ABSTRACT
Older patients, suffering from numerous diseases and taking multiple medications are the rule rather than the exception in primary care. A manifold of medical conditions are often associated with poor outcomes, and their multiple medications raise additional risks of polypharmacy. Such patients account for most healthcare expenditures. Effective approaches are needed to manage such complex patients in primary care. This paper describes the results of a scoping exercise, including a two-day workshop with 17 professionals from six countries, experienced in general practice and primary care research as well as epidemiology, clinical pharmacology, gerontology and methodology. This was followed by a consensus process investigating the challenges and core questions for multimorbidity research in primary care from a clinical perspective and presents examples of the best research practice. Current approaches in measuring and clustering multimorbidity inform policy-makers and researchers, but research is needed to provide support in clinical decision making. Multimorbidity presents a complexity of conditions leading to individual patient's needs and demanding complex processes in clinical decision making. The identification of patterns presupposes the development of strategies on how to manage multimorbidity and polypharmacy. Interventions have to be complex and multifaceted, and their evaluation poses numerous methodological challenges in study design, outcome measurement and analysis. Overall, it can be seen that complexity is a main underlying theme. Moreover, flexible study designs, outcome parameters and evaluation strategies are needed to account for this complexity.
KEY MESSAGE
Multimorbidity is resulting in complex (health) care situations, while evidence-based clinical decision support for care for patients with multimorbidity is lacking.
Both interventions to handle patients with multimorbidity and study designs evaluating these interventions are bound to be complex, and hence need to be flexible.
Hard end points such as hospitalization and mortality are common; patient-centred and holistic measures—such as the assessment of health related quality of life—are essential, but users should be aware of the underlying multidimensional constructs.
INTRODUCTION
Mr Curran is a single farmer aged 62 years, who has been living by himself, in a rural community in western Ireland. After a lifetime of herding sheep and cattle in rough terrain, he developed osteoarthritis of the knees. This condition limits his mobility. The resulting lack of activity led to significant weight gain. The increase in weight together with a 40-year history of smoking contributed to the onset of chronic obstructive pulmonary disease (COPD), type 2 diabetes, atrial fibrillation and hypertension. These diseases were only discovered when Mr Curran suffered an acute cardio-embolic stroke (residual dysarthria and hemiparesis) and associated chronic kidney disease. Other diagnoses detected during his hospitalization include hyperlipidaemia, hyperuricaemia and finally benign prostatic hyperplasia (BPH).
In a relatively short time, Mr Curran has evolved from a fiercely independent man to a person struggling to navigate his way through a healthcare system that appears to him to be increasingly complex and fragmented. His numerous prescriptions now include 13 medications (see ); he has had 42 primary care consultations over the past year with nurses, his family doctor, speech therapist, occupational therapist, and physiotherapist. In addition, Mr Curran has to be present regularly at three different outpatient clinics in a hospital centre that is a 1.5-h drive away by car but he is unable to drive anymore. Furthermore, he has been admitted twice to hospital in the last year. ‘Frustrated,’ ‘worried,’ ‘confused’ are the words Mr Curran uses to describe himself in his journey through healthcare.
Table 1. Medication regimen of Mr Curran.
Mr Curran’ details have been altered to protect his anonymity, but he constitutes a typical case of multimorbidity based on a real patient. Patients like him are the rule rather than the exception in primary care (Citation1–3). Multiple health conditions often result in the prescribing of complex regimes including multiple drugs with the potential for drug-drug and drug-disease interactions. Further risks associated with multimorbidity and polypharmacy are over or under treatment, as well as decreased (medication) adherence (Citation4–7). Evidence-based clinical decision support for such patients is sparse (Citation8), and the mere application of current disease-oriented clinical practice guidelines may actually have harmful consequences (Citation9,Citation10). In addition, multiple chronic medical conditions are associated with poor outcomes: decreased quality of life, psychological distress, longer hospital stays, more postoperative complications and higher mortality (Citation11–14). Healthcare costs increase exponentially with the number of chronic diseases (Citation15,Citation16), which altogether constitutes complex problems that clinicians and researchers are attempting to solve.
These complex problems pose several challenges (Citation17,Citation18). Effective approaches to managing these patients in primary health care are needed and have to be tailored to different health care systems (Citation19). Furthermore, selecting adequate research methodologies for studying diagnosis and treatment of such complex patients is not easy (Citation20).
THE SCOPING APPROACH
An International Workshop on Methodological Research Strategies in Multimorbidity was held in Frankfurt/Main (Germany) on 4–5 February 2011. Seventeen participants from six countries (Canada, Germany, Ireland, the Netherlands, Spain and the UK) represented general practice and primary care research including epidemiology (n = 12), clinical pharmacology (n = 1), gerontology (n = 2) and methodology (n = 2). A scoping exercise was used to examine the extent, range and nature of relevant research activities regarding multimorbidity research in primary care. Different definitions for the term ‘scoping’ exist, but it is an accepted method to ‘map’ relevant information in interest. Advantages of scoping are that topics can be dealt with within a relatively broad range, and concepts underpinning a research area can be mapped quickly (Citation21). Scoping is especially useful when it is difficult to visualize the range of available material.
Fifteen experts presented experiences from different field studies and their theoretical and epidemiological backgrounds (for full programme of the expert meeting see: http://www.allgemeinmedizin.uni-frankfurt.de/forschung2/int_workshop.html). Group discussions were audio-taped and later summarized and reframed by three of the speakers (CM, MvdA, MB). Thirteen of the participants (all authors) re-evaluated the results and conducted an iterative process of exploration including a narrative literature review to consider the current state of understanding in multimorbidity research. We used a case example to elucidate further the key issues of the workshop. This paper presents the participants’ consensus, after the expert meeting, analysis of the audio tapes, additional narrative review and written consensus rounds.
Our scoping exercise resulted in three main research areas illustrated by current research projects and elaborated on our patient, Mr Curran. The first area of focus is the various operationalizations of multimorbidity concepts and their consequences on measurement and clustering of multimorbidity. The second area involves the description of possible strategies on how to manage multimorbidity and polypharmacy in ongoing intervention studies. These provide examples to discuss methodological challenges in the third part.
Classifications and patterns
Systematic reviews showed that a wide variety of methods has been used in studies on the prevalence and patterns of multimorbidity revealing a great variation in estimates, making direct comparisons meaningless (Citation22,Citation23). As a result, significant challenges exist in the definition, classification and measurement of multimorbidity. Measures to quantify multimorbidity vary from a simple count of the number of diseases or clusters to calculating disease scores, such as the Charlson Index and the Cumulative Illness Rating Scale, which both assess the presence and weight for the severity of conditions (Citation24,Citation25). If morbidity data are not available, classes of drug prescriptions from routine dispensing data are sometimes used as a proxy for the occurrence of chronic diseases—e.g. CDS: Chronic Disease Score (Citation26). Specific populations and contexts require further revisions and adaptations such as the development of the medication based CDS (med-CDS) adapted for Germany. The med-CDS aims at assessing multimorbidity in elderly patients to predict health-related outcomes (i.e. mortality, hospitalization) and at comparing patient populations by medication data (Citation27).
The above-mentioned measures are used for research purposes or to inform policy-makers, but provide little or no support to clinical decision making in daily general practice. Clinicians caring for older patients with multimorbidity such as Mr Curran, tend to group conditions that are either causally related or intertwined complications. Concepts of ‘disease-related’ or ‘causal’ multimorbidity reflect the needs in clinical decision making (Citation28–30); a recent approach is the example of ‘cardiovascular multimorbidity.’ This is used to describe the often related morbidities of cardiovascular disease (CVD), diabetes mellitus and chronic kidney disease (CKD) (see Box 1). Apart from causally related disease clusters, patients frequently present combinations of diseases for which the GP has no reasonable explanation of a pathophysiologic relationship, also called ‘general susceptibility’ (Citation29).
The classification of ‘causal’ multimorbidity has led to the development of the term ‘cardiovascular multimorbidity’ to describe coexisting CVD, diabetes and chronic kidney disease (CKD) (Citation14,Citation31). The rationale for this classification is based on the shared pathophysiologic background, common interventions, and congruence with daily clinical experience. The risk of developing an additional disease from the spectrum of cardiovascular morbidity is often increased (e.g. the risk of a cardiovascular event is increased in patients with diabetes or CKD). In patients with established CVD, diabetes is associated with a significantly increased risk of cardiovascular mortality and morbidity as is CKD (Citation32–36). The level of cardiovascular multimorbidity has been an independent predictor of prognosis for patients with established CVD; in such patients the presence of CKD carries a mortality risk similar to that of diabetes (Citation14). Therefore, patients with cardiovascular multimorbidity do not simply have an accumulation of conditions but rather a complex interplay of risk factors that accelerate specific outcomes of cardiovascular events, or death. Mr Curran's hypertension and atrial fibrillation put him at a high risk of developing chronic heart failure (CHF), which may have a worse prognosis because of his diabetes, CKD, and other comorbidities (Citation37). Alternatively, a treatment with ACE inhibitors is effective in hypertension, to reduce the risk of developing CHF in diabetics and slow the progression of a diabetic CKD in a synergistic manner (Citation37).
Interventions
Multimorbidity. GPs need specific strategies to handle patients with multimorbidity. Some of them have been studied, to address multimorbidity in general practice (Citation38). The first type of strategy is based on the implementation of single interventions that may be beneficial for many medical conditions, such as the prescription of physical exercise (Citation39–42) or more complex psychosocial interventions (Citation43). Other strategies have focussed on specific clusters of conditions that may benefit from concordant management, such as cardiovascular risk management (Citation44,Citation45). In an attempt to maximize care for more complex patients, other approaches are based on different models of structured healthcare, for instance, the Chronic Care Model (Citation46,Citation47). All these kinds of strategies rely on the assumption that one single intervention, as complex as it may be, would operate on a common pathway. For many patients, however, this intervention may not be feasible, and a patient-centred prioritization of competing needs might actually be one of the key issues (Citation48). In Box 2, we provide examples of ongoing interventions using Mr Curran's case.
PR1MaC, a multifaceted intervention integrating chronic disease rehabilitation services (lifestyle intervention, self-management training, and motivational interviewing by multiple professionals) in primary care practices in Canada (NCT01319656).
After a consultation during which Mr Curran and his general practitioner (GP) explored the possible link between lifestyles and health status, he was referred (including the patient's medical history) to the PR1MaC team so that he could receive support in his own environment. Soon thereafter, Mr Curran meets with the PR1MaC nurse at his usual clinic, for a one-hour discussion about how he is coping daily with his medical conditions. They discuss how to integrate the required specific actions into Mr Curran's daily habits and how to overcome the barriers to self-care he is experiencing. Mr Curran is asked to record his symptoms before the next medical visit, so that he can discuss these with his GP and obtain specific advice. Before agreeing to meet again for a 30-min follow-up visit, Mr Curran accepts the nurse's offer to meet a dietician and a physical activity therapist in the upcoming weeks to discuss a suitable exercise regimen adapted to his condition.
PraCMan, ‘primary care practice-based care management for chronically ill patients’ (ISRCTN56104508) (Citation49): a complex, multifaceted intervention on multimorbidity to reduce the likelihood of (re-)hospitalization in Germany.
Mr Curran clearly has an increased risk of future hospitalizations because of the complexity and instability of his health status; he was already admitted to the hospital twice during the last year. Mr Curran is identified through the family practice based on his multimorbidity and his history of (avoidable) hospitalizations and receives a comprehensive assessment of his medical and non-medical care needs and resources. He is subsequently monitored by a trained healthcare assistant (e.g. by telephone calls based on a structured checklist). The frequency of these calls depends on his needs, and will be increased, for instance if his health status becomes unstable, so that any symptom deteriorations can be detected early allowing timely intervention and prevention of hospitalization.
SMOOTH-Turn sepsis to life! ‘Sepsis survivors monitoring and coordination in outpatient health care,’ (ISRCTN61744782): a transitional collaborative care programme to support clinical complex patients after severe sepsis in Germany.
Having survived a sepsis—due to a pneumonia exacerbating the COPD and requiring an artificial respirator—Mr Curran is followed actively and in a structured way for a year. His GP has obtained structured information from the intensive care unit in accordance with a discharge management scheme. Mr Curran and his GP have received evidence-based interactive training on sepsis sequels and self-management by a liaison physician and a nurse case manager. The case manager is monitoring Mr Curran by telephone to track his clinical and psychosocial situation. Liaison physicians provide clinical support to the GP.
Polypharmacy. Polypharmacy is one of most frequent consequences of multimorbidity, resulting in serious problems. Studies have shown that about 6.5% of all hospital admissions are caused by adverse drug reactions (Citation50–52), and between 30% and 70% of these admissions are seen as preventable (Citation53,Citation54). Rational prescribing for patients with multimorbidity relies on a patient-centred, instead of a disease-oriented approach with clear therapeutic objectives avoiding inappropriate medications and underprescribing, as well as the prioritization of available therapies (Citation55–58). However, implementation in daily routine is difficult as the translation from study conclusions to practical guidance is lacking. Another issue is the often inadequate communication with patients (Citation59,Citation60). Interventions to address these issues frequently consist of several components, such as PIL and PRIMUM (see Box 3), and provide examples for discussing methodological challenges.
PIL, ‘Polypharmacy Intervention Limburg’ (NTR 2154): a collaborative approach of the GP, the pharmacist, and specialists to improve the quality of life in elderly patients through optimized chronic drug therapy in the Netherlands.
The nurse practitioner (NP) visits Mr Curran at his home and makes an inventory of all drugs, indications according to Mr Curran's own knowledge, and his medication problems, such as side-effects and difficulties with adherence. The GP and the pharmacist review the patient's medication according to the NP's documentation and further electronic information. This review is checked with other medical specialists involved. Afterwards, the review is discussed with Mr Curran and implemented step-by-step. Mr Curran is followed-up for one year.
PRIMUM, ‘prioritizing multimedication in multimorbid patients’ (ISRCTN99526053): a general practice based complex intervention to improve the medication appropriateness in elderly patients in Germany.
A healthcare assistant of the general practice assesses the medication-related problems and therapeutic preferences of Mr Curran by a checklist, reconciles his medication and enters drug data into a web-based drug information system. The GP optimizes the medication supported by computerized alerts on drug-drug interactions, inappropriate dosages, etc. in consideration of the problems identified by the checklist. Finally, the GP discusses the adjusted medication with Mr Curran during a consultation.
Methodological challenges
As exemplified in Boxes 2 and 3, interventions on multimorbidity and polypharmacy are multifaceted, frequently with interacting components, often directed at the organization level while measuring outcomes at patient-level, i.e. interventions are complex (Citation61). Additionally, the study population of patients with multimorbidity is often complex, but at least heterogeneous. Attempting to evaluate those interventions in these patients’ points towards several methodological challenges highlighted in the following:
Study design. The best type of evidence for the effectiveness of an intervention is information obtained from a large, well-conducted randomized controlled trial (RCT). However, the evaluation of complex interventions, such as those used to address multimorbidity and polypharmacy (see Boxes 2 and 3) pose specific challenges (Citation61).
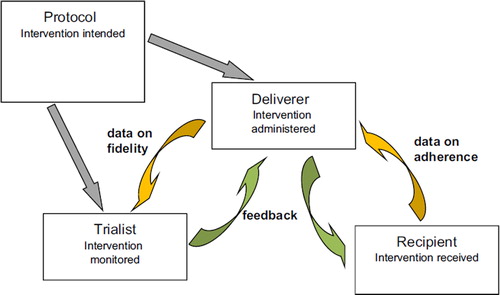
The multi-component nature of these interventions is part of the design as it aims to be: flexible but at the same time reproducible. Flexibility is needed to adapt to different requirements of the intervention and study protocol, and conditions of the deliverer (e.g. general practitioner) (see ). However, the intervention needs to be reproducible, because otherwise the evaluation or implementation of this intervention would be impossible (Citation62).
This tension means that protocols defining the required aspects (such as content and timing) of each component must be clearly specified not only for the intervention itself, but also for the control used in the evaluation (Citation62). These protocols should be the basis of the delivery of the intervention during the evaluation and the implementation phases and should determine how flexible each component can be.
Finally, measuring the fidelity in delivery of the protocol is crucial to determine what is actually being evaluated. To establish the feasibility of evaluating this intervention in a trial, evidence of fidelity should be collected during the pilot phase together with recruitment, compliance, and attrition rates (Citation63).
Outcome measurement. The choice of outcome measures depends on the relevance for patients and appropriateness to detect pre- and post-intervention differences. Furthermore, it depends on the main research question, feasibility, and methodological issues, such as study design and setting. Mortality, hospitalizations and disease-specific outcomes—typical endpoints in clinical trials to prove for instance the efficacy of drugs—are less frequently used as primary outcomes in interventions on polypharmacy: these endpoints are often not feasible because large study populations or long follow-up times are needed. Moreover, these outcomes are sometimes less appropriate: studies have shown that older people may prefer higher quality of life over prolonged survival (Citation64–66).
Some relevant and feasible outcomes for intervention studies on multimorbidity and polypharmacy in primary care are quality of life, quality of care, and medication appropriateness. Health-related quality of life measures (HR-QOL) are patient-related outcomes (PROMs), based on complex constructs that pose specific challenges to their use in older aged patients (see Box 4). Apart from quality of life measurements, a broad variety of patient-reported outcomes are available, such as patient autonomy (Citation67), and psychological well-being (Citation68). An example of quality of care measurement is the Patient Assessment of Chronic Illness Care (PACIC) (Citation69,Citation70) that is based on the Chronic Care Model (CCM) (Citation47). The development of this generic questionnaire aims at assessing whether the quality of care received is in line with the CCM and from the patient's perspective. This is particularly essential in the case of multiple chronic conditions (Citation2,Citation71).
Most measures for disease-specific health-related quality of life (HR-QOL) have been developed for younger populations. Particularly in patients with multimorbidity, quality of life may thus be systematically underestimated (Citation72). Psychometrically-sound generic quality of life measures that incorporate age-specific quality of life dimensions have only been developed recently (e.g. WHO-QOL-OLD) (Citation73). From a psychological perspective, quality of life of older people is considered a multidimensional construct that includes objective indicators and subjective evaluations related to developmental processes of growth, maintenance, and resiliency, as well as management of loss (Citation74,Citation75). For Mr Curran the crucial question is not only to learn about his medical conditions, but also deal with his everyday needs or to be able to organize his new life and his medical requirements (e.g. medication) and perceive stability or even improvement in daily life. Environmental Gerontology adds to this holistic perspective the insight that quality of life unfolds in terms of person-environment exchange processes, such as belonging and agency (Citation76,Citation77). Whereas processes of belonging (e.g. attitudes, attachment) are particularly linked to the maintenance of one's integrity and identity, processes of agency (e.g. adaptation, compensation) are linked to outcomes of independence and autonomy (Citation78). Both processes have to be considered for a better understanding of older patients’ prioritization of treatment goals in multimorbidity, adherence to complex drug regimes in polypharmacy and the maintenance of subjective well-being in the face of multiple chronic health conditions. For Mr Curran that could mean to follow up some (adapted) identity-relevant familiar patterns of behaviour or to maintain parts of his former life, e.g. being out and about in the countryside again or doing some gardening in front of his house, which might help to reduce some aspects of his perceived frustration and bring some stability back into his life.
Apart from the previously mentioned patient-reported outcomes, there are other ways to establish study effects. In the case of complex interventions regarding polypharmacy in primary care, the Medication Appropriateness Index (MAI) is a useful outcome measure (see Box 5).
The perception of inappropriateness is highly variable within clinical and theoretical disciplines and often lacks solid evidence. Polypharmacy is often well justified, and medications rated as inappropriate in older patients (e.g. amiodarone) are elsewhere part of established therapy guidelines or do not have an alternative. Of Mr Curran's 13 medications, each is approved for at least one of his conditions, and doses prescribed match their label restrictions. It seems desirable, therefore, to use an integrated approach describing the entire prescription at different levels of appropriateness. The MAI consists of 10 items on prescription quality for each prescription (e.g. dose, duration, indication, interactions, practicability, and costs) but also provides a quality indicator for the entire medication regimen (Citation79). The MAI usually uses data from chart review (Citation80,Citation81), but underprescribing and effectiveness of the medication cannot be reliably detected unless the prescription history and some clinical parameters are known. For example, only if we knew the actual lactate dehydrogenase of Mr Curran, could we decide whether the low dose of his simvastatin prescription is ineffective. Therefore, the accuracy of the MAI ratings increases with the degree of detail in the chart. Thus, the MAI is less relevant when analysing larger secondary data sources for appropriateness.
Process evaluation. The choice of (clinically) relevant outcome parameters is challenging, but so is their analysis. The evaluation of complex interventions requires not only an answer to the pragmatic question of whether an intervention works, but also an answer to the explanatory question of how it works (Citation61). The assessment of potential moderators and mediators may be helpful to explain intervention effects or to investigate the reasons for an intervention's failure to yield the expected outcome. Moderators are baseline variables that only modify the relationship between an intervention and its outcome (Citation82,Citation83). In contrast, mediators occur on the causal pathway between an intervention and its outcome (see ). Evaluation of such process variables can help improve interventions. For example, in the PRIMUM trial (see Box 3) identifying medication adherence as a strong mediator could change the complex intervention to maximise the improvement in this intermediate outcome and thus the final outcome. Future process evaluations may utilize an approach similar to Baron and Kenny’s mediation modeling, but which explicitly allows for unmeasured confounding (Citation84).
DISCUSSION
Given the high prevalence and impact of both multimorbidity and polypharmacy there is an urgent need for the development of more effective interventions based on a solid theoretical framework that take into account both the opportunities offered by conditions with shared pathophysiology and the need for making patient-centred decisions for competing demands. ‘Complexity’ seems to be the agenda of developments in epidemiology, clinical knowledge, research methodology, outcome measurement, and in studies regarding new approaches to health care. Complexity means that in multimorbidity research, environmental, psychosocial and biological factors interfere with the relationship between intervention and outcomes, which is often not easily understood (Citation85). In complex multimorbid patients like Mr Curran there is no simple cause-effect relationship. In addition to healthcare needs, they have complex individual needs regarding the maintenance of subjective well-being.
In the further development of patient-centred care for those with multimorbidity and polypharmacy in primary care, we should take into account the previous geriatric and rehabilitation experiences in this field. Despite their research-based knowledge on multimorbidity, the differences in population in primary care, as well as the context (e.g. longitudinality) have to be valued.
One limitation of this investigation was our purposive sampling of experts. Despite the multidisciplinary nature of our workshop, we lacked for instance the patient perspective due to limitation to the number of participants. However, the number of group members was sufficient to render reliable composite judgements, and in larger groups, members might actually be reluctant to express views (Citation86,Citation87).
In this paper, we presented selected studies to exemplify methodological choices necessary in this field. Similarly, not all studies and developments in this field could be discussed in depth; we limited ourselves to examples that were most relevant to clinical care.
Conclusion
Overall, we see that complexity is a main underlying theme: multimorbidity presents a complexity of conditions leading to more complex individual needs and subsequently complex interventions. In addition, interventions have to be complex to change appropriately the actions of the healthcare team. Moreover, flexible study designs, outcome parameters, and evaluation strategies are needed to account for this complexity.
ACKNOWLEDGEMENTS
The authors acknowledge the input of Ferdinand M. Gerlach, Mareike Leifermann, Anne Namyst and Anja Paesel to the workshop as contributors and thank Gisela Kassner and Beate Braungart for their organizational and administrative support of the workshop; and Phillip Elliott for the final review of the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The workshop was possible thanks to the financial support of the Association of Friends and Patrons of the Johann Wolfgang Goethe University.
References
- Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3:223–8.
- Glynn LG, Valderas JM, Healy P, Burke E, Newell J, Gillespie P, et al. The prevalence of multimorbidity in primary care and its effect on health care utilization and cost. Fam Pract. 2011;28:516–23.
- van den Akker M, Buntinx F, Metsemakers JF, Roos S, Knottnerus JA. Multimorbidity in general practice: Prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J Clin Epidemiol. 1998;51:367–75.
- Fialova D, Topinkova E, Gambassi G, Finne-Soveri H, Jonsson PV, Carpenter I, et al. Potentially inappropriate medication use among elderly home care patients in Europe. J Am Med Assoc. 2005;293:1348–58.
- Field TS, Gurwitz JH, Harrold LR, Rothschild J, DeBellis KR, Seger AC, et al. Risk factors for adverse drug events among older adults in the ambulatory setting. J Am Geriatr Soc. 2004;52:1349–54.
- Kuijpers MA, van Marum RJ, Egberts AC, Jansen PA. Relationship between polypharmacy and underprescribing. Br J Clin Pharmacol. 2008;65:130–3.
- Steinman MA, Landefeld CS, Rosenthal GE, Berthenthal D, Sen S, Kaboli PJ. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516–23.
- Campbell-Scherer D. Multimorbidity: A challenge for evidence-based medicine. Evid Based Med. 2010;15:165–6.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. J Am Med Assoc. 2005;294:716–24.
- Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351:2870–4.
- Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu AL, Maltais D. Multimorbidity and quality of life in primary care: A systematic review. Health Qual Life Outcomes. 2004;2:51.
- Fortin M, Bravo G, Hudon C, Lapointe L, Almirall J, Dubois MF, et al. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual Life Res. 2006; 15:83–91.
- Fortin M, Bravo G, Hudon C, Lapointe L, Dubois M-F, Almirall J. Psychological distress and multimorbidity in primary care. Ann Fam Med. 2006;4:417–22.
- Glynn LG, Buckley B, Reddan D, Newell J, Hinde J, Dinneen SF, et al. Multimorbidity and risk among patients with established cardiovascular disease: A cohort study. Br J Gen Pract. 2008;58:488–94.
- Thorpe KE, Howard DH. The rise in spending among Medicare beneficiaries: The role of chronic disease prevalence and changes in treatment intensity. Health Aff (Millwood). 2006;25:w378–88.
- Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–76.
- Heath I, Rubinstein A, Stange KC, van Driel ML. Quality in primary health care: a multidimensional approach to complexity. Br Med J. 2009;338:b1242.
- Plsek PE, Greenhalgh T. Complexity science: The challenge of complexity in health care. Br Med J. 2001;323:625–8.
- Bodenheimer TS, Berry-Millett R. Care management of patients with complex health care needs. Synth Proj Res Synth Rep. 2012;pii:52372.
- Valderas JM, Mercer SW, Fortin M. Research on patients with multiple health conditions: Different constructs, different views, one voice. J Comorbidity. 2011;1:1–3.
- Arksey H, O’Malley L. Scoping studies: Towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32.
- Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: Toward a more uniform methodology. Ann Fam Med. 2012;10:142–51.
- Valderas JM, Glynn LG, Ferrer-Menduina X, Johnson R, Salisbury C. Multi-morbidity, rule or exception? A systematic review and meta-regression of prevalence studies of multi-morbidity. Fam Med. 2011;43(Suppl. 1). Available at http://www.stfm.org/fmsup/napcrg/fmconferencesupplement.cfm?confid = 135 (accessed 3 June 2013).
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
- Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–6.
- von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203.
- Freitag M, Quinzler R, Beyer M, Dahlhaus A, Doering A, Freund T, et al. Diagnosenselektion für einen neuen medikationsbasierten. Chronic Disease Score (BMBF-FZ: 01ET1004B). Available at http://www.egms.de/static/en/meetings/fom2011/11fom095.shtml (accessed 3 June 2013).
- Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. 2009;7:357–63.
- van den Akker M, Vos R, Knottnerus JA. In an exploratory prospective study on multimorbidity general and disease-related susceptibility could be distinguished. J Clin Epidemiol. 2006;59:934–9.
- van Weel C, Schellevis FG. Comorbidity and guidelines: Conflicting interests. Lancet 2006;367:550–1.
- Lanzer P, Zuehlke H, Jehle P, Silber RE. Cardiovascular multimorbidity, emerging coalescence of the integrated panvascular approach. Z Kardiol. 2004;93:259–65.
- Glynn LG, Reddan D, Newell J, Hinde J, Buckley B, Murphy AW. Chronic kidney disease and mortality and morbidity among patients with established cardiovascular disease: A West of Ireland community-based cohort study. Nephrol Dial Transplant 2007;22:2586–94.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34.
- Mather HM, Chaturvedi N, Fuller JH. Mortality and morbidity from diabetes in South Asians and Europeans: 11-year follow-up of the Southall Diabetes Survey, London, UK. Diabet Med. 1998;15:53–9.
- Miettinen H, Lehto S, Salomaa V, Mahonen M, Niemela M, Haffner SM, et al. Impact of diabetes on mortality after the first myocardial infarction. The FINMONICA Myocardial Infarction Register Study Group. Diabetes Care 1998;21:69–75.
- Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM. Cause-specific mortality in a population with diabetes: South Tees Diabetes Mortality Study. Diabetes Care 2002;25:43–8.
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847.
- Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database of Syst Rev. 2012:CD006560.
- Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011:CD001800.
- Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011:CD000333.
- Kanning M, Schlicht W. A bio-psycho-social model of successful aging as shown through the variable ‘physical activity’. Eur Rev Aging Phys Act. 2008;5:79–87.
- Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database of Syst Rev. 2009:CD00432009.
- Harkness E, Macdonald W, Valderas J, Coventry P, Gask L, Bower P. Identifying psychosocial interventions that improve both physical and mental health in patients with diabetes: A systematic review and meta-analysis. Diabetes Care. 2010;33:926–30.
- Cooper A, O’Flynn N. Risk assessment and lipid modification for primary and secondary prevention of cardiovascular disease: Summary of NICE guidance. Br Med J. 2008;336:1246–8.
- Sanz G, Fuster V. Fixed-dose combination therapy and secondary cardiovascular prevention: Rationale, selection of drugs and target population. Nat Clin Pract Cardiovasc Med. 2009;6:101–10.
- Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care 2005;11:478–88.
- Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: Translating evidence into action. Health Aff (Millwood). 2004;20:64–78.
- Morris RL, Sanders C, Kennedy AP, Rogers A. Shifting priorities in multimorbidity: A longitudinal qualitative study of patient’s prioritization of multiple conditions. Chronic Illn. 2011;7:147–61.
- Freund T, Wensing M, Mahler C, Gensichen J, Erler A, Beyer M, et al. Development of a primary care-based complex care management intervention for chronically ill patients at high risk for hospitalization: A study protocol. Implement Sci. 2010;570.
- Campbell SE, Seymour DG, Primrose WR, Lynch JE, Dunstan E, Espallargues M, et al. A multi-centre European study of factors affecting the discharge destination of older people admitted to hospital: Analysis of in-hospital data from the ACMEplus project. Age Ageing. 2005;34:467–75.
- Hanlon JT, Schmader KE, Ruby CM, Weinberger M. Suboptimal prescribing in older inpatients and outpatients. J Am Geriatr Soc. 2001;49:209.
- Thomson LA, Winterstein AG, Sondergaard B, Haugbolle LS, Melander A. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007;41:1411–26.
- Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: A prospective analysis of 3695 patient-episodes. PLoS One 2009;4:e4439.
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. Br Med J. 2004; 329:15–9.
- Crooks J. Rational therapeutics in the elderly. J Chronic Dis. 1983;36:59–65.
- Steinman MA, Hanlon JT. Managing medications in clinically complex elders: ‘There’s got to be a happy medium’. J Am Med Assoc. 2010;304:1592–601.
- Vestal RE. Drug use in the elderly: A review of problems and special considerations. Drugs 1978;16:358–82.
- Vestal RE. Aging and pharmacology. Cancer 1997;80: 1302–10.
- Schaeffer D, Mueller-Mundt G, Haslbeck J. Bewältigung komplexer Medikamentenregime bei chronischen Erkrankungen—Herausforderungen aus Sicht der Gesundheitsprofessionen. Veroeffentlichungsreihe des Instituts für Pflegewissenschaft an der Universität Bielefeld (IPW), P07–134 2007. Avaliable at http://www.uni-bielefeld.de/gesundhw/ag6/downloads/ipw-134.pdf (accessed 3 June 2013).
- Stevenson FA, Cox K, Britten N, Dundar Y. A systematic review of the research on communication between patients and health care professionals about medicines: The consequences for concordance. Health Expect. 2004;7:235–45.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: The new Medical Research Council guidance. Br Med J. 2008; 337:a1655.
- Perera R, Heneghan C, Yudkin P. Graphical method for depicting randomised trials of complex interventions. Br Med J. 2007;334:127–9.
- Yudkin P, Perera R, Glasziou P. Complexity of the intervention, in randomized clinical trials of nonpharmacological treatments. In: Boutron I, Ravaud P, Moher D, editors. Randomized clinical trials of nonpharmacological treatments. Boca Raton: CRC Press; Taylor & Francis Group; 2012. pp. 27–42.
- Mollenkopf H, Walker A. Quality of life in old age - international and multi-disciplinary perspectives. Social Indicators Research Series, 2007.
- Lawton MP, Moss M, Hoffman C, Kleban MH, Ruckdeschel K, Winter L. Valuation of life: A concept and a scale. J Aging Health 2001;13:3–31.
- Meropol NJ, Egleston BL, Buzaglo JS, Benson AB, III, Cegala DJ, Diefenbach MA, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113:3459–66.
- Vernooij-Dassen MJ, Osse BH, Schade E, Grol RP. Patient autonomy problems in palliative care: Systematic development and evaluation of a questionnaire. J Pain Symptom Manage. 2005;30:264–70.
- Hills P, Argyle M. The Oxford happiness questionnaire: A compact scale for the measurement of psychological well-being. Pers Individ Dif. 2002;33:1073–82.
- Gensichen J, Serras A, Paulitsch MA, Rosemann T, Koenig J, Gerlach FM, et al. The patient assessment of chronic illness care questionnaire: Evaluation in patients with mental disorders in primary care. Community Ment Health J. 2011;47:447–53.
- Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the patient assessment of chronic illness care (PACIC). Med Care. 2005;43: 436–44.
- Goetz K, Freund T, Gensichen J, Miksch A, Szecsenyi J, Steinhaeuser J. Adaptation and psychometric properties of the PACIC short form. Am J Manag Care 2012;18:e55–60.
- Hickey A, Barker M, McGee H, O’Boyle C. Measuring health-related quality of life in older patient populations: A review of current approaches. Pharmacoeconomics. 2005; 23:971–93.
- Power M, Quinn K, Schmidt S. Development of the WHOQOL-old module. Qual Life Res. 2005;14:2197–214.
- Baltes PB. On the incomplete architecture of human ontogeny. Selection, optimization, and compensation as foundation of developmental theory. Am Psychol. 1997;52:366–80.
- Lawton MP. Three functions of the residential environment. In: Pastalan L, Cowart M, editors. Lifestyle and housing of older adults: The Florida experience. New York: The Haworth Press; 1989. pp. 39–50.
- Wahl H-W, Mollenkopf H, Oswald F, Claus C. Environmental aspects of quality of life in old age: Conceptual and empirical issues. In: Mollenkopf H, Walker A, editors. Quality of life in old age—international and multidisciplinary perspectives. Dordrecht: Springer; 2007. pp. 101–22.
- Wahl H-W, Oswald F. Environmental perspectives on aging. In: Dannefer D, Phillipson, C, editors. International handbook of social gerontology. London: London; 2010. pp. 111–24.
- Wahl HW, Iwarsson S, Oswald F. Aging well and the environment: Toward an integrative model and research agenda for the future. Gerontologist. 2012;52:306–16.
- Hanlon JT, Schmader KE, Samsa GP, Weinberger M, Uttech KM, Lewis IK, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045–51.
- Bregnhoj L, Thirstrup S, Kristensen MB, Bjerrum L, Sonne J. Combined intervention programme reduces inappropriate prescribing in elderly patients exposed to polypharmacy in primary care. Eur J Clin Pharmacol. 2009;65:199–207.
- Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100:428–37.
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82.
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry 2002;59:877–83.
- Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010;19:237–70.
- Kernick D. A theoretical framework for multimorbidity: From complicated to chaotic. Br J Gen Pract. 2012;62:659–62.
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Marteau T. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2:188.
- Walker AE, McLeer SK. Small group processes relevant to data monitoring committees in controlled clinical trials: An overview of reviews. Clin Trials 2004;1:282–96.