Abstract
Invasive mycoses are a major problem for immunocompromised individuals and patients in intensive care units. Morbidity and mortality rates of these infections are high because of late diagnosis and delayed treatment. Moreover, the number of available antifungal agents is low, and there are problems with toxicity and resistance. Alternatives for treating invasive fungal infections are necessary. Nanostructured systems could be excellent carriers for antifungal drugs, reducing toxicity and targeting their action. The use of nanostructured systems for antifungal therapy began in the 1990s, with the appearance of lipid formulations of amphotericin B. This review encompasses different antifungal drug delivery systems, such as liposomes, carriers based on solid lipids and nanostructure lipids, polymeric nanoparticles, dendrimers, and others. All these delivery systems have advantages and disadvantages. Main advantages are the improvement in the antifungal properties, such as bioavailability, reduction in toxicity, and target tissue, which facilitates innovative therapeutic techniques. Conversely, a major disadvantage is the high cost of production. In the near future, the use of nanosystems for drug delivery strategies can be used for delivering peptides, including mucoadhesive systems for the treatment of oral and vaginal candidiasis.
Fungal diseases
Fungal infections are a growing public health problem, mainly related to the advances of modern medicine in prolonging the lifespan and the quality of life of patients under severe clinical conditions.Citation1 A range of new broad-spectrum antibiotics made it possible to successfully treat infections of many microorganisms, which had previously been fatal. This resulted in prolonged survival of patients highly susceptible to infection. Thus, fungal infections emerge as leading causes of morbidity and mortality in immunocompromised and intensive care unit patients.Citation2
In recent decades, bacteria and fungi have developed considerable resistance to many traditional and modern synthetic drugs.Citation3 In this context, nanoparticles (NPs) can also overcome the drug resistance mechanisms, related to decreased absorption, increased drug efflux from microbial cells, biofilm formation, or intracellularity.Citation4 Finally, NPs deliver the highest dose of antimicrobial agents specifically to the site of infection, thus overcoming drug resistance with less adverse effects on the patient.Citation5
Pathogenic fungi
Mycoses are among the most difficult global diseases to be controlled. Some conditions can be a predisposition to invasive mycoses, such as immunosuppression, neoplasia, and some chronic diseases. Oral candidiasis and vaginal candidiasis are the most common fungal diseases.Citation6 These superficial mycoses affect 25%–30% of human population.Citation4 Candida albicans is also involved in denture stomatitis pathogenesis, a disease very common in older individuals. Other fungal diseases can be less frequent, but much more severe, such as asthma with fungal sensitization, allergic bronchopulmonary aspergillosis, invasive aspergillosis, chronic pulmonary aspergillosis, pneumocystosis, meningeal cryptococcosis, mucormycoses, or invasive candidiasis.Citation7 Invasive fungal infections (IFIs) are less predominant, but their morbidity and mortality rates are high, killing about 1.5 million people per year.Citation8 A total of ten genera of fungi have a high prevalence in infections, including Aspergillus, Candida, Cryptococcus, Blastomyces, Coccidioides, Histoplasma, Paracoccidioides, Penicillium, Pneumocystis, and Rhizopus. However, 90% of deaths are caused by Candida, Cryptococcus, Aspergillus and Pneumocystis.Citation8 Bitar et alCitation9 observed a higher incidence of candidemia (43.4%), followed by Pneumocystis jirovecii pneumonia (26.1%), invasive aspergillosis (23.9%), cryptococcosis (5.2%), and mucormycosis (1.5%) in IFIs through a retrospective study conducted in France in 2001–2010. Among fungal infections, candidiasis is the most common fungal infection worldwideCitation10 and an important cause of morbidity and mortality in bloodstream and other invasive infections among hospitalized patients in many countries of the world.Citation11 C. albicans is the main etiology of candidiasis, but other species, such as Candida glabrata, Candida parapsilosis, or Candida krusei, are emerging as causes of nosocomial infections.Citation12–Citation14
Cryptococcus neoformans is the third most common cause of infectious complications in the central nervous system in AIDS patients:Citation15 1 million new cases of cryptococcal meningitis occur each year causing ~600,000 deaths.Citation16 Aspergillus fumigatus is the most common cause of invasive mycoses by filamentous fungi, with mortality rates of 40%–90%.Citation17,Citation18
Antifungal drugs
Antifungal resistance is an increasing threat for the effective treatment of invasive mycoses, making their therapy difficult, expensive, or even impossible.Citation10 The current treatment approaches for IFIs are fairly limited and include three main classes of drugs: polyenes (amphotericin B [AmB]), azoles (fluconazole, isavuconazole, itraconazole, posaconazole, and voriconazole), and echinocandins (anidulafungin, caspofungin, and mycafungin).Citation18 To obtain good clinical results in the treatment, early and appropriate treatment is required, but the activity of current antifungal agents is not predictably against emerging yeasts and filamentous fungi and can cause undesirable side effects.Citation19 Older antifungal agents, such as AmB, despite their toxicity, are very important in the treatment of IFIs as they have a broad-spectrum and low resistance rates.Citation20
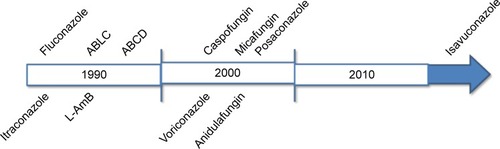
Recent advances in antifungal chemotherapy with broad-spectrum triazoles and echinocandins provide more effective and less toxic alternatives to conventional polyenes. Despite this, IFI mortality rates remain high, and there is a growing need for new therapeutic options.Citation21 However, the rate of discovery of antifungal drugs is unlikely to be sufficient for the future demands, since few drugs are currently being discovered. In the early 1990s, two new antifungal drugs were approved by the US Food and Drug Administration (FDA), namely, fluconazole and itraconazole.Citation22 Still in the 1990s, lipid formulations of AmB, amphotericin B lipid complex (ABLC, in 1995), amphotericin B colloidal dispersion (ABCD, in 1996), and liposomal AmB (L-AmB, in 1997) were all approved. In the 2000s, caspofungin (in 2001) and voriconazole (in 2002)Citation23 were also approved. Micafungin was the second echinocandin antifungal agent approved by the FDA in 2005 and anidulafungin was the third to be approved in 2006.Citation24 Posaconazole was approved in 2006 as oral suspension, and in 2013 and 2014 for use in tablets and intravenously, respectively.Citation22 More recently, in March 2015, the FDA approved isavuconazoleCitation25 ().
Figure 1 Time course of discovery of antifungal drugs.
Abbreviations: ABLC, amphotericin B lipid complex; ABCD, amphotericin B colloidal dispersion; L-AmB, liposomal amphotericin B.
Given the current panorama of microbial resistance and lack of new drugs, NPs appear to aid in the treatment of various diseases, including mycoses.Citation26 NPs can be defined as ultradispersed supramolecular structures with submicrometer size ranging from 10 µm to 1,000 µm. The drug may be dissolved, entrapped, encapsulated, or bound to a matrix of NPs, which acts as a reservoir for particulate systems and therefore plays an important role as a drug delivery system for clinical applications, particularly in oncology.Citation27 Many studies have currently demonstrated the efficacy of anti-fungal agents incorporated into NPs for combating fungal infections.Citation6–Citation8 The production of NPs through nanotechnology has revolutionized the delivery of drugs. Today, there is a consensus that nanotechnology represents a miniaturization of objects, as well as the preparation of nanomaterials with physical and chemical properties that drastically differ from those of bulk materials because they are on a nanoscale. Until the early 1970s, the administration of pharmaceutical suspensions intravenously was considered impossible due to the risk of embolism. The current development of suspensions of NPs containing drugs (eg, nanomedicines or nanopharmaceuticals) is the use of NPs for treating, diagnosing, and preventing diseases. Through these, it is possible to increase the therapeutic index of various drugs by improving activity, reducing toxicity, and targeting them selectively toward diseased tissues and cells.
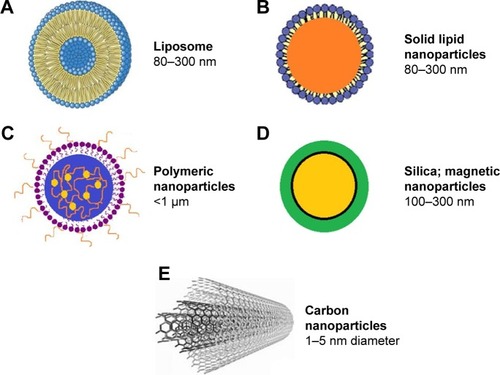
A noteworthy problem in the treatment of many diseases, including invasive mycoses, is the delivery of the drug to the target site, since the conventional drugs have limitations such as restricted efficacy, poor biodistribution, and lack of selectivity. The solution to this problem is the use of a drug delivery control system that can overcome these limitations and drawbacks. The therapy based on a delivery system is important to solve problems, regarding the balance between high drug concentrations and toxic effects. A major technological breakthrough in medicine has been the reduction in the particle size from micrometers to nanometers.Citation28 Through small dimensions, NPs can target specific sites within the body as cells and tissues are permeable to them. Therefore, NPs can deliver the active drug to sites where conventional drugs do not reach, thus minimizing unwanted side effects. The therapeutic potential of NPs as carriers of drugs depends on their hydrodynamic size, shape, quantity, surface chemistry, route of administration, length of stay in circulation, and reaction with the immune system. Nanostructures exhibit unique physicochemical and biological properties, which makes them a favorable material for biomedical applications.Citation26,Citation29 Nanoscale structures, or nanosized structures, can be used to carry drugs such as liposomes, synthetic and natural polymers, inorganic and metal NPs, dendrimers, silica, and carbon materials, as well as magnetic NPs (MNPs)Citation30,Citation31 ().
Lipid-associated formulations
Lipid formulations involve the association of an antifungal drug, such as AmB or nystatin, with a lipid delivery system to reduce toxicity.Citation33,Citation34 Three different lipid formulations of AmB have been introduced in the clinical setting. The lipid composition and molecular structure of these formulations vary considerably with unique pharmacokinetic profiles. Although there is evidence of the safety of these formulations, the impact of their unique structure and pharmacokinetic differences for specific clinical efficacy is unproven.Citation35
AmB deoxycholate (D-AmB) is a polyene macrolide available for clinical use since its initial FDA approval in 1959.Citation36 AmB is produced through a fermentation process by soil actinomycete Streptomyces nodosus. The AmB has broad spectrum of action and has been considered the gold standard of antifungal therapy for many years, despite being associated with a high incidence of adverse effects related to infusion and nephrotoxicity.Citation36,Citation37 D-AmB still has a place in the antifungal therapy but newer drugs (eg, AmB associated with lipid formulations, fluconazole and voriconazole, or caspofungin and micafungin) are being used as first-line treatment options.Citation38 Fluconazol represented a major advance in the treatment of invasive candidiasis because of its broad activity, excellent tolerability, and favorable pharmacokinetics. Since its introduction, fluconazole has been widely used for the treatment and prophylaxis of candidiasis, except for those infections caused by C. krusei, C. glabrata, or other species with reduced susceptibility or resistance to this drug.Citation39
Lipid complex and colloidal dispersion
In the late 1990s, almost 40 years after the first formulation of D-AmB, three AmB-based lipid formulations, namely ABLC (Abelcet®), ABCD (Amphotec®), and L-AmB (AmBisome®) were developed to reduce nephrotoxicity without compromising antifungal efficacy.Citation20,Citation38 ABLC (Abelcet®; The Liposome Company, Princeton, NJ, USA) received initial approval in the UK in April 1995 and was the first lipid-based formulation approved by the FDA in December 1995. ABCD was previously marketed as both Amphocil® and Amphotec® and was initially approved in the UK in 1994 and by the FDA in December 1996.Citation27 The first lipid-based formulation developed was ABLC by associating AmB with a lipid–drug delivery vehicle. ABLC consists of AmB in complex with two phospholipids at 1:1 drug-to-lipid molar ratio. Both phospholipids, l-α-dimyristoylphosphatidylcholine and l-α-dimyristoylphosphatidylglycerol, are present at 7:3 molar ratio. ABLC is characterized by lipid-stabilized AmB aggregates, which appear as ribbon-like structures, with length ranging from 1.6 nm to 11.1 nm, and because of its size, circulating AmB serum concentrations are lower when compared to D-AmB.Citation35 ABCD consists of 1:1 molar ratio of AmB and cholesterol sulfate, a highly organized structure formed by a natural metabolite of cholesterol. A noncovalent complex of AmB and cholesteryl sulfate forms a tetramer consisting of a hydrophilic and a hydrophobic part. These add-in spiral arms form a disk-like structure with a diameter of ~122 nm and thickness of 4 nm.Citation40 Although ABCD reduces the availability of AmB in the kidneys reducing the nephrotoxicity, this drug concentration increases in the endothelial reticulum system,Citation41,Citation42 as well as the ABLC formulation.Citation35 Both ABCD and ABLC are quickly endocyted by the endothelial reticulum system and distributed into the tissue.Citation43 ABLC formulations demonstrated efficacy against fungi such as Fusarium solani,Citation44 Candida dubliniensis,Citation45 A. fumigatus,Citation46 Aspergillus quadrilineatus,Citation47 C. neoformans,Citation48 and Rhizopus oryzae.Citation49 lists the activity of ABCL and ABCD formulations against different fungi.
Table 1 Effect of AmB formulations ABLC and ABCD with different fungal species
ABCD exhibits dose-limiting, infusion-related toxicities;Citation35 consequently, the dosages administered should not exceed 3–4 mg/kg/d. ABCD formulation was not effective in the treatment of paracoccidioidomycosis with a dosage of 3 mg/kg/d, the failure of which can possibly be due to dosage, duration, or poor effectiveness of this lipid preparation,Citation50 although Hanson and StevensCitation51 reported in vitro activity against Paracoccidiodes brasiliensis. This formulation is not suitable as a prophylactic antifungal agent for neutropenic patients due to adverse effects related to infusion.Citation52 ABCD was found at high concentrations in the lungs after treatment, which does not happen with L-AmB, thus being a possible alternative for lung infections.Citation53 The prophylactic use against pulmonary mycoses by AmB nebulization has been reported.Citation54,Citation55 Other drugs, such as itraconazole, in colloidal dispersion could also be suitable for nebulization.Citation56
Liposomes
Liposomes are other type of lipid formulations, consisting of unilamellar or multilamellar layers on the membrane of lipids such as phospholipids, surrounded by aqueous compartment.Citation60,Citation61 The liposomes can carry hydrophilic drugs in the aqueous core and increase penetration through the lipophilic membranes, as well as lipophilic drugs, which are inserted into the lipid bilayer, increasing their solubility in aqueous body fluids.Citation68 Liposomes provide a better protection than other lipid formulations against external degradation by enzymes. In addition, they are biocompatible and biodegradable.Citation62,Citation63
Conventional liposomes have some limitations, such as little instability and difficult to be stored for long periods and rapid uptake by the RES, thereby decreasing their half-life in circulation.Citation69 To solve these problems, extensive research has been developed to modify the surface of liposomes, to optimize their size, and to understanding their mechanisms of action. New generation liposomes are characterized by high mechanical stability, ability to induce or to inhibit the immune system, longer bypass, high loading efficiency, ease of interaction with the cell membrane, and increased target specificity. The milestone in the development of the new generation of liposomes is to control drug release.Citation68
Indeed, the progress in pharmacology has introduced a number of potent therapeutic agents requiring drug carriers that are selective and bioresponsive. Advances in the technology of liposomes as drug delivery systems include long-circulating liposomes, for example, liposomes prepared with hydrophilic polymers on their surface (eg, polyethylene glycol), reduce both uptake by reticuloendothelial system and toxicity of the encapsulated drug.Citation64,Citation70 This camouflage allows liposomes to exhibit the abovementioned functions. However, there is the disadvantage of an inhibited cellular absorption, limiting their uptake by macrophages and tumor cells. Hatakeyama et alCitation71 developed cleavable polyethylene glycol (PEG)-lipids to solve the problem of cellular uptake inhibition, since PEG systems are separated in response to the target tissue microenvironment. The target specificity is achieved by anchoring targeting ligands that bind to the desired receptors.Citation72 The number of plates or cross-linking lipids controls the rate of drug release from liposomes.Citation73 Approximately 50 years after the discovery of liposomes, the FDA approved 13 liposome-based products for human use, which includes one formulation containing AmB for the treatment of fungal infections.Citation68
L-AmB presents significantly lower toxicity compared to other AmB formulations, and it is effective in the treatment of severe invasive mycoses, including mucormycosis,Citation74 fusariosis,Citation75 cryptococcal meningitis,Citation76,Citation77 coccidioidal meningitis,Citation78,Citation79 blastomycosis,Citation80 and pulmonary aspergillosis.Citation81,Citation82 However, in 2013, Ariano et alCitation83 reported that L-AmB may not be adequate to control lung infections by Blastomyces dermatitidis. Al Nakeeb et alCitation82 found that lipid formulations of AmB can induce dose-dependent reduction in lung injury markers and circulating fungal biomarkers. The recommended therapeutic dosages are 3–6 mg/kg/d.Citation35 A clinical dose of L-AmB 3 mg/kg/d may cause complete suppression of both galactomannan and levels of 1,3-β-d-glucan in most patients with invasive aspergillosis.Citation82
The literature reports some problems associated with administration of L-AmB, such as hepatotoxicity,Citation84 progressive leukoencephalopathy,Citation85,Citation86 and also development of lysosomal storage disease.Citation87 Treatment failures have also been reported.Citation88,Citation89 The prophylactic use of L-AmB in immunocompromised patients is still a challenge. Mihara et alCitation90 report that prophylaxis with aerosolized L-AmB was not effective in animal model. Therefore, prospective studies are needed to compare this formulation with triazoles. In addition to AmB, other antifungal agents are carried by liposomal delivery systems, such as nystatin.Citation33,Citation34 L-AmB could be useful for the treatment of cryptococcosis,Citation91 including species of Aspergillus,Citation92 C. dubliniensis.Citation45
L-AmB also has activity against fungal biofilms. Schinabeck et alCitation94 were the first to describe Candida biofilm infection of catheters in animal models treated with L-AmB to block the infection. In addition, L-AmB was effective to eradicate Candida biofilm in a continuous catheter flow model,Citation95 and Ramage et alCitation96 showed that L-AmB kills C. albicans biofilms rapidly and effectively in a dose-dependent manner.
The need to improve treatment outcomes for IFI increased interest in exploring an alternative antifungal strategy. The administration of AmB in aerosol, which has been widely used, to provide the drug directly to the site of infection or fungal colonization, has the potential to maximize their spectrum of activity while minimizing systemic toxicity that is associated with parenteral administration. Aerosol AmB is used (usually as a prophylactic strategy) in high-risk patients.Citation97 The literature reports few studies regarding delivery systems based on aerosol for fungal infections, including AmB to prevent pulmonary aspergillosis,Citation98 C. neoformans,Citation89 and C. albicans.Citation99
shows the studies with L-AmB and L-AmB formulations associated with other conventional antifungal drugs for the treatment of IFIs in immunocompromised patients, including studies on in vitro activity of L-AmB against different fungi.
Table 2 Liposomes in fungal diseases
In addition to the liposomal preparation of AmB, there are AmB-polyaggregates with similar efficacy to that of D-AmB and L-AmB in the treatment of a murine-disseminated infection by C. glabrata.Citation120 Souza et alCitation129 tested an alternative delivery system to D-AmB, the NANO-D-AmB that has antifungal efficacy against P. brasiliensis with lower levels of cytotoxicity compared to that of D-AmB formulation both in vivo and in vitro, thus confirming a better delivery of AmB.
NPs based on solid lipid nanoparticles and nanostructured lipid carriers
Solid lipid nanoparticles (SLNs) emerged as a new class of colloidal drug carriers at the beginning of the 1990s, and their application has been widely exploited as drug delivery in the area of pharmaceutics, clinical medicine, and therapy. Polymeric NPs (PNPs) have the advantage of promoting chemical modifications, but there are some limitations such as polymer degradation, high cost, and difficult approval by regulatory authorities.Citation130 Thus, the attention of several research groups has been focused on an alternative to PNPs, that is, the SLNs.Citation131 SLNs provide physical stability as incorporated drugs do not suffer degradation, have controlled release, and excellent tolerability. Therefore, they can be used by different routes of administration, such as parenteral,Citation132 peroral,Citation133 dermal,Citation134 ocular,Citation135 pulmonary,Citation136 and rectal.Citation137
SLNs are a generation of drugs where the liquid lipid (oil) has been replaced by a solid lipid, mainly composed of a dispersed lipid in physiological water or aqueous surfactant solution (). Replacement of liquid lipid by solid lipid represents a milestone for drug controlled release because the mobility of the drug within the solid lipid is usually lower than within the liquid oil, which makes this system performance attractive for pharmaceutical products.Citation131
Figure 3 Nanoparticles based on solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs).
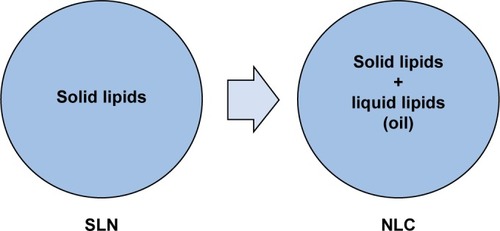
The most advanced forms of SLNs are nanostructured lipid carriers (NLCs), lipid–drug conjugates, and polymer lipid hybrid nanoparticles (PLNs). Therefore, NLCs, introduced at the millennium’s turn, are made of a solid lipid matrix that traps the liquid lipid in their nanocompartments,Citation138 which decreases some of the problems associated with SLNs, such as limited drug-loading capacity, expulsion of the drug during storage, suitability of drug release, and physical stability of long-term suspension.Citation131 Lipid–drug conjugates were developed to increase the drug-loading capacity, whereas PLNs are hybrids of liposome and PNPs developed to carry poorly water-soluble drugs with high encapsulation efficiency and loading capacity and to control the release of drugs. Moreover, PLNs show excellent serum stability and a wide spectrum of different target cells.Citation139,Citation140 The presence of a solid lipid matrix can cause problems in the production of SLNs, since this matrix system is subject to crystallization during its formation, resulting in some drawbacks such as a low encapsulation efficiency and a poor expulsion of the stored drug.Citation141–Citation143 shows some studies on SLN transport systems in antifungal therapy for the therapy of fungal infections. However, scientific evidence on infection treated with the SLN system is scarce.
Table 3 Antifungal drugs-loaded nanoparticles based on solid lipids (SLNs) and nanostructured lipid carriers (NLCs)
NLCs are produced by a lipid mixture of liquid and solid phases with increased content of NPs.Citation27 Mathpal et alCitation144 have recently conducted a study using a spraying technique for pulmonary delivery of AmB-NCL and concluded that through this technique the drugs are better distributed throughout the lung tissue. Several antifungal agents were also tested in different SLN and NLC delivery systems, such as itraconazole-loaded SLNs,Citation130,Citation145 itraconazole-loaded NLC,Citation146,Citation147 miconazole nitrate-loaded NLC,Citation148 econazole nitrate-loaded NLC,Citation149 and voriconazoleCitation150 (). PNPs and nanosuspensions would present clear advantages over lipid formulations, since they have a longer shelf life at room temperature and low production costs.Citation151
Polymeric NPs
PNPs are polymeric colloidal systems, which have a diameter <1 µm, in which the drug can be dissolved, coated, encapsulated, or dispersed.Citation29,Citation159 Polymer degradation, high cost, and difficult approval by regulatory authorities are some of the disadvantages.Citation131 PNPs are stable in the gastrointestinal environment and protect encapsulated drugs against gastrointestinal pH, degradation enzymes, and efflux pumps, maintaining the stability of the drugs in this unfavorable environment.Citation160 The use of polymers to form PNPs provides flexibility due to their physicochemical properties (eg, size, surface charge, and hydrophobicity), allowing a controlled drug release. In addition, it is possible to modulate the surface properties or use different polymer conjugates on the surface of PNPs.Citation161 The possibility to add antibodies, peptides, or small molecules to the polymer surface allows tissue-specific interactions with cell receptors or components.Citation162 Moreover, PNPs enable the encapsulation of a broad range of therapeutic drugs and molecules, such as DNA and small interfering RNA.Citation160
PNPs are classified into two categories: nanospheres and nanocapsules. Nanocapsules are vesicular systems in which the drug is inside an aqueous or oily cavity surrounded by a polymeric membrane, whereas nanospheres are matrix systems in which the drug is physically and uniformly dispersed in the matrix.Citation163 These delivery systems have been developed primarily for parenteral, oral, or ocular administration. There are several polymers for preparing PNPs, such as poly-ε-caprolactone,Citation164 polyacrylamide,Citation165 polyacrylate,Citation166 DNA,Citation167 chitosan,Citation167–Citation169 and gelatin.Citation170 After a polymerization reaction, drugs may be immobilized on the surface of the PNPsCitation171 or encapsulated in their structure during the polymerization processing.Citation172 The release of the drug may occur by desorption, diffusion, or erosion of PNPs in the target tissue.Citation29 However, during the storage time, aggregation of NPs can occur and form precipitates. Other chemical stability problems regarding the polymer or other raw materials have been described, which obstruct their industrial applicability.Citation173
Inorganic NPs, including gold, iron oxide, silver, or silica, among others, are investigated in preclinical and clinical studies for the treatment, diagnosis, and detection of many diseases. Moreover, many inorganic compounds serving as the material for making NPs have been widely used in clinical practice for several therapeutic applications.Citation174 One example of therapeutic compounds that act as antibacterial agents is silver ions.Citation175–Citation177 Inorganic NPs offer diagnostic and therapeutic opportunity that other PNPs or not, cannot offer.Citation174
PNPs have some problems arising from residues of organic solvents used in the production process, such as cytotoxicity of the polymer and complex production for industrial application. In many production processes, the concentration of NPs is low not exceeding 2%,Citation178 which compromises their use. Thus, the development of solid dosage forms of NPs is a point of interest in research. Examples of antifungal agents and metal particles associated with PNPs used as drug delivery systems are shown in .
Table 4 Polymeric and other nanoparticles with antifungal activity
Dendrimers
Dendrimers present synthetic polymeric architectures with low polydispersion and controlled surface features. Dendrimers have three main architectural components, namely, core, dendrons, and surface-active groups.Citation196,Citation197 There are some ways to connect biologically active compounds to dendrimers: the drug can be encapsulated in the internal structure of the dendrimersCitation198 or chemically linked or physically adsorbed onto the surface of them.Citation199 The choice of the immobilization method will depend on the characteristics of the drug.
Several families of dendrimers have been widely studied regarding their use in biomedical sciences. Most well-known dendrimers include polyamidoamines, polypropyleneimines, poly-l-lysines, carbosilanes, and phosphorous dendrimers. Their properties are often not satisfactory because of the high cytotoxicity of the nanomolecules and their low solubility and biocompatibility. Thus, dendrimers are often subjected to various modifications in order to improve their features: dendrimer conjugate with PEG,Citation200 carbohydrates,Citation201 or acetyl groupsCitation202 to reduce the cytotoxicity. The compounds bound to dendrimers can improve the surface activity as well as their biological and physical properties. Several specific ligands can be adsorbed, including folic acid,Citation203 antibodies,Citation204 target cyclic peptides containing arginine-glycine-aspartic acid,Citation205 and PEG.Citation206
There are few studies on the antifungal activity enhanced by dendrimers. Polyamidoamine dendrimers were shown to improve the solubility of clotrimazole and enhance its antifungal activity against different species of Candida.Citation207 According to Janiszewska et al,Citation208 the antifungal activity of dendrimeric lipopeptides causes morphological changes in fungal cells and inhibition of enzyme activity candidal 1,3-β-d-glucan synthase. Staniszewska et alCitation209 reported in vitro effects of the dendrimer D186 on the virulence factors of C. albicans, where there was a reduction in adhesive properties and potential of the pathogenic yeast.
Other delivery systems
The antifungal activity in other delivery systems, such as carbon nanotubes, MNPs, and silica NPs, has been less studied. Carbon nanotubes have been one of the most exploited biomedical applications of NPs in the world. Benincasa et alCitation210 showed that AmB conjugated to carbon nanotubes presented an excellent activity against clinical isolates of Candida spp. The antimicrobial activity against bacteria and fungi (C. albicans) was also demonstrated by scanning electron microscopy, showing that microbial cells were wrapped or entrapped by carbon nanotube networks.Citation211 Reduced graphene oxide nanosheets have antifungal activity against Aspergillus niger, Aspergillus oryzae, and Fusarium oxysporum.Citation212 In 2014, Ciu et alCitation213 showed graphene oxide as a novel two-dimensional nanomaterial for applications in health biomedical with antifungal properties and low cost.
Hussein-Al-Ali et alCitation214 demonstrated the antimicrobial activity of MNPs loaded with ampicillin to form a nanocomposite decreases the activity of C. albicans. Niemirowicz et alCitation215 also reported an inhibition of the growth of C. albicans by using MNPs that can be removed from human plasma, blood, serum, and abdominal and cerebrospinal fluids.
Conclusion and future prospects
There is a clear need to find new therapeutic alternatives for IFIs as the number of drugs is reduced and there is an increased resistance to antifungal agents, mainly in emerging fungi such as non-C. albicans species. Moreover, many of the current drugs show toxicity. Thus, a major disadvantage of the polyene antifungal agents, such as D-AmB, is their clinically significant toxicity, although the development of lipid formulations of AmB has reduced this problem.Citation35 Lipid formulations of AmB preserve renal function and survival of critically ill patients suffering from IFIs. However, these formulations are very expensive and are not globally available.Citation216
Studies involving nanotechnology and medically important fungi have demonstrated improvements in the antifungal properties, such as bioavailability, toxicity, and target tissue, for some drugs, such as AmB, which can facilitate innovative therapeutic approaches. Nanotechnology offers the possibility of multifunctional systems to meet the many different biological and therapeutic requirements.Citation85 The ultimate therapeutic goal will be to select a drug that can effectively cure the disease without causing side effects.Citation217 In the near future, the use of nanosystems for drug delivery can be attractive strategies for delivering peptides, nuclear acids, or drugs.Citation218 In addition, mucoadhesive systems can promote a more specific targeting and retention of the delivery system in humans, such as mucosal surfaces, gastrointestinal tract, lung, genitourinary tract, nasal, and ocular systems. In combination with excellent technological platforms, nanotechnological strategies can increase the bioavailability of antifungal drugs.Citation188
Acknowledgments
This work was financially supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tec-nológico (CNPq), and Programa de Apoio ao Desenvolvimento Científico – Faculdade de Ciências Farmacêuticas – UNESP (PADC-FCF).
Disclosure
In the past 5 years, Guillermo Quindós has received grant support from Astellas Pharma, Gilead Sciences, Pfizer SLU, Schering Plough, and Merck Sharp and Dohme. He has been an advisor/consultant to Merck Sharp and Dohme and has been paid for talks on behalf of Abbvie, Astellas Pharma, Esteve Hospital, Gilead Sciences, Merck Sharp and Dohme, and Pfizer SLU. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript, apart from those disclosed.
References
- PerlrothJChoiBSpellbergBNosocomial fungal infections: epidemiology, diagnosis, and treatmentMed Mycol200745432134617510856
- AlangadenGJNosocomial fungal infections: epidemiology, infection control, and preventionInfect Dis Clin North Am201125120122521316001
- WHOOptions for ActionGenevaWHO Publication2012
- Sardi JdeCPitanguiNDSRodríguez-ArellanesGTaylorMLFusco-AlmeidaAMMendes-GianniniMJSHighlights in pathogenic fungal biofilmsRev Iberoam Micol2014311222924252828
- PelgriftRYFriedmanAJNanotechnology as a therapeutic tool to combat microbial resistanceAdv Drug Deliv Rev20136513–141803181523892192
- DorganEDenningDWMcMullanRBurden of fungal disease – IrelandJ Med Microbiol201564pt 442342625596121
- ChanderJStchigelAMAlastruey-IzquierdoAFungal necrotizing fasciitis, an emerging infectious disease caused by Apophysomyces (Mucorales)Rev Iberoam Micol2015322939825576377
- BrownGDDenningDWGowNALevitzSMNeteaMGWhiteTCHidden killers: human fungal infectionsSci Transl Med20124165165r v13
- BitarDLortholaryOLe StratYPopulation-based analysis of invasive fungal infections, France, 2001–2010Emerg Infect Dis20142071149115524960557
- WHOAntimicrobial Resistance Global Report on Surveillance 2014GenevaWHO2014
- RuhnkeMAntifungal stewardship in invasive Candida infectionsClin Microbiol Infect201420suppl 6111824661820
- VoltanARFusco-AlmeidaAMMendes–GianniniMJSCandiduria: epidemiology, resistance, classical and alternative antifungals drugsSOJ Microbiol Infect Dis20142217
- SchaalJVLeclercTSolerCEpidemiology of filamentous fungal infections in burned patients: a French retrospective studyBurns201541485386325681957
- QuindósGEpidemiology of candidaemia and invasive candidiasis. A changing faceRev Iberoam Micol2014311424824270071
- LinXHeitmanJThe biology of the Cryptococcus neoformans species complexAnnu Rev Microbiol2006606910516704346
- ParkBJWannemuehlerKAMarstonBJGovenderNPappasPGChillerTMEstimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDSAIDS200923452553019182676
- LinSJSchranzJTeutschSMAspergillosis case-fatality rate: systematic review of the literatureClin Infect Dis200132335836611170942
- XieJLPolviEJShekhar-GuturjaTCowenLEElucidating drug resistance in human fungal pathogensFuture Microbiol20149452354224810351
- ChenSCPlayfordEGSorrellTCAntifungal therapy in invasive fungal infectionsCurr Opin Pharmacol201010552253020598943
- ChandrasekarPManagement of invasive fungal infections: a role for polyenesJ Antimicrob Chemother201166345746521172787
- PetrikkosGSkiadaARecent advances in antifungal chemotherapyInt J Antimicrob Agents200730210811717524625
- AllenDWilsonDDrewRPerfectJAzole antifungals: 35 years of invasive fungal infection managementExpert Rev Anti Infect Ther201513678779825843556
- AsciogluSChanKAUtilization and comparative effectiveness of caspofungin and voriconazole early after market approval in the U.SPLoS One201491e8365824427277
- ChandwaniSWentworthCBurkeTAPattersonTFUtilization and dosage pattern of echinocandins for treatment of fungal infections in US hospital practiceCurr Med Res Opin200925238539319192983
- McCormackPIsavuconazonium: first global approvalDrugs201575781782225902926
- WeissigVPettingerTKMurdockNNanopharmaceuticals (part 1): products on the marketInt J Nanomedicine201494357437325258527
- CalixtoGBernegossiJFonseca-SantosBChorilliMNanotechnology-based drug delivery systems for treatment of oral cancer: a reviewInt J Nanomedicine201493719373525143724
- CouvreurPNanoparticles in drug delivery: past, present and futureAdv Drug Deliv Rev2013651212322580334
- WilczewskaAZNiemirowiczKMarkiewiczKHCarHNanoparticles as drug delivery systemsPharmacol Rep2012641020103723238461
- LiakosIGrumezescuAMHolbanAMMagnetite nanostructures as novel strategies for anti-infectious therapyMolecules2014198127101272625140449
- BaumgartnerJBertinettiLWiddratMHirtAMFaivreDFormation of magnetite nanoparticles at low temperature: from superparamagnetic to stable single domain particlesPLoS One201383e5707023520462
- AstrucDElectron-transfer processes in dendrimers and their implication in biology, catalysis, sensing and nanotechnologyNat Chem20124425526722437709
- Carrillo-MuñozAJQuindósGTurCIn-vitro antifungal activity of liposomal nystatin in comparison with nystatin, amphotericin B cholesteryl sulphate, liposomal amphotericin B, amphotericin B lipid complex, amphotericin B desoxycholate, fluconazole and itraconazoleJ Antimicrob Chemother199944339740110511410
- QuindósGCarrillo-MuñozAJRuesgaMTIn vitro activity of a new liposomal nystatin formulation against opportunistic fungal pathogensEur J Clin Microbiol Infect Dis200019864564811014634
- HamillRJAmphotericin B formulations: a comparative review of efficacy and toxicityDrugs201373991993423729001
- CornelyOAAspergillus to zygomycetes: causes, risk factors, prevention, and treatment of invasive fungal infectionsInfection200836429631318642109
- Laniado-LaborínRCabrales-VargasMNAmphotericin B: side effects and toxicityRev Iberoam Micol200926422322719836985
- MoenMDLyseng-WilliamsonKAScottLJLiposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infectionsDrugs200969336139219275278
- GómezJGarcía-VázquezEHernándezAEspinosaCRuizJNosocomial candidemia: new challenges of an emergent problemRev Esp Quimioter201023415816821191553
- WorkingPKAmphotericin B colloidal dispersion. Pre-clinical reviewChemotherapy199945suppl 1152610394017
- GrollAHMickieneDPiscitelliSCWalshTJDistribution of lipid formulations of amphotericin B into bone marrow and fat tissue in rabbitsAntimicrob Agents Chemother200044240841010639371
- FieldingRMSingerAWWangLHBabbarSGuoLSRelationship of pharmacokinetics and drug distribution in tissue to increased safety of amphotericin B colloidal dispersion in dogsAntimicrob Agents Chemother19923622993071605595
- PatelRAmphotericin B colloidal dispersionExpert Opin Pharmacother20001347548811249532
- ArneyKLTiernanRJudsonMAPrimary pulmonary involvement of Fusarium solani in a lung transplant recipientChest19971124112811309377931
- QuindósGCarrillo-MuñozAJArévaloMPIn vitro susceptibility of Candida dubliniensis to current and new antifungal agentsChemotherapy200046639540111053905
- SivakOBartlettKRisovicVAssessing the antifungal activity and toxicity profile of amphotericin B lipid complex (ABLC; Abelcet) in combination with Caspofungin in experimental systemic aspergillosisJ Pharm Sci20049361382138915124198
- PolacheckINaglerAOkonEDrakosPPlaskowitzJKwon-ChungKJAspergillus quadrilineatus, a new causative agent of fungal sinusitisJ Clin Microbiol19923012329032931452721
- DiamondDMBauerMDanielBEAmphotericin B colloidal dispersion combined with flucytosine with or without fluconazole for treatment of murine cryptococcal meningitisAntimicrob Agents Chemother19984235285339517927
- VidovicAArsic-ArsenijevicVTominDProven invasive pulmonary mucormycosis successfully treated with amphotericin B and surgery in patient with acute myeloblastic leukemia: a case reportJ Med Case Rep2013726324299522
- DietzeRFowlerVGSteinerTSPecanhaPMCoreyGRFailure of amphotericin B colloidal dispersion in the treatment of paracoccidioidomycosisAm J Trop Med Hyg199960583783910344661
- HansonLHStevensDAComparison of antifungal activity of amphotericin B deoxycholate suspension with that of amphotericin B cholesteryl sulfate colloidal dispersionAntimicrob Agents Chemother19923624864881605618
- TimmersGJZweegmanSSimoons-SmitAMvan LoenenACTouwDHuijgensPCAmphotericin B colloidal dispersion (Amphocil) vs fluconazole for the prevention of fungal infections in neutropenic patients: data of a prematurely stopped clinical trialBone Marrow Transplant200025887988410808210
- VogelsingerHWeilerSDjananiAAmphotericin B tissue distribution in autopsy material after treatment with liposomal amphotericin B and amphotericin B colloidal dispersionJ Antimicrob Chemother20065761153116016627591
- RuijgrokEJFensMHABakker-WoudenbergIAJMvan EttenEWMVultoAGNebulization of four commercially available amphotericin B formulations in persistently granulocytopenic rats with invasive pulmonary aspergillosis: evidence for long-term biological activityJ Pharm Pharmacol200557101289129516259757
- HusainSCapitanoBCorcoranTIntrapulmonary disposition of amphotericin B after aerosolized delivery of amphotericin B lipid complex (Abelcet; ABLC) in lung transplant recipientsTransplantation201090111215121920881664
- YangWTamJMillerDAHigh bioavailability from nebulized itraconazole nanoparticle dispersions with biocompatible stabilizersInt J Pharm20083611–217718818556158
- HostetlerJSClemonsKVHansonLHStevensDAEfficacy and safety of amphotericin B colloidal dispersion compared with those of amphotericin B deoxycholate suspension for treatment of disseminated murine cryptococcosisAntimicrob Agents Chemother19923612265626601482133
- TkatchLSKusneSEiblingDSuccessful treatment of zygomycosis of the paranasal sinuses with surgical debridement and amphotericin B colloidal dispersionAm J Otolaryngol19931442492538214317
- VukmirRBKusneSLindenPSuccessful therapy for cerebral phaeohyphomycosis due to Dactylaria gallopava in a liver transplant recipientClin Infect Dis19941947147197803637
- HerbrechtRLetscher-BruVBowdenRATreatment of 21 cases of invasive mucormycosis with amphotericin B colloidal dispersionEur J Clin Microbiol Infect Dis200120746046611561801
- NoskinGAPietrelliLCoffeyGGurwithMLiangLJAmphotericin B colloidal dispersion for treatment of candidemia in immunocompromised patientsClin Infect Dis19982624614679502471
- MosesAERahavGBarenholzYRhinocerebral mucormycosis treated with amphotericin B colloidal dispersion in three patientsClin Infect Dis1998266143014339636875
- HunstadDACohenAHSt GemeJWSuccessful eradication of mucormycosis occurring in a pulmonary allograftJ Heart Lung Transplant199918880180410512529
- BowdenRChandrasekarPWhiteMHA double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patientsClin Infect Dis200235435936612145716
- CapillaJClemonsKVSobelRAStevensDAEfficacy of amphotericin B lipid complex in a rabbit model of coccidioidal meningitisJ Antimicrob Chemother200760367367617646202
- WeilerSUberlacherESchofmannJPharmacokinetics of amphotericin B colloidal dispersion in critically ill patients with cholestatic liver diseaseAntimicrob Agents Chemother201256105414541822850517
- SunHYCacciarelliTVSinghNMicafungin versus amphotericin B lipid complex for the prevention of invasive fungal infections in high-risk liver transplant recipientsTransplantation201396657357823842191
- MadniASarfrazMRehmanMAhmadMAkhtarNAhmadSLiposomal drug delivery: a versatile platform for challenging clinical applicationsJ Pharm Pharm Sci201417340142625224351
- DaiYZhouRLiuLLuYQiJWuWLiposomes containing bile salts as novel ocular delivery systems for tacrolimus (FK506): in vitro characterization and improved corneal permeationInt J Nanomedicine201381921193323690687
- CorcoranTEVenkataramananRMihelcKMAerosol deposition of lipid complex amphotericin-B (Abelcet) in lung transplant recipientsAm J Transplant20066112765277317049064
- HatakeyamaHAkitaHHarashimaHThe polyethyleneglycol dilemma: advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumorsBiol Pharm Bull201336689289923727912
- AhmadIAllenTMAntibody-mediated specific binding and cytotoxicity of liposome-entrapped doxorubicin to lung cancer cells in vitroCancer Res19925217481748201511445
- JooKIXiaoLLiuSCrosslinked multilamellar liposomes for controlled delivery of anticancer drugsBiomaterials201334123098310923375392
- IbrahimASAvanessianVSpellbergBEdwardsJELiposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzaeAntimicrob Agents Chemother200347103343334414506054
- OrtonedaMCapillaJPastorFJPujolIGuarroJEfficacy of liposomal amphotericin B in treatment of systemic murine fusariosisAntimicrob Agents Chemother20024672273227512069988
- OlsonJAAdler-MooreJPJensenGMSchwartzJDignaniMCProffittRTComparison of the physicochemical, antifungal, and toxic properties of two liposomal amphotericin B productsAntimicrob Agents Chemother200852125926817967910
- SloanDJParrisVCryptococcal meningitis: epidemiology and therapeutic optionsClin Epidemiol2014616918224872723
- ClemonsKVSobelRAWilliamsPLPappagianisDStevensDAEfficacy of intravenous liposomal amphotericin B (AmBisome) against coccidioidal meningitis in rabbitsAntimicrob Agents Chemother20024682420242612121913
- ClemonsKVCapillaJSobelRAMartinezMTongAJStevensDAComparative efficacies of lipid-complexed amphotericin B and liposomal amphotericin B against coccidioidal meningitis in rabbitsAntimicrob Agents Chemother20095351858186219273680
- ClemonsKVStevensDATherapeutic efficacy of a liposomal formulation of amphotericin B (AmBisome) against murine blastomycosisJ Antimicrob Chemother19933234654728262869
- OlsonJAAdler-MooreJPSchwartzJJensenGMProffittRTComparative efficacies, toxicities, and tissue concentrations of amphotericin B lipid formulations in a murine pulmonary aspergillosis modelAntimicrob Agents Chemother20065062122213116723574
- Al NakeebZPetraitisVGoodwinJPetraitieneRWalshTJHopeWWPharmacodynamics of amphotericin B deoxycholate, amphotericin B lipid complex and liposomal amphotericin B against Aspergillus fumigatusAntimicrob Agents Chemother20155952735274525712363
- ArianoREMitchelmoreBRLagacé-WiensPRZelenitskySASuccessful treatment of pulmonary blastomycosis with continuously infused amphotericin B deoxycholate after failure with liposomal amphotericin BAnn Pharmacother2013476e2623673538
- Akyol ErikciAOzyurtMTerekeciHOzturkAKarabudakOOncuKOesophageal aspergillosis in a case of acute lymphoblastic leukaemia successfully treated with caspofungin alone due to liposomal amphotericin B induced severe hepatotoxicityMycoses2009521848618498301
- SatoMRda SilvaPBde SouzaRAdos SantosKCChorilliMRecent advances in nanoparticle carriers for coordination complexesCurr Top Med Chem201515428729725579344
- LiuJSChangYYChenWHChenSSAmphotericin B-induced leukoencephalopathy in a patient with cryptococcal meningitisJ Formos Med Assoc19959474324347549570
- MichotJMGubavuCFournEVery prolonged liposomal amphotericin B use leading to a lysosomal storage diseaseInt J Antimicrob Agents201443656656924787480
- HospenthalDRRogersALMillsGLDevelopment of amphotericin B liposomes bearing antibody specific to Candida albicansMycopathologia1988101137453281020
- GilbertBEWydePRWilsonSZAerosolized liposomal amphotericin B for treatment of pulmonary and systemic Cryptococcus neoformans infections in miceAntimicrob Agents Chemother1992367146614711510442
- MiharaTKakeyaHIzumikawaKEfficacy of aerosolized liposomal amphotericin B against murine invasive pulmonary mucormycosisJ Infect Chemother201420210410824462443
- Alonso-VargasRGonzález-AlvarezLRuesgaMTIn vitro activity of a liposomal nystatin formulation (Nyotran) against Cryptococcus neoformansRev Iberoam Micol2000173909215762799
- OakleyKLMooreCBDenningDWComparison of in vitro activity of liposomal nystatin against Aspergillus species with those of nystatin, amphotericin B (AB) deoxycholate, AB colloidal dispersion, liposomal AB, AB lipid complex, and itraconazoleAntimicrob Agents Chemother19994351264126610223948
- Lopez-BeresteinGBodeyGPFrankelLSMehtaKTreatment of hepatosplenic candidiasis with liposomal-amphotericin BJ Clin Oncol1987523103173806172
- SchinabeckMKLongLAHossainMARabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapyAntimicrob Agents Chemother20044851727173215105127
- SeidlerMSalvenmoserSMüllerFMLiposomal amphotericin B eradicates Candida albicans biofilm in a continuous catheter flow modelFEMS Yeast Res201010449249520345899
- RamageGJoseASherryLLappinDFJonesBWilliamsCLiposomal amphotericin B displays rapid dose-dependent activity against Candida albicans biofilmsAntimicrob Agents Chemother20135752369237123422915
- DrewRPotential role of aerosolized amphotericin B formulations in the prevention and adjunctive treatment of invasive fungal infectionsInt J Antimicrob Agents200627suppl 1364416713192
- RothCGebhartJJust-NublingGvon Eisenhart-RotheBBeinhauer-ReebICharacterization of amphotericin B aerosols for inhalation treatment of pulmonary aspergillosisInfection19962453543608923045
- GilbertBEWydePRLopez-BeresteinGWilsonSZAerosolized amphotericin B-liposomes for treatment of systemic Candida infections in miceAntimicrob Agents Chemother19943823563598192464
- Lopez-BeresteinGMehtaRHopferRMehtaKHershEMJulianoREffects of sterols on the therapeutic efficacy of liposomal amphotericin B in murine candidiasisCancer Drug Deliv19831137426544116
- Lopez-BeresteinGFainsteinVHopferRLiposomal amphotericin B for the treatment of systemic fungal infections in patients with cancer: a preliminary studyJ Infect Dis198515147047103973417
- WeberRSLopez-BeresteinGTreatment of invasive Aspergillus sinusitis with liposomal-amphotericin BLaryngoscope1987978 pt 19379413613794
- PontaniDRSunDBrownJWInhibition of HIV replication by liposomal encapsulated amphotericin BAntiviral Res19891131191252472114
- KatzNMPiercePFAnzeckRALiposomal amphotericin B for treatment of pulmonary aspergillosis in a heart transplant patientJ Heart Transplant19909114172313415
- TollemarJDurajFEriczonBGLiposomal amphotericin B treatment in a 9-month-old liver recipientMycoses19903352512522267002
- AntunesAMTeixeiraCCorvoMLPerdigotoRBarrosoEMarcelinoPProphylactic use of liposomal amphotericin B in preventing fungal infections early after liver transplantation: a retrospective, single-center studyTransplant Proc201446103554355925498088
- SelleslagDA case of fusariosis in an immunocompromised patient successfully treated with liposomal amphotericin BActa Biomed200677suppl 2323516918066
- FisherEWTomaAFisherPHCheesmanADRhinocerebral mucormycosis: use of liposomal amphotericin BJ Laryngol Otol199110575755771875144
- LimKKPottsMJWarnockDWIbrahimNBBrownEMBurns-CoxCJAnother case report of rhinocerebral mucormycosis treated with liposomal amphotericin B and surgeryClin Infect Dis19941846536548038329
- OgawaTTakezawaKTojimaISuccessful treatment of rhino-orbital mucormycosis by a new combination therapy with liposomal amphotericin B and micafunginAuris Nasus Larynx201239222422821592699
- MunckhofWJonesRTosoliniFAMarzecAAngusPGraysonMLCure of Rhizopus sinusitis in a liver transplant recipient with liposomal amphotericin BClin Infect Dis19931611838448307
- CokerRJVivianiMGazzardBGTreatment of cryptococcosis with liposomal amphotericin B (AmBisome) in 23 patients with AIDSAIDS1993768298358363759
- KaratzaAADimitriouGMarangosMSuccessful resolution of cardiac mycetomas by combined liposomal Amphotericin B with Fluconazole treatment in premature neonatesEur J Pediatr200816791021102318205013
- TungerOBayramHDegerliKDincGCetinBCComparison of the efficacy of combination and monotherapy with caspofungin and liposomal amphotericin B against invasive candidiasisSaudi Med J200829572873318454223
- BucklerBSSamsRNGoeiVLTreatment of central venous catheter fungal infection using liposomal amphotericin-B lock therapyPediatr Infect Dis J200827876276418664989
- KashimotoSTakemotoKYamamotoYKanazawaKIn vivo activity of liposomal amphotericin B against Exophiala dermatitidis in a murine lethal infection modelJpn J Antibiot201063326527220976882
- Ruíz-CendoyaMPastorFJCapillaJGuarroJTreatment of murine Fusarium verticillioides infection with liposomal amphotericin B plus terbinafineInt J Antimicrob Agents2011371586120947310
- InoueAHaradaHIwataSIntraventricular cryptococcoma successfully treated with liposomal amphotericin B and voriconazole: a case reportNo Shinkei Geka201240977778422915699
- SharmaLCFalodiaJKallaKEsophageal histoplasmosis in a renal allograft recipientSaudi J Kidney Dis Transpl201324476476723816727
- BrazzolaPRossiMRHigh weekly doses of liposomal amphotericin B as secondary prophylaxis after cerebral aspergillosis in a paediatric patientMed Mycol Case Rep2013311324567890
- VilleSTalarminJPGaultier-LintiaADisseminated mucormycosis with cerebral involvement owing to rhizopus microsporus in a kidney recipient treated with combined liposomal amphotericin b and posaconazole therapyExp Clin Transpl20161419699
- KleinotieneGPosiunasGRaistenskisJLiposomal amphotericin B and surgery as successful therapy for pulmonary Lichtheimia corymbifera zygomycosis in a pediatric patient with acute promyelocytic leukemia on antifungal prophylaxis with posaconazoleMed Oncol201330143323307250
- HandEORamanathanMRSafety and tolerability of high-dose weekly liposomal amphotericin B antifungal prophylaxisPediatr Infect Dis J201433883583625222303
- StormLLauschKRArendrupMCMortensenKLPetersenEVertebral infection with Candida albicans failing caspofungin and fluconazole combination therapy but successfully treated with high dose liposomal amphotericin B and flucytosineMed Mycol Case Rep201466925379389
- RousseauNPicotSBBienvenuALErythropoietin combined with liposomal amphotericin B improves outcome during disseminated aspergillosis in miceFront Immunol2014550225352847
- FujitaMYanagisawaJHiratsukaMA case report of pulmonary aspergillosis in lung transplant recipient successfully treated with inhalation administration of liposomal amphotericin BJpn J Antibiot2013661374323777015
- GodetCGoudetVLaurentFNebulised liposomal amphotericin B for Aspergillus lung diseases: case series and literature reviewMycoses201558317318025690951
- HanadaSUrugaHTakayaHNebulized liposomal amphotericin B for treating Aspergillus empyema with bronchopleural fistulaAm J Respir Crit Care Med2014189560760824579838
- SouzaACNascimentoALde VasconcelosNMActivity and in vivo tracking of amphotericin B loaded PLGA nanoparticlesEur J Med Chem20159526727625827397
- MukherjeeSRaySThakurRSDesign and evaluation of Itraconazole loaded solid lipid nanoparticulate system for improving the antifungal therapyPak J Pharm Sci200922213113819339221
- PardeshiCRajputPBelgamwarVSolid lipid based nanocarriers: an overviewActa Pharm201262443347223333884
- NayakAPTiyaboonchaiWPatankarSMadhusudhanBSoutoEBCurcuminoids-loaded lipid nanoparticles: novel approach towards malaria treatmentColloids Surf B Biointerfaces201081126327320688493
- MuchowMMaincentPMullerRHLipid nanoparticles with a solid matrix (SLN, NLC, LDC) for oral drug deliveryDrug Dev Ind Pharm200834121394140518665980
- Abdel-MottalebMMANeumannDLamprechtALipid nanocapsules for dermal application: a comparative study of lipid-based versus polymer-based nanocarriersEur J Pharm Biopharm2011791364221558002
- AttamaAASchickeBCPaepenmüllerTMüller-GoymannCCSolid lipid nanodispersions containing mixed lipid core and a polar heterolipid: characterizationEur J Pharm Biopharm2007671485717276663
- LiuJGongTFuHSolid lipid nanoparticles for pulmonary delivery of insulinInt J Pharm20083561–233334418281169
- SznitowskaMGajewskaMJanickiSRadwanskaALukowskiGBioavailability of diazepam from aqueous-organic solution, submicron emulsion and solid lipid nanoparticles after rectal administration in rabbitsEur J Pharm Biopharm200152215916311522481
- MüllerRHRadtkeMWissingSASolid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparationsAdv Drug Deliv Rev200254suppl 1S131S15512460720
- ZhangLChanJMGuFXSelf-assembled lipid-polymer hybrid nanoparticles: a robust drug delivery platformACS Nano2008281696170219206374
- Salvador-MoralesCZhangLLangerRFarokhzadOCImmunocompatibility properties of lipid-polymer hybrid nanoparticles with heterogeneous surface functional groupsBiomaterials200930122231224019167749
- MüllerRHRungeSARavelliVThünemannAFMehnertWSoutoEBCyclosporine-loaded solid lipid nanoparticles (SLN®): drug-lipid physicochemical interactions and characterization of drug incorporationEur J Pharm Biopharm200868353554417804210
- TanSWBillaNRobertsCRBurleyJCSurfactant effects on the physical characteristics of Amphotericin B-containing nanostructured lipid carriersColloids Surf A Physicochem Eng Asp20103721–37379
- KovacevicASavicSVuletaGMüllerRHKeckCMPolyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): effects on size, physical stability and particle matrix structureInt J Pharm20114061–216317221219990
- MathpalDGargTRathGGoyalAKDevelopment and characterization of spray dried microparticles for pulmonary delivery of antifungal drugCurr Drug Deliv201512446447125808185
- MohantyBMajumdarDKMishraSKPandaAKPatnaikSDevelopment and characterization of itraconazole-loaded solid lipid nanoparticles for ocular deliveryPharm Dev Technol201520445846424490828
- PardeikeJWeberSHaberTDevelopment of an Itraconazole-loaded nanostructured lipid carrier (NLC) formulation for pulmonary applicationInt J Pharm20114191–232933821839157
- LimWMRajinikanthPSMallikarjunCKangYBFormulation and delivery of itraconazole to the brain using a nanolipid carrier systemInt J Nanomedicine201492117212624833900
- SanapGSMohantaGPDesign and evaluation of miconazole nitrate loaded nanostructured lipid carriers (NLC) for improving the antifungal therapyJ Appl Pharm Sci2013314654
- KeshriLPathakKDevelopment of thermodynamically stable nanostructured lipid carrier system using central composite design for zero order permeation of Econazole nitrate through epidermisPharm Dev Technol2012183111
- SongSHLeeKMKangJBLeeSGKangMJChoiYWImproved skin delivery of voriconazole with a nanostructured lipid carrier-based hydrogel formulationChem Pharm Bull (Tokyo)201462879379825087631
- LemkeAKiderlenAFKayserOAmphotericin BAppl Microbiol Biotechnol200568215116215821914
- JainSJainSKharePGulbakeABansalDJainSKDesign and development of solid lipid nanoparticles for topical delivery of an anti-fungal agentDrug Deliv201017644345120486871
- GuptaMVyasSPDevelopment, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasisChem Phys Lipids2012165445446122309657
- RavaniLEspositoEBoriesCClotrimazole-loaded nanostructured lipid carrier hydrogels: thermal analysis and in vitro studiesInt J Pharm2013454269570223792467
- MendesAISilvaACCatitaJAMCerqueiraFGabrielCLopesCMMiconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: improving antifungal activityColloids Surf B Biointerfaces201311175576323954816
- GuptaMTiwariSVyasSPInfluence of various lipid core on characteristics of SLNs designed for topical delivery of fluconazole against cutaneous candidiasisPharm Dev Technol201318355055921810069
- VaghasiyaHKumarASawantKDevelopment of solid lipid nanoparticles based controlled release system for topical delivery of terbinafine hydrochlorideEur J Pharm Sci201349231132223557842
- CassanoRFerrarelliTMauroMVCavalcantiPPicciNTrombinoSPreparation, characterization and in vitro activities evaluation of solid lipid nanoparticles based on PEG-40 stearate for antifungal drugs vaginal deliveryDrug Deliv20162331047105625005582
- MeloSCunhaSFialhoSLFormas farmacêuticas poliméricas para a administração de peptídeos e proteínas terapêuticos [Polymeric delivery systems for the administration of therapeutic peptides and proteins]Rev Ciênc Farm Básica Apl2012334469477 Portuguese
- PridgenEMAlexisFFarokhzadOCPolymeric nanoparticle technologies for oral drug deliveryClin Gastroenterol Hepatol201412101605161024981782
- ValenciaPMPridgenEMRheeMLangerRFarokhzadOCKarnikRMicrofluidic platform for combinatorial synthesis and optimization of targeted nanoparticles for cancer therapyACS Nano2013712106711068024215426
- YuMKParkJJonSTargeting strategies for multifunctional nanoparticles in cancer imaging and therapyTheranostics20122134422272217
- des RieuxAFievezVGarinotMSchneiderYJPréatVNanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approachJ Controll Release20061161127
- BilensoyESarisozenCEsendaǧliGIntravesical cationic nanoparticles of chitosan and polycaprolactone for the delivery of Mitomycin C to bladder tumorsInt J Pharm20093711–217017619135514
- BaiJLiYDuJOne-pot synthesis of polyacrylamide-gold nanocompositeMater Chem Phys20071062–3412415
- TurosEShimJYWangYAntibiotic-conjugated polyacrylate nanoparticles: new opportunities for development of anti-MRSA agentsBioorg Med Chem Lett2007171535617049850
- MaoHQRoyKTroung-LeVLChitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiencyJ Control Release200170339942111182210
- RejinoldNSMuthunarayananMMuthuchelianKChennazhiKPNairSVJayakumarRSaponin-loaded chitosan nanoparticles and their cytotoxicity to cancer cell lines in vitroCarbohydr Polym2011841407416
- GonçalvesJODuarteDADPlaaGLUse of chitosan with different deacetylation degrees for the adsorption of food dyes in a binary systemClean Soil Air Water2014426767774
- SaraogiGKGuptaPGuptaUDJainNKAgrawalGPGelatin nanocarriers as potential vectors for effective management of tuberculosisInt J Pharm20103851–214314919819315
- LuoGYuXJinCLyP-1-conjugated nanoparticles for targeting drug delivery to lymphatic metastatic tumorsInt J Pharm20103851–215015619825404
- Mora-HuertasCEFessiHElaissariAPolymer-based nanocapsules for drug deliveryInt J Pharm20103851–211314219825408
- SchaffazickSRGuterresSSDe Lucca FreitasLPohlmannARCaracterização e estabilidade físico-química de sistemas poliméricos nanoparticulados para administração de fármacos. [Physicochemical characterization and stability of the polymeric nanoparticle systems for drug administration]Quim Nova2003265726737 Portuguese
- AnselmoACMitragotriSA review of clinical translation of inorganic nanoparticlesAAPS J20151751041105425956384
- PintoRJBAlmeidaAFernandesSCMAntifungal activity of transparent nanocomposite thin films of pullulan and silver against Aspergillus nigerColloids Surf B Biointerfaces201310314314823201731
- SilvaSPiresPMonteiroDRThe effect of silver nanoparticles and nystatin on mixed biofilms of Candida glabrata and Candida albicans on acrylicMed Mycol201351217818422803822
- MonteiroDRNegriMSilvaSAdhesion of Candida biofilm cells to human epithelial cells and polystyrene after treatment with silver nanoparticlesColloids Surf B Biointerfaces201411441041224257686
- MehnertWMaderKSolid lipid nanoparticles – production, characterization and applicationsAdv Drug Deliv Rev2001472–316519611311991
- VakilRKnilansKAndesDKwonGSCombination antifungal therapy involving amphotericin B, rapamycin and 5-fluorocytosine using PEG-phospholipid micellesPharm Res20082592056206418415047
- WangCHWangWTHsiueGHDevelopment of polyion complex micelles for encapsulating and delivering amphotericin BBiomaterials200930193352335819299011
- AmaralACMarquesAFMuñozJEPoly(lactic acid-glycolic acid) nanoparticles markedly improve immunological protection provided by peptide P10 against murine paracoccidioidomycosisBr J Pharmacol201015951126113220136827
- XuNGuJZhuYWenHRenQChenJEfficacy of intravenous amphotericin B-polybutylcyanoacrylate nanoparticles against cryptococcal meningitis in miceInt J Nanomedicine2011690591321720503
- PatelNRDamannKLeonardiCSabliovCSize dependency of PLGA-nanoparticle uptake and antifungal activity against Aspergillus flavusNanomedicine (Lond)2011681381139521651442
- Cunha-AzevedoEPSilvaJRMartinsOPIn vitro antifungal activity and toxicity of itraconazole in DMSA-PLGA nanoparticlesJ Nanosci Nanotechnol20111132308231421449386
- Roy ChoudhurySGhoshMMandalASurface-modified sulfur nanoparticles: an effective antifungal agent against Aspergillus niger and Fusarium oxysporumAppl Microbiol Biotechnol201190273374321350853
- ShimYHKimYCLeeHJAmphotericin b aggregation inhibition with novel nanoparticles prepared with poly(ε-caprolactone)/poly(N,N-dimethylamino-2-ethyl methacrylate) diblock copolymerJ Microbiol Biotechnol2011211283621301189
- HaghighiFMohammadiShRMohammadiPEskandariMHosseinkhaniSThe evaluation of Candida albicans biofilms formation on silicone catheter, PVC and glass coated with titanium dioxide nanoparticles by XTT method and ATPase assayBratisl Lek List201211312707711
- ShaoKWuJChenZA brain-vectored angiopep-2 based polymeric micelles for the treatment of intracranial fungal infectionBiomaterials201233286898690722789719
- Van De VenHPaulussenCFeijensPBPLGA nanoparticles and nanosuspensions with amphotericin B: potent in vitro and in vivo alternatives to Fungizone and AmBisomeJ Control Release2012161379580322641062
- MohammedNSanoj RejinoldNMangalathillamSBiswasRNairSVJayakumarRFluconazole loaded chitin nanogels as a topical ocular drug delivery agent for corneal fungal infectionsJ Biomed Nanotechnol2013991521153123980500
- MathewTVKuriakoseSPhotochemical and antimicrobial properties of silver nanoparticle-encapsulated chitosan functionalized with photoactive groupsMater Sci Eng C201333744094415
- SantosSSLorenzoniAFerreiraLMClotrimazole-loaded Eudragit® RS100 nanocapsules: preparation, characterization and in vitro evaluation of antifungal activity against Candida speciesMater Sci Eng C Mater Biol Appl20133331389139423827586
- BashaMAbd El-AlimSHShammaRNAwadGEDesign and optimization of surfactant-based nanovesicles for ocular delivery of ClotrimazoleJ Liposome Res201323320321023607316
- SantosSSLorenzoniAPegoraroNSFormulation and in vitro evaluation of coconut oil-core cationic nanocapsules intended for vaginal delivery of clotrimazoleColloids Surf B Biointerfaces201411627027624503350
- AzevedoMMRamalhoPSilvaAPTeixeira-SantosRPina-VazCRodriguesAGPolyethyleneimine and polyethyleneimine-based nanoparticles: novel bacterial and yeast biofilm inhibitorsJ Med Microbiol201463pt 91167117324913563
- LazniewskaJMilowskaKGabryelakTDendrimers-revolutionary drugs for infectious diseasesWiley Interdiscip Rev Nanomed Nanobiotechnol20124546949122761054
- WuLFickerMChristensenJBTrohopoulosPNMoghimiSMDendrimers in medicine: therapeutic concepts and pharmaceutical challengesBioconjug Chem20152671198121125654320
- D’EmanueleAAttwoodDDendrimer-drug interactionsAdv Drug Deliv Rev200557152147216216310283
- MenjogeARKannanRMTomaliaDADendrimer-based drug and imaging conjugates: design considerations for nanomedical applicationsDrug Discov Today2010155–617118520116448
- GajbhiyeVVijayaraj KumarPKumar TekadeRJainNKPharmaceutical and biomedical potential of pegylated dendrimersCurr Pharm Design200713415429
- ZiembaBJanaszewskaACiepluchKIn vivo toxicity of poly(propyleneimine) dendrimersJ Biomed Mater Res A201199226126821976451
- KolhatkarRBKitchensKMSwaanPWGhandehariHSurface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeabilityBioconjug Chem20071862054206017960872
- SinghPGuptaUAsthanaAJainNKFolate and Folate-PEG-PAMAM dendrimers: synthesis, characterization, and targeted anticancer drug delivery potential in tumor bearing miceBioconjug Chem200819112239225218950215
- WänglerCMoldenhauerGEisenhutMHaberkornUMierWAntibody-dendrimer conjugates: the number, not the size of the dendrimers, determines the immunoreactivityBioconjug Chem200819481382018361514
- WaiteCLRothCMPAMAM-RGD conjugates enhance siRNA delivery through a multicellular spheroid model of malignant gliomaBioconjug Chem200920101908191619775120
- LopezAIReinsRYMcDermottAMTrautnerBWCaiCAntibacterial activity and cytotoxicity of PEGylated poly(amidoamine) dendrimersMol Biosyst20095101148115619756304
- WinnickaKSosnowskaKWieczorekPSachaPTTryniszewskaEPoly(amidoamine) dendrimers increase antifungal activity of ClotrimazoleBiol Pharm Bull20113471129113321720026
- JaniszewskaJSowińskaMRajniszANovel dendrimeric lipopeptides with antifungal activityBioorg Med Chem Lett20122231388139322230049
- StaniszewskaMBondarykMZielinskaPUrbańczyk-LipkowskaZThe in vitro effects of new D186 dendrimer on virulence factors of Candida albicansJ Antibiot (Tokyo)201467642543224690909
- BenincasaMPacorSWuWPratoMBiancoAGennaroRAnti-fungal activity of amphotericin B conjugated to carbon nanotubesACS Nano20115119920821141979
- OliviMZanniEDe BellisGInhibition of microbial growth by carbon nanotube networksNanoscale20135199023902923934344
- SawangphrukMSrimukPChiochanPSangsriTSiwayaprahmPSynthesis and antifungal activity of reduced graphene oxide nanosheetsCarbon N Y2012501451565161
- CuiJYangYZhengMFacile fabrication of graphene oxide loaded with silver nanoparticles as antifungal materialsMater Res Express20141445007
- Hussein-Al-AliSHEl ZowalatyMEHusseinMZGeilichBMWebsterTJSynthesis, characterization, and antimicrobial activity of an ampicillin-conjugated magnetic nanoantibiotic for medical applicationsInt J Nanomedicine2014913801381425143729
- NiemirowiczKSwiecickaIWilczewskaAZGrowth arrest and rapid capture of select pathogens following magnetic nanoparticle treatmentColloids Surf B Biointerfaces2015131293825942700
- RoemerTKrysanDJAntifungal drug development: challenges, unmet clinical needs, and new approachesCold Spring Harb Perspect Med201445 pii a019703
- OyafusoMHCarvalhoFCChiavacciLAGremiãoMPDChorilliMDesign and characterization of silicone and surfactant based systems for topical drug deliveryJ Nanosci Nanotechnol201515181782626328446
- LiuXChenXLiYWangXPengXZhuWPreparation of superparamagnetic Fe 3O 4@alginate/chitosan nanospheres for candida rugosa lipase immobilization and utilization of layer-by-layer assembly to enhance the stability of immobilized lipaseACS Appl Mater Interfaces20124105169517822985256