different studies used a strain of L. lactis engineered to deliver IL-5 and Hsp-65, respectively, and the GM strain was able to attenuate or prevent collagen-induced arthritis in mice.Citation151,Citation152
In addition, LAB have been used to express antigenic proteins for the treatment or prevention of other autoimmune diseases such as Sjogren’s syndrome, a disorder that mainly affects the lacrimal and salivary glands. A strain of L. lactis genetically modified to express enterotoxigenic E. coli colonization factor antigen I (CFA/I) was evaluated in a murine model of Sjogren’s syndrome and the ability of the GM strain to reduce the progression of the disease was demonstrated.Citation153
Metabolic disorder
Obesity is a global public health problem and treatments to reduce this problem have a very high therapeutic potential. A GM lactococci that produce human leptin, a hormone produced mainly by the adipocyte, which informs the brain of the state of adipose reserves was developed. In obese mice (ob/ob), leptin deficiency leads to massive obesity. The aim of the work was to measure the effects of nasal administration of a strain of L. lactis secreting human leptin in ob/ob mice. First, a strain of L. lactis that efficiently secreted biologically active human leptin was constructed. Then, it was determined whether intra-nasal administration of LL-Lep could inhibit food intake and weight gain in ob/ob mice. It was observed that daily administration of this GM strain of L. lactis to these obese mice significantly reduced weight gain and food intake.Citation154 These results demonstrate that leptin is produced in an active form by L. lactis and that this strain can be successfully used to regulate body weight and food intake.
Current status of the use of GM LAB and Bifidobacteria in clinical trials
Although different groups have reported the use of GM LAB and Bifidobacteria to treat different diseases in humans and animals, few studies have advanced to clinical trials. This is certainly due to the fact that it is necessary to integrate certain important features in the successful development of such GMM as live biotherapeutics, such as transient presence in the host gut (e.g. humans), a biocontainment strategy, biomarkers of activity and compliance with FDA (Food and Drug Administration, United States) requirements.
One of the few human clinical trials properly conducted considering all these factors were by Steidler et al. Indeed, data obtained by this group in a Phase 1 clinical trial conducted with a human IL-10-secreting GM strain of L. lactisCitation155 showed that the containment strategy used to construct the strainCitation156 was not only safe and effective but also that mucosal delivery of IL-10 by a GMM is feasible in humans.Citation157 In addition, a Phase 2a clinical trial in patients suffering from Crohn’s disease (a type of IBD) confirmed that the primary endpoints of the study were met with this GM L. lactis strain expressing IL-10: i.e., safety and tolerability of the GM strain, environmental containment of the GM organism, and assessment of strain-associated biomarkers. Unfortunately, concerning the disease progression endpoints, the clinical results did not reveal a statistically significant difference in mucosal healing compared to the placebo group (https://clinicaltrials.gov/ct2/show/NCT00729872). However, a new study from the same group demonstrated in another Phase 1b clinical trial that the use of a strain of L. lactis expressing another therapeutic molecule (i.e., human trefoil factor 1,Citation158) showed that this GMM was effective in treating oral mucositis, a major inflammation and ulceration of the membranes lining the oral cavity, throat and esophagus that is among the most commonly reported adverse events associated with cancer chemotherapy. Interestingly, preliminary data demonstrated the positive efficacy of this GM strain against oral mucositis in 25 patients compared to placebo.Citation159 Finally, our group is currently developing a biosafety strategy to use a GMM expressing human elafin in human clinical studies (Ref.Citation160 and unpublished data).
Discussion and conclusions
In the last years, the interest in the use of LAB and bifidobacteria to deliver molecules of interest has increased considerably, resulting in significant advances that are gathered in this review. In spite of this progress, many questions remain unanswered, notably concerning the immune response generated in the host by the native antigens of the LAB used as a vector or the mode of oral or intranasal administration. Currently, the latter seems to be the most adapted to induce a good immune response at the systemic and mucosal levels, but the health aspect of these administrations remains to be ensured.
Therapeutic applications have evolved in such a way that we can reasonably envisage the use of GM LAB in the treatment of human pathologies in the coming years. Biological containment systems have been developed to prevent the dissemination of these GM LAB. This strategy has allowed the implementation of some Phase I and Phase II clinical trials (see above) which is an essential step in the future use of these very promising tools.
Author Contributions
All the authors contributed equally to this work. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mokoena MP. Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules. 2017;22(8):1255. doi:10.3390/molecules22081255.
- Turroni F, van Sinderen D, Ventura M. Genomics and ecological overview of the genus Bifidobacterium. Int J Food Microbiol. 2011;149(1):37–22. doi:10.1016/j.ijfoodmicro.2010.12.010.
- Stiles ME, Holzapfel WH. Lactic acid bacteria of foods and their current taxonomy. Int J Food Microbiol. 1997;36(1):1–29. doi:10.1016/S0168-1605(96)01233-0.
- Hugenholtz P, Chuvochina M, Oren A, Parks DH, Soo RM. Prokaryotic taxonomy and nomenclature in the age of big sequence data. ISME J. 2021;15(7):1879–1892. doi:10.1038/s41396-021-00941-x.
- Klein G, Pack A, Bonaparte C, Reuter G. Taxonomy and physiology of probiotic lactic acid bacteria. Int J Food Microbiol. 1998;41(2):103–125. doi:10.1016/S0168-1605(98)00049-X.
- Lukjancenko O, Ussery DW, Wassenaar TM. Comparative genomics of Bifidobacterium, Lactobacillus and related probiotic genera. Microb Ecol. 2012;63(3):651–673. doi:10.1007/s00248-011-9948-y.
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi:10.1038/nrgastro.2014.66.
- Helander HF, Fändriks L. Surface area of the digestive tract – revisited. Scand J Gastroenterol. 2014;49(6):681–689. doi:10.3109/00365521.2014.898326.
- Corr SC, Gahan CCGM, Hill C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. Fems Immunol Med Mic. 2008;52(1):2–12. doi:10.1111/j.1574-695X.2007.00359.x.
- Cesta MF. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol Pathol. 2006;34(5):599–608. doi:10.1080/01926230600865531.
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(S4):S45–S53. doi:10.1038/nm1213.
- Moradi-Kalbolandi S, Majidzadeh-A K, Abdolvahab MH, Jalili N, Farahmand L. The role of mucosal immunity and recombinant probiotics in SARS-CoV2 vaccine development. Probiotics Antimicrob Proteins. 2021;13(5):1239–1253. doi:10.1007/s12602-021-09773-9.
- Szatraj K, Szczepankowska AK, Chmielewska-Jeznach M. Lactic acid bacteria-promising vaccine vectors: possibilities, limitations, doubts. J Appl Microbiol. 2017;123(2):325–339. doi:10.1111/jam.13446.
- Wyszynska A, Kobierecka P, Bardowski J, Jagusztyn-Krynicka EK. Lactic acid bacteria–20 years exploring their potential as live vectors for mucosal vaccination. Appl Microbiol Biotechnol. 2015;99(7):2967–2977. doi:10.1007/s00253-015-6498-0.
- Plavec TV, Berlec A. Safety Aspects of Genetically Modified Lactic Acid Bacteria. Microorganisms. 2020;8(2):297.
- Siegers K, Entian KD. Genes involved in immunity to the lantibiotic nisin produced by Lactococcus lactis 6F3. Appl Environ Microbiol. 1995;61(3):1082–1089. doi:10.1128/aem.61.3.1082-1089.1995.
- Djordjevic GM, Klaenhammer TR. Inducible gene expression systems inLactococcus lactis. Mol Biotechnol. 1998;9(2):127–139. doi:10.1007/BF02760814.
- Nouaille S, Ribeiro LA, Miyoshi A, Pontes D, Le Loir Y, Oliveira SC, Langella P, Azevedo V. Heterologous protein production and delivery systems for Lactococcus lactis. Genet Mol Res. 2003;2:102–111.
- Bermúdez-Humarán L, Corthier G, Langella P. Recent advances in the use of Lactococcus lactis as live recombinant vector for the development of new safe mucosal vaccines. Recent Res Dev Microbiol. 2004;8:147–160.
- Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, Ehrlich SD, Sorokin A. The Complete Genome Sequence of the Lactic Acid Bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 2001;11(5):731–753. doi:10.1101/gr.169701.
- Wegmann U, O’Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C, Ventura M, Goesmann A, Gasson MJ, Kuipers OP, et al. Complete Genome Sequence of the Prototype Lactic Acid Bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol. 2007;189(8):3256–3270. doi:10.1128/JB.01768-06.
- Bermúdez-Humarán LG. Lactococcus lactis as a live vector for mucosal delivery of therapeutic proteins. Hum Vaccin. 2009;5(4):264–267. doi:10.4161/hv.5.4.7553.
- Wells JM, Mercenier A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol. 2008;6(5):349–362. doi:10.1038/nrmicro1840.
- Leloir Y, Gruss A, Ehrlich SD, Langella P. Direct Screening of Recombinants in Gram-Positive Bacteria Using the Secreted Staphylococcal Nuclease as a Reporter. J Bacteriol. 1994;176(16):5135–5139. doi:10.1128/jb.176.16.5135-5139.1994.
- de Ruyter Pg, Kuipers OP, Beerthuyzen MM, van Alen-boerrigter I, de Vos WM, de Ruyter PG. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J Bacteriol. 1996;178(12):3434–3439. doi:10.1128/jb.178.12.3434-3439.1996.
- van Asseldonk M, de Vos Wm, Simons G, de Vos WM. Functional analysis of the Lactococcus lactis usp45 secretion signal in the secretion of a homologous proteinase and a heterologous α-amylase. Mol Gen Genet. 1993;240(3):428–434. doi:10.1007/BF00280397.
- Di Lorenzo A, Varcamonti M, Parascandola P, Vignola R, Bernardi A, Sacceddu P, Sisto R, de Alteriis E. Characterization and performance of a toluene-degrading biofilm developed on pumice stones. Microb Cell Fact. 2005;4(1):4. doi:10.1186/1475-2859-4-4.
- Piard JC, Hautefort I, Fischetti VA, Ehrlich SD, Fons M, Gruss A. Cell wall anchoring of the Streptococcus pyogenes M6 protein in various lactic acid bacteria. J Bacteriol. 1997;179(9):3068–3072. doi:10.1128/jb.179.9.3068-3072.1997.
- Song J, Zhao L, Song M. A lactococcus lactis-vectored oral vaccine induces protective immunity of mice against enterotoxigenic escherichia coli lethal challenge. Immunol Lett. 2020;225:57–63. doi:10.1016/j.imlet.2020.06.007.
- van der Vossen Jm, van der Lelie D, Venema G, van der Vossen JM. Isolation and characterization of Streptococcus cremoris Wg2-specific promoters. Appl Environ Microbiol. 1987;53(10):2452–2457. doi:10.1128/aem.53.10.2452-2457.1987.
- Clow F, Peterken K, Pearson V, Proft T, Radcliff FJ. PilVax, a novel Lactococcus lactis -based mucosal vaccine platform, stimulates systemic and mucosal immune responses to Staphylococcus aureus. Immunol Cell Biol. 2020;98(5):369–381. doi:10.1111/imcb.12325.
- Rezaei M, Rabbani-Khorasgani M, Zarkesh-Esfahani SH, Emamzadeh R, Abtahi H. Lactococcus-based vaccine against brucellosis: igG immune response in mice with rOmp16-IL2 fusion protein. Arch Microbiol. 2021;203(5):2591–2596. doi:10.1007/s00203-021-02241-6.
- Aliramaei MR, Khorasgani MR, Rahmani MR, Esfahani SHZ, Emamzadeh R. Expression of Helicobacter pylori CagL gene in Lactococcus lactis MG1363 and evaluation of its immunogenicity as an oral vaccine in mice. Microb Pathog. 2020;142:103926. doi:10.1016/j.micpath.2019.103926.
- de Castro Cp, Souza BM, Mancha-Agresti P, Pereira VB, Zurita-Turk M, Preisser TM, de Castro CP, da Cunha VP, Dos Santos JSC, Leclercq SY, et al. Lactococcus lactis FNBPA+ (pValac:e6ag85a) Induces Cellular and Humoral Immune Responses After Oral Immunization of Mice. Front Microbiol. 2021;12:1222.
- Mohseni AH, Taghinezhad-S S, Keyvani H. The First Clinical Use of a Recombinant Lactococcus lactis Expressing Human Papillomavirus Type 16 E7 Oncogene Oral Vaccine: a Phase I Safety and Immunogenicity Trial in Healthy Women Volunteers. Mol Cancer Ther. 2020;19(2):717–727. doi:10.1158/1535-7163.MCT-19-0375.
- Diaz-Dinamarca DA, Hernandez C, Escobar DF, Soto DA, Muñoz GA, Badilla JF, Manzo RA, Carrión F, Kalergis AM, Vasquez AE, et al. Mucosal vaccination with lactococcus lactis-secreting surface immunological protein induces humoral and cellular immune protection against group b streptococcus in a murine model. Vaccines. 2020;8(2):146. doi:10.3390/vaccines8020146.
- Xu P, Wang Y, Tao L, Wu X, Wu W. Recombinant lactococcus lactis secreting viral protein 1 of enterovirus 71 and its immunogenicity in mice. Biotechnol Lett. 2019;41(6–7):867–872. doi:10.1007/s10529-019-02695-1.
- Lei H, Gao T, Cen Q. Cross-protective immunity of the haemagglutinin stalk domain presented on the surface of Lactococcus lactis against divergent influenza viruses in mice. Virulence. 2021;12(1):12–19. doi:10.1080/21505594.2020.1857162.
- Zhou BY, Sun JC, Li X, Zhang Y, Luo B, Jiang N, Liu MC. Analysis of Immune Responses in Mice Orally Immunized with Recombinant pMG36e-SP-TSOL18/ Lactococcus lactis and pMG36e-TSOL18/ Lactococcus lactis Vaccines of Taenia solium. J Imm Res. 2018;2018:1–12. doi:10.1155/2018/9262631.
- Singh SK, Plieskatt J, Chourasia BK, Singh V, Bolscher JM, Dechering KJ, Adu B, López-Méndez B, Kaviraj S, Locke E, et al. The Plasmodium falciparum circumsporozoite protein produced in Lactococcus lactis is pure and stable. Journal of Biological Chemistry. 2020;295(2):403–414. doi:10.1074/jbc.RA119.011268.
- Davarpanah E, Seyed N, Bahrami F, Rafati S, Safaralizadeh R, Taheri T, Bueno LL. Lactococcus lactis expressing sand fly PpSP15 salivary protein confers long-term protection against Leishmania major in BALB/c mice. PLoS Negl Trop Dis. 2020;14(1):e0007939. doi:10.1371/journal.pntd.0007939.
- Torkashvand A, Bahrami F, Adib M, Ajdary S. Subcutaneous immunization with recombinant Lactococcus lactis expressing F1S1 fusion protein induces systemic and mucosal immune responses in BALB/C mice. Biochem Moler Bio. 2019;7:196.
- Kim EB, Piao da C, Son JS, Choi YJ. Cloning and characterization of a novel tuf promoter from Lactococcus lactis subsp. lactis IL1403. Curr Microbiol. 2009;59(4):425–431. doi:10.1007/s00284-009-9455-2.
- Xuan B, Park J, Yoo JH, Kim EB. Oral Immunization of mice with cell extracts from recombinant Lactococcus lactis Expressing SARS-CoV-2 Spike Protein. Curr Microbiol. 2022;79(6):1–8. doi:10.1007/s00284-022-02866-w.
- Temprana CF, Argüelles MH, Gutierrez NM, Barril PA, Esteban LE, Silvestre D, Mandile MG, Glikmann G, Castello AA, et al. Rotavirus VP6 protein mucosally delivered by cell wall-derived particles from Lactococcus lactis induces protection against infection in a murine model. PloS one. 2018;13(9):e0203700. doi:10.1371/journal.pone.0203700.
- Kuipers OP, Beerthuyzen MM, de Ruyter Pg, Luesink EJ, de Vos WM, de Ruyter PGGA. Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J Biol Chem. 1995;270(45):27299–27304. doi:10.1074/jbc.270.45.27299.
- Mierau I, Kleerebezem M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol. 2005;68(6):705–717. doi:10.1007/s00253-005-0107-6.
- Morello E, Bermudez-Humaran LG, Llull D, Sole V, Miraglio N, Langella P, Poquet I. Lactococcus lactis, an efficient cell factory for recombinant protein production and secretion. J Mol Microbiol Biotechnol. 2008;14(1–3):48–58. doi:10.1159/000106082.
- Benbouziane B, Ribelles P, Aubry C, Martin R, Kharrat P, Riazi A, Langella P, Bermúdez-Humarán LG. Development of a Stress-Inducible Controlled Expression (SICE) system in Lactococcus lactis for the production and delivery of therapeutic molecules at mucosal surfaces. J Biotechnol. 2013;168(2):120–129. doi:10.1016/j.jbiotec.2013.04.019.
- Carvalho RD, Do Carmo FLR, de Oliveira Junior A, Langella P, Chatel J-M, Bermúdez-Humarán LG, Azevedo V, de Azevedo MS. Use of wild type or recombinant lactic acid bacteria as an alternative treatment for gastrointestinal inflammatory diseases: a focus on inflammatory bowel diseases and mucositis. Front Microbiol. 2017;8:800. doi:10.3389/fmicb.2017.00800.
- Tavares LM, De Jesus LCL, Da Silva TF, Barroso FAL, Batista VL, Coelho-Rocha ND, Azevedo V, Drumond MM, Mancha-Agresti P. Novel strategies for efficient production and delivery of live biotherapeutics and biotechnological uses of Lactococcus lactis: the lactic acid bacterium model. Front BioengiBiotech. 2020;8:1269. doi:10.3389/fbioe.2020.517166.
- Miyoshi A, Jamet E, Commissaire J, Renault P, Langella P, Azevedo V. A xylose-inducible expression system for Lactococcus lactis. FEMS Microbiol Lett. 2004;239(2):205–212. doi:10.1016/j.femsle.2004.08.018.
- de Azevedo Ms, Rocha CS, Electo N, Pontes DS, Molfetta JB, Goncalves ED, de Azevedo MSP, Azevedo V, Silva CL, Miyoshi A. Cytoplasmic and extracellular expression of pharmaceutical-grade mycobacterial 65-kDa heat shock protein in Lactococcus lactis. Genet Mol Res. 2012;11(2):1146–1157. doi:10.4238/2012.April.27.14.
- Hollmann A, Saviello M, Delfederico L, Saraiva TD, Barh D, Jain N, Tiwari S, Chandra S, Gupta K, Zambare V, et al. Tight controlled expression and secretion of Lactobacillus brevis SlpA in Lactococcus lactis. Biotechnol Lett. 2012;34(7):1275–1281. doi:10.1007/s10529-012-0887-6.
- Le Loir Y, Gruss A, Ehrlich SD, Langella P. A Nine-Residue Synthetic Propeptide Enhances Secretion Efficiency of Heterologous Proteins in Lactococcus lactis. J Bacteriol. 1998;180(7):1895–1903. doi:10.1128/JB.180.7.1895-1903.1998.
- Morello E, Nouaille S, Cortes-Perez NG, Blugeon S, Medina LF, Azevedo V, Gratadoux JJ, Berm�dez-Humar�n LG, Le Loir Y, Langella P, et al. Inactivation of the ybdD Gene in Lactococcus lactis increases the amounts of exported proteins. Appl Environ Microbiol. 2012;78(19):7148–7151. doi:10.1128/AEM.01076-12.
- Nouaille S, Morello E, Cortez-Peres N, Le Loir Y, Commissaire J, Gratadoux JJ, Poumerol E, Gruss A, Langella P. Complementation of the Lactococcus lactis secretion machinery with Bacillus subtilis SecDF Improves Secretion of Staphylococcal Nuclease. Appl Environ Microbiol. 2006;72(3):2272–2279. doi:10.1128/AEM.72.3.2272-2279.2006.
- Poquet I, Saint V, Seznec E, Simoes N, Bolotin A, Gruss A. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol Microbiol. 2000;35(5):1042–1051. doi:10.1046/j.1365-2958.2000.01757.x.
- Frees D, Ingmer H. ClpP participates in the degradation of misfolded protein in Lactococcus lactis. Mol Microbiol. 1999;31(1):79–87. doi:10.1046/j.1365-2958.1999.01149.x.
- Cortes-Perez NG, Poquet I, Oliveira M, Gratadoux JJ, Madsen SM, Miyoshi A, Corthier G, Azevedo V, Langella P, Bermúdez-Humarán LG, et al. Construction and characterization of a Lactococcus lactis strain deficient in intracellular ClpP and extracellular HtrA proteases. Micro Bio. 2006;152(9):2611–2618. doi:10.1099/mic.0.28698-0.
- Iwaki M, Okahashi N, Takahashi I, Kanamoto T, Sugita-Konishi Y, Aibara K, Koga T. Oral immunization with recombinant Streptococcus lactis carrying the Streptococcus mutans surface protein antigen gene. Infect Immun. 1990;58(9):2929–2934. doi:10.1128/iai.58.9.2929-2934.1990.
- Wells JM, Wilson PW, Norton PM, Gasson MJ, Le Page RW. Lactococcus lactis: high-level expression of tetanus toxin fragment C and protection against lethal challenge. Mol Microbiol. 1993;8:1155–1162.
- Norton PM, Brown HW, Wells JM, Macpherson AM, Wilson PW, Le Page RW. Factors affecting the immunogenicity of tetanus toxin fragment C expressed in Lactococcus lactis. FEMS Immunol Med Microbiol. 1996;14(2–3):167–177. doi:10.1111/j.1574-695X.1996.tb00284.x.
- Robinson K, Chamberlain LM, Schofield KM, Wells JM, Le Page RW. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol. 1997;15:653–657.
- Seegers JFML. Lactobacilli as live vaccine delivery vectors: progress and prospects. Trends Biotechnol. 2002;20(12):508–515. doi:10.1016/S0167-7799(02)02075-9.
- Allain T, Mansour NM, Bahr MMA, Martin R, Florent I, Langella P, Bermúdez-Humarán LG. A new lactobacilli in vivo expression system for the production and delivery of heterologous proteins at mucosal surfaces. FEMS Microbiol Lett. 2016;363(13):fnw117. doi:10.1093/femsle/fnw117.
- Rush CM, Hafner LM, Timms PL. Vehicles for antigen delivery to the female urogenital tract. Advances in Mucosal Immunology, Pts a and B. 1995;371:1547–1552.
- Pouwels PH, Leer RJ, Boersma WJA. The potential of Lactobacillus as a carrier for oral immunization: development and preliminary characterization of vector systems for targeted delivery of antigens. J Biotechnol. 1996;44(1–3):183–192. doi:10.1016/0168-1656(95)00140-9.
- Xue Y, Zhang B, Huang H-B, Li J-Y, Pan T-X, Tang Y, Shi C-W, Chen H-L, Wang N, Yang G-L, et al. Immunoprotective effects of invasive Lactobacillus plantarum delivered nucleic acid vaccine coexpressing Trichinella spiralis CPF1 and murine interleukin-4. Vet Parasitol. 2021;298:109556. doi:10.1016/j.vetpar.2021.109556.
- Ding G, Bai J, Feng B, Wang L, Qiao X, Zhou H, Jiang Y, Cui W, Tang L, Li Y, et al. An EGFP-marked recombinant lactobacillus oral tetravalent vaccine constitutively expressing α, ε, β1, and β2 toxoids for Clostridium perfringens elicits effective anti-toxins protective immunity. Virulence. 2019;10(1):754–767. doi:10.1080/21505594.2019.1653720.
- Wang S, Geng N, Zhou D, Qu Y, Shi M, Xu Y, Liu K, Liu Y, Liu J. Oral immunization of chickens with recombinant Lactobacillus plantarum vaccine against early ALV-J infection. Front Immunol. 2019;10:2299. doi:10.3389/fimmu.2019.02299.
- Huang H, Jiang Y, Zhou F, Shi C, Yang W, Wang J, Kang Y, Cao X, Wang C, Yang G, et al. A potential vaccine candidate towards chicken coccidiosis mediated by recombinant Lactobacillus plantarum with surface displayed EtMIC2 protein. Exp Parasitol. 2020;215:107901. doi:10.1016/j.exppara.2020.107901.
- Wang J, Jiang H, Yang R, Zhang S, Zhao W, Hu J, Jiang Y, Yang W, Huang H, Shi C, et al. Construction and evaluation of recombinant Lactobacillus plantarum NC8 delivering one single or two copies of G protein fused with a DC-targeting peptide (DCpep) as novel oral rabies vaccine. Vet Microbiol. 2020;251:108906. doi:10.1016/j.vetmic.2020.108906.
- Wang L, Xia T, Guo T, Ru Y, Jiang Y, Cui W, Zhou H, Qiao X, Tang L, Xu Y, et al. Recombinant Lactobacillus casei expressing capsid protein vp60 can serve as vaccine against rabbit hemorrhagic disease virus in rabbits. Vaccines. 2019;7(4):172. doi:10.3390/vaccines7040172.
- Kuczkowska K, Copland A, Øverland L, Mathiesen G, Tran AC, Paul MJ, Eijsink VGH, Reljic R. Inactivated Lactobacillus plantarum carrying a surface-displayed Ag85B-ESAT-6 fusion antigen as a booster vaccine against Mycobacterium tuberculosis infection. Front Immunol. 2019;10:1588. doi:10.3389/fimmu.2019.01588.
- Shonyela SM, Shi C, Yang W, Cao X, Yang G, Wang C. Recombinant Lactobacillus plantarum NC8 strain expressing porcine rotavirus VP7 induces specific antibodies in BALB/c mice. Acta Biochim Biophys Sin (Shanghai). 2021;53(6):707–718. doi:10.1093/abbs/gmab050.
- Pan N, Liu B, Bao X, Zhang H, Sheng S, Liang Y, Pan H, Wang X. Oral delivery of novel recombinant lactobacillus elicit high protection against staphylococcus aureus pulmonary and skin infections. Vaccines. 2021;9(9):984. doi:10.3390/vaccines9090984.
- Zhang Z, Huang H-B, Jiang Y-L, Liu J, Gao X, Liu Y, Yang W-T, Shi C-W, Wang D, Wang J-Z, et al. Immunological evaluation of invasive Lactobacillus plantarum co-expressing EtMIC2 and chicken interleukin-18 against Eimeria tenella. Parasitol Res. 2020;119(9):2885–2895. doi:10.1007/s00436-020-06745-w.
- Bai Y, Wang G, Qi H, Wang Y, Xu C, Yue L, Hou X, Yu L. Immunogenicity of 987P fimbriae of enterotoxigenic Escherichia coli surface-displayed on Lactobacillus casei. Res Vet Sci. 2020;128:308–314. doi:10.1016/j.rvsc.2019.12.016.
- Li L, Wang M, Hao J, Han J, Fu T, Bai J, et al. Mucosal IgA response elicited by intranasal immunization of Lactobacillus plantarum expressing surface-displayed RBD protein of SARS-CoV-2. Int J Biol Macromol. 2021;190:599–608. doi:10.1016/j.ijbiomac.2021.08.232.
- Wells JM, Wilson PW, Norton PM, Gasson MJ, Lepage RWF. Lactococcus lactis: high-level expression of tetanus toxin fragment C and protection against lethal challenge. Mol Microbiol. 1993;8:1155–1162.
- Robinson K, Chamberlain LM, Schofield KM, Wells JM, LePage RWF. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol. 1997;15:653–657.
- Maassen CBM, Laman JD, den Bak-glashouwer MJH, Tielen FJ, van Holten-neelen J, Hoogteijling L, Antonissen C, Leer RJ, Pouwels PH, Boersma WJA, et al. Instruments for oral disease-intervention strategies: recombinant Lactobacillus casei expressing tetanus toxin fragment C for vaccination or myelin proteins for oral tolerance induction in multiple sclerosis. Vaccine. 1999;17(17):2117–2128. doi:10.1016/S0264-410X(99)00010-9.
- Reveneau N, Geoffroy M-C, Locht C, Chagnaud P, Mercenier A. Comparison of the immune responses induced by local immunizations with recombinant Lactobacillus plantarum producing tetanus toxin fragment C in different cellular locations. Vaccine. 2002;20(13–14):1769–1777. doi:10.1016/S0264-410X(02)00027-0.
- Cortes-Perez NG, Lefevre F, Corthier G, Adel-Patient K, Langella P, Bermudez-Humaran LG. Influence of the route of immunization and the nature of the bacterial vector on immunogenicity of mucosal vaccines based on lactic acid bacteria. Vaccine. 2007;25(36):6581–6588. doi:10.1016/j.vaccine.2007.06.062.
- Rochat T, Gratadoux -J-J, Gruss A, Corthier G, Maguin E, Langella P, van de Guchte M. Production of a heterologous nonheme catalase by lactobacillus casei: an efficient tool for removal of H 2 O 2 and Protection of Lactobacillus bulgaricus from Oxidative Stress in Milk. Appl Environ Microb. 2006;72(8):5143–5149. doi:10.1128/AEM.00482-06.
- Watterlot L, Rochat T, Sokol H, Cherbuy C, Bouloufa I, Lefevre F, Gratadoux -J-J, Honvo-Hueto E, Chilmonczyk S, Blugeon S, et al. Intragastric administration of a superoxide dismutase-producing recombinant Lactobacillus casei BL23 strain attenuates DSS colitis in mice. Int J Food Microbiol. 2010;144(1):35–41. doi:10.1016/j.ijfoodmicro.2010.03.037.
- Chang TLY, Chang C-H, Simpson DA, Xu Q, Martin PK, Lagenaur LA, Schoolnik GK, Ho DD, Hillier SL, Holodniy M, et al. Inhibition of HIV infectivity by a natural human isolate of Lactobacillus jensenii engineered to express functional two-domain CD4. P Natl Acad Sci USA. 2003;100(20):11672–11677. doi:10.1073/pnas.1934747100.
- Wang Z, Yu Q, Gao J, Yang Q. Mucosal and systemic immune responses induced by recombinant Lactobacillus spp. expressing the hemagglutinin of the avian influenza virus H5N1. Clini and Vaccine Immu. 2012;19(2):174–179. doi:10.1128/CVI.05618-11.
- Pavan S, Hols P, Delcour J, Geoffroy M-C, Grangette C, Kleerebezem M, Mercenier A. Adaptation of The Nisin-Controlled Expression System In Lactobacillus Plantarum: A Tool To Study In Vivo Biological Effects. Appl Environ Microbiol. 2000;66(10):4427–4432. doi:10.1128/AEM.66.10.4427-4432.2000.
- Shaw DM, Gaerthe B, Leer RJ, Van Der Stap J, Smittenaar C, Heijne Den Bak-Glashouwer M-J, Thole JER, Tielen FJ, Pouwels PH, Havenith CEG, et al. Engineering the microflora to vaccinate the mucosa: serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology. 2000;100(4):510–518. doi:10.1046/j.1365-2567.2000.00069.x.
- Bermúdez-Humarán LG, Kharrat P, Chatel J-M, Langella P. Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines. Microb Cell Fact. 2011;10(S1):1–10. doi:10.1186/1475-2859-10-S1-S4.
- Kruger C, Hu YZ, Pan Q, Marcotte H, Hultberg A, Delwar D, van Dalen PJ, Pouwels PH, Leer RJ, Kelly CG, et al. In situ delivery of passive immunity by lactobacilli producing single-chain antibodies. Nat Biotechnol. 2002;20(7):702–706. doi:10.1038/nbt0702-702.
- Hidalgo-Cantabrana C, Delgado S, Ruiz L, Ruas-Madiedo P, Sanchez B, Margolles A. Bifidobacteria and their health-promoting effects. Microbiology Spectrum. 2017;5(3):5.3. 21. doi:10.1128/microbiolspec.BAD-0010-2016.
- Mauras A, Chain F, Faucheux A, Ruffié P, Gontier S, Ryffel B, Butel M-J, Langella P, Bermúdez-Humarán LG, Waligora-Dupriet A-J, et al. A new Bifidobacteria Expression SysTem (BEST) to produce and deliver interleukin-10 in Bifidobacterium bifidum. Front Microbiol. 2018;9:3075. doi:10.3389/fmicb.2018.03075.
- Shirakawa T, Kitagawa K. Antitumor effect of oral cancer vaccine with Bifidobacterium delivering WT1 protein to gut immune system is superior to WT1 peptide vaccine. Hum Vaccin Immunother. 2018;14(1):159–162. doi:10.1080/21645515.2017.1382787.
- Hu B, Kou L, Li C, Zhu L-P, Fan Y-R, Wu Z-W, Wang -J-J, Xu G-X. Bifidobacterium longum as a delivery system of TRAIL and endostatin cooperates with chemotherapeutic drugs to inhibit hypoxic tumor growth. Cancer Gene Ther. 2009;16(8):655–663. doi:10.1038/cgt.2009.7.
- Yu Z, Huang Z, Sao C, Huang Y, Zhang F, Ma G, Chen Z, Zeng Z, Qiwen D, Zeng W, et al. Oral immunization of mice using Bifidobacterium longum expressing VP1 protein from enterovirus 71. Arch Virol. 2013;158(5):1071–1077. doi:10.1007/s00705-012-1589-z.
- Takei S, Omoto C, Kitagawa K, Morishita N, Katayama T, Shigemura K, Fujisawa M, Kawabata M, Hotta H, Shirakawa T, et al. Oral administration of genetically modified Bifidobacterium displaying HCV-NS3 multi-epitope fusion protein could induce an HCV-NS3-specific systemic immune response in mice. Vaccine. 2014;32(25):3066–3074. doi:10.1016/j.vaccine.2014.03.022.
- Yamamoto S, Wada J, Katayama T, Jikimoto T, Nakamura M, Kinoshita S, et al. Genetically modified Bifidobacterium displaying Salmonella-antigen protects mice from lethal challenge of Salmonella Typhimurium in a murine typhoid fever model. Vaccine. 2010;28(41):6684–6691. doi:10.1016/j.vaccine.2010.08.007.
- Castellsague X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008;110(3):S4–S7. doi:10.1016/j.ygyno.2008.07.045.
- Schiller JT, Castellsague X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26:K53–K61. doi:10.1016/j.vaccine.2008.06.002.
- Metzger DW. IL-12 as an adjuvant for the enhancement of protective humoral immunity. Expert Rev Vaccines. 2009;8(5):515–518. doi:10.1586/erv.09.13.
- Bermudez-Humaran LG, Langella P, Miyoshi A, Gruss A, Guerra RT, de Oca-luna RM, et al. Production of Human Papillomavirus Type 16 E7 Protein i Lactococcus lactis. Appl Environ Microb. 2002;68(2):917–922. doi:10.1128/AEM.68.2.917-922.2002.
- Bermudez-Humaran LG, Cortes-Perez NG, Le Loir Y, Alcocer-Gonzalez JM, Tamez-Guerra RS, de Oca-luna RM, et al. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J Med Microbiol. 2004;53(5):427–433. doi:10.1099/jmm.0.05472-0.
- Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. Journal of Neuroimmune Pharmacology. 2020;15(3):359–386. doi:10.1007/s11481-020-09944-5.
- Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25. doi:10.1016/j.addr.2020.12.011.
- Mezhenskaya D, Isakova-Sivak I, Gupalova T, Bormotova E, Kuleshevich E, Kramskaya T, et al. a live probiotic vaccine prototype based on conserved influenza a virus antigens protect mice against lethal influenza virus infection. Biomedicines. 2021;9(11):1515. doi:10.3390/biomedicines9111515.
- Gupalova T, Leontieva G, Kramskaya T, Grabovskaya K, Kuleshevich E, Suvorov A. Development of experimental pneumococcal vaccine for mucosal immunization. PloS one. 2019;14(6):e0218679. doi:10.1371/journal.pone.0218679.
- Thinbanmai T, Lulitanond V, Mayo B, Lulitanond A, Panya M. Cloning and expression of enterovirus 71 capsid protein 1 in a probio Bifidobacterium pseudocatenulatum. Lett Appl Microbiol. 2019;68(1):9–16. doi:10.1111/lam.13089.
- Jung K, Saif LJ, Wang Q. Porcine epidemic diarrhea virus (PEDV): an update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020;286:198045. doi:10.1016/j.virusres.2020.198045.
- Hou X-L, L-y Y, Liu J, Wang G-H. Surface-displayed porcine epidemic diarrhea viral (PEDV) antigens on lactic acid bacteria. Vaccine. 2007;26(1):24–31. doi:10.1016/j.vaccine.2007.10.065.
- Li X, Zhang B, Zhang D, Liu S, Ren J. The construction of recombinant Lactobacillus casei vaccine of PEDV and its immune responses in mice. BMC Vet Res. 2021;17(1):1–10. doi:10.1186/s12917-021-02885-y.
- Yu M, Wang L, Ma S, Wang X, Wang Y, Xiao Y, et al. Immunogenicity of eGFP-marked recombinant Lactobacillus casei against transmissible gastroenteritis virus and porcine epidemic diarrhea virus. Viruses. 2017;9(10):274. doi:10.3390/v9100274.
- Lahiri A, Bhowmick S, Sharif S, Mallick AI. Pre-treatment with chicken IL-17A secreted by bioengineered LAB vector protects chicken embryo fibroblasts against Influenza Type A Virus (IAV) infection. Mol Immunol. 2021;140:106–119. doi:10.1016/j.molimm.2021.10.003.
- Liu L, Zhang W, Song Y, Wang W, Zhang Y, Wang T, et al. Recombinant Lactococcus lactis co-expressing OmpH of an M cell-targeting ligand and IBDV-VP2 protein provide immunological protection in chickens. Vaccine. 2018;36(5):729–735. doi:10.1016/j.vaccine.2017.12.027.
- Fleckenstein JM, Kuhlmann FM, Sheikh A. Acute Bacterial Gastroenteritis. GastrO Clin. 2021;50(2):283–304. doi:10.1016/j.gtc.2021.02.002.
- Gorain C, Khan A, Singh A, Mondal S, Mallick AI. Bioengineering of LAB vector expressing Haemolysin co-regulated protein (Hcp): a strategic approach to control gut colonization of Campylobacter jejuni in a murine model. Gut Pathog. 2021;13(1):1–16. doi:10.1186/s13099-021-00444-2.
- Ahmadi Rouzbahani H, Mousavi Gargari SL, Nazarian S, Abdollahi S. Protective immunity against enterotoxigenic escherichia coli by oral vaccination of engineered lactococcus lactis. Curr Microbiol. 2021;78(9):3464–3473. doi:10.1007/s00284-021-02601-x.
- de Castro C P, Mendes Souza B, Mancha-Agresti P, Bastos Pereira V, Zurita-Turk M, Melo Preisser T, et al. Lactococcus lactis FNBPA+ (pValac: e6ag85a) induces cellular and humoral immune responses after oral immunization of mice. Front Microbiol. 2021;12:1222.
- Gottstein B, Pozio E, Noìˆckler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev. 2009;22(1):127–145. doi:10.1128/CMR.00026-08.
- Abdellahi L, Iraji F, Mahmoudabadi A, Hejazi SH. Vaccination in leishmaniasis: a review article. Iran Biomed J. 2022;26(1):1. doi:10.52547/ibj.26.1.35.
- Hugentobler F, Di Roberto RB, Gillard J, Cousineau B. Oral immunization using live Lactococcus lactis co-expressing LACK and IL-12 protects BALB/c mice against Leishmania major infection. Vaccine. 2012;30(39):5726–5732. doi:10.1016/j.vaccine.2012.07.004.
- Varo R, Chaccour C, Bassat Q. Update on malaria. Medicina Clínica(English Edition). Medicina clinica. 2020;155(9):395–402. doi:10.1016/j.medcli.2020.05.010.
- Singh SK, Roeffen W, Mistarz UH, Chourasia BK, Yang F, Rand KD, et al. Construct design, production, and characterization of Plasmodium falciparum 48/45 R0. 6C subunit protein produced in Lactococcus lactis as candidate vaccine. Microb Cell Fact. 2017;16(1):1–11. doi:10.1186/s12934-017-0710-0.
- Singh SK, Plieskatt J, Chourasia BK, Fabra-García A, Garcia-Senosiain A, Singh V, et al. A reproducible and scalable process for manufacturing a Pfs48/45 based Plasmodium falciparum transmission-blocking vaccine. Front Immunol. 2021;11:606266.
- Singh SK, Plieskatt J, Chourasia BK, Singh V, Bengtsson KL, Reimer JM, et al. Preclinical development of a Pfs230-Pfs48/45 chimeric malaria transmission-blocking vaccine. NPJ Vaccines. 2021;6(1):1–11. doi:10.1038/s41541-021-00383-8.
- Mills RM. Chagas disease: epidemiology and barriers to treatment. Am J Med. 2020;133(11):1262–1265. doi:10.1016/j.amjmed.2020.05.022.
- Quintana I, Espariz M, Villar SR, González FB, Pacini MF, Cabrera G, et al. Genetic engineering of Lactococcus lactis co-producing antigen and the mucosal adjuvant 3’ 5’-cyclic di adenosine monophosphate (c-di-AMP) as a design strategy to develop a mucosal vaccine prototype. Front Microbiol. 2018;9:2100. doi:10.3389/fmicb.2018.02100.
- Ebrahimzadeh F, Shirdast H, Taromchi A, Talebkhan Y, Haniloo A, Esmaeilzadeh A, et al. Induction of immunogenic response in balb/c mice by live and killed form of recombinant Lactococcus lactis displaying EG95 of echinococcus granulosus. Iran Biomed J. 2021;25(4):284. doi:10.52547/ibj.25.4.284.
- Bermudez-Humaran LG, Langella P, Cortes-Perez NG, Gruss A, Tamez-Guerra RS, Oliveira SC, et al. intranasal immunization with recombinant lactococcus lactis secreting murine interleukin-12 enhances antigen-specific th1 cytokine production. Infect Immun. 2003;71(4):1887–1896. doi:10.1128/IAI.71.4.1887-1896.2003.
- Bermudez-Humaran LG, Cortes-Perez NG, Lefevre F, Guimaraes V, Rabot S, Alcocer-Gonzalez JM, et al. A novel mucosal vaccine based on live lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. Journal of Immunology. 2005;175(11):7297–7302. doi:10.4049/jimmunol.175.11.7297.
- Angkasekwinai P. Th9 cells in allergic disease. Curr Allergy Asthma Rep. 2019;19(5):1–9. doi:10.1007/s11882-019-0860-8.
- Ai C, Zhang Q, Ren C, Wang G, Liu X, Tian F, et al. Genetically engineered Lactococcus lactis protect against house dust mite allergy in a BALB/c mouse model. PloS one. 2014;9(10):e109461. doi:10.1371/journal.pone.0109461.
- Chan CJ, Yong YS, Song AAL, Abdul Rahim R, In LLA, Lim RLH. Lactococcus lactharbouring Ara h 2.02 alleviates allergen-specific Th2-associated responses in sensitized mice. J Appl Microbiol. 2020;128(3):862–874. doi:10.1111/jam.14524.
- C-y W, Bermúdez-Humarán LG, Yue F, Li M, Zhang L-P. Intranasal administration with recombinant Lactococcus lactis expressing heme oxygenase-1 reduces hyperoxia-induced lung inflammation in rat pups. Biotechnol Lett. 2015;37(6):1203–1211. doi:10.1007/s10529-015-1795-3.
- Martin R, Chain F, Natividad JM, Natividad JM, Sokol H. Effects in the use of a genetically engineered strain of Lactococcus lacti delivering in situ IL-10 as a therapy to treat low-grade colon inflammation. Hum Vaccin Immunother. 2014;10(6):1611–1621. doi:10.4161/hv.28549.
- Hanson ML, Hixon JA, Li W, Felber BK, Anver MR, Stewart CA, et al. Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology. 2014;146(1):210–21. e13. doi:10.1053/j.gastro.2013.09.060.
- Carvalho RD, Breyner N, Menezes-Garcia Z, Rodrigues NM, Lemos L, Maioli TU, et al. Secretion of biologically active pancreatitis-associated protein I (PAP) by genetically modified dairy Lactococcus lactis NZ9000 in the prevention of intestinal mucositis. Microb Cell Fact. 2017;16(1):1–11. doi:10.1186/s12934-017-0624-x.
- Del Carmen S, de Moreno de Leblanc A, Perdigón G, Bastos Pereira V, Miyoshi A, Azevedo V, et al. Evaluation of the anti-inflammatory effect of milk fermented by a strain of IL-10-producing Lactococcus lactis using a murine model of Crohn`s disease. J Mol Microbiol Biotechnol. 2011;21(3–4):138–146. doi:10.1159/000333830.
- LeBlanc JG, Del Carmen S, Miyoshi A, Azevedo V, Sesma F, Langella P, et al. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J Biotechnol. 2011;151(3):287–293. doi:10.1016/j.jbiotec.2010.11.008.
- DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449–2462. doi:10.1016/S0140-6736(18)31320-5.
- Liu K-F, Liu X-R, G-L L, S-P L, Jin L, Wu J. Oral administration of Lactococcus lactis-expressing heat shock protein 65 and tandemly repeated IA2P2 prevents type 1 diabetes in NOD mice. Immunol Lett. 2016;174:28–36. doi:10.1016/j.imlet.2016.04.008.
- Cook DP, Cunha JPMCM, Martens P-J, Sassi G, Mancarella F, Ventriglia G, Sebastiani G, Vanherwegen A-S, Atkinson MA, Van Huynegem K, et al. Intestinal delivery of proinsulin and IL-10 via Lactococcus lactis combined with low-dose anti-CD3 restores tolerance outside the window of acute type 1 diabetes diagnosis. Front Immunol. 2020;11:1103. doi:10.3389/fimmu.2020.01103.
- Preisser TM, da Cunha VP, Santana MP, Pereira VB, Cara DC, Souza BM, Miyoshi A. Recombinant Lactococcus lactis carrying IL-4 and IL-10 coding vectors protects against type 1 diabetes in NOD mice and attenuates insulitis in the STZ-induced model. J Diabetes Res. 2021;2021:1–15. doi:10.1155/2021/6697319.
- Sand IK. Classification, diagnosis, and differential diagnosis of multiple sclerosis. Curr Opin Neurol. 2015;28(3):193–205. doi:10.1097/WCO.0000000000000206.
- Rezende RM, Oliveira RP, Medeiros SR, Gomes-Santos AC, Alves AC, Loli FG, Guimarães MAF, Amaral SS, da Cunha AP, Weiner HL, et al. Hsp65-producing Lactococcus lactis prevents experimental autoimmune encephalomyelitis in mice by inducing CD4+LAP+ regulatory T cells. J Autoimmun. 2013;40:45–57. doi:10.1016/j.jaut.2012.07.012.
- Szczepankowska K. Oral administration of Lactococcus lactis expressing synthetic genes of myelin antigens in decreasing experimental autoimmune encephalomyelitis in rats. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2015;21:1587. doi:10.12659/MSM.892764.
- Kasarello K, Szczepankowska A, Kwiatkowska-Patzer B, Lipkowski AW, Gadamski R, Sulejczak D, Łachwa M, Biały M, Bardowski J. Effect of recombinantLactococcuslactis producing myelin peptides on neuroimmunological changes in rats with experimental allergic encephalomyelitis. Folia Neuropathologica. 2016;3:249–258. doi:10.5114/fn.2016.62534.
- Littlejohn EA, Monrad SU. Early diagnosis and treatment of rheumatoid arthritis. Primary Care: Clinics in Office Practice. 2018;45(2):237–255. doi:10.1016/j.pop.2018.02.010.
- Maddaloni M, Kochetkova I, Hoffman C, Pascual DW. Delivery of IL-35 by Lactococcus lactis ameliorates collagen-induced arthritis in mice. Front Immunol. 2018;9:2691. doi:10.3389/fimmu.2018.02691.
- Gusmao-Silva G, Aguiar SLF, Miranda MCG, Guimaraes MA, Alves JL, Vieira AT, et al. Hsp65-producing Lactococcocus lactis prevents antigen-induced arthritis in mice. Front Immunol. 2020;11:562905.
- Akgul A, Maddaloni M, Jun SM, Nelson AS, Odreman VA, Hoffman C, Bhagyaraj E, Voigt A, Abbott JR, Nguyen CQ, et al. Stimulation of regulatory T cells with Lactococcus lactis expressing enterotoxigenic E. coli colonization factor antigen 1 retains salivary flow in a genetic model of Sjögren’s syndrome. Arthritis Res Ther. 2021;23(1):1–16. doi:10.1186/s13075-021-02475-1.
- Bermudez-Humaraan LG, Nouaille S, Zilberfarb V, Corthier G, Gruss A, Langella P, Issad T. Effects of intranasal administration of a leptin-secreting lactococcus lactis recombinant on food intake, body weight, and immune response of mice. Appl Environ Microb. 2007;73(16):5300–5307. doi:10.1128/AEM.00295-07.
- Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by lactococcus lactis secreting interleukin-10. Science. 2000;289(5483):1352–1355. doi:10.1126/science.289.5483.1352.
- Steidler L, Neirynck S, Huyghebaert N, Snoeck V, Vermeire A, Goddeeris B, Cox E, Remon JP, Remaut E. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol. 2003;21(7):785–789. doi:10.1038/nbt840.
- Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, van Deventer SJH, Neirynck S, Peppelenbosch MP, Steidler L, et al. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4(6):754–759. doi:10.1016/j.cgh.2006.03.028.
- Vandenbroucke K, Hans W, Van Huysse J, Neirynck S, Demetter P, Remaut E, Rottiers P, Steidler L. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology. 2004;127(2):502–513. doi:10.1053/j.gastro.2004.05.020.
- Caluwaerts S, Vandenbroucke K, Steidler L, Neirynck S, Vanhoenacker P, Corveleyn S, Watkins B, Sonis S, Coulie B, Rottiers P, et al. AG013, a mouth rinse formulation of Lactococcus lactis secreting human Trefoil Factor 1, provides a safe and efficacious therapeutic tool for treating oral mucositis. Oral Oncol. 2010;46(7):564–570. doi:10.1016/j.oraloncology.2010.04.008.
- Motta J-P, Bermudez-Humaran LG, Deraison C, Martin L, Rolland C, Rousset P, Boue J, Dietrich G, Chapman K, Kharrat P, et al. Food-grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci Transl Med. 2012;4(158):158ra44. doi:10.1126/scitranslmed.3004212.

