Abstract
In September 2019, The Lancet published details of two large Phase III double-blind placebo-controlled studies (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52) confirming the clinical efficacy of the biologic dupilumab in simultaneously blocking both IL-4/IL-13 signalling in chronic rhinosinusitis with nasal polyps (CRSwNP). The studies demonstrated that dupilumab (Dupixent®, Sanofi and Regeneron) 300mg subcutaneously administered was clinically effective when added for patients with moderate to severe CRSwNP already maintained on the standard intranasal steroid mometasone furoate. Duration of treatment ranged from injections either 2 weekly for 24 weeks (SINUS-24) or every 2 weeks for 52 weeks or finally every 2 weeks for 24 weeks stepping down thereafter to every 4 weeks for a further 28 weeks (SINUS-52). Rapid improvements in all important parameters of disease burden were seen with such improvement maintained even where the frequency of injections was decreased. In patients with co-existent asthma, lung function and asthma control scores improved. This is consistent with the one airway hypothesis of shared T2 inflammatory programmes driving both disease syndromes. The studies formed the basis for FDA registration and clinical launch in the US, and EMA approval in Europe. Dupilumab presents a significant new treatment option in an area of urgent unmet therapeutic need in CRSwNP. Should dupilumab prove to be as effective in the real-life clinical environment as it has been in the studies, then a paradigm shift from sinonasal surgery to medical treatment of CRSwNP may need to occur in the ENT community. Questions in relation to best patient selection, combined upper and lower airway therapeutic pathways, long-term safety along with health economics and cost constraints ought now to be addressed.
Introduction
Chronic rhinosinusitis (CRS) is the broad term used to define sinonasal inflammation. It is characterised clinically by nasal obstruction, and/or excess mucus with or without loss of smell function or facial pain. The overall duration of symptoms must be 12 weeks or more.Citation1 The current estimated treatment cost of CRS is estimated at $22 billion USD per annum for CRS in the US,Citation2 yet therapeutic approaches are often ineffective, particularly within the more severe inflammatory endo-phenotypes.Citation3 In such cases, rapid disease recurrence is common after all forms of treatment.Citation4 Poor quality of life, surprisingly worse than that with chronic heart failure or Parkinson’s disease, is often found.Citation5 The prevalence of CRS in Europe is estimated at 13% of the population.Citation6
CRS is conveniently subdivided between CRS with or without nasal polyps (CRSwNP and CRSsNP, respectively). CRSwNP is a distinct clinical and immunological entity within which there is a sub-type or syndrome termed non-steroidal anti-inflammatory drugs (NSAIDS)-exacerbated respiratory disease (N-ERD). In N-ERD, the airway reacts swiftly to aspirin and other NSAIDS. CRSwNP is often more difficult to treat when associated with N-ERD with a tendency to rapid recurrence of polyps following surgery and is associated with more difficult to treat asthma.Citation1,Citation7 N-ERD prevalence can be as high as 30% in patients with CRSwNP and asthma cohorts.Citation8 Such patients are twice as likely to undergo repeat sinus surgery.Citation9
Treatments so far for CRSwNP have been grossly inadequate and therefore an urgent unmet clinical need exists. Therapeutic strategies involving the monoclonal antibody dupilumab (Dupixent®, Sanofi, Paris, France and Regeneron, NY, US) that can effectively inhibit key inflammatory signalling pathways in CRSwNP by inhibiting IL-4 and IL-13 signalling is finally available.
This review outlines the basic immunobiology of CRSwNP in the context of the IL-4/13 axis. The recent studies demonstrating the clinical efficacy of blocking this axis in CRSwNP by the biologic dupilumab are evaluated. In concluding, the review seeks to place this drug in a correct therapeutic context.
Type 2 Inflammation
In the broadest sense, airway mucosal immunology can be divided simply into Th1 and Th2 inflammatory subsets.Citation10,Citation11 Th1 inflammation is primarily in relation to bacterial, viral and fungal host-defence with a predominance of Th1 and Th17 cells alongside innate lymphoid cells (ILCs) type 1 and 3 together with the signatory key canonical cytokine interferon-γ and TNF. Classic macrophage activation, generation of opsonising antibodies and killing of intracellular pathogens occurs. In contrast, Th2 inflammation is driven by Th2 T cells and type 2 innate lymphoid cells (ILC2s) with excess eosinophils and IgE. T follicular helper cells (Tfh) are a specialized subset of CD4+ T cells that play a critical role in the development of antigen-specific B cell immunity and facilitate antibody production. Th2 inflammation, in order to incorporate the role of other cells that contribute to this inflammatory endotype, is now collectively termed T2 inflammation. The pivotal type 2 cytokines IL-4, IL-5 and IL-13 are effector molecules and are produced not just by Th2/ILC2s, but also by structural cells and granulocytes (eosinophils, mast cells and basophils). Such T2-high inflammation is the common endotype in Caucasians with CRSwNPCitation12 and N-ERD-associated disease often predicts more severe T2 inflammatory states with high blood and tissue eosinophils and local mucosal IgE levels. Intranasal steroids, so far, the mainstay of treatment, are often ineffective in treating such excessive inflammation-driven mucosal disease. High dose systemic steroids have been shown to be more effective, at least in a temporary way,Citation13 but continuous use is associated with significant risks.Citation14 Blocking multiple signalling events before the initiation of key inflammatory signalling programmes that can simultaneously influence multiple downstream signalling pathways is thus an attractive therapeutic strategy. IL-4/IL-13 are both potent mediators of the induction phase of T2 inflammation as well as drivers of the effector phase of the T2 inflammatory cascade.
IL-4 and IL-13
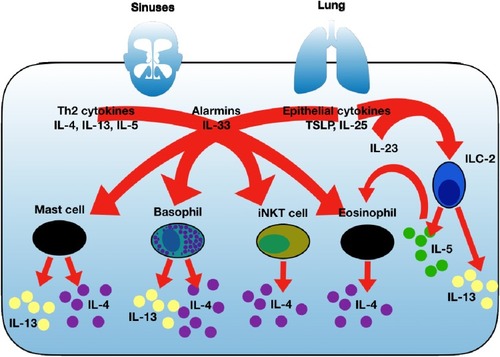
IL-4 was identified in 1982 as B cell growth factor.Citation15 It is a cytokine that functions as a potent regulator of immunity. IL-4 determines the CD4+ cell polarization to effector Th2 cells.Citation16 IL-4 is essential for immune cell survival both in healthy and Th2-driven inflammatory disease states,Citation17,Citation18 including eosinophil trafficking into tissue.Citation19 IL-4 determines B cell immunoglobulin class switching to IgE production.Citation20 IL-13 was discovered just over a decade later in 1993.Citation21,Citation22 It soon became apparent that IL-4 and IL-13 are structurally and functionally related cytokines.Citation23 IL-13 is also an effector cytokine for immunoglobulin B cell class switchingCitation22 and drives eosinophilic states via the induction of the key eosinophil chemoattractant IL-5 and eotaxin synthesis.Citation24,Citation25 IL-13 has a further distinct role in the production of mucusCitation26 and is an important regulator of tissue modelling.Citation26 IL-4 and IL-13 production and secretion occur under basal conditions given their important homeostatic roles in immune regulation and physiological function ranging from as far wide as embryogenesis to neuronal function and cell metabolism. Excess IL-4/IL-13 production is seen as part of the critical immune defence against parasitic infection. In inflammatory airway states such as CRSwNP and T2-driven asthma, immune over-interpretation of environmental threats by such examples as allergens, pollutants and microbes, leads to an excessive and dysregulated response with excessive inflammation and subsequent abnormal tissue repair termed remodelling. An excess of IL-4/IL-13 in association with T2-high inflammatory states become established with auto-inflammatory loops sustaining such disease states. Cellular sources of IL-4 and IL-13 are summarised in . ILC2s are a rich source of IL-13 but only secrete IL-4 under limited circumstances. Despite an important role for IL-4/13 in remodelling, structural cells do not produce significant amounts of IL-4/13.Citation27
Figure 1 Summary of key T2 cytokines and cells with established roles in sustaining T2 inflammation and key sources of IL-4 and IL-13. IL-4 and IL-13 are secreted from several cellular sources and along with other key T2 cytokines such as IL-5, IL-33, IL-25 and TSLP can lead to further IL-4 with or without IL-13 secretion depending on the cell type. This leads to immune amplification and auto-inflammatory loops that sustain mucosal disease and contribute to airway disease severity. ILC2 cells, particularly in response to IL-33 secrete large amount IL-13 as well as IL-5.
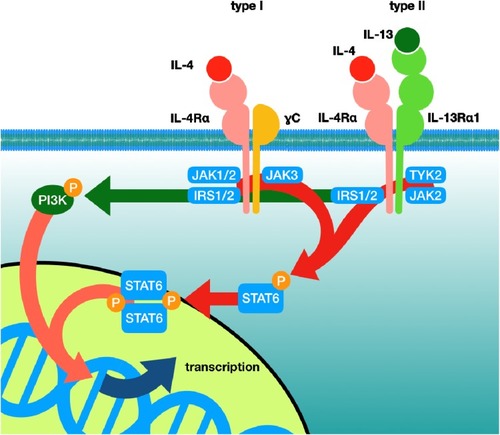
IL-4 and IL-13 Signalling
IL-4 can signal either via the type 1 or type 2 receptor. This has been reviewed in several articles.Citation28,Citation29 The main signalling pathways are illustrated in a simplified manner in . The type 1 receptor is composed of the IL4-Rα and γC receptor subunits. The type 2 receptor is composed of IL4-Rα and IL-13Rα1. IL-4 has a very high affinity for IL4-Rα with less affinity for γC and IL-13Rα1. IL-4 can signal through either the type 1 or type 2 receptor. IL-13 signals solely via the type 2 receptor by binding IL-13Rα1.The cell surface density of γC and IL-13Rα1 thus will have significant influence on which receptor combination and which signalling pathway will dominate in cells.Citation30 Whilst IL4-Rα and IL-13Rα1 are expressed on a wide range of cells, γC expression is confined mainly to blood-derived immune cells. IL-4 binding the IL4-Rα/γC complex leads to JAK1/3 phosphorylation which phosphorylates the tyrosine residues on IL-4-Rα. Such phospho-tyrosines can now generate “docking” sites on the transcription factor STAT6 or IRS-2, which are the main mediators of nuclear transcription in relation to IL-4/IL-13 signalling. IL-4 binding to IL4-Rα/γC leads to preferential IRS-2 over STAT6 signalling. IRS links to activation of the PI3K signalling pathway. IL-4 and IL-13 on binding the type 2 receptor preferentially induce STAT6 activation.Citation31 IL-13 has only moderate affinity for IL-13Rα1. On activation, IL-13/IL-13Rα1 recruits IL4-Rα with subsequent activation of JAK1 or JAK2/TYK2 leading to the recruitment and activation of STAT6, but can also involve STAT3, with subsequent gene transcription. Given that the affinity of IL-4 for IL4-Rα is markedly higher than IL-13 has for IL-13Rα1, IL-4 and IL-13 must compete for the type 2 cell surface receptor. The degree of each receptor activation on the cell surface will determine thus exactly to what degree each JAK/STAT/IRS pathway is activated and thereby the cell response to inflammation and hence disease endotype. Generally, the receptor subunits have low basal expression with a view to fulfilling cellular homeostatic roles. Infection and inflammation can up-regulate receptor expression. Accordingly, receptor density and the kinetics of receptor co-association or assembly along with the local concentration of IL-4 and IL-13 in the local micro-milieu will determine the exact cell response to these cytokines.
Dupilumab
Dupilumab is a human monoclonal IgG4 subclass antibody.Citation32 By specifically binding to the IL-4Rα receptor subunit, dupilumab blocks both IL-4 and IL-13 signalling, thereby modulating an array of downstream cytokine/chemokine release and activity, cell function and IgE synthesis. Inflammation and tissue remodelling programmes, at least in theory, should be in consequence modulated and regulated. The bioavailability of dupilumab is approximately 64% following subcutaneous administration.Citation32
LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52
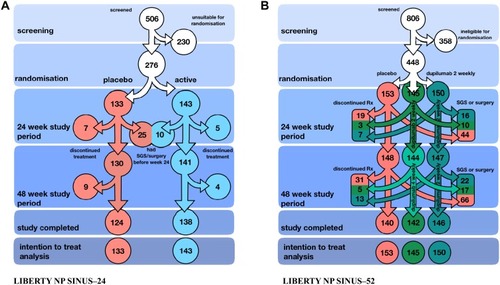
LIBERTY NP SINUS-24 (of 24 weeks duration) and LIBERTY NP SINUS-52 (of 52 weeks duration) are two pivotal Phase 3 studies that evaluated the clinical efficacy of dupilumab (Dupixent) 300mg given subcutaneously in CRSwNP.Citation33 In total, 724 patients were randomised to receive either dupilumab or placebo. The initial dosing frequency was twice weekly. The studies aimed to evaluate what additional improvement in nasal disease occurred when dupilumab was added to a standard intranasal steroid regime of daily mometasone furoate spray. The trial arms are illustrated in (LIBERTY NP SINUS-24) and 3B (LIBERTY NP SINUS-52). There were two primary endpoints as measures of clinical improvement at 24 weeks: decrease in nasal polyp score (NPS) as assessed via direct nasal endoscopic examination video recording with blinded review and decrease in nasal obstruction as assessed by collecting the monthly average of daily patient scores.
There were several secondary endpoint assessments. Daily patient-reported total symptom score of nasal congestion, loss of smell, and anterior or posterior rhinorrhoea were recorded. The Lund–Mackay radiological scoring system (again in a blinded manner) was used to grade the disease in radiological terms before and after treatment. A maximum score of 24 is possible and generally correlates with disease severity. The SNOT-22 is a symptom-based rhinosinusitis questionnaire with a higher score corresponding to more severe symptoms. It was used to provide information on both nasal symptoms and the burden of disease on general health from CRS. The highest score is 110. Severity is defined as mild when 8–20 inclusive, moderate when >20-50 and severe when >50.Citation34 All patients were of moderate-severe disease. Olfaction was assessed with the University of Pennsylvania Smell Identification Test (UPSIT), a scratch and sniff objective suprathreshold identification test scored out of 40 where a higher score indicates better olfaction.
The results presented are overwhelmingly positive. In SINUS-24, the least squares mean difference in NPS of treatment arm versus placebo was –2.06 (95% CI –2.43 to –1.69; p<0.0001) and in SINUS-52 was –1.80 (–2.10 to –1.51; p<0.0001). This was alongside significant and marked improvement in the severity of nasal congestion. The difference in nasal congestion score was −0.89 (–1.07 to –0.71; p<0.0001) in SINUS-24 and −0.87 (–1.03 to –0.71; p<0.0001) in SINUS-52. Impressively, all secondary endpoints improved early on with treatment. An improved radiological difference in all sinuses was seen and the Lund–Mackay CT scores least squares mean difference was –7.44 (–8.35 to –6.53; p<0.0001) in SINUS-24 and –5.13 (–5.80 to –4.46; p<0.0001) in SINUS-52.Citation33 Improvement in smell function was noteworthy with 74% and 79% participants defined as having anosmia on UPSIT testing pre-dupilumab but only 24% and 30% remaining anosmic at 24 weeks in SINUS-24/SINUS-52, respectively. There was no relevant change observed in the placebo group. With all these symptom improvements, the finding of overall improvement in SNOT-22 was not surprising.
What perhaps was surprising was that there was no clinical subgroup such as those having co-existent asthma, N-ERD or previous sino-nasal surgery that responded any better to dupilumab. This is a contrast to an earlier Phase 2A proof-of-concept study of dupilumab.Citation35 In this earlier study with a population of 60 patients, dupilumab when used alongside mometasone furoate nasal spray showed significant improvement in both clinical, endoscopic and radiological parameters of disease. Only 8 out of the self-reported 19 N-ERD study volunteers received dupilumab. As expected, the small number of N-ERD volunteers had more severe disease at baseline. Again in this earlier study, subgroup analysis suggested that dupilumab was particularly efficacious in the more severe difficult to treat N-ERD patients.Citation36 Reassuringly, there have been no safety concerns with dupilumab either in the CRSwNP focussed studies or those related to asthma and atopic eczema.Citation37 Nasopharyngitis, headache, epistaxis, and injection-site irritation along with CRSwNP and asthma exacerbation were the more frequent adverse events noted in the study and were all worse within the placebo group. Conjunctivitis seen more often in the dupilumab-atopic eczema studies occurred only in 7 patients receiving dupilumab and one in the placebo arm.Citation33 In SINUS-52, dupilumab was further administered with one treatment group continuing the 2-weekly schedule whilst another group received dupilumab every 4 weeks up to 52 weeks (B). At week 52, only the endpoints of polyp size, nasal obstruction score and the SNOT-22 were considered. Patients in both treatment arms continued to show progressive improvement up to week 52 regardless of 2 weekly or 4 weekly dosing, but numerically 2-weekly dosing appeared to confer greater clinical benefit. Dupilumab significantly decreased exposure to systemic steroids and the need for surgical intervention. Discontinuation of dupilumab after 24 weeks alas resulted in progressive worsening of sinonasal disease back to baseline severity. The improvements in co-associated asthma was expected given that three previous asthma studies with dupilumab using either 200mg or 300mg every 2 weeks confirmed impressive reductions in asthma exacerbations rates and oral corticosteroid use.Citation38
Potential Mechanisms of Dupilumab in CRSwNP
Despite the licenced use of dupilumab in eczema, asthma and now CRSwNP, the exact mechanism of action by dupilumab remains uncertain. There are several possibilities. If dupilumab predominantly suppressed IL-4 signalling by its association with IL-4Rα, then suppression of Th2 differentiation would down-grade key steps that establish T2 inflammation such as IgE production, mast cell priming and activation and some auto-inflammatory loops that support eosinophilia. Alternatively, if dupilumab preferentially inhibited IL‐4Rα from associating with the IL‐13Rα1 subunit then inhibition of more IL-13 driven disease will prevail. In knock-out mice for either IL-4Rα vs IL-13Rα1, IL4-Rα/γC determines Th2 cell responses whilst the IL4-Rα/IL-13Rα1 type II receptor was critical for the induction of allergen‐induced lower airway hyper-responsiveness and excess mucus production.Citation39 This would suggest that aspects of disease pathogenesis in CRSwNP and associated asthma that are both shared and different will be differentially modulated by dupilumab. Real-life data on phenotype-based dupilumab clinical outcomes are therefore eagerly awaited.
Inflammation and Remodelling
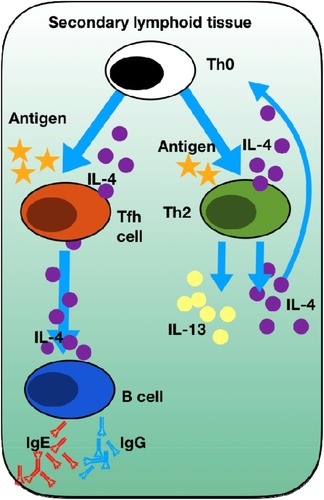
The working assumption must be that dupilumab, by blocking both IL-4 and IL-13 signalling, attenuates inflammation and reverses tissue remodelling towards a normal mucosal state. In SINUS-52, pre-set “exploratory” biomarkers of total serum IgE, eotaxin-3, periostin (a marker of IL-13 signalling) and TARC all decreased with dupilumab at both 24 and 52 weeks.Citation33 Concentration of ECP, eotaxin-3, IgE total and IL-5 decreased measured at 24 weeks in nasal secretions. In both SINUS-24 and SINUS-52, blood eosinophils increased but did eventually return to baseline levels, but never declined. Analysis of nasal polyp tissue homogenates from volunteers described in the early dupilumab study from 2016Citation35 demonstrated significant reductions in IgE and we therefore assume mast cell and basophil activity. Fall in local tissue IgE is probably one of the most significant findings. The role of local airway mucosal and polyp-derived IgE has been neglected to such an extent so far that anti-IgE blocking strategies with omalizumab (Xolair®, Novartis) have been denied to “non-allergic” asthma and in CRSwNP was completely ignored. Proof-of-concept studies and observational studies convincingly demonstrate nasal polyp regression and clinical improvement with IgE neutralisation with omalizumab.Citation40,Citation41 IL-4 signalling through IL4-Rα strongly expressed on B cells results in binding of STAT6 to the IL-4-responsive element of the epsilon gene promoter and, in synergy with CD40 engagement from T cells, results in activation and transcription of the IgE locus, driving IgE switching.Citation42 High local concentrations of IgE, specific for a range of antigens including allergens, bacterial superantigensCitation43,Citation44 and autoantigens,Citation45,Citation46 are a characteristic feature of eosinophilic nasal polyps. Various lines of evidence support that IgE switching occurs in-situ because of the pro-Th2 environment. Class switch recombination is generally restricted to secondary lymphoid tissue but structures reminiscent of ectopic lymphoid follicles have been observed within nasal polyp tissueCitation47,Citation48 along with epsilon germline transcripts and epsilon circle transcripts.Citation49,Citation50 Local IgE production has been found to correlate with numbers of IL-4-producing Tfh cells in nasal polyps and blocking IL-4 in-vitro inhibits IgE production following co-culture of nasal polyp-derived TfH cell with naïve B cells.Citation51 IL-4 role in establishing T2 inflammation is illustrated in .
Figure 4 Summary of IL-4 in establishing T2 inflammation. Naive T helper cells (Th0) can only undergo differentiation into Th2 cells upon antigen presentation by dendritic cells in the presence of IL-4. This is a key step in the establishment of Th2 mediated inflammation. Tfh cells in the presence of antigen and IL-4 undergo activation to produce further IL-4 that is essential for the induction of B cell-dependent production of IgE and IgG.
Previous studies demonstrated that direct blockade of IL-4 inhibits IgE production in miceCitation52 and in-vitro human cell culture.Citation51 Indeed, early clinical trials of dupilumab for atopic dermatitis reported significant reductions in total IgE in serum.Citation53 The critical role of IL-4/IL-13 signalling in antibody class switch recombination by B cells in this way explains the impact of dupilumab on de novo IgE production. IgE inhibition leads also to down-regulation of auto-amplificatory loops with a consequential general “knock-down” of T2 inflammation including serum eosinophil levels.Citation54,Citation55 Analysis of nasal secretions and polyp biopsy tissue pre and post dupilumab in the initial Phase 2A study is interesting and provides valuable mechanistic insights.Citation35,Citation56 Reductions in markers of eosinophilic inflammation in tissue (ECP and eotaxin-3) are for this reason expected with dupilumab. PARC (CCL18), produced by antigen-presenting cells in response to IL4/IL13 signalling, was also less post dupilumab, giving confirmation of direct IL-4/13 signalling inhibition. Levels of IL-6, IL-1β and surprisingly eotaxin-1, IL-4, IL-5, IL-10, IL-17, IL-33, and TNF-α and TARC did not change significantly compared with baseline pre-dupilumab values.Citation56 Given that inflammatory signalling pathways are never stand-alone and are considerably integrated, it is striking that a broader down-regulation of inflammatory signalling proteins was not seen.
IL-25 is upstream of other T2 cytokines and an amplification factor for the T2 inflammatory cascade.Citation57 TSLP is a “master amplifier” of T2 inflammation and is strongly expressed in nasal polyp epithelial cells in T2-high inflammatory endotypes.Citation58 TSLP can drive PGD2 production. PGD2 is a potent driver in N-ERD.Citation59 PGD2 is the major prostaglandin produced by mast cells and is a potent recruiter of Th2 cells, eosinophils, and basophils. It is therefore surprising and rather disappointing that the study did not measure IL-25, TSLP and PGD2. Such reductions nevertheless in other key markers of type 2 inflammation and the biomarker PARC directly linked to IL-4/IL-13 signalling is proof of the principle supporting the predicted anti-inflammatory effects of dupilumab.
When reviewing the CRSwNP population group inflammatory data before and after dupilumab data,Citation35 it must be remembered that the mean SNOT-22 score pre-treatment was less than 50 which is by definition moderate diseaseCitation34 and the patient group in the clinical study only had a mean serum eosinophil count of 0.41 x109/l.Citation35,Citation56 This is only just above the normal blood eosinophil range. This data does thus not directly report inflammatory response in the severe more hyper-eosinophilic states found in severe CRSwNP, and as such raises the key questions if such severe patients were studied alone would the response to dupilumab be different? It is disappointing that no attempt was made to identify high vs low T2 inflammatory CRSwNP endotypes’ clinical response to dupilumab or model any predictor variables into the data analysis to try and evaluate what factors predicted a better treatment response in SINUS-24 and SINUS-52. Given the role of IL-4 and particularly IL-13 in remodelling, the lack of focus on tissue structure and pathways of airway remodellingCitation27 including mucin and collagen expression is disappointing.
Dexpramipexole leads to eosinophil depletion via the maturational arrest of eosinophilopoiesis in bone marrow.Citation60 In a recent study of dexpramipexole in CRSwNP, where complete blood and nasal polyp tissue eosinophil depletion was achieved, no reduction in nasal polyp size or improvement in clinical symptoms was seen.Citation61 This has re-ignited the debate on the exact role of eosinophils in airway inflammation and tissue remodellingCitation62 since mepolizumab that blocks IL-5 has been associated with polyp volume reduction and clinical improvement with CRSwNP in two studies so far.Citation63,Citation64 It may be that it is IL-5 acting via basophils and possibly mast cells rather than eosinophils that contribute to CRSwNP.Citation65 Thus, the finding that tissue IL-5 and blood eosinophil levels did not change with dupilumab but markers of eosinophilic tissue inflammation (such ECP) and trafficking (eotaxin-3) did decrease, raises the question whether eosinophils are a relevant biomarker of disease at all for selection of patients with a view to dupilumab therapy in CRSwNP.
Predicting biomarkers that associate with treatment outcomes in airway disease so far has been difficult. For example, predictors of response to IgE blockade with omalizumab (Xolair®-Novartis/Genentech) in severe allergic asthma were surprisingly blood eosinophils rather than serum IgE.Citation66 It is thus disappointing that major groups of eosinophilic asthmatics were not considered for anti-IgE treatment in the last 13 years or so. Interestingly, a real-life study of omalizumab in treating CRS from our own group also showed that serum eosinophils and not IgE predict treatment response.Citation67 Further urgent work to understand how to match the right patient for dupilumab is necessary. It is tempting to speculate that serum IgE is a better predictor of excess IL-4/IL-13-driven disease.
Mast cells, basophils and arachidonic acid metabolic pathways have a significant role in N-ERD, where severe, often treatment refractory CRSwNP can occur, but are often forgotten as potential areas for therapeutic strategies. No measurements of either tryptase or leukotriene such as LTC4 (a potent inducer of mucus secretion) were undertaken in the dupilumab studies. IL-4 can up-regulate Cys-LT1 receptor expression on both eosinophils and mast cells.Citation68
It is noteworthy that the SINUS-24 and SINUS-52 studies did not show the N-ERD group having greater clinical improvements compared to the non-N-ERD group. This conflicts with data reporting sub-group analysis from the earlier, albeit less powered, study of dupilumab in CRSwNP, where N-ERD-CRSwNP demonstrated greater clinical improvement.Citation36 Such contrasting findings are an important reminder that disease heterogeneity exists. Further work on understanding how to predict which patient and disease traits will respond to any biologic with integration of potential biomarkers to help determine such is required.
Improvement in Olfaction
One of the most striking findings from the dupilumab studies was the effect on olfaction which was objectively assessed for the first time in a trial of a biologic in CRSwNP. Hyposmia or anosmia is a characteristic finding of CRS and one of the cardinal symptoms in the diagnosis according to the EPOS2020 criteria.Citation1 It is one symptom which the sufferers of this condition attach great importance to. Although this symptom has been qualitatively assessed in the trial of the monoclonal antibody mepolizumab against IL-5,Citation63 the SINUS-24 and SINUS-52 trials were the first to demonstrate this objectively through the use of the UPSIT supra-threshold identification “scratch and sniff” test.
Smell loss is almost universal in CRSwNP. The exact pathophysiological mechanisms underlying olfactory loss are poorly understood, but at least two mechanisms have been shown to play a role: the rather obvious problem of obstruction of access to the olfactory mucosa neuroepithelium rich in globose basal cells, sustentacular cells and olfactory sensory neurons (OSNs) in the upper nasal cavity through mucosal oedema or polyp mass, but also a direct cytotoxic inflammatory effect to the olfactory mucosa from the disease process itself.Citation69 Although the exact nature of the olfactory effectiveness of dupilumab is not known, the documented improvement in polyp size, mucosal oedema and reduction in markers of inflammation may go some way to explaining the impact of dupilumab on olfaction. The thus-far unique anti-IL-4Rα activity of the molecule may confer additional olfactory benefits due to its effects on mucus production and basal cell regeneration and function. Olfactory basal stem cells give rise to specialized epithelial cell subtypes that include the key secretory and ciliated cells. The olfactory pathway from nostril to olfactory receptor passes through a layer of specialised olfactory mucus secreted from the Bowman’s glands of the olfactory mucosa. IL–13 is present in excess in such olfactory mucusCitation70 and has been shown at least in the murine models as fundamental to airway goblet cell metaplasia and mucus-production.Citation26 Inflammatory nasal mucus would lead to alteration of the amount and type (and thus biochemical and biophysical properties) of mucus which may trap olfactants, preventing access to the olfactory receptors within the olfactory mucus. Such altered mucus may also alter the specific transport properties of the olfactory mucus and reduce the “partition effect” of the olfactants moving through it. Basal cells in CRSwNP express an impressive array of IL4/IL-13 responsive genes compared to normal tissue basal cells.Citation71 Early work (albeit in just one individual) suggests that IL4-Rα blockade with dupilumab could revert secretory cells towards a transcriptome associated with healthy cells.Citation71 In particular, the genes involved in Wnt signalling were inhibited. IL-4 induced Wnt signalling drives T2 polarisation of different cells.Citation72 Dupilumab may here block the T2 polarising signal to cells preventing T2 cellular reprogramming.
There may hence be another unique effect of dupilumab on olfaction function due to its anti-IL-4/IL–13 effect. IL–13 has been posited as well to influence airway basal cells in the lungs.Citation73 Throughout their lifespan, OSNs in olfactory respiratory epithelium are constantly regenerating from a heterogeneous population of basal stem cells.Citation74 Although these globose and horizontal basal cells are pluripotent sources of neural cells and not necessarily the basal cells examined by Rock et al,Citation73 there remains the intriguing and welcome possibility that IL–4Rα blockade may improve the regeneration of mature OSNs from their stem cells and therefore improve olfactory function.
Outstanding Questions
Study data collection and analysis in LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52 was rigorous and robust with primary efficacy and subsequent secondary analyses all well-defined using analysis of covariance (ANCOVA) to compare one variable in several groups whilst considering the variability of other variables (covariates).Citation33 Regrettably, it is not possible for these authors to ascertain exactly what percentage of patients improved from the treatment compared to the number treated in each group, as the data collected are given as a continuous variable rather than a dichotomous variable. The data presented are overall encouraging and it is hoped that we finally have an intervention to treat CRSwNP. However, there are still several outstanding issues to address.
Given that CRSwNP is a broad and complex heterogenous disease in terms of both phenotypes and endotypes of disease, sub-classification into just T2 inflammatory disease is too simplistic to predict which patients will have clinically relevant disease response to biologic treatments. There will be T2 inflammatory subsets with varying treatment response to dupilumab. It is disappointing that patient individual data listings are not available, as with this it will be possible to interrogate the data more closely and ascertain exactly how many individuals were “high” responders and very importantly how many were “low” responders. It would be important to look at what biomarkers associated with such responder groups along with individual patient traits and clinical patterns. For example, in a model of allergic rhinitis, it was the “high” nasal IL-13 group that responded better overall to an anti-IL-13 blocking approach.Citation75 Furthermore, variations in IL4-Rα structure will exist and have the potential to contribute to alteration of drug binding. Such variants of IL4-Rα occur in CRSwNP and may contribute to variations in disease severity and treatment response.Citation76 In severe asthma, individuals with SNPs in the IL-4Rα gene have more severe disease and increased inflammation.Citation77 IL-13 gene SNPs that predispose to excess eosinophilia (the latter an indicator of more severe disease) have been identified in CRSwNP.Citation78
Raised blood eosinophil counts are common in more severe CRSwNP phenotypes and high counts often predict more severe and difficult to treat disease.Citation79 Very high counts can indicate EGPA or risk of progression to EGPA.Citation80 The mean blood eosinophil counts were less than 0.5 x 109/L in SINUS-24 and SINUS-52. Treatment with dupilumab was associated with a transient increase in mean eosinophil counts at around 16 weeks of treatment. This is not a new finding as dupilumab therapy in asthma was also associated with a rise in blood eosinophils of approximately 10% from baseline.Citation81 The peak in count was again seen as late as 16–20 weeks from the start of therapy. The obvious concern is whether dupilumab could unmask clinically silent EGPA and if there is an eosinophil baseline count above which dupilumab should be avoided? Given EGPA is still a rare syndrome, the finding of the dupilumab arm declaring two cases of EGPA and one additional case of high eosinophilia and systemic symptoms must suggest that dupilumab cannot just be given to anyone with CRSwNP. Guidelines on patient selection, blood eosinophil limits and monitoring guidance should now be determined.
A recent study concluded that dupilumab in the real-life setting is less cost-effective than surgical intervention.Citation82 It must be remembered that when undertaking any cost-benefit analysis with a biologic the integration of multiple health variables must be undertaken. If for example the cost–benefit is for both CRSwNP and associated asthma, if dupilumab treats both diseases, the cost-savings from treating two difficult diseases is greater, with potentially less hospital attendance, infrequent or no surgery, less medication use and overall better health outcomes, leading to less abstinence from work. Such overall health factors must be considered. The cost of regular dupilumab against regular surgery will not be the right modelling approach to consider.
Multidisciplinary Care Model for Delivery of Biologics
Dupilumab therapy cannot be given to every patient with CRSwNP. Yet, there is no definite guidance on how to undertake patient selection, dosing regimens (2 weekly or 4 weekly) or model of care delivery, and exactly who will administer and monitor therapy, treatment response and safety.Citation83 Most ENT surgeons unfortunately have limited training in mechanisms of airway disease and mucosal immunology. Pulmonologists still ignore the upper airway and have no or limited training in sinonasal disease. We therefore propose a multidisciplinary approach to CRS, as is the case in our institution. Given that nearly all patients with severe asthma have CRSCitation84 it is easy to justify a shared clinic between an ENT surgeon and pulmonologist to see these complex patients. As with asthma,Citation85 a protocol-based assessment of CRS is needed. Every aspect of the airway should be assessed using objective measures of airflow, smell function, endoscopic polyp grade scoring, exclusion of systemic disease and any infection predisposition evaluated by an immunologist if indicated. Mental health status and quality of life should be assessed with measures taken to understand patient expectations. Sinonasal disease must be optimised. If surgical intervention with FESS is indicated, then this must be prioritised, with patients given standard medical treatment to optimise sinonasal mucosal function before and afterward. The latter approach is still needed even if a patient was to proceed to biologic therapy. It has been shown with difficult asthma that real-world results with biologics are often better than that reported in clinical studies and this is presumed to be because of clinical time taken to optimise asthma and treat associated disease traits to improve patient wellbeing before commencing biologics.Citation86
Given that the “one airway” concept has been undoubtedly validated with not just dupilumabCitation33,Citation35,Citation87 but also other emerging biologics for CRSwNP,Citation88 with both CRS and asthma responding together, it is imperative that a biologic treatment that will treat CRS and any associated asthma be chosen. This must be a joint decision between the ENT surgeon and pulmonologist in an MDT setting.
Conclusion
Dupilumab is the first biologic to receive a licence both in the US and Europe to treat CRSwNP. For the first time, clinicians have the opportunity to treat this difficult and troublesome disease syndrome. It remains to be seen if such therapy with a double-block approach of inhibiting IL-4/IL-13 signalling with dupilumab in the “real-world” setting will lead to the same degree of clinical improvement and stability in the more severe CRSwNP endo-phenotype patients that dominate tertiary rhinology clinics. It also remains to be seen the exact cost of such treatment approaches given that these are long-term and potentially life-long therapies. If dupilumab can treat CRSwNP and severe asthma together, then such an approach may indeed prove cost-effective and sustainable. As with any new medication, hypervigilance and monitoring of patients will be needed until further safety data are available. It is essential that the right drug is given to the right patient, and as more biologics that target key points of the T2 inflammatory cascade appear on the horizon, identification of disease traits and associated biomarkers that predict treatment response with a particular biologic will become ever more important. A multidisciplinary approach with ENT surgeons working alongside pulmonologists must become the norm and not the exception.
Abbreviations
N-ERD, non-steroidal anti-inflammatory exacerbated respiratory disease; CRS, chronic rhinosinusitis; CRSwNP, chronic rhinosinusitis with nasal polyps; CRSsNP, chronic rhinosinusitis without nasal polyps; ECP, eosinophilic cationic protein; EGPA, eosinophilic granulomatous polyangiitis; EMA, European Medicines Agency; FDA, Federal Drug Agency; IgE, immunoglobulin E; IL, interleukin; ILCs, innate lymphoid cells; IRS, insulin receptor substrate; JAKs, janus kinases; KO, knock-out; MDT, multidisciplinary team; PI3K, phosphoinositide-3-kinase; PGD2, prostaglandin D2; PARC, pulmonary and activation-regulated chemokine; RANTES, regulated on activation, normal T cell expressed and secreted; STAT, signal transducer and activator of transcription proteins; SNPs, single nucleotide polymorphisms; TARC, Thymus and activation-regulated chemokine; Tfh, T follicular helper; TNF, tumour necrosis factor; TSLP, thymic stromal lymphopoietin; TYK2, tyrosine-protein kinase.
Disclosure
HHK has undertaken paid consultancy work for Sanofi and Novartis and received lecture fees and support for conference attendance from GSK. HHK has undertaken paid lecture commitment work for AstraZeneca. The authors report no other conflicts of interest in this work.
References
- FokkensWJ, LundVJ, HopkinsC, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464.
- SmithKA, OrlandiRR, RudmikL. Cost of adult chronic rhinosinusitis: a systematic review. Laryngoscope. 2015;125(7):1547–1556.25640115
- LiaoB, LiuJX, LiZY, et al. Multidimensional endotypes of chronic rhinosinusitis and their association with treatment outcomes. Allergy. 2018;73(7):1459–1469.29331025
- DeCondeAS, SolerZM. Chronic rhinosinusitis: epidemiology and burden of disease. Am J Rhinol Allergy. 2016;30(2):134–139.
- SolerZM, WittenbergE, SchlosserRJ, MaceJC, SmithTL. Health state utility values in patients undergoing endoscopic sinus surgery. Laryngoscope. 2011;121(12):2672–2678.22034223
- HastanD, FokkensWJ, BachertC, et al. Chronic rhinosinusitis in Europe–an underestimated disease. A GA(2)LEN study. Allergy. 2011;66(9):1216–1223.21605125
- KowalskiML, AgacheI, BavbekS, et al. Diagnosis and management of NSAID-Exacerbated Respiratory Disease (N-ERD)-a EAACI position paper. Allergy. 2019;74(1):28–39.30216468
- RajanJP, WineingerNE, StevensonDD, WhiteAA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: a meta-analysis of the literature. J Allergy Clin Immunol. 2015;135(3):676–681.25282015
- StevensWW, PetersAT, HirschAG, et al. Clinical characteristics of patients with chronic rhinosinusitis with nasal polyps, asthma, and aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2017;5(4):1061–1070.e1063.28286156
- CoffmanRL. Origins of the T(H)1-T(H)2 model: a personal perspective. Nat Immunol. 2006;7(6):539–541.16715060
- RobinsonDS, HamidQ, YingS, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326(5):298–304.1530827
- TomassenP, VandeplasG, Van ZeleT, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137(5):1449–1456.e1444.26949058
- Van ZeleT, GevaertP, HoltappelsG, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. 2010;125(5):1069–1076.20451040
- HoxV, LourijsenE, JordensA, et al. Benefits and harm of systemic steroids for short- and long-term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin Transl Allergy. 2020;10:1.31908763
- HowardM, FarrarJ, HilfikerM, et al. Pillars article: identification of a T cell-derived B cell growth factor distinct from interleukin 2. J. Exp. Med. 1982. 155: 914-923. J Immunol. 2013;190(3):864–873.23335802
- SwainSL, WeinbergAD, EnglishM, HustonG. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145(11):3796–3806.2147202
- SederRA, PaulWE, DavisMM, Fazekas de St GrothB. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176(4):1091–1098.1328464
- HsiehCS, HeimbergerAB, GoldJS, O’GarraA, MurphyKM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci U S A. 1992;89(13):6065–6069.1385868
- WebbDC, McKenzieAN, KoskinenAM, YangM, MattesJ, FosterPS. Integrated signals between IL-13, IL-4, and IL-5 regulate airways hyperreactivity. J Immunol. 2000;165(1):108–113.10861042
- PeneJ, RoussetF, BriereF, et al. IgE production by normal human B cells induced by alloreactive T cell clones is mediated by IL-4 and suppressed by IFN-gamma. J Immunol. 1988;141(4):1218–1224.3135324
- MintyA, ChalonP, DerocqJM, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362(6417):248–250.8096327
- McKenzieAN, CulpepperJA, de Waal MalefytR, et al. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci U S A. 1993;90(8):3735–3739.8097324
- ZurawskiG, de VriesJE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15(1):19–26.7907877
- Wills-KarpM, LuyimbaziJ, XuX, et al. Interleukin-13: central mediator of allergic asthma. Science (New York, NY). 1998;282(5397):2258–2261.
- GrunigG, WarnockM, WakilAE, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282(5397):2261–2263.9856950
- ZhuZ, HomerRJ, WangZ, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779–788.10079098
- SamitasK, CarterA, KariyawasamHH, XanthouG. Upper and lower airway remodelling mechanisms in asthma, allergic rhinitis and chronic rhinosinusitis: the one airway concept revisited. Allergy. 2018;73(5):993–1002.29197105
- McCormickSM, HellerNM. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine. 2015;75(1):38–50.26187331
- GandhiNA, PirozziG, GrahamNMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425–437.28277826
- JunttilaIS, CreusotRJ, MoragaI, et al. Redirecting cell-type specific cytokine responses with engineered interleukin-4 superkines. Nat Chem Biol. 2012;8(12):990–998.23103943
- HeimMH. The Jak-STAT pathway: cytokine signalling from the receptor to the nucleus. Journal of Receptor and Signal Transduction Research. 1999;19(1–4):75–120.10071751
- ShirleyM. Dupilumab: first Global Approval. Drugs. 2017;77(10):1115–1121.28547386
- BachertC, HanJK, DesrosiersM, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650.31543428
- TomaS, HopkinsC. Stratification of SNOT-22 scores into mild, moderate or severe and relationship with other subjective instruments. Rhinology. 2016;54(2):129–133.27017484
- BachertC, MannentL, NaclerioRM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315(5):469–479.26836729
- LaidlawTM, MullolJ, FanC, et al. Dupilumab improves nasal polyp burden and asthma control in patients with CRSwNP and AERD. J Allergy Clin Immunol Pract. 2019;7(7):2462–2465.e2461.30954643
- BraddockM, HananiaNA, SharafkhanehA, ColiceG, CarlssonM. Potential risks related to modulating interleukin-13 and interleukin-4 signalling: a systematic review. Drug Saf. 2018;41(5):489–509.29411337
- AgacheI, SongY, RochaC, et al. Efficacy and safety of treatment with dupilumab for severe asthma. Allergy. 2020.
- RamalingamTR, PesceJT, SheikhF, et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9(1):25–33.18066066
- GevaertP, CalusL, Van ZeleT, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131(1):110–116.23021878
- BidderT, SahotaJ, RennieC, LundVJ, RobinsonDS, KariyawasamHH. Omalizumab treats chronic rhinosinusitis with nasal polyps and asthma together-a real life study. Rhinology. 2018;56(1):42–45.29288573
- GouldHJ, BeavilRL, VercelliD. IgE isotype determination: epsilon-germline gene transcription, DNA recombination and B-cell differentiation. Br Med Bull. 2000;56(4):908–924.11359628
- ZhangN, HoltappelsG, GevaertP, et al. Mucosal tissue polyclonal IgE is functional in response to allergen and SEB. Allergy. 2011;66(1):141–148.20659077
- ChenJB, JamesLK, DaviesAM, et al. Antibodies and superantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2017;139(4):1195–1204.e1111.27658758
- JeffeJS, SeshadriS, HamillKJ, et al. A role for anti-BP180 autoantibodies in chronic rhinosinusitis. Laryngoscope. 2013;123(9):2104–2111.24167818
- TanBK, LiQZ, SuhL, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128(6):1198–1206.e1191.21996343
- GevaertP, HoltappelsG, JohanssonSG, CuvelierC, CauwenbergeP, BachertC. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60(1):71–79.15575934
- PatadiaM, DixonJ, ConleyD, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24(1):11–16.20109310
- BabaS, KondoK, Toma-HiranoM, et al. Local increase in IgE and class switch recombination to IgE in nasal polyps in chronic rhinosinusitis. Clin Exp Allergy. 2014;44(5):701–712.24931597
- GevaertP, Nouri-AriaKT, WuH, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013;68(1):55–63.23157682
- ZhangYN, SongJ, WangH, et al. Nasal IL-4(+)CXCR5(+)CD4(+) T follicular helper cell counts correlate with local IgE production in eosinophilic nasal polyps. J Allergy Clin Immunol. 2016;137(2):462–473.26329514
- FinkelmanFD, KatonaIM, UrbanJF Jr, et al. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141(7):2335–2341.2459206
- BeckLA, ThaciD, HamiltonJD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–139.25006719
- NogaO, HanfG, BrachmannI, et al. Effect of omalizumab treatment on peripheral eosinophil and T-lymphocyte function in patients with allergic asthma. J Allergy Clin Immunol. 2006;117(6):1493–1499.16751018
- van RensenEL, EvertseCE, van SchadewijkWA, et al. Eosinophils in bronchial mucosa of asthmatics after allergen challenge: effect of anti-IgE treatment. Allergy. 2009;64(1):72–80.19076931
- JonstamK, SwansonBN, MannentLP, et al. Dupilumab reduces local type 2 pro-inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy. 2019;74(4):743–752.30488542
- LamEP, KariyawasamHH, RanaBM, et al. IL-25/IL-33-responsive T2 cells characterize nasal polyps with a default T17 signature in nasal mucosa. J Allergy Clin Immunol. 2015.
- LiaoB, CaoPP, ZengM, et al. Interaction of thymic stromal lymphopoietin, IL-33, and their receptors in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. Allergy. 2015;70(9):1169–1180.26095319
- BuchheitKM, CahillKN, KatzHR, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137(5):1566–1576.e1565.26691435
- PanchSR, BozikME, BrownT, et al. Dexpramipexole as an oral steroid-sparing agent in hypereosinophilic syndromes. Blood. 2018;132(5):501–509.29739754
- LaidlawTM, PrussinC, PanettieriRA, et al. Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope. 2019;129(2):E61–e66.30284267
- KariyawasamHH, RobinsonDS. The role of eosinophils in airway tissue remodelling in asthma. Curr Opin Immunol. 2007;19(6):681–686.17949963
- BachertC, SousaAR, LundVJ, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol. 2017;140(4):1024–1031.e1014.28687232
- GevaertP, VanBN, CattaertT, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128(5):989–995.21958585
- DenburgJA, SilverJE, AbramsJS. Interleukin-5 is a human basophilopoietin: induction of histamine content and basophilic differentiation of HL-60 cells and of peripheral blood basophil-eosinophil progenitors. Blood. 1991;77(7):1462–1468.1706953
- HumbertM, TailleC, MalaL, Le GrosV, JustJ, MolimardM. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur Respir J. 2018;51(5).
- SahotaJ, BidderT, LivingstonR, et al. Chronic rhinosinusitis and omalizumab: eosinophils not IgE predict treatment response in real-life. Rhinol. 2018;1:147–153.
- MellorEA, AustenKF, BoyceJA. Cysteinyl leukotrienes and uridine diphosphate induce cytokine generation by human mast cells through an interleukin 4-regulated pathway that is inhibited by leukotriene receptor antagonists. J Exp Med. 2002;195(5):583–592.11877481
- BoesveldtS, PostmaEM, BoakD, et al. Anosmia-A clinical review. Chem Senses. 2017;42(7):513–523.28531300
- SolerZM, YooF, SchlosserRJ, et al. Correlation of mucus inflammatory proteins and olfaction in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019.
- Ordovas-MontanesJ, DwyerDF, NyquistSK, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560(7720):649–654.30135581
- KoopmansT, GosensR. Revisiting asthma therapeutics: focus on WNT signal transduction. Drug Discov Today. 2018;23(1):49–62.28890197
- RockJR, RandellSH, HoganBL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Models Mech. 2010;3(9–10):545–556.
- GraziadeiGA, GraziadeiPP. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979;8(2):197–213.469573
- NicholsonGC, KariyawasamHH, TanAJ, et al. The effects of an anti-IL-13 mAb on cytokine levels and nasal symptoms following nasal allergen challenge. J Allergy Clin Immunol. 2011;128(4):800–807.21719078
- KabeschM, SchedelM, CarrD, et al. IL-4/IL-13 pathway genetics strongly influence serum IgE levels and childhood asthma. J Allergy Clin Immunol. 2006;117:269–274.16461126
- WenzelSE, BalzarS, AmplefordE, et al. IL4R alpha mutations are associated with asthma exacerbations and mast cell/IgE expression. Am J Respir Crit Care Med. 2007;175(6):570–576.17170387
- PalikheNS, KimSH, ChoBY, et al. IL-13 gene polymorphisms are associated with rhinosinusitis and eosinophilic inflammation in aspirin intolerant asthma. Allergy Asthma Immunol Res. 2010;2(2):134–140.20358028
- AslanF, AltunE, PaksoyS, TuranG. Could eosinophilia predict clinical severity in nasal polyps? Multidiscip Respir Med. 2017;12:21.28835819
- KhouryP, GraysonPC, KlionAD. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat Rev Rheumatol. 2014;10(8):474–483.25003763
- CastroM, CorrenJ, PavordID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496.29782217
- GAS, AWW, JYT, et al. Cost utility analysis of dupilumab versus endoscopic sinus surgery for chronic rhinosinusitis with nasal polyps. Laryngoscope. 2020.
- FokkensWJ, LundV, BachertC, et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy. 2019;74(12):2312–2319.31090937
- KariyawasamHH, RotirotiG. Allergic rhinitis, chronic rhinosinusitis and asthma: unravelling a complex relationship. Curr Opin Otolaryngol Head Neck Surg. 2013;21(1):79–86.23241653
- RobinsonDS, CampbellDA, DurhamSR, PfefferJ, BarnesPJ, ChungKF. Systematic assessment of difficult-to-treat asthma. Eur Respir J. 2003;22(3):478–483.14516138
- RobinsonDS, KariyawasamHH, HeaneyLG. Phase three studies of biologics for severe asthma: could do better? Eur Respir J. 2017;50(3).
- WenzelS, CastroM, CorrenJ, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44.27130691
- KariyawasamHH. Chronic rhinosinusitis with nasal polyps: insights into mechanisms of disease from emerging biological therapies. Expert Rev Clin Immunol. 2019;15(1):59–71.30370785