?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This article reports the green fabrication of cerium oxide nanoparticles (CeO2 NPs) using Olea europaea leaf extract and their applications as effective antimicrobial agents. O. europaea leaf extract functions as a chelating agent for reduction of cerium nitrate. The resulting CeO2 NPs exhibit pure single-face cubic structure, which is examined by X-ray diffraction, with a uniform spherical shape and a mean size 24 nm observed through scanning electron microscopy and transmission electron microscopy. Ultraviolet-visible spectroscopy confirms the characteristic absorption peak of CeO2 NPs at 315 nm. Fourier transform infrared spectroscopy reflects stretching frequencies at 459 cm−1, showing utilization of natural components for the production of NPs. Thermal gravimetric analysis predicts the successful capping of CeO2 NPs by bioactive molecules present in the plant extract. The antimicrobial studies show significant zone of inhibition against bacterial and fungal strains. The higher activities shown by the green synthesized NPs than the plant extract lead to the conclusion that they can be effectively used in biomedical application. Furthermore, reduction of cerium salt by plant extract will reduce environmental impact over chemical synthesis.
Introduction
Green synthesis of metallic oxide nanoparticles (NPs) is an emerging field of nanoscience and technology. Synthesis methodology and surface modification play a critical role in the physical, chemical, electrical, and optical properties of nanomaterials. Cerium oxide (CeO2) is a semiconductor with a band gap energy of 3.0–3.9 eV and large excitation energy. In bulk form, CeO2 is a singular rare earth oxide and does not show any crystallographic modifications by temperature increase up to its melting point.Citation1–Citation5 Its various applications such as acting as a catalyst; use in sensor, solid oxide fuel cells, and sunscreen cosmetics; and its O2 storage capacity, role in CO2 solar conversion, and antibacterial applications are due to its idiosyncratic ability to alter the oxidation states.Citation6–Citation10
Ordinarily, CeO2 NPs are fabricated by physical and chemical methods including hydrothermal,Citation11 flame spray pyrolysis,Citation12 thermal decomposition,Citation13 aqueous precipitation,Citation14 and coprecipitation technique.Citation15 In contrast, green synthesis of metallic oxide NPs offers several advantages such as cost-effectiveness, large-scale commercial production, and pharmaceutical applications. The CeO2 NPs are considered less toxic toward cell lines, when compared to TiO2 and ZnO.Citation16 Biosynthesis of CeO2 NPs using plant extracts such as Gloriosa superba L. and Hibiscus sabdariffa has been reportedCitation1 with promising results.
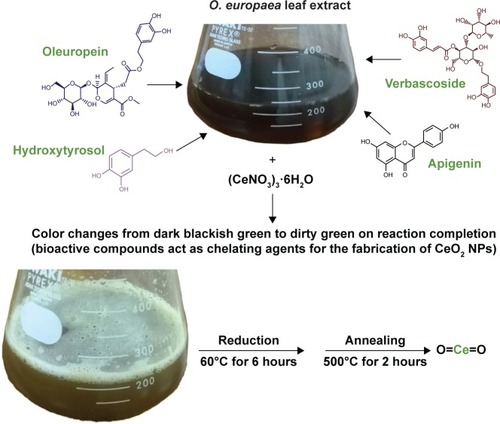
Olea europaea belongs to the family Oleaceae and is native to tropical and warm temperate regions of the world.Citation17 Mediterranean region serves as the major cultivation area, accounting for about 98% of the world’s olive production.Citation18 O. europaea exhibits antioxidant, anti-inflammatory, antiatherogenic, anticancer, antimicrobial, and antiviral activities, along with hypolipidemic and hypoglycemic potential.Citation19 Bioactive compounds principally present in olive leaves are oleuropein, followed by hydroxytyrosol, the flavone-7-glucosides of luteolin and apigenin, and verbascoside.Citation20,Citation21
This study reports green synthesis of CeO2 NPs utilizing O. europaea leaf extract as an effective chelating agent without any acid or base treatment. The synthesized CeO2 NPs are further characterized for their antibacterial and antifungal potential.
Experimental procedures
Collection and processing of plant material
The authenticated O. europaea leaves were procured from Barani Agricultural Research Institute, Chakwal, Pakistan. Fresh leaves were washed with distilled water and air-dried under shade at room temperature to avoid photodissociation of bioactive compounds. For extract preparation, 20 g of dried leaves was ground and suspended in 200 mL of distilled water. The mixture was placed in a shaking incubator at 50°C for 2 hours at 50 rpm. Thereafter, it was filtered using Whatman No 1 filter paper and the filtrate was stored at room temperature for further usage.
Fabrication of CeO2 NPs by O. europaea leaf extract
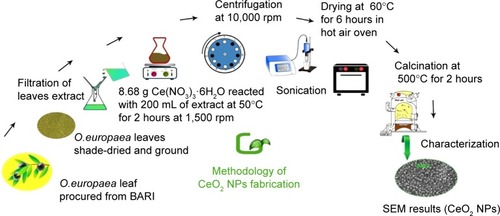
For green synthesis of CeO2 NPs, 8.68 g of Ce(NO3)3 ⋅ 6H2O was added to 200 mL of O. europaea leaf extract and stirred on a magnetic hotplate at 50°C, 1,500 rpm for 2 hours. The blackish brown CeO2 NPs were collected by centrifugation at 10,000 rpm (GR BioTek, Orpington, England) for 10 minutes. The NPs were washed repeatedly with deionized water and finally dried in hot air oven at 60°C for ~6 hours and further annealed in Gallenkamp furnace (Apeldoorn, the Netherlands) at 500°C for 2 hours. The yellow-colored NPs obtained were stored in an air-tight jar at room temperature. A schematic diagram for the fabrication of CeO2 NPs using O. europaea leaf extract is shown in .
Characterization of CeO2 NPs
Scanning electron microscopy (SEM)
The size and shape of synthesized CeO2 NPs were studied using JEOL-JSM-6490LA SEM (JEOL, Tokyo, Japan) operating at 20 kV with a counting rate of 2,838 cps.
Transmission electron microscopy (TEM)
To further investigate the internal morphology, TEM analysis of green fabricated CeO2 NPs was performed by TEM (model no JEOL-1010) operating at 80 kV.
X-ray diffraction (XRD; crystallographic structure)
To examine the crystallographic structure of green synthesized CeO2 NPs, XRD analysis was carried out using X’PertCitation3 Powder (PANalytical) with nickel monochromator in the range of 2θ from 20° to 80° using Cu Kα radiation of wavelength 1.5406 Å. Operating voltage of 40 kV with 30 mA current was provided at room temperature. To calculate the theoretical size of CeO2 NPs, Scherrer’s equation (D=0.9λ/β cosθ) was employed, where D is the average crystalline domain size perpendicular to the reflecting planes, λ the X-ray wavelength (1.5406 Å), β the angular full width at half maximum in radians, and θ is the diffraction angle (2θ is the measured angle of diffraction in degrees) or Bragg’s angle. Furthermore, the lattice constant was measured using the following equation:
Fourier transform infrared (FTIR) spectroscopy
For the identification of unknown bio-active compounds in plant extract, utilization of capping agents from plant extract and confirmation of production materials (CeO2 NPs), FTIR spectroscopic analysis was performed using KBr pellet methodology (model SHIMADZU FTIR, Kyoto, Japan) in the wavenumber ranges 400–4,000 cm−1. FTIR spectra for CeO2 NPs, residue (as synthesized), and O. europaea leaf extract were recorded separately and explained comparatively.
Ultraviolet (UV)-visible spectroscopy
The optical characterization of green fabricated CeO2 NPs was made through UV-visible spectroscopy in the range of 290–400 nm with a Shimadzu spectrophotometer (model UV-1800, Kyoto, Japan) operating at a resolution of 1 nm. Three milligrams of synthesized CeO2 NPs was dissolved in 10 mL of deionized H2O and this solution was sonicated for 20 minutes. Subsequently, the liquid sample was subjected to UV-visible spectrophotometry.
Thermal gravimetric analysis (TGA)
To examine the thermal properties and capping action of bioactive compounds for tailoring CeO2 NPs, we performed TGA (model Diamond TGA; PerkinElmer, Waltham, USA) under nitrogen environment from 25°C to 800°C at 10°C/minute.
Antibacterial activity of CeO2 NPs
The antibacterial activities of green synthesized CeO2 NPs were studied against Gram-positive (G+ve) (Staphylococcus aureus ATCC 6538) and Gram-negative (G−ve) (Escherichia coli ATCC 15224, Pseudomonas aeruginosa ATCC 15442, Klebsiella pneumoniae ATCC-BAA 1706) strains by disk diffusion method with slight modification.Citation1 In brief, the bacterial strains were cultured in nutrient broth (Sigma-Aldrich Co., St Louis, MO, USA) at 37°C until the culture reached 1.5×108 colony forming units (CFU) per milliliter. About 20 mL of autoclaved molten nutrient agar was poured into the Petri dishes and allowed to cool. All of the four bacterial cultures were swapped over solidified agar medium. Disks were loaded with 20 µg/5 µL CeO2 NPs solution. A solution of cefixime or roxithromycin (4 mg/mL each) was used as positive control and deionized H2O as negative control. The plates were incubated at 37°C for 24 hours and the zones of inhibition (ZOIs) around the disks were measured thereafter.
Antifungal assay
Antifungal activity was determined against Mucor species (FCBP-0300), Aspergillus flavus (FCBP-0064), Fusarium solani (FCBP-434), and Aspergillus niger (FCBP-0198) by disk diffusion method. Sabouraud dextrose agar (pH 5.7) (Sigma-Aldrich Co.) was autoclaved and poured in Petri plates ensuring sterile conditions. Fungal lawns were prepared by inoculating spores on the surface of the growth media. Thereafter, disks were loaded with CeO2 NPs solution 20 µg/5 µL through micropipette. Clotrimazole 10 µg/disk was used as positive control, while deionized water served as negative control. The plates were incubated at 25°C for 24–48 hours and ZOI was measured.
CFU counting assay
To examine the percentage reduction in bacterial count with time, we carried out CFU counting assay. The experimentation was performed in sample tubes, each having 2 mL of sterilized (autoclaved) nutrient broth. The first sample tube containing 2 mL of nutrient broth acted as sterile negative control and the second tube was impregnated with cefixime (as positive control), while the remaining four samples were inoculated with S. aureus (G+ve), and E. coli, P. aeruginosa, and K. pneumoniae (G−ve), respectively, having a concentration of 107 and 109 CFU/mL each, and then placed in a shaking incubator at 37°C. Thereafter, the bacterial culture tubes were loaded with CeO2 NPs solution (20 µg/5 µL). After an interval of 30 minutes, 10 µL from each sample was collected and spread over the already prepared agar plate surface. To estimate the bacterial colony in liquid, McFarland turbidity standards were applied, and their values were calibrated before and after incubation. Positive and negative control tests were performed on E. coli only. Later on, the plates were incubated at 37°C and the percentage reduction with time in bacterial count was calculated by the following approximation:Citation22
Statistical analysis
Results are calculated as the mean of at least three individual experiments presented with standard deviation (SD). The statistical analysis of the results was carried out with Student’s t-tests by using Statistical Package for the Social Sciences software package version 19.0 (IBM Corporation, Armonk, NY, USA) and considered significant at P-values <0.05.
Results and discussion
Biosynthesis process using O. europaea leaf extract (visual observations)
Synthesis of NPs using plant extracts has several advantages over other conventional biosynthesized routes (based on fungi, bacteria, etc). It is because the green extract is more ecofriendly and environment friendly, and fewer cytotoxic reducing agents are required for tailoring NPs. It is also commonly observed that maintaining microbial cultures under optimum conditions is time consuming and costly. In bulk (at an industrial level), safe mode synthesis of NPs, microbial-based NPs production has its own limitations.Citation23,Citation24
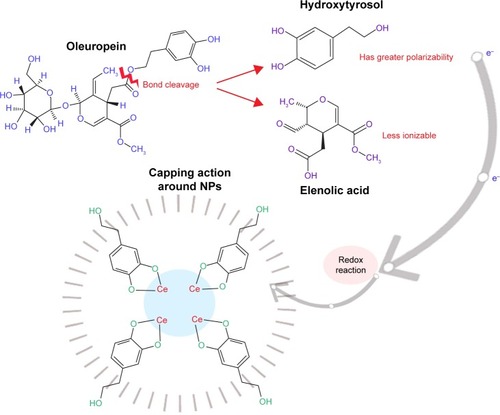
O. europaea aqueous extract contains a variety of molecules that might be effectively utilized as chelating agents in the green synthesis of CeO2 NPs. Upon addition of (CeNO3)3 6H2O, the biologically active compounds of the plant extractCitation21 act as reducing agents for the nanofabrication process. Oleuropein is the most abundant bioactive compound in O. europaea aqueous extract, which breaks down into highly polarizable and reactive hydroxytyrosol.Citation21 It may take part in the redox mechanism in tuning CeO2 NPs, as shown in . shows the change in color from dark blackish green to dirty green, indicating the formation of CeO2 NPs. The blackish brown CeO2 NPs were collected by centrifugation at 10,000 rpm for about 10 minutes. Thereafter, the NPs were washed repeatedly with deionized water to eliminate the uncoordinated biomolecules from the extract, and finally dried in hot air oven at 60°C for ~6 hours and further annealed at 500°C for 2 hours. The yellow-colored NPs obtained were stored at room temperature. The entire recovery process is presented in and .
SEM analysis
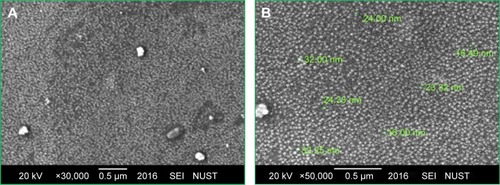
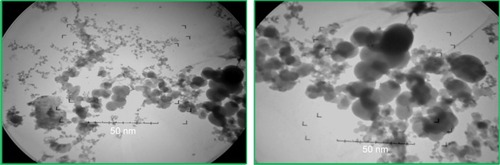
depicts the SEM images of green synthesized CeO2 NPs. It is obvious from the SEM micrograph that the CeO2 NPs are highly homogeneous and symmetrical in morphology with spherical shape, and the findings clearly represent a mean size of 24 nm. Similar morphological shape with uniform average grain size of less than 30 nm was also reported previously.Citation10,Citation25,Citation26
TEM analysis
It is clear from the TEM micrograph that the green synthesized CeO2 NPs exhibit spherical morphology with highly homogeneous symmetry and an average particle size of 24 nm ().
XRD (crystallographic structure) analysis
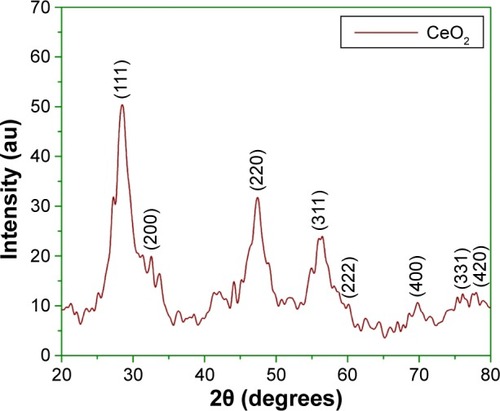
shows the XRD profile of green fabricated CeO2 NPs. It shows that the CeO2 NPs possess single-phase cubic fluorite structure without any additional peaks. Presence of several broad Bragg peaks corresponds to (111), (200), (220), (311), (222), (400), (331), and (420) orientations and they are precisely well indexed to Joint Committee on Powder Diffraction Standards (JCPDS) (card no 34-0394). This later crystallographic phase reflects that each cerium site is encountered by eight oxygen sites in face-centered cubic arrangement, while each oxygen has the geometry of tetrahedron cerium site. Using Debye–Scherrer approximation and from the broadening of peaks, the approximate crystal-lite size of CeO2 NPs was found to be 6 nm, which shows that each grain of size 24 nm was composed of a cluster of 6 nm crystallites. The observed value was found to be close to that reported previously.Citation25 The calculated value of lattice constant “a” is 0.585 nm, while the unit cell volume “V” is measured to be 0.20 nm.
FTIR spectroscopy analysis
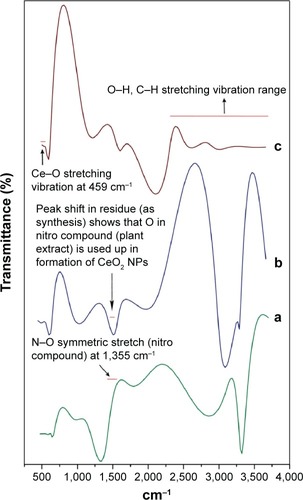
Comparative FTIR results illustrate significant absorption peaks in the wave number range 400–4,000 cm−1 (). The range between 2,500 and near 4,000 cm−1 is assigned to O–H and C–H stretch. The plant extract exhibiting N–O symmetric stretch at 1,355 cm−1 was found to be less in the residue after CeO2 NPs were isolated, reflecting that Ce is actively oxidized to CeO2 by the nitro compounds in extract chemistry. The onset of a broad intense band that can be viewed as one tends toward higher vibrations (2,800 cm−1) can be ascribed to O–H stretching range due to the presence of surface-adsorbed water molecules from the surroundings.Citation27 The spectra due to stretching frequency of Ce–O can be seen at 459 cm−1, which indicates the fabrication of CeO2 NPs. Similarly, Ce–O stretching band at 451 cm−1 and at 450 cm−1 was reported by Arumugam et alCitation10 and Goharshadi et al,Citation28 respectively.
UV-visible spectroscopy studies
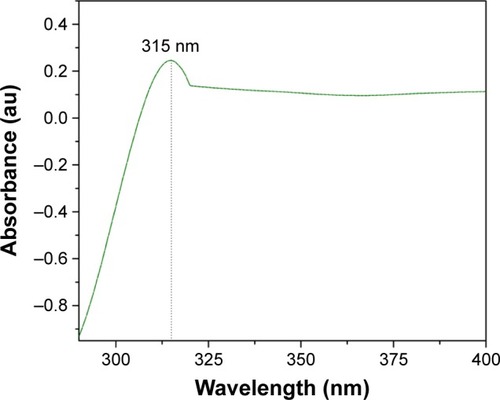
illustrates the UV-visible absorption spectra of CeO2 NPs, which demonstrates the presence of a single intensified peak at 315 nm that was also closely observed in previous studies.Citation25 The position of the absorption peak confirms that green synthesized CeO2 NPs possess fluorite cubic morphology,Citation26 as justified in and .
TGA studies
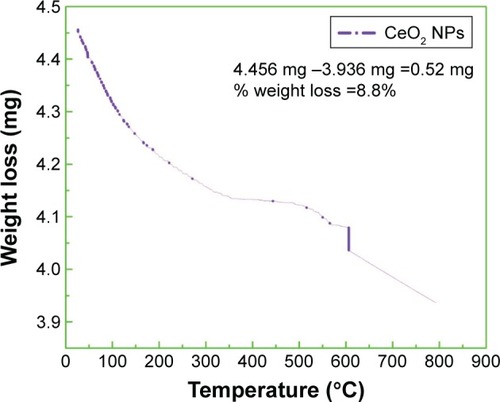
TGA of biosynthesized CeO2 NPs was performed to study the capping action of biomolecules and the thermal behavior of the synthesized CeO2 NPs, which is shown in . TGA represents different stages of decomposition. Weight loss occurring in the first step at temperatures from 25°C to 115°C was ascribed to the loss of surface water molecules.Citation29 The minor weight loss that occurred in the second step at temperatures from 50°C to 200°C was due to the combustion of bioactive surface organic components acting as capping agents. The slight weight loss occurring at around 600°C was because of oxygen loss at high temperature.Citation30 Our experimental observations show total weight loss of 0.52 mg (8.8%) of the sample.
Antibacterial analysis of CeO2 NPs
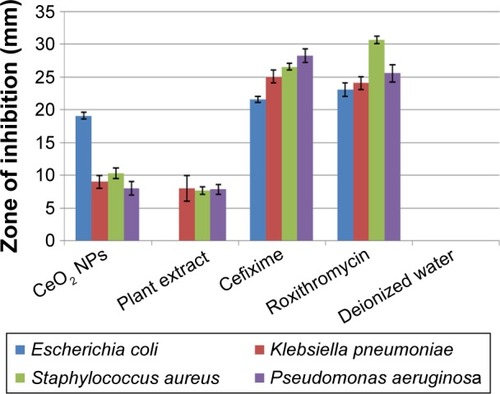
The antibacterial assay was performed against G+ve and G−ve bacterial entities using CeO2 NPs sample loaded at a concentration of 20 µg/05 µL on disks. reflects the measurements in size of ZOI around CeO2 NPs poured disks. In the case of K. pneumoniae, S. aureus, and P. aeruginosa, a ZOI of 9, 10, and 8 mm was observed, respectively, which shows mild to moderate antibacterial behavior. Promising ZOI of 19 mm was recorded against E. coli; the prominent difference in ZOI might be due to the unique cell membrane structure that helps the bacterium to resist antimicrobial agents. Besides all these, other features such as the rate at which the NPs diffuse also play an important role against various bacterial strains.Citation31 shows that G+ve bacteria are more susceptible to CeO2 NPs than G−ve bacteria; similar trends were reported before.Citation10 Green synthesized NPs have proven efficiency and comparatively low genotoxic and cytotoxic behavior toward healthy cells, when compared to NPs synthesized by various chemical methods.Citation32 CeO2 NPs fabricated by way of chemical methods were also found to be cytotoxic to somatic cells.Citation25 O. europaea leaf extract also takes part in inhibiting K. pneumoniae, S. aureus, and P. aeruginosa, with ZOI of 8, 7.6, and 7.8 mm, respectively; its potential activity has also been reported in previous studies.Citation33,Citation34 Bioactive compounds from the leaf extract, such as oleuropein, are also found to be magically active in inhibiting several bacterial pathogens.Citation34
Figure 10 Antibacterial analysis of CeO2 NPs, plant extract, cefixime/roxithromycin (positive control), and deionized water (negative control), taking their mean values with ±SD.
Abbreviations: NPs, nanoparticles; SD, standard deviation.
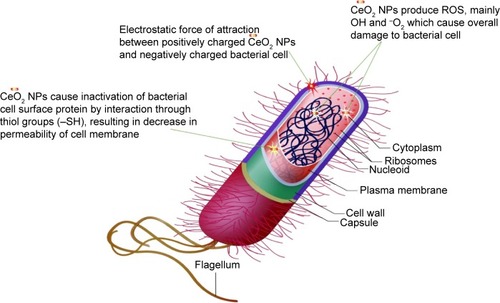
From the results of XRD, SEM, and TEM, the average CeO2 NP size was found to be 24 nm, with the smaller crystallite size being 6 nm. Higher surface area due to smaller crystal size results in greater antibacterial activity.Citation35 The antibacterial activity of CeO2 NPs primarily depends upon the electrostatic attraction between positively charged NPs and negatively charged bacterial cell surface and is crucial for the activity of NPs as a bactericidal agent. The interaction is also responsible for reactive oxygen species (ROS) generation, which ultimately leads to bactericidal effect.Citation36,Citation37 ROS include the highly reactive hydroxyl radical (OH), singlet oxygen (1O2), and the least toxic superoxide anion radical (−*O2), contributing to the major oxidative stress in biological systems.Citation38 ROS production is closely related to the efficiency of a photocatalyst, depending on the generation rate, rate of migration, and energy levels of the photoexcited electron–hole pairs. In the present study, such an increase in ROS levels is perhaps linked to the electronic and microstructure properties like grain size, specific surface area, pore size, and so on. The mechanism of photoexcited generation of ROS can be given as follows:Citation10
Mostly, it is believed that NPs release ions which interact with the thiol (−SH) group of the protein residing at the bacterial cell surface,Citation39 resulting in denaturation of surface protein along with loss of cell membrane permeability, ultimately causing cell death. The rough surface for metallic NPs also intensifies their mechanical bactericidal capabilities of E. coli.Citation40 In our experimentation, UV analysis showed strong, intensified CeO2 NPs absorption peaks at 315 nm. It is a well-known fact that Ce, as compared to its bulk, forms low band gap energy which results in its photoexcitation at room temperature.Citation25 This phenomenon will also assist in mass production of ROS inside bacterial cellular environment, and intoleration of ROS due to CeO2 NPs results in malfunctioning of microbial biosynthetic machinery, causing lethality. In is presented a possible schematic diagram summarizing the above fact.
Antifungal assay analysis of CeO2 NPs
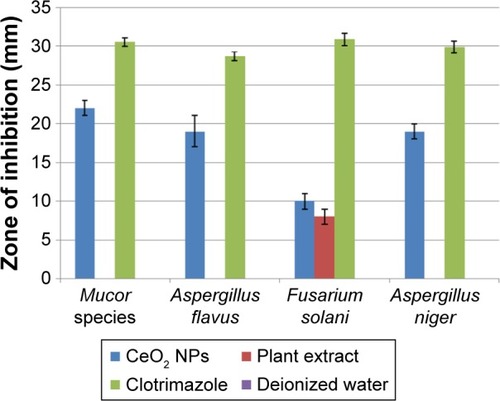
The antifungal properties of green synthesized CeO2 NPs have been shown in . A. flavus and A. niger show greater CeO2 NPs susceptibility with ZOI of 19 mm than F. solani for which the ZOI is 10 mm. The fungicidal activity of CeO2 NPs was found to be maximum in the case of Mucor species with ZOI of 22 mm, showing great competency as an antifungal agent, like other antifungal candidates.Citation41 The exact mechanism of the antifungal activity of CeO2 NPs is not understood, but the antimicrobial potential is probably due to the electromagnetic interaction and ROS generation,Citation36 like the production of lethal hydroxyl radical (OH) when present in the immediate vicinity of the lipid membrane. ROS cause oxidative deterioration of cell membrane lipids,Citation42 denaturing the cell membrane permeability with leakage of potassium ions, ultimately causing cell death.Citation39
Analysis of CFU counting assay
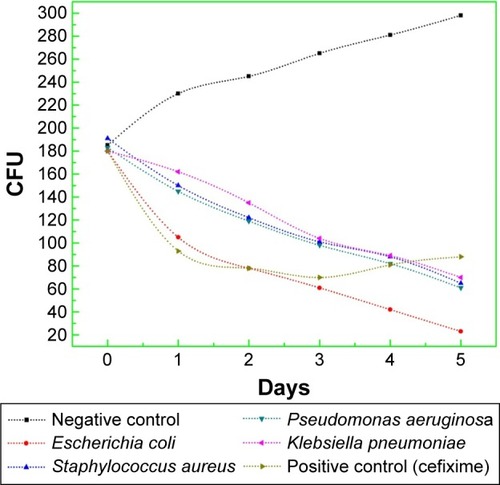
The results of progression in bacterial growth with time are documented in . It is obvious that for all samples, the bacterial population was in lag phase during the first 20 minutes of the experiment, but with the addition of CeO2 NPs (20 µg/5 µL), persistent decline in growth was observed. Reduction in viability of E. coli was markedly maximum, while for all other bacterial strains, the growth inhibition rate with time was almost similar. So, it is very clear that due to extremely small particle size and pure phase crystallinity with high surface area ( and ), CeO2 NPs exhibit prominent bacteriostatic and bactericidal activity. This might be due to intolerance of metallic CeO2 NPs by bacterial metabolic machinery, lethal ROS generation, and greater cytotoxicity toward microbial pathogens.Citation36,Citation37 Future work regarding the molecular level interaction of CeO2 NPs in bacterial growth inhibition needs more exploration.
Conclusion
The leaf extract of O. europaea is proved to be an effective chelating agent for the green synthesis of highly homogeneous, ecofriendly, and extremely small sized CeO2 NPs. Both XRD and SEM findings confirm the cubic fluorite structure of CeO2 NPs without any impurities and with a mean crystallite size of 6 nm and an average grain size of 24 nm. FTIR results reveal discharge of bioactive compounds from the leaf extract and Ce–O stretching mode at 459 cm−1. This study shows that CeO2 NPs exhibit mild to moderate antibacterial activity against both G+ve and G−ve strains, especially high susceptibility was shown by E. coli. CeO2 NPs exhibited promising inhibitory effect against Mucor species, ensuring their antifungal properties. It can be deduced from this study that CeO2 NPs synthesized through green chemistry have great potential for future antimicrobial therapies. The follow-up study will focus on differential cytotoxic behavior of photosynthesized CeO2 NPs to healthy and cancer cells; more specifically, the size-dependent anticancer and antimicrobial properties will be examined.
Acknowledgments
The authors acknowledge Sidra Iftikhar (Department of Mathematics, University of Wah, Pakistan) for providing technical assistance regarding SEM and XRD operations.
Disclosure
The authors report no conflicts of interest in this work.
References
- ThovhogiNDialloAGurib-FakimAMaazaMNanoparticles green synthesis by Hibiscus Sabdariffa flower extract: main physical properties – university of South AfricaJ Alloys Compd2015647392396
- ChandrakalaHNRamarajBShivakumaraiahSiddaramaiahOptical properties and structural characteristics of zinc oxide cerium oxide doped polyvinyl alcohol filmsJ Alloys Compd2014586333342
- ChenJCChenWCTienYCShihCJEffect of calcination temperature on the crystallite growth of cerium oxide nano-powders prepared by the co-precipitation processJ Alloys Compd20104961–2364369
- LeeB-HNakayamaTTokoiYSuzukiTNiiharaKSynthesis of CeO/TiO nanoparticles by laser ablation of Ti target in cerium (III) nitrate hexahydrate (Ce(NO)⋅6HO) aqueous solutionJ Alloys Compd2010509412311235
- GangopadhyaySFrolovDDMasunovAESealSStructure and properties of cerium oxides in bulk and nanoparticulate formsJ Alloys Compd2014584199208
- KorsvikCPatilSSealSSelfSSuperoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticlesChem Commun (Camb)2007101056105817325804
- ChandarKNJayavelRSynthesis and characterization of CTAB passivated cerium oxide nanoparticles prepared by co-precipitation routePhysica E2014584851
- DarroudiMHoseiniSJOskueeRKHosseiniHAGholamiLGerayliSFood-directed synthesis of cerium oxide nanoparticles and their neurotoxicity effectsCeram Int201340574257430
- RenuGDivya RaniVVNairSVSubramanianKRVLakshmananVKDevelopment of cerium oxide nanoparticles and its cytotoxicity in prostate cancer cellsAdv Sci Lett201261725
- ArumugamAKarthikeyanCHameedASHGopinathKGowriSKarthikaVSynthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial propertiesMater Sci Eng C Biol Appl201549408415
- YuS-HCölfenHFischerAHigh quality CeO nanocrystals stabilized by a double hydrophilic block copolymerColloids Surf A Physicochem Eng Asp20042431–34952
- ChanneiDInceesungvornBWetchakunNPhotocatalytic activity under visible light of Fe-doped CeO nanoparticles synthesized by flame spray pyrolysisCeram Int201239331293134
- LinHLWuCYChiangRKFacile synthesis of CeO nanoplates and nanorods by [100] oriented growthJ Colloid Interface Sci20103411121719833346
- SreeremyaTSThulasiKMKrishnanAGhoshSA novel aqueous route to fabricate ltrasmall monodisperse Lipophilic Cerium oxide NanoparticlesInd Eng Chem Res2012511318326
- GodinhoMJGonçalvesRFSantosLPSVarelaJALongoELeiteERRoom temperature co-precipitation of nanocrystalline CeO and CeGdO powderMater Lett2006618–919041907
- GeorgeSPokhrelSXiaTUse of a rapid cytotoxicity screening approach to engineer a safer zinc oxide Nanoparticle through iron DopingACS Nano201041152920043640
- SoniMGBurdockGAChristianMSBitlerCMCreaRSafety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foodsFood Chem Toxicol200644790391516530907
- UN Food and Agriculture OrganizationYearbook ProductionRomeFAO199548118119
- OmarSHOleuropein in olive and its pharmacological effectsSci Pharm201078213315421179340
- Japón-LujánRLuque-RodríguezJMLuque de CastroMDDynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leavesJ Chromatogr A200611081768216442552
- Benavente-GarcíaOCastilloJLorenteJOrtuñoADel RioJAAntioxidant activity of phenolics extracted from L. leavesFood Chem2000684457462
- ManeerungTTokuraSRujiravanitRImpregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressingCarbohydr polym20087214351
- AhmedSAhmadMSwamiBLIkramSA review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertiseJ Adv Res201671172826843966
- ThakkarKNMhatreSSParikhRYBiological synthesis of metallic nanoparticlesNanomedicine20106225726219616126
- AbbasFIqbalJJanTDifferential cytotoxicity of ferromagnetic Co doped CeO nanoparticles against human neuroblastoma cancer cellsJ Alloys Compd201564810601066
- KhanSBFaisalMRahmanMMJamalAExploration of CeO nanoparticles as a chemi-sensor and photo-catalyst for environmental applicationsSci Total Environ2011409152987299221570707
- GaoJZhaoYYangWPreparation of samarium oxide nanoparticles and its catalytic activity on the esterificationMater Chem Phys20037716569
- GoharshadiEKSamieeSNancarrowPFabrication of cerium oxide nanoparticles: Characterization and optical propertiesJ Colloid Interface Sci2011356247348021316699
- MaensiriSMasingboonCLaokulPEgg white synthesis and photoluminescence of platelike clusters of CeO2 nanoparticlesCryst Growth Des200775950955
- SureshRPonnuswamyVMariappanREffect of annealing temperature on the microstructural, optical and electrical properties of CeO2 nanoparticles by chemical precipitation methodApp Surf Sci2013273457464
- FaikONMehmetYDenisaFAntonFFloricaDAlexandraPMolecular mechanism and targets of the Antimicrobial activity of metal NanoparticlesCur Top Med Chem2015151615831588
- LimaRSeabraABDuránNSilver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticlesJ App Toxicol20123211867879
- AzizNHFaragSEMousaLAAbo-ZaidMAComparative antibacterial and antifungal effects of some phenolic compoundsMicrobios19989337443549670554
- FurneriPMMarinoASaijaAUccellaNBisignanoGIn vitro antimycoplasmal activity of oleuropeinInt J Antimicrob Agents200220429329612385687
- HameedASHKarthikeyanCSasikumarSKumarSKumaresanSRaviGImpact of alkaline metal ions mg2+, ca2+, Sr2+ and Ba2+ on the structural, optical, thermal and antibacterial properties of ZnO nanoparticles prepared by the co-precipitation methodJ Mater Chem B201314359505962
- XiaTKovochichMLiongMComparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress propertiesACS Nano20082102121213419206459
- BurelloEWorthAPA theoretical framework for predicting the oxidative stress potential of oxide nanoparticlesNanotoxicology20115222823521609138
- LiYZhangWNiuJChenYMechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide NanoparticlesACS Nano2012665164517322587225
- TongG-XDuF-FLiangYPolymorphous ZnO complex architectures: Selective synthesis, mechanism, surface area and Zn-polar plane-codetermining antibacterial activityJ Mater ChemB201214454463
- WangXYangFYangWYangXA study on the antibacterial activity of one-dimensional ZnO nanowire arrays: effects of the orientation and plane surfaceChem Commun (Camb)2007424419442117957306
- StylianouMKulesskiyELopesJPGranlundMWennerbergKAntifungal application of Nonantifungal drugsAntimicrob Agents Chemother20145821055106224277040
- HowlettNGAverySVInduction of lipid peroxidation during heavy metal stress in Saccharomyces cerevisiae and influence of plasma membrane fatty acid unsaturationAppl Environ Microbiol1997638297129769251184