Abstract
Background
The aim of this study was to compare the distribution characteristics and ocular pharmacokinetics of norvancomycin (NVCM) in ocular tissues of the anterior segment between continuous topical ocular instillation and hourly administration of eye drop in rabbits.
Methods
Sixty rabbits were randomly divided into two groups: continuous topical ocular instillation drug delivery (CTOIDD) group and eye drop (control) group. In the CTOIDD group, NVCM solution (50 mg/mL) was perfused to the ocular surface using the CTOIDD system at 2 mL/h up to 10 h and the same solution was administered at one drop (50 μL) per hour for 10 h in the control group. Animals (N=6 per time-point per group) were humanely killed at 2, 4, 6, 10, and 24 h to analyze their ocular tissues and plasma. The concentrations of NVCM in the conjunctiva, cornea, aqueous humour, iris, ciliary body and plasma were measured by HPLC with photodiode array detector. The pharmacokinetic parameters were calculated by Kinetica 5.1.
Results
The highest concentrations of NVCM for the CTOIDD group and control group were 2105.45±919.89 μg/g and 97.18±43.14 μg/g in cornea, 3033.92±1061.95 μg/g and 806.99±563.02 μg/g in conjunctiva, 1570.19±402.87 μg/g and 46.93±23.46 μg/g in iris, 181.94±47.11 μg/g and 15.38±4.00 μg/g in ciliary body, 29.78±4.90 μg/mL and 3.20±1.48 μg/mL in aqueous humour, and 26.89±5.57 μg/mL and 1.90±1.87 μg/mL in plasma, respectively. The mean NVCM levels significantly increased at all time-points in cornea, iris, and ciliary body (p<0.05) in the CTOIDD group. The AUC0–24 values in the CTOIDD group were 27,543.70 μg·h/g in cornea, 32,514.48 μg·h/g in conjunctiva, 8631.05 μg·h/g in iris, 2194.36 μg·h/g in ciliary body and 343.9 μg·h/mL in aqueous humour, which were higher than for the eye drop group in all tissues.
Conclusion
Since continuous instillation of NVCM with CTOIDD could reach significantly higher concentrations and was sustained for a longer period compared with hourly administration of eye drop, CTOIDD administered NVCM could be a possible method to treat bacterial keratitis.
Introduction
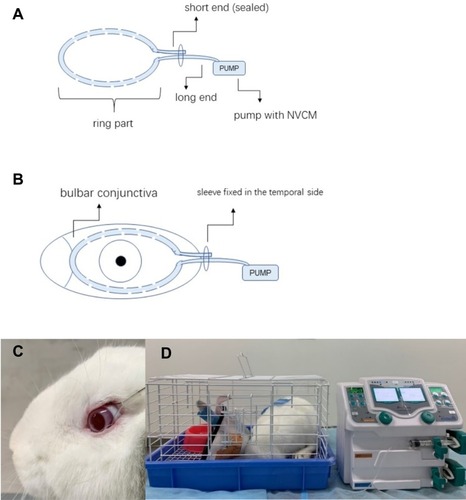
Topical ocular administration of eye drops is a common method used to treat anterior segment diseases of the eye, especially chronic ocular diseases. Topical ocular administered drugs bypass the liver, thus avoiding the need for higher systemic doses with the oral route.Citation1 However, with diseases such as ulcerative keratitis and acute conjunctivitis, higher therapeutic drug levels are required, as regular dose intervals of eye drops (four times per day) cannot meet the requirements owing to poor retention on the drug on the ocular surface because of tear turnover, and the innate protective mechanisms of the eye.Citation2–Citation4 Therefore, fortified dropsCitation5 and subconjunctival and intracameral injections are chosen in severe cases. Administration of drops every 15 min is clinically impractical and hourly administration can decrease patient compliance and increase health-care expenses.Citation6–Citation8 On the other hand, injections are invasive and associated with a risk of injury.Citation9 Various methods, such as punctum plugs, drug-eluting contact lenses, ocular implants, and nanocarrier systems, have been explored to prolong the pre-corneal time and improve the drug concentration in the ocular tissues for treating anterior ocular segment diseases.Citation10–Citation12 However, limiting factors of these methods are biodegradation, an inability to precisely control drug delivery, and idiopathic harm to the eyeball.Citation7,Citation13-15 The continuous topical ocular instillation drug delivery (CTOIDD) system in our study consists of a cribriform polyvinyl chloride tube with a ring shape and an attached drug pump (). This device allows for automatic, controllable, and continuous instillation of drug solution to the ocular surface.
Figure 1 Illustration of the CTOIDD system model (A); the tube section was fixed on the conjunctiva of a rabbit eye (B, C); the pump section was connected with the tube and delivered the norvancomycin to the ocular surface. Rabbits were kept in individual cages with food and water provided during drug administration (D).
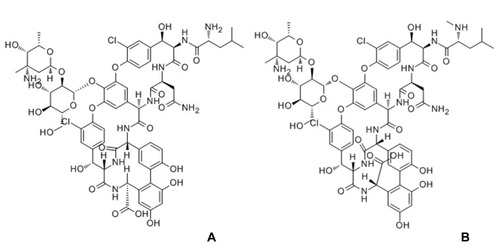
Norvancomycin (NVCM), a glycopeptide antimicrobial agent, differs from vancomycin only in that the –NHCH3 at the peptide amino-terminal of vancomycin is replaced by –NH2. It has comparable anti-bacterial spectrum and antibacterial properties to those of vancomycin, used for Gram-positive infections (). Its solubility is greater than 100 mg/mL in water solvent, which is similar to vancomycin. NVCM was independently developed by Chinese scientists and is a cheaper alternative to vancomycin. NVCM is approved by the China Food and Drug Administration and is widely used in China to treat bacterial infections of Gram-positive cocci and bacilli. especially infections of methicillin-resistant Staphylococcus.Citation16–Citation18
To date, there have been no pharmacokinetic studies on continuous instillation of NVCM to the ocular surface. This study was designed to compare the distribution characteristics and pharmacokinetics of NVCM between a continuous topical ocular instillation and hourly administration of eye drops (control group) in rabbits.
Materials and Methods
Animals
Adult male and female New Zealand white rabbits weighing 2.0–2.5 kg were obtained from the Department of Laboratory Animals, Xiangya School of Medicine, Central South University. All animal experiments were conducted in accordance with the statement of the Association for Research in Vision and Ophthalmology. The statement for the Use of Animals in Ophthalmic and Vision Research was approved by the Animal Ethics Committee of the Central South University (approval no. 2019sydw0045). All of the healthy rabbits, with no ocular disease upon full examination, were assigned randomly to two groups for the drug delivery study.
Drug Administration
Sixty rabbits were randomly divided into the CTOIDD group (n=30) and the eye drop group (n=30). Males and females were randomly grouped. The rabbits were anesthetized with intramuscular injection of xylazine hydrochloride (2 mL: 0.2 g; Huamu Animal Health Products Co., Jilin, China; 0.1–0.2 mL/kg). NVCM hydrochloride solution (50 mg/mL) was prepared as follows: 400 mg NVCM hydrochloride (Huabei Pharmaceutical Co., China) dissolved in 8 mL 0.9% saline. NVCM was freshly prepared and used within 2 days. In the CTOIDD group, a 0.7 mm diameter polyvinyl chloride tube was circumflected and the both ends were travelled across a plastic sleeve. The short end was sealed and the long end was connected to a pump (Biyang Corp., Changsha, China) loaded in NVCM hydrochloride solution. The polyvinyl chloride tube ring had 10 pores of 0.3 mm diameter in each hemicircle (). The eyes of each rabbit were anesthetized topically with oxybuprocaine hydrochloride eye drops (20 mL: 80 mg; Santen Pharmaceutical Co., Japan), three times at 3 min intervals. The ring around the limbus was sutured in the bulbar conjunctiva (). NVCM solution was instilled at a rate of 2 mL/h from the pump through the pores to the eye for 10 h. The NVCM solution was seen to be distributed and instilled in the ocular surface. All rabbits, wearing Elizabethan collars, were kept in individual cages with food and water provided during drug administration (). In the eye drop group, 50 mg/mL NVCM solution was administered at one drop (50 μL) per hour for 10 h.
Obtaining Tissue Samples
The rabbits from both groups (n=6 for CTOIDD group, n=6 for eye drop group) were killed by administrating 3 mL lidocaine and 3 mL air intravenously at 2, 4, 6, 10, and 24 h, respectively, after initial administration. Blood plasma and ocular tissues including conjunctiva, cornea, aqueous humour, iris, and ciliary body were collected promptly after death. All the samples were weighed, stored at −20°C, and processed for analysis.
Drug Analysis Procedures
The concentration of NVCM in the samples was determined by high-performance liquid chromatography with a photodiode array detector (HPLC-PDA). The HPLC-PDA analyses were performed using an Ultimate 3000 system (Thermo Fisher Scientific, San Jose, CA, USA), including a DAD-3000 detector, a TCC-3000 column oven, a WPS-3000 sampler, a HPG-3400RS pump, a Thermo Xcalibur Roadmap, and a Chromeleon Xpress version 2.4 workstation. Liquid chromatographic separation was performed on an Elite C18 Hypersil octadecylsilyl silicon column (2.1 mm×150 mm i.d., 5 μm particle size; Thermo Fisher Scientific), at 40°C. The mobile phase consisted of a combination of phase 91% solvent A (20 mmol/L sodium dihydrogen phosphate, pH 3.2) and phase 9% solvent B (acetonitrile). The isocratic flow rate was 0.250 mL/min. The total running time was 12.0 min for each injection. The detection wavelength was 205 nm. Data were calculated with the Xcalibur 2.0 system.
Frozen rabbit ocular tissue and plasma samples were thawed at ambient temperature. Each weighted solid ocular tissue (bulbar conjunctiva, cornea, iris, ciliary body) was added to 500 μL pure water and homogenized using a Bio-Gen PRO200 Homogenizer (ProScientific, USA). Fluid samples were also added to 500 μL pure water. A volume of 200 μL of the mixture was transferred to a 1.5 mL centrifuge tube, with the addition of with 20 μL aliquots of vancomycin internal standard (IS) 60 μg/mL standard solution and mixed. After thorough vortex mixing for 30 s, 30 μL 50% trifluoroacetic acid was added and then vortex mixed for 30 s. The mixture was stored at 4°C for 10 min. The mixture was centrifuged at 14,000 rpm for 5 min. The upper layer was removed and dried with a miVac (Genevac) at 45°C. The dried residue was dissolved with 200 μL mobile phase. After centrifugation (14,000 rpm for 5 min), 2 μL of the clear supernatant was injected into the liquid chromatography system.
Pharmacokinetics and Statistical Analysis
NVCM concentrations were analysed for pharmacodynamics in different tissues as calculated by Kinetica 5.1 software (Thermo Fisher Scientific, Waltham, MA, USA) using a model of non-compartmental analysis. The pharmacokinetic parameters included were maximum concentration (Cmax), time to maximum concentration (Tmax), and the area under the concentration–time curve from 0 to 24 h (AUC0–24).
Statistical analysis was performed using SPSS 24.0 (IBM Corp., Armonk, NY, USA). All of the NVCM concentration results were presented as mean ± SD. One-way analysis of variance was used to compare the overall difference between the CTOIDD group and eye drop group. An independent samples t-test was used to compare the differences between the two groups at each time-point. AUC0–24 comparison was performed using thet-test. Differences were considered statistically significant at p<0.05.
Results
Analytical Method Validation
Linearity
Standard calibration curves were prepared by spiking blank serum with the appropriate volume of one of the above-mentioned working solutions to produce the standard curve points equivalent to 0, 1.67, 3.12, 6.25, 12.5, 25.0, 50.0, and 100.0 μg/mL of NVCM. Each sample also contained 20 μL aliquots of vancomycin (IS 60 μg/mL). The following preparation and assay procedures were the same as described in the “Drug analysis procedures” subsection. In each run, a blank serum (no IS) was also analyzed. Least squares linear regression was used to determine the plasma concentration from the peak area ratios (NVCM vs vancomycin). The equation is:
Y =0.01019+0.0106*X, R2=0.9993. The instrumental lower limits of detection and quantitation were 0.50 μg/mL and 1.00 μg/mL, respectively.
Specificity and Selectivity
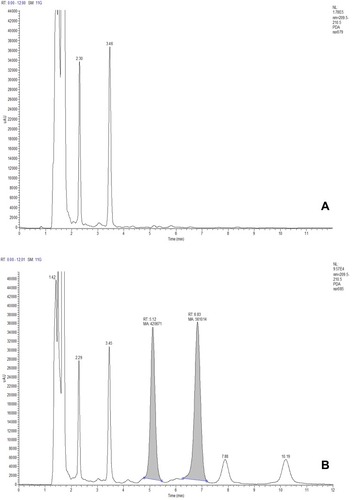
shows the chromatograph of blank plasma and that of blank plasma spiked with NVCM and vancomycin. The NVCM’s retention time was 5.1 min and that of vancomycin was 6.8 min. No disturbance in samples was found in this condition.
Recovery, Precision, and Stability
The recovery of the extraction procedure for NVCM and the internal standard were calculated by comparing the peak area obtained after extraction with that of an aqueous drug solution of corresponding concentration without extraction. Five samples were tested. The tested data are shown in .
Table 1 Mean Recovery Rates of Norvancomycin and Vancomycin in Plasma
The NVCM plasma working standards prepared above, at concentrations of 3.12, 12.5, and 50 μg/mL, were used to determine the recovery, inter-day and intra-day precision, and stability in storage of the method ( and ).
Table 2 Intra-Day Precision and Inter-Day Precision
Table 3 Stability of Long-Term Storage
Concentration of Norvancomycin in the Two Groups
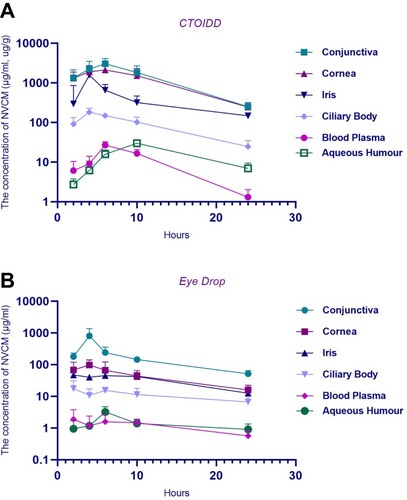
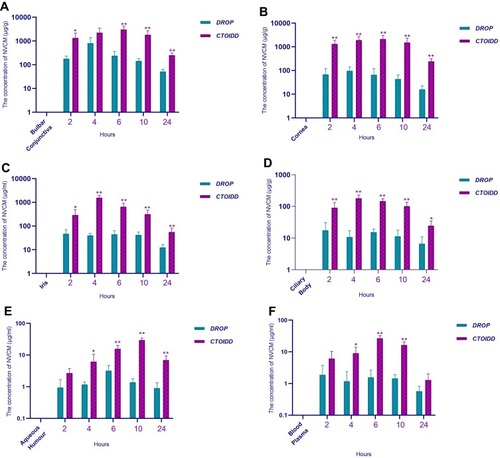
The mean concentrations of NVCM in different tissues in the two groups at five time-points are shown in . The concentration–time graphs are shown in . The NVCM concentrations in ocular tissues and blood plasma were significantly different between the two groups. The concentration levels of NVCM in conjunctiva tissue in the CTOIDD group were higher significantly at 2, 6, 10, and 24 (p=0.02 at 2 h, p=0.001 at 6 h, p=0.005 at 10 h, p=0.001 at 24 h) and not significantly different at 4 h (p=0.085) in comparison to the control group (). The concentration levels of NVCM in corneal tissue in the CTOIDD group were significantly higher at all time-points (p=0.002 at 2 h, p=0.003 at 4 h, p=0.003 at 6 h, p=0.006 at 10 h, p=0.000 at 24 h) than in the control group. NVCM concentration was shown to be very limited in the cornea tissue in the control group (). NVCM concentrations were also higher significantly at all time-points in iris and ciliary body tissues in the CTOIDD group (iris: p=0.033 at 2 h, p=0.000 at 4 h, p=0.002 at 6 h, p=0.004 at 10 h, p=0.007 at 24 h; ciliary body: p=0.007 at 2 h, p=0.000 at 4 h, p=0.000 at 6 h, p=0.002 at 10 h, p=0.012 at 24 h) than in the control group (). NVCM concentration reached peak concentration rapidly at 4 h and declined quickly later, while more moderate fluctuation was observed in the control group. The concentration levels of NVCM in aqueous humour in the CTOIDD group were higher significantly at 4, 6, 10, and 24 h (p=0.041 at 2 h, p=0.001 at 6 h, p=0.000 at 10 h, p=0.003 at 24 h) and not significantly different at 2 h (p=0.054) in comparison to the control group (). NVCM concentration levels measured in blood plasma revealed significant differences between the two groups at 4, 6, and 10 h (p=0.018 at 4 h, p=0.000 at 6 h, p=0.000 at 10 h) and negligible differences at 2 and 24 h (p=0.107 at 4 h, p=0.064 at 6 h) ().
Table 4 Norvancomycin Concentrations in the Anterior Segment Tissue and Blood Plasma in OTCIDD and Eye Drop Groups
Figure 4 Concentration–time curve of norvancomycin concentration in conjunctiva (A), cornea (B), iris (C), ciliary body (D), aqueous humour (E), and blood plasma (F) in the two groups. The aread under the concentration–time curve from 0 to 24 h were compared using the t-test. Values are given as the mean±SD (n=6), **p<0.01.
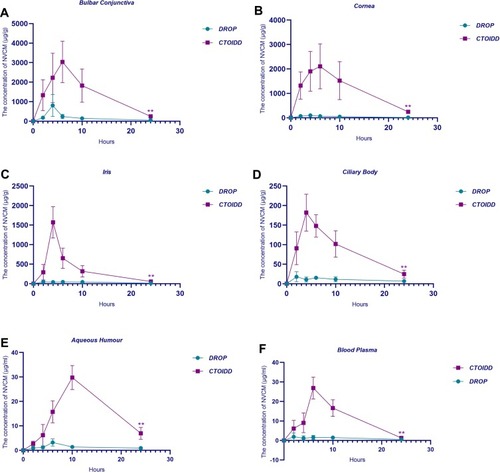
Figure 5 Comparison of the levels of norvancomycin at different time-points between the two groups in conjunctiva (A), cornea (B), iris (C), ciliary body (D), aqueous humour (E), and blood plasma (F). One-way analysis of variance was used to compare the overall difference between the CTOIDD group and eye drop group. Values are given as the mean±SD (n=6), *p<0.05, **p<0.01.
Relative Relationship of Norvancomycin Level in Ocular Tissue and Plasma Within Groups
The relative relationship of NVCM in ocular tissue and plasma, including conjunctiva/cornea, iris/cornea, and plasma/cornea ratios in the CTOIDD and eye drop groups are shown in . NVCM concentration–time profiles in ocular tissue and blood plasma in the CTOIDD group and eye drop group are shown in .
Table 5 Concentration Ratio of Conjunctiva/Cornea, Iris/Cornea, and Plasma/Cornea in OTCIDD and Eye Drop Groups
Pharmacokinetic Study
The pharmacokinetic parameters of NVCM in ocular tissue in the two groups are shown in . The AUC0–24 of all tissues in the CTOIDD was significantly higher than in the eye drop group (). In the CTOIDD group, the Cmax values were 3033.92 μg/g (conjunctiva), 2105.45 μg/g (cornea), 1570.19 μg/g (iris), 181.94 μg/g (ciliary body), 29.78 μg/mL (aqueous humour), and 26.89 μg/mL (blood plasma). The time-points of Tmax were 6 h (conjunctiva), 6 h (cornea), 4 h (iris), 4 h (ciliary body), 10 h (aqueous humour), and 6 h (blood plasma), respectively. The AUC0–24 values were 32,514.48 μg·h/g (conjunctiva), 27,543.70 μg·h/g (cornea), 8631.05 μg·h/g (iris), 2194.36 μg·h/g (ciliary body), 343.94 μg·h/mL (aqueous humour), and 234.36 μg·h/mL (blood plasma), respectively.
Table 6 Pharmacokinetic Parameters of Norvancomycin in Rabbit’ Anterior Segment Tissue in OTCIDD and Eye Drop Groups
In the control group, the Cmax values were 806.99 μg/g (conjunctiva), 97.18 μg/g (cornea), 46.93 μg/g (iris), 17.75 μg/g (ciliary body), 3.2 μg/mL (aqueous humour), and 1.9 μg/mL (blood plasma). The Tmax time-points were 4 h (conjunctiva), 4 h (cornea), 2 h (iris), 2 h (ciliary body), 6 h (aqueous humour), and 2 h (blood plasma), respectively. The AUC0–24 values were 4505.89 μg·h/g (conjunctiva), 1075.80 μg·h/g (cornea), 813.68 μg·h/g (iris), 252.16 μg·h/g (ciliary body), 31.92 μg·h/mL (aqueous humour), and 29.22 μg·h/mL (blood plasma), respectively.
Discussion
Conventional topical eye drops have limitations because of poor retention on the ocular surface due to tear turnover, and low drug bioavailability in ocular tissue. Thus, frequent application (half hourly or hourly)Citation19 of eye drops would be required to improve the drug concentration in a short time when treating acute severe anterior segment diseases, which generally leads to poor patient compliance.Citation20,Citation21 NVCM has been considered the antibiotic of choice for the treatment of infections caused by methicillin-resistant Gram-positive bacteria.Citation17,Citation18 In this study, we compared the distribution characteristics and pharmacokinetics of NVCM between continuous topical instillation via a CTOIDD system and hourly administration of eye drops.
The ocular surface consists of tear film, conjunctiva (bulbar, palpebrae, and fornix), and cornea. The cornea and conjunctiva are the tissue barriers that limit ocular drug absorption after the instillation of eye drops. There are two main routes for topical eye drop absorption: trans-corneal and trans-conjunctival.Citation22 Topically administrated drugs are diluted or even washed away when they come into contact with the tear film in the pre-corneal area. The cornea is composed of five layers: the epithelium, Bowman’s layer, the stroma, Descemet’s membrane, and the endothelium. The cornea can be viewed as three distinct layers when discussing drug delivery: the corneal epithelium, stroma, and endothelium.Citation8 The epithelium, with its tight junctions, restricts drug permeation,Citation23 especially for hydrophilic and large molecules. The stroma beneath limits lipophilic drugs; however, the innermost endothelium is not a strict barrier.Citation24 The surface area of the conjunctiva is nine and 17 times larger than that of cornea in rabbits and human, respectively,Citation25 which may be another factor contributing to the greater absorption of hydrophilic drugs via conjunctival routes, as the conjunctiva has leakier and more numerous tight junctions than the cornea.Citation26 Furthermore, the conjunctiva is composed of multi-layered vascularized connective tissue, which is responsible for drug absorption and disposition to the sclera, iris, and ciliary body, and sparingly to the retina and choroid. In addition, the trans-conjunctival route is one of the pathways that leads to systemic drug absorption.Citation26–Citation28
In our study, the NVCM concentrations in the ocular tissue and blood plasma in rabbits were measured by the established HPLC-PDA method. HPLC determination showed a good linear correlation and was validated via extraction recovery, intra- and inter-day methodological recovery, and stability of NVCM in serum.
Our results showed that NVCM levels of ocular tissues in the CTOIDD group were much higher at all time-points, except for in the conjunctiva at 4 h and the aqueous humour, withno significant difference from the control group. It was demonstrated that delivering NVCM administered by the CTOIDD method increased the level of NVCM in anterior segment tissue significantly compared to when the drug was administered topically in an hourly manner. NVCM weighs 1435.19 Da and has hydrophilic characteristics with a solubility of more than 100 mg/mL in solvent water. Thus, in the control group, the NVCM concentrations present in corneal tissue were low even when NVCM solution was instilled at one drop/hour. The CTOIDD system used in our experiment allows NVCM solution to be delivered continuously, thereby elevating the concentration in the tear film and counteracting the effects of high turnover of tear film to some extent, as the rate of drug delivery by CTOIDD is faster than tear production, which is unattainable by frequent eye drop application. CTOIDD achieves higher levels of drug delivery to the anterior segment of the eye by prolonging the residence time of drug in the ocular surface. This prolonged residence time was also seen to influence the trans-conjunctival route, where the concentrations in the iris, ciliary body, and blood plasma in the CTOIDD group showed higher values as a result of greater absorption by the conjunctiva.
In the CTOIDD group, the mean NVCM concentrations were found in the following samples, from highest to lowest in order: conjunctiva, cornea, iris, ciliary body, aqueous humour, and blood plasma. This order is same as in the control group during the 24 h study. The differences between conjunctiva and cornea were closer in the CTOIDD group, but wider in the control group. The ratios of conjunctiva/cornea at all time-points were lower and the ratios of iris/cornea were slightly lower in the CTOIDD group. Trans-conjunctival routes play an important role in drop absorption, mainly because NVCM is hydrophilic and the corneal epithelium favours lipophilic drugs.Citation28,Citation29 However, continuous NVCM delivery can narrow the difference between these two routes. For systemic absorption, the levels in plasma were higher in the CTOIDD group. This can be explained by the fact that more drug absorption occurs through the trans-conjunctival route and through the nasolacrimal route into the circular system. However, the ratio of plasma/cornea showed a lower value in the CTOIDD system. The rate and extent of trans-corneal routes increased faster than the systemic absorption in the CTOIDD group.
The pharmacokinetic study in the two groups revealed that, comparing the main pharmacokinetic parameters between the CTOIDD and control groups, NVCM preservation in the cornea and conjunctiva in the CTOIDD group during the 24 h study was about 25.60 and 7.22 times greater than in the control group, and around 10 times higher in the aqueous humour, iris, and ciliary body. The Cmax of cornea and conjunctiva in the CTOIDD group reached 2105.45 μg/g and 3033.92 μg/g, respectively. In the CTOIDD group, the conjunctiva and cornea reached their peak concentration at 6 h, aqueous humour at 10 h, and iris and ciliary body at 4 h. We observed that the NVCM level in the cornea, iris, and ciliary body at 24 h in the CTOIDD group was more than the peak concentration in the control group. No significant ocular pathological changes or adverse events were found in the CTOIDD or the eye drop group. This study demonstrates that the CTOIDD system can provide and maintain higher levels of NVCM to the anterior segment in the eye and can potentially be used for acute and severe anterior segment diseases, such as ulcerative keratitis and bacterial conjunctivitis, where expedient high drug concentrations are required and need to be sustained for a relatively long time.
Studies on topical delivery device systems, including polymeric micelles,Citation30 contact lenses,Citation31–Citation33 resorbable conjunctival devices, punctum plugs, and mini tablets,Citation9 have reported improved drug uptake over conventional drug formulations. However, these devices were limited to a certain total drug dosage, not controllable, with a limited time of action and accidental loss.Citation15 In addition, microneedles that can be inserted into the cornea,Citation34–Citation36 trans-corneal ultrasound-media drug delivery,Citation37 and trans-corneal iontophoresis techniques have been reported.Citation38–Citation40 Even though these methods are non-invasive or minimally invasive, to increase the ability of the drug to travel across the biological barrier, and further research is required to confirm their safety and efficacy.
The limitations in this experiment are as follows. Differences exist between human and the rabbit eye model, such as rabbits having smaller eyeballs and more rapid blood circulation. However, rabbits are generally used as surrogates for drug devices and pharmacokinetic studies as their eye anatomy and physiology resemble those in humans.Citation41 In addition, the NVCM level in solid ocular tissue cannot be measured in vivo at different time-points on the same rabbit by the HPLC-PDA method because the solid ocular tissues can only be obtained when the animal is humanely killed. Generalization of these data from rabbits to humans would be influenced by lower rabbit tear volumes and blink rate, which may affect the dynamics of NVCM dissolution, and the release and ocular absorption of the impregnated drugs. Since the eyes of rabbits have proptosis, we fixed the CTOIDD with a suture to prevent the tube from dropping out in this study. More advanced versions of the CTOIDD could be optimized to be placed in the cul-de-sac non-invasively and effortlessly, with a portable pump, when applied in human subjects.
In summary, this preliminary study suggests that continuous topical instillation via CTOIDD improves NVCM concentration, and the convenience of the CTOIDD system (without the need to frequently administer antibiotic drops) relieves nursing needs. Although CTOIDD has advantages over the current clinical methods of topical eye drop delivery of NVCM into rabbit eyes, further research is warranted to evaluate the efficacy of the CTOIDD system in animal disease models.
Conclusion
The current study provided promising results for the potential application of the CTOIDD system to prolong the dosing interval, increase compliance, and improve the efficacy of NVCM in anterior segment ocular tissue in comparison with the hourly eye drop method. Thus, the CTOIDD system is a possible alternative method to deliver drugs to the anterior segment of the eye and to provide possible management of severe anterior segment disease.
Disclosure
Dr Muhammad Ahmad Khan report grants from Aier Eye Hospital, during the conduct of the study. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors would like to thank Dr SG Liu for guidance on the HPLC-PDA method. We also thank the Advanced Research Center of Central South University for help with conducting the HPLC-PDA, Aier School Of Ophthalmology of Central South University, and the Department of Laboratory Animals, Xiangya School of Medicine of Central South University, for their great help and support. The study was funded by Hunan Provincial Science and Technology Department (No. [2014] 14).
References
- AwwadS, AHAMA, SharmaG, et al. Principles of pharmacology in the eye. Br J Pharmacol. 2017;174(23):4205–4223. doi:10.1111/bph.1402428865239
- Di PrimaG, LicciardiM, Carfì PaviaF, Lo MonteAI, CavallaroG, GiammonaG. Microfibrillar polymeric ocular inserts for triamcinolone acetonide delivery. Int J Pharm. 2019;567:118459. doi:10.1016/j.ijpharm.2019.11845931247275
- AgrahariV, MandalA, AgrahariV, et al. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res. 2016;6(6):735–754. doi:10.1007/s13346-016-0339-227798766
- SoutoEB, Dias-FerreiraJ, López-MachadoA, et al. Advanced formulation approaches for ocular drug delivery: state-of-the-art and recent patents. Pharmaceutics. 2019;11(9):460. doi:10.3390/pharmaceutics11090460
- SharmaA, TaniguchiTJ. Review: emerging strategies for antimicrobial drug delivery to the ocular surface: implications for infectious keratitis. Ocul Surf. 2017;15(4):670–679. doi:10.1016/j.jtos.2017.06.00128602948
- ZhaoX, ShenX, ZhangX, ChenS, LuH, WangM. Comparative study of ocular pharmacokinetics of gatifloxacin between continuous lavage and hourly topical instillation in rabbits. Cornea. 2018;37(11):1457–1462. doi:10.1097/ICO.000000000000171230124589
- SapinoS, ChirioD, PeiraE, et al. Ocular drug delivery: a special focus on the thermosensitive approach. Nanomaterials. 2019;9(6):884. doi:10.3390/nano9060884
- ShenJ, LuGW, HughesP. Targeted ocular drug delivery with pharmacokinetic/pharmacodynamic considerations. Pharm Res. 2018;35(11):217. doi:10.1007/s11095-018-2498-y30255364
- MoosaRM, ChoonaraYE, Du ToitLC, et al. A review of topically administered mini-tablets for drug delivery to the anterior segment of the eye. J Pharm Pharmacol. 2014;66(4):490–506. doi:10.1111/jphp.1213124635554
- BachuR, ChowdhuryP, Al-SaediZ, KarlaP, BodduS. Ocular drug delivery barriers—role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics. 2018;10(1):28. doi:10.3390/pharmaceutics10010028
- GoteV, SikderS, SicotteJ, PalD. Ocular drug delivery: present innovations and future challenges. J Pharmacol Exp Ther. 2019;jpet.119.256933. doi:10.1124/jpet.119.256933
- JanagamDR, WuL, LoweTL. Nanoparticles for drug delivery to the anterior segment of the eye. Adv Drug Deliv Rev. 2017;122:31–64. doi:10.1016/j.addr.2017.04.00128392306
- YellepeddiVK, PalakurthiS. Recent advances in topical ocular drug delivery. J Ocul Pharmacol Ther. 2016;32(2):67–82. doi:10.1089/jop.2015.004726666398
- YousryC, ElkheshenSA, El-laithyHM, EssamT, FahmyRH. Studying the influence of formulation and process variables on Vancomycin-loaded polymeric nanoparticles as potential carrier for enhanced ophthalmic delivery. Eur J Pharm Sci. 2017;100:142–154. doi:10.1016/j.ejps.2017.01.01328089661
- BertensCJF, GijsM, van den BiggelaarFJHM, NuijtsRMMA. Topical drug delivery devices: a review. Exp Eye Res. 2018;168:149–160. doi:10.1016/j.exer.2018.01.01029352994
- JiangZ, LeiX, ChenM, et al. Three structurally-related impurities in norvancomycin drug substance. J Antibiot (Tokyo). 2017;70(2):158–165. doi:10.1038/ja.2016.11527703158
- LiJ, HeS, YangZ, LuC. Pharmacokinetics and cerebrospinal fluid penetration of norvancomycin in Chinese adult patients. Int J Antimicrob Agents. 2017;49(5):603–608. doi:10.1016/j.ijantimicag.2017.01.01428366660
- WuY, KangJ, WangQ. Drug concentrations in the serum and cerebrospinal fluid of patients treated with norvancomycin after craniotomy. Eur J Clin Microbiol Infect Dis. 2017;36(2):305–311. doi:10.1007/s10096-016-2803-927738856
- American Academy of Ophthalmology Cornea/External Disease Panel. Preferred Practice Pattern® Guidelines.Bacterial Keratitis. San Francisco, CA: American Acadamy of Ophthalmology; 2013 Available from: www.aao.org/ppp. Accessed 217, 2020.
- DubaldM, BourgeoisS, AndrieuV, FessiH. Ophthalmic drug delivery systems for antibiotherapy—a review. Pharmaceutics. 2018;10(1):10. doi:10.3390/pharmaceutics10010010
- DuxfieldL, SultanaR, WangR, et al. Ocular delivery systems for topical application of anti-infective agents. Drug Dev Ind Pharm. 2016;42(1):1–11. doi:10.3109/03639045.2015.107017126325119
- UrttiA. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58(11):1131–1135. doi:10.1016/j.addr.2006.07.02717097758
- GaudanaR, AnanthulaHK, ParenkyA, MitraAK. Ocular drug delivery. AAPS J. 2010;12(3):348–360. doi:10.1208/s12248-010-9183-320437123
- Abdul NasirNA, AgarwalP, AgarwalR, et al. Intraocular distribution of topically applied hydrophilic and lipophilic substances in rat eyes. Drug Deliv. 2016;23(8):2765–2771. doi:10.3109/10717544.2015.107729226289215
- WatskyMA, JablonskiMM, EdelhauserHF. Comparison of conjunctival and corneal surface areas in rabbit and human. Curr Eye Res. 1988;7(5):483–486. doi:10.3109/027136888090318013409715
- HosoyaKI, LeeVHL, KimKJ. Roles of the conjunctiva in ocular drug delivery: a review of conjunctival transport mechanisms and their regulation. Eur J Pharm Biopharm. 2005;60(2):227–240. doi:10.1016/j.ejpb.2004.12.00715939235
- RamsayE, RuponenM, PicardatT, et al. Impact of chemical structure on conjunctival drug permeability: adopting porcine conjunctiva and cassette dosing for construction of in silico model. J Pharm Sci. 2017;106(9):2463–2471. doi:10.1016/j.xphs.2017.04.06128479360
- RamsayE, Del AmoEM, ToropainenE, et al. Corneal and conjunctival drug permeability: systematic comparison and pharmacokinetic impact in the eye. Eur J Pharm Sci. 2018;119:(March):83–89. doi:10.1016/j.ejps.2018.03.034
- BararJ, JavadzadehAR, OmidiY. Ocular novel drug delivery: impacts of membranes and barriers. Expert Opin Drug Deliv. 2008;5(5):567–581. doi:10.1517/17425247.5.5.56718491982
- MandalA, BishtR, RupenthalID, MitraAK. Polymeric micelles for ocular drug delivery: from structural frameworks to recent preclinical studies. J Control Release. 2017;248:96–116. doi:10.1016/j.jconrel.2017.01.01228087407
- Alvarez-LorenzoC, Anguiano-IgeaS, Varela-GarcíaA, Vivero-LopezM, ConcheiroA. Bioinspired hydrogels for drug-eluting contact lenses. Acta Biomater. 2019;84:49–62. doi:10.1016/j.actbio.2018.11.02030448434
- RibeiroAM, FigueirasA, VeigaF. Improvements in topical ocular drug delivery systems: hydrogels and contact lenses. J Pharm Pharm Sci. 2015;18(5):683–695. doi:10.18433/J3H60P.26670365
- ShiY, LvH, FuY, et al. Preparation and characterization of a hydrogel carrier to deliver gatifloxacin and its application as a therapeutic contact lens for bacterial keratitis therapy. Biomed Mater. 2013;8(5). doi:10.1088/1748-6041/8/5/055007
- JiangJ, GillHS, GhateD, et al. Coated microneedles for drug delivery to the eye. Invest Ophthalmol Vis Sci. 2007;48(9):4038. doi:10.1167/iovs.07-006617724185
- MoffattK, WangY, Raj SinghTR, DonnellyRF. Microneedles for enhanced transdermal and intraocular drug delivery. Curr Opin Pharmacol. 2017;36:14–21. doi:10.1016/j.coph.2017.07.00728780407
- Thakur SinghRR, TekkoI, McAvoyK, McMillanH, JonesD, DonnellyRF. Minimally invasive microneedles for ocular drug delivery. Expert Opin Drug Deliv. 2017;14(4):525–537. doi:10.1080/17425247.2016.121846027485251
- LafondM, AptelF, Mestas J-LLC. Ultrasound-mediated ocular delivery of therapeutic agents: a review. Expert Opin Drug Deliv. 2017;14(4):539–550. doi:10.1080/17425247.2016.119876627310925
- ChristopherK, ChauhanA. Contact lens based drug delivery to the posterior segment via iontophoresis in cadaver rabbit eyes. Pharm Res. 2019;36(6):87. doi:10.1007/s11095-019-2625-431004227
- Eljarrat-BinstockE, RaiskupF, Frucht-PeryJ, DombAJ. Transcorneal and transscleral iontophoresis of dexamethasone phosphate using drug loaded hydrogel. J Control Release. 2005;106(3):386–390. doi:10.1016/j.jconrel.2005.05.02016026884
- GratieriT, SanterV, KaliaYN. Basic principles and current status of transcorneal and transscleral iontophoresis. Expert Opin Drug Deliv. 2017;14(9):1091–1102. doi:10.1080/17425247.2017.126633427892757
- DengF, RantaV-P, KidronH, UrttiA. General pharmacokinetic model for topically administered ocular drug dosage forms. Pharm Res. 2016;33(11):2680–2690. doi:10.1007/s11095-016-1993-227431864